Below is the uncorrected machine-read text of this chapter, intended to provide our own search engines and external engines with highly rich, chapter-representative searchable text of each book. Because it is UNCORRECTED material, please consider the following text as a useful but insufficient proxy for the authoritative book pages.
TV SOURCES AND CHARACTERIZATION OF INDOOR POLLUTION This chapter addresses several chemical pollutants with respect to their sources, concentrations, and indoor-outdoor relationships. In addition, with the aim of characterizing the general quality of the indoor environment, it considers temperature, humidity, unwanted sound, and electromagnetic radiation, such as the radiofrequency, infrared, ~risible, ultraviolet, and x-ray portions of the spectrum. In the case of some pollutants, information on health effects is scanty, at bent. To the extent possible, the health effects of such pollutants are discussed here. Detailed discussion of the health effects of other pollutants, on which more information is available, is to be found in Chapter VII. Radioactivity and formaldehyde emitted indoors from building product. are discussed in the first two sections of this chapter. Consumer products, a generic Source of indoor pollutants of many types, are discussed next. The chapter proceeds with sections on asbestos and fibrous glass {which occur in different forms in many indoor environments), combustion processes (especially of unrented cooking and heating appliances), and tobacco smoke {a hiahlv complex and ublaultous mixture of pollutants). Several indoor air pollutants can be _ . . _ _ , _ _ recognized by their odors. Such odors are often the first indications of deterioration in air quality and may themselves affect people's well-being adversely; hence, they are treated as a distinct category of pollutant in this chapter. Air temperature, radiant temperature, and air velocity and humidity affect the quality of the indoor environment through physiologic and sensory responses, so the thermal environment is also discussed in a separate section. Other physical factors of the indoor environment, such as noise and electromagnetic radiation, are d iscussed briefly in a f inal section. The diversity of subjects discussed in this chapter is evident. Some of the pollutants considered here may be associated with ~roluntery behavioral patterns, such as tobacc~o-amoicing, whereas others may be related to involuntary and unavoidable exposure, such as exposure to substances emitted from building materials. me reader should not infer any order of priority among the pollutants discussed here. An effort to attach priorities would require judgment" on exposures and effects, 57
So and the order of discussion is not intended to indicate the application o f such j udgment . RADIOACTIVITY INTRODUCTION Radioactivity and ionizing radiation occur naturally throughout the biosphere, bath because of the presence of primordial radioactive elements and their decay products in the earth and because of natural processes (primarily cosmic radiation) that produce radionuclides or direct radiation fields. These natural sources expose humans to radiation both outdoors and in buildings. The magnitudes of various contributions to total radiation dose vary from place to place and between outdoors and indoors, and the type of radiation dose depends on the radiation source. At one extreme, the coemic-radiation field delivers a dose to the entire body; this dose is not affected greatly by the presence of a building and may be characterized prissily on the basis of altitude. At the other extreme, airborne alpha-emitting radionuclides may deliver doses specifically to the lungs, and their concentrations indoors may be strongly affected by the nature of building materials and other sources, such as soil and water, and by building operations, such as ventilation. As an intermediate case, the gamma-radiation field arising from radionuclides that are fixed in place typically exposes the whole body and is affected by radionuclide concentration, proximity, and shielding. . In the discussion that follows, we refer to radioactivity concentrations and radiation fields and, by inference, to radiation doses from sources that are.inside and outside the body. Radioactivity is given in curies' 1 Ct - 3.7 x 101° becquerele, so 1 psi ~ 0.037 Bq. Radiation fields can be specified in terms of energy flux' but it is more conventional in the present context to use units of dose rate, in which case the type of radiation has to be indicated. We use the red as the unit of (absorbed) dose when specify5ing q~-radiation fields (1 red - 0.01 J/kg, so 1 mead ~ 1 x 10~ J/kg). For gamma doses, the dose in reds is numerically equal to the dose equi~raler~- (D!:) in rema. A distinction must be drawn between the Tissue dose,. that actually received by tissue and therefore including self-shielding by the body, and the fair dose,. that deposited in air in the space under consideration. It is useful to atomize the dose-rate contribution in the United States from radiation arising outside buildings. Three recent summaries are those of the National Council on Radiation Protection and asurements34 and the U.N. Scientific Committee on the Effects of Atomic Radiation,.' which depended heavily on Oakley38 for U.S. data, and the 1980 BEIR report of the National Research Council. Is External radiation, that arising from sources outside the body, may be divided into two categories, cosmic and terrestrial. The average tissue dose rate outdoors from cosmic radiation is approximately 28 mrads/yr; the dose rate indoor e is slightly reduced by overhead
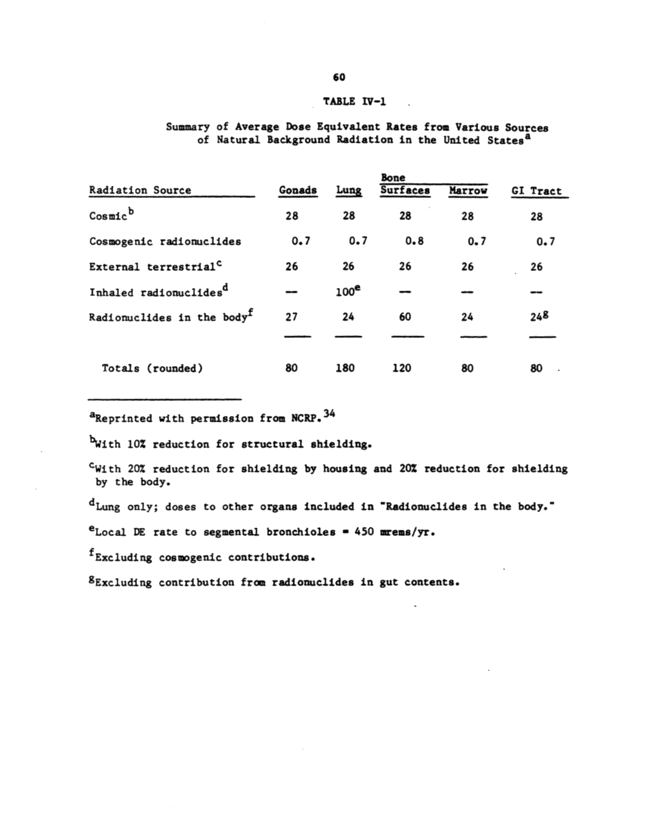
as shielding (the NCRP report amounted a 10% reduction in average exposures). This contribution has a substantial altitude dependence, increasing from about 26 mrads/yr at sea level to about 50 mrads/yr at 1,600 a, the altitude of Denver. The average outdoor population- weighted tissue dose rate from terrestrial radionuclides--due principally to gamma rays from potassium-40, the thorium-232 series, and the uranium-238 series--is approximately 35 mrad~/yr. This dose rate varies substantially because of geographic variations in the distribution of these radionuclides. For estimating average terrestrial dose rates, the NCRP assumed that indoor done rates were 20% lower than outdoor rates. (It also assumed that the tissue dose was 20% less than the air dose.) Internal radionuclides contribute important beta and gamma doses (about 15 mrads/yr to cast of the body, primarily from potassium-40) and an important alpha dose (even if that to the lungs from radon and its progeny is excluded). The alpha dose arises primarily from internally deposited uranium-238 and -234, radium-226 and -228, and polonium-210 and varies greatly with body organ. One of the larger contributions, about 3 mrads/yr, is the polonium-210 alpha dose to the cells lining the bone surfaces. However, alpha particles have a greater biologic effectiveness than gamma rays, so the absorbed alpha dose contributes a DE some 10 times greater than that of the same (absorbed) dose of gamma radiation. Table IV-1 shows estimate. of various contributions to DE rates, in millirems per year, which are numerically equal to tissue dose rates (in millirads per year) for gamma and beta radiation. For alpha radiation, a quality factor of 10 was assumed (based on relative biologic effectiveness), although 20 is now recommended.' The value given for lung dose from inhaled radionuclides assumed a radon-222 concentration in air of 0.15 nCi/~3 (and slightly less than equilibrium amounts of its radioactive decay products, or progeny). The resulting DE has the largest value in the table. Nonetheless, this value appears more appropriate for outdoor than for indoor air, in which higher radon concentrations are found. All indoor dose rates from natural radiation sources are affected by buildings, and those from inhaled radionuclides are affected most strongly. The only natural airborne radionuclides of importance are radon and its progeny, principally the series beginning with radon-222, the alpha-decay product of radium-226 (a member of the uranium-238 series). Radon is a noble gas that can move from the site of its formation, giving it a substantial opportunity to reach air that is inhaled by humans. The short-lived decay products of radon--polonium, . . . . . ~ . . . . . _ ~ ~ · _ lead, and b~mutn--are chemically active ana thus can De co~ec~ea An the lungs, either directly or through particle" to which they attach. The most important dose arises from alpha decay of the polonium isotopes. The decay sequence beginning with radium-226 is shown in Figure IV-1, and, from the biomedical point of view, effectively.ends with lead-210, because of its half-life of about 20 yr. Because the alpha energy associated with decays of the short-lived products to lead-210 poses the main risk, progeny concentrations are often expressed as the associated potential alpha-energy concentration. (PAEC) in air. The unit conventionally used for PAEC is the working
60 TABLE IV-1 Summary of Average Dose }equivalent Rates from Various Sources of Natural Background Radiation in the limited Statesa Bone Radiation Source Gonads Lung Surfaces Marrow GI Tract Cosmleb 28 28 28 28 28 Cosmogenic radiormclides 0.7 0. 7 0.8 0.7 0.7 External terrestrials 26 26 26 26 26 Inhaled radionuclideed 1 OOe ~ Radionuclides in the bodyf 27 24 60 24 248 Tombs ~ rounded ~ 80 IS0 120 80 80 aReprinted with permission from NCRP.34 blfith 10: reduction for structural shielding. CWith 20X reduction for shielding by housing and 20X reduction for ahielting by the body. d Lung only; doses to other organs included in "Radionuclides in the body. eLocal DO rate to segmental bronchioles - 450 areme/yr. fExcluding cos~geni~ contribution. "Excluding contribution from ra~itomclides in gut contents.
61 s~u lull ~SY 1~r 2 "eV - Pa '.~lUX,' 1.2 ~ 2 3 ~V '..U lUtIl ~ 25Y tO., .,'_ 4J - eV , ' ·'~h ~X,) 24 d 02.01 ~V "~h Ib} B0 a 106' 4e _ 47 - V . ~ ~ 1 1 ~1 r ~JC" 1500 ~ 48 - V sesnn 382d SS - V , ·~Po (~) "-o ~' 3.0S~ 16 >< 10~s 60 ~V _7 ~ ~Y . t.~s, lC) ~ '. ,. 19? 04 3.3 - Y , "~~ IRaS)' "` tl" 268 mm ~t ~ 07. ~O - V CO1 ~V "~' I~E,' 50d ~1 2 - eV 86 - o l~bF) 138 d ~S 3 - eV '~ teaG) | s~ 1 . FIGllRE: IV-} Principal decay scheme of uranium-238 to radon-222 to lead-206, showing alpha ar~d beta decay; decay energies in millions of electron volts. Reprinted with permi,'lon {r~om National Council on Radiation Protection and Measurements. P-
62 level (WL), defined as 1.3 x 105 MeV/L, the PANIC if radon-222 at 100 nCi/~3 is present with equilibrium amounts of its progeny. Done (and DE) rates may be inferred from the PAEC on the basis of relatively complicated modeling, provided that the progeny particle size distribution and other factors are prescribed. The character of a building may affect occupant radiation exposure in three principal ways: the building serves as a container for indoor~generated radon and its associated progeny, whether from building materials, underlying soil, or water and Gas; the building materials contain natural gamm=-emitters (potassium-40, the thori~232 series, and the uranium-238 series); and the building shields occupants from cosmic or external terrestrial radiation. The last two effect" tend to cancel one another. The building structure may, in unusual circumstances, also protect occupants from outdoor radon-urooenY . _ ~ _ ~ _ _, _ _ _, concentrations. However, the indoor concentration 18 ordinarily larger than the outdoor, and outdoor-generated radon usually contributes a "mall additive term to indoor concentrations. If this term is ignored, the steady-state indoor radon concentration for a f iced indoor radon source strength is inversely proportional to the air-exchange rate, the rate at which the indoor air is exchanged for outdoor air. The air-exchange rate for most U.S. buildings is around 1/h, with O.S/h to 1 . 5/h typical for residences (windows closed) . The air-exchange rate and other removal mechanisms also affect the ratios of radon-progeny concentration to radon concentration. Lack of removal implies activity ratios of 1, but substantially lower values have been observed. An equilibrium factor (F} is often defined as the ratio of the actual Pm3C to the PAE;C that would be associated with a specific radon concentration if the progeny were in equilibrium with this concentration. This section characterizes indoor airborne radionuclides and radiation, su~arizes measurements of actual concentrations or radiation f ields, briefly Indicates con~crol measures, and suggests subjects for further research. The major emphasis is on radon and its progeny. The radionuclides in this decay chain, even at typical outdoor concentrations, cause larger radiation doses to internal organs than all other airborne radionuclides. Furthermore, the radon and progeny concentration"may be substantially higher indoor-, particularly in building with low air-exchange rates. In addition. building oc`:upants receive external whole-body-radiation from radionuclides fixed in building materials and soil, and these doses are also given subetantial treatment. This radiation arises principally from several primordial radionuclides--potassium-40 and Sobers of the thorium-232 and uranium-238 decay series--with concentrations of around 0.1 pCi/g or greater in rocks, soil, and derivative building materials. There are also the decay chains in which radon-220, radon-222, and their progeny occur. \
63 SC}URCES OF RADICXlilCLIDES AT RADIATION Building Mater ials Radionuclide Content. Few measurements and no wide~scale surveys of the radionuclide content of U.S. building materials have been made. Surveys of materials in Europe are summarized in UltSCEAR 1977, AMP. 50) which gives activity concentrations of potassium-40, radium-226, and thorium-232. As examples, average values for the concrete Ample groups examined range from 0.9 to 2.0 pCi/g for radium-226, 0.8 to 2.3 pCi/g for thoriu~n-232, and 9 to 19 pCi/g for potassium-40. By comparison, the ranges for brick are about 50% higher; those for cement are similar, except for potas~ium-40 (which is SO. less); and those for natural plaster are lower by about a factor of 5. Available U.S. data {Table IV-2) show concentrations in the same range, assuming that the series radionuclides are sufficiently close to equilibrium to permit comparison. In a number of cares, U.S. workers have examined the radionuclide contents of concrete in the course of selec~cing materials for low-background facilities for use in radiation- counting; 2' the values obtained are consistent with the European da=, although somewhat lower. The observed concentrations are also within the range of values typical for major rock types and "oils. Concentrations for building materials not derived from crustal components, such as wood, are much lower . Measurement programs have recently been initiated to characterize the radionucl~de contents of building mater ials as a basis for understanding the resulting effect on the indoor radiation environment. Kahn et al.25 have reported measurements of concentrations in various building material" in the Atlanta area; potassi~-40, radium-226 progeny, and thorium-232 progeny concentrations for samples of concrete, brick, and tile are given in Table TV-2. Lawrence Berkeley Laboratory has begun to survey concretes and other materials as part of a program on indoor air quality; radionuclide contents for concrete and rock-bed samples from a number of areas are given in the table. I' Considerably greater radionuclide concentrations may be found in building materials that contain residues from industrial processed. The principal example of such materials in the United States in concrete blocks incorporating phosphate slag Sequentially calcium silicate), a byproduct of phosphate production. As discussed by Roessler et al.,.2 this slag contains most of the radium-226 and uranium~238 found in the phosphate ore. For the electric furnace Process used in Florida, concentrations in the ore are about 60 pCi/g, and the slag has similar concentrations. A plane In A'zioame Musing Florida and Tennessee phosphate ores) sold slag to companies in Alabama, Mississippi, Tennessee, Georgia, and Kentucky. The concrete produced by these companies has radium-226 concentrations estimated. and in some cares measured, to be about 20 pCi/g.25 Phosphogypaum (essentially calcium sulfate produced by treatment of phosphate ores with sulfuric acid) may also be used for building materials, particularly wallboard. In this treatment, radium-226 follows the
6. 0 c ~ ~2 v A: so of l ~ 0 ~ ~ v ~ ~ o ~ In . 1 0 Jo c ~ 0 ~ o ~ As ~ ~ ~ ~ ~ a, of ~ o e ~ ~ 0 ~ ~ a: C ~a 0 ~ ~ a so ~ ~ ~ O b MU U: 0 b ~ O V ~ a 1 a o. C C ~ ,~ 4~ ~,4 0 ~ ~ ~ ~ ~ 0 _' a,' ~ ~ ~ ~ 0 ~ 0 ~ oo 5: ~ ~ ~ ~ ~ ~ ~ ~ O^ N 1 ~ Jo ~ ~ 0 ~ ~ 0 .1 ~ V ~ '0 ~ ~ K TIC C C ~ _ 3 ~ G C. ~ :' ~ V V ~ al ~ ~ 0 In e a: ~ z z ~ ~ v ~ ·- 0 e~ _~ 0 ~ ~ ~ ° ~ ~ · (U ~ D `0 ~ ~ :^ V ~ ~ e 0 0 ~ 0 ~ ~= · ~ ~ ~ · ~ ; ~ ~ I ~ I a) ~J ~ h · · · O O :> · L O a 0 E :^ x ~ a' 0 0 ~ 0 . . · .c _1 ~ 0 ~ _' ~ ~ ~ 3 0 ~i ~ ~ I ~ ~ ~ 0 ~ 0 . ~ D · O O ^ O ~ O o ~ ~ s O ~ 0 :: D b a~ ~r, ~ .~ ~ S C~J ~~~ -~U O +e V ~ ~ V ~ 0 O tV ~J ~ (tJ O CL C~ ~ ~ ~ ~ ~ ~ dV C~ ~ ~ ~ ~ ~ ~ ~ C ~ ~ ~ ~ C ~ ~ . - O O ~ ~ O ~ O u3 0 en c~ o e' o ' O
65 calcium, leading to tens of picocuries per grue in the gypsum but such gypsum has not been used on ~ large scale in U.S. wallboard. In contrast, concrete that incorporates phosphate slag may have been used in approximately 100,000 homes.2S Finally, awe fly ash from coal-fired power plants has been used in cement production, and tote use may continue. Heretofore, it has not been thought to contribute substantially to the radionuclide content of the resulting building material. IS Bnanation measurements on fly-ash concretes are now being performed at Lawrence Berkeley Laboratory. Radon Emanation. The effective radon-222 ser~eration rate in building ma~ceriale depends on the radi~-226 content, which varies widely, and on tbe percentage of radon formed that does not rain lodged in the matrix of the material. Radon that is not fixed in place may mc've through the matrix by diffusion or, if the material contain e large air spaces, by convection. Diffusive movement depends on the diffusion length of the ~nateris1 in question and on its thickness. The extent to which these processes occur depends not only on the material ' s characteristics, but also on environmental conditione-- pressure, temperature, and moisture content. A rule of thumb Wartimes cited {e.g., UNSCEAR.~) is that 1% of the radon-222 generated from materials in walls and ceilings escapee into the adjacent air space. However, recent measurements at Lawrence Berkeley "bora~cory and elsewhere have indicated that a considerably higher fraction can escape, e.g., from concrete. Ingersoll et al. cited eacape-to- production ratios of 0.08-0.25 for radon-222 from concrete. (Radionuclide contents for the sample groups examined are indicated in Table IV-2. ) Of most direct interest for indoor sir quality is the actual emanation rate, often given as picocuries per square meter per second and sometimes as picocuries per gram per second. Measurements for various materials give emanation rates over a wide range. For exe ~ le. Euro Ian gypsum board and bricks yield radon-222 at about 0.3 x 10- pCi/m -a, whereas rates for European concretes range from 0.001 to 0.2 pCi/m2-~.2. 32 Preliminary measurements of radon-222 emanation rate per unit mast for sample groups of concrete from U.S. metropolitan areas (Table IV-2) give averages that range from 0.4 to 1.2 pCi/kg-h (0.8 pCi/lcg-h yields approximately 0.03 pCi/~-s for O.l-~thick concrete). Several rock samples from solar-beat storage beds averaged 0.5 pCi/kg-h, although radium-226 contents were considerably higher t ban those for the concrete samples. I' The resulting indoor radon-222 concentrations depend on the amount of such material in the structure, the interior volume, and the air~exabange rate. For an air-exchange rate of lih and a ratio of indoor emanating surface to indoor volume of 0.5 m~/m3, an emanation rate of 0.03 pCi/m2-e corresponds to a radon-222 concentration of about 0.04 nCi/m3. If the equilibrium factor is 0.5, this would yield a PANIC of about 0.0002 WL. Direct measurement of emanation rates of materials made with industrial byproducts (such ens phosphate-alag concrete is underway. but results are not available. Because these materials may contain 20 times a. much radion-226 as a typical concrete, radon-222 contributions
66 of up to several nanocuries per cubic - ter of radon-222 and a corresponding increase in the PANIC could be expected if the same emanation ratio pertains. Measurements of emanation rate vary by more than an order of magnitude, I' no it is difficult to use radium content to predict the contribution of a particular material to indoor radon content. For this reason, more comprehensive information on diffusible fraction, diffusion length, etc., and their dependence on material or environmental factors is required before we can characterize building materials on the basis of radionuclide content. If this information becomes available, radionuclide Contents may then be helpful in characterizing indoor concentrations on a broad scale, e.g., by geographic area. Ilowever, the dependence of diffusion and emanation r ates on environmental factors, such as pressure and temperature, and on the Moisture content of the material may limit the possibility for such characterization. In some cases, radon-220 (.thoron.) and its progeny, ordinarily present at much lower concentrations than radon-222 and its progeny, may assume importance, particularly when mechanisms exist for transporting emanating radon-220 rapidly into the air space of interest. In comparison with the half-life of radon-222, the much shorter half-life of thoron, 55 s, caches the measured radioactivity in curies to be a characteristic of secondary interest. However, the PAEC still gives a relatively direct indication of possible dose to the lung. One WL of radon-222 progeny has the same PAEC as that associated with progeny in eguilibrium with thoron at 7 nCi/~3. To the extent that uranium-238 and thorium-232, which have similar half-lives, have similar activities In source materials, the PAEC from their progeny, radon-220 and -222, can reach similar values if rapid transport mechanisms exist. This may occur, for example, in solar buildings that sweep air through rock or concrete thermal-atorage beds. A few efforts have begun to measure thoron emanation rates, but results are not yet available. Gamma Radiation. The energies and intensities of photons f rom decay of natural radionuclide. have been well characterized. The external dose from radionuclides in building materials is due to the gamma rays emitted and depends on the geometry of the structure and attenuation by the materials, as well as the gaama-ray energies. A simple expression may be derived for the -ray air dose in a hole in an infinite uniform medium:25 X',,, ~ (2.43 Vrad/h) (1i;UCU + EThcq~h + EKCK} . where Cu. CTh, and CR are the concentrations (in picocuries per g ram) of uraniu~238 and it's progeny, thor lum-232 and its progeny, and potassium-40, respectively, and Eu, ETh, and E': are the average ga~a-ray energies per disintegration of the same radionuclides (including disintegration of the progeny for the uranium and thorium -eeriest. Used Eu ~ 1.72 Mev, E',h ~ 2.36 MeV, and ER a 0.156 MeV, 25 t" ~ 4 ECU + 5.7CTh + 0.38CR, in microrads per
67 hour. The stated dose contributions from the uranium and thorium series are slightly less than those cited elsewhere. e.g., by Krisiuk et al., 27 who may have used older information on decay schemes. For the radionuclide contents cited in Table IV-2, the three terms in the expression for to contribute comparable amount". (An analogous expression for the dose from a flat plane is cited in the section on soil. ~ For an actual structure, the geometry is complex and Salaried; in addition, the building materials may attenuate the external radiation dose from other sources. Moreover, radon-222 and its progeny may be present in the material at less ton equilibrium values, thereby decreasing the corresponding gamoa-ray dose. The radon-222 escape-to~production ratio is most often in the range of low to 0.25, causing a small reduction in the value of X. The effects of geometry and attenuation cannot be so simply characterized. Dose-rate expressions from various workers, pertaining to a variety of structures, have been summarized.3' Some of these expressions account for reduction of the dose rate from outdoor sources. Moeller _ al.' described a computer program suitable for analysis of varied geometries. The infinite-geometry case yields air dose rates of about 8 wads/in for a potassiumr40 concentration of 8 pCi/g and uranium-238 and thorium-232 series concentrations of 0.5 pCi/g. An infinitely thick slab of such material would contribute about half thin dose rate at its surface. As discussed earlier, ~ typical outdoor tissue dose rate from terrestrial radionuclides is 35 mrads/yr or 4 prads/h. {Owing to shielding by the body, the tissue dose rate is about 20% less than the air dose rate.} Soil and Groundwater Radionuclide Content. Radionuclide concentrations of major rock types and soil have been summarized.'. U.S. soil values of 0.6, 1.0, and 12 pCi/g have been stated for uraniu~-238, thorium-232. and potasstum-40, respectively, on the basis of 200 measurements of g~mm^-ray dose rate cited by Lowder et al.'° These values vary by a factor of around 3 from place to place. Values for crustal rocks'. typically lie within this Dame range, but are considerably higher for some formations. For example, the phosphate rocks of Florida contain the uranium-238 Series at tens of picocuries per gram, but normal amounts of thoriu~-232s commercial uranium ore bodies in the United States have uranium-238 concentrations of hundreds of picocur ies per gram and higher. Radon Emanation and Transport. The uranium-238 series, typically presenS in soils and rocks at concentrations of about 1 pCi/g, includes radium-226, the source of radon-222. The actual radon-222 emanation rate from the ground depends, as for building materiels, on the percentage of diffusible radon, diffusion length, and other transport mechanisms (including groundwater) in the soil. A review of available
68 measurements Of radon-222 indicates a mean emanation rate from the soil of 0.42 psi/m -~.~. Given this value for the ground under a one-story house, and assuming that the Elena ted radon finds its way into the indoor air, the soil could account for indoor radon-222 at about 1 nCi/~3 at a typical air~exchange rate of 1/h. Because emanation rates very by at least a factor of 10 from place to place, this potential contribution can also be expected to vary substantialiv Bong u. S . building. . . ~ ~ _, ~ _ _ _,# The soil as ~ source of radon-222 can be characterized directly by emanation measurements or, if disequilibrium and transport mechanisms (including groundwater) are known, indirectly by measurements of members of the uranium-238 series. Because of the relative ease of measuring gamer rays, the indirect methods may be more appropriate for large-scale surveys intended to characterize the contribution of soil radon by geographic area. Gamma-ray source measurements may also be lest sensitive to changes in pressure, temperature, and moisture content than emanation-rate measurements (see UNSCEAR.' and ~ . NC"~. Moreover, variations in emanation rate may correlate with factors that affect air-exchange rates and may thus complicate assessment of the importance of soil as a source of indoor radon. The mechanisms by which radon may be transported into buildings have been studied little. Soil-gas measurements, which have yielded results of 100-2,000 pCi/L (Xraner;26 Scottish and unpublished measurements by Lawrence Berkeley Laboratory), may be relevant to this question, because they may help in characterizing the radon content of air trapped beneath building-. Emanation rates themselves are useful only for placing an upper limit on the potential of soil as an indoor source. However, a more detailed understanding of radon transport in soil could provide a basis for using emanation data to estimate the amount of radon that may accumulate beneath houses and be transported indoors. Such collection and transport mechanisms any be greatly affected by changes in barometric pressure, soil moisture content, temperature gradients, and wind. The actual pathway by which radon enters a building from the soil appears to vary substantially with building design and construction practice. In houses with concrete basements that are closed to the outdoors, radon may enter by diffusion through the basement floor, by convection within basement walls, and by movement through cracks, designed openings, or penetrations in either of these components. Even in communities where numerous measurements have been performed, it has not been possible to determine the relative importance of these mechanisme.i 2 In some mining communities, sealing of cracks has proved relatively successful in reducing indoor radon content, but the effectiveness of this method in general has not been evaluated. The movement of radon from the point of entry to other parts of the building depends on interns1 construction and building use. men in buildings with ventilated crawlepaces, the radon concentration in the crawlapace air may be considerably [higher than outdoors, and ~ substantial amount of Me radon emanating from the soil may reach the interior space by transport from the crawIspace.
69 More comprehensive information on how radon is transported is needed for the development of techniques to prevent radon fro. entering buildings and for establishment of a correlation between the radium-226 content in soil and the indoor radon content attributable to this source. Gamma Radiation. The go dose from radionuclides in sot1 flay be expressed in a fashion analogous to that for building suteriale, the air dose rate (urad/h) at 1 m above the ground due to natural. emitters uniformly distributed in the soil has been given as Xplane - 1.82CU + 2.82CTh + 0.179CR for Cu. CTh, CR in p per gram. ~ More current data on decay schemes May alter this slightly. As noted above, concentrations of natural redionuclides in soil and rock vary from place to place, causing comparable variations in dose rates. The air dose rate is estimated at 2.6 ~rade/h on the coastal plain (the Atlantic and Gulf coastal areas}, 10.2 prade/h on the Colorado Plateau, and 5.2 urade/h on the rest of the contiguous United States {NC",3. based on nuclear-plant site surveys'. The materials in a building can provide significant shielding of occupants from go rays from local radionuclide concentrations, but the radionuclide content of the ~teriale may Ire than compensate for this shielding. Radon from Utilities Water. Measured concentrations of radon-222 in well water in Maine . , ~ and New Hampshire average 53,000 and 101,000 pCi/L, respectively. ~ ' More recent measurements have been performed in Maine. ~. Lawrence Berkeley Laboratory has found concentrations of 100-7, 500 pCi/L in tapwater from wells or underground reacrvoirs associated with houses and has correlated use of such water with increases in indoor radon content. Radon-222 in water can quickly transfer to air, winch efficiencies of 30-90t, depending on water use; ~ ~ a concentration of 10,000 pCi/L can raise average indoor radon-222 content by about 1 nCi/~3. It is not known how widespread such water concentrations are, nor how closely they correlate with high radium content in surface soils and rocks. Natural Gas. Concentrations of radon-222 in natural gas in the Houston area have been found to average approximately 50 pCi/L at STP. I Concentrations in distribution lines at various points in the United States were found to average about 20 pCi/L.22 The resulting concentrations in U.S. residences due to natural~gas combustion have been estimated to be less than 0.1 nCi/m3, even with unrented burners.
70 INDOOR CO~RATIONS AND RADIATION FLUS Airborne Radionuclides Radon Concentrations. Data from several sources tabulated by UNSCEAR.t indicated that indoor radon-222 concentrations vary by two orders of magnitude, with average values of about 1 nCi/~3. Such a large range is not surprising, considering that the studies included various types of buildings, building Materials, underlying materials, and ventilation conditions and used many measurement techniques. More recent measurements have conf irmed this wide variation . A wide variation is expected even for conventional housing, because the air-exchange rate typically ranges from about 0.5/h to I.5/h in such buildings, and further variation in air-exchange rates occurs because of window or door openings and Mechanical ventilation system. The soil under a house can be expected to be the principal contributor to the indoor radon concentration in most cases. As noted earlier, a typical soil emanation rate, if the radon goes into the interior of a bouse with an sir exchange rate of 1/h, would contribute radon-222 at about 1 nCi/~3 . Inasmuch as Soil emanation rates and ef fictive capture by the house vary by an order of magnitude and air-exchange rates vary widely, a large range of indoor concentrations would result. As indicated in Table Tv-3, bomes monitored in New York and New Jersey were found to have an annual average radon-222 concentration of 0.3-3.1 nCi/m3 in the living space, with a geometric mean of about 0.8 nCi/~3.' Similar measurements in Austria yielded a geometric mean of 0.42nCi/m3.~. In these studies the mean indoor concentrations were 3-4 times as great as local outdoor concentrations. Spot measurements of homes in the San Francisco area, made during the surmer with windows closed and with an average air-exchange rate of 0.4/h, showed concentrations averaging 0~3 nCi/m3. Is Spot measurements in Illinois showed a substantis1 incidence of concentrations greater than 5 nCi/m3; six of 22 houses had concentrations of 10 nCi/~3 or ave. .' High radon222 concentrations have been found in urani~mining areas and in buildings that use materials high in radium. In houses monitored in Bancroft, Ontario, 501 of the ample had concentrations greater than 3 nCi/~3, over 25% had concentrations greater than 7 nCi/m3, and about 696 had concentrations greater than 15 nCi/m3. 2 ~ High concentrations have also been found in homes in mining areas in the United State-:. at Grand Junction, Colorado, PAElCe corresponding to radon-222 at up ~~ hundreds of nanocuries per cubic meter have Been measured. In a Prey of several Swedish houses built with alu~shale concrete, the average radon-222 concentration was 7 nCi/m3, .. more recent cats showed average concentrations of 15 nCi/m3 or Ore for residences built entirely of alum shale.47 Radon-222 concentrations of 0.6-22 nCi/m3 have been found during spot n`~urements of energy-efficient homes, many of which had low air-exc-~ange rates, these measurements were taken with windows closed, and the sir-exchange rates were measured simultaneously. i. Concentrations and air~exchange rates have also been measured in
71 U C E O ~ o ~ ~ S ~ ~ V ~ ~ O V 3 V Eli -, V ~ ^ O V V CD ~ O `= ~ ~ o 3 0 ~ O ~ ~ O ~ ~ 0~ L ~ ~ - C C ~ ~ ~ ~ ~ ~ - ~ ~ O ~ Sit 0 A, C ~ ,~ CL.a O ~ _ L _ C 0 ~ tO 3 to C . | ~ h 0 ~ - 4 eC 00 ~ ~ ~ ~ c: ~ ~ ~ ~ :^ ~ ~ V Cal 81 ~ o _ ~ ~ c 0 c 0 · O ~ ~ ~ ~ ~ V ~ to ~ 0 ~ ~ _~ :> ~ O ~ ~ ~ ~ ~ ~ O E^= Cal V 0 C: I: .. ~ · 0 0 0 ~ ~ r_ Z ~ ~ c~ e~ ~ _ 1 ~ ~ c o o {V D . . ~ 00 ^ O O p ° C~ Oe I ~ I O O O -: ~ Q O O I C~ ~ O e e e O O O :~ v O _ C C _ O tJ O Q 00 0 e~ ~ 3 O ~ 0 1 ~ ~ O C D O _' _' I e v ~ t~' ~ ~ ~ 8 ~ ~ ~ ;= J V e e ~ e C ~ E O o ~ O ~ O O y _ O ~ · e e _1 73 0 C-) | e e O O O t ~ ~ L) ~: 1 0 0 `_' _ _ U. ·. ~ ~ O ·0 O dV ~ O ~ ~ _4 ~s: a~ _4 ~ s~ ~ O e :^ 0 ~ ~ 0 c - c5 .5 ~ 73 0 ~ a' ~ ~ _ ~ ~ ~ ~ ~ CC C: O O ~ ~ ' C; ~ O v e 8, 0 ~ 3 c: ~ ~ 1 ~ ~ ~ l~ _ C V (V _~ ~ e V ~ O O ~ C 0 3 Z ~4 ~ ~5 U~ ~ O C~ tIS e CL ~ O ~ ~ Z ~ U~ :;) CJ) C:) CO 0 O S 3 V 1 3 0 Cc) o _ ~ ~0 0 e' ~4
72 c Cal o ~ ~ 0 ~ ~ , 0 N N O ~ ~ A: _ ~ ~ ~ ~ ~ CL -0 ~ b ~ 1U ILl dU 0 HI Cat c e ~ ~ ~ ~ ~ C ~ ~ ~ ~ ~ ~ o. e c ~ 0 c e ~ ~ ~ ~ C v c 0 0 0 ~ ~ ~ ~ 0 0 ~ 0 _ ~ ~ 0 0 v 0 v 0 as ~ ~ c ~ 3 0 , ~ v ~ ~ C 1 _. b b ~ ~ so 0 ~ C ·^ Cot V 0` V L' V ~ 0 ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ a0 C ~ C b C ~ C b £: ~ C eo ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ O ~ O ~ O ~ O ~ O 0 :> V b ~ ~ Va b eo 0 ~ O · 0 O ~ ~ `0 ~ `0 Z" :: ~ ~ 0 D O _. ~ O f: I C~ O ~ ^ ~ ~ O O ~d ~ O _' b e ~ a :^ ~ O ¢: c: _ ~ ~ ~ ~ ~ ~ ~ ~ ~ o0 00 V O O O ~ O O O O O b O ~ e · ~ e e ~ hd O O O O O O C C 0 O O ~ V l ~ ~ ~ O al I ~ ~ I a~ O . ~ ~ . V ~ 5= C _ ~ s ~ :e ~ ~ ~ 9 ·- ^a ~ o 0 - C ~ ~ . - o ~ ~ ~ ~ ~ "e ^V ~ V ~ ~ V V. g ° g ~ ~ ~ oe o ~ ~ ~ ~ ~ 0 D ~
73 conventional houses in 13ngland5 and in housce at 1311iot Lake, Ontario;.' the measured radon concentrations were consistent wi" those observed for Conventional houses elsewhere. Radon-Pr ~ any Concentrations. Radon-progeny concentrations are often measured as potential alpha-energy concentrations {PANIC}, given in working levels. Indoor concentrations of radon-222 progeny were measured at the Environmental Measurements Laboratory {13ML) in New York City.' The concentrations were 0.02 WI' in the EML basement and about Q.O1 WL in the building's fifth floor, both with progeny activity ratios of polonium-218, lead-21a, and bismuth-214 of about ls0.5:0.3. For 21 New York and New Jersey houses, the mean annual-average PABC for progeny of radon-222 was about 0.004 WL in the living space, with a range of values from one house to another of 0.002-0.013 WL; equilibrium factors averaged slightly above 0.6 in the living space. ' Measurements in Florida houses built on reclaimed phosphate land yielded average radon-progeny concentrations of about 0.01 WL, but the range extended to above 0.05 WL.~' Mouses in Grand Junction, Colorado, in which remedial action has been recommended, had Paps ranging from 0.02 to 1 AL. Sets of control houses monitored in Florida and Colorado had an average PAE:C similar to that in New York and New Jersey (see fable IV-31. Measurements have also been performed in homes in the vicinity of uraniu~mining operations. ~ 2 A few measurements of individual radon-progeny concentrations have been made, often to correlate such concentrations with possible removal processed.' 36 These processes constitute potential control techniques . Some work has been done on characterizing the particle size distribution of indoor radon progeny, as well as the dependence of concentrations and distributions on various characteristics, including location, particulate mase concentration, air~exchange rate, and air-mixing rate. The fraction of radon progeny that is unattached to particle., as well as the size distribution of attached progeny, was measured at the EML building and in homes. ' ~ Such measurements have also been performed in uranium mines. The diffusion coefficients of radon progeny have been measured,.) and their interactions with particles have been examined theoretically. ~ ' Lawrence Berkeley Laboratory has performed ~ few measurements of radon progeny in solar homes in New Mexico and found PA]3Cs of about O .00 5 WL (J.G. Ingersoll, personal communication) . The simplest models of indoor radon and radon-progeny concentrations use a set of simple equations connecting the indoor radon source strength, outdoor concentrations, and rate of air exchange, assumed to be the only removal mechanism (other than radioactive decay). An example is a computer program of Rusuds,2. ~ _ ~ , . ~ ~ _ ~ _ _ ~ am_ ~ _ ~ ~ ~ ~ ~ which permits step variations in atr-exonange rate. novels may ale simulate diffusion of radon into a house, 33 but transport has not been modeled in any compreben`3ive way. Models have been made of radon-progeny diffusion and attachment processes.' so and of the effect of such processes on progeny concentrations and unattached fractions. I' 2. l' Clever, no realistic Gels of radon Ad progeny
74 behavior in buildings, by which actual concentrations (or the effect of control measurers might be simulated, has been attempted. More experimental information will evidently be required to develop and validate such models. Ganma-Radiation Fluxes and Shielding Effects from Building Materials As discussed above, go radiation from terrestrial radionuclides may arise from both building materials and nearby soil and rock, although the radionuclide content of these two 'sources may vary significantly. Moreover, the structural materials shield occupants both from gamma rays from soil and rock and, to ~ lesser degree, from cosmic rays. As a result, the building may affect external dose rates of occupants in various ways and degrees. Given information on a particular building, the net effect may be calculated in a way similar to that used by Moeller et al.,33 based on the -ray doe-rate expressions given. above ana on estimation or snlelalag expects. In come cases, the structure may have little effect on terrestrial or cosmic dose rates. Exclusive use of materials that do not contain substantial radioactivity, such as wood, has the effect of shielding the terrestrial go flux (tissue dose, about 35 mre - /yr) by about 20 or 301 and has little effect on Me cosmic-ray dose (about 28 mrems/yr). A concrete foundation (~lab floor or basement) would have no effect on the cosmic-ray dose and, if its radionuclide content were similar to that of surrounding soil or rock, little effect on the terrestrial dose. That is, although concrete substantially attenuates gamma radiation from the soil or rock, it contributes a g~-ray flux that compensates for this reduction. However, if a building also use" concrete in the walls and ceilings and has a radionuclide content similar to that of local soil and rock, an approximate doubling of the terrestrial dose rate would occur. As some compensation, concrete walls and ceilings would tend to shield occupants from cosmic rays in many cases by only about 20%, but by larger fractions for large buildings. Ordinarily, then, building materials with crystal components whose radionuclide contents are similar to those of local soil and rock may increase external dose rates for occupants by up to tens of millirem per year or nay decrease cosmic-ray rates by a somewhat souller amount. For building materials and surrounding soil or rock that contain higher radionuclide contents, the dose-rate differences between outdoors and indoors would be correspondingly larger. CON rROL TE:CHNIQ13ES From the few available indoor measurements of radon-222 progeny, it appears that variations of 0.01 WL from one building to another, depending on air-exchange rates and on building or ground materials, are not unusual. The full range of values for conventional houses is considerably larger than this, and measures that reduce the
75 air-exchange rate can be expected to change it further. A progeny concentration of 0.01 WL, if experienced two~tl~irds of the time, corresponds to an exposure of about 0.3 WI`M/yr--less by about a factor of 10 than the occupational limit of 4 WLM/yr. (Exposure of a person to 1 WL for 170 h, a working month, yields one working-level month, or 1 WLM.) But variations in external dose rate due to ordinary building materials are around 10 mre~/yr, less than one-hundredth of the whole-body occupational dose limit of ~ re~/yr. If these occupational limits correspond to similarly valued risks, it appears that the effect of the structure on radon-progeny exposures (given in woricing-level ninths per year) is far more important than the effect on external whole-body dose rates (given in r ems per year}. Health effects are discussed elsewhere, but this simple comparison indicated one basis for emphasizing methods for controlling radon-progeny exposures. Of these methods, only material substitution may be used for control of gamma-ray dose rates, particularly where materials have unusually high radionuclide contents. Techniques for controlling indoor concentrations of radon-222 or its progeny include measures that decrease radon sources, reduce transport from sources, remove radon or its progeny from indoor air, or exchange indoor air for outdoor air. The easiest technique to implement in many cases is to increase the air~exchange rate--for example, by opening windows or installing fans. For reasons of comfort or energy ef f iciency, other methods, sometimes equally straightforward, may often be preferable. In general, not enough is known about the cost, effectiveness, and applicability of various measures for a judgment of their importance in t},e general building shock. Material Selection or Site Preparation In construction of a building, the use of materials whose radon-222 emanation rates are low affects the source strength directly. However, in situations where the surrounding soil and rock contribute most of the radon, opportunities for controlling the source strength are limited, especially because the diffusion length of radon-222 is relatively large and radon source strength is not of ten a Or iter ion for s ite selection. Attention to building materials or site materials (underlying and surrounding soil ~ in new construction has a substantial effect in cases where the emanation rate from either of these may be unusually high . Replacing such materials (on a remedial basin ~ is often difficult or expensive, so other measures may be favored. Reducing Transpor t The principal means of reducing the transport of radon to building interiors are the sealing of materials that have high emanation rates and, for the case of transport f rom surrounding soil, the plugging of cracks and holes through which air with a high radon-222 content (e.g., soil gas) moves. Materials may be sealed by epoxy resins or other
76 coatings with up to 901 effecti~renese. ~ ~ Sealing surfaces, filling holes with impervious materials, and stopping transport by installing plastic or other barriers have proved effective in Base cases that required remedial action (see, for example. Atomic Energy Control Board If, but they all require integrity of the barrier for long-term reduction of transport. The general applicability or effectiveness of these measures as long-term passive controls is not known. It should be noted that confinement of radon by diffusion or convection barriers also permits buildup of radon and its progeny behind the barrier, causing an increase in gamma radiation from building materials. Nevertheless, this increase appears less important than the associated decrease in airborne radon-222 and its progeny.' Transport may also be reduced by ventilating crawl~paces or basements or {in new constructions by designing transport routes that bypass Blab floors or basements. Removal of Progeny from Indoor Air Methods for removing radon-222 progeny from indoor sir include filtration with fiber, electrostatic, or charcoal filterer mixing of indoor air to cause deposition within the structure or ventilation system, and space-charging to remove progeny tone. Filtration systems are effective in reducing airborne particulate mass concentrations. However, depending on the system, they may thereby ratse the concentration of unattached progeny ions, especially polonium-218; for some particle size distributions, this would raise the ratio of lung dose to PAEC. Nazaroff't observed a subetantis1 decrease in PAEC from operation of the furnace fan twhich thereby activated the system's filter), but the unattached fraction was not measured. -flub _ al.t' and Jonassen23 have performed related experiments on air-mixing, ventilation, and filtration. Finally, in many measurement techniques, charged radon-222 progeny are collected by voltage differentials, but it does not appear that this principle can easily be applied as a control measure. Exchange of Indoor and Outdoor Air Use of air-to-air heat exchangers to remove indoor air while conserving potentially lost energy is being investigated by Lawrence Berkeley Laboratory. Preliminary resulted indicate that this method is effective, in at least one configuration, in reducing radon-222 a--d progeny concentrations. mid method is particularly attractive because it can be applied in both new and existing buildings and because it is effective in reducing concentrations of other indoor contaminants. RESEARCH NEEDS Substantial research efforts are needed in three subjects: the characterization of radon sources and of the indoor concentrations and
77 behavior of radon and its progeny, the development and testing of control techniques, and the modeling of radon and its progeny in structures. These effort. need to be supported by de~relopacnt of measurement instrumentation, followed by an evaluation of indoor concentrations, control measures, and building energy-conser~ration measures, among other factors. In addition, evaluative efforts will require further work on the health effects of radon, which have not been discussed beret Programs to characterize building materials by radon emanation rate or radionuclide content should be more widespread and coo plate. It is even more important to survey soil and groundwater with respect to radionuclide content, radon emanation, and radon transport. A rapid effort should be undertaken to determine the feasibility of geologic or geographic characterization of soil. As part of efforts to characterize materials, attention should be given to the effects of moisture, pressure, and temperature. Community water supplies should also be surveyed. Studies of indoor radon and progeny concentration. should be undertaken with two major purposes: to learn the range and distribution of radon and its progeny in the building stock, and to understand the behavior of radon and ins progeny in buildings. me first purpose requires surveys of many building of a variety of types and in various geographic areas. These surveys may be implemented by associating there with other large-aca~e efforts, such as those for energy-conservation retrofits or for insurance purposes. Hey may measure either radon concentration. or potential alpha-energy concentrations (PAECa), but the former may be measured more easily and may in fact be preferable, in that an adequate understanding of progeny behavior could be used to infer PAECs in a way that lends itself to generalization . This interpretative basis must be developed through intensive measurements to characterize indoor radon and progeny behavior. Such intensive work at only ~ few sites would serve a. ~ basis not only for improving measurement techniques but also for developing control techniques. Particular attention must be given to progeny-particle interaction" and removal processes. Results of intensive investigations would be validated by lens-detailed field measurements at a larger number of sites . Ultimately, the results would serve as basis for estimates of health effects. Many measurement programs will have to be supported by instrumentation development. More convenient portable instruments for field source measurements based on alpha-acintillation techniques or on sodium iodide gamma-ray detectors could be developed. Further work on integrating devices for large-scale surveys of Door concentrations is warranted, as is development of simple and quick progeny monitors, presumably based on semiconductor detectors. For intensive investigation of progeny behavior at a few sites, more versatile special-purpose systems must be designed to measure infiltration rate, radon, individual radon progeny, particle concentrations. and environmental conditions automatically.
78 Substantial efforts to develop and study control techniques are required. The effects of techniques to clean the sir {rather than control the source) would have to be studied in the manner indicated above for detailed investigations of progeny behavior. These measurement programs must be accompanied by corresponding modeling efforts. Models that characterize sources {on a geologic and geographic basis) and transport (by site and building type) are needed. Although models for physical processes involving radon progeny have begun to be developed, much more work is needed, especially for understanding progeny-particle interactions and control techniques. Models of indoor-air quality that use ache source and progeny models appropriately could then be developed. Finally, the models of indoor-air quality could be combined with models of the building stock to represent current radon and progeny concentrations and the effects of changes in building design and of potential control measures. Models of indoor-air quality and the building stock will be necessary for any indoor air pollutant and for evaluation of potential strategies f or controlling indoor -se r quali ty. REFERENCES 1. Atomic Energy Control Board [Canada]. Workshop on Radon and Radon Daughters in Urban Communities Associated with Uranium Mining and Processing , Elliot Lake , Ontar to, March 7 , 1978. Ottawa, Ont ., Canada: Atomic Energy Control Board, 1979. 2. Atomic Energy Control Board [Canada]. Second Workshop on Radon and Radon Daughters in Urban Communities Associated with Uranium Mining and Processing, Bancroft , Ontario, March 12-14, 1979. Ottawa, Ont., Canada: Atomic Energy Control Board, 1980. 3 . Auxier , J. A., W. B. Shinpaugh, G. D. Kerr , and D. J. Christian. Preliminary studies of the effects of sealants on radon emanation from concrete. Health Phys. 27:390-392, 1974. 4. Beck, }1. L., J. A. DeCampo, and C. V. Gogolak. In Situ Ge (Li ~ and NaI(Tl) Gamma-Ray Spectrometry. U.S. Department of Energy, Bealth and Safety Laboratory Report }lASL-258. Washington, D.C.: U. S. Department of Energy, 1972. Available f rom National Technical Information Service , Spr ingf ield , vat, as HASI - 258 . 5. Cliff, K. D. Assessment of airborne radon daughter concentrations in dwellings in Great Britain. Phy';. Med. Biol. 23:696-711, 1978. 6 . Culot, M. V. J., R. J. Schiager , and B. G. Olson. Radon Progeny Control in Buildings. Final Report. U. S. Atomic Energy Commission Report C00~22734. Fort Collins, Col.: Colorado State University, 1973. 277 pp. 7. Culot, M. V. J., R. J. Schiager, and B. G. Olson. Prediction of increased gamma fields after application of a radon barrier on concrete surfaces. Bealth Phys. 30:471-478, 1976. 8. George, A. C. Indoor and outdoor measurement`; of natural radon and radon daughter decay products in New York City air, pp. 741-750. In J. A. S. Adam;, W. M. powder, and T. F. Gesell, Eds. The Natural
79 Radiation Environment II. Proceedings of the Second International Symposium on the Natural Radiation Environment, August 7-11, 1972, Houston, Texas, U.S.A. Available from National Technical Information Service, Springfield, Va., as CONF-720805-P-2. 9. George, A. C., and A. J. Breslin. man =;_~_ ~ ~ ~ `~`e u~oUt1On or ambient radon and radon daughters in residential buildings in the New Jersey-New York area, pp. L27;~-1292 ~ includes discussion) . In T. F. Gesell and W. M. Lowder, Eds. Natural Radiation Environment III. Vol. 2. Proceedings of a Symposium Held at Houston, Texas, April 23-2B, 1978 . Oak Ridge , Tenn.: U. S. . Department of Energy, Technical Information Center, 1980. 10. Gesell, T. F. Some radiological health aspect. of radon-222 in liqulf fed petroleum gas, pp. 612-629. In R. E. Stanley and A. A. Moghissi, Eds. Noble Gases. U. S. Energy Research and Development Administration Report CONF-730915. Washington, D.C.: O.S. Government Printing Office, 197S. 11. Gesell, T. F., and H. M. Prichard. The contribution of radon In tap water to indoor radon concentrations, pp. 1347-1363. In Natural Radiation Environment III. Vol. 2. Proceedings of a Symposium Beld at Houston, Texas, April 23-28, 1978. Oak Ridge, Tenn.: U.S. Department of Energy, Technical Information Center , 1980. Gesell, T. F., H. M. Prichard, and C. T. Hess. Epidemiologic Implications of Radon in Public Water Supplies. Paper presented at Specialist Meeting on the Assessment of Radon and Daughter Exposure and Related Biological Effects, Rome, Italy, March 3-7, 198U. 13. Gulmond, R. J., Jr., W. H. Ellett, J. E. Fitzgerald, Jr., S. T. Windham, and P . A. Cuny . Indoor Radiation Exposure due to Radium-226 in Florida Phosphate Lands. Washington, 13.C.: U. S. Environmental Protection Agency Report No. EPA 520/4-78-013. Revised printing. Washington, D.C.: U.S. Government Printing Office, 1979. [2llJ pp. 14. Hess, C. T., R. E. Casparius, S. A. Norton, and W. F. Brutsaert. Investigations of natural levels of radon-222 in groundwater in Maine for assessment of related health effects, pp. 529-546. In Natural Radiation Environment III. Vol. 1. Proceedings of a Symposium Held at Houston, Texas, April 23-28, 1978. Oak Ridge, Tenn.: U. S . Department of Energy, Technical Information Center , 1980 . 1 5. Hollowell, C. D., et al. Building Ventilation and Indoor Air Quality. Annual Report. Lawrence Berkeley Laboratory Report No. LBL 10390. Berkeley, Cal.: Lawrence Berkeley Laboratory, 1979. 16. Hollowell, C. Ingersoll, D. L. Rr inkel , and W. W. Nazaroff. Radon in Energy Ett icient Residences. Lawrence Berkeley Laboratory Report LBL-9560 . Berkeley, Cal. : Lawrence Berkeley Laboratory, 1980. 17. Holub, R. F. , R. F. Droullard, W.-L. Ho, P. R. Hopke, R. Parsley, and J. J. Stukel. The reduction of airborne radon daughter concentration by plateout on an air-mixing fan. Health Phys. 36:497-504, 1979. . D., J. V. Berk, M. L. Boeael. P. A. Hillis. J. G.
80 18. Ingersoll, J. G., B. D. Stitt, and G. H. Zapalac. A Survey of Radionuclide Contents and Radon Emanation Rates in U.S. Building Materials. Lawrence Berkeley Laboratory Report LBL-11771. Berkeley: Lawrence Berkeley Iaboratory, University of California, 1981. 19. International Commission on.Radiological Protection. Recommendations of the International Commission on Radiological Protections New York: Pergamon Press, 1977. 20. Jacobi, W. Activity and potential o-energy of 222radon and 222radon-daughters in different air atmospheres. Health Phy';. 22: 441-450, 1972. 21. Jades F. MacLaren Limited. Investigation and Implementation of Remedial Measures for the Reduction of Radioactivity Found in Bancroft, Ontario, and Its Environs. Report to Atomic Energy Control Board [Canada] . Willowdale , Ont., Canada : James F. MacLaren Limited, 1979. 104 pp. (unpublished) Johnson , R. ~ ., Jr ., D. E . Bernhardt , N. S . Nelson , and B. W . Galley. Radiological health significance of radon in natural gas, pp. 532-539 . In R. E. Stanley, and A. A. Moghiss~, Eds. Noble Gases. U. S. Energy Research and Development Administration Report CONF-730915. Washington, D.C.: U.S. Government Printing Office, 1975. 23. Jonassen, N. Measurement of Radon and Radon Daughters. Paper presented at Specialist Meeting on the Assessment of Radon and Radon Daughter Exposure and Related Biological Effects, Rome, Italy, March 3-7, 1980. 24. Jonassen, N., and J. P. McLaughlin. Exhalation of radon-222 from building materials and walls, pp. 1211-1224. In T. F. Gesell and W. 3~. Lowder, Eds. Natural Radiation Environment III. Vol. 2. Proceedings of a Symposium Beld at Bouston, Texas, April 23-28, 1978. Oak Ridge, Tenn.: U.S. Department of Energy, Technical Information Center, 1980. Kahn, B., G. G. Eichholz, and F. J. Clarke. Assessment of the Critical Populations at Risk due to Radiation E::cposure in Structures. Atlanta: Georgia Institute of Technology, School of Nuclear Engineering, 1979. Kraner, B. W., G. L. Schroeder, and R. D. Evans. Measurements of the effects of atmospheric variables on radon-222 flux and soil~gas concentrations, pp. 191-215. In J. A. S. Adams, and W. A. Lowder, Eds. The Natural Radiation Environment. Chicago: University of Chicago Press, 1964. Krisiuk, E. H., E. P. Lisachenko, S. I. Terasov, V. P. Shams, and N. I. Shalak. A Study on Radioactivity in Building Materials. Leningrad: Ministry of Public Bealth of the U.S.S.R., Leningrad Research Institute for Radiation Bygiene, 1971. . Kusuda, T., S. Silberstein, and P. E. McNall. Modeling of radon and its daughter concentrations in ventilated spaces. J. Air Pollut. Control Assoc. 30:1201-1207, 1980. Lloyd, R. D. G~-ray emitters in concrete. Health Phys. 31: 71-73, 1976. 3 0. Lewder , W. M., W. J. Condon, and H . L. Beck . Field spectrometr ic investigations of environmental radiation in the U.S.A. In J. A. S.
81 Adams, and W. M. Loader, Eds. The l~tura1 Radiation Environment. Chicago: University of Chicago Press, 1964. 31. Lucas. H. F. A fast and accurate survey technique for both _ _ _ , . radon-222 and radium-226. In J. A. S. Adams, and W. M. Lowder, Eds. The Natural Radiation Environment. Chicago: University of Chicago Press, 1964. 32. McLaughin, J. P., and N. Jonassen. The effect of pressure drops on radon exhalation from walls, pp. 1225-1236. In T. F. Gesell and W. M. Lowder, EdS. Natural Radiation Environment III. Proceedinas of a Symposium Held at Houston, Texas, April 23-28, 1978. Oak Ridge, Tenn.: U.S. Department of Energy, Technical Information Center, 1980. , 33. Moeller, D. W., D. W. Underhill, and G. V. Gulezian. Population dose equivalent from naturally occurring radionuclides in building materials, pp. 1424-1443. In T. F. Gesell and W. M. Lowder, Eds. Natural Radiation Environment III. Vol. 2. Proceedings of a Symposium Held at Houston, Texas, April 23-28, 1978. Oak Ridge, Tenn.: U.S. Department of Energy, Technical Information Center, 1980. 3 4. National Council on Radiation Protection and Measurements. Natural Background Radiation in the United States. NCRPM Report No. 45. Washington, D.C.: National Council on Radiation Protection and Measurements, 1975. 163 pp. 35. National Research Council, Committee on the Biological Effects of Ionizing Radiations. The Effects on Populations of Exposure to Low Levels of Ionizing Radiation : 1980 . Washington, D.C.: National Academy Press, 1980. 524 pp. 3 6 . Nazarof f, W. W., M. L. Boegel, C. D. Mollowell , and A. D. Roseme . The Use of Mechanical Ventilation with Beat Recovery for Controlling Radon and Radon-Daughter Concentrations. Lawrence Berkeley Laboratory Report LBL-10222. Paper presented at Third Workshop on Radon and Radon Daughters in Urban Communities Associated with Uranium Mining and Processing, Port Hope, Ontario, Canada, March 12-14, 1980. 37. Nuclear Energy Agency. Exposure to Radiation from the Natural Radioactivity in Building Materials. Paris: Organization for Economic Cooperation and Development, Nuclear Energy Agency, 1979. 38. Oakley, D. T. Natural Radiation Exposure in the United States. U.S. Environmental Protection Agency Report ORP/SID 72-1. Washington, DeCe UeSe Environmental Protection Agency, Office of Radiation Programs, Surveillance and Inspection Division, 1972. 77 pp. Available from National Technical Information Service, Springfield, Va., as PB-235 795. 39. PorstendOrfer, J., A. Wicke, and A. Schraub. The influence of exhalation, ventilation, and deposition processes upon the concentrations of radon (Rn-222), thoron (Th-222), and their decay products in room air . Health Phys. 34: 465-473, 1978. 40. Raabe, O. G. Concerning the interactions that occur between radon decay products and aerosols. Health Phys. 17 :177-185, 1969. 41. Raghunath, B., and P. KotrapE,". Diffusion coefficients of decay products of radon and thoron. J. Aerosol Sci. 10 :133-138, 1979.
82 42. Roessler, C. E., Z. A. Smith, W. E. Bolch, and R. J. Prince. Uranium and radium-226 in Florida phosphate materials. Bealth Phys. 37: 269-277, 1979. 4 3 . Rundo, J., F. Markun, and N. J. Plondke. Observation of high concentrations of radon in certain houses. Health Phys . 36: 729-739, 1979 . 44. Scott, A. G. The source of radon in Elliot I,ake. In Workshop on Radon and Don Daughters in Urban Communities Associated with Uranium Mining and Processing, Elliot Lake, Ontario, March 7, 1918. Ottawa, Ont., Canada: Atomic Energy Control Board, 1979. 45. Smith, D. Ventilation rates and their influence on equilibrium fraction. In Second Workshop on Radon and Radon Daughters in Urban Communities Associated with Uranium Mining and Processing, Bancrof t , Ontar lo, March 12-14 , 1979 . Ottawa , Ont ., Canada : Atomic Energy Control Board, 1979. 4 6. Steinhausler, F., W. Hofmann, E. Pohl, and J. Pohl-Ruling. Local and temporal distribution pattern of radon and daughters in an urban environment and determination of organ dose frequency distributions with demoscopical methods, pp. 1145-1162 {includes discussion). In To F. Gesell and W. M.. powder, Eds. Natural Radiation Environment III. Vol. 2. Proceedings of a Symposium Held at Houston, Texas, April 23-28, 1978. Oak Ridge, Tenn.: U.S. Department of Energy, Technical Information Center, 1980. 47. Swedjemark, G. A. Radioactivity in Bouses Built of Aerated Concrete Based on Alum Shale. Paper presented at Specialist Meeting on the Assessment of Radon and Radon Daughter Exposure and Related Biological Effects, Acme, Italy, March 3-7, 1980. 47. Swedjemark, G. A. Radon in dwellings in Sweden, pp. 1237-1259 (includes discussion). In T. F. Gesell and W. M. Lowder, Eds. Natural Radiation Environment III. vol. 2. Proceedings of a Symposium Held at Bouston, Texas, April 23-28, 1978. Oak Ridge, Tenn.: U.S. Department of Energy, Technical Information Center, 1980. 49. United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation. Report to the General Assembly, with Annexes. New York: United Nations, 1977. 725 pp. 50. wilkening, M. H., W. E. Clements, and D. Stanley. Radon 222 flux measurements in widely separated regions, pp. 717-730. In J. A. S. Adams, W. M. Lowder, and T. F. Gesell, Eds . The Natural Radiation Environment Il. Proceedings of the Second International Symposium on the Natural Radiation Environment, August 7-11, 1972, Bouston, Texas, U.S.A. 51. Wrenn, M. E:., M. Eisenbua, CO Costa-Ribeiro, A. J. Hazle, and R. D. Siek. Reduction of radon daughter concentrations in mines by rapid mixing without makeup sir. Bealth Phys. 17:405-414, 1969. FORMALDEHYDE AND OTHER ORGANIC SUBSTANCES The infiltration of outdoor air is one source of formaldehyde and other organic substances in the indoor environment, but the primary
83 sources are in the indoor environment it~elf--building materials, combustion appliances, tobacco smoke, and a large variety of consumer products. A buildup of formaldehyde may be exacerbated in buildings that have been subjected to energy-efficiency measures intended to reduce infiltration and, thus, energy consumption. Emission rates for formaldehyde and other organic pollutants emitted in the indoor environment are generally unknown. Analytical techniques have been applied mostly in measuring concentrations in indoor air. Very little work has been done in measuring Specific source strengths or ranking emission sources, except on a broad relative basis. FORMALDEHYDE - In general, indoor formaldehyde concentrations exceed those outdoors. The contribution of formaldehyde in outdoor air to indoor a ir appears to be minor. A recent National Research Council report deals in great detail with the sources and effects of formaldehyde and other aldehydes. ~ s The reader is referred to that report for a more comprehensive treatment than is feasible here. Sources and Emission Rates Insulation. Urea-formaldehyde (UF) foam is used as thermal insulation In the side walls of existing buildings, especially single-family residential buildings. UF foam i" a convenient substance for retrofitting existing buildings, because it is injected directly into wall cavities through small holes that are then -sealed up. UF foams were developed in 1933 and first used as an insulating material in the 1960s. UF foam has been used for thermal insulation in Europe for many year", but is relatively new in the United States. In the early 1970s, interest in and use of this material increased as the cost of energy mounted and resulted in increased demand for residential insulation. A production peak occurred in 1977, when the demand for insulating products created shortages of other insulating materials, such as cellulose and fiberglass. I' Approximately 170,000 houses were insulated in 1977. The need for efficient thermal insulation in housing has increased dramatically in the last few years. Bout 150,000 houses a year are now being insulated with UF foam. Industry representatives reportedly believe that more than 200,000 homes will be insulated with UF foam during 1980. i' Installation involves mixing partially polymerized UF resin with a surfactant ~ foaming agent ~ and an acid catalyst under pressure that forces air into the mixture to create a foam. The foam hardens within minutes and cures and dries completely within a few days. Building codes in the United States, concerned with the fire-safety aspects of UF-foam insulation, rate it as a combustible material. The codes require that UF foam, when used on the inside of buildings, must be protected by a thermal barrier of fire-resistant material. In England
84 . and Bbiland, UF insulation materials are certified for use only in masonry cavities of buildings. , If the ingredient. of OF-foam insulation are improperly formulated or mixed, formaldehyde may be released into the building. Long et al. 12 enumerated come of the factor. that affect the release of formaldehyde from UF foam: Excessive formaldehyde in the res in~concentrate solution . Excessive acid catalyst in the foaming agent. Excess foaming agent (surfactant). Foaming during periods of high humidity and high temperature. Foaming with cold chemicals (optimal temperature, 50-80°F). Improper use of vapor barriers. Improper use of foams (in ceilings, etc.~. Owing to the diversity of time factors and the complexity of their interrelationabips, the quantity and rate of formaldehyde relesase from a house insulated with UF foam is difficult to predict. Particleboard and P1YWOOd. The superior bonding properties and low cost of formaldehyde polymers make them the resins of choice for the production of building material., especially plywood and particleboard. Among the various formaldehyde resins used in building materials--urea-formaldebyde, phenol-formaldehyde, and melamine- formaldehyde--urea-formaldebyde resin is the most colon adhesive used in indoor plywood and particleboard. Plywood is composed of several thin sheets of wood glued together with UF resin. Particleboard is made by saturating small wood shavings with UF resin and pressing the resulting mixture, usually at high temperature, into the final form. Particleboard can emit formaldehyde continuously for a long time (several months, or even yeare}. In buildings in which these wood products are used for partition walls or furniture, formaldehyde may reach concentrations Sufficient to cause eye and upper respiratory ire itation . In cases of extensive use of these products where air~exchange rates are low, the concentration can reach 1 ppm or more. This is due in part to the high surface-to-,rolume ratio of particleboard and ply used as building materials. The emission rate depends on a number of factor=~tbe original manufacturing process, quality control of fabrication. porosity, ambient temperature, humidity, cutting of the board for final use, etc. UF resins contain some free formaldehyde s in addition, the resin may hydrolyze and release free formaldehyde at high temperature and humidity. The phenol-formaldebyde resins used for wood products that require greater moisture resistance {i.e., exterior plywood) do not release formaldehyde as readily as products bound with UF resin. Pbenol-formaidebyde resins are not generally used for indoor wood products, because of their higher cost. Combustion Appliances. Recent studier have reported on combustion-generated indoor air pollutants, specifically contaminants from 988 stoves and heating system in residentis1 buildings.
85 Laboratory studies have shown that gas stoves emit substantial quantities of aldebydes and that formaldehyde is the major component of the aldehydes measured (Schmidt and Glitzy, G. W. Traynor, Lawrence Berkeley Laboratory, personal communications. Reported formaldehyde emission from a single gas stove under test conditions has been measured as approximately 15,000 and 25,000 ug/b for each top burner and the oven, respectively (Traynor, personal communication). Tobacco Smoke. Tobacco smoke is a source of several chemicals. including several aldehyde. other than formaldehyde. It may contribute formaldehyde to the indoor environment. The amoker's exposure to these chemicals results principally from smoke inhaled directly into the lungs (mainstream smoke). The smoke that is not directly inhaled into the lungs enters the space surrounding the smoke (sidestream smoke ~ . It is the sidestream smoke that is the major contributor to indoor pollution. Analysis by Osborne et al. :. indicated that acrolein was an important component of tobacco Smoke; this f inding was con f irmed by Jermini _ al., 9 whose studier were conducted on a smoking machine in an environmental chamber. Formaldehyde and acetaldehyde have also been identif fed in cigarette smoke. Harke et al. ' ~ measured concentrations of nicotine, carbon monoxide, acrolein, and other aidebyde. (expressed a. acetaldehyde) in the air of an unventilated room in which a series of experiments with a smoking machine bad been performed. Subatantis1 concentrations of all four compounds were observed with this extremely low ventilation. Other Sources. Several products that are potential sources of formaldehyde emission are mentioned below. Because there is no information on the rates or quantities of such emission, it is not known which are important sources. UF resins are used in the paper industry to increase the wet strength of various grades of paper. Paper products typically treated with UF resins are grocery bags, wax paper, facial tissues, napkins, paper towels, and disposable sanitary products. Formaldehyde polymers are used extensively in the manufacture of floor coverings and as carpet backing. UP resins are used in the textile industry as binder" for pigments, fire retardants. or other materials to cloth and to impart stiffness, wrinkle resistance, and water repellency to fabr ice. Fertilizers and pesticides used for co' - ercially grown plant" also may use~aldehydes and, theoretically, could contribute to the aidehyde content of ambient air locally. Urea-formaldebyde fertilizers are used not only to obtain a more uniform release rate than is possible with soluble sources of nitrogen but also to minimize the hazards of water pollution by nitrates leached out of the soil. These compounds have been used with field crops, turfgrass, pine seedlings, and geranium. The extent of their indoor use and the amounts and rates of formaldehyde release are unknown. Formaldehyde is used in numerous places, such as biologic laboratories, hospitals, and hobby and craft areas.
86 Concentrations Indoor monitoring data for U.S. bomes are few; there are limited monitoring data for European homes, particularly in the northern European countries, and they show higher indoor formaldehyde concentrations than in the United States. Table IV-4 satirizes recent monitoring data. Most of the measurements of organic substances in the indoor environment have been made on aldehydes--specifically on formaldehyde. Studies of the indoor-outdoor relationships of formaldehyde a bow that indoor concentrations generally exceed outdoor. There have recently been several studies measuring indoor formaldehyde concentrations in which emission was from particleboard and plywood furnishings and UF-foam insulation in houses. Measurements in Denmark, 2 Sweden (J. Sundell, personal communications T. Lindvall, personal communication}, West Germany (B. Seifert, personal communication ; M. Deimel, personal communication), and the United States (P. A. Breysse, personal communications have shown that indoor concentration" often exceed 0.1 ppm and may even exceed the tben~established 8-h time~weighted average safe exposure limit {3 ppm) for workroom air.23 In the 23 Danish houses, the average formaldehyde concentration {the predominant source was particleboard) was 0.62 mg/m3 (about 0.5 ppm}, and the range was 0.08-2.24 mg/m3 (about 0.07-1.9 pp~. 2 As a result of occupants' complaints, formaldehyde was measured in snore than 200 mobile homes in the United States; the concentration" reported ranged from 0.03 to 2.4 ppm (about 0.04-2.9 mg/m3} {Breys~e, University Of Washington, personal communication). A study of formaldehyde concentrations in new office trailers with air-exchange rates as low as 0.16/h found formaldehyde concentrations of 0.15-0.~:~: pen, S in contract with outdoor concentrations of less than 0.01 ppe. Formaldehyde concentrations build up in mobile homes, not only because of emission from some building material. used in their construction, but also because mobile homes are often more tightly constructed than conventional homes, thus decreasing ventilation. Aldebydes ('measured by the MBTH metbod) were monitored in a study of 19 homes across the United States. ~. Indoor concentrations of aldehydes were always higher than outdoor concentrations, typically by a factor of 6 and guise often by a factor of about 10. Figure Iv-2 shows the data collected in this study from a gas~cooking bome with one smoker. Although the source "trengebs were not determined in this study, the highest concentrations were observed in the two mobile homes' in general, the plywood and particleboard appeared to be the primary sources. In a more recent study, formaldehyde and total aliphatic aldehydes (formaldehyde plus other aliphatic aldehydes) were measured at several energy~efficient research houses at various geographic locations in the United States. i~ Results showed that, at low infiltration rates ~ <O. 3 air change per hour, or ach), indoor formaldehyde concentration" often exceeded 0.1 ppm, whereas outdoor concentrations typically remained at O.Q16 ppm {20 ~/m~i or less. Apical air~exchange rates for single-family residential buildings are between
Sampling Site Danish residences Netherlands residences built wi shout formal dehyde releasing materials 87 TABLE IV-4 Summary of Aldehyde Measurements in Nonoccupational Indoor Environments a Concentration, b ppm Range Mb an . 1.8 (peak) 0.08 (peak) 0.03 Method of Analysis Unspecif fed Unspecif led Residences in Demark, 2.3 (peak) 0.4 Unspecified Netherlands, and F ederal Republi c of Germany Two mobile homes in 0.1-0.8b 0.36 BTH bubblers Pittsburgh, Pa. Sample residence in 0.5 (peak)b 0.15 MBTH bubblers Pittsburgh, Pa. Mobile homes registering 0-1. 77 0. 1-0. 44 Chromotropic complaints in state acid ~ single of Washington impinger) Mobile homes registering 0-3. 0 0. 4 Chromotropic complaints in Minnesota acid (30-min sample Mobile homes registering 0. 02-4. 2 0. 88 Chromotropi~ complaints in Wisconsin acid Public buildings and 0~0.21 Pararosaniline energy-ef ficient homes and chromo- occupied and unoccupied ~ tropic acid 0-0. 23b MBTH bubblers aReprinted from National Research Council. ]5( P- 5 i3) bFormaidehyde, unless otherwise indicated .
88 0-<~ ~=D-D< <-._. - = ___ ~ ''~- ; - .~ ~ o |~88 o ~ ~ ~ 4< ! by ~ ~ ~ ~ I . 1 . I- 8 ~ ° ~ 8 ~ ~ ~ 8 ~ ° ~ ~ ~ ~ ~ ~ _ _ ~ _ I - /~d 's~o'zw~uo~ o , So V a, v o :^ ~ t o o V _ _ _ o 8 E _ ~ q: ~ S -A ~ o o o v - ~5 - ~ ~ - o By v Ad He o L, to As E o Pe o ~0 ~5 v S it: o s Cot
89 0.6 and 1 ach. Figure IV-3 is a histogram showing the frequency of occurrence of formaldehyde and total aliphatic aldehyde concentrations measured at an energy-efficient house with an average air~exchange rate of 0. 2 ach. Data taken at an energy~efficient house in Mission Viejo, California, are shown in Table IV-~. As shown, when the house did not contain furniture, formaldehyde content was 80 ug/m3; when furniture was added, formaldehyde rose almost threefold. A further increase was noted when the house was occupied, very likely because of such activities as gas cooking. When occupants opened windows to increase ventilation, the formaldebyde content dropped substantially. Although high, aldehyde contents observed in the majority of the energy-efficient dwellings monitored were generally below 200 ~/m3. Indoor and outdoor formaldehyde and aldehyde concentrations were found to be about the same at a public school in Columbus, Ohio, and a large medical center in Long Beach, California, and were well below 0.} ppm (120 ug/m31. Both buildings have high ventilation rates, this probably explains the low indoor concentrations, essentially equivalent to outdoor concentrations. Because many of the data reported from these field-monitoring studies involved houses whose occupants had complained of indoor air quality, these findings may not be representative of all bomes. However, when data from a random sample in Wisconsin are compared with those f ram the Washington mobile-home sample, most of the dif ferences in aldebyde concentrations can be explained by differences in the age of the homes. The mobile homer in the complaint sample are much newer than those in the random sample. Tabershaw Associates2~ analyzed the complaint data for mobile homes in Washington, and found that there was no statistically valid relationship between the severity of symptoms reported by occupants and the concentration of formaldehyde and that, regardless of the actual exposure concentration, all persons in the mobile homes reacted in substantially the same manner. Foreign (particularly Danish and Swedish) houses monitored for formaldehyde appear to have much higher concentrations than U.S. houses. These findings probably represent differences in construction and, hence, cannot be considered as representative of U.S. houses. Andersen et al. 2 formulated a mathematical model that estimates the indoor a ir concentration of formaldehyde . Although use of Danish studies may not be appropriate for O.S. houses, the treatment of Andersen_ al. illustrates the many variables that must be considered. By conducting climate-chamber experiments, Andersen et al. found the equilibrium concentration of formaldehyde from particleboard to be related to temperature, water-vapor concentration in the air, ventilation, and the amount of particleboard present. From this work, a mathematical model was established for room-air concentration of formaldehyde. When thin mathematical formulation was applied to the room--a~pling results, a correlation coefficient of 0.33 we. found between the observed and predicted concentrations--not a particularly good predictive ability. The autbor~ then modified tbe adjustable constants by calculating them for each room on the basis of monitoring results. The modified values led to a correlation coeff icient of 0. 88--a considerable improvement in predictive ability.
so 30r, ~ , , , ~,`00. cam tHCHO' Icon V ECHO ~ 6 lo > to ~Ouldco, /~ aided O- 1~4ndot, j r sIdehydes __ _ l_ _ 120 Concentrotion (~g/m3) ItO ~0 FIGURE IV-3 Indoor and outdoor formaldehyde and other aldehyde ~ :-~:~ centrations at single-famil; house in Maryland. Histogram showing the frequency of occurrence of formaldehyde and total aliphatic aldehydes at an energy-effigient haste with 0.2 ach. Reprinted from National Research Council. P- 5 J
91 ~c' +1 +1 +1 +1 to ~ ~ ~ O ~ ~ cat ~ rat I_ of v - - ~ ¢¢ JJ ~ ^ o ~ ~ ~ ~ to :^ of ~ o s +1 +1 +1 +1 s ~ ~ ~ of ~ ~ to ~ ~ ~ - ~ ~ ~ < ~ ~ Do o ~ ~ 0 v ~ ~ o To 1 ~ :~. ~. ~ ~ 1 to En ; ~ To :- ~ ~ ~ 0 I, v ~ o ~ v ~ O ~ 3 .0 0 O ~ Zip 0 0 V o o o o C C' o A: A: 0 V 0 ^ S' V ~ ~ ~ C V 0 - 0 O O o A: ~0 v o A: to to 1 U~ - U~ c, o v ~ ce c~ ~ to o E CL oo ~0 0V a, O ~ s: V 0 ~ CL ~ `: 0 C) S o o a, v 0 CO cn s~ - ~ o,o o ~s O V ~ oo =: ~ k o ~5 0 ~ o E 0 0 V ~ ~ O V . <) ~0 - ~U S s~ C~ ~0 :- v C~ C) oe v ~0 <U x c) es - 21 ·^ v o v ~0 C~ C) CO X 3 ~ O <: 3 C CL o ~ S"
92 Fornaldehyde release from interior particleboard occurs at a decreasing rate with an increase in product age. Eventually the rate of formaldehyde evolution decreases to an imperceptible point. The time necessary for this phenomenon to occur (perhaps several years) depends on the atmospheric conditions to which the board has been subjected, an well as the degree of cure of the resin. The Ire unstable groups degrade first, followed by the more stable free methylol groups. A 1977 field study in which field tests and a mathematical model were used to determine the half-life of formaldehyde in particleboard typically used in Scandinavian home construction reported it to be about 2 yr when the ventilation rate in the home is 0.3 ach (C.D. Hollowell, personal communication). Suta has analyzed the effect of home age on formaldehyde concentrations in Danish houses. These data give the following relationship of formaldehyde concentrations as a function of house age when no corrections are made for other pertinent factors, such as the amount of particleboard in the home, temperature, humidity, and ventilation: C ~ O. . SOe~0 IDA, where C 5 formaldehyde concentration (ppm) and A ~ home age {mo). On the basis of this formula, the half-life of formaldehyde concentration is 58 no. We difference between half-life value. derived from test data and those from house-~nitoring may result partly from the fact that particleboard is often added to older homes for repair and improvement. Monitoring data for the 65 randomly selected mobile hates in Wisconsin show a similar decrease in formaldehyde concentration with increasing home age (M. Woodbury, personal cormunica~cion}. In these studies, the reported formaldehyde half-life was 69 mo, which is quite similar to that found in the Denmark study. Monitoring data on 45 complaint mobile homes in Wisconsin also showed a decrease in formaldehyde concentration with increase in home age; the indicated half-life in this sample wan 28 mot When these data are combined, the formaldehyde half-life becomes 53 ma, or approximately 4.4 yr. Residences are not all expected to have the same formaldehyde concentration. A" suggested earlier, there is variation even Hong homes of the same age, depending on the amount and type of particleboard, plywood, and UF-foam in~ula~cion used in the construction, as well as on temperature, humidity, and ventilation . Therefore, monitored concentrations from a sample of similar honked will be characterized by a frequency distribution that can be approximated by a known statistical distribution, which, in turn, can be used to estimate the range of human exposures to formaldehyde in the res idential environment . The average atmospheric formaldehyde concentration appears to be approximately 0.2 ppm in both mobile homes and homes insulated with UP foam. Little information is available on conventional houses that do not contain UF-foam insulation or that were not designed to be energy-ef f icient . The average formaldehyde concentration in conventional houses appears to be 0.01-0.1 ppm and may be only slightly higher than outdoor concentration". Houses containing more
93 particleboard would fall on the high side of this concentration range, and houses with no particleboard would fall on the low side. Outdoor atmospheric formaldehyde concentrations are generally much lower than 0.1 ppm in U.S. cities. Examples of annual average concentrations are 0.05 pop for Low Angel, 2. 22 0.004-0.007 pep for four New Jersey cities,. 0.04 ppm for Winconsin cities (Baarahan, personal communications, and le.. than 0.03 ppm in Raleigh, North Carolina, and Pasadena, California.. Formaldehyde concentrations at four Swiss locations ranged from 0.007 to 0.014 ppm; these concentrations are about one-fifth the corresponding indoor Swiss concentrations.23 A mean value of 0.004 ppm has been reported for mainland Europe.' Thus, formaldehyde concentrations in mobile homes and for homes insulated with UF-foam resin are oonsiderably higher than those of the corresponding outdoor atmosphere. Control Techniques Several measures have been used in attempts to correct problems associated with formaldehyde release from building materials, including: Ventilation (opening doors and windows). Mechanical ventilation coupled with the use of heat exchangers. The use of impregnated charcoal in furnace or air-oonditioning filters. · The evaporation of household ammonia in closed and overheated rooms to neutralize formaldehyde, followed by ventilation. · In j eat ion of apron is into insulation through holes in walls to neutral i ze formaldehyde . · Spraying of air filters or floors with a specified odor absorbent provided by the manufacturer. · Use of a smacking agent. available from the manufacturer. ~ Application of vinyl wallpaper or a low-permeability paint to inter for walls . · Removal of all or some of the insulation from the home. Although many manufacturers claimed that they had successfully used these remedial measures, no studies have shown that the measure. will reliably control the release of formaldehyde gas. OTHER OR~ITC SUBSTANCES In addition to formaldehyde t many other organic contaminants can be present in indoor environments. Very little work has been done to identify or measure organic contaminants that may be harmful. Nevertheless, these compounds rosy provide a partial explanation for complaints registered by people in indoor environments where it is determined that formaldehyde and other indoor-pollutant concentrations are low or undetectable.
94 The experimental tasks associated with characterizing organic contaminants in indoor environments are formidable. The contaminants are usually present as complex mixtures of many compounds at low concentrations. Enough work has been done to outline broadly the nature of the problem and the available information. Sources and Emission Rates Four ma jor sources of organic contaminants can be identif led. People emit one category of organic contaminants termed ~bioeffluents. Building materials can also emit organic contaminants. The other two categories, personal consumer products (including insecticides, pesticides, and herbicides) and tobacco-smoking, are discussed later in this chapter . 8ioeffluents. Humans, through normal biologic processes, emit a category of organic substances known as ~bioeffluentse ~ Wang 2 ~ studied bioef fluents in a school auditorium seating over 400 people . Many organic contaminants were observed, but the major ones associated with people were methanol, ethanol, acetone, and butyric acid. As an example, the absolute concentrations and the emission rate per person during a class lecture are shown in Table IV-6. Emission rates of bioeffluents increased sharply during a class examination, considered by Wang to be a period of stress. The findings of Wang were corroborated in part by Johansson,~° who studied two schoolrooms in Sweden. He observed that acetone and ethanol were associated with the presence of schoolchildren. No emission rates were reported. Building Materials. Such products as adhesives. paints, and sealants contain solvents and other agents that can be released daring and immediately after application. The health hazards associated with these short-term releases of organic contaminants are acknowledged in the warning labels regarding the use of adequate ventilation, which are commonly appl fed to these products . Less well understood is the potential for long-term emission of organic contaminants from building materials. Slow release of residual solvents and other agents (e.g., catalysts. surfactants, and plastic monomers) is one possibility, as is the -gradual production of contaminants by degradation (e.g., air oxidation, photoinitiated reactions, and retropolymerization reactions) . Preliminary data indicate that concentrations of organic compounds in new buildings are generally higher than outdoor concentrations. ~' I' Figure I1r-4 shows comparative gas chromatograms of an indoor air sample and an outdoor air sample taken simultaneously at the same building. Classes of compounds consistently observed in indoor air include hydrocarbons, alkylated aromatic compounds, and chlorinated hydrocarbon solvents. Table IV-7 lists organic compounds identified in several office buildings at concentrations at least 5 times greater than outdoor concentrations . "
9s TABLE I;~-6 Average Concentrations and Emission Rates of Organic Bioeffluents in a Lecture Class (389 People at 9:30 a.m. ~ Emi ss ion Rate, Bioeffluent Concentration, Pub mg/day per person Acetone 20.6 + 2.8 50.7 + 27.3 Ace taldehyde 4.2 + 2.1 6.2 + 4.5 Acetic acid 9.9 + 1.1 3.6 + 3.6 Allyl alcohol 1.7 + 1.7 19.9 + 2.3 Amyl alcohol 7.6 + 7.2 21.9 + 20.8 Butyric acid 15.1 + 7.3 44.6 t 21.5 Diethy lke tone Ethyl acetate Ethyl alcohol Methyl alcohol Phenol Toluene aData from Wang. 5.7 + 5.0 - 8.6 + 2. 6 22.8 + 10.0 54.8 + 29.3 - 4.6 + 1.9 1.8+ 1.7 20.8 + 11.4 25.4 ~ 4~8 44.7 + 21 ~ 5 74.4 ~ 5~0 9.5 ~ 1~5 7.4 + 4.9
Indoor J o 1 · · · 1 1 1 ~ ~ I Outdoor ,¢: ~,_~ lo ~ 1 ~ ~ ~ 1 ~ ~ ~ ~ 96 ~1 Let 20 . , . ~ 30 . . . . . . . . . . 40 , ...., ~ Low Ti me ( minutes ) o o L ' 50 _ 100 150 Temperature FIGURE IVES Comparison of chromatograms of samples taken inside and outside an office for trace organic substances. From C. D. Hollowell, pe re o nal c or ic a tio n .
97 TABLE IV-7 Organic Substances Detected in Officesa Substance Hydrocarbons: n-Hexane n-Heptane n-Oct ane n-Nonane _-Unde cane 2-Hethylpentane 3-Me achy lpe ntane 2, 5-Dimethylheptane Me thy lcyclopentane Ethylcyclohexane Methylcyclohexane Pentamethylheptane Aromatics: Benzene Xylene. s Toluene e Halogenated hydrocarbons Trichloroethane Trichloroethylene Tetrachloroethylene Miscellaneous Hexanal Methy le thylke tone aData from Schmidt _ a1.18 OSHA Permissible Exposure 500 500 500 500 100 200 350 100 100 200 e
98 In general, concentrators of specific organic compounds in nonindustrial indoor environments are weal below occupational exposure limits established by OSHA. The health hazard from the effects of o rganic compounds at concentrations observed in indoor environments cannot now be assessed. It is important to note that OSHA criteria were establ ished for the industr ial environment, where high exposures to single compounds are encountered. The possibility cannot be overlooked that cumulative exposure to several compounds at low concentrations, or synergistic effects, may explain the complaints of building occupants . REFERENCES 3. 5. 1. Altshuller, A. P., T. A. Bellar, and S. P. McPherson. Hydrocarbons and Aldehydes in the Los Angeles Atmosphere. Presented at Air Pollution Control Association Annual Meeting, May 2, 1962, Chicago, Illinois . Cincinnati: U. S. Department of Health, Education , and Welfare, Division of Air Pollution, Public Health Service, 1962. Andersen, I ., G. R. Lundqvist , and L. Molhave . Indoor air pollution due to Shipboard used as a construction mater ial . Atmos . Environ . 9: 1121-1127, 1975. Cauler, R. Some problems of atmospheric chemistry. In Compendium of Meteorology. Baltimore: Waverly Press, Inc., l9Sl. I. Cleveland, W. S., T. E. Graedel, and B. Rleiner. Urban formaldehyde: Observed correlation with source emissions and photochemistry. Atmos. Environ. 11:357-360, 1977. Fanning, L. Z. Formaldehyde in Office Trailers. Lawrence Berkeley Laboratory Report No. LBID-084. Berkeley, Cal.: Lawrence Berkeley Laboratory, Energy and Environment Division, 1979. 7 pp. 6 . Hans t, P . ~ ., W. E . Wilson , R. K . Patterson , B . W. Gay, Jr ., L . W. Chaney, and C. S . Burton. A spectroscopic study of California smog, pp. 17-70. In Proceedings of the 6th Annual Symposium. Trace Analysis and Detection in the Environment, 29 April-1 May, 1975 . Edgewood Arsenal Special Report EO~76001. 7. Harke, H.-P. The problem of passive smoking.. Munch. Med. Wochenschr . 112: 2328-2334, 1970 ~ in German; English sundry} 8 . Harke, H.-P., A. Baars, B. Frahm, H. Peters, and CO Schultz . Pass ive smoking . Concentration of smoke constituents in the air of large and small rooms as a function of number of cigarettes smoked and t ime . Int. Arch . Arbeitsmed . 29: 323-339, 1972 . ~ in German) 9 . Jermini, C., A. Weber ~ and E. Grand Jean. Quantitative determination of various gas-phase Components of the side-stream smoke of cigarettes in the room air as a contribution to the problem of passive-smoking . Int. Arch. Occup. Environ. Health 36 :169-181, 1976. (in German; English summary) 10 . Johansson, I . Determination of organic compounds in indoor air with potential reference to air quality. Atmos. Environ. 12:1371-1377, 1978 .
99 11. Lin, C.-I., R. N. Anaclerio. D. W. Anthon, L. Z. Fanning, and C. D. Hollowell. Indoor/Outdoor Measurements of Formaldehyde and Total Aldehydes. Presented at the 178th National Meeting of the American Chemical Society, Washington, D.C., September 9-14 , 1979 . Abstract No. 112 in Abstracts of Papers. 178th American Chemical Society National Meeting . Vol . ~ . Washington, D.C.: American Chemical Society, 1979 . 1 2 . Long , K . R., D . A. Pierson , S . T . Brennan , C . W. Frank , and R. A . Hahne. Problems Associated with the Use of Urea-Formaldehyde Foam for Residential Insulation. Part I: The Effects of Temperature and Humidity on Formaldehyde Release from Urea-Formaldehyde Foam Insulation. Oak Ridge National Laboratory Report No. ORNL/SUB-7559/I . Washington, D.C.: U.S. Government Printing Office, 1979. 89 pp. 13. M'Slha~re, L. Indoor air pollution due to building materials, pp. 8 9-110 . In P . O. Fanger, and O. Valbj,drn, Eds. Indoor Climate. Effects on Human Comfort, Performance, and Health In Residential, Commercial, and Light-Industry Buildings. Proceedings of the First International Indoor Climate Symposium, Copenhagen, August 30 September 1, 1978 . Copenhagen: Danish Building Research Institute, 1979 . 1 4 e Moschandreas , D. J ., J . W. C . Stark , J . E. McFadden, and S. S . Morse. Indoor Air Pollution in the Residential Environment. Vol. 1. Data Collection, Analysis, and Interpretation. U.S. Environmental Protection Agency Repor t No. EPA 600/7-78-229a . Research Triangle Park , N.C .: U. S. Environmental Protection Agency, Environmental Monitoring and Support Laboratory, 1978. 201 pp. 15 . Nat tonal Research Counc il. Cocci ttee on Aldehydes . Formaldehyde and Other Aldehydes . Washington, D.C.: National Academy Press, 1981. ~ 354; pp. 1 6 . Osborne , J. S ., S . Adamek , and M. E. Hobbs . Some components of gas phase of cigarette smoke . Anal. Chem. 28: 211-2IS, 1956. 1 7. Schmidt, A., and H. GiStz . Die Entstehung von Formaldehyd bet der Verbrennung van Erdgas in Haushaltsgeraten. GWF--Gas/Erdgas 118:112-115, 1977. (in German) 18¢ Schmidt, H. E., C. D. Hollowell, R. R. MikSch, and A. S. Newton. Trace Organics in Offices. Lawrence Berkeley Laboratory Report 11378. Berkeley, Cal.: Lawrence Berkeley Laboratory, 1980. 19. Sheldrick, J. E., and T. R. Steadman. Product/Industry Profile and Related Analysis on Formaldehyde and Formaldehyde~Containing Consumer Products. Part II. Products/Industry Profile on Urea Formaldehyde. Report to U.S. Consumer Product Safety Cononisszon. Columbus, Ohio: Battelle Columbus Division, 1979. [241 pp. 20. Stahl, Q. R. Preliminary Air Pollution Survey of Aldehydes. A Literature Review. National Air Pollution Control Administration Publication No. APTD 69-24. Raleigh, N.C.: U.S. Department of Health, Education, and Welfare, National Air Pollution Control Administration, 1969.
100 21. Tabershaw, I. R., H. N. Doyle, L. Gaudette, S. H. La~, and 0. Wang. A Review of the Formaldehyde Problems in I50bile Homes. Report to National Part~cleboara Association. Rockville, Md.: Tabershaw Occupational Medicine Associates, P.~., 1979. 19 pp. 22. V.S. Department of Health, Education, and Welfare, National Air Pollution Control Administration. Air Quality Cr iteria for Hydrocarbons. DREW Publications No. (HSM) 72-7516. Washington, D.C.: U.S. Government Printing Off ice, 1970. 118 pp. 23. U.S. Department of Labor, Occupational Health and Safety Administration. Occupational Safety and Health Standards. Subpart Z--Taxic and hazardous substances. Code of Federal Regulations, Title 29, Part 1901.1, July 1, 1980. 24. Wang, T. C. A study of bioeffluents in a college classroom. ASHAE Trans. 81 (Pt. 1~:32-44, 1975. 25. Wanner, B. U., A. Deuber, J. Satish, M. Meter, and H. Sommer. Air pollution in the vicinity of streets, pp. 99-107. In M. W. Benarie, Ed. Proceedings of the 12th International Colloquium on Atmospheric Pollution, 1976. Amsterdam: Elsevier, 1976. CONSUMER PRODUCTS A wide array of activities and products that affect air quality can be found in contemporary residential spaces. The full range is of ten wider than is found in most occupational or public spaces. We are concerned here with the air-pollution aspects of such activities and consumer products. Pollutants emitted into the indoor environment by consumer products are usually dissipated by dilution and surface deposition. For a given amount of such a pollutant, the rate of discharge, the volume of the space, the ventilation rate or infiltration rate, and the presence of occupants determine the sever ity and duration of human exposure. The specif ics of control strategies are discussed in Chapter ~X, but it will be clear that even an identical activity could produce radically aif f Brent exposures in dif ferent spaces, depending on the application and eff icacy of control strategies. Consumer products introduce pollutants into the indoor air in a number of forms and ways. For example, spray paint and sprayed-on oven cleaners introduce an aerosol of the chemical products whenever they are used. There are many spray products; it has been estimated that the average U.S. residence at any given tine contains IS aerosol sprays. Aerosols can also be produced indirectly, as in grinding, sand ing, cleaning, and some hobby activities. Other consumer products and the activities associated with them introduce pollutants by evaporation or sublimation of solvents or active ingredients, such as paint solvents, cleaners;, bleaches, and disinfectants. Still other products--e . g ., plastics, paints, and textiles with artif icial f ibers and conditioners--release, or ~outgas, · small amounts of volatile chemicals over long periods. The association of emission with these types of products or activities is shown in Table IV-8. The table represents a compilation of kinds of emission encountered in a search
101 TABLE IV-8 Types of Emission of Indoor Air Pollutants Associated with Various Activities and Consumer Products Intentional Unintentional Evaporation Activity or Aerosol Aerosol or Unintentional Product Production Production Sublimation ()utgassing . Cleaning X X X X Painting X X X Polishing X X X Stripping X X Ref inishing X X X Hobbies and craf ts X X X X Deodorizer X X Inser ticide X X Disinfectan~c X X Personal groom- X X X ing product Plas tic X
102 of the literature and in lists in consumer catalogs; it in clearly i ncomplete . AEROSOL-PRODUCING PRODUCTS . Pressurized aerosol cans have found widespread use in a great variety of applications. Aerosol cans typically contain a propellant gas under a relatively low pres~ure--about 40 psi (3 kg/cm27--with a vapor pressure at normal room temperature that allows some of the propellant to be in equilibrium in the liquid phase. Until recently, dichlorodifluoromethane and trichlorofluoromethane (fluorocarbon-12 and fluorocarbon-l!) were used widely as propellants, but their effect on the atmospheric ozone layer has led to a prohibition of this application in the United States. Propane, butane, nitrous oxide, methylene chloride, and others are currently used as propellants. Most propellant gases are biologically inactive or active only at high concentrations. Some are extremely flammable and could reach explosive concentrations in enclosed spaces. Active ingredients vary from product to product, and a complete list of propellants and active ingredients rarely appears on a can. Cleaning agents include sodium or potassium hydroxide in oven-cleaners, ammonium hydroxide in window-cleaners, and tetrachloroethylene and petroleum-derived solvents in spot-cleaners. Spray paints often contain toluene, xylene, methylchloride, and other volatile organic substances, as well as pigments and a vehicle. Dra~nieks 2 ~ measured the organic chemicals present in indoor air of a high-rise apartment in which aerosol products had been used. The use of a scented oven-cleaner released at least 13 organic chemicals into the res idential space . An unscented aerosol deodorant and a scented aerosol furniture polish released similarly large numbers of chemical species throughout the residence. Cote et al. 7 surveyed the composition of a range of aerosol products for propellants and active ingredients. Mokler et al. 23 reported that under Worst reasonable conditions. some aerosol products introduce respirable particles smaller than 6 am in aerodynamic diameter at a concentration of over 50 mg/m3--10 times the threshold limit value for daily average exposure in an industrial environment for biologically inactive nuisance dust. 3 The same investigators found that the conditions of discharge did not have a great effect on the size characteristics. 2. There was no evidence of animal toxicologic effects after a series of studies of cosmetic aerosols. I' Marier and co-workers 2 ~ exposed 20 human subjects to a namer of aerosol products daily for four consecutive weeks. These products included deodorants , hair spray , frying-pan spray, room-freshener, insect repellent, window-cleaner, insecticide. furniture polish, bathroom-cleaner, and a depilatory. All products were used according to the manufacturer's instructions; after 3 wk of exposure. all subjects were evaluated for cardiac function, respiratory function, and hematologic and clinical biochemical characteristics. None of the tests showed evidence of toxic effects, and no fluorocarbon was found
103 at any time in any blood sable. All the spray products used fluorocarbon as propellant. In an epidemiologic study of 3, 800 people in Tucson, Ar izona, Lebowitz reported in 1976 that he found respiratory ef feats associated with frequent use of aerosols. I' In a followup study 5 yr after the f irst one, he found that use of aerosol products was lower by a factor of 10, and the association between aerosol use and respiratory effects was no longer seen. I' Stewart et al.32 evaluated physiologic responses to different propellant gases (isobutane, propane, fluorocarbon-12, and fluorocarbon-l!). They were inhaled at concentrations of 250, 500, and 1,000 ppm for periods of 1 min to 8 h. None of the subjects showed a decrease in pulmonary function or a change in cardiac rhythm as a result of these exposures. Deliberate inhalation of some aerosol sprays often leads to serious consequences, or even death. Lipid sprays have been shown under such circumstances to lead to acute functional and anatomic disintegration of alveolar surfactant. The resulting alveolar collapse can cause fatal hypoxemia. In one preliminary study, 23 the statistical association found between the indoor use of spray adhesives and congenital malformations was suff icient to warrant further study. PARTICLES PRODUCED AS A BYPRODUCT The major production of particles in the indoor environment is undoubtedly due to tobacco-smoking and food-preparation (which are considered elsewhere, as is malfunctioning heating equipment) . In the course of cleaning of floors and furniture, there is likely to be a resuspension of dust particles that had previously settled. Maintenance and cleaning activities have been identif fed as a source of asbestos fibers in spaces that contain accessible asbestos. Resuspension of settled dust in the absence of ventilation or air-cleaning effectively increases the exposure of occupants.20 Even minimal exposures to asbestos fibers in hobby materials' have been found to result in identifiable asbestos bodies in the lung, and the asbestos fibers found in mesothelioma have been ascribed to minimal exposures in the pursuit of hobbies.5 Hobby and home craft activities can cause substantial production of particles. 2 2 The use of lead glazes by potters can lead to high lead intakes, and an unknown fraction of this intake will be from inhalation. The use of solder and flux in stained-glass fabrication and jewelry-n~aking results in the aerosolization of lead, cadmium, and flux. Is The effects of inhalation of lead and cadmium are known better from occupational exposures, but some residential exposure must be expected. Some phases of woodwork ing result in the production of airborne wood dust from sawing, grinding, and sanding . Acheson and co-workers studied the rink of nasal cancer in woodworkers in the furniture industry ~ and concluded that exposure to wood dust, rather than varnish or polish, caused an increased risk of nasal cancer. For
104 occupational exposures, the risk of nasal cancer was an much as 500 t Ames the normal incidence. PRODUCTS AND ACTIVITIES ASSOCIATED WITH EVAPORATION OR SUBLIMATION . Solvents are used in the indoor environment for ~ variety of purposes--in clothing- and furniture-cleaners; as cart iers for polishes, paints, and varnishes; and as chemical strippers in the ref inishing of furniture--and they are inherent in many adhesives, personal grooming products, disinfectants, and deodorizers. Most of the chemical species involved are used in the occupational environment, and threshold limit values have been adopted for that environment. 2 ~ Table IV-9 contains a nonexhaustive list of substances that evaporate or sublimate from solvents and household products and their occupational exposure limits. ~ The containers of many of the product" containing substances listed in Table 1~-9 do not list their constituents or concentrations, a [though hazard warnings are generally provided . The indoor a ir concentrations are rarely measured; even when they are, there is d isagreement concerning the typical conditions. An example of this; is provided by two reports on methylene chlor ide . The f irst deals with exposures to methylene chloride in paint-removers in home workshops. ~ ~ One person developed acute myocardial infarction in three separate episodes after the use of methylene chloride for furniture-stripping in a basement workshop. The lest exposure was fatal. In the case of a healthy younger man, it was accidentally discovered that he had a high carboxyhemoglobin (COMb) concentration (6-81) on each of several mornings after 2-h exposures to paint-removers at home on the previous evening. Controlled exposures of volunteers to methylene chloride at 50, 250, and 986 ppm for 3 h produced COHb at concentrations of 2%, 4. See, and let, respectively, measured 1 h after the end of exposure. In the second study, 3 0 volunteers were exposed to methylene chloride at 4S0 ppm for 26 mini the authors considered that a typical exposure for the use of spray paint with methylene chloride as propellant. They found "a clinically insignificant increased in COBb 7 h after exposure. These two reports represent a range of reactions to methylene chloride--from nearly undetectable to accidentally detected to fatal--that depends on individual vulnerability, conditions of use, and criteria used for detection. Measurements of actual concentrations of chemicals associated with household products are scarce; where they are reported, they can be very high, as in a report of benzene at 130 pum found in a double garage during furniture-~tripping.3' The use of mercury compounds as fungicides in latex paints gives rise to long-term emission of mercury into the indoor spaces in which such paints have been applied. Taylor'. used a radioactive tracer and found that 20-2S% of the mercury was lost in the first 90 d after application. Foote ~ ~ measured background ambient atmospheric mercury concentrations in a number of homes at slightly over 2 ng/m3 and in rooms recently painted with latex paint at up to 3~000 ng/m ~ In
105 TABLE IV-9 Threshold Limit Values of Various Substancesa Chem] Acetone Ammonia Benzene Carbon tetrachioride Chl vine Me thanol Tri chl oroethane Methylene chloride Ozone Trichloroethylene Turpentine Xylene Toluene TLV-]WA' b TLV-]TEL ~ c mg/m Gum 2,400 18 30 65 3 260 1,900 700 0.2 535 560 435 375 3,000 27 130 9 310 2,380 870 0.6 800 840 655 560 Source Lacquer solvent Cleaner Adhesive, spot cleaner, paint remover Spot cleane r, dry cleaner Cleaner Paint, spot cleaner Cleaning f luid Paint remover Copying machine, electro- static air cleaner Dry-cleaning agent Paint, f inish Solvent, paint carrier, shoe dye Solvent, paint carrier, dry cleaning aData from American Conference of Governmental Industrial Hygienists. 3 bThreshold limit value--time-weighted average. Threshold limit value--short-term exposure limit.
106 rooms painted 3 yr before, he found concentrations of 68 ng/m3. The threshold limit value adopted by the American Conference of Governmental Industrial Hygienists 3 for 8-h workdays is 50,000 ng/m3 . S. ibbett ~ al . ~ ~ found room-air concentrations of around 1, 000-2, 000 ng/m in rooms with fresh latex paint--also well below the TLV and not likely to produce clinical symptoms. In comparison, spilt mercury from broken clinical thermometers i~ produced mercury-vapor concentrations In room air of about 5 ug/m3, continuous exposure to which might produce clinical symptoms. Residential or commercial use of insecticides, pesticides, and herbicides both inside and outside has the potential for contaminating the indoor environment. Some organochlorinated or organophosphated pesticides have specific agricultural applications, but others are used widely in urban areas by both private property-owners and municipalities. The organic-based compounds can find their way indoors by various routes. Some are applied directly indoors for rodent and insect control. The long-term effectiveness of many of these compounds is achieved by prolonged sublimation. Various studies have found many of these compounds indoors.. ~ ~' ~. In spite of the widespread use of residential and commercial insecticides, pesticides, and herbicides, no systematic survey has been done to identify indoor concentrations for the numerous compounds likely to be present. SOME MECHANISMS OF BIOMEDICAL EFFECTS . The wide variety of the chemicals in consumer products makes it difficult to anticipate all the possible adverse health effects. Some classes of chemicals have common characteristics that cause them to attack particular organ systems. Various environmental chemicals have structural similarities that suggest that they may have similar effects on the myocardium; these chemicals have a lung-tissue half-life that could represent a long-term hazard. Some examples are polyhalogenated hydrocarbons used as insecticides and industrial chemicals, such as polychlorinated and polybrominated biphenyls (PCB and PBB). These may produce sudden death. The polyhalogenated hydrocarbons bind to estrogen receptors and are estrogenic in animal systems. This may increase cholesterol and triglyceride concentrations and so increase the risk of coronary heart disease (CHD), of mortality from CHD, or of CHD-related death. ~ s Myocardial fibers may also be damaged by toxic agents, such as ozone. 27 Coronary-arterial-disease mortality has also been shown to be related to concentrations of suspended particulate matter in the external environment. 27 Thus, there may be many sources of cardiotoxic agents in the indoor environment. ~ Organophosphorus pesticides, such as parathion, lead to clinical symptoms resembling strong cholinergic stimulation due to a nc~=versible blockade of cholinesterase and leading to an accumulation of =.ndogenous acetylcholine.~, Thin a:-umulation disrupts the transmission of impulses from nerve firers to muscles. (In the case of parathion, skin may be the route of entry. ~ Chronic effects on
107 cerebrospinal fluid may also occur. Other disturbances of the nervous system may occur through exposure to such chemicals as PCB, which may be ingested and may be stoked in fatty tissue. PCB inhibits growth in cultured cells and interferes with the activity of a variety of enzymes. 35 Pesticides occur in indoor environments through the spraying of pesticides or herbicides and through the contamination of items brought into the home, including foods and flowers. 2 S Solvents, especially chlorinated hydrocarbons, may damage the kidneys and liver. 38 Although the skin acts as an effective barrier, serious toxicologic effects may result from exposure of the skin to such substances as some organic phosphates, lead compounds, and acid. Dermatitis--especially in the form of dry, scaly or fissured reactions--can be caused by recurrent contact with solvents, emulsifying substances, dehydrators, or detergents. Acidic or alkaline gases and aerosols are readily dissolved in the aqueous protective film of the eye, on the mucous membranes of the nose and mouth, and on skin that is moist with sweat. Such exposure may also erode teeth and change hair structure . 3 3 These mechanisms have been demons~crated; because the exposures described can occur in nonoccupational indoor spaces, their potential impact should be considered. However, the importance of such exposures is still difficult to evaluate. SUMMARY AND CONCLUSIONS Among the sources of pollutants in the indoor a ir that are due to consumer products or hobby or craft activities, many can harm exposed occupants. Such products usually bear labels with hazard warnings and instructions for use that, if followed carefully, will reduce pollutant e xposures to a point that is presumably acceptable for healthy users . Willful abuse, as in the case of direct inhalation of aerosol products or careless use of solvents in enclosed spaces, can result in acute or delayed disorders or even death. The prohibition of fluorocarbons as propellants in aerosol spray products has resulted in substitution of other propellants that may be found to be toxicologically less des irable . The recognition of the carcinogenicity of vinyl chloride and benzene has resulted in the banning of these chemicals front consumer products, but a number of chemicals with ser ious toxic potential continue to be used. Many consumer products are used only intermittently by a g iven person, and those in different households are likely to use different products for a g iven purpose . For safety, most products are formulated to avoid acute discomfort or irritation and because such acute effects will reduce marketability. Risks of long-term or delayed adverse health effects are not as likely to become apparent to the consumer and are not as likely to be incorporated in hazard warnings on products. If the constituents of the consumer products are known, it may be possible to use a combination of occupational threshold limit values and likely exposure concentrations and exposure durations in making assessments of the impact of consumer products on indoor air quality.
108 Disclosure of product composition, assessment of acute and chronic consequences, and labeling with composition, directions for use, and hazard warnings specific for a particular formulation amount to one of several strategies that should be evaluated. Monitoring or sur~relllance techniques now in use by such responsible agencies as the Consumer Product Safety Commission, the Environmental Protection Agency, and the Centers for Disease Control are more likely to discover acute consequences than delayed adverse health effects. The assessment of the human exposure and adverse health consequences due to the storage and use of consumer products is made difficult by the irregular, sporadic, and highly variable exposures, scarcity of measurements, and limited knowledge about composition of many of the products. Except for studies of accidental poisoning, epidemiologic assessments are about completely impossible, owing to the episodic and irregular nature of exposures. It will be necessary to rely on knowledge of and experience with the use of the constituent. of consumer products in the workplace. Assessment of the impact of consumer products on nonoccupational indoor air quality must, then, be based on constructed risks and potential exposures. Labeling of consumer products with lists of constituents, instructions for safe use, and hazard warnings is often inadequate, and in any case it may be disregarded by the user and is ineffective when the products are handled by children. REFERENCES . 1. Acheson, E. D., R. H. Cowdell, E. Hadfield, and R. G. Macbeth. Nasal cancer in woodworkers in the furniture industry. Br. Med. J. 2:587-596, 1968. - 2. American Conference of Governmental Industrial Hygienists. Documentation of ache Threshold Limit Values for Substances in Workroom Air. Cincinnati: American Conference of Governmental Industrial Hygienists, 1977. 3 . Amer lean Conference of Governmental Industrial Hygienists . TLVa . Threshold Limit Values for Chemical Substances in Workroom Air Adopted by ACGIM for 1980. Cincinnati: American Conference of Governmental Industrial lIygieni~ts, 1980. 93 pp. 4. Burch, G. E. Toxic agents, cardiovascular disease, and the polluted home. Am. Heart J. 87:679-680, 1974. 5. Chen, W., and N. K. Mottet. Malignant mesotheliama with minimal asbestos exposure . Bum. Pathol . 9: 253-258, 1978 . 6. Churg, A., and M. L. Warnock. Analysis of the cores of asbestos bodies from meanders of the general population: Patients with probable low~degree exposure to asbestos. An`. Rev. Respir. Dis. 120: 781-786, 1979. 7. Cote, W. A., W. A. Wade, ITI, and J. E. Yocom. A Study of Indoor Air Quality. Final Report. U.S. Environmental Protection Agency Report No. EPA-650/4-74-042. Washington, D.C. s U.S. Environmental Protection Agency, 1974. 282 pp.
109 8 . Davies, J. E., W. F. FAmundson, and A. Raffonelli. The role of house dust in human DDT pollution. Am. J. Public Bealth 65 (1~: 53-57, 1975. Davis, J. H., J. E. Davies, A. Raffonelli, and G. Reich. The investigation of fate' acrylonitrile intoxications, pp. 547-555. In W. B. Deichmann, Ed. Pesticides and the Environment: A Continuing Controversy. Chronic Toxicology, Ecological Effects, Carcinogenesi=, Mutagenesis, Teratogenesis, Drug Interactions. Vol. II. New York: Intercontinental Medical neck Corporation, 1973. 10. Fag an, D. G., J. B. Forrest, G. Enhdrning, M. Prey, and J. Guy. Acute pulmonary toxicity of a commercial fluorocarbon-lipid aerosol. B~stopathology 1: 209-223, 1977. 11. Foote, R. S. Mercury vapor concentrations inside buildings. Science 177: 513-514, 1974. 12. Fritsch, A. J., Ed. The Household Pollutants Guide. Garden City, N. Y.: Anchor Press/Doubleday, 1978. 309 pp. 13. Holleman, J. h., M. G. Ryon, and A. S. Harpoons. Chemical Contaminants in Nonoccupationally Exposed U. S. Residents. U. S. Environmental Protection Agency Report No. EPA-600/1-80-001. Research Triangle Park, N.C.: U.S. Environmental Protection Agency, Health Effects Research Laboratory, 1980. 150 pp. 14 . Koplan, J . P ., A. V. Wells, H. J . P. Diggory, E. L. Baker , and J . Liddle. Lead absorption in a community of potters in Barbados. Int. J. Epidemiol. 6: 225-229, 1977. . Kronoveter, K. J., and C. R. Meyer. Industris1 hygiene study in stained glass workshop, pp. 28-35. In M. McCann and G. Barazani, Eds. Proceedings of the SOEH Conference on Bealth Hazards in the Arts and Crafts. Washington, D.C.: Society for Occupational and Environmental Health, 1980. . Leary, J. S., W. T. Keane, C. Fc~ntenot, E. F. Feichtmeir, D. Schultz, B. A. Koos, L. Hirsch, E. M. Lavor, C. C. Roan, and C. lI. Hine. Safety evaluation in the home of polyvinyl chloride resin str ip containing dichlorvos (DDUP) . Arch. Environ. Health 29: 308-314, 1974. 17. Lebowitz, M. D. Aerosol usage and respiratory symptomatology. Arch. Environ. Health 31: 83-86, 197 6. 18. Lebowitz, M. D. The Effects of Cosmetic Aerosols on Respiratory Physiology. Final Contract Report. Washington, D.C.: U.S. Food and Drug Administration, 1980. 19. Lovelace Foundation. Inhalation toxicological studies of aerosolized products. Final Contract Report. Washington, D.C.: U.S. Food and Drug Administration, 1979. Available from National Technical Information Service as PB 89 108509. Graedel. Measurements and models of indoor aerosol size spectra. Atmos. Environ. 7:827-842, 1973. 21. Marier, G., B. HacParland, G. S. W:berg, H. Buchwald, and P. Dussault. Blood fluorocarbon levels following exposure to household aerosols. Can. Med. Assoc. J. 111:39-42, 1974. . McCann, M., and G. Barazani, Eds. Proceedings of the SOEH Conference on Health Hazards in the Arts and Crafts. Washington, D.C.: Society for Occupatione1 and En`,ironmente1 Bealth, 1980. 232 PP 2 0 . Lum, R. M. . and T. E .
110 _. Makler, B. V., B. A. Hong, and M. J. Snow.-Respirable particulates generated by pressurized consumer products. I. Experimental method and general characteristics. Am. Ind. Hyg. Assoc. 3. 40:330-338, 1979. 24. Mokler, B. V., B. A. Hong, and M. J. Snow. Respirab}e particulates generated by pressurized consumer products . IT . Inf luence of experimental conditions. Am. Ind. [Iyg. Assoc. J. 40 :339-347, 1979. 25. Morse , D. L., E. L. Baker , and P. J. Iandrigan. Cut f lowers : A potential pesticide hazard. Am. J. Public Health 69:53-56, 1979. 26. Moschandreas, O. J., Ed. Indoor Air Pollution in the Residential Environment. Vol. II. Field Monitoring Protocol, Indoor Episodic Pollutant Release Experiments and Numerical Analyses, pp. 198-220. U.S. Environmental Protection Agency Report No. EPA-600/7-78-229b. Research Triangle Park, N.C.: U.S. Environmental Protection Agency, Environmental Monitoring and Support Laboratory, 1978. 27. Shy, C., J. Goldsmith, J. Hackney, M. Do Lebowitz, and D. Menzel. Statement on the health effects of air pollution. ATS Newsletter, 4:22-62, 1978. Sibbett, D. J., R. B. Moyer, and G. H. Molly. Emission of mercury from latex paints. Presented Division of Water, Air and Waste Chemistry, Americal Chemical Society Boston, Mass., April 1972. 29. Silberg, S. L., D. R. Ransom, J. A. Lyon, and P. S. Anderson, Jr. - Relationship between spray adhesives and congenital malformations. South. Med. J. 72:1170-1173, 1979. 30. Stevenson, M. F., G. L. Cooper, and M. B. Chenoweth. Effect on carboxyhemoglobin of exposure to aerosol spray paints with methylene chloride. Clin. Toxicol. 12:551-561, 1978. 31. Stewart, R. D., and C. L. Hake. Paint-remover hazard. JO Am. Med. Assoc. 235:398-401, 1976. 32. Stewart, R. D., P. E. Newton, E. D. Baretta, A. A. Herrmann, H. V. Forster, and R. J. Soto. Physiological response to aerosol propellants. Environ. Health Perspect. 26:275-285, 1978. 33. Stokinger, H. E. Mode of action of toxic substances, pp. 13-26. In W. M. Gafafer, Ed. Occupational Diseases. A Guide to Their Recognition. U.S. Department of Health, Education and Welfare, Public Health Service Publication No. 1097. Washington, D.C.: U.S. Government Printing Office, 1964. 34. Taylor, C. G. The loss of mercury from fungicidal paints. J. Appl. Chem. 15:232-236, 1965. 35. U.S. Department of Health Education, and Welfare, National Heart, Lung and Blood Institute (NHLBI). Working Group on Beart Disease Epidemiology. DREW (NIE) Publication No. 79-1667. Washington, D.C.: U.S. Department of Bealth, Education, and Welfare, 1979. 36. Waldbott, G. L. Health Effects of Environmental Pollutants. Saint Louis: The C.V. Mosby Company, 1973. 316 pp. 37. Westerman, E. Accumulation of environmental agents and their effects in the body, pp. 16-27. In D. H. X. Lee and D. Minard, Eds. Physiology, Environment and Man. ;~.~-w York: Academic Press, Inc., 1970. 38. World Health Organization. Health Hazards of the Human Environment. Geneva: World Health Organization, 1972. 387 pp.
111 39 . Young , R. J., R. A. Rinsky, P. F. Infante, and J . lt. Wagoner . Benzene in consumer products . Science 199: 248, 1978. ASBESTOS Asbestos ~ is a collective term for the f ibrous or asbestiform types of various minerals. Characteristics of flexibility, strength, and durability have brought these mineral f ibers into numerous and varied applications involving potential exposure of large populations. Both widespread use and increasing investigations of the health effects of asbestos exposure have created intense interest in asbestos as an environmental contaminant. The health effects and toxicologic impact of mineral fibers are covered in detail in Chapter VII. Because the asbestiform minerals have been used in numerous construction materials, consumer products, and appliances, the nonoccupational environment has become an area for investigation of a sbes tos contaminat ion and human exposure . A potential f or contamination from some types of these materials in structures during construction, renovation, demolition, and even normal use has been demonstrated. Repair and maintenance of household appliances, furnaces, stoves, and asbestos-cement pipes can also result in release of fibers into the air. DEFINITION OF ASBESTOS "Asbestos" is applied to chemically varied, naturally occurring mineral silicates of the serpentine and amphibole classification that are separable into fibers that are flexible and incombustible and usually have large length-to-diameter ratios. These asbestos, or asbestiform, mineral fibers have high tensile strength and desirable thermal and electric insulating properties and resist chemical -~- degradation. Asbestos minerals with commercial and exposure importance are serpentine chrysotile and the amphibole group of asbestos minerals: apposite, crocidolite, anthophyllite, and actinolite-tremolite.. S2 IMMORTAL CHARACTERISTICS OF ASBESTIFORM MINERAL FIBERS The characteristics of durability, airborne lifetime, and f iber d imension are especially important in determining the potential of exposure and biolog ic ef f ect . Durability Asbestiform f ibers retain physical integrity in nearly all uses and applications and within human tissue. 1. S2
112 Potential to Remain Airborne This potential strongly affects exposure probability. Settling velocity depends heavily on fiber diameter and to a lesser extent on fiber length.. 3' Settling in still air in a 3-m-high room, a fiber 5 am long and 1 am in diameter will remain airborne for approximately 4 h. A fiber of the same length with a O.l-um diameter will remain airborne for up to 20 ho. Such settling times could be prolonged in turbulent air, and, like other suspended particles, fibers can be transported by air currents. Disruptive mechanical forces cause predominantly longitudinal cleavage of f ibers or f iber bundler into a larger population of particles with smaller diameters and increased persistence in the air. ~ However, the extent of fiber cleavage by natural forces in the environment is unknown. Fiber Dimensions The deposition and retention of fibers in the respiratory tract depend on fiber dimensions, breathing conditions, and airborne fiber concentration. Most fibers retained in human lungs are shorter than 5 Em, and have diameters less than approximately 2.5 pa,: 2 ~ ~ 2 ~ ~ O but some thin fibers up to 200 Em have been found in lung samples.~° ASBES=S PRODUCTION AND APPLICATION lo_ The characteristics of durability, flexibility, strength, and resistance to wear bring the asbestiform minerals into thousands of appl ications . They are used an roof ing and f loor ing products, textiles, papers and felts, friction materials, filters and gaskets, cement, panels, pipes, sheets, coating materials, and thermal and acoustic insulation. 38 Asbestos production began late in the nineteenth century, when it was used as thermal insulation for steam engines. Worldwide production is now nearing 5 million tons/yr, with chrysotile the principal f iber type. ~ 2 Production . Approximate consumption of asbestos in the United States was 600,000 tons in 197942 and is expected to be 400,000-900,000 tons in 2000. Over 90% of the asbestos used in the United States is imported, and over 90% of the imported asbestos is Canadian chrysotile. Over 70% of the asbestos is used in the construction industry. 2
113 Stray Application . . ~ Of all the uses in the construction industry, the spray application of asbestos onto structural surfaces i. the most important in the development of potential contamination situations. Such sprayed material is usually friable or susceptible to damage and disintegration By nana pressure . Sprayed mater iai has been applied extensively to steel work to retard deformation during fire and to other structural surfaces for thermal and acoustic insulation, decoration, or condensation control. Spray application of asbestos fibers began in the 1930s and allowed the rapid covering of irregular surfaces without the use of mechanical support or extensive preparation. Early spray applications in the United States were mainly for decoration and acoustic insulation. In 1950, the Underwriters Laboratories approved the use of sprayed asbestos where concrete had been required for prevention of deformation of steel from fire in multistory buildings. This approval brought about an intense use of sprayed asbestos material in new construction. 2 ~ 2 ~ However, evidence of the health hazards of asbestos exposure wan accumulating. In 1972, the New York City Council banned asbestos spray application because of the health hazard to spray operators, other construction workers, and the general public. After failure of attempts at on-site asbestos-contamination control,S ° the EPA, in 1973, banned sprayed asbestos application for structural insulating or f ireproof ins. ~ ~ Decorative materials and some heavy mix materials were not included in the ban. In JU1Y 1978, the EPA banned Paragon application or materials, except those In wnlcn the asbestos Elders are encapsulated with a bituminous or resinous binder during spraying and that are not friable after drying. ·' A rough estimate of the total amount of asbestos-containing materials sprayed over the 28-yr period is 500, 000 tons. ~ 6 Although the spraying of asbestos~containing materials in construction ceased, such friable material in existing structures remains a widespread asbestos-f iber source with potential for indoor contamination (see Lumley; ~ ' Sawyer:' ~ and H. in Brown, tic LA, personal communications. ASBESTOS CONTAMINATION OF THE ENVIRONMENT Fiber release depends on both material cohesiveness and the disruptive energy applied. The majority (85% or more) of asbestos in current use is immobilized in strong binding materials, such as cement or tiles; however, any asbesto=~containing materials will release fibers when sufficiently disrupted, and the hard materials will liberate f ibers if ground, sanded, or cut. The remaining asbestos--including that in insulation, troweled asbestos; plaster, and pipe lagging--releases fibers upon minor disturbance. - These friable materials are the most important source of asbestos contamination in structures. Friable materials can be found on open and visible ceilings, walls, and structural members and on hidden surfaces accessible to maintenance, renovation, or ventilatory air flow.
114 The proportion of asbestos in such material is generally 10-30% by weight, but may vary from trace amounts to nearly 100%.~. Other f ibrou~ components include f ibrous glass, mineral wools, and cellulose. Friable materials can also contain vermiculite, talc, perlite, diatomaceous earth, organic f ibers, clays, quartz, gypsum, and various adhesives. Environmental contamination f rom asbestos~containing materials can occur in three general ways: fallout, contact disruption, and reentrainment of previously released but settled f ibers. ~ s Fallout is, except for very friable material, negligible. Contact disruption and reentrainment are activity~dependent and can result tin substantial contamination and exposure. Fibers enter occupied spaces at a relatively low rate, depending on material friability and exposed surface area. Variations in the fallout rate are due to structural vibration, air movement, and changes in cohesiveness. Fallout can result in the accumulation of surface deposits of f ibers over long periods; such accumulations are then available for later disturbance and reentrainment. Any asbestos-containing material will release f ibers if the energy applied to it is adequate. Contact may be intentional during demolition, renovation, or vandalism, unavoidable during maintenance, or accidental during routine activity. Fiber release depends on probability and energy of contact. Contamination is episodic and local and can be intense.3' The disturbance of released and accumulated fibers can cause repeated cycles of settling and resuspension. Such reentrainment contamination may occur after any disturbance, but can be important in custodial activities. ENVIRONMENTAL SAMPLING FOR ASBESTOS . Analysis of Bulk Materials Identification of asbestos is relatively simple with mineralogic specimens that are generally uniform in type and composition. With samples of construction materials, identification is more difficult. The amount of asbestos may be small, and construction materials may contain other fibrous components with a variable collection of nonf ibrous components. The primary method for asbestos identification in bulk materials is polarized-light microscopy (PIM). X-ray diffraction (XRD) -= used for quantitative analysis of f iber type. ~ ~ ~ ~ Transmission electron microscopy has only lionized application. ~ ~ The petrographic microscope is a transmitted-polarized-light instrument widely used for identification and characterization of substances based on their optical and crystallographic properties. The techniques are established, and the equipment is inexpensive. However a high degree of skill and experience is required of the microscopist. ~ ~ ~ ~ ~ ~
115 X rays diffracted by crystalline material produce a characteristic pattern. The technique usually yields information with a high degree of diagnostic reliability and a printed record. I`c is usually used to confirm results of petrographic microscopy. X-ray diffraction requires a large investment in equipment, references, mineral standards, and technical expertise. X-ray diffraction of bulk construction material cannot def ine particle shape and may fail to detect concentrations of asbestos much below S% . Moreover, other silicates or crystalline phases can interfere with asbestos identification. ~ ~ ~ ~ Specific and accurate fiber identification can be achieved by examination of the structure of individual particles, especially in conjunction with electron diffraction or energy~dispersive x-ray analysis. The extrapolation of precise electron-microscope data, however, to bulk sample information is ineff icient and costly. Its use in ident~f ication is usually conf ined to resolving ambiguities raised by petrographic microscopy and x-ray cliff Faction. The most important use of such analytic techniques is the identif ication and analysis of inorganic particles in tissue. ~ ~ ~ 6 Errors in asbestiform-mineral analysis have potentially serious consequences and are not uncommon. False-negative results will lead to a continuation of unnecessary environmental contamination, and false-positive results can precipitate unnecessary action. Errors arise from analyst inexperience and from the use of phase-contrast microscopy, rather than the appropriate polarized-light instrument.' 7 Measurement of Airborne Asbestos A pump is used to draw a measured volume of air through a membrane f ilter. The pump and f liter are either stationary or carried on a person, with the sampling orif ice in the respiratory zone. Common sampling rates are 2.0 L/min for personnel monitoring and 10 L/min for general environmental sampling. Sampling times vary from minutes to many hours, depending on anticipated fiber concentrations. Filter segments can be examined by var ious methods and observers for comparison or verification and can be stored indefinitely. Estimation of the amount of asbestos collected on the sampling filters is performed by one of two methods: counting fibers by optical microscopy with a phase-contrast light microscope and counting fibers by electron microscopy. The standard technique for fiber enumeration is specified by the National Institu~ce for Occupational Safety and Health (NIOSH) for determination of airborne asbestos in occupational settings.iS 19 Air is pumped through a membrane filter (effective pore size, 0.8 ~m). A filter segment is examined with a microscope that has phase-contrast illumination at a magnification of 400-450. Particles longer than 5 Em and with a length-to-width ratio (aspect ratio) greater than 3 are counted. Results are presented as the numbers of fibers per milliliter of air. This method enumerates only particles of defined aspect ratio and length. It is not capable of accurate identification of asbestos. Both the resolution limits of the
116 optical microscope and the S-pm length limit preclude enumeration of some smaller fibers, which may be present and in numbers greater by an order of magnitude or more. ~ ~ ° However, the short fibers that are not counted may be less hazardous than the longer ones that are. ~ ~ ~ ~ Electron microscopy is the definitive method for both identifying and counting small asbestos particles. When it is combined with selected-area electron diffraction (SAED) and energy absorption, accurate identification of a particle is possible 27 Laboratories vary in techniques of sample preparation, magnif ication, and mass estimation, and comparison of results has been discouraging. 36 furthermore, there are no standards for interpretation of exposure data derived this way. Investigation of asbestos contamination by electron microscopy is both expensive and extremely time-consuming. Results are usually given in nanograms per cubic meter for mass estimations, but can also appear as f ibers per milliliter where only enumerations is performed. ~ ~ 2 7 ~ ~ - _ Other Contamination Assessment Methods The most relevant estimate of asbestos hazard is based on the concentration of airborne, respirable asbestos f ibers in the immediate environment of building occupants. Under the usual conditions and with the standard NIOSH optical-microscopy technique, this task is difficult. The technique was originally intended for use in areas of recognized contamination, such as asbestos production facilities. The airborne contaminant was known, and relatively high concentrations were readily measured. In this setting, the optical technique is effective and appropr late . However, in the assessment of exposure situations in other, nonoccupational structures, it has become apparent that it is not entirely satisfactory. ~ 7 The optical-microscopy method is truncated in its limit of resolution both physically and by regulation. The 5-~n lower limit of counted particles will preclude the enumeration of many small fibers in the sample environment. 3° Fiber emission is nearly always local, sporadic, and act~vity-related. Routine air-sa~pling commonly fails to describe this contamination situation. 3' ~ ~ An alternative approach to assessment by air sampling involves a subjective observational ranking system to provide guidance in potential contamination situations. ~ ~ ~ 9 This process evaluates relevant contamination factors that can contribute to total contamination potential. Rating systems are an approach to a complex ~ ~ _ _ _ process involving f iber aerodynamics, material characteristics, structure effects, and human activity. However, they are not exact, are subject to variations in factor estimation, and cannot be easily evaluated with existing methods. They do not provide a precise benchmark for selecting appropriate corrective actions, but can provide consistent guidance in assessment and evaluation. An adequate system should reflect factors that influence material fallout, direct material disturbance, and reentrainment of fibers. These modes of contamination are functions of material characteristics,
117 structure conf figuration, and user activities. One example is a 8y8t~ that considers eight factors that influence exposure. i~ Tt is predictive to the extent of estimating the probability of contamination and exposure potential. The factors are: . . Total asbestos content of mater Sal . Friability of material. Condition of material. Extent of damage of material by water. Accessibility of material to activity and contact. Surface area of material exposed. Activity or movement in environment. Plenum or other airstream effects. The factors are individually weighted and used in a formula to generate a single score. Contamination evaluation by this met}x~d will provide guidance in two ways: {1) It indicates corrective action t scores exceeding a given value indicate that hazard potential is substantial. (2) It establishes relative priority, the most useful feature of the scaling system; the bigher the "core, the greater the need for corrective action, and Scores can be used to establish a logical sequence of corrective actions within indoor spaces or buildings . ASBESTOS AIR DATA Studies have been performed in structures containing various types of asbestos material (classified as either friable or bonded) and under a series of activity conditions. Tables IV-10 and IV-ll list airborne- fiber concentration data obtained by optical microscopy (with the HIOSE standardized technique tS) and electron microscopy. Nearly all the data were collected in nonoccupational settings in apartment buildings and private homes and in offices and school.. Both surveillance and reenactment studies are included. The airborne-fiber concentrations determined by optical microscopy range from zero in background, quiet, and some routine activity to over 100 fiber/1 in stripping dry-spray-applied ceiling material. Fi ~ r contamination teas an expected relationship to activity and proximity. The data in Table IV-ll obtained by transmission electron microscopy are expressed as nanograms per cubic meter. Thus, they do not distinguish between fibrous and nonfibrous asbestos, nor between long and short fibers. Estimation of the health significance of mass~concentration data f ram Table IV-ll is impossible . Obese is a lack of metbodolog to standardize ion, exposure standards, and applicable epidemiologic information that relates f iber number concentration to mass concentration. The electron-microacapy results vary from zero to nearly 2,000 ng/m3. There is a progression in contamination from background through routine and custodial activity. Exposure probabilities can be estimated to some degree by consideration of modes of contamination and activity in a building.
118 CO A: Cal ~ ~ Cal en · e e C' ~ O O ClOCQ ~ 1 ~ 1 1 C. ~ O O O ~ O ~ ~ ~ ~ O V ~ id · e e e e ~ ~ e e e ~ ~ e 0: 0 O O O O O O ~ O ~ O O O O C~ {) ~ ~ O ~ O ~ ~D `:! V o ·- CO ~ O O 1 Ct ~ O O ~ O O ~ O ~ 00 - ' ~ ~ ~ · e e e e ~ · · · ~ · . e S-l e' - 1 O O O O C~ _4 ~ O C~ O _~ _4 O V U: 0 o CD D ~0 ~: o :- ~5 ~ o o ~ o ~ :: ~ o ) ·. Fs oo v v o a, 0 c' s" ~ oo ~ ~ ~ v ~ o ~ c e' ·- :^ 0 :^ E ~ c ~ ~ ~ v ~ a, ^ C tov V ~ so oo O ~ ~ e- ^ v ~ ~ "v ~ o ~ ~ ~ ~ S" ~ ~ ~ ~ ~ ~ V ~ ~ o 0 0 cn ~ ~ Ll CD _ O V ~ ~ ~ ~ ~ ~ ~ ~ = - O C) c1 _' o ~ ~ 0 ~ ~ :^ ~ ~ ~ "o ~ C: ~ O ~ 3 ~ ~ ~ ~ ~ ~ 8 t.O ~ ~ ~ ~ ~ CO ~ Ct5 :^ ~ O ~ ~ :' V O CJ ~ V ~ ~ _1 O ~ ~ 0 O ~ ~ ~ ~ a ~ ~ ~ :~ V ~ C 0 ~ ~ _' £ _~ ~ 3 V ~ .V ' X ~ ~ O x:
~9 lo em ~ em ~ ~ ~ ~ ~ em em . am 1 or · · · · · . a, ~ 0 ~ ~ ~ I_ 1 1 · ~ ol c~ · · ~ ~ ~ To . lo em em ~ lo em em · 1 · e · · ~0 Cal ~ CO Us ~ ~ on ~ ~ Cal ~ ~ Cal rid ~ ~ ~ ~ 0 ~ ~ ~ O · ~ ~ ~ e ~ ~ · · · rid 0 ~ ~ cat ~ ~ ~ 0 0 ~ ~ ID O 0 .. O t be 1 _. ~ fill o :- ~ ~ ~ ~ ~ ~ ~ ~ v v ~ v S" I D tV V ~ ~ ~ ~ ~ C O ~ ~ ~ ~ ~ ~ ~ V ~ O V ~ L? == ~ ~ ~ ~ ~ ~ ~ ~ ~ V ~ ~ s~ ~ ~ ~ ~ O O _4 ct ~ ~ ~ 3 ct ~ :~ ~ ~ v 3 ~ O O b0 ~ ~ ~ ~ 3 E ~ D ~ ~ v ~ ~ v ~ ~ O V e3 <t ~~ ~ e ~ ·~ ~ E o = - C~ - C) ~ ~ ~ == "~ ~ ~V o ~ ~ ~ == ~ "C ~ O C ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ C ~ C ~ ~ C V ~ ~ ~ ~ ~ t0 Ct ct ~ ~ to v v ~ ~ ~ ~ ~ ~ ~ ~ E ~ ~ ~ 3 ~ ~ ~ ~ v :> ~ ct ~ ~ ~ ~ ~ ~ ~ ~ Q ~ ~ ~ ~ _ ~ ~ ~ ~ ~ ~ v ~ ~ ~ O ~ ~ ct ct ~ ~t ~ ~ ~ ~ ct ~ ~ ~ :, ~ ~ :^ ~ "c ~ ct 3 ~ ~ C =, ~., p,, _~ ~ S~ ~ ~ ~ ~ 0 ~ ~ D _ ~ ~ ~ ~ ~ ~ O ~J v u ~ D o V ~ 0 : c~ =~s ~ u: :~: ~ 0 cL o 1 1 ~ ~ ~ ~ 3 O O ~ D O Ca) {V S~ ~ C~ ~ V V ·- = - C) ~ ~ ~ ~ . V X V _/ O O O ~ ~ C;
120 TABLE IV-11 Alzborne Asbestos: Elect roe-Microscopy Data location and Activity Mean, ng/m3 n Range, ng/m3 Reference Urban outdoors: 48 U.S. cities <10 IB7 23 N. Y. City 17 22 2-65 25 N. J. schools 14 3 3-30 25 Indoors: F riable-asbes tos, structural surface: Of fice building 79 3 40-110 36 N. Y. City schools 99 5 9-135 24 Mas a. schools 151 5 38-260 24 N. J. schools 217 27 9-1, 950 25 Office buildings 2. 5-200 116 0-B00 24 Custodial activity: N. J. apartment 296 ~ -- 36 Conn. school 643 2 IB6-1, 100 36 N. J. school 1, 950 1 -- 25
121 Quiet or background conditions may represent the usual extent of activity in areas with low probability of either contact or reentrainment of asbestos. Under these conditions, contamination is low. Routine activities in a structure containing sprayed-asbestos surfaces will not usually result in detectable f iber concentrations. Routine activity can result in intense contamination in some situations. A school population's routine activity in a building with accessible asbestos surfaces may cause environmental contamination. Increased fallout, occasional contact, and reentra~nment may all con~cribute to the highly variable fiber concentrations found under these cond i tions . Custodial work can cause disturbance and reentrainment of accumulations of asbestos fibers. Reentrainment can be high during custodial activity, depending on cleaning methods. Maintenance work may involve direct contact with asbestos surfaces. Such activities may result in marked fiber dissemination. 36 Uncontrolled removal of sprayed-asbestos surfaces during renovation not only causes high fiber concentrations for the duration of the work, but also increases the released-fiber burden In the structure. In such cases, exposures involve the renovation worker and the routine building-user as well. Before a decision on building renovation to remove asbestos, the potential contamination during and after renovation must be evaluated. Both contact and reentrainment release mechanisms are involved, and very high concentrations occur during actual contact. S TANDARDS Estimation of the hazard associated with the airborne f iber concentrations of Table IV-10 can be only approximate. No exposure standards to evaluate hazard have been developed for the general indoor environment. The only existing standard is that of the Occupational Safety and Health Administration (OSHA). Other reconenendation~ for occupational exposure limits are those of NIOSH and the Amer lean Conference of Governmental Industrial Hygienists (ACGIH). Although these apply to and were intended for only occupational exposures, their consideration in the general indoor environment may have some merit: · The standard optical-microscopy method is used, and the air data of Table IV-10 are comparable with the standard limits. · The occupational exposure limits represent a distillate or summary of both exposure and epidemiologic information. Recently proposed changes reflect additional relevant epidemiologic evidence. The use of the occupational exposure limits is considered by some investigators to be acceptable for approximate ng the exposure hazard.'. Table IV-12 outlines the occupational exposure limits from 1972 to the present . Compar ison of the data in Table IV-10 with the exposure 1 imits of Table IV-12 demonstrates that Boone activities can exceed
122 TABLE IV-1 2 Occupational Asbesto s Exposure Limits Limit Time-Weighted Ceiling, Average (8 hid), f ibers/ml f ibers/ml OSHA original, 197246 5.0 10.0 OSHA present, 197645~46 2.0 10.0 OSHA proposed, 197543 0.5 5.0 NIOSH revised, 197741 0.1 0.5 ACGIH adopted, 1980 1 Amos ite 0. 5 -- Chrysotile 2.0 -- Crocidoli te 0. 2 -- Other forms 2.0 --
123 present time-weighted average {TWA) OSHA limits. Occasional events--such as removal, renovation, and vandalism {contact-mode categories)--exceed the 15-min excursion limit of 10 fibers/ml. The present TWA and ceiling limits were set by the 1972 regulations to become effective in 1976. 4 6 The more recent limits, the 1975 proposed and the 1977 revised recommended, reflect the increasing awareness in asbestos-disease epidemiology and are more stringent. With each successive regulation, the range of activities that could be considered hazardous becomes more inclusive. The activities remaining outside the 1977 limits are only in categories of quiet and nonspecific routine. Occupational standards can potentially be exceeded during renovation, maintenance, and custodial activities that disturb applied material or accumulated fibers. The optical-microscopy data indicate that contamination can sometimes exceed concentrations considered hazardous. Exposures occur in existing structures, and the population involved is large and varied in age, occupation, and behavior. Children attending schools that contain friable asbestos material constitute a population of special concern. The schoolchildren population differs from other nonoccupational populations in age, population density, and behavior. Any exposure would occur early in their life, leaving a long period for development of asbestos-related diseases. A large number of students can be exposed at one time to asbestos that is released from asbestos-containing materials in the school building. The school population is also very active. Friable asbestos-containing materials can be damaged during routine activities and as a result of capricious behavior. Many cases of badly damaged asbestos-containing materials have been found in schools. 3 7 REGULATIONS - Most asbestos handling, control, and disposal in the structural environment are subject to regulation by the EPA and the OSHA. Other federal agencies that regulate asbestos in various settings are the Mine Safety and Health Administration (MSHA), Consumer Product Safety Commission (CPSC), Food and Drug Administration (FDA), and Department of Transportation (DOT). In accordance with section 112 of the Clean Air Act ("National Emission Standards for Hazardous and Air Pollutants"), the EPA promulgated regulations on asbestos in 1973 (40 CFR 61, Subpart B. "National Emission Standard for Asbestos". 48 These regulations apply to the renovation or demolition of friable asbestos materials and to the spraying of asbestos. They specify procedures for removal and stripping of friable sprayed-asbestos fireproof ing and insulation materials. The required work practices include EPA notification, material-wetting, containment, container labeling, and disposal of the removed material in an approved landfill. Fiber concentrations are not specified, but the regulations require that there be no visible emission outside the structure.
124 Actions taken by the CPSC have banned the use of asbestos textiles in general-use garments, asbestos in artificial-fireplace materials, and asbestos~containing speckling and taping compounds. ' ~ Voluntary actions by manufacturers controlled the use of asbestos in hand-held hair~ryers. CONTROL OF CONT - INATION ~TE=I" there a potentially hazardous situation has Men identified in a structure, asbestos-contamination control or elimination methods are indicated." .t There are four approaches to corrective action: preventive management (a specific management system initiated to prevent disturbance of asbestos-containing material, with no direct action taken on the material itself), removal and disposal by burial, encapsulation (asbestos-containing material is coated with a sealant) and enclosure (asbestos-containing material is separated from the building environment by barriers, such as sealed suspended ceilings, The corrective methods can be used separately or in combination, and each has its own advantages. Removal eliminates the source of exposure to asbestos. Both enclosure and encapsulation are containment methods; because the asbestos material remains in the building, enclosure and encapsulation should be considered as temporary control methods, for use until the building is renovated or demolished. The surface cuff asbestos~containing material- can be damaged--causing the f r table f ragments to be released--by inadvertent or uninformed maintenance, repair, or renovation. A management system should be implemented to control and activity be either structure personnel or - . _ _ _ ~ ~ ~ ~ _ ~ _ . . . ~ ~ _ _ _ _ ~ _ _ _ _ ~ . _ ~ _ ~ . _ ~ . . ~un~`d~u`~O any ne~e~a~y WC,~'C Mu he pe~lw~ulau unu=z In conditions to protect involved personnel and other building users and to prevent contamination of the building environment. Renovation or demolition should include elimination of friable asbestos~containing materials under safe conditions and protection for the worker, the building users, and the ct~munity. Corrective action for surfaces considered very hazardous should be planned with priority appropriate to their contamination potential. Removal is the ultimate solution and will end contamination potential. Encapsulation with either penetrating or bridging sealants will enhance the cohesiveness of the material, eliminate fallout, and protect from minor damage. The technology of encapsulating asbestos has recently been the subject of intense interest, and effective sealants are now available. Management and control should be initiated for all areas that contain friable asbestos material, no matter what other action is planned. An asbestos management system can be implemented immediately, has low cost, and is highly effective in exposure control.37 .' SU~Y - Asbestos is a widespread component of the structural environment. Release of asbestiform mineral fiber'; from `;tructural components
125 depends on the cohesiveness of the asbestos-~ntaining material and the intensity of the disturbing force. Asbestos~containing material that is friable is most readily released in structures. }ligh-energy or machine disruption is necessary for the release of fibers from hard or bounce asbestos~containing materials. Durability and aerodynamic capability have combined deco produce a persistent and important contaminant for human exposure. Contam~.ation can be due to alight fiber release in fallout, relatively great release by contact or direct material disruption, or reentrainment of fallen and accumulated ~ ibers. Most contamination is episodic, activity-related, and local. Documented concentrations have been compared with existing and proposed Occupational standards. Extensive disruption of asbestos~containing material results in substantial hazard potential. Removal, renovation, and demolition of friable material and machining of hard asbestos-containing material result in a high degree of environmental contamination in the vicinity of the disturbance. Maintenance work or custodial care involving either friable material or accumulated fibers can cause airborne conta~n~nation that should be considered hazardous. REVERENCES 1. American Conference of Govern~ental.Industrial Hygienists. TLVs. Threshold Limit Values for Chemical Substances in Workroom Air Adopted by ACGIN for 1980. Cincinnati: American Conference of Governmental Industrial Hygienists, 1980. 93 pp. 2. Asbestos Information Association of North America. Asbestos-- General Information. Washington, D.C., 1975. 3. Assuncso, J. , and M. Corn. The effects of milling on diameters and lengths of fibrous glass and chry~otile asbestos fibers. Am. Tnd. Hyg. Assoc. J. 36:811-819, 1975. 4. Bragg, G. M., L. van Zuiden, and C. E. Hermance. The free fall of cylinders at intermediate Reynold ' s numbers. Atmo=. Environ. 8: 755-764, 1974. 5. British Occupational Hygiene Society, Committee on Bygiene Standards. Bygiene standard for chrysotile asbestos dust. Ann. Occup. Hyg . 11: 47 - 49, 1968. 6. Campbell, W. J., R. L. Blake, L. L. Brown, E. E. Cather, and J. J. Sjoberg. Selected Silicate Minerals and Their Asbestiform Varieties. Mineralogical Def initions and Identification- Characterization. Bureau of Mines Information Circular 8751. College Park , lad .: U. S. Department of Interior , Bureau of Mines, College Park Metallurgy Research Center, 1977. 64 pp. 7. Consumer Product Safety Commission. Consumer patching compounds and artif icial emberizing materials (embers and ash) containing respirable free-form asbestos. Fed. Reg. 42 :63354-63365, December 15, 1977. 8. Consumer Product Safety Commission. General use garments containing asbestos are banned hazardous substances. Code of Federal Regulations, Title 16, Part ISOO.17 (a7), 1970.
126 9. 13. Cement, J. M., R. D. Zumwalde , and K. M. Wallingford. Discussion paper: Asbestos f iber exposures in a hard gold mine. Ann. N. Y. Acad. Sci. 271:345-352, 1976. I. Dreessen, W. C., J. M. Dallavalle, T. I. Edwards, J. W. Miller, and R. R. Sayers. A study of Asbestosis in the Asbestos Textile Industry . Public Health Bulletin - No. 241. Washington, D. C .: U. S . Treasury Department, Public Health Service, 1938. 126 pp. Ferris, G., Jr. Asbestos Numerical Rating System. Boston: Commonwealth of Massachusetts Special Leg islature Commission on Asbestos, September 18, 1978. Fonaimare, A., et al. Quantitative study of the deposition of asbestos in the lung and pleura of subjects with diverse exposures. In Proceedings of the Symposium on the Pathology of Asbestos, Rouen, France, October 28, 1975. Langer, A. M. Approaches and constraints to identification and quantitation of asbestos f ibers. Environ. Health Perspect. 9: 133-136, 1974. 14. Langer, A. A., R. Ashley, V. Baden, C. Berkley, E. C. Hammond, A. D. Cackler, C. J. Magg~ore, W. J. Nicholson, A. N. Rohl, I. B. Rubin, A. Sastre, and I. J. Selikoff. Identification of asbestos in human tissues. J. Occup. Med. 15: 281-295, 1973. 1 5. Le. idel, N. A., S . G . Bayer , R. D. Zumwalde , and K . A. Busch. USPHS/NIOSH Membrane Filter Method for Evaluating Airborne Asbestos Fibers. DHEW (NIOSH) Publication No. 79-127. Cincinnati: U.S. Department of Health, Education, and Welfare, National Institute for Occupational Safety and Health, 1979. 89 pp. 16. Levine, R. J., Ed. Asbestos : An Information Resource, p. C-9. DREW Publication No. (NIH) 79-1681. Washington, D.C.: U.S. Government Printing Of f ice, 1978. [190 ~ pp. 17. Lumley, K. P. S., P. G. Harries, and F. JO O'Relly. Buildings insulated with sprayed asbestos: A potential hazard. Ann. Occup. Hyg . 14: 255 - 257, 1971. 18. McCrone, ~ . C . Evaluat ion of asbestos in insulat ion. Am. Lab . 11~127:19-31, 1979. 19. ~tillipore Corp. Monitor ing Airborne Membrane Filter . Application Procedure. Bedford, Mass.: Millipore Corp., 1972. 20. Murphy, R. L., B. W. Levine, F. J. Al Bazzaz, J. J. Lynch, and W. A. Burgess. Floor tile installation as a source of asbestos exposure. Am. Rear. Respir. DiS. 104: 576-580, 1971. 21. New York City Council. Air Pollution Control Code . Local Law, Section 1403.2-9.11(B). New York: New York City Council, 1971. 22. Nicholson, ii. J., A. N. RohI, and E. F. Ferrand. Asbestos air pollution in New York City, pp. 136-139. In H. M. Englund and W. T. Leery, Eds. Proceeedings of the Second International Clean Air Congress. New York: Academic Press, Inc., 1971. 23 . Nicholson, W. J., A. N. Rohl, R. N. Sawyer , E. Swoszowski , and J . D. Rodaro. Measurement of Asbestos in Ambient Air. Final Report. Contract CPA 70-92. National Air Pollution Control Administration, 1971. 24. Nicholson, A. J., A. N. Rohl, and I. Weisman. Asbestos contamination of building air supply systems. Paper No. 29-6 in Proceedings. International Conference on Environmental Sensing and
127 Assessment. Vol. II. Institute of Electrical and Electronics Engineers Ann. No. 75CH1044-~-29-6. Piscataway, N. J.: Institute of Elect r ical and Electronics Eng ineers , Inc ., 1976. Nicholson , ~ . J ., E . J . Swoszowsk i , Jr ., A. N. Robl , J. D. Tc~daro, and A. Adams. Asbestos contamination in United States schools from use of asbestos surfacing materials. Ann. N.Y. Acad. Sci. 330:587-596, 1979. . Pooley, F. D. Electron microscope characteristics of inhaled chrysotile asbestos fibre. Br. J. Ind. Med. 29:146-153, 1972. 27. Pooley, F. D. The identification of asbestos dust with an electron microscope microprobe analyses. Ann. Occup. Hyg. 18:181-186, 1975. 28. Prust, R. S. Future problems to be anticipated: Demolition, repair and disposal. Ann. N.Y. Acad. Sci. 330:545-547, 1979. 29. Reitze, W. B., W. J. Nicholson, D. A. Boladay, and I. J. Selikoff. - Application of sprayed inorganic f iber containing asbestos: Occupational health hazards. Am. Ind. Hyg. Assoc. J. 33 :118-191, March 1972. 30. Rohl, A. N., A. M. Langer, I. J. Selikoff, and W. J. Nicholson. Exposure to asbestos in the use of consumer speckling, patching~and taping compounds. Science 189:551-553, 1975. 31. Rohl, A. N., A. M. Langer, and A. G. Wylie. Mineral characterization of asbestos~containing spray f inishes, pp. 59-64. In D. S . Envirorunental Protection Agency. Asbestos Containing Material in School Buildings: A Guidance Document. Part I. U.S. Environmental Protection Agency, Office of Toxic Substances Publication No. COOO90. Washington, D.C.: U.S. Environmental Protection Agency, 1979. 32. Rohl, A. N., and R. N. Sawyer. Airborne Fiber Levels in Asbestos Abatement Projects. To be presented at International Symposium on Indoor Air Pollution, Health and Energy Conservation, Amherst, Mass. ~ October 14, 1981, sponsored by Harvard University School of Public Health, Energy and Environmental Policy Center. 33. Sawyer, R. N. Asbestos exposure in a Yale building. Analysis and resolution. Environ. Res. 13 :146-169, 1977. 34. Sawyer, R. N. Indoor asbestos pollution: Application of hazard criteria. Ann. N.Y. Acad. Sci. 330:579-586, 1979. 35. Sawyer, R. N. Yale art and architecture building: Asbestos Contamination: Past, Present, and Future . Institute of Elects ical and Electronics Engineers, Inc., Ann. No. 75CE1044-1-20-5. Piscataway, N.J e: Institute of Electrical and Electronics Engineers, Inc., 1976. 3 6. Lawyer , R. N., and C. M. Spooner. Sprayed Asbestos~ontaining Material in Buildings. A Guidance Document. Part 2. U. S. Environmental Protection Agency Report No. EPA-450/2-78-014. Research Triangle Park, N.C.: t3.S. Environmental Protection Agency, 1978. 3 7. Sawyer , R. N ., and E . J. Swoszowsk i , Jr . Asbestos abatement in schools: Observations and experiences. Ann. N. Y. Acad. Sci . 330:765-776, 1979. 38. Spell, S., and J. P. Leineweber. Asbestos minerals in modern technology. Environ. Res. 2 :166-208, 1969. . Stanton, M. F., and C. Wrench. Mechanisms of me';othelioma induction with asbestos and f ibrous glass. J. Nat. Cancer Inst. 48: 797-821, 1972 .
128 40. Timbrell, V. Inhalation and biological effects of asbestos, pp. 429-445. In T. T. Hercer, P. E. Morrow, and W. Stober, Ede. Assessment of Airborne Particles. Fundamentals, Applications, and Implications to Inhalation Toxicity. Proceedings of the Third Rochester Tnternat$onal Conference on Environmental -toxicity. Springf Geld, Ill.: Charles C Thomas , Publisher , 1972. 41. U.S. Department of Bealth, Education, and Welfare, National Institute for Occupational Safety and Bealth. Revised Recommended Asbestos Standard. DHFW {NIOSI1) Publication No. 77-169, 1977 . Washington, D.C.: U.S. Government Printing Office, 1977. 96 pp. 42. U.S. Department of Interior, Bureau of Hines. Mineral Industry Surveys. Asbestos. Washington, D.C.: U.S. Department of Interior, Bureau of Mines, 1979. 43. U.S. Department of Labor, Occupational Satety and Bealth Administration. Occupational exposure to asbestos. Notice of proposed rulemaking. Fed. Reg. 40:47651-47665, October 9, 1975. 44. U.S. Department of Labor, Occupational Safety and Health Administration. Occupational safety and health standards. Emergency standard for exposure to asbestos dust. Fed. Reg. 36:23207-23208, December 7, 1971. U.S. Department of Labor, Occupational Safety and Bealth Administration. Occupatione1 safety and health standards. Recodification of air contaminant standards. Fed. Reg. 40:23072-23073, May 28, 1975 (29 CER 1910.1001~. 46. U.S. Department of Labor, Occupational Satety and Health Administration. Occupational safety and health standards. Standard for exposure to asbestos dust. Fed. Reg. 37:11318-11322, June 7, 1972. 47. U.S. Environmental Protection Agency. National emission standards for hazardous air pollutants. Amendments to asbestos standard. Fed. Reg. 43:26372-26374, June 19, 1978. 48. U.S. Environmental Protection Agency. National emission s-~ds tor hazardous air pollutants. Asbestos. Fed. Reg. 38:~820~23, 8829-8830, April 6, 1973. 49. U.S. Environmental Protection Agency, Office of TOXIC Substance=. Asbestos Containing Material in School Buildings: A Guidance Document, Part 1. U.S. Environmental Protection Agency, office or Toxic Substances Publication No. C0009U. Washington, D.C.: U. S. Environmental Protection Agency, 1979. 50. VIllecco, M. Spray fireproofing facet; control'; or ban as research links asbestos to' cancer. Archit. Forum 133~57 :50-52, 1970. S1. Wagner, J. C., G. Berry, J. W. Skidmore, and V. Timbrell. The effects of the inhalation of a-be';to'3 in rats. Br. J. Cancer 29:252-269, 1974. 52. Zoltai, T. Asbestiform and acicular mineral fragments. Ann. N.Y. Aced. Sci. 330:621-643, 1979. FIBROUS GLASS Fibrous glass Is a man-made inorganic fiber with widespread application and distribution in the fabrication, textile, and construction industries. It is used in thermal insulation {for
129 structures, appliances, and pipe), acoustic insulation, textiles, plastic-material reinforcement. tire cord, yarns, matting, and filters. It is a common component of the structural environment. Although the production of fibrous glass is a half~century old, interest in potentially her ious adverse health effects has occurred f airly recently. DEFINITION The term Fibrous glass. generally includes particles composed of glassy material with a length-to-width (or length-to~diameter) ratio that exceeds 3. The composite elements of the glassy material form an amorphous structure and are not well ordered or crystalline, as in the asbestiform minerals. Contemporary conventional fibrous~glass production blends silica sand, limestone, and soda ash as raw materials in a continuous process. Before 1950, glassy fibrous materials, commonly known as Mineral wools,. were co~ranonly produced by pelting the slag of ore-smelting processes {slag wool) or naturally occurring rocks (rock wool). Mineral-woo] production began serious development in this country after 1920 and reached a peak in the 1950s, before modern continuous glans processes reduced the use of slag and rook as primary materials. ~ ~ ~ ~ There are two major categories of fibrous~lass products: continuous-f ilament glass and glass wool. Continuou~-f Lament glass is used in textiles and fabrics s as reinforcement in plastics, rubber, and paper; and in numerous other applications. It is produced by extruding molten glass through dimensioned orifices to yield fibers with fairly well~defined d iameters. Continuous glass f ibers can be selectively sized to provide the strength, hardness, or thermal properties desired for the intended application. Most continuous-filament operations produce fibers roughly 6 am in diameter, with some glass reinforcing fibers having Diameters of over 10 am (see Pundsack;~3 Smith;~. and W. Rietze, Johns Manville Corporation, personal communication). The Frost important example of glass wool is the f ibrous~glass thermal-insulation material used extensively in construction and equipment fabrication. Contemporary glass-wool production is a continuous processing of raw materials through melting, fiberization. and packaging. Fiberization devices use rotors or high-velocity gas jets either alone or in combination to produce particles of a desired d imens ion range. The d iameter is important in the~mal-insulation production, because the effectiveness of the material varies inversely with the fiber diameter. The fiber population produced in this way does not have the well~def fined diameter of the continuous-f ilament process. A glass-wool fiberization system produces a range of sizes following a frequency distribution characteristic of the process. t' Most commercial f ibrous~g lass insulation teas mean f iber diameters in the range of 4. 0-9. 0 Am. For special applications, a small percentage of glass wool is produced at mean diameters of 1.0 Am or less. I' i'
130 CONCERN OVER POTENTIAL ADVERSE HEALTH EFFECTS The increasing concern over the potentially serious health effects o f f ibrous-glass exposure is a consequence of a number of factors: · Interest in the carcinogenicity of asbestiform mineral f ibers has raised questions concerning a possible similar ef feet of other fibrous materials, including fibrous glass. I' · The f ibrogenicity and carcinogenicity of f ibrous glass had been demonstrated in animal inoculation studies in which mesothelio~s were produced by intrapleural and intraperitoneal implantation of fibrous glass. ~9 22 · Some studies of the mechanism of carcinogenicity associated with fibers have indicated that fiber dimensions are more important than physical or chemical properties. The demonstration of the apparent influence of particle size and shape indicated the possibility of common mechanisms and effects among fibrous materials, specifically asbestos and f ibrous glass. · Glass f ibers 1. O An or less in diameter are termed "microf ibers . In animal studies, f ibers of pathologic importance are in the microfiber range, with diameters less than approximately 0.S an. I' Fibers considered respirable have diameters of approximately 3.5 ~ or less. ~ 20 Furthermore, the aerodynamic capability and potential for respiration increase mainly as a function of decreasing f iber diameter. 2 0 21 This implicates microfibers as the most suspect in pathologic importance, with respect to cellular effect, respirability, and aerodynamic capability. ~ Microfibers are being intentionally produced for special applications, and there is incidental microf iber production in some glass-wool processes. · The use of f ibrous glass is widespread and increasing . There is heavy consumption of f ibrous glass by the construction industry, including friable materials that readily release fibers in the s tructural environment . The substitution of f ibrous glass for asbestos and the demand for f ibrous-~lass insulation products for energy conservation will increase the use of these f riable materials. The forms, uses, and distribution of fibrous-glass materials implies a substantial impact if the material has marked adverse health effects. There is concern that human exposure to f ibrous glass may cause d isease. However, studies of mortality and morbidity and radiographic examinations have failed to demonstrate discernible hazard in occupa- tional populations with exposure to fibrous-glass particles. ~ ~ ~ 2 2 3 Glass microf ibers could be expected to have more a irborne persistence, respirability, and cellular effect. However, no human epidemiologic data support the concept of microfiber pathogenicity. The industrial use of microfibers is relatively small, and there have been few accumulated years of human exposure.. However, in the older mineral-wool production, with a wide distribution of f iber dimensions, there has been a substantial population of - particles that meet the microfiber definition. In the mineral~wool industry, the accumulated
131 exposure experience is large in numbers and years. 13 The health significance of microfiber exposure in human populations is not known. I~ORTANCE OF CHARACTERISTICS OF FIBROUS GLASS The potential for environmental contamination and exposure is influenced by the dimensions of f ibrous-gla~s particles . 80th airborne persistence and respirabili~cy of the environmental contaminant are less than those of asbestos, because of the relatively large diameters of the glass particles. Emission rates of f ibrous glass depend on the proximity of the source mater ial, general character istics of cones iveness and f Liability, and the intensity of the force causing the disruption. Studies of the general environment, including space ventilation systems, have demonstrated extremely low concentrations, less than 3 fibers/L. ~ Glass-fiber concentrations in occupational and production environments vary widely with the nature of the production process. Most studies have shown normal production-facility concentrations well below 1 fiber/ml as measured by the OSHA-NIOSE standard method for airborne asbestos. S-7 l. As would be anticipated from ceramic considerations, studies in microfiber production facilities have documented airborne concentrations orders of magnitude higher than those in conventional fibrous-glass processes.. I The more friable forms, such as thermal insulation, are potential sources for environmental contamination. Studies of concentrations of a irborne f ibrous particles during removal of friable insulation mater ial have shown high concentrations of airborne f ibrous glass in the vicinity of worker activity. During the removal of friable spray-applied material (208 chrysotile asbestos and 70% fibrous glass), fiber counts in excess of 100 fibers/ml were encountered. Is ANALYSI S _ The polarized-light microscope can be an effective instrument for identification of fibrous glass in construction materials. Characteristic shape, transparency, and lack of birefringence distinguish fibrous glass from asbestos mineral fibers. Modern fibrous glass usually appears as isotropic particles of fairly uniform diameter, rod-like appearance, and high length-to-diameter ratio. Some mineral-wool products may have highly variable dimensions, teardrop shapes, and spherical glass ~shot. of relatively large diameters. ti i STANDARDS . . _ A TLV/TWA of 10 mg/m3 for f ibrous glass or dust has been listed by the ACGIM. 2. This had been listed as a nuisance particle, with the occupational exposure limit for f ibrous glass Warren as 30 x 10 particles/ft3, or 10 mg/m3 of air. ~ In a recent industry-wide
132 survey, concentrations of airborne particulate matter were generally less than 2.S mg/m3. s CONTROL In consideration of the uncertainties of carcinogenicity, relevance of the exper imental tumor-production studies in animals, and the character istics of glass-f iber exposures of people, i t appears prudent to reduce microfiber exposure to the lowest possible point permitted by available technology. REFERENCES 1. American Conference of Governmental Industrial Hygienists. Industrial Ventilation. A Manual of Recommended Practice, p. 13-13. 1 4 th ed . Lans ing , MiCh .: Amer lean Conference of Governmental Industrial Hygienists, 1977. 2. American Conference of Governmental Industrial Hygienists. ILVs. Threshold Limit values for Chemical Substances in Workroom Air Adopted by ACGTH for 1980, p. 19. Cincinnati: American Conference of Governmental Industrial Hygienists, 1980. 3 . Balzer, J . L. Environmental data ; airborne concentrations found in var ious operations, pp. 83-89 . In U. S. Department of Health, Education, and Welfare, National Institute for Occupational Safety and Health. Occupational Exposure to Fibrous Glass. Proceedings of a Symposium. BEN Publication No. (NIOSH) 76-151. Washington, D.C.: U.S. Government Printing Office, 1976. Dement, J . M. Environmental aspects of f ibrous glass production and utilization, pp. 97-109 . In U.S . Department of Health, Education, and Welfare, National Institute for Occupational Safety and Health. Occupational Exposure to Fibrous Glass. Proceedings of a Symposium. HEW Publication No. {NIOSH} 76-151 . Washington, D.C.: U.S. Government Pr inting Of f ice, 1976 . Esmen, N., M. Corn, Y . Ha~ad, D. Whittier , and N. Rotsko. Summary of measurements of employee expo';ure to airborne dust and f iber in ~ ixteen facilities producing man-made mineral f ibers. Am. Ind. Hyg. Assoc. J. 40 :108-117, 1979 0 Esmen , N. A., Y . Y. Hand ~ M. Corn, D. Whittier , N. Kotsko, M. Haller, and R. A. Kahn. Exposure of employees to man-made mineral f ibers: Mineral wool production. Environ. Res. IS: 262-277, 1978. ,. Fowler, D. P., J. L. Balser, and W. C. Cooper. Exposure of insulation workers to airborne fibrous glass. Am. Ind. Hyg. ABSOC. J . 32: 86-91, 1971. 8. Gross, P ., J. Tuma, and R. T. P. deTreville . Lungs of workers exposed to fiber glass;. A study of their pathologic changes and their dust content. Arch. Environ. Health 23: 67-76, 1971. 9 . Hill, J. W., W. S. ~itehead, J . D. Cameron, and G. A. Bedgecock . Glass fibbed: Absence of pulmonary hazard in production workers. Br . J. Ind. Med. 30 :174-179, 1973. I:
133 10. Ronzen, J. L. Results of environmental air-~pling studies conducted in Owens~Corning Fiberglas manufacturing plants, pp. 115-120 . In U. S . Department of Health, Education, and Welfare, National Institute for Occupational Safety and Health. Occupational Exposure to Fibrous Glass. Proceedings of a Symposium. B]3W Publication No. (NIOSH) 76-151. Washington, D.C.: U. S. Government Pr inting Of f ice, 1976 . 11. McCrone, W. C. Evaluation of asbestos in insulation. Am. Lab. 11 (12~:19-31, 1979. 12. Naar, A. N. M., T. Ditchek, and P. A. Scholtens. The prevalence of radiographic abnormalities in the chests of fiber glass workers. J. Occup. Med. 13: 371-376, 1971. 13 . Pundsack, F. L. Fibrous qlass--manufacture, use, and physical properties, pp. 11-18. In U.S. Department of Health, Education, and Welfare, National Institute for Occupational Safety and Health. Occupational Exposure to Fibrous Glass. Proceedings of ~ Symposium. HEW Publication No . (NIOSH) 76-151 . Washington , D.C.: U.S. Government Printing Office, 1976. 14. P=hI, A. N., A. IS. Langer, and A. G. Wylie. Mineral character ization of asbestos-containing spray f inishes, pp. 59-64 . In U. S . Environmental Protection Agency, Of f ice of Toxic Subs Lance" . Asbestos Containing Mater ial in School Buildings: A Guidance Document . Part 1. Washington , D.C.: U.S. Government Printing Of f ice, 1979 . 15. Sawyer, R. N. Asbestos exposure in a Yale building. Analysis and resolution . Environ . Res . 13 :146-169, 1977 . 16 . Smith, H. V. History, processes, and operations in the manufactur ins and uses of f ibrous glass-~one company ' ~ experience, pp. 19-26. In U.S. Department of Health, Education, and welfare, National Institute for Occupational Safety and Health. Occupational Exposure to Fibrous Glass . Proceedings of a Sympos ium. HEW Publication No. (NIOSH) 76-151. Washington, D.C.: U.S. Government Printing Office, 1976. 17. Stanton, M. F. Fiber carcinogenesis: Is asbestos the only hazard? J . Nat. Cancer Tnst . 52: 633-634, 1974 . 18. Stanton, M. F. Some etiological considerations of f ibre carcinogene~is, pp. 289-294 . In P. Bogovski, G. Gilson, V. Timbrell, and J. C. Wagner, Eds. Biological Effects of Asbestos. Scientif ic Publications No. 8 . Lyon, France: International Agency for Research on Cancer, 1973. 1 9 . Stanton , M. F., and C . Wrench . Mechanisms of mesothelio~ induction with asbestos and f ibrous glass . J . Nat . Cancer last. 48: 797-821, 1972. 2 0. Ti~brel1, V. Aerodynamic considerations and other aspects of glass f iber, pp. 33-50 . In U. S . Department of Health, Education, and Welfare, National Institute for Occupational Safety and Health. Occupational Exposure to Fibrous Glans. Proceeding" of a Symposium. HEW Publication No. (NIOS~) 76-}51. Washington, D.C.: U.S. Governmen t Pr int ing Of f ice, 19 76 .
134 21. Sawyer , R. N., and C. M. Spooner. Sprayed Asbestos~Containing Material in Buildings. A Guidance Dc~c~ent. Part 2. O. S . Environmental Protection Agency Report No. EPA-450/2-78-014. Research Triangle Park, NoCe UeS. Environmental Protection Agency, 1978. 2 2. Wagner , J. C ., G. Berry, and V. Timbrell. Me~otheliomata In rats after inoculation with asbestos and other materials. Br. J. Cance r 2 8: 173-185, 1973. 23. Wright, G. h. Airborne fibrous glass particles. Chest roentgeno<3rams of persons with prolonged exposure. Arch. Environ. Health 16 :175-1810 1968. COMI3tJSTION SOURCES Whenever Invented combustion takes place indoors or venting systems attached to stoves, boilers, or heaters are malfunctioning, a wide range of combustion products can be discharged directly into the indoor atmosphere. This section summarizes essential information pertaining to indoor sources of combustion products and their effects on indoor air quality. The emphasis is on residential buildings, where research efforts on indoor air quality have been concentrated, but results of limited studies of combustion-generated pollution in other types of buildings are also presented. In general, the data presented here represent isolated situations whose characteristics are highly specific to the site and the combinations of activities that produced the ef fects; therefore, transfer of these findings to other situations must be done only with extreme care. Smoking (which is discussed elsewhere), is the most widely encountered source of combustion products indoors. Besides smoking ~ the primary sources of combustion byproducts in residential buildings are usually space heater';, gas stoves, and gas water heaters. Exhaust from automobiles in attached garages can also be a source of combustion byproducts in buildings, as can wood fires, oil and kerosene lamps, and candles. The major pollutants associated with indoor combustion are carbon monoxide, nitric oxide, nitrogen dioxide, aldehydes and other organic compounds, and f ine particles. These combustion products usually occur in low concentrations, compared with the major combustion products-- carbon dioxide and water vapor. Inefficient combustion from Invented or poorly vented space heaters, f fireplaces, and lamps can also emit carcinogenic hydrocarbon particles. Carbon dioxide and water vapor are also produced as a result of normal metabolic processes of building occupants and add to the burden associated with gas appliances. Humans produce 30-60 g of carbon dioxide and a similar amount of water vapor per hour . An Invented space heater rated at 10, 000 Btu/h, or about 2,500 kcal/h, produces around 750 g of carbon dioxide per hour. Accordingly, depending on occupant density, space limitations. and the extent of ventilation and infiltration, carbon dioxide concentration in the indoor atmosphere can r ise substantially above the normal range of 0. 03-~. 06~. Respiration is affected when the concentration of carbon
135 dioxide in the air risen above 1.5%, and concentration above 3% can cause headache, dizziness, and nausea. Above about 6-8%, carbon dioxide causes stupor to the degree that exposed persons are unable to take steps for self-preservation. ~ The Threshold Limit value in the occupational environment is 0.51 i--a value which teas also been applied to submarine crews, whose incidence of illness increased after long-term exposure to concentrations of 0~5-18~' 22 2S In the residential environment under occupied conditions, carbon dioxide concentrations are typically 0.07-0.20~. RES IDENTIAL WINGS Space Beating There are many documented cases of health problems and even deaths resulting from excessive carbon monoxide released by Invented or improperly vented heating systems; 2 ~ 6 ~ ~ 2 7 however, few systematic studies bave provided detailed measurements of indoor asexuality problems in such house" . Most space heating in U. S . houses is by externally vented heating systems {central furnaces or space heaters). When the beating system is properly designed, maintained, and f unctioning, combustion products that could directly affect indoor air quality do not enter the indoor environment. However, if a negative pressure develops in the interior space or if there is a faulty exhaust system (e.g., a cracked heat exchanger or blocked flue), there can be direct and serious degradation of indoor air quality. Some space beating of homes is generally provided by unvented gas and kerosene heaters. This type of heating is more commonly used in rural areas and warm climates, such as the southern United States, and is especially dangerous, because it emits its combustion products directly into the living space. In their study of indoor air quality in several homes in Rotterdam, Biersteker and associates determined that sulfur dioxide concentrations indoors are not normally affected by beating systems that are kept in good condition. 3 Bc>wever, in one older home with a faulty beater, the indoor concentration was 3.8 times the outdoor concentration. In a 1969-1970 study of indoor~outdoor air quality in the United State=, Yoco~n and co-workers selected four homes, two public buildings, and two office buildings for analysis.28 29 One of the two homes with coal heating bad an antiquated central beating system with a leaky flue. Sulfur dioxide in this home approached 1 ppm and carbon monoxide exceeded 50 ppm over periods of 1 b and longer. coinciding with periods when coal was added to the fire and the fire was Stoked. As part of a pilot study to assess indoor air quality in bu ildings, Hollowell and co-workers abowed that a gas-f ired beating system In one home, although vented to the outside, produced higher nitric oxide and nitrogen dioxide concentrations indoors than outdoors. 9 In this work and in the earlier work of Yocom et al. 2 ~ 2 9 and Bier~teker et al. , 3 no attempt we" made to measure the emission rate" of the
136 combustion source or the rates at which pollutant gases entered the indoor environment. In a 1973-1974 study of indoor sources of sir pollutants, Cote and co-workers determined the emission rates of carbon monoxide, nitric oxide, and nitrogen dioxide from an unven ted gas-fired space heater.' Table IV-13 shows the emission data obtained. Y^manaka and co-workers measured nitrogen oxides from various Invented and vented space heaters commonly used in Japan.2' Results on both radiant and convection types of Invented kerosene heaters, as well as on various water heaters and gas stoves, were reported. Nitrogen dioxide emission from the radiant kerosene-fired space heater averaged 46 ~/kcal (~.011 g/MJ); that from the convection type averaged 251 u9/kcal (0.060 g/MJ). In all the studies noted, the purpose war to measure emission of ~typical. units. The units tested were not necessarily representative of the entire class of devices from which they were selected, nor was there any attempt to conduct an exhaustive study of their combustion character istics . Homeowners in many parts of the country are returning to the use of wood as a heating fuel, because of the increasing cost of oil and natural gas . Th is trend i'; especially strong in the northeastern state., which have depended largely on oil as a heating fuel. (me price of No. 2 fuel ot1 increased by a factor of 6 or more between 1965 and 1980. ~ Wood stoves and f ireplaces are vented to the outdoor atmosphere, but a number of circumstances can cause combustion products to be emitted to the indoor atmosphere: improper installation (e.g., insuf f icient stack height), cracks or leaks in or poor f itting of s tovepipe, negative air pressure indoors, downdraf ts, and accidents, as when a log rolls out of the fireplace. Although much is known about the combustion products of fuels used for space heating, little is known a put the impact of the emission from wood stoves and fireplaces on indoor air quality--a subject urgently in need of investigation. Combustion products of wood are highly irritating to the eyes, nose, and respiratory system and thus provide a warning to occupants that combustion products are present. Table Iv-14, from the work of Duncan and co-workers, shows the tides of pollutants associated with wood burning. ? ~ , , _ _ _ _ ~ A field monitoring program designed to compare indoor and outdoor pollution in 10 residences and two office buildings was undertaken in the Boston metropolitan area. Three of the monitored residence. used either a wood stove or ~ fireplace in the course of the sampling period. Increased indoor concentrations of total suspended particles (TSP), respirable particles, and benzo[~]pyrene were observed during periods of wood-burning. The average indoor TSP concentrations during days with wood-burning were about 3 times the corresponding concentrations during days without wood-burning. Indoor 24-h benzolalpyrene concentrations during days with wood-stove use were an average of 5 timer higher than those during non-wood-burning periods. The authors concluded that the increased indoor concentrations of TSP,
137 TABLE IV-1 3 Pollutant-Gas Emission from Unvented Gas-Fired Space Heatersa Pollutant Emission Pollutant Emission Heat Input, Factors, ~ g/kcal Rates, mg/h Operation keel /h NO NO2 CO NO NO2 CO ~ ~ , Low f lame, steady state 2, 800 76.4 46.4 632 214 130 1, 770 High flame 6, 200 135 43. ~ 319 837 aBased on Cote et al 6 . 272 1 ~ 982
138 TABLE IV-14 Emission f ram Residential Wood-Fired Stovesa Emi ssion, lb/cordb _ ubstance Emitted home Aver Criteria pollutants Particles 3~93 30.3 SOx 0. 5-1. 5 0.7 NO 0.7-2.6 1.6 Hydrocarbons 1-146 41.6 Carbon monoxide 3001, 220 598. 3 Noncri teria pa llutants Polycyclic organic 0. 6-1. 22 0.9 materials Formaldehyde 0. 3~1 0. Acetaldehyde 0.1-0. 3 0. 4 Phenols 0. 3~8 3.3 Acetic aci d 5-48 21.1 Aluminum -- 1. 3 Calcium -- 10.2 Chlorine -- 0.1 Iron Magnesium -- 2. 0 Manganese ~ 1. 6 Phos phorus -- 1.0 Potassium -- 3. 6 Silicon -- 1.6 Sodium ~~ 0. 7 Ti tanium ~~ 0. 02 aBased on Duncan et al. 7 bThe relationship used to convert from lb/ton to lb/cord was: 1 lb/ton ~ 1.65 lb/~ord.
139 respirable particles, and benzofalpyrene attributed to wood-burning may have long-term health implications. Gas Stoves: Pollutant Emission Rates and Concentrations - The early pilot studies of Yocom and co-workers, reported in 1974, showed that, on the basis of relative indoor-outdoor concentrations of carbon monoxide, Invented gas stoves definitely contribute to the deterioration of indoor air quality. 28 A brief study by the EPA showed that peak nitrogen dioxide concentrations up to 1 ppm (about 1,880 ~/m3) and 1-h averages of O.25-0.50 ppm (about 470-940 ~/m3) are reached in a closed kitchen with no external ventilation.. Wade and co-workers in 1973-1974 studied four homes equipped with gas stoves to determine the concentrations of nitric oxide, nitrogen dioxide and carbon monoxide and their impact on indoor air quality. 24 Sampling was carried out for 2-wk periods in each home, simultaneously at four sampling locations--three indoors and one outdoors. Table IV-15 presents the principal data from one home included in this study. The following were the main conclusions of the study: · Emission from gas stoves contributes nitrogen dioxide, nitric oxide, and carbon monoxide to the indoor atmosphere of houses where such stoves are used. Kitchen concentrations of these gases responded rapidly to stove use and, for a given house during a given season, there was a rough correlation between average nitrogen dioxide concentrations and average stove use. · Nitrogen dioxide and nitric oxide were produced in roughly equal amounts in the homes where testing was conducted. Indoor concentrations of these pollutants were invariably higher than those outside. · Normal stove operations frequently resulted in nitrogen dioxide concentrations in the kitchens averaging over 100 ~/m3 for the 2-wk sampling per iods . · Comparison of samplings carried out in the spring-su~mer of 1973 and the fall-winter of 1973-1974 showed that in the colder weather, when the house was closed up more often, pollutant concentrations were more uniformly distributed in the various rooms of the house than In the warmer months. · A diffusion experiment conducted in one of the houses showed that the half-life of nitrogen dioxide was coly one-~hird that of carbon monoxide and nitric oxide, indicating that nitrogen dioxide decays through reaction or adsorption, in addition to normal dilution from air exchange. This effect was observed in some of the other houses by comparing the relative concentrations of nitrogen dioxide and the other pollutants In various parts of the house. Moschandreas and his associates carried out a 2-yr air sampling program to characterize the indoor residential air environment. 12 Indoor air quality was monitored for continuous periods of
140 o I of ~ ~ o - ~ ~ - + o' .. ~ ~ e e e ~ I I I ~ ~ ~ ~ ~ 1 1 1 0 V I: so :> 1 a: O _ b O ~ ~ _ 4~~ ~C) | I e ~ ~ ~ O O O _ ~ 1 _ ~ ~ Cal Cal ~ O 0 _ ~ -. _ 0 ~ CL C' O O ~ O ~ ~ O ~ O aa ~ ~ ~ A 0 us ~0 1~ ~ ~ _~ ~ ·^ ~ 3 ~ O _ ~ ~ en "v ~1 V , 0 of - - O V a ~ - , C 1 o 1 ~ be to ~ ~ O b bO O ~ ~ u~ ~ ~ ~ O ~ D v O ~ ~ _. 0 C L' ~ ~ +^ 0 1 ~ -^ 3 O ~ ~ ~ ^ e' a, ~° ? c ~o '-o ~c~ I C ~ - d ~ =_~ ° ~ O O ~ ,~ ~ ~ a~ C 0 ~ O ~ O O ~ O ~ _ ~ o C s~ ~ ooc~ =~ - =~ - o =° ~ 'u|~ ~ ~ - | C~ ~ ~ C V ~ o S~ ~ ~ ~ O O ~ O _ t V L. ~ ~ ~ ~ ~ - + ~ ~ O ^ ~ ~ ~4 ~ ~ _. ~ ~o O ~ -t ~ ~ tn ~ 1 1 0 +^ -^ ~ ~ ~ 0 cn 0 ~ 0 a-^ o so o > - 0 q5 000 0 0 0 0 00 ~ _10 ~ ~O ZZ~ Z=O ZZO ~= ~ 1 1 ~10~= a0 1 . ^ - ~ - - o - ~ ~ - ~ O o~ ~ ~` ~ ~ o' .c V ~ ~ ^ so 0 ~ ~ ~ - " o ~ o ~ ~ ~ 1 ~ ~ ~ C ~ 3 - = o£ - ~ - 0 3 - 0 ~S v O ~ 1 - 1 ~ ~ 1 ~ ~ ~ V o, ~ o~ - 4 _ o' ~ a, v ~_ - - ~ ~ ~ 0 ~ ~ ~ ~ ~ C~ co ~ cn
141 approximately 14 d in each of five detached dwellings. two semidetached dwellings (townhouses), six apartment units, two mobile homes, and one school. Three of tbe dwellings were referred to as ~experimental, ~ because they were designed to conserve energy. The remaining dwellings were referred to either as ~conventional. or by structural type. In addition, the residences were divided according to their cooking and heating f uel . These structures are in f ive metropolitan areas: Baltimore , Washington , D.C., Chicago, Denver , and Pittsburgh. me dwellings in Baltimore, Washington, D.C., and Chicago were monitored twice to obtain seasonal variation.. Conclusions front the study pertaining to pollutants associated with combustion products {carbon monoxide, nitric oxide, and nitrogen dioxide) follow: Indoor CO concentrations are generally higher than corresponding outdoor levels in all residences monitored. High indoor concentrations may be attributed to . . . indoor CO emission sources, such as gas-fired cooking appliances, attached garages, faulty furnaces, and cigarette smoking. . . . The complexity of the dynamics involved in the establishment of an indoor-outdoor relationship is clearly illustrated in the interpretation of the data base generated for NOe From the perspective of NO indoor variation and under real-life conditions, three types of indoor environments have emerged: 1) houses with electric cooking and heating appliances; 2 ~ houses that are heated by gas furnaces, yet serviced by electric cooking appliances; and 3 ~ houses that are furnished with gas cooking and heating equipment. In houses equipped with gas cooking appliances, observed indoor NO levels are cons istently higher than observed outdoor levels . Houses with gas furnaces but elects ic cooking appliances display h igher NO indoor levels than outdoor levels, most of the time. However, there are time intervals interspersed throughout the monitoring period during which the observed NO outdoor levels surpass corresponding indoor levels. Indoor NO concentrations in totally electric homes are almost always lower than corresponding outdoor concentrations.... The residential environment often provides a shelter from high outdoor NO2 levels. The three classes of residences identified in the interpretation of the NO data also manifest themselves in the study of NO2. The data base collected for this project indicates that the hourly average indoor concentrations of NO2 are almost always lower than the cor responding ambient levels in totally elects ic houses . Houses equipped with gas furnaces and electric cooking appliances also shelter their occupants, but to a lesser extent during peak ambient NO2 levels. Totally gas residences do not appear to provide such protection. . .
142 W i th the large data base generated by the study, Moschandreas and Stark2 ~ formulated, documented, and validated a numerical prediction model (see Chapter VI). A series of numerical simulations with the model showed that, under some conditions involving gas appliances, indoor carbon monoxide, nitric oxide, and nitrogen dioxide concentrations increase substantially. Under these same conditions (with the oven in use for 2 consecutive hours), carbon monoxide may reach concentrations over 3 5 ppm. Ibe inf luence of gas stoves on indoor nitrogen dioxide content was confirmed by Palmes and associates, who used integrating ~personal. monitors. ~ s Melia and associates u see this same type of sampler in an epidemiolog ic study to show that nitrogen dioxide concentrations were signif icantly higher in homes with gas stoves than in those with electric stoves. ~ ° Puxbaum measured nitric oxide and nitrogen dioxide in a kitchen and an adjoining room in a home with a gas stove and gas hot-water appliance and compared indoor and outdoor concentrations. He also compared the burning of natural and ~town" gas and found that the concentrations of both nitrogen dioxide and nitric oxide were signif icantly higher during the natural-gas runs than during the town~gas burning. ~ 7 In their 1973-1974 study, Cote and co-workers measured rates of pollutant.emission from gas appliances. Table IV-16 presents their data from various burner configurations and operating modes for a new and an old stove . (These stoves produce low but continuous emiss i on f rom the pilot light, whereas current trends are toward low-heat-input gas pil ot lights and novas ignition systems, which will reduce this source of indoor air pollution. ~ More recently, Traynor and co-workers studied, in detail, emission from a gas-fired stove. 23 Table IV-17 summarizes some of the data from this work. In the case of nitrogen dioxide, there was generally good agreement between the Cote and Traynor studies. No laboratory tests on electric stoves were carr fed out for comparison in these studies, although, in an earlier field study, Hollowell et al. did observe small increases in kitchen ozone concentrations with electric-stove use. 9 Both Cote et al. and Hollowell et al. measured gaseous pollutant emission from top burners while water-filled pans of different materials were being heated. Cote et al. noted some minor effects on gas-flame emission, especially with a low flame.. For example, carbon monoxide concentrations were higher when an aluminum pan was in place, and nitrogen dioxide concentrations were marginally lower with all types of pans under low-f lame conditions. Hollowell and co-workers, however, could f ind no appreciable differences in gaseous emiss ion, regardless of the types of pans used . In the study by Cote and co-workers, the efficiency of an exhaust hood in removing stove-generated pollutants was determined. 6 Removal efficiencies varied from 4% with the hood fan off to 49% with the fan at its highest speed setting . (The low removal eff iciency with the fan off was the result of natural ventilation through the hood. ~ Pa recirculating hood with activated charcoal, as might be expected, had
143 8 . Cal _ o ~ ID ~ ~ ID o ~ ~ _ ~ _ . ,_ ~ ~ C , _ . _ _ o CO · ~ or 0 o ~ _ _ ~ ~ _ _ ~ m~ r_ 6= Ol 1 - At_ - = _ Z U) V so C: t: E O Ct ~ Lo v CO to o ~ ~ o ~ Go undo =0` C ~ ~ O ~ I- Cal t0 ~ ~ ~ O ~ O ~ O O ~ ~ ~ U~ ~ ~ ~ ~o Ll o0 Ct C~ U' _' O E ~ ~ ~ 0 _ ~ O ~ ~ O ~ ~ ~ ~ ~ ~ ~ O ~ V L) ~ U~ '_ ~ ~ r~ ,, C' CO ::' O ~ ~ O ~ ~ O O _ V · · · · · · _ C) ~~ C~ I_ _ ~ O ~ ~ O ~ O ~ ~ - m ~ ~ f_ =4 tZ. ~ _ _I 0 a, ~: ;L~ V _ Ct CL V 1 ~ ~ ~ ~ O O O O O O O O '000 000 0 C~ U~ V ~ ~ ~ Ct V Ct C~ = - ~ O ~ cn ~ = c~ -5 C~ C' O z - o o U' ~0 V C: O _ ~ V m0 0 :- Ct E U~ C C~ ·S ~ E ~ e ~ ~ ~ Ct _ _ ~ V ,_d ".d ~ s V . - V ~: t0 cn ~ oo ~n oo ~ t0 _ o0 ~ ~ :^ 0O _ ~: V ~: ~; V ~ ~5 =: ^ ~ s ^ ^ 0 ~ ~ ^ 0 ~ ~ ~ v ~ s~ ~ v _ ~ ~ a, _ ~ ~ a' ~ ~ t: C: v ~ s" ^ o ~ ~ c o ~ ~ ~ _I D D ~ _I D D _ :> O P. ~4 ~ O _ Ct o ~ I o~ v ~ S4 ~ ~ ~ ~ O _ O ~ ~ V V V CO ~ O tq v a' u, ~ Ce) u) ~V 3 0 ~ 0 ~ ~ D ~ V L1 ~ ~ O s0 _ S c: S" V ~ Sad V V 3 3 _ O Z
144 v ~ 0 · ~ c: o X :' Zip ~ =- C ~ o co a, CO 0 e a 0 o v U. 1 a, C' 0 En 6 Cal ca . P. O X ~ Z to =: Ct C) - ~0 D C o ~: o ~D 0 V C Ct V o o o o _ ~ '_~ 0~ ^ == ~ 1 ^ O m0^ O O ~ C~ "O ~ O ~ ~ O O e _~ O ~ O o _d 1 1 c~ 1 ~ ~ _1 1 1 ~ ~1 1 0= 1 1 ~ O =0 0 C~ ~ _1 ~ · e . ~ ~ ~ _/ C~ ~ O C~J O O _4 ___ __ _ ___ o . - O O _1 ~ 0o C~ O C~ 0 ~ 0 0 0 o C~ C~ . ~ ~ ~ ~ ~ ~ u~ ~ ~{ o o o a O O 0 1 =0 0 - ^O O O ~ ~ C~ 1 ~ ~ t_ ~ 1 0 '1 1 1 L~ ~ ~ ~ ~ O _ _ _ _ _ _ O O O O o o · a, C' C~ - C~ O0 ~ C~ ~ · - O o ~ C~Z ~ O O C,) C' 2 C~ ~ :~ o C~ o 1 o . o - _' O · · J 00 · E - . - 0 o o o oc ~ C _ ~ o o C~ o o CO v ~S ~D O ~n ~ ~ ~o _ ~ v o CD C~ ~1 C'' Io E~ ~5 v O CL o ~o Ct ~ o D L V o Ct 3 0 ~C ~5 V 0 ~0 CL O C~ ~)
145 little effect on gaseous pollutants; however, such a device may partially control organic gases and vapors responsible for odors and aerosols released during cooking. The effect of kitchen ventilation on carbon monoxide, nitric oxide, and nitrogen dioxide was also included in the study by Traynor et al.,23 and their data on nitrogen dioxide are presented In Figure IV-5. Other Indoor Combustion Sources - Severa' other combustion sources can affect indoor air quality. Although they tend to be s ite-specif ic and not as common as domestic heating and gas-stove operation, several are worthy of mention. Water Heaters and Clothes Driers. In two homes with gas water heaters, Traynor observed that indoor nitrogen dioxide concentrations were greater than outdoor {G. W. Traynor, Lawrence Berkeley Laboratory, personal communication). Initially, passive monitors were used to measure average indoor concentrations over 1 wk. Both homes were found to have increased indoor concentrations and were investigated in more detail with a continuous analyzer. In both cases, nitrogen dioxide entered the living space from the flue collar on top of the water heater, despite the fact that the appliances were designed to vent the combustion products outside. Similar considerations apply to gas clothes dryers. Automobiles. or ivino a car into or out of a basement or attached garage can strongly affect indoor air quality, depending on the configuration of the house and the routes for entering and leaving the garage. In a number of documented cases, occupants have inadvertently left a car running in a basement garage from which the resulting carbon monoxide dr if ted into the associated house or apartment and caused s ickness or death of occupants . The most critical situation would occur in a basement garage dur ing cold weather, when the Stack effect of a heated house tends to draw air in from the garage and distribute the pollutants captured in the garage throughout the house. A variant of this situation is an apartment house or off ice building with a basement garage that has stairwells or elevator shaf ts that can d istr ibute pollutants throughout the building . Little information is available on the impact on indoor air quality of automotive exhaust emitted in garages. Yocom and associates". sampled one house with an attached garage and concluded that the design of the house (split-level) caused automotive emission from the garage to have a greater impact on carbon monoxide concentration than the gas stove . Charcoal Broiling. Charcoal cooking is usually an outdoor activity; however, it is sometimes done in a f fireplace, and some f ireplaces even include a charcoal cooker. Depending on how such cooking is carried out and how well the fireplace draws, the resulting emission can enter the indoor environment. There appear to be no data
146 2.0 1.5 Q. 1-0 0.5 Range of recommended 1 hr air quality standard o Gas Oven on ~ t80°C (350° F) NO2 MU' /V v - //\Un~ 3-~ 1 \~ \ 0.25 ach \ \~ ach ~- - 7.0 ach Q 1 3,000 Cal 2,000 _ 1 ,000 1 HOURS 2 FIGURE IV-5 Nitrogen dioxide concentrations in test kitchens reported by Traynor et al. as functions of use calf gas oven with different kitchen exhaust rates.
147 on the direct impact of charcoal broilers on indoor air quality, but a recent study by Brookman and Birenzvige on exposure to air pollutants from Undomestic combustion sources provides some idea of how great these exposures might be.S They used personal samplers to measure hourly carbon monoxide and particulate matter in the outdoor environment while people were operating gas lawn mowers and chain saws and cooking with charcoal. A sampler adjacent to a charcoal cooker recorded that hourly carbon monoxide exposures ranged from 8 to 38 ppm. Analysis of the particulate filters for polynuclear aromatic organic matter showed that it was below the detection concentration of the screening method used. Hobbies. A variety of hobbies involve combustion processes, for example, heating and soldering with an LPG torch and brazing or welding with an oxyacetylene torch. Depending on the location, extent, and duration of such activities, they could have an impact on indoor air quality. Under some circumstances, the exposure of those pursuing such hobbies to harmful contaminants could be substantial. COMMERCIAL BUILDINGS Only limited information is available on the ef feats of combustion sources on indoor air quality in commercial buildings. Nevertheless, any of the sources mentioned above can affect the quality of indoor air in commercial buildings as they do in res idential buildings . Spengler and co-workers determined carbon monoxide concetrations in several enclosed ice-skating rinks in Massachusetts 2 o where gasoline- powered ice resurfacing machines are used; the concentrations were found to exceed 50 ppm regularly. Large public garages where traffic congestion commonly occurs in confined spaces constitute another example of indoor air-quality problems in commercial buildings. There are many documented cases of drivers and passengers who experienced acute health effects (presumably from carbon monoxide) at times of heavy garage traffic. The most critical situation occurs at the end of the workday or at the completion of a major sporting event, when hundreds of cars line up with their engines running. Stankunas and associates carried out a study for the American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) to measure carbon monoxide in several parking garages. 2 ~ They developed a model for calculating in-garage carbon monoxide concentrations on the basis of such variables as ventilation volume, outdoor ambient carbon monoxide concentration, initial carbon monoxide concentration in the garage, and garage volume. REFERENCES - 1 e Amer ican Conference of Governmental Industrial Hygienists ~ TL\t Threshold Limit Values for Chemical Substances in Workroom Air Adopted by ACGIH for 1979. Cincinnati: American Conference of Governmental Industrial Hygienists, ~ 979 ~ 94 PP.
148 A. Amiro, A. Carbon monoxide presents public health problem. J. Environ. Health 32 :83-88, 15~69. 3. Biersteker, K., H. de GrBaf, and C. A. G. Nass. Indoor air pollution in Rotterdam homes. Int. J. Air Water Pollut. 9: 343-350, 1965. 4. Billings, C. E. Atmosphere, pp. 35-63. In J. F. Parker, Jr., and V. R. West, Eds. Bioastronautic'; Data Book . 2nd ed . National Aeronautics and Space Administration. Publication No. NASA SP-3006. Washington, D.C.: U.S. Government Printing Off ice, 1973. 5. BrooRman, E. T., and A. Birenz~ige. Exposure to Pollutants f rom Domestic Combustion Sources: A Preliminary As';es';ment. U. S. Environmental Protection Agency Report too. EPA-600/7-80-084. Washington, D.C.: U.S. Environmental Protection Agency, Office of Research ant Development, 1980. 51 pp. 6. Cote , W. A., W. A. Wade, III , ant' J. E. Yocom. A Study of Indoor Ai r Quality. Final Report. U. S . Environmental Protection Agency Report No. EPA-650/4-74-042. Washington, D.C.: U. S. Environmental P.otectic:)n Agency, 1974. 282 pp. 7. Duncan, J . R., K. M. Morkin , and M. P. Schmierbach . Air Quality Impact Potential from Residential Wood Burning Stoves. Paper 80-7. 2, presented at 73rd Annual Meeting of the Air Pollution Control Association, Montreal, Quebec, June 2Z-27, 1980. 8. Eaton, W. C., J. N. Howard, Jr., R. M. Burton, F. Benson, and G. H. Ward. A Preliminary Study of Indoor Air Pollution in a Home Using a Gas Stove. Part T: Oxides of Nitrogen. U.S. Environmental Protection Agency, Human Studies Laboratory, 1972. 9. Mollowell, C. D., R. J. Budnitz, G. D. Case, and G. W. Traynor. Co~nbustion-Generated Indoor Air Pollution. I. Field Measurements 8/75-10/75. Lawrence Berkeley Laboratory Report LBL-4416. Berkeley, Cal.: Lawrence Berkeley Laboratory, 1976. 2S pp. Available from National Technical Information Service , Spr ~ngf ield , Va., as LBL4416. 10. Melia, R. J. W., C. Florey, S. C. Darby, E. D. Palmes, and B. D. Goldstein. Differences in NO2 levels in kitchens with gas or electric cookers. Atmos. Environ. 12 :1379-1381, 1978. . . , 1 1. Moschandreas , D. J ., and J. W. C. Star k . The Residential Environment and Energy Conser`,ation: Predicting Indoor Air Quality. Paper 78-60. 4, presented at the 71st Annual Meeting of the Air Pollution Control Association, Houston, Texas, June 1978. 1 2. Moschandreas, D. J ., J. W. C . Stark , J. E. Morse. Indoor Air McFadden, and S. S . Pollution in the Residential Enviroment. VO1. 1. Data Collection, Analysis and Interpretation. t3. S. E:nvironmenta1 Protection Agency Report NO. EPA-600/7-78-229a. Research Triangle Park, N.C.: U.S. Environmental Protection Agency, Environmental Monitoring and Support Laboratory, 1978. 201 pp. 13. Moschandreas, D. J., J. Zabransky, and D. J. Pelton. Comparison of Indoor-Outdoor Concentrations of Atmospheric Pollutants. Final Report for the Electric Power Research Institute. GEORGE Technologies Inc., Contract No. EP 130~-1. May l9HO. 14. Moschandreas, D. J., J. Zabransky, and H. E. Rector. The effects of woodburning on the indoor residential air quality. Environ. Int. (in press, 1961)
149 1 5. Palmes , E. D., C. Tomazyk , and J. DiMattio. Average NO2 concentration in dwellings with gas or electric stoves. Atmos. Environ. 11: 869-872, 1977. 16. Plot kin, S., and R. Kapplow. Food poisoning and carbon monoxide poisoning . N. Y. State J. Med . 52: 2409-2411, 1952. 17. Paxbaum, B. Indoor Air Pollution by Combustion Sources--Influence of the Gas Type on NOX Emissions. Paper presented at Euroanaly~es III, Dublin, Ireland, August 20-25, 1978. 1 8. Rench, J., and E. P. Savage. Carbon monoxide in the home environment. J. Environ. Bealth 39:104-106, 1976. i9. Schaefer, K. E., Ed. Preventive Aspects of Submarine Medicine. Undersea Biomed. Res. 6(Suppl.~:S-l--S-246, 1979. 20. Spengler, J. D., K. R. Stone, and F. W. Lilley. High carbon monoxide levels measured in enclosed skating rinks. J. Air Pollut. Control Assoc. 28:776-779, 1978. 21. Stankunas, A. R., P. T. Bartlett, and K. C. Tower . Contaminant Level Control in Parking Garages. Conference DV 80-5, No. 3, REP 223, presented at the Meeting of the American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASERAEl, Denver, Colorado, June 23-27, 1980. 22. Tansey, W. A., J. M. Wilson, and K. E. Schaefer. Analysis of health data from 10 years of Polaris submarine patrols. Undersea Biomed. Res. 6(Suppl.~:S-217--S-246, 1979. 23. Traynor, G. W., D. W. Anthon, and C. D. Bollowell. Indoor Air Quality : Gas Stove Emissions . Berkeley, Cal.: Lawrence Berkeley Laboratory, 19 79. 2 4 . Wade, W. A., I I I, W. A. Cote, and J. E . Yocom. A Study of Indoor Air Quality. J. Air Pollut . Control Assoc. 25: 933-939, 197S. 25. Waligora, J. N., Ed. The Physiological Basis for Spacecraft Environmental Limits. National Aeronautics and Space Administration Reference Publication 1045. Washington, D. C.: National Aeronautics and Space Administration, 1979. 2 6. Yamanaka , S ., B. Hirose , and S. Takada. Nitrogen oxides emissions for domestic kerosene-f ired and gas-f ired appliances. Atmos . Environ. 13: 407-412, 1979. 27. Yates, M. W. A preliminary study of carbon monoxide gas in the home. J. Environ . Health 29: 413-420, 1967. 28. Yocom, J., W. Cote, and W. Clink. A Study of Indoor~Outdoor Air Pollutant Relationships . Vol . 1. Summary Report. Washingon , D.C.: National Air Pollution Control Administration, 1974. 29. Yocom, J., W. Cote, and W. Clink. A Study of Indoor~Outdoor Air Pollutant Relationships. Vol. 2. Supplementary Study. Washington, D. C.: National Air pollution Control Administration . 1974. TOBACCO SMOKE Nearly everyone is exposed to tobacco Smoke at come time or othere Indirect exposure (i.e., exposure of nonsmokers) is referred to as "passive exposure,. Impassive smoking,. or Involuntary smoking.. The extent of the exposure is determined by the number of smokers one
150 associates with, their smoking habits, and the characteristics of the environment in which exposure occurs. Actual population exposures to tobacco smoke are therefore quite variable. For some, passive exposure to cigarette, pipe, or cigar smoke may occur routinely at work, in transit, or at home. For others, passive exposure may result only from infrequent encounters with smokers in public facilities. Over 2, 000 compounds have been identif fed in cigarette smoke; many a re established carcinogens that appear primarily in the particulate phase . I t is reasonable to assume that passive tobacco-smoke exposure is many people's principal source of exposure to many of these compounds . Despite the overwhelming evidence on health effects on smokers, the impact of tobacco-smoke exposure on nonsmokers is not well documented. (The health effects of gases and particles emanating from tobacco combustion are summarized in Chapter VIT,) Tobacco smoke irritates the eyes, nose, and throat and is annoying to nonsmokers , even in the presence of Adequate ~ ventilation . Annoyance increases with increasing smoke contamination and increasing dryness of air. Aside from the irritation and annoyance that it causes, smoking in confined spaces increases annoyance from odors and particle accumulation. Aldehydes and ketones produced by burning tobacco give rise to odors. Particles that adsorb and release organic vapors can be odor sources long after the tobacco is extinguished, and the lingering odors can be smelled by those not desensitized. This section discusses indoor exposure to pollution resulting f ram cigarette-smoking. The factors of concern here are the number of people exposed to cigarette smoke, the composition of the gases and particles emitted, and the concentrations of pollutants encountered. BACKGROUND The number of people exposed to passive smoking, who might also be termed "involuntary smokers, is not known. However, given the number of people who smoke in the United States, some involuntary inhalation of tobacco combustion products from smoke-contaminated atmospheres by nonsmokers is unavoidable . Pass ive exposure to tobacco smoke will inevitably occur in any number of public or private activities. In 1978, an estimated 54 million persons smoked 615 billion cigarettes. The prevalence of regular cigarette-smoking in the adult population declined from 42% in 1964 to 33% in 1978. Figure IV-6 plots the annual consumption, from 1950 to 1978, of cigarettes and filter-tip cigarettes per person aged 18 and over. The adult per capita consumption for 1978 is estimated at 3,965, which is the lowest recorded consumption since 1958. Surveys show that fewer men are smoking each year, but more women are smoking, particularly teen-agers. Figure IV-7 presents the proportion of U.S. men, women, and teen-agers that reported being regular smokers in 1974 and 1975. Th is f igure implies that one of every three persons between the ages of 17 and 64 regularly smokes cigarettes. Association with adult males 3 5-44 yr old increases the likelihood that a person will be passively
lS1 `0 55 . ,,,,,, , ~ . 6V 65 70 . . 4000 ~ l 3000 2000 tooo- · · Ie~ ~ . .1~. .e . · Total · · · ~ ~ To · · Filte~ip ~0 55 60 65 70 Year 40ao 3000 2000 100~) FIGURE IV-6 Annual consumption of cigarettes and filter-tip cigarettes per person aged 18 yr and over, 19501978. 1978 per capita consumption of cigarettes was 3,965, the lowest since 1958. Open circle, preliminary estimate. Reg~{ntAd from U. S . Department of Health, Education, and Welfare. P- 5
152 40 In 30 A o c ~ 20 a flu ~0 o 50 40 in O 30 10 o Females t2-14 1 _ _ ~ ~\~ All' _ 15-16 17-18 Males L 12 1415-16 17-18 ~ . _ it, 2 t -24 25-34 35-44 45-54 55-64 >65 AGE, YEARS _ ~ :_ 21-24 25-34 3544 AGE, YEARS 45-5455-64 >65 FIGURE IV-7 Percentage of regular smokers in population. Teenagers, 1974w 1 fAdult~i~plp.75A_~8atA_l~o~m U. S. Department of Health, Education,
153 exposed to cigarette smoke. In 1974, the percentages of girls and boys 15-16 yr old who smoked were essentially the same. The percentage of teen-aged boys smoking, however, has been dropping since 1970, whereas the percentage of teen-aged girls smoking has increased dramatically. Because cigarette smoke is ubiquitous, passive exposure to it will be encountered in off ices, industrial facilities, homes, public sporting events , restaurants, transportation facilities, and innumerable other locations. Professional and technical workers have the lowest percentage of cigarette-smokers; laborers, craftsmen, and Other blue-collar workers have the highest. Of the males in ~blue-collar. occupations, 47% were smokers in 1975, whereas 369e of the males in "white-collar ~ employment were smokers . Females showed the opposite relationship; 34% of the ~white-collar ~ females and 32% of blue-collar female workers smoked. In the 1975 survey, 409 of the women in the sample worked outside the home; of these, 33% were cigarette-smokers, compared with 27% of the housewives. Information on passive exposure of nonsmokers to tobacco smoke has never been systematically obtained, except in a limited number of epidemiologic investigations (see Chapter VII ~ . Data on populations exposed at home, at work, or in other locations must be estimated from s urveys on smok ing prevalence and epidemiolog ic s tudies . The National Center on Smoking and Health (NOSH) has conducted the most extensive surveys on American smoking habits. Marital status, educational achievement, and income are demographic var tables associated with differences in smoking prevalence. NCSH information indicates that divorced or separated persons smoke more than those in any other marital-status group. For men, the highest prevalence occurs in middle-income and high-school-educated groups. For women, there is a more direct relationship between income and smoking, with more smoking in the higher-income groups. The demographics of smoking prevalence do not indicate exactly what groups are passively exposed to tobacco smoke or where or when they are likely to be exposed. We do not have information on the percentage of the 80 million workers in the United States who are employed in an environment f ree of tobacco smoke. We do not know what percentage of America's 80 million residential units have smokers, but the results of some surveys are summer ized below. Some 54 million adult Amer icans smoke ~ 33% ), so we can infer that the number of homes and other residential units with smokers is - substantial. Summaries of prevalence of homes with smokers taken from a study on air-pollution health effects support this contention. Table IV-18 summarizes the response to a questionnaire in the children's study. On the basis of 8,493 questionnaire responses obtained across six cities (7596 response rate), 70% of the homes reported having at least one smoker. The percentages reporting at least one smoker ranged from 63% in rural Portage, Wisconsin, to 76% in a middle-income community in St. Louis. Lebowitz and Burrowsi 3 reported that 53.8% of the children in their Tucson study had smokers in their homes. Schilling et al. 2S reported that an average of 63% of the sampled homes in two Connecticut towns had smokers . Substantial reg tonal variations in the percentage of homes with smokers may be expected,
154 TABLE IV-18 Percentage of Homes Reporting One or More Smokersa Location St. Louis, Mo. Steubenville, Oh. Kingston-Harriman, Tenn. Watertown, Mas s. Topeka, Kans. Portage, Wis. Tucson, Ariz. Two towns, Conn. "Data from Ferris et al., No. Responses 1~922 1~808 810 838 1~663 1~452 676 376 Proportion of Homes with Smokers, % 76e 1 74~2 71~8 69~8 63~3 62~5 53~8 63~3 8 Lebowitz and Burrows,l7 and Schilling et al. 25
155 owing to geographic var iations in social, demographic, and religious variables that are associated with differences in smoking prevalence. Further stratif ication of the response" to the question of parental smoking by level of parental education confirm earlier NC SE surveys. In the Harvard six-city study, ' of the homes in which neither parent had a high-school education, over 80% had at least one smoker . In homes in which one parent had graduated from college, only 50% had smokers . CONTAMINANTS IN SHORE A distinction can be made between mainstream smoke and sideseream smoke in the contaminants evolved f tom tobacco combustion . Both smokers and nonsmokers are, of course, exposed to ~ idestream smoke . Mainstream smoke is undiluted and is pulled through the tobacco into a smoker Is lungs. Sidestream smoke is directly from the burning tobacco . Depending on smok ing behavior, burning temperature, and type of f ilter, the composition of mainstream smoke exhaled by a smoker varies substantially. From 50% to more than 90% of water-soluble compounds are removed f rom the mainstream smoke by the smoker, depending on the depth of inhalation and the time of breath-holding. The insoluble compounds show equal variability over a range of 20-703. A typical cigarette-smoker inhales mainstream smoke 8-10 times, for a total of 24-30 s of a total 12-min burn time for a cigarette. ~t There is no dispute that the concentrations of almost all cons~cituents are far greater in mainstream than in sidestream smoke. }Iowever, given approximately 24 to 1 disparity in burning time (i.e., the sidestream smoke is produced during 96% of the total smoking timed and the d ifference in combustion conditions, it is not surprising that sidestream smoke is more important to the involuntary, passive smoker. The sidestream smoke can also be enriched in many compounds. Sidestream smoke and mainstream smoke have been characterized by many investigators.2~. ' i. I' The mean particle size of fresh mainstream smoke is slightly greater than that of s idestream smoke . Exhaled-smoke particles are larger, on the average, than those in fresh mainstream or sidestream smoke, because of water absorption and coagulation. The same processes modify the size distribution of smoke particles as they age. If particles begin with a mass mean diameter less than 0.2 - , they grow by agglomeration and water absorption to a mass mean diameter approaching 1 An within minutes. The passive smoker by no means receives a lung dose of smoke equivalent to that of the smoker. Several investigators have estimated the exposure of the nonsmoker in a smake-f illed environment as one-hundredth to one-tenth of the smoker's exposure. 12 l' 22 23 These estimates were based primarily on measurements of carbon monoxide, suspended particulate matter, or nicotine concentration extrapolated from reported values for mainstream smoke. Substantial evidence indicates that many substances are increased in sidestrea~n smoke . Therefore, direct compar ison on the basis of particle mass or concentration of a specific gas may not adequately describe a passive smoker 's exposure to cigarette smoke.
156 The sidestream-to-main';tream ratios Is :m) of specif ic compounds range from 0.7 to 46. The ratios of vapor-phase compounds vary more than those of particulate-phase compounds. Although the composition of mainstream smoke from nonflitered cigarettes is quite different from that from filtered cigarettes, the compositions of their sidestream smoke are essentially the same. Table IV-l9 presents a compilation of the concentrations of some substances found in cigarette smoke, with s:m ratios. Values are given in mass per cigarette. Unless otherwise noted, the values refer to nonfiltered cigarettes. Sidestream cigarette smoke, because of the length of the burn and the burn temperature, is a more important source of local air contamination with many substances--such as carbon monoxide, nicotine, ammonia, and aldehydes--than mainstream smoke. Cigarette-smoking in enclosed areas increases concentrations of particles and gases. Increased concentrations of carbon monoxide, n icotine, n itrosamines, and benzopyrene are among the most f Sequent . Pollution measurements of al r contaminated with tobacco stake can be conveniently d ivided among controlled chamber exper iments and actual exposure conditions. In many of the controlled-setting experiments, carbon monoxide concentrations exceeding the 1-h NAAQS of 35 ppm have been reported (see summary table on chamber studies of cigarette-smoke exposure in U.S. Surneon General's Report, Smoking and Health, t) . Without ventilation, indoor carbon monoxide concentrations are proportional to the amount of tobacco burned and inversely proportional to room volume. These studies indicate that carbon monoxide concentrations of 50-100 ppm can be obtained and that increased ventilation substantially reduces concentrations (see Figure TV-8 ~ . Chapter 11 of the 1979 Surgeon General's report tabulates the results 0 f many of the chamber exper iments {Table IV-l9 ~ . The experiments performed in controlled environments generally involved heavier smoking than is normally encountered. Of more interest are the observations of carbon monoxide and other pollutants in normal indoor locations . Several studies are summer ized in Table IV-20 . In general, the carbon monoxide concentrations were less than those in the controlled exper iments, probably because of the heavier smoking. Taverns, bars, nightclubs, and restaurants have been the more f requently reported locations in assessments of the air~quality impact of cigarette-amoking. Concentrations less than 35 ppm for an hourly average have been reported, primarily because of mechanical or natural ventilation. However, studies have indicated that the 8-h NhAQS of 9 ppm could be exceeded in public facilities that permit cigarette- smoking. Elliot and Rowe, reported that carbon monoxide concentrations in public assemblies of 2,000-14,000 people were between 9 and 25 ppm--up to 4 times higher than background. As a more direct measure of exposure, several investigators have measured COHb in nonsmokers' blood after their exposure to cigarette smoke. The results were as expected: modest increases in COMb.
157 TABLE IV-1 9 Composition of Mainstream and Sidestream Concent rat ion, ma/ cigare t tea Mainstream Sidestream Smoke Smoke Ratio, Characteristic or Compound (1 ) (2 ~ 2:1 Reference General characteri~tics: Duration of smoke 20 S50 27.5 19 production, s Tobacco burned 347 2 411 2 1.2 15 Particles, no. per 1. OS x 101 3.5 x 101 3.3 24 cigare tte Particles: Tar ~ chl orof o rm ext ract ~ 20. 8 44 .1 . 2. 1 16 10. 2b 34. 5b 3.4 16 Nicotine 0. 92 1. 69b 1.8 16 0.46b 1.27 2.8 16 Benzo ~ a ~ pyrene 3. 5 x 10 5 1. 3S x 10 4 3. 9 10 4.4 x 10-5 1.99 x 10- 4. 5 18 Pyrene 1. 3 x 10 4 3. 9 x 10 4 3.0 10 2. 70 x 10-4 1.011 x 10-3 3.7 18 Fluoranthene 2.72 x 10 4 1.255 x 10 3 4.6 18 Benzota~fluorene 1.84 x 10 4 7.S1 x 10 4 4.1 18 Benzo~b~cifluarene 6.9 x 10 S 2.51 x 10 43 3.6 18 Chrysene, benz~aJanthracene 1.91 x 10 4 1.224 x 1O4 6.4 18 Benzotb/k/~]fluoranthrene 4.9 x 10 S 2.60 x 10 4 5.3 18 Benzote~pyrene 2.5 x 10 5 1.35 x 1O5 5.4 18 Perylene 9.0 x 10-6 3.9 x 10- 4.3 18 Dibenz~a,]]anthracene 1. ~ x 10 S 4.1 x 10 54 3. 7 18 Dibenz~a,h~anthracene, 3.1 x 10 5 1.04 x 10 3.4 18 ideno- ~ 2, 3-ed ~ pyrene _ -S Benzo~ghi~perylene 3.9 x 10 5 9.8 x 10 2.5 18 Anthanthrene 2.2 x 10 5 3.9 x 10 5 1.8 18 Phenols ~ total ~ O. 228 0. 603 4 2.6 13 Cadmium 1. 2S x 10 4 4. S x 10 3.6 27 Gases and vapors: Water 7. sc 298d 39 7 13 Carbon monoxide 18.3 86.3 4.7 32 - 72.6 - 26 Ammonia 0.16 7.4 46.3 20 Carbon dioxide 63.5 79.5 1.3 20 N0 0.014 0.051 3. 6 20 Hy~rogen cyanide 0.24 0.16 0.67 31 Acrolein 0.084 -- 31 - 0.825 - - 26 Formaldehyde 1.44 26 Toluene 0.108 0.60 5.6 32 Ace tone 0. 578 1. 45 2. 5 32 Polonium~210, pCt O. 04-0. 10 0. 10-0. 16 1-4 7 aUnless otherwise noted. bFiltered cigarettes. C3. 5 mg in particulate phase; rest in vapor phase. d5. 5 mg in particulate phase; rest in vapor phase.
158 25 SI40KERS 50 . . . . . . . ILV t | =t 11~ CO Pa 20 ~0~: 500 C '/~ 1000 Cf~ ~ ~ t 30 40 50 71~5 (ttilO) 25 N()'JSMOKEllS 8 - 6 CO ppm 3 2 O . . 0 10 20 SO AMBIENT ~ OU0TY ST - ~~ CF" 100 CF~ 7 1' 250 CFM7 500 750 CF" 1 000= Cf~ 30 40 SO 60 1114L (nor)) FIGURE IV-8 Calculated buildup of carbon monoxide under vario s conditions of ventilation ant smoking. Calculated for a room of 3,000 ft. with 25 smokers on the lef ~ and for 25 nonsmokers on the right. ILV is the threshold limit value for carbon monoxide (50 ppm). CFM is ventilation in cubic feet per minute. Reprinted with permission from Galuskinova.
159 Harked showed a COHb increase from 0.9% to 2.1% after a 2-h exposure to carbon monoxide at 30 ppm. Aronowt reported similar results in patients exposed to smoke from 15 cigarettes smoked over 2 h in a 30.8-m3 room without ventilation. It is possible that passive smoking was the reason that apportion of the nonsmoking population was reported by Stewart et al. 38 (in a national COHb survey) to have COMb of over 1.51. But, given the range of carbon monoxide concentrations reported in smoke-filled indoor locations, it is unlikely that cigarettes alone contribute signif icantly to increased COHb in the nonsmoking population. Because carbon monoxide is slow to be removed from the environment, reducing fresh-air supplies to office buildings or public facilities that permit smoking would necessarily increase carbon monoxide concentrations. Several other constituents of tobacco smoke have been measured indoors, including nicotine, acrolein, benzolalpyrene, nitrosamines, and aldehydes. Under heavy-smoking conditions, acrolein is the only gaseous substance that has been shown to exceed threshold limit values established for industrial environments. Acrolein is found at I-20 ppb in bars and restaurants ; even to nonsensitive persons, these concentrations can cause annoying odors and eye and nose irr itation. The indoor concentrations of benzo [a] pyrene where smoking occurs are more ambiguous. Galuskinova9 reported concentrations of 0.2-4.6 ~/m3 in restaurants described as ~smoky.. These high concentrations may have been due to cooking; Elliot and Rowe, reported concentrations of 7-22 ng/m3 in the presence of total suspended particles at 224-480 ug/m3 in public arenas with smokers. The nicotine concentration in air is an excellent indicator of cigarette smoke. A typical cigarette contains 2 mg of nicotine. Hinds and Firsts measured nicotine at 1-10.3 ~/m3 in a number of public facilities, including cocktail lounges, transportation waiting rooms, trains, and buses . The submarine contains a unique environment for observing human exposure to cigarette smoke. Cano et al.S found nicotine in the urine of nonsmokers when the submar ine environment had nicotine at 15-35 ~g/m3, and the nonsmokers' urinary nicotine concentration was only 1% of that of the smokers. It appears unlikely that the threshold limit value (500 ~/m3) for exposure to nicotine in industrial environments would be exceeded in indoor locations with ventilation, although only a few studies have been reported. Total suspended particles (TSP] and the fractions of respirable suspended particles (RSP) have been measured in the indoor environment in the presence of tobacco smoke. TSP concentrations of 50-400 ~/m3 have been reported for integrated samples taken in public arenas, lounges, bus stations, and airplanes (see Table IV-20. There are few reported measurements of RSP in the vicinity of tobacco-smoking. Repace and Lowrey2i reported on 2-min RSP samples taken in 20 indoor environments where smoking is permitted {outdoor measurements were also reported). The indoor RSP concentrations ranged from 86 to 697 ~g/m3. These results are consistent with residential measurements of 24-h RSP concentrations reported by Spengler et al. 29 Daily indoor concentrations of RSP frequently
160 o in . - ~ ~ o o ~ o Moo Cal ~ Cal ~ ~ ~ ~ 8 ~ cot ~ cot cut ~ cat ~ ~ 0 ~ ~ 0 "$ I I I I I I ~ ~ e e | t O ~ 1 1 1 1 1 ~ ~~ - ~ i ~ . o '1 1 ' 1 :~ ~ do O _I v O C Cl ol ~4 O C owe 0 0 0 8 8 E" Em 8 - u: ~ . cad cad _ cad ~ ~ ~ om ~ _ ~ ~ ~ ~ ~ ~ ~ t0 6 ~ 6 ~ ~ ~ ~ 0 oo ^~ ~_ ~ e ~ ~ ~ ~ e ~ o-_ _ =~ C A: ~ to ~ 60 . CL_ ~ ~ C C ~ Da ~ ~ ~ C - _d _1 ~ ~ ~ C ~ ~ 60 ~ ~ C ~ CL E ~ E! ~J dV ~ dV ~ ~ O ~ ~ c Z > £ ~ ~ ~ ~ O O ~ O I ~ ~ _ . _~ ~ ~ ~ ~ e ~ ~ ~~~ ~ _t t~ ; ~ _1 _~ ~ _1 · _ ~: O e0 ~ 1 0 ~ :^ ~ . S~ · 60 O O ~ tJ ~ ~ O -t V V 00 ~ C V ~ O 0 3 ~ ~ ~ O D I I ~ O 1 1 1 1 ~ ~ 1 1 1 ~ 1 1 1 V ~S ~ I I ~ I ~ 0` 1 1 1 1 I J ~ _~ _~ ~ 00 ~ ~ O C ~ ~ o ~1 ~ 3 ~ ' C~ s~ V ~ ~ ~ ~ a_ O (V I t I ~ 0 1 1 1 I C I ::. 1 ~ 1 1 ~ 1 1 1 1 ~ - 1 V ~ e 0 ~ C 0 ~ ~ e v os 0 ~ ~ l: ~ ~ ~ ~ V x: 0 ~ ~ ~ ~ ~ C~ _ 0 n1 ~ 8 ~ :: C~ ~ ~ V 0 ~D ~ _' ~ ~ ~ 3 0 ~ ~ ~ `0 ~ ~ O O O ~ ~ ~ ~ ~ ~ 0 0 O ~ ~ ~ · 0 ~ ~ ~= _ ~ ~ ~ ~ CL c: ^ 0 ~ :' ~ ~ ~ op ~ ~ ^ ~ ~ e ~J ~C ~ V v ~ ~ E ~ ~ ~ e c ~ ~1 ~ ~ ~ ~ v ~ ~ ~ v e ou ~ ~ ~ s ~ ~ ~ ~ `: s~ oo ~ ~ ~ ~ ~ 0 ~ ~ ~ ~ ~ ~ 0 ~ ~ ~ 0 ~ ~ ~ ~ ~ ~ ~ Z f: C~ ~ C~ C~ ~ Z ~ E~ ~ ~ <: s ~ _ C~ ~
161 o - - o V ~ o v Coo v - c Cal o o v v U. so o A: Cal 3 a V V ~ Cat 3 c~ O ~ E O <: ~ ~: o - v - - v :> V ~ C: O O ~ V V _ ~ V 60 O ~ O C~ t ^ 0 ~: V C ~ ~ a <; ~ s: C' ~ e ~ · ~ ~ 0 +1+1 0 ~ 1 . . 1 Ol I I O O I I ~ 0 0 e ~ c~ - tD ~ E C O ~ · · O O O h' ~ O C~ L) 8 ~ ~ ~ E ~ I o + ~ + I E E ~ ~ =~ a. t~ ~ ~, O ~ ~ ~ ~ ~ ~ ~ _ ~ 1 0 O ~ 0 ~ ~ ~ ~ ~ C~ _ U~ C~ · D _4 ~ ~ _ ~ _ ~ _' _ 0 0 00 ~ a0 o0 O0 E _ _ ~ ~ _' 0 C) V ~ <U V 1 1 1 1 1 1 1 1 1 · · ~ c e . ^o ^o ^ 0 ^e :: ~ 1 ~ ~ ~ _. ~ _' ~ ~ ^ dV - C ~ ~ v ~ v ~ v ~ CL ~ ~ `: ° e 8 ~ t. ~ 8. a, ~ O ~ O O 0 0 _I ~ _1 a~ _1 ~ 0 ~ 0 V ~ V V ~ V 54 ~ ~ ~ ~ ~ ~ ~ ~ 3 C ~ ~ "C ~ ~ ~ ~ ~ ~ ~ ~ ~ O O _ ~ ~ ~ _ ~ _ v ~5 O E E E E ~ O ~ O ~ O ~ _~ p: _ ~ V ~ ~ ~ C: CS 1 1 1 ~ 0 1 u~ o 0 o 1 ~ 1 ~ ~ 1 ~ 0 CD v v ~4 ~L oo o0 E e ~ ~ 0 ~ _' V · ~ ~ ~ V V V _I ~ ~J ~ ~ o 3 D · ~ O ~ V ~ ~ ~ ~ ~ C ~ ~ ~ ~ ~ ~ ~ O _ q5 ~, V V ~ ~ ~ ^ ~ Ct ~ ~ ~ O V a' Y - - O Y =, ·~~ ~ ~ h O O C~ i4 ~ O C~ C
o v ~n o s" ~ ~ v C: ~ o o c~ c: o o o - v v c) u: E ~n C o o t) C V O D e ~o 162 o CL C~ o o ~ o o C) 0 o ~ C) _ ~ o. CL ~ D X _ ~ ~ ~ ~ C~ 1 1 1 1 ~ ~ 1 ~ 1 · 1 · ~ 1 1 1 1 1 ~ 1 1 · 1 ~ 1 o0 ~ G1 1 O . O ~ 1 - 4 - - "E ~ ~ ~ 6 ~ ~ _ ___ ~ ~ O O o4 OC OC 00 o0 00 ~~ O C~ C) O ·e ~ ~ E E ~ ~ ~ D ~ ~ o 5 ~ ~ O ~ cri O ~ c~ ~ ~ 0 CJ ~ ~ ~ E ~ O _ ~ ~ O ~ _ CL ~ C~ CL . o o O ~1 0 ~ CL CL 6 ~ O O O O O O O X ~ ~ 0 C~ CL V e · e · e e ~ r_ a C~J ~ Ck. ~4 0 e ~ I e O ~ ~ 1_ 0o ~0 c~ 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 ~ 1 1 1 1 1 1 ce cn bC 0c ~ ~ C ct ~ ~ ~ 5 0 S O c' eS V _ ~ ~ C~ ~ CL I I I I t I I I I I I I 1 1 1 1 1 1 1 1 1 1 1 1 - _ ~ E V ~ O E ~ . o _ O 00 tV ~ F O ~ so ~ _ a _ ~ ~ e oo ~ ~ ° ~ v oo oe ~ 0 v ~ e v ~ . 0 ~ ~ ~ ~ so_ 0 ~ e ~ _ a, O ~ O _ ~ D ~ a: V _' O ~ ~ D _ ~ _1 3 c: _1 ^ 1 - ~ ~ ~ v ~ _ ~V _ V _ Ce ~ O ~ ~ {V t~ _ 4,) h ~ V e ~ ~ ~ ~ ~ ~: ~ v ~ C: ~ ~ ~ ~ ~n ~ ~ a, s~ C ~ ~ ~ 3 3 ~ t~ ~ ~ ~ 3 ~ ^ [z: ~ ~ E _ V Y ~ ~ ~ O a ~o E E co ~ a' v :1 o ~ ~ ~ ~ oo a, o~ _ ~ 4) ~ o O ~ _ ~ O v V O ~ ~ D _ ~ :' ~ O ~ ~ ~ c: ~ ~ a <: ~ c~ u~ ~ :: c~ 0 D z ~ ~ ~ c; <: ~ C _ ~V ~ ~ ~ ~ ~ ~ ~ _~: C~? cn cn
163 v U. o ~ o o ~ V ~ 0 o o c o v C~ U o C~ o o V V C V :, O D ~ O <: ~ O O ~ V V %_ o5 ~ 0 O ~o e 1 C . ~: ~ C~ 1 1 1 1 o ~: E~ ~ e _ ~ _ 0 0 so ~ 00 e~ o 0 c; ~ 0 £ 1 C~ ~ O O E ~ =~ ~ ~ I ¢~ ~ ~ ~ C~ ~ J CL · ~ · ~D L~ V 1 - 1 O ~ - Ct oc :^ ~ ~ 900 ~ V V C :^ ~ ~ 3 't s" ~ C o ~ U: ~ :~ ~3 D :' ~ ~ ~ ~ O D _ ~ v v ^ - V ~ ~ O C ~ V V V C o 1 1 ~ ~J ~ 1 1 ~ v v ~ ~ cL. ce ~ - ~ ~ cc cc ~ ^ v L ~ o ~ ~ ~ v .= 3 ~ ~ ~ v 0 ~ ~ ~ n eC ~ so ~ S~ C ~ := ~: 0 Ct "C o ~ E~ CL 1 ~ "C O ', ~ ·^ e ~ 3 0 . ~ c . v ~ ~ ~ ~ ~ c :: _ ~ ct 0 _ _ _ _ ~ ~ ·- ~ 1 v ~ ~ .. v 0 ~ cn 0 J: 0 ~ . .^ oe ~ ~ ~ a: 0 ~ 1 1 ~ ~ e" ~ I l ev CD ~ O V ~D ~ Ct ~ S" ~ ~ ~ v :^ ~ u, tn ~ v . q5 s~ ~ ~ so v ~ ~ E ~ _ . ~ ~ O V V - s" 0 ~ e c: v <: ~ c: . v c~ eo c~ ~ ~ ~ ~ . ~ v u, := ~
164 exceeded 200 ug/m3 in homes with cigarette-smokers. Aggregating the data obtained from a study of 69 homes in six cities reveals that the indoor and outdoor concentrations in the 38 nonsmoking homes are essentially equivalent (24 ug/m3 indoors versus 22 ~/m3 outdoors ~ . In the 22 homes with only one cigarette-smoker, the mean concentration indoors was 43 ~/m3; the nine homes with two or more smokers had a mean concentration of 75 ~/m3. These data, collected over a 3-yr period, are presented in Figure IV-9, which shows monthly mean RSP concentrations outside and inside homes without smokers, with one smoker, and with two or more smokers. The data clearly illustrate the contribution of cigarette-smokinq to indoor particle concentrations. (The effects of pollution control on indoor concentrations of particles generated from tobacco-burning are discussed in Chapter IX.) In recent investigations of personal exposures to respirable particles by Spengler et al., 2 8 passive smoking was shown to be an important source. Volunteers in Topeka, Kansas, carried portable monitors for 12-h periods on 15 sampling days. The mean RSP concentration of samples where participants reported passive cigarette- smoke exposure for some time dur ing the day was 40 ug/m3 . The nonsmoking, nonexposed participants had an overall mean concentration of approximately 22 ng/m3, and the outdoor concentrations averaged less than 15 ug/m3. CONCLUSIONS l Tobacco-smoking indoors can contribute to or cause increased concentrations of respirable particles. nicotine, carbon monoxide, acrolein, and many other substances in the smoke. Many exper imental and "real-life measurements hale demonstrated that where ventilation i s low or nonexistent, indoor pollutant concentrations can exceed ambient-air quality standards and industrial standards. The indoor concentrations have been shown to depend on the number of smokers, how the tobacco is smoked (cigarette , pipe , or cigar ), the room volume , the volume of fresh-air makeup, the eff iciency of the air-cleaning apparatus, and the effectiveness of air mixing in the room. The absorbing characteristics of building and furnishing materials can af feet the concentration . I t has been demonstrated that the use of makeup air ~ for ~rentila~cion) that has lower concentrations of the contaminants effectively lowers the concentrations of carbon monoxide and other pollutants in tobacco smoke. Nevertheless, high respirable and total suspended particle concentrations have been noted even in the presence of Adequate ~ ventilation . Daily concentrations of respirable particles can exceed proposed ambient-air quality standards for TSP in homes with smokers. Although it needs to be documented, tobacco smoke may be the most important source of exposure of nonsruak ing populations to tenon [a] pyrene, nicotine, and other compounds in nonindustrialized areas. It must be noted, however, that there are other important sources, both indoors and outdoors, of many of the pollutants produced
165 120 tOO Cal ~ 40 80 60 20 2 or more smokers per home 1 smoker per home no smokers I\ outdoor / REV >"~~~' - \ _ ~ ~ _ I-__ ~ ~ _-_ \ '',`~',\/\ / \_-~v O l l Nov Jan Mar May Jul Sep Nov Jan Nlar 1976 1977 t 978 FIGURE IV-9 Monthly mean respirable particle concentrations. O. outside. X, inside nonsmoker homes. 1, inside homes with one smoker. Solid circles, Preside homes with two or more smokers. Sample represents 80 homes across six cities (approximately 1~129 homes per city ). Reprinted with permission from Spengler et al.
166 by the burning of tobacco. And it is important to point out that d irect exposure of a smoker is an order of magnitude greater than the passive exposure of a nonsmoker. REFERENCES 1. Aronow, W. S. Effect of passive smoking on angina pectoris. N. Eng1 . ~ . Med . 299: 21-24, 1978 . 2 . Brunnemann , K. D., J . D. Adams, D. P . S. Ho, and D. Hof fmann. The influence of tobacco smoke on indoor atmospheres. II. Volatile and tobacco specif ic nitrosamines in main- and sidestream smoke and their contribution to indoor pollution, pp. 876-880 . In 4th Joint Conference on Sensing of Environmental Pollutants, New Orleans, Louisiana , November 6-11 , 1977 . Washington, D.C.: American Chemical Society, 1978. 3. Brunneman, K. D.' and D. Hoffmann. Chemical studies on tobacco smoke. XXIV. A quantitative method for carbon monoxide and carbon d Oxide in cigarette and cigar smoke. J. Chromat. Sci. 12:70-75, 1974 . 4 . Brunnemann , K. D., and D. Hof fmann. Chemical studies on tobacco smoke. LIX. Analysis of volatile nitrosamines In tobacco smoke and polluted indoor environments, pp. 343-356 . In F. A. walker, M. Castegnaro, L . Gr iciote, and R. E. Lyle, Eds . Environmental Aspects of N-Nitroso Compounds. lARC Scientific Publications No. 19. Lyon, France: International Agency for Research on Cancer, 1978. 5 . Cano, J . P ., J . Catal in , R. Badre , C . Dumas , A . viala , and R. Guillerme. Determination de la nicotine par chromatagrophie en phase gazeuse. II. Applications. Ann. Pharm. Fr. 28~11~:633-640, 1970 . 6. Corn, M. Characteristics of tobacco sidestream smoke and factors influencing its concentration and distribution in occupied spaces. Scand. J. Respir. Dis. Suppl. 91:21-36, 1974. 7. Elliot, L. P., and D. R. Rowe. Air quality during public gatherings. J. Air Pollut. Control Assoc. 25:635-636, 197S. 8. Ferris, B. J., Jr., F. E. Spiezer, J. D. Spengler, D. Dockery, Y. M. M. Bishop, M. Wolfson, and C. Humble. Effects of sulfur oxides and respirable particles on human hearth O Methodology and demography of populations in study. Am. Rev. Respir. DiS. 120: 767-779, 1979. Galuskino~ra, V. 3,4-Benzpyrene determination in the smoky atmosphere of social meeting rooms and restaurants. ~ contribution to the problem of the noxiousness of so-called passive smoking. Neoplasma 11: 465-468, 1964 . (in Czech) 1 0 . Gr immer , G ., H . B5hnke , H . and H . -P . Harke . Pass ive smok ins : Measuring of concentrations of polycyclic aromatic hydrocarbons in rooms after machine smoking of cigarettes. Int. Arch. Occup. Environ. Health 40:83-92, 1977. (in German; English swmary) Harke J H.-P . The problem of Impassive smoking . ~ Munch. Med. Wochenschr . 112: 2328-2334, 1970 . (in German; English summary}
167 12. Binds, W. C., and M. W. First. Concentrations or nicotine and tobacco smoke in public places. N. Engl. J. Med. Z92: 844-845, 1975 . 13. Moegg, U. R. Cigarette smoke in closed spaces. Environ. Health Perspect. 2 :117-12e, 1972. 1 4. Johnson, W . R., R. W. Hale , J. W. Neelock , H. J. Grunts , and D. H . Powell. The distr ibution of products between mainstream and s~destream smoke. Tob. Sci. 175 (21) ~;~-45, October 12, 197~. 15. Keith, C. H., and J. C. Derrick. Measurement ot the particle size distribution and concentration of cigarette smoke by the "conituge.. J. Colloid. Sci. 15:340-356, 196U. 16. Kotin, P., and H. L. Falk. The role and action of environmental agents In the pathogenesis of lung cancer. lI. Cigarette smoke. Cancer 13:250-262, 1960. Lebowitz, M. D., and B. Burrows. Respiratory symptoms related to smoking habits ot family adults. Chest 69:48-5U, 1976. 18. Neurath, G., and H. Ehmke. Apparatur zur Untersuchung des ' Nebenstromrauches. Belts. Tabaktorsch. 2:117-121, 1964. (in German; English summary) 19. Neurath, G., and H. Horstmann. E~ntluss des FeuchtigReitsgehaltes von Cigaretten aut die Zusammensetzung des Rauches und die Glutzonentemperatur. Be~tr. Tabaktorsch. 2:~-10U, 1973. (in German; English summary) 20. Partenov, Y. D. Polonium-210 in the environment and in the human organism. At. Energy Rev. 12/1:75-143, 1974. 2 1. Repace , J . L., and A. H . Lowrey . Indoor a i r pollut Ion , tobacco smoke, and public health. Science 208: 464-472, 1980. 2 2. Russell , M. A. H., P. V. Cole , and E. Brown. Absorption by non-smokers of carbon monoxide from room air polluted by tobacco smoke. Lancet 1:576-579, 1973. Russell, M. A. H., and C. Feyerabend. Blood and urinary nicotine In non-smokers . Lancet 1 :179-181, 197 5. ;~4. Scassellatz Storzolini, G., and A. Savino. Evaluation of a rapid Index of ambient contamination by cigarette smoke, In relation to the composition of the gas phases ot the smoke. RIv. Ital. Ig. A: 43-55, 1S'6 ~ . ;25. Schilling, R. S. F., A. D. Lethal S. L. Hut, G. J. Beck, J. B. Schoenberg, and A. Bouhuys. Lung function, respiratory disease, and, smoking in families. Am. J. Epidemiol. 106 :274-283, 1977. ;26. Schmeltz, I., and D. Hoffmann. Chemical studies on tobacco smoke , XXXVITI . The physiochemical nature of cigarette smoke, pp. 13-34. In E. L. Wynder, D. Hotimann, and G. B. Gori, Eds. Smoking and Health. 1. Moditying the Risk for the Smoker. Proceedings of the 3rd World Conference on Smoking and Health. Washington, DeCe UeSe Government Printing Ottice, 1976. . Seehoter, F., D. Hanssen, H. Rabitz, and R. Schroder. Uber den ~rerble't des Wassers beam Abrauchen. i. M~tte~lung. Belts. Tabaktorsch. 3: 491-503, 1966. ~ in German; English summary) ;2 8. Spengler , J. D., D. W. Dockery, M. P. Reed, T. Tosteson, and P. Qulnlan. Personal Exposure to Respirable Particles. Paper 80-61.:,b, presented at 73rd Annual Meeting of the Alr Pollution Control Association, June 22-27, Montreal, Quebec, 198(' .
168 29 . Spengler, J . D., D. W. Dockery, W. A. Turner, J . M. Wolfson, and B. G. Ferris, Jr. Long-term measurements of respirable sulfates and particles inside and outside homes . Atmos . Environ . 15: 23-30, 1981. 30. Stewart, R. D., E. D. Baretta, L. R. Platte, E. B. Stewart, J. H. Kalbfleisch, B. Van Yserloo, and A. A. Risen. Carboxyhemoglob~n levels in American blood donors. J. Am. Med. Assoc. 229 :1187-1195, 1974 . 31 . U . S . Department of Health, Education, and Welfare, Public Health Service. Smoking and Health. A Report of the Surgeon General. DREW Publication No. (PHS) 79-50066. Washington, D.C.: U.S. Government Printing Office, 1979. [1250] pp. 3 2. Weber-Tschopp, A., T. Fischer, and E. Grand jean. Physiological and psychological effects of passive smoking. Inn. Arch. Occup. Environ. Health 37: 211-288, 1976. ~ in German; English summary) ODORS Some substances in the indoor environment make their presence known primarily by their ability to evoke odor sensations These substances generally arise from humans or their activities. A room full of people, for example, will invariably have Occupancy odor. ~ A characteristic odor like this generally emerges from a mixture of many organic substances, each present at a low concentration. Some of the individual constituents might cause greater concern if present alone at much higher concentrations; as building blocks of ~ composite, relatively benign odor, they receive attention only as odorants. Thus, the smell of the indoor environment is a measure of environmental quality. The remarkable sensitivity of olfaction encourages this approach. That is, treating low-concentration organic contaminants on the basis of their olfactory impact usually places stringent requirements on the quality of the indoor air. With some notable exceptions (e.g., the presence of carbon monoxide or mercury vapor), an indoor environment that is odorless contains air of healthful quality. Completely odorless conditions occur indoors only rarely. Weak odors may go unnoticed or may be tolerated, particularly after persons have had the opportunity to remain in a space for a while. The lability of olfaction, evident in this phenomenon of adaptation, may impair the credibility of the nose as an air~uality indicator. Its sensitivity, often as good as or better than that of the most sensitive instruments, offers some compensation for its functional instability. This section deals with how odors arise, how human beings perceive them, and how their status as ~perceived. contaminants determines the means to cope witch them. The str ingent air~uality standard effectively imposed by high olfactory sensitivity makes odorants a target of particular interest in any effort to keep indoor air quality high in an energy-conscious society.
169 SOURCES Occupancy Odor . Most buildings exist to hold people, in some cases many people e With the exception of churches, structures built specifically to hold many people were relatively rare ur.~il the nineteenth century. Before then, people avoided crowded places because of possible contamination--it was believed that crowded places, with their odors, served as breeding grounds of disease. The odors were often blamed for the spread of infection.2' This attitude makes some sense, in view of the imperceptibility of the actual agents of contagion. Even at the end of the nineteenth century, many people still found it difficult to accept the notion that contagion is primarily fingerborne, rather than airborne . Belief in the airborne route carr fed no evident penalty for the layman, merely some inconvenience. Hence, the notion that bad- smellinq air indoors signaled unhealthful conditions could carry on undisturbed. Through the burning of incense, churches had long seemed to give credence to the idea that a good (i.e., pleasant) odor would purify the air and thereby protect against illness. Such a practice actually reflected a predominant view of pre-nineteenth-century medicine. Only in the second half of the nineteenth century did the toxicity of the body effluvia responsible for occupancy odor receive scientific attention. In a common type of laboratory experiment on the matter. animals breathed air previously breathed by other animals, or received liquid injections of condensed organic materials from previously breathed air. In the experiments on ~rebreathing,. the animals sometimes developed infections or other difficulties; but, despite such occurrences, experimenters could not point indisputably to any harmful effects of the organic materials in previously breathed air. 12 Experiments on injections of condensed materials yielded essentially the same result: no consistent hazard. At the close of the nineteenth ~ ~ On the basis of both a review of available literature and their own experiments, Billings et al. 12 concluded that century, the issue seemed more or less settled. [it is] very improbable that the minute quantity of organic matter contained in the air expired from human lungs has any deleterious influence upon men who inhale it in ordinary rooms, factor rooms. . . . `~= "~-VllI~VL ~ ~~_~ -] I__ and, hence, it is probably unnecessary to take this into account in providing for the ventilation of such rant A;IF_ ~ ~~..~A At, ~~ ~ 11-ventilated rooms in persons not accustomed to them is not due to the excess of carbonic acid, nor to bacteria, nor, in most cases, to dusts of any kind. The two great causes of such discomfort, though not the only ones, are excessive temperature and unpleasant odors.... The cause of the unpleasant, musty odor which is perceptible to most persons on passing from the outer air into a crowded , unventilated room is unknown; it may, in part, be due to volatile products
170 of decompos ition contained in the expired air of persons having decayed teeth, foul mouths, or cer tain d isorders of the digestive apparatus, and it is due, in part, to volatile fatty acids given off with, or produced from, the excretions of the skin, and from clothing soiled with such excretions. It may produce nausea and other disagreeable sensations in specially susceptible persons, but most men soon become accustomed to it, and cease to notice it, as they will do with regard to the odor of a smoking-car, or of a soap factory, after they have been for some time in the place. The direct and indirect effects of odors of various kinds upon the comfort, and perhaps also upon the health , of men are more considerable than would be indicated by any tests now known for determining the nature and quantity of the matters which give rise to them. (pp. 24, 26-27) This statement would prompt little dispute today. A quarter-century after the experiments of Billings and colleagues, a New York State commission focused on ventilation requirements for occupied classrooms. " Tests of such varied functions and indexes as comfort, body temperature, intellectual performance, motivation, respiration, metabolism, condition of the nasal mucosa, f requency of colds, blood pressure, hematocrit, appetite, and rate of physical work uncovered no cause for medical concern under normal conditions of occupancy. This 8-yr effort reinforced notions that control of occ~pan;-y odor should figure prominently in indoor-air quality control, but the justification had to rest on grounds of comfort, rather than on grounds of health . The f its ~ truly quant itative studies of ventilation requirements started with the premise that ventilation primarily must control occupancy odor. Tobacco Smok ing. Throughout the twentieth century, the air in occupied rooms has co~runonly been smoky. Mainstream cigarette smoke contains approximately 3,000 gaseous constituents, 76 and these and associated particulate matter may both constitute health hazards for occupants . To add insult to inj ury, tobacco smoke forms the most annoying and persistent indoor odor nuisance. SO A survey of professional ventilating engineers placed it well ahead of the next two most disturbing indoor odorous contaminants, occupancy and cooking odors. Its severity as a nuisance derives from its properties: it is an apparent allergen for some persons; it is an eye, nose, and throat irritant for most persons; it is an odorant; it is a soiling agent; and it is a stimulus for chest discomfort in persons with angina pectoris. s' 7 Its ~tar. content causes it to adsorb strongly to surfaces. After adsorption, it desorbs slowly and thereby promotes so-called secondary sources of odor . In general, such sources concentrate previously airborne odorants on their surfaces (e.g., air-conditioning coils ~ or in their interstices (e.g., fabric). When the adsorbed odorants desorb, they often have an odor character somewhat different from that of the parent contaminant, and this generally seems true of tobacco smoke.S.
171 Tobacco-smoke odor increases in intensity and unpleasantness immediately after active smoking has ceased and after the particulate- vapor complex has adsorbed to surfaces. ~ 9 Figure IV-10 demonstrates how odor increased in intensity during a period after cigarettes were extinguished in an unventilated room. During this time, the odor character changed from pungent and burnt to stale and sour. This presumably reflected some chemical instability of the airborne matter. The contaminants of mere occupancy also seem somewhat unstable; but, unlike tobacco odor, occupancy odor diminishes rapidly and dramatically with time (see Figure IV-10 ~ . Cooking. One brand of cigarettes differs from another in type and blend of tobacco, type of additives sprayed on the tobacco or paper, and various other character istics, such as porosity of paper and temperature of the ember. 42 In spite of these variations, all c inarettes ~ ive r ise to an odor readily identif table perceptually as _ tobacco odor. i9 In similar fashion, cooking gives rise to perceptually character istic odors . These do not possess quite the s imple integr ity as a perceptual class as does tobacco-smoke odor . Some cook ing odors const itute more ser ious nu isances than others. Some (e.g. , cabbage odors are generally considered disagreeable, whereas others {e.g., baking odors) are generally considered inoffensive. Some vapors (e.g. ~ those from deep frying) adsorb tenaciously to surfaces and thereby become long-term contaminants. During initial generation, such vapors may evoke pungency, as well as odor; whether the pungency der ives f ram organic gases generated by the reaction of the oil with the food, from inorganic gases generated by combustion, or from particulate matter remains unknown, but deserves attention. ~ ° Bathroom and Waste Odors. In many buildings, exhaust hoods remove cooking odors at the site of generation. In some residences, cooking odors may have no such easy route of egress. Ductless range hoods, equipped with aluminum-mesh filters and carbon filters, sometimes serve as substitutes for exhaust ducts and true exhaust fans. Similarly, ductless bathroom ~ventilators. have begun to see some use in homes. Nevertheless, most commercial, institutional , and industrial buildings exhaust bathroom air to the outside. In theory, recirculation of properly filtered bathroom air in nonresidential buildings offers an opportunity for great energy savings . In practice, the putative need to eliminate moisture before recirculation and the possible, if only occasional, breakthrough of contaminants through any filtration system limit the prospects for recirculation. ~. People will tolerate inadequate control of cook ing odors (e . g ., operation of a ductless range hood with a spent carbon filter ~ much more readily than i nadequate control of bathroom odors . Odor Control. Elimination of the odor source, local exhaust, . general ventilation, and f titration (usually adsorption) are the principal ways of controlling indoor matadors physically. In some places, such as bathrooms and smoking areas, generation of malodor may exceed the limits of physical control and persons in charge of
172 5 4 ~Vaieric Acid _ z 3 LU a - o o 2 1 o Trot Odor S - y for _ . ~ . I I I ~ - 1 1 1 80 160 240 320 400 480 560 TIME AFTER SOURCE OF ODOR REMOVED (min.) FIGURE IV-10 Decay of odor in still air in an unventilated chamber af ter an open f task of Valerie acid had been removed, five cigarettes had been smoked, and a amber of nonsmoking occupants had lef t the chamber ~ "body odor" ). Odor judgments were made by observers who entered momentarily from time to 79 time. Reprinted with permission from Yaglou and Witheridge.
173 maintenance resort to commercially marketed odor counteractants.S. Such products generally comprise a fragrance base made up of many aroma chemicals and possibly a single proprietary tactile. ingredient. Typically, a manufacturer claims that the product has eliminated the objectionability or diminished the intensity of some standard malodor in laboratory Scents. The claim invariably has some validity, because all odorants, including n~lodoran.s, can influence the perception of other odors. Thin rule forms the foundation of practical perfumery. I' Basic research in olfaction supports the notion that a gas-phase mixture of components generally has an odor less intense than the total of the separate odors of the unmixed components, 20 perhaps less intense than some of its constituents alone--an extreme case of perceptual hypoadditivity. I 23 file matter of alterations in odor quality (character ~ has received little attention in the scientif ic laboratory. The perfumer knows through experience how to manipulate or blend malodorants to produce acceptability. Hence, some fruity- smelling natural essences (always mixtures of constituents) may contain some subsurface putrid-smellinq constituents. The perfumer may therefore blend a ~deodorizing. fragrance that will assimilate a putrid-~melling contaminant into a fruity complex. When unable to anticipate the particular malodorous quality of interest, ache perfumer generally blends a fragrance with a nondescript, unnameable odor character. Such a broad-band masker may assimilate some malodors r eadily, but at the very least adds perceptual unwise ~ to the maldodorous environment. Eventually, the usual or frequent occupants o f a space may come to smell the malodorant through the olfactory noise. Persons who ride airplanes often, for instance, eventually find that the smoking area ells strongly of both tobacco nuke and marking agent. It might seem that deodorizing products are used commercially only in special locations (e.g., bathrooms) or only on ache occasion. of uncontrollable malodorous emission {e.g., in the case of water do - ge to upholatery}. In fact, such products, under the generic name of ~reodorants, ~ appear in virtually every cleaning product (e.g., degreaser., detergents, soaps, and fabric shampoos} and in many other materials {e.g., fabrics, plastics, floor finishes, and carpets). Reodorants are sometimes used to cover up undesirable ambient odors (e.g., mildew in damp spaces) and often to cover up the intrinsic odors of manufactured products themselves (e.g., formaldehyde in permanent-press fabrics). No matter what their purpose, Deodorants and odor counteractants have become permanent parts of the indoor environment . ~ ~ Building Mater ials and Furnishings Almost any ob ject indoors may serve as a pr imary or secondary source of odor. Accordingly, the list of indoor odorous contaminants could go on indefinitely. In fact, Jarke.. identified over 200 organic constituents in residences under conditions designed to minimize active generation of contaminants. behave and M¢ller.°
174 identif fed a similar number and noted that only six of 46 dwelling seemed odorless. The identities of the more colon indoor contaminants led Jarke to conclude that notable more or less permanent sources include food. plants, bodies, dry-cleaned clothes, cosmetics, household products, attached garages, heating systems, new furniture, carpeting, and redecorated surfaces. Chemical contamination is apprently much higher in new than in old homes. ' ° New materials require a considerable amount of time to ~cure. before the off-gassing of volatile odorous substances diminishes to an imperceptible point. One of the got notor ious of the odorous contaminants in new buildings is formaldehyde. ' It emanates from shipboard. panel adhesive, carpet backing, vinyl wall-covering, resin-treated fabrics, and urea- formaldehyde foam insulation. In some Danish homes, the concentration of gas-phase formaldehyde has exceeded threshold limit values for occupational exposure. 9 Although formaldehyde has an odor, it has developed a reputation as an olfactory anesthetic. s 2 The reason for its putative anesthetic properties has not been fully studied. Conceivably, its irritant properties play a role in the anesthetic phenomenon. Cain and Murphy 2 C have reported that an irritant can immediately diminish and actually block olfaction. This effect seems to occur through interplay between sensory activity in the trigeminal nerve system and sensory activity in the olfactory nerve system. The trigeminal nerve mediates all cutaneous sensations of the face , as well as the pungency, irritation, warmth, cooling, and pain that can arise from chemical stimulation of mucosal tissue in the nose, mouth, and eyes. The evidence suggests that the interaction between odor and irritation takes place in the central nervous system and, hence, that it would hold true for virtually all combinations of Odorous and irritating stimuli. It seems relevant that various successful deodorizers have contained ire itants or pungent materials . For example, one commonly used deodorizer of the wick type contained formaldehyde, and other types of deodorizers contained other aldehydes. Ozone, a pungent gas, has long had a reputation as a deodorizer, even at concentrations too low to eliminate malodors through oxidation. Is The ~fresh-air. smell that deodorizers sometimes are claimed to produce can stem from the pungency produced by these substances at low concentrations in the product. Fresh Arctic air generally contains noticeable amounts of ozone. Hence, the association of pungency with afresh air. has some basis in common experience. Nevertheless, the deliberate addition, even at low concentrations, of products that are irritating has questionable justif ication ~ Odors That Enter from Outdoors In theory, ventilation dilutes and displaces contaminants that are generated indoors . Br inging in odor-contaminated air for use in ventilation to reduce odors obviously can defeat the purpose. Odorous outside air is encountered in areas of great industrial pollution or
175 where micrometeorologic conditions allow entrainment of emitted substances from local sources into intake vents. In recognition of the possibility that the air used for ventilation sometimes fails to meet normal standards of quality, the American Society of Heating, Refr iterating and Air{:onditioning Engineers (ASHRAE) specif led, in Standards for Natural and Mechanical Ventilation, ~ that intake air Should meet both objective and subjective criteria for cleanliness. A draft revision of this standard contains a list of notable outdoor contaminants, some odorous and some nonodorous, and concentrations not to be exceeded {see Table IV-21~. Adherence to these criteria will not guarantee the odorlessness of air, but will probably minimize dif f Faculties in polluted regions . Odors may also be generated in the air delivery system itself. Berglund and Lindvall ~ ~ discovered cases where the air supplied to a room had a higher degree of odorant contamination than the air already in the room. That can occur when air-to-air heat exchangers, installed to conserve energy, allow exchange of organic mater ials between exhaust and makeup air. Another source may be the accumulation of odorous mater ials on roughing f Liters and cooling coils and in humidif iers in the intake sys tem. MEASUREMENT OF ODOR , Odors have various attr ibutes : intensity, quality (character ), affective charge (acceptability~objectionability), and duration. Most environmental odorants are mixtures of substances . Hence, intensity and character generally represent the net action and nonlinear perceptual combination of various constituents. Figure IV-ll depicts a gas chromatogram of a sample oF odorous air (perspiration odor). In this case, the odorous sample is split into two streams after it passes through the column of the chromatograph. 32 One stream goes to a flame ionization detector and the other to a sniffing port, where the experimenter can note the odor character associated with the various chromatogram peaks. Such an odor-annotated chromatogram, called an ~odorogram,. can help in deciding how many peaks (constituents) seem odor-relevant, whether a few constituents seem particularly redolent of the unfractionated sample, and whether there is a relationship between the height of a peak and the magnitude of its odor. Often, a barely detectable peak is related to a strong odor and an enormous peak to a barely detectable odor. This situation reflects the nonuniform sensitivity of the nose; some substances stimulate at much lower concentrations than others. Table IV-22 shows, for instance, that ethyl acrylate stimulates at a concentration six orders of magnitude below that of ethylene and five orders of magnitude below that of acetone. Odor science has long sought, with limited success, to account for such large disparities in stimulating efficiency. Is 28
116 TABLE IV-21. Ambient-Air Quality Standards for Notable Contaminants [Jnregulated by Federal Clean Air Acta Long-Term Standard Shore;-Term Standard Coneaminantb Concentration Period Concentratlon Period~ * Ace tone * Acrole i; Ammo ni a Be ryl lium Cadalum Cal cium oxide ~ lime Carbon disulf ide Chlorine Chromi~m Cresol Dichloroethan~ Ethyl acetate Formal dehyded * Hydrothloric acit Hydroge n s~ f ide Me~captans Me rcury * Methyl alcohol * He thy le ne chl oride Nickel Nitrogen nonoxide Phenol* Sulf ates Sulf uric acid* Vanad ium Zinc 7 ~'g/m3 __ Oe S ~ag/m3 O. 01 ~ g/53 2.0 ug/m __ 0. 1S mg/~3 0~1 mg/m 1.5~g/m3 Oa 1 mg/m 2~0 ~g/53 14 mg/m 24 h 24 olg/m 25 l~g/m3 7 mg/m3 ~ yr 30 ~ 24 h 24 h 24 h 24 h 24 h 24 h 24 h O. 4 mg/m3 40~50 ~ g/m3 24 h __ 2 ~ g/'Q3 1~5 c~g/~3 20 mg/m 50 mg/~}3 2 ~g/m 0.5 mg/m 0. 1 mg~m3 4 ),g/m 12 1,8/m 50 ~g/m * 100 ~8<,~23 Trichloroethylene 2 mg/m S mg/~3 2 ~ g/~3 50 ~8/m3 100 ~8/~3 30 min C ' C 24 h 24 h 1 yr 24 h 24 h 24 h 24 h 1 yr 24 h 1 yr 24 h 1 yr 24 h 24 h 1 yr 24 h 20~30 ~ g/~5 0.45 mg/' 0.3 mg/m __ 6e 0 mg/~}3 42 mg/m 3 120 ug<m 3 mg/m 42 ug/~3 20 pa/m __ 4. 5 mg/m3 150 mg/m __ 1 mg/m3 __ C 30 min 30 min 30 min 30 ~n C 30 min 1 h 1 h 30 min 30 min 30 min 200 ~g/m3 30 min 16 t~g/m3 30 min Reprinted with permission from ANSI/ASHRAE. 1 Concentrations listed should be corrected to s tanda rd condi t ions--2 5°C and 760 ~n Hg. Contaminants marked with an asterisk have odors ae concentrations sometimes found in outdoor air; concentrations listed do not necessarily result ir~ absence of odor. C, ce fling (maximal allowable concentration). dAn industry organization has appealed the air quality limits of 120 ~g/m3 as shown in Tables 2 and 4 of Standard 62-1981. The appea1 is under considera- tion. If any change in Standard 62-1981 results from the appeal, all original recipients will be informed by ASHRAE.
177 600 1 200 KOVATS INDEX 1400 t600 1800 2000 rid - I , I l I ~ I l o ~ = = '- E '' ~ t ~ z !2 ad ~ At, I '- I 1 2 ., ~ o ~W PERSPIRATION I 1 1 1 ~ 1 1 1 1 1 0 9 18 27 REtENTION TIME {min.) FIGURE IV-ll Odor-annotated gas chromatogram, called an "odorogram." Test vapor was human perspiratf on. Annota- tions refer to odor qualities noted by an observer when various constituents (represented by peaks) eluted from the chromatogra~)i~ column. Reprinted with permission from Dravnieks.
178 TABLE IV-2 2 Odor Threshold and Quality of Various Petrochemicalsa Threshold Concentration, ppm 50: 100: Recog- Recog- Compound Absolute nition nition Quality Acetone 20. 0 32. 5 140 Sweet-f rutty 2, 6-Butanol 0. 30 1.0 2. 0 Rancid-sweet Di-N-butylamine 0.08 0. 27 0.48 Fishy-amine Diethylamine 0. 02 0.06 0.06 Musty-fishy-amine Ethyl acetate 6.3 13.2 13.2 Sweet-ester Ethyl acrylate 0. 0002 0.00030 0.00036 Sour-pungent Ethylene 260 400 700 Olefinic N-Ethyl morpholine 0.08 0.25 0.25 Ammo niaca1 Isobutyl acetate 0.35 0.50 0.50 Sweet-ester Isobutyl acrylate 0. 002 0. 009 0. 012 Sweet-musty Methanol 4.26 53. 3 53.3 Sour-sharp Methylethylketone 2.0 5. 5 6.0 Sweet-sharp 2-Methyl-5-ethyl 0.006 - 0. 008 0.010 Sour-pungent . - pyrlc gene 2, 4-Pentanedione 0.01 0.020 0.024 Sour-rancid Propanal 0.009 0.040 0.080 Sweet-ester Propioni~ acid 0.028 0.034 0.034 Sour Propylene 22.5 67.6 67.6 Sharp-amine aData f rom Hellman and Small. 46
179 Sample Collection P rocedures used to collect environmental samples for odor analys is have begun to approach standardization. Figure IV-12 displays a Cole ector that contains the porous polymer material Tenax GC. ' s Use of this adsorbent material avoids the need for solvents in collection and anal ysis of air samples . A 2-L sample drawn through the collector typically retains organic materials in a quantitatively faithful fashion if their molecules have more than about six carbon atoms. For analysis of its contents, the collector is connected to a gas chromatograph, where flash-heating desorbs the contents into a stream of inert carrier gas. New techniques, such as high-pressure capillary-column chromatography, allow greater resolution and sensitivity than could be achieved with packed columns of the sort used to analyze the sample in Figure IV-11. Unfortunately, there is some - incompatibility between capillary injection and the odorogram procedure. Psychophysical Analysis The standardization that has evolved in the collection of samples has extended also to psychophysical procedures for evaluation of environmental odors. The choice of procedures obviously depends on the question of interest . A var. iety of techniques are used in the laboratory, and a considerable number of techniques compete for attention in the field. Nevertheless, techniques that have recently emerged from activities of the ASTM Committee on Sensory Evaluation seem reasonably stable and precise and therefore legitimate for use as standard techniques. This section highlights these methods for the assessment of important attributes of odors. Odor Character. The odor of any substance can be described precisely by only one term--the name of the substance itself. Stated otherwise, the only precise name for the odor of a lemon is Lemon odor, ~ for the odor of a rose, "rose odor, n and for the odor of a goat, Goat odor . ~ Unlike colors, tastes, and sounds, odors have never given rise to their own glossary. ~, Moreover, people often find it difficult to retrieve the names of even familiar odors, not to mention describing unfamiliar ones. This situation motivated the derivation of a list of 146 descriptors to aid in the characterization of odors (Figure IV-13. 33 It seems necessary, if unwieldy, and has led to surprising reliability in a multila~oratory comparison. 3C Earlier systems of odor classification, generally hierarchic, with a small number of major categor ies and associated subdivisions never proved practical . ~ s Data derived from the list of 146 odor descriptors can have particular use in an effort to track down the source of a malodor, particularly an episodic malodor. The list enables persons influenced by the same malodor to express possible consensus regarding its character. Without such uniform terminology, even articulate persons often give such impoverished qualitative descriptions of matadors as ~stinky, n nrotten,. ~yocky,~ and "foul. n
180 s tent GO \ / Glass Woo ~ / GC Port 1 'A B Co I rector FIGURE IV-12 Tenex-filled collector for organic contaminants. Insert shows details of Connecting the end of the collector to the injection port of a gas chromatograph. Length of collector, 200 ma; outride diameter, 3.1 mm. Reprinted with permission frae Jarke. L
181 .. - ~. ......~. ~ . ~ # . ~ ~ ~ ~ ~ #e ~ #e ~ # _ ~ _ _ _ _ _ ~ · C. ~ · C. ~ ~ ~ · ~ · · · ·' ' i '. ~ ~ t: t j ! ~ I, ~ t · · ~ ., · ! · ' ' ~ ~ ·., -',, ,! ·. - ' .i x ~ 3 ~ '. . ~, ~ ~ ~ ~ IS88~2_~. . _ i ; i 1 · : : ' 1 : I : : : i . . i 5 i ~ i , . ~ 1 1 . · · '. ! ~ 1 ' '. . . . . . . 2 2 ' 2 It ~ ' ~ ' . . ~ . . . : . : . ,: ', , ,i i.! '. s i e i ~ I i ~ ·1911111lEc ,_ I,! i a ~ ~ ~ ~ .... ~ ~ . C i , I I I , I al 5 , _ ~ ~ 8 i° §~1i i,5!1~5,!, , ~S88~8SSSRS _ _ . . - . e · · ~ - - ~ ~ - ~ 1' 1 ~ I , I , i'!ii'l3 S ~ . 7 . ~ ~ ~ S~ - EEilI8EEli91 , . ; .. s" . ~ . .1 . . ~ #~ d 1 ~ ~ lI'sItlillll . . ~ . ~ ~ ~ ~ . . . _ _ _ _ _ _ _ _ _ _ _ - ~ ~ ~ ~ ~ ~ ~ o o · j · :: j · : .: : i ~ · : ! . i ,;;;; i . ! i i;;; t I i .i i '.; ~ ~ . i 2 2 ~ 2 2 ~ ~ i ! ~ i ~ ~ ! ~ i . ~ ! ! ' ii I - ., ~ j A i, ~ j ! .~i ~ 3 ~ ~ 3 ~ ~ . sIllIlillIs S ~ ~ ~ ~ ~ 8 ~ ~ o o |~lS'l'8i _ _ _ _ _ _ ~ o . . ~ · Ch ~ : · : : : : .; . . . . ~ . ; . .. . , y . ~ ~ .8 s . . ~ . ~ ~ · 3 ji r ~ ~ 0 0 ~ ~ ~ i 39 0 ~i o - IalIlill i i I ..,; . .. i ... , .. e, , ~ . i ,.~d i, i li i ~ ~ -. i''''!' Blitell' ;: 1:: ~ ! . ~ : : : : : ,, : - 4a : : : : ~ : . . · i : : : : ; ~ : : 2 · ~ ! : : · : . . .,, ~ '. · : : : : ~ : : . -, . ~ · ~ . : . ~ .: ~ -2 ~ ~ ~ ~ Y ~ sillill' o 00 o ~t ~L ~ C S ~ O C~ ~ S C) ~ ~t 0 1 a: ~5 p~ O C' ~ Z
182 Odor Intensity. From the standpoint of environmental engineering, intensity Is the most important attribute of an odor. It also permits relatively precise measurement by matching. A recently adopted ASTM butanol reference scale has already served well in this capacity. 2 The reference scale entails the use of an olfactometer that sets up eight concentrations of butanol spanning a range of subjective intensity from very weak to very strong. As Figure IV-14 shown, an observer seeks to f ind a nozzle on a lazy Susan that matches the intensity of a test odor. ~ ~ Figure TV-15 gives an example of some results obtained In this fashion. ~ ~ Intramodality matching, such as that used with the butanol reference scale, is the most fundamental and secure psychophysical operation. The widespread, successful use of intramodality matching in vision and hearing ensures its basic validity.S. Nevertheless, its use requires some knowledge of psychophysics in general and olfactory psychophysics in particular. For example, adaptation is important in determining how many judgments a person can make in a given period. 24 Inattention to time-dependent changes in sensitivity can severely distort the outcome of the matching operation . The NEC report Odors from Stationary and Mobile Sources' s presented other techniques for assessing odor intensity. Some involve the use of a matching odorant other than butanol. ~ ~ 29 Others involve only numerical judgments. The potential value of numerical judgements is derived from the possibility that they reflect tile true form of the psychophysical function. Such judgments imply, for instance, that the perceived magnitude of butanol var ies with concentration, not proportionally, but with approximately the 2/3 power of concentration. This relation, expressed in the equation S - 0. 261C0 66 (where S is perceived magnitude and C is concentration), can make it possible to convert a concentration of butanol chosen during matching to a numerical perceived magnitude. ' ~ The psychophysical technique of magnitude estimation used to derive the equation falls into a class of ratio scaling techniques. These commonly used techniques assume that sub jects can j udge ratio relations among sensa~cions . 6 ~ Al though the equation above has been proposed as a standard function, its parameters can vary systematically with conditions of stimulation, type of numerical scaling procedure, etc. Hence, the standard function requires specification of standard means of data acquisition. Some category scaling procedures--wherein subjects use a scale of five categories, seven categories, and so on--typically fail to produce a function that conforms to the equation. In principle, however, a category scale with a properly chosen number (and hence range) of categories should produce such a relation and might thereby bring the outcome of category scaling and ratio scaling into register. The issue of why these two classes of procedures generally produce different results has escaped simple resolution throughout sensory research. S. Odor Acceptability. The acceptability-objectionabilitY or pleasantness-unpleasantness of an odor can be assessed by essential I. my
183 1 -Bumno' \ Butanol Vapor \ Distributor Make Up Air \ I Distributor \ I Make U,^~' Air to 1-Butanol Vessel At/ Unknown Odor FIGURE IV-14 A subject using the butanol olfa~tometer (lazy Susan configuration) to find an odor that matches an unknown stimulus. As customarily arranged, the device delivers concentrations that range from 16 to 2, 000 ppm. Concentration changes by a factor of 2 from port to port. Print with permission frown Flavor Quality: 0b jective Measurement e pe 104 jig 103 ~ ·~ I I T I 1111 I I I IJr~ I _ F~R~tNE ~ ~5 at' 1 - IOOOE=~ ~ ~ ~ //.HEXANOL- ~ ~0 BUT - ETHER ~ ~ K} lo2 ,03 '04 R£LATlYE CONCENTRATION o2 Id FIGURE IV-15 Psychophysical functions for five odorants. Functions were obtained by matching butanol (note lef t ordinate ~ to various concentrations of each ado rent. Right ordinate shows odor intensity derived via the psychophysical func tion f or butanol. Data f ram Dravnieks and Laf fort.
184 the same techniques as its intensity. Far instance, Lindval1 and Svensson56 used Itching to hydrogen sulfide {rotten-egg odor} to assess the unpleasantness of combustion-toilet emiss ion . Many investigators have used numerical scaling, sometimes ratio scaling, 3' and sometimes category scaling. 3° The category scales have typically been bipolar, ranging, for instance, from -3 (very unpleasant ~ to +3 (very pleasant) . ~ Unfortunately, such a scale seems often to misrepresent the variability of acceptability and objectionability in the extremes of the hedonic continuum. Accordingly, some persons have used a line-marking or line-producing technique. 62 This generally involves a bipolar scale, but without f ixed categor ies, and seems to yield a Fore realistic picture of response variability than does category scaling. 2 S Other techniques of continuous, rather than categoric, judgment may behave just as well as line-marking. with respect to response variability. Odor Threshold . The measurement of odors has of ten compr ised . . merely the measurement of the concentration (or dilution ) necessary to achieve some criterion of detectability. The literature on olfaction contains well over 1,000 threshold values for odorants. All too commonly, these were obtained by techniques devised specifically for particular investigations; .~ 72 for this reason, the threshold value for a particular odorant may vary widely, even by order'; of magnitude, from one investigation to another. Specific factors contributing to this vat lability include the means of stimulus presentation to the odor-panel never, judgmental factors, and the implicit or explicit definition of threshold. Few experimenters have sought to verify the reliability of the vessels or olfactometers used to present odorous stimuli. Furthermore, each device customarily uses arbitrarily chosen conditions (e.g., flowrate, temperature, humidity, and solvent), and this often precludes comparison with results obtained with other devices or, more important, may preclude a comparison with typical environmental conditions. The psychophysical means of eliciting information on detectability can alter apparent sensitivity by a factor of 100 or more.Ss Presenting a pair of stimulus samples--one containing an odorant at fixed, weak concentration and the other containing only air--and forcing the subject to choose one of the two in each of scores or hundred. of trials will maximize detection. (This forced-choice procedure could also offer one odorous sample and two blanks, etce) But presenting various concentrations randomly with no direct comparison stimulus ~ ~blank. ~ invites variability. One sub ject may set low criterion and say ~yes. to almost anything. Another subject may behave much more conservatively. Such differences between subjects have given incentive to erect a new psychophysics of signal detection. Because of its time-consuming demand" in data collection, the theory of signal detection is not popular with researchers. That theory challenges the concept of threshold and instead specifies, in probabilistic and relatively bias-free terms, the detectability of any given signal. In this respect, it highlights the probabilistic nature of all thresholds. Despite the layman's view, ~threshold. hardly
185 refers to a concentration below which a normal person can never detect odor. Even concentrations well below ~threshold. may be detectable with sufficient frequency to cause concern about the validity of the threshold concentration. Complications involved in the interpretation of existing threshold data and pitfall" in the collection of new data raise the question of whether Thresholds, or comparable indexes of detection, offer greater benefits than suprathreshold matching. Nevertheless, some per&one will undoubtedly choose threshold measurement over matching. A relatively new ASTM procedure at least offers some standardization.' It recommends three-alternative forced-choice presentations wherein one nozzle of an air-dilution olfactometer presents an odorous stimulus at ~ given dilution and two companion nozzles present odorless air. Testing begins at a very low concentration and progresses to higher concentrations until the subject detects the stimulus reliably. Although the method contains arbitrary ingredients, it combines various important features designed to minimize response bias; and it allows a relatively speedy, if gross, estimate of the degree of dilution that sample of odorous air can withstand before it ceases to be detected readily. ODOR CONTROL The control of odor should follow the same strategy as the control of virtually any other type of indoor contaminant. The first step should involve good housekeeping and prevention of the source. This will obviously fail in the many cases where the mere presence and normal activities of occupants inevitably give rise to Odorous organic materials. A second step would eliminate airborne contaminants, such as kitchen odors, by local exhaust. In principle, the removal of odorous contaminants can follow one or more of the classical strategies: oxidation (e.g., incineration), scrubbing (e.g., spray-washing), chemical conversion (e.~., chlorination), filtration, and dilution. ' ° The first three of these a re used almost exclus ively in the control of industr ial odorous emission . For odors in residential, commercial, and in~ti~cutional spaces, filtration and dilution are the methods of choice. The use of deodorizers is justified only under unusual and temporary conditions. Ventilation Before the development of mechanical ventilation, the entrance of outside air through windows was the means of both thermal and contaminant control. The need for thermal control often dictated the demand for outside air. 21 Mechanical ventilation system, however, allowed separate control of the temperature and contaminants. The amount of ventilation air necessary for control of con taminant$ h istor ically has been a matter of contention . Some persons argued for ventilation rate'; that would render the air odorless. This strategy
186 rested on the premise that odor-laden air necessarily contained harmful organic contaminants. Similarly, some argued for rates that would maintain carbon dioxide at a concentration only twice that of the ambient a Or . ~ ~ Here again, the strategy rested on the notion that conservative control of a measurable (or, in the case of odors, perceptible ~ contaminant, even a rather innocuous one, would take care of unknown, but possibly harmful, airborne substances. This strategy . , ~ ~ ~ . . . ~ _ ~ . _ _ ~ . _ ~ ~ . _ _ lea do unreasonavly ups` venc~lac~un Taut . To achieve a Triter ion of approximate odorlessness, the ventilation rate must generally exceed 30 f t /m~n (about 14 L/S) per occupant ~ even during nonsmoking occupancy. The New York State Commission on Ventilation found that the concentration of carbon dioxide in a normally ventilated schoolroom correlated only weakly with odor. " Hence, the use of one contaminant seemed unable to pred ict the concentrations of all contaminants . The correlation rule may actually work reasonably wel 1 under conditions of active control of the delivery of ventilation air. S3 Nevertheless, the New York State commission found that a rate of 10-15 ft3<min (about 4.7-7.1 L/s) per student in a classroom with about 250 ft (9 m3) per student sufficed to control odor and carbon dioxide concentration reasonably well; furthermore, it seemed acceptable on the teas is of cr iter ia of comfort, health, and performance . In Winslow' s ' ~ words: The chemical vitiation of the air of an occupied room (unless poisons or dusts f tom industr ial processes or defective heating appliances are involved) is of relatively slight importance. The organic substances present, manifest as body odors, may exert a depressing effect upon inclination to work and upon appetite; therefore occupied rooms should be free from odors which are obvious to anyone entering from without. perceived by those who have been in the room while they have been accumulating.) Objectionable effects of this sort have only been (Such odors are never demonstrated, however, with a carbon dioxide content of over .2 per cent, which would correspond to an air change of less than 6 cubic feet per person per minute. (pp. 77-78) As mechanical ventilation systems became more common, there was more interest in discovering how the odor of a room would vary with changes in the proportion of total supply air that consisted of ventilation (outdoor) air. Figure IV-16 depicts a functional relation that Houghten and colleagues. 7 erected from judgments in a junior- high-school classroom. The function, derived from judgments of visitors who entered the occupied classroom briefly, intersects the line equal to a judgment of 2 Unnoticeable [odor] but not objectionably at a ventilation rate of about 11 ft3/min (about 5.2 L/s ~ per student . This outcome seemed to confirm the f indings of the New York State commission. Experiments performed at the Harvard School of Public Health shortly after the study of Houghten and colleagues implied that ventilation requirements per occupant would vary with the amount of space (volume} available to each occupant. Yaglou and colleagues 7
187 L/SEC. PERSON 4 5 ~ 4 C) i 3 6 7 8 9 4 6 8 10 12 14 16 1 8 OUTDOOR AIR SUPPLY (CFr' / PERSON ) FIGURE IV-16 Relat ion between odor intens ity and ventilation air in junior-high-school classrooms. Observers entered the occupied classroom from Correlatively odor-free corridor. Adapted from Houghten et al.
188 of Harvard, like Boughten et al., derived functions that related odor to ventilation rate and added such variables as relative crowding, hygiene, and age. Each variable had some influence, but crowding (i.e., occupant density in an experimental room) had the most noteworthy influence. Figure IV-17 depict ventilation requirements decided by various criteria versus air space per occupant. Function C represents requirements according to an odor criterion of .moderate. (a rating of 2 on a scale of 0-5~. It reflects the intersection of this perceived extent of odor with the various combinations of ventilation rate and air space per person in the functions for occupancy odor depicted in Figure IV-18. Although admittedly incomplete, the work of Yaglou et al. stands as the most definitive investigation of ventilation requirements ever performed. It seemed to f igure at least implicitly in the recommendations of ASHORE Standard 62-73, a standard based on professional consensus. ' As Figure IV-l9 ebows, the recommended ventilation rates for a diverse group of residential and commercial spaces follows much the same curvature as the function of Yaglou et al. Almost all the recommended rates fall above the function--an unsurprising feature, inasmuch as moat spaces have a bigher odor load than that imposed merely by sedentary occupancy. Cigarette-smoking, for instance, leads to much higher ventilation reguirement-. Unfortunately, the study shown in Figure IV-20 proffered the only "borough look at the ventilation Beguilements necessary to control the odor of fresh tobacco smoke.77 In many of the spaces represented in Figure IV-17, smoking or so'De physical activity might occur. Curve D in Figure TV-17 formed one attempt to account for the requirements in spaces with such activities. The function, a SO% upward transposition of curve C, apparently has no experimental justification. but may nevertheless serve well as a rough guide for ventilation requirement.. ' 3 See Chapter IX for more complete d iscuss ion of ventilation standa rds . Al r-Cleaninc: The principles of contaminant dilution and displacement, achieved through ventilation, offer the simplest means of indoor contaminant control. In some instances, however, ventilation alone proves inefficient or ineffective. Other means of control can then assist. Outdoor air sometimes contains unwanted concentration'; of contaminants, and recirculation of indoor air becomes desirable. For instance, the outdoor air may contain enough sulfur dioxide to damage sensitive electronic equipment. Filtration of one Fort or another can reduce the concentrations of the contaminant in the incoming air or, sometimes more productively, can reduce the concentrations of the various contaminants generated indoors and thereby reduce reliance on the use of contaminated outdoor air. 5 0 In principle, filtration of recirculated air can achieve indoor air quality that exceeds that of outdoor a r. But in practice, filtration leaves some contaminants (e.g., cation monoxide and carbon dioxide) unattenuated. Only very expensive procedures will eliminate these contaminants. Thus, an
189 Al R SPACE PER PERSON (m3 ) 40 o At? , 30 g E - ~ 20 6 a: o O 10 3 5 10 15 20 - - - - - - - - - - _ _ 200 400 600 800 AIR SPACE PER PERSON {cu. ft.} 20 15 . 10 ~ 6 cr o o o FIGURE IV-17 Relation between ventilation rate and air space per occupant according to four criteria: A, maintenance of oxygen; B. control of carbon dioxide ~ <O. 6% ); C, control of body odor under sedentary conditions (no smoking); and D, control of odor when occupants we re slightly active and when smoking was permitted . Lower cur,, derived from data of Yaglou et al. Adapted from Viessman.
190 AIR SPACE PER PERSON (m3) 10 IS .g g tO tS 20 AS 30 1 100 ADULT SUS.I EC TS tm PER Pt,8?so 150 200 2S0 300 400 BOO AIR SPACE PER PERSON tCU. FT.) FIGURE IV-18 Odor intensity versus net air space per person for ventilation relies of 5-30 cfm ~ 2. 5-15 L/s ). Adapted from Yaglou et al.
191 ~ SPACE PER PERSON (m3J o 0 0 0 ~0 20 ~0 ~0 ~0 2Ck 10 o : \ 0 0 0\ 0. 0 oN ID ~ O 0 0 0 ~ coo om _ ooo . 0 O .~..1 ~ 0 ~ 0 o ~ oo 0 0 . ~ · ., · ~ ~ . 100 200 5X 100D 2000 AIR SPACE PER PERSON {CU. FS. ) 20 ~0 15 ~ 10 ~ 20 ~ FIGURE IV-l9 Top, points depict rates of ventilation recommended for various residential and commercial spaces by ASHRAE Standard 62-73 versus air space per person (logarithmic scale). Air spade per parson was derived from estimates of occupancy per 1,000 ft (93 m ~ of floor area (ir~corporated into the standard) and from estimate of ceiling height. Excluded were spaces, such as theaters, where ceiling height might vary considerably from one space to another. Bottom, points depict rates of ventilation recommended for new buildings by ASHRAE Standard 90-75. The points represent the so-called minimal ventilation rates of Standard 62-73. Lines in top and bottom portions are function C from Figure IV-17.
192 s >-4 _ in at UJ he 3 _ - A Lo CL 1 0 5 - USEC SMOKER 10 1 5 20 25 30 I I I 1 Acc - able Smo - ' _ I Oberon ~Non~oker' 20 30 40 50 60 OUTSIDE Al R SUPPLY {ctm per smoker} 0 10 FIGURE IV-20 Variation in odor intensity with fresh air supply when nine persons, including six smokers, occupied Yagiou's chamber and smoked cigarettes at a rate of 24 per hour. Functions labeled -emokera. amd "nonsmokere" depict judgments of occupants. Function labeled "obeervers" depicts Judgments of persons who77 entered briefly from an otor-free room. Adapted from Yaglou.
193 engineer or designer cannot rely entirely on recirculation, but must deliver came minimal quantity of outdoor air. According to ASHRAM guidelines, ' the minimum should equal 5 ft3/m$n (about 2.4 L/~} per person when the filtration system has high efficiency. This quantity of air guarantee adequate control of carbon dioxide with a subetanelal margin of safety. In a space that might normally demand outdoor air at, say, 20 ft3/min (about 9.4 L/~) pet occupant to control the concentrations of organic materials generated during occupancy, use of a high-efficiency filtration system could save considerable amounts of the energy generally used to heat or cool ventilation air. The characteristic way to clean indoor sir involves the use of granular filter media and, if necessary, particle filters. ~ Particle filtration will prove necessary in the presence of cigarette-emoking. Some portion of tobacco-~moke odor is presumably eliminated by such f iltration. Particle filters protect the granular filter. Activated carbon is the Host coon type of granular medium for control of airborne organic matter. It removes most odorous material and generally renders the air ~fresh-amelling.. its adsorbent property leads to actual retention of the contaminant up to a Maturation point, when the f ilter bed must be replaced. Activated carbon ts available in many varieties, depending on starting materials and production conditions. Efficiency varies considerably, and life span may be unpredictable. Such technical vagaries detract from the use of activated carbon by nonspecialists, and tic currently is little used in ordinary ventilation systems. Other adsorbent materials, such as porous polymers, have been developed for gas chron~atography," and perhaps these now-costly materials will become cheap enough for use in ventilat ion . Finally, activated carbon or activated alumina can be impregnated with other materials to increase efficacy against particular contaminants. Activated alumina impregnated with postassium permanganate, developed specifically for odor control, offers the roost readily available commercial alternative to activated charcoal.45 It operates by adsorption and oxidation and thereby capitalizes on an empirically observed rule that a malodorant may lose its malodorous properties when oxidized. RESEARCH NEEDS ventilation Codes A surrey of building codes in the 1960" uncovered a tenfold variation in the listed ventilation rates. i. Such variation reflects in part the change in modes of thought regarding ventilation requ irement~ through the years . Some codes apparently arose f tom local notions of Good engineering practice. and had seen little or no recision in decades. In the late nineteenth century, the Amer3can Society of Heating and Ventilating Engineers recommended 30 f t /min per occupant. AS mentioned above, this Prague arose from the notion that ventilation should keep the indoor concentration of carbon dioxide
194 at less than twice its outdoor concentration. Forty years later, the Society recommended a rate of 10 ft3/min per occupant. Although the studies of the New York State commission and other researchers had made it clear that a rate as low as 10 ft3/min per occupant would not lead to harm, the members of the Society's committee apparently felt the need for considerably more data. W. H. Driscoll, a member of the committee, noted: " When we finally decided that we would take 10 cfm per person as the minimum it was a sheer compromise, merely an attempt to finish the work of the Committee and get the report before the Society. There was a difference of opinion as to whether the 30 cu ft that have been set up as a standard since time immemorial should be adopted, or whether no cubic feet, for which there was very aggressive support, not necessarily within the Committee but from outside of the Committee, on the theory that no scientific studies had ever been made to support the necessity for the introduction of any outdoor air as a ventilation requirement. (p. 160) The experiments of Yaglou et al. 7' added some critical information, but this did not necessarily become part of the thinking of code-makers. For instance, some codes still specify ventilation requirements in terms of air changes per hour, irrespective of density of occupancy. Yaglou et al. had suggested a need for great sensitivity regarding density of occupancy. Indeed, Yaglou and Witheridge's experiment prompted the comment: 73 One result of this investigation, for which I think we can be particularly grateful to the authors, is that it effectively explodes the myth of air change based on room volume as a measure of ventilation . I cons ider it a real step forward if the idea of air change on a room volume basis can be eliminated from our thinking . (p. 432 ~ I rrespective of the reasons for variation in ventilation codes, its mere existence implies that a reduction of rates in some of the localities now requiring high rates would probably arouse few complaints. Despite variation from one location to another, both code-makers and ventilating engineers have generally sought to Supply ventilation air liberally. Mast codes specify rates for conditions of full occupancy; but full occupancy may occur only rarely. The increasing cost of energy has prompted an unprecedented desire for uniformity in American ventilation codes. The bottom part of Figure IV-l9 shows the so-called minimal ventilation rates promulgated in ASHRAE Standard 62-73.' These values, like the recommended values shown above them, capture much of the curvature of the recommendations of Yaglou _ al. m e minimal values actually cluster around the Yaglou _ al. function. When energy first became particularly expensive, it seemed desirable to reduce the ~recommended. ventilation rates to these minimal values, at least on a trial basis. These were therefore incorporated into the general standard, ASERAE 90-75, Energy
l9S Conservation in New Building Design. 5 . The various model-code groups (Building Officials and Codes Administrators International, International Conference of Building Officials, and Southern Building Code Congress International} have since cooperated with the National Conference of States on Building Codes and Standards (NCSBCS ~ in drawing up an energy-conservation code, Code for Energy Conservation New Building Construction. ~ ~ It too incorporated the minimal rates of the ASHRAE ventilation standard. An NCSBCS survey of building codes'. conducted in May 1979 revealed widespread adoption of energy conservation in ventilation {Table IV-23. Virtually all large jurisdictions have conformed or will soon conform to conservation standards through adherence to one or another model code, a separate state code rooted in a model code, or ASERAE Standard 90-7S. At the time of the survey, only five jurisdictions had failed to take overt action toward the adoption of an energy-ef f icient code . Energy Efficiency, Comfort, and Health Both the recommended and minimal ventilation rates in ASHRAE Standard 62-73 comprise consensus values. Except, perhaps, for spaces with unexpectedly high rates of cigarette-smoking, the recommended values probably serve well. In some spaces with heavy smoking or cooking, the minimal values will probably serve very poorly. Without a f oundation of modern research on a var. iety of contaminants, the justif ication of the minimal values will rest heavily on considerations o f energy, rather than health . The research must seek to spec if y rates and strategies that will achieve both energy ef f iciency and healthf ul conditions. This will eventually require strict control over the internal atmosphere. Without guidance, some engineers will shut down intake-air dampers to save fuel and will thereafter rely on infiltration alone for fresh air. This strategy actually reduces, rather than increases, the engineer 's active control over the building. The dimensions of ventilation research are roughly the same now as in the time of the work of Yaglou _ al. Ventilation dilutes contaminants . I ts ef f icacy depends on the na tore of indoor contaminants, the s ize of a space, environmental var tables , furnishings, duration of occupancy, aesthetic standards of occupants of or visitors to the space, and sensitivity of occupants . Modern methods available for exper imentation include the newly standarized psychophysical methods discussed here and monitoring equipment not available during earlier research on ventilation (e.g., continuous carbon monoxide analyzer, continuous carbon dioxide analyzer, gas chromatograph, mass spectrometer, particle-mass monitor, condensation nucleus counter, and electric aerosol analyzer) . With these various tools, it is now possible to characterize the indoor environment in both a psychophys ical and a phys icochemical manner . Such characterization has now begun in North America and Europe. 22
196 TABLE IV-23 NCS8CS Survey of Energy-Conservation Codesa Jurindietion Energy- Conservation Code Jurisdiction Energy- Conservation Code Alabama S BCCI Nebraska ~ ICBO Alaska (ASHRAE 90-7'~ Nevada MCEC Arizona (State code)* New Hampshire MCEC Arkansas (State code ~ New Jersey BOCA California State code New Mexico ICBO * Colorado MCEC * New York State code Connecticut State code North Carolina State code Delaware (MCEC ~ North Dakota ICBO District of (City code) Ohio MCEC Colu~bi a * Oklahoma None Florida State code Oregon ICBO Georgia MCEC Pennsylvania (ASHRAE 9075 Hawsti ICBO Rhode Island ASHRAE 90-75 Idaho ICBO * South Carolina SBCCI Illinois (State code ~ South Dakota (MCEC Indiana MCEC Tennessee MCEC Iowa MCEC Texas (MCEC Kansas State code Utah MCEC Kentucky MCEC Vermont (ASHORE 90~75 ~ Loui siana (}lCEC ~ Virginia BOCA, ASH RAE 90-7 5 Maine (State code ~ Washington S tate code Maryland (ASHRAE 90~75 ~ West Virginia None * Hassachusetts MCEC Wisconsin State rode Michigan ASHRAE 90~75 Wyoming ICBO Minnesota ASHRAE 90~75 American Samoa MCEC Mississippi (MCEC) Guam IC8O Missouri (MCE:C) Puerto Rico MCEC Montana b£CEC at ~ denotes that legislation is pending. ASHRAE 90~75 ~ ASlIRAE STANDARD 90~75. BOCA - Model Code, Building Officials ~ Code Administrators International, Inc. ICBO -Model Code, Interna~cional Conference of Building Officials. MCEC - Model Code for Energy Conservation in New Building Construction. SBCCI ~ Mode] Code, Southern Building Code Congress International Inc. Asterisk denotes obvious incorporation of energy~conserving aspects of a model code or ASHRAE 90-75; codes or pending codes for California, Maine, and North Carolina Who include some such aspects.
197 REFERENCES 1. American National Standards Institute, and Society of Beating, Refrigerating and Air~Conditioning Engineers, Inc. ANSI/ASERAE S tandard 6 2-1981 e -A ~ ~ · ~ ~ ~ ~ ~- ~ ~ ~ New York: American society of Heating, Refrigerating and Air~Conditioning Engineer., Inc., 1981. 48 pp. 2. Anerican Society for Testing and Materials. ASTM E 544-75. Standard Recommended Practicer for Referencing Suprathreshold Odor Intensi ty . Philadelphia: Amer loan Society for Testing and Materials, 1975. 3. American Society for Testing and Materials. ASTM D 1391. Standard Test Method for Measurement of Odor in Atmospheres {Dilution Method). Philadelphia: Anerican Society for Testing and Materials, 1978. 4. American Society of Heating, Refrigerating and Air~Conditioning Engineers . Control of odors and gaseous contaminants, pp. 3 3.1-33 .8. In ASHRAE Handbook and Product Directory. 1980 Systems. New York: American Society of Heating, Refrigerating and Air~Conditioning Engineers, Inc., 1980. 5. American Society of Heating, Refrigerating and Air~Conditioning Engineers. ASHRAE Standard 90-75. }:nergy Conservation in New Building Design. New York: American Society of Heating, Refrigerating and Air~Conditioning Engineers, Inc., 1975. 53 pp. 6. American Society of Heating, Refrigerating and Air~Conditioning Engineers. ASERAE Standard 62-73. Standards for Natural and Mechanical Ventilation . New York: Amer ican Society of Heating, Refrigerating and Air~Conditioning Engineers, Inc., 1973. 17 pp. 7. Amerine, M. A., R. M. Pangborn, and E. B. Roessler . Principles of Sensory Evaluation of Food. New York: Academic Press, Inc., 1965. 602 pp. 8 . Andersen, I . Formaldehyde in the indoor environment--Health implications and the setting of standards, pp. 65-87 (includes d iscuss ion J . In P . O. Fanger and O. Valb join, Eds . Indoor Climate. Effects on Human Comfort, Performance, and Health in Residential, Commercial, and Light-Industry Buildings. Proceedings of the First International Indoor Climate Symposium, Copenhagen. August 30-September 1, 1978. Copenhagen: Danish Building Research Institute, 1979 . 9 . Andersen, T ., G. R. Lundqvist, and L. Molhave . Indoor air pollution due to Shipboard used as a construction material. Ados. l~n~riron. 9 :1121-1127, 1975. 10. Berglund, B. Quantitative and qualitative analysis of industrial odors wits human observers . Ann. N.Y. Acad . Sci . 237: 35-51, 1974 . 1 1. Berglund, B., and T. Lindvall. Olfactory evaluation of indoor air qual ity, pp . 141-1S7 . In P . O . Fanger and O. Valb j0(rn, Eds . Indoor Climate. Effects on Human Comfort, Performance, and Health in Res identia1, Commercial, and Light-Industry Buildings . Proceedings of the First International Indoor Climate Symposium, Copenhagen, August 30-September 1, 1978. Copenhagen: Danish Building Research Institute, 1979. venom tor Acceptable Indoor Air Quality.
198 12. Billings, J. S., S. W. Mitchell, and D. H. Bergey. The condition of expired air and its effects upon animal life. Smithsonian Contributions to Knowledge 29 (989) :~-81, 1895. 13. Billot, M., and F. it. Wells. Perfumery Technology. Art: Science: Industry. Chichester, England: Ellis Horwood Limited, 1975. 353 pp. 14. Brauer, R. L., and R. L. Ruehner. me Variability of ventilation Codes. In Odors and Odorants: The Engineering view. ASHRAM Symposium Bulletin CH-62-2, January 27-30, 1969. 15. Cain, W. S. History of research on smell, pp. 197-229. In E. C. Carterette and M. P. Friedman, Eds. Handbook of Perception. Vol. 6A. Tasting and Smelling. New York: Academic Press, Inc., 1978. 16 . Cain, W. S . Interactions among odors, environmental factors and ventilation, pp. 257-274. In P.O. Fanger and 0. Valbj~rn, Eds. Indoor Climate. Effects on Human Comfort, Performance, and Health in Residential, Commercial, and Light-Industry Buildings. Proceedings of the First International Indoor Climate Symposium, Copenhagen, August 30-September 1, 1978. Copenhagen: Danish Building Research Institute, 1979. 17. Cain, W. S. Physical and cognitive limitations on olfactory processing in human beings, pp. 287-302. In D. Muller-Schwarze and M. M. Mozell, Eds. Chemical Signals in Vertebrates. New York: Plenum Press, 1977. 18. Cain, W. S. To know with the nose: Keys to odor identification. Science 203:467-470, 1979. 19. Cain, W. S. Sensory attributes of cigarette smoking. In G. B. Gori and F. G. Bock, Eds. Banbury Report Three. A Safe Cigarette? Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratories, 1980. 20. Cain, W. S. The odoriferous environment and the application of olfactory research, pp. 277-304. In E. C. Carterette and M. P. Friedman, Eds. Handbook of Perception. Vol. 6A. Tasting and Smelling. New York: Academic Press, 1978. 21. Cain, W. S . Ventilation and odor control: Prospects for energy savings. ASHRAE Trans. 85(Pt. 1) :784-793, 1979. 22. Cain, W. S., L. G. Berglund, R. A. Duffee, and A. Turk. Ventilation and Odor Control. Prospects for Energy Efficiency. Final Report of Phase I of Energy Efficient Ventilation Standards: Requirements for Odor Control. Lawrence Berkeley Laboratory Report LBL-9578. Berkeley, Cal.: Lawrence Berkeley Laboratory, Energy and Environment Division, 1979. 23. Cain, We Se, and M. Drexler. Scope and evaluation of odor counteraction and masking . Ann. N.Y. Acad. Sci. 237:427-439, 1974. 24. Cain, W. S., and T. Engen. Olfactory adaptation and the scaling of odor intensity, pp. 127-141. In C. Pfaffmann, Ed. Olfaction and Taste. Proceedings of the m ird International Symposium. New York: me Rockefeller University Press, 1969. 25. Cain, W. S., and F. Johnson, Jr. Lability of odor pleasantness: Influence of mere exposure. Perception 7:459-465, 1978. 26. Cain, W. S:' and C. L. Murphy. Interaction between chemoreceptive modalities of odor and irritation. Nature 284:255-257. 1980. 27. Chapin, C. V. me Sources and Modes of Infection. New York: John Wiley & Sons, 1910. 399 pp.
199 28. Davies, J. T. Olfactory theories, pp. 322-350. In L. M. Be~dler, Ed. land boo k of Sensory Physiology. Vol. INt. Chemical Senses. Part 1. Olfaction. Berlin: Springer-Verlag, 1971. 29. Degobert, P. Hedonic and intensity ranking of different malodours by category estimation and paired comparison, pp. 107-121. In J. H . A. Krooze , Ed . Preference Behaviour and Chemoreception. London: Information Retrieval Ltd., 1979. 30. Doty, R. L. An examination of relationships between the pleasantness, intensity, and concentration of 10 odorous stimuli. Percept. Psychophys. 17:492-496, 1975. 31. Dravnieks, A. Correlation of odor intensities and vapor pressures with structural properties of odorants, pp. 11-28. In Re A. Scanlan, Ed. Flavor Quality: Objective Measurement. American Chemical Society Symposium Series, No. 51. Washington, D.C.: American Chemical Society, 1977. 32. Dravnieks, A. Evaluation of human body odors: Methods and interpretations. J. Soc. Cosmet. Chem. 26:S51-571, 1975. 33. Dra~nieks, A. Fundamental considerations and methods for measur ing air pollution odors, pp. 429-436. In J e LeMagnen and P. MacLeod, Eds. Olfaction and Taste. VI. London: Information Retrieval Ltd., 1978. 34. Dra~nieks, A. Measurement of odors in an indoor environment, pp. 127-139. In P.O. Fanger, and O Valbj0rn, Eds. Indoor Climate. Effects on Human Comfort, Performance, and Health in Residential, Commercial, and Light-Industry Buildings. Proceedings of the First International Indoor Climate Symposium, Copenhagen, August 30- September 1, 1978. Copenhagen: Danish Building Research Institute, 1979. 35. Dra~nieks, A. Organic Contaminants In Indoor Air and Their Relationship to Outdoor Contaminants. Phase I, ASHRAE Research Project 183. IIT Research Institute. New York: American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc., February 1977. (unpublished) 36. Dravnieks, A., F. C. Bock, J. J. Powers, M. Tibbetts, and Me Ford. Comparison of odors directly and through profiling. Chem. Senses Flavor 3:191-22S, 1978. 37. Dra~nieks, A., and P. Laffort. Physico-chem~cal basis of quantitative and qualitative odor discr imination in humans, pp . 142-148. In D. Schneider, Ed. Olfaction and Taste. Id. Stuttgart: Wissenschaftliche Verlagsgesellschaft M}3H, 1972 . Engen, T. Psychophysics. 1. Discrimination and detection, pp. 11-46. In J. W. Fling and L. A. Riggs, Eds. Woodworth and Schlosberg ' s Exper imental Psychology. 3rd ed . New York: Bolt , Rinehart and Winston, Inc., 1972. Engen, T., and D. H. McBurney. Magnitude and category scales of the pleasantness of odors. J. Exp. Psychol. 68:435-440, 1964. 40. Evans, C. D., K. Warner, G. i. List, and J. C. Cowan. Room odor evaluation of oils and cooking fats. J. Am. Oil Chem. Soc. 49:578-582, 1972. 41. Fazzalari, F. A., Ed. Compilation of Odor and Taste Threshold Values Data. Philadelphia: Amer ican Society for Testing and Mater i als, 1978. 497 pp. 38. 39.
200 56. 42. Gori, G. B. L^w-risk cigarettes: A prescription. Science 194 :1243-1246, 1976. 43. Haggard, H. W. Devils, Drugs, and Doctors. The Story of the Science of Healing from Medicine-Man to Doctor. New York: Harper Brothers, 1929. 405 pp. 44. henna, G. F., and R. L. Kuehner. Critical Factors in Odorant Measurement and Control. ASHRAE Symposium Bulletin CH-69-2:66-72. January 27-30, 1969. 4 5 . Hanna , G . ~ ., ~ . L. Knehner , J . D. Karnes , and R. Garbowicz . A chemical method for odor control. Ann. N.Y. Acad. Sci. 116:663-675. 1964. 46. Bellman, T. A., and F. H. Small. Characterization of the odor properties of 101 petrochemicals using sensory methods. J. Air Pollut. Control Assoc. 24:979-982, 1974. 47. Houghten, F. C., B. B. Trimble, C. Gutberlet, and M. F. Lichtenfel';. Classroom odors with reduced outside air supply. ASEVE Trans. 41: 253-267, 1935. 4 8. Jarke, F. B. Organic Contaminants in Indoor Air and Their Relation to Outdoor Contaminants. Final Report of ASHRAE Research Project 183, December 1979. 49. Jellinek, J. S. The Use of Fragrance in Consumer Products. New York: John Wiley & Sons, Inc., 1975. 219 pp. 50. Kalika, P. W., J. K. Bolco~e, and W. A. Cote. The re-use of interior air. ASElfiAE J. 12~11~:44-48, 1970. 5 1. Klauss , A. K ., R. B. Tull , L. M. Roots , and J . R . Pf af f lin. Hi story of the c hang ins concepts in ventilation requirements. ASHRAE J . 12 (6~: 51-55, 1970. 52. Xulka, R. Odor control by modif ication. Ann. N. Y. Acad. Sci . 116: 676-681, 1964. 5 3. Kusuda, T. Control of ventilation to conserve energy while maintaining acceptable indoor air quality. ASHRAE Trans. 82 (Pt.1):1169-1181, 1976. 54. Leonardos, G., and D. A. Kendall. Questionnaire study on odor problems of enclosed space. ASHRAE Tran=. 77(Pt. 1~:101-112, 1971. 55. Lindvall, T. On sensory evaluation of odorous air pollutant intensities. Measurements of odor intensity in the laboratory and in the field with special reference to effluents of sulfate pulp f actor ies . Nord . Byg . Tidskr . Suppl . 2 :1-181, 19 70 . I.indvall, T., and L. T. Svensson. Equal unpleasantness stitching of malodorous substances in the community. J. Appl. Psychol. 59: 264-269, 1974. 57. Lundqvist, G. R. The effect of smoking on ventilation requirements, pp. 275-292 (includes di`;cu';sion). In P.O. Fanger, and 0. Valbj'$rn, Eds . Indoor Climate . Ef feats on Human Comfort, Performance, and Health in Residential, Commercial, and Light-Industry Bu ildings . Proceedinq'; of the First Interna~cional Indoor Climate Symposium, Copenhagen, August 30-September 1, 1978. Copenhagen: Danish Building Research Institute, 1979. 58. Quarks, L. E. Sensory Proce';~;e';. The New P';ychophy~;ics. New York: Academic Press, Inc., 1974.
201 59. McCord, C. P., and W. N. Witheridge. Odors. Physiology and control. New York: McGraw-Hill Book Company, Inc., 1949. 405 pp. 60. M0lhave, L., and J. Miller. The atmospheric environment in modern Danish dwellings -Measurements in 39 flats, pp. 171-186. In P. O. Fanger and O. Valbj0rn, Eds. Indoor Climate. Effects on Human Comfort, Performance, and Health in Residential, Commercial, and Light-Industry Buildings. Proceedings of the First Tnternational Indoor Climate Symposium, Copenhagen, August 30-September 1, 1978. Copenhagen: Danish Building Research Institute, 1979 61. Moskowitz, H. R., A. Dra~nieks, W. S. Cain, and A. Turk. Standardized procedure for expressing odor intensity. Chem. Senses Flavor 1:235-237, 1974. 62. Moskowitz, H. R., A. Dravnieks, and Lo A. Rlarman. Odor intensity and pleasantness for a diverse set of odorants. Percept. Psychophys. 19:122-128, 1976. 63. National Conference of States on Building Codes and Standards. Code for Energy Conservation in New Building Construction. McLean, Va.: National Conference of States on Building Codes and Standards, 1977. 64. National Conference of States on Building Codes and Standards. Survey of Energy Efficient Building Codes. McLean, Va.: National Conference of States on Building Codes and Standards, 1979. 65. National Research Council, Committee on Odors frown Stationary and Mobile Sources. Odors from Stationary and Mobile Sources. Washington, D.C.: National Academy of Sciences, 1979. 491 pp. 66. New York State Commission on Ventilation. Ventilation. New York: E. P. Dutton & Company, 1923 . 67. Stevens, S. S. Psychophysics: Introduction to Its Perceptual, Neural, and Social Prospects. New York: John Wiley ~ Sons, Inc., 1975. 329 pp. 68. Turk, A. Absorption, Chapter 8. In A. C. Stern, Ed. Air Pollution . 3rd ed. Vol. 4. Engineering Control of Air Pollution. New York: Academic Press, Inc., 1977. 69. Turk , A., and R. A. Bownes. Absorption can control odors. Chem. Eng. 58~5) :156-158, l9Sl. 70. Turk, A., R. C. Haring, and R. W. Okey. Odor control technology. Environ. Sci. Technol. 6: 602-607, 1972. 71. U.S. Department of Health, Education, and Welfare, Public Health Service. Smoking and Health. A Report of the Surgeon General. DREW Publication No. (PHS) 79-50066. Washington, D. C.: U. S. Government Printing Off ice, 1979. [1250 ~ pp. 72. van Gemert, L. J., and A. H. Nettenbreier , Eds. Compilation of Odour Threshold Values in Air and Water. Voorburg, Netherlands: National Institute for Water Supply, 1977. 73 . Viessman, W. Ventilation control of odors. Ann. N. Y. Acad . Sci . 116: 630-637, 1964. 74. Winslow, C.-E. A. Fresh air and ventilation. New York: E. P. Out ton & Company, 1926. 182pp. 75. hitheridge, W. N., and C. P. Yaglou. Ozone in ventilation--Its possibilities and limitations. ASHVE Trans. 45:509-522, 1939. (includes discussion) 76. Wynder, E. L., and D. Hoffmann. Tobacco and health. A societal challenge. New Engl. J. Med. 300 894-903 ~ 1979e
202 77. Yaglou, C. P. Ventilation requirements for cigarette Smoke. ASHAE Trans . 61: 25-32, 1955. 7 8 . Yaglou, C . P., E. C. Riley, and D. I . Coggins. Ventilation requirements. ASHVE Trans. 42:133-162, 1936. (includes discussion) 79. Yaglou, C. P., and W. N. Witheridge. Ventilation requirements (Part 2~. ASHVE Trans. 43:423-436, 1937. (includes discussion) TEMPERATURE AND HUMIDITY The atmosphere not only has an important role in respiratory gas exchange (supplying oxygen and accepting carbon dioxide ), but also serves as the heat-exchange medium surrounding the human body. Atmospheric pressure, including its constituents and especially water-vapor pressure, environmental temperature, and the rate of air movement all affect rates of heat loss and heat gain. A change in the rate of heat loss or heat gain ultimately has an effect on body heat content and body temperature. Human body temperatures are maintained within very narrow ranges either by involuntary physiologic responses controlled by the thermoregulatory system or by behavioral adjustments that modify the thermal environment toward thermal equilibrium. The physiologic responses~are proportional to deviations from preferred body temperature, especially those associated with the hypothalamic region of the brain. Behavioral adjustments are proportional to deviations in thermal sensation and thermal comfort from thermoneutral and acceptable, respectively. In general, people prefer to use the behavioral adjustments, rather than having to rely on the physiologic responses. The thermal state of the human body can be described by the heat balance equation: S = M - E W - R - C, in which S = M = _ W = R - - _ rate of storage of body heat, rate of metabolic heat production, rate of evaporative heat loss, rate of external work done, rate of radiant heat loss, and rate of convective heat loss. (1) All the above terms are usually expressed in watts per square meter of skin surface area. Body surface areas in adults range from 1.6 to 2.2 m2 and can be estimated by Equation 2 f ram height and weight: in wh ich AD m H AD = 0.202 m0·425HO.725 = body surface area, m2, = body weight, kg, and = body height, m. (2)
203 Except for short periods, Equation 1 should result in values of S v ery near zero. Any deviation f role zero results in lower ing or raising of body temperature (hypothermia or hyperthermia, r especti~rely), with adverse consequences for health and well-being . The metabolic heat generated in the body varies from a minimum of 45 W/m2 to a maximum that varies between 600 and 900 W/m2 for shor t per iods . . Heat losses in excess of 45 W/m2 in the resting state tend to produce hypothermia in some people. Depending on She individual, evaporative heat loss must be at least 100-300 W/m to prevent hyperthermia under conditions of sustained hard work. The range of deep-body temperatures that can be encountered is shown in d iagrammatic form in Figure IV-21. A range of ambient temperatures with the appropriate responses is shown in Figure IV-22, and a range o f metabolic rates associated with some typical activities is presented in Figure IV-23. As is evident f rom the heat-balance equation, many factors determine the heat balance, and they must always be evaluated s imultaneously. Energy-conservation strategies may involve lower or higher air temperatures, higher or lower vapor pressures, and higher or lower air velocities. Interruptions in energy supplies can result in sharply higher or lower air temperatures, which can have additional adverse health effects. HEAT EXCHANGE WITH THE INDOOR ATMOSPHERE . A complete assessment of heat exchange between man and his thermal environment can be found in a recent review by Gagge and Nishi.. The following is a limited overview. Whenever the value of S differs from zero In the heat-balance equation, body temperature will change. At an average body weight of 40 kg/m2 and a weighted average specific heat of 36.5 Wh.°C.m 2, it follows that, for example, with S at 36.5 W/m2 for 1 h, the average body temperature will rise by 1°C. The rate of metabolic heat production, M, cannot be reduced to below about 40 W/m, but can rise to as high as 800 W/m2 during maximal exercise . Heat exchange by convection (C ), by radiation (R), and by evaporation (E) Is affected by ambient temperature Ta' by air velocity, and by clothing insulation . For a nude person, convective heat exchange in still air (velocity, V, less than 1 m/s) is approx imated by: he (Ts - Ta in which C ho As Ta (3) convective heat exchange, W/m2, convective-heat-transfer coeff icient, W M-2 oc-1, mean weighted skin temperature, °C, and ambient air temperature, °C.
106 104 102 100 98 96 94 92 38 37 36 35 ~ l 34 33 204 of °C 108 42 4t 40 39 ~ Hem Exit Moder - e Normal ~ Ran. I at R - t Early Morning Cold War Hyp~rnis Vow H~ Exit GAP - are Hy - ~~ - RECTAL TEMPERATURE FIGURE IV-21 Range of recta] temperatures encountered in different conditions. Temperatures designated as "hyperthermia" and "hypo- thermia" are associated with increased risk to health and should be avoided. -
205 F C - 50 120 ~ 110 100 90 80 70 60 50 - 40- 30 - 20- - 40 3 - 20 lo - o - -10 BEHAVIORAL REGULATION THERMAL COMFORT BEHAVI ORAL R EGULATION PHYSIOLOGIC THERMOR£GULATION FIGURE IV-22 The range for thermal comfort a6suees minimal clothing at the high end and substantial clothing at the low end and thus includes some behavioral regulation. The zones designated "behavioral regulation" require modification by means other than clothing. "Phys- tologic thermoregulation" indicates the limits of ahort-term physio- logic regulation in a healthy person at rest with minimal clothing.
206 lm2 300 r Sawing Wood by Hand 280 260 240 220 200 180 t60 140 120 100 80 60 40 _ Sleeping M ETA80 Ll C RATES Table Tennis Slow Walking Typing r FIGURE IV-23 Metabolic heat production rates associated with various activities. Adapted from Berenson and Robertson.
207 lhe convective-heat-transfer coefficient depends on position and posture and on air velocity. Reasonable estimates of he are about 4 W.m~2.oC~1 in still air and 11.6 V0- 5 in air with a velocity of over 0.2 m/s.~ me human body also exchanges heat by radiation. The radiant environment is usually characterized by a mean radiant temperature (Tr) or by an effective radiant field (fir) in watts per square meter. Equation 4 shows the conversion between these terms: - Hr = hr(Tr ~ Ta)~ in which he = radiant-heat-transfer coefficient. (4) A reasonable approximation of hr is 4. 5 ~ ·m~2 ~ oC~1 at a skin temperature of 34 °C and an ambient temperature of 29 °C. It i s often convenient, especially when Tr and Ta are relatively close together , to combine radiation and convection into an overall neat-transfer coefficient, h, and to use the operative temperature, To, for the thermal environment. Operative temperature is the temperature of an environment with uniform air and wall temperature that exchanges heat with the body at the same rate as with the complex environment that it describes. The combined heat-transfer coeff icient thy in still air is about 8-10 W.m~2~°C~l. The use of clothing reduces the heat exchange with the environment. This insulation, IClo, is usually expressed in clo units; 1 clo unit corresponds to insulation at O .15 5 m2. oC.~~l. The reciprocal of Iclo Is hCl, the conductance of clothing . I f clothing ef f iciency i s FCl, the n [cl = hcl/(hc1 ~ h) - 1/~1 + 0.155hIClo), t5, and the total radiant and convective heat transfer with clothing becomes: R ~ C = Fclh(~s ~ To) (6) FC1 varies f rom 1 for a nude man down to O . 25 for heavy winter clothing, including an overcoat. The temperature at which man feels thermoneutral at rest var. ies with clothing . At FC1 = 1, thermoneutrality occurs at 28°C; at Fc3. = 0. 25, it occurs at 15°C. Figure IV-24 further illustrates the relationsn~p between clothing and temperature for thermoneutrality. At temperatures above thermoneutrality, or when increased metabolic heat production exceeds loss of "dry heat to the environment, sweat is secreted and the resulting evaporative heat loss (E) restores overall thermal equilibrium. For every gram of sweat that evaporates from the skin, 0.68 Wh of heat is lost from the skin. The maximal evaporative heat loss is limited by the maximal amount of sweat that can be secreted (about 1,500 g/h) and by the evaporative power of the environment. The maximal rate of evaporation, EmaX' f rom a completely wetted skin is given in Equation 7:
208 OPERATIVE TEMPERATURE (°C) 2.0 - o ~ 1.5 _\ 2 o Id \ ~ 1.0 I o 0-5 - . a 1 8 20 22 24 26 28 l l l _~^ l it: ~~ W_ W~ _ _ ~ _ _ ~ _ _ _ ~ _ _ - ~~e _ _ 64 68 72 76 80 84 OPERATIVE TEMPERATURE (°F) FIGURE IV-24 Ef feet of interaction of operative temperature and clothing insulation on thermoneutrality, and ambient temperatures accepted by 80X of people when sedentary at minimal air velocity.
209 EmaX ~ 2.2bC(p~k ~ Pdp)Fpcl' . - (7) in which ho EmaX ~ maximal evaporative rate, W/m2, 2.2 ~ I`ewis relation, °C/Torr, convective heat transfer coefficient, W M-2 .oc1 saturated water-vapor pressure at skin, water-vapor pressure in the atmosphere, Torr, and Nishi permeation factor. ~2 pa k dp FpC1 = For most types of porous clothing, Nishi and Gagged have shown exper imentally that ~1 ~ 1/ (1 ~ O . 143~IClo ~ . (8) I f the evaporative heat loss required for thermal equilibrium (Ereq) is less than EmaX, the skin will be less than 100% wet. If W is the percent of skin surface area wetted by sweat, then W ~ Er eg/Emax (9) Increasing values of W produce increasing discomfort. The value of EmaX in decreased by adding clothing, by increasing water-`rapor pressure, and by lowering air velocity. Lowering the humidity, ~- reducing clothing, and increasing air velocity reduce W and physiologic s train and increase thermal comfort. PHYSIOLOGIC RESPONSES TO THE THERMAL ENVIRONMENT In environments in which the body tends to lose or gain heat, body temperature changes. Such changes produce physiologic responses aimed at keeping body temperature constant. Men body temperature falls, s kin temperature tends to fall f irst, and then (more gradually) deep-body temperature . The f irst physiologic response is a reduction in blood flow to the extremities and to the skin in general. Peripheral vasoconstric.tion reduces the convective heat transfer between the skin and the trunk core and causes rapid lowering of the temperature of hands and feet while reducing the loss of heat from the core. Normal skin blood flow is at about 250 m1/min and can easily be reduced to 50 m1/min or less . I f further reduction occurs in body temperature, the thermoregulatory system causes an increase in metabolic heat production through involuntary shivering, which can add 100-150 W/m2 to the basal 40 W/~2. I f, however, the thermal environment causes body temperature to rise, the initial physiologic response is an increase in blood flow to the extremities and the skin. ~asodilatation increases the convective heat transfer between the skin and the trunk core. The normal skin blood flow of 250 ml/min can be increased to as much as 3,000 m1/min. Effective overall thermal conductance between skin and core is about 18 W ~ 2 "C-1, with ~ low of 6 W =-2~°C~1 in the cold and a high
210 of 100 W =-2~°C~1 in the heat. Increased body temperature also causes the secretion of sweat over most of the skin surface area at rates proportional to the ~ody-temperature increase. Above the sweating threshold, a further 1°C rise in mean body temperature produces sweat secretion at 200-600 ~ M-2 ·h~l, corresponding to evaporative heat loss at 150-400 W/m . For a more complete and quantitative review of human thermoregulation, the reader is referred to Stolwijk and Hardy. IS The involuntary physiologic responses just described are usually associated with feelings of thermal discomfort. Relationships among ambient temperature, thermal pleasantness, comfort, and temperature sensation are illustrated in Figure IV-25. Prediction of thermal sensation, thermal comfort, and thermal acceptability must take into account all the factors involved in heat production, heat loss, and heat transfer; and it must do it simultaneously. In a nearly steady state, this is fairly feasible, but it becomes more complex when body temperature or ambient temperature is changing. Berglund and Gonzalez 2 evaluated the effect of slowly changing ambient temperature and water-vapor pressure and found that environmental temperature changes of O . 6°C/h from a 25°C starting point were quite acceptable, as were changes in the water-vapor pressure, long as the instantaneous water-vapor pressure was kept below 16 Torr. Internal body temperature has a considerable effect on comfort sensation. When the body interior is hyperthermic, as after vigorous exercise, a low air temperature even at high velocity (cold draft) is felt as comfortable and pleasant, although the same cold draf t would be extremely uncomfortable to a person who is already slightly hypothermic f rom a previous exposure to cold . A hypothermic person would prefer very warm air, which in turn would be felt as very uncomfortable by someone in a hyperthermic state. There is general agreement that age, gender, and physical f itness do not affect preferred ambient temperature, if metabolic heat production and clothing insulation are constant. When people are exposed to thermal conditions outside the comfort range, the extent of their discomfort is affected by age. gender, and physical fitness, even if activity and clothing are controlled. The extent of discomfort is closely correlated with physiologic thermoregulatory responses. Those who have a vigorous involuntary response, such as sweat secretion on body warming, exper fence more intensive and earlier discomfort than those whose sweat secretion begins at higher body temperature. When a space has thermal nonuniformities (such as vertical temperature gradients) or radiant nonuniformities (Such AS rat lent temperature In one direction 10°C higher than in another direction}, such nonuniformities can be perceived as uncomfortable, even if the average temperature Is in the comfortable zone. In a cool or thermoneutral environment, increased air movement is felt as uncomfortable; but at temperatures above thermoneutrality, increased air movement is desirable and extends the comfort zone to higher ambient temperatures.
211 E oE c 3 E 0 ~ c' ~ a ~ ,,, ~ 0 0 , e ~ ~ 0 in 3 ° \ \ a~ \' \ ~ ID C \\ 5 =e° 5e-~~ 0 ~ ~ 0/^ . ~ C E _ · 1 · · · O O ~ _ o ~ _. ~ 0 E , E ~ i 5 ' c ~ '"° °, n 5 ~ ~ ~ C ~ ~ ~ _ O ~ 0 co v ~ 0
212 HEALTH CONSEQUENCES OF EXTREMES OF TEMPERATURE AND HUMIDITY Adverse health effects of extremes of temperature and humidity are in several categories. AS extremes of temperature and humidity, we designate operative temperatures below 18°C and above 30°C and humidities below 4 Torr and above 16 Torr water-vapor pressure, for people in conventional clothing and at rest. Any temperature or water- vapor pressure that requires physiologic responses makes demands on the reserves of the systems that regulate cardiovascular function or fluid balance. In healthy people, these responses reduce such reserves, thus perhaps interfering temporarily with work capacity or limiting athletic achievement. In diseased persons, such as those with cardiovascular disease, such reserves are very small or nonexistent; such persons can be seriously threatened by even small excursions of temperature or water-~apor pressure. Assessments of health consequences of extremes of temperature and humidity tend to be based on two kinds of observations: observations of acutely ill people whose status can be correlated with temperature measurements in their hospital rooms, and observations in epidemiologic studies of whole populations, with daily outdoor temperatures correlated with daily mortality or with the number of emergency-room visits. In the first case, the number of patients observed in relatively small , and each patient has a different disease state; it is difficult co draw very detailed conclusions from such observations. In the second case, very large populations can be observed, but i t is d if f icult to evaluate actual exposure and to assess the pre-existing disease of those who have died. Clinical reports of particular sensitivity of patients with congestive hear ~ failure to hot and humid environments have been presented by Burch and co-workers. 3 -. Ellis, ~ Schuman, ~. and Oech~li and Buechley ~ ' have reported on excess mortality in populations exposed to unusually hot weather. This excess mortality occurred particularly among the aged, the hypertensives, diabetics, an patients with arter iosclerotic and other cardiovascular disease and chronic respiratory disease. Exposure to low temperatures can produce accidental hypothermia, especially in the aged (Watts; ~. McNicol and Smithy. Low winter temperatures are associated with increased mortality,' although few studies have specifically addressed this association. REFERENCES l. Berenson, P. J., and W. G. Robertson. Temperature, pp. 65-148. In J. F. Parker, Jr. and V. R. West, Eds. Bioastronautics Data Book. 2nd ed. National Aeronautics and Space AdministratiOn Publication No . NASA SP-3006 . Washington , D.C.: U. S . Government Printing Office, 1973. 2 . Berglund, L. G., and R. R. Gonzalez . Application of acceptable temperature drifts to built environments as a mode of energy conservation. ASURAE Trans. 84 (1) :110-121. 1978.
213 3 . Burch, G . E. The influence of environmental temperature and relative humidity on the rate of water loss through the skin in congestive heart failure in a subtropical climate. Am. J. Med. Sci. 211: 181-188, 1946. 4. Burch, G. E., and A. Ansari. Artificial acclimatization to heat in control subjects and patients with chronic congestive heart failure a ~ bed rest. Am. J. Med. Sci . 256 :180-194, 1968. 5. Burch, G. E., and N. DePa~quale . Influence of air conditioning on hospitalized patients . J . Am. Med. Assoc. 170 :160-163, 1959. 6. Burch, G. E., and G. C. Miller. The effects of warm, humid environment on patients with congestive heart failure. South. Med. J . 62: 816-822, 1969 . 7. Ellis, F. P. Mortality from heat illness and heat-aggravated illness in the United States. Environ. Res . ~ :1-58, 1972. 8 . Gagge, A. P., and Y. Nishi. Beat exchange be~cween human skin sur face and thermal environment, pp . 69-92 . In D. B. R . Lee, ~ . L . Falk, S. D. Murphy, and S. R. Geiger, Eds. Handbook of Physiology. Section 9. Reactions to Environmental Agents e Bethesda, Hd.s American Physiological Society, 1977. 9. Hardy, J. D., J. A. J. Stolwi jk, and A. P. Gagge. Man, p. 342. In G. C. Wh~ttow, Ed. Comparative Physiology of Thermoregulation. Vol. II . Mammals. New York: Academic Press, Inc., 1971. 1 0 . McNicol, M. W., and R. Smith. Accidental hypothermia. Br . Med. J . 1: 19-21, 1964; 11. Nishi, Y., and A. P. Gagge. Direct evaluation of convective heat transfer coeff icient by naphthalene sublimation . J . Appl. Physiol . 29: 830-838, 1970. 1 2 . Hishi , Y ., and A. P . Gagge . Moisture permeation of clothing--A factor governing thermal equilibrium and comfort. ASHRAE Trans. ?6 (Pt. I) :137-14S, 1970. 1 3 . Oechall , F. W., and R. W. Buechley. Excess mortality associated with three L:os Angeles September hot spells. Environ. Res. 3: 277-284, 1970. 14. Schuman, S. H. Patterns of urban heat-wave deaths and implications for prevention: Data from New 'cork and St. Louis during July, 1966. Environ. Res. 5: 59-75, 1972. 15. Stolwijk, J. A. J. ~ and J. D. Hardy. Control of body temperature, pp. 4S-68 . In D. ~ . A. Lee, H. L. Falk, S. D. Murphy, and S. R. Ge iger, Eds . Handbook o f Phys iology. Section 9 . Reactions to Environmental Agents . Bethesda, Md.: American Physiological Society, 1977 . 16. Watts, A. J. Hypothermia in the aged: A study of the role of cold-sensitivity . E~,riron . Res . ~ :119-126, 1972 . CHARACTERI ZATION OF ADDITIONAL PHYSICAL INDOOR POL~t~rANTS . . . . . _ The indoor pollutants thus far descr ibed have been def ined primarily as airborne contaminants. Other forms of pollution do not depend on mass concentration, such as sound and electromagnetic radiation . Electromagnetic radiation occurs in the radiotrequency .
214 infrared, visible light, ultraviolet, and x-ray portions of the electromagnetic spectrum. The freguencies of the electromagnetic radiation discussed here range from 104 Ez {radiofrequency) to 1015 Hz {ultraviolet). Table IV-24 s''mn~rizes the frequency distribution for each of these portions of the spectrum and of audible sound, which is transmitted via the vibration of air molecules. SOUND AND NOISE Phvaical Character i8tiC8 The audibility of sound depends on intensity and frequency, with a maximal human response in the region near 3 x 103 Ez. A sound with predominant frequencies below 100 Hz or above 104 Hz may require ~ million times more energy to have the same audibility as a sound with a predominant frequency of 3 x 103 Ez. A method of weighting the pressure exerted by the sound waves at different frequencies has been developed to compensate for these variations. The decibel values (which constitute a logarithmic intensity scale) cited herein are measurements with level A weighting, the scale that most closely matches the response of the human ear. The difference. in the treatment of the intensity content of a sound are alight and do not change substantially from one source to another."' Sounds in the indoor environment are generated both outside and inside the occupied space. Table IV-25 gives examples of sound intensities in the outdoor environment. Table Iv-26 lists sound intent ities produced by typical household appliances in the indoor environment. Sound intensities are usually measured by a meter satisfying the requirements of American National Standards Institute Specification SI.4-1971 {for sound meters). PsYchoPhysiolosic Effects The possible effects of sound include permanent and temporary 108S of hearing, cardiovascular disease, sleep disruption, and paychologic effects. :. The physiologic and psyabologic responses to sound may be transitory; ~. however, there i'; insufficient information on the effects of sound by itself or in combination with other stressore. Sound at intensities that are found to be objectionable will affect productivity and decrease enjoyment of the environment. ~. '~e EPA has identified sound intensities that, if not exceeded, should protect against some of the adverse effects of sound. ~. These values are expressed in term of maximal 8-h <?5 dB) and 24-h (70 dB) averages required to protect against hearing loss. 'were are also yearly average long-range environmental- goals of SS dB outdoors and 45 dB indoors, which are recommended to avoid activity interference or annoyance. t2 Were is still debate in professional circles about the maximal intensities of short~duration environmental sound that can be
215 TABLE IV-24 Radiation Wavelengthe and Frequencies Type of Radia~cion Wave length Frequency, Hz Ultraviolet Ultraviolet C 0.19~0.28 vm 1.07 x 1014-1.58 x 10~5 Ultraviolet B 0.28-0.315 um 9.5 s 1014-1.07 s 124 Ultraviolet A 0.315-0.4 ym 7.5 s 101 -9.5 s 10 Visible light 0.4-0.7 llm 4 29 s 1ol4_7 5 x 1ol4 Infrared 4 14 Near infrared 0.7-1.4 um 2.14 x 101 -4.29 ~c 10 Infrared 1.4-3 pm 1.00 ~c 1011-2.14 ~c 10 4 Far infrared 3-1,000 um 3 x 101 -1.00 ~c 10} Radio frequency ~ 1 1 Microwave 1-1,000 ~ 3 x 1O7-3 x 10 Ves~y high frequency 1-10 m 3 x 10 -3 x 10 High frequency 10-100 m 3 ~c 10 -3 ~ 10 Medium frequency 100~1, 000 m 3 x 1O4-3 x lO5 Low frequency 1,000~10,000 m 3 x 10 -3 x 10 Very low frequency 10,000-30,000 m 1 x 10 -3 x 10 Sount, audible 0.016-20.0 o~ 15-2 x 104
216 TABLE IV-2 5 Examples of Outdoor Day-Night Average Sound Intensities at Various Locations a Location Apartment next to freeway Downtown with some construction Urban high-dens ity apartment Urban row housing on major avenue Old urban residential area Wooded res idential area Agricultural cropland Rural residenelal area Wil de rnes s ambient aData from Council on Enviror~ental Quali~cy. 4 TABLE IV-2 6 Average Sound Intensity, dB(A) 88 79 78 68 59 51 44 39 35 Examples 0 f Sound Intensities Generated Indoors by Household Appliancesa Appl lance Blende r Garbage disposer Window air conditioner Re f rigerator Vacuum cleaner Hair dryer Mixe r aData fray Jones e 8 Average Sound Intensity, dB (A) 80-90 80 60 45 70-75 78 82
217 considered safe. However, above 110 do, sound is so intense that most people experience pain or a tickle in their ears. ~. Although it is difficult to determine the exact day and night indoor sound intensities, studies have indicated that an intensity of 6 0 . 4 dB with a s tandard deviation of 5 . 9 dB can be expected in a typical urban residential area, with instantaneous intensities exceeding 80 dB. 12 An exacted intensity of 60.4 do is below the 70 dB recommended by the EPA to prevent hearing loss, but it is well above the intensity recommended to aneroid interference and annoyance. Therefore, day and night sound intensities in the 100-site EPA survey may contribute to speech interference, reduced worker productivity, and annoyance.iotpp. 66-69) A high intensity of background noise in urban areas stemming primarily from transportation appears to affect the developing fetus. Women exposed to aircraft noise have a higher proportion of low-birthweight children, who are at higher risk of mortality and both physical and mental effects.~°(PP 110-111) This association cannot be separated from the social status of the women (a codetermining variable), inasmuch as many members of the lower social classes live in Noisy areas. Exposure to high intensities of sound affects communication and learning, including the acquisition of language.~°(P 115) Adaptation or resignation to annoyance may occur, and there do not appear to be groups of people that are particularly sensitive. After-effects of noise have been noted at home and at work, and noise appears to influence aggressiveness and minimize voluntary helping behavior.~otPp. 120-12 RADIOFREQUENCY AND MICROWAVE RADIATION (104 to 3 x 1011 HZ . Phys ical Character istics Although the physical characteristics of all electromagnetic radiation are similar, the frequency is inversely proportional to wavelength, and the effects of the longer wavelengths, such as radiofrequency radiation, are radically different from those of the shorter-wavelength ioniz ing radiation, such as x rays and gamma rays . The photon energy in radio waves is so small that there is no ionization when it is absorbed in an organism. Is Table IV-27 summarizes the radiation properties of some common nonionizing-radiation systems and their expected far-field power densities. Energy radiated by these systems can be additive, provided that the frequencies are within the same octave band. For the purposes of this report, the densities of all radio- frequency energies generated outdoors are defined as Background power densities, ~ and those of radiofrequencies generated indoors as Generated power densities.. Some radiofrequency energy is generated in the indoor environment. In general, all electric equipment produces come radiofrequency radiation. However, all but a few electric devices radiate energy at
218 3 O 0 U. so 1 C Cal :^ 0 ~ 0 - CI ~e ~ ~ ~ ~ 8 V _ ~ ~ ~ V 3 __ A_ 3 ~ 3 - ~' ~ o o o Us o · · · · ~ O O O O O lo O o O O O Us . O Us Cal v 8 ~ O O ^O O Cal - ~ O . lo ~ _ lo lo O O ^O _ ~ em 8 ~ - 0 3 V ~ ~ ~ ~o v - _ 3 ~ Gm 0 _ 0 ~ V~ ~ _ a~ - 0 v 0 - o CL 0 V o ·- ~0 3 ~ ~ 3 ~ O ~ ~ O O 3 - o 3 o ~ ~ o Cd V ~ 3 ~' eS ,,~ e e e c 0 ~ x a_ c~ E" 3 - 3 ~3 ~ ~ 3 3 0 S eJ 000 ~ O S 0 000 ^0 O ~ U~~ 0 0 0 O V CL N N a,1 00 O N !~ 5 ~ ~ O N ~ O N N ~ O '~ O ~ 0 2 ~ C' ~ 0 v 0 0 1 ~ C~ 00~^ 0 . ~ ~ U~ - a: o0 0 O ~ ~ ~ 0 :: ~ 0 ~ 0 O ' V V V ~ l,, . ~ _' a. ~ ~ V0 ~ ~ ~ ~ ~ O ~ ~ ~ ~ ~ ~0 O cn C~ V V O o0 _1 ~ _1 c: O O ~ ~ ~< ~ ~ :. 0 cn a~ - ~" ~ ~ ~ ~ ~ S. b ~ ~ ~ e 0 k ~ ~ e. =} .¢ ~ ~
219 well below the American National Standards Institute recommended exposure limits, even in combination with one another. One ma jor exception is the microwave oven. Under normal operating conditions, a residential microwave oven radiates approximately 1 mW/cm2 at the Real on the door. However, If the door is defective, values in excess of 1 W/cm2 can be achieved. PsYchophYs ioloq ic Ef f eats Effects of radiofrequency radiation can be divided into two major categories: t~ thermal effects (when the radio-wave energy is converted into heat) and nonthermal effects (which cannot be directly explained by thermal equivalents). Biologic effects depend on the f requency and the intensity of the radiation; the duration of exposure; the dielectric constant, temperature, and thermal conductivity of the irradiated tissue; the ability of the tissue to dissipate heat; and the dimensions of the body. Absorption of microwave radiation by body tissues results in an increase in temperature, often producing internal burns due to local hot spots caused by nonuniformity in the f ield. The eyes and testes were found to be the most sensitive. I' Specific effects at the cellular or molecular level were postulated more than a decade ago without resolution of the importance of these effects with respect to biologic damage. 3 The possibilities of nonthermal effects, such as rearrangements within macromolecules and subcellular structures, have been under investigation for many years, but further studies will be necessary to clarify the issues. It Is relatively clear that metabolic and functional disturbances at the cellular level can be caused by microwave radiation, but the mechanisms of these ef fects are not yet well understood. Table IV-28 characterizes the relative rates of absorption by the human body; however, it is difficult to determine the exact effect of each frequency. Because the radiofrequency energy generated indoors is low, the ma jor emphasis should be on outdoor sources. Indoor radiofrequency f ields are generally lower than outdoor. Osepchuck has discussed sources of microwave and other forms of radiofrequency energy . FAR-~NF~D AND INF=~D =DIATION {3 x 1011 HZ to 4 .3 x 1014 HZ Physical Character istics . The infrared energy spectrum ranges from far-infrared (3 x 10 Hz to 1014 Hz), through infrared (1.0-2.14 x loll Hz), to near- infrared (2.14-4.29 x 1014 Hz). Infrared radiation is produced naturally by the sun and by all common heating and artificial-light sources. The incandescent lamp is one of the major sources of infrared radiation and the most common artificzal-light source in the indoor environment. Of the total input wattage of an incandescent lamp,
220 TABLE IV-28 Relative Absorption of Radiofrequencies by Hen Bodya Frequency, 106 Hz <400 400-1, 000 1,000~3,000 3, 000~10, 000 Haximn1 Absorption by Human Body, <50 50~100 20-100 >50 aData from U. S. Department of Health, Education, and Welf are . 13
221 75-808 is converted to near-infrared and infrared radiation.' The ACGIE has adopted ~ TLV of 10 mW/ce2 for infrared radiation in the workplace. The power density 2 ~ from ~ 100-W lamp is approximately 0.6 mW/cm2 for the total infrared spectrum. Sunlight on the earth's surface produces a flux of about 70 mW/cm2, of which about half is infrared. Psychophvaiologic Effects Depending on its wavelength, infrared is absorbed in the surface of the skin (wavelengths larger than 2 ye) or can penetrate asveral millimeters (waveleng the between O.7 and 1.5 And. Safety standards in industrial environments are based on the risk that infrared radiation may induce cataracts in the eyes of persons exposed to excessive infrared radiation, such as glassblowers or open-hearth steelworkers.~. Excessive infrared radiation is most easily controlled by shielding the source with reflecting metallic foils. VISIBLE RADIATION Physical Character tStiC8 Radiation in the near-infrared and visible spectrum is produced by many sources, both natural and artificial. Our sense of sight, feeling of well-being, and comfort are all. to a great extent, influenced by Risible and near-infrared radiation. Psychophysiologic Effects Retinal burns from observation of the sun have been described throughout history. Chorioretin-1 burns rarely occur from exposure to artificial light, because the normal aversion to high-brightnese light sources (the blink reflex) provide. adequate protection , unless the exposure is hazardous within the duration of the blink reflex. Many factors at feet the usefulness of visible light . Among the most important are discomfort glare and disability glare. Light sources can cause ~ reduction in contrast of an image, owing to scattered visible radiation, by adding a uniform veil of luminance to the object. This effect, commonly called .veiling luminance,. may cause a reduction in visual performance without physical damage. Discomfort glare is a sensation of annoyance or pain caused by brightness in the field of view that is greater than that to which the eyes are adapted. It has been shown that the threshold of discomfort glare changes as ~ function of age.2 Although discomfort glare does not necessarily interfere with visual performance, it can cause eye strain and contribute to fatigue. Disability glare and ocular stray light influence one's ability to perform a task by artificially veiling
222 the contrast of the visual target. It is therefore a great contributor to eye fatigue. ULTRAVIOLET RADIATION (0 . 75-1. S8 x 1015 Ez; - wavelength, 0. 19-0.400 =) Physical Characterstics Ultraviolet radiation is divided into three wavelength categories: ultraviolet-A (W-A), 0 . 315-0 .400 - ; ultraviolet-B (W-B), 0.28-0.315 - ; and ultraviolets (W~C}, 0.19-0.28 - . All fluorescent lamps emit W-A, but not W-B or ARC. High-inten~ity d ischarge lamps produce W-A, W-B, and some ARC. Incandescent lamps produce small amounts of W-A, and essentially no W-B and ARC. Ultraviolet radiation is measured with specialized radiometric photome ters . Psychophysiologic Ef feats UV-B and W~C are known photocarcinogens. s Doses of W-B and W~C 10 times the human minimal erythema dose (MET)) have initiated squamous cell carcinomas, and chronic continuous exposure to W-A can also have a carcinogenic effect. s The ACGIH recommends limits on workplace ultraviolet exposure that depend on wavelength and on the duration of exposure.' For W-A, the intensity should not exceed 1 mW/cm for more than 1,000 a, nor s hould the dose exceed 1 J/cm2 i f g iven in less than 1, 000 s . For W-B and Wee, the dose should not exceed about 3-10 =/cra2 in any 8-h period. The degree of hazard seems to be associated with the erythemal efficiency of each frequency. ~ s SUMMARY Ionizing and nonionizing electromagnetic radiation occurs in the indoor environment. This radiation can be harmful, and one cannot always sense its presence. Sound can generally be heard and in some cases felt. Excessive sound can cause deterioration of hearing acuity and, if extremely intense or prolonged, cause deafness. Background sound in the urban residential environment can exceed the recommended intensities and result in interference and annoyance. Sound of 70-80 do, commonly found in indoor environments, can inhibit task performance and possibly contribute to aggressive human behavior. ~ Infrared, far-infrared, and radiofrequency radiation produce no visible or audible evidence of their presence. However, infrared radiation does provide sensory indication of its presence by heating of human tissue. Far-infrared and radiofrequency radiation, however, provide no indication of their presence, unless their power levels are
223 so high as to increase skin temperature. Heating of human tissue occurs because of the infrared output of incandescent lamps. However, the detrimental effects of this heating have not been fully investigated. Surveys have shown that in several cities 98% of the people are exposed to less than 1 ~W/cm2 from broadcasting transmitters.' However, ultrahigh-frequency television transmitters can radiate radiofrequency pollution to adjacent buildings at 5-200 W/cm2 . Ultraviolet-A, visible light, and near-infrared radiation can produce surface heating of human and animal tissue. These frequencies are of concern because of their ability to affect human performance. The veiling reflections caused by most artificial lighting systems can have substantial influence on human visual performance. Reduction of veiling reflections can increase visual performance and decrease the energy consumed by lighting systems. Transient adaptation (dilation during or immediately after eye movements) i. caused by sudden changes in the visual spectrum power. Transient adaptation contributes to eye fatigue and decreased visual performance. REFERENCES 7. 1. American Conference of Governmental Industrial Hygienists. ILUs. Threshold Limit Valuen for Chemical Substances in Workroom Air Adopted by ACGIH for 1980 . Cincinnati: Amer ican Conference of Governmental Industrial Hygienists, 1980. 93 pp. 2. Bennet, H. J. Discomfort Glare: Demographic variables, p. 6. IER] Special Report No. 118, 1976. 3. Cleary, E. Biological Effects and Health Implications of Microwave Radiation. Symposium Proceedings. Richmond, Virginia, September 17-19, 1969. U. S. Department of Health, Education, and Welfare, Bureau of Radiological Health Publication No. BRH/DBE 70-2. Washington, D.C.: U.S. Government Printing Office, 1971. 265 pp. 4. Council on Environmental Quality. Noise, pp. 533-576 . In Environmental Quality--1979. The Tenth Annual Report of the Council on Environmental Quality. Washington, D.C.: U.S. Government Printing Office, 1980. 5. Cunningham-Dunlop, S., and B. H. Kleinstein. Wavelength dependence, pp. 51-61. In Carcinogenic Properties of Ionizing and Nonionizing Radiation. Vol. I. Optical Radiation. DREW (NIOSH) Publication No. 78-122. Washington, D.C.: U.S. Government Printing Office, 1977. Geen, R. G., and E. C. O'Neal. Activation of the cue-elicited aggression by general arousal. J. Pers. Soc. Psychol. 11:289-292, 1969. Janes, D. E., Jr. Radiation surveys--Measurement of leakage emissions and potential exposure fields. Bull. N.Y. Acad. Med. 55:1021-1041, 1979. 8. Jones, H. W. Noise in the Human Environment. Edmonton, Alberta: Environmental Council of Alberta, 1979. 9. Kaufman, J. E., and J. F. Christensen, Eds. IES Lighting Handbook. The Standard Lighting Guide. 5th ed. New York: Illuminating Engineering Society, 1972.
224 1U. National Research Council, committee on Apprales1 ot Societe1 Consequences of Transportation Noise Abatement. Noise Abatement: Policy Alternatives for Transportation. Wanton, D.C.: National Academy of Sciences, 1977. 206 pp. osepobuck, J. M. Sources and bB8tC characteristics of microwave/RF radiation. Bull. N.Y. Acad. Ned. 55:976-998, 1979. Schultz, T. J. Noise Assessment Guidelines. (Technical Background for Norse Abatement In BUD's Operating Programs.) U.S. Department of Housing and Urban Development Report No. TE/NA 172. Washington, D.C.: U.S. Government Printing Office, 1971. 210 pp. 13. Salty, S. W., and D. G. Brown. Radio Frequency and Radio HIcrowave Radiation Levels Resulting from Mhn-Made Sources in the Washington, D.C. Area, pp. 1-13. U.S. Department of Bealtn, Education, and Welfare Pub. No. (FDA)72-8015. Washington, D.C.: U.S. Government Printing O$tice, 197Z. 14. U.S. Environmental Protection Agency, Ot$lce of Nolse Abatement and Control. Tnformatzon on Levels of Environmental Noise Red taite to Protect Public Health and Weltare With an Adequate Margin of Safety. O.S. Environmental Protection Agency Report No. 550/9-74-004. Washington, D.C.: U.S. Government Printing Office, 1974. tZ141 pp. 15. Vogelman, J. H. Physical characteristics of microwave and other rad~ofrequency radiation, pp. 7-10. In S. F. Cleary, Ed. Biological Effects and Health Implication. of Microwave Radiation. Symposium Proceedings. Richmond, virgins, September 17-19, 1969. U.S. Department of Health, Education, and Weltare, Bureau of Radiological Bealth Publication No. BRE/DBE 70-2. Washington, D.C.: U.S. Government Printing Office, 1971. 16. Wallace, J., P. M. Sweetnam, C. G. Warner, P. A. Graham, And A. L. cocbraner An ep~demtological study of lens opacities among steel workers. Br. J. Ind. Mea. 28:265-Z71, 1971. 17. World Health Organization. Health Bazards In the Human Environment. Geneva: World Health Organization, 1972. 387 pp.