




























Below is the uncorrected machine-read text of this chapter, intended to provide our own search engines and external engines with highly rich, chapter-representative searchable text of each book. Because it is UNCORRECTED material, please consider the following text as a useful but insufficient proxy for the authoritative book pages.
52 5.1 Introduction This chapter describes the laboratory testing and model- ing methods used to reduce the dissolved metals concentra- tion in stormwater. The first objective of this work was to develop a standard protocol for evaluating effectiveness of various processes for treating highway runoff. As reported in Chapter 4, adsorption is the most promising technology for removal of metal ions from highway runoff, and the most appropriate adsorption media identified were oxide minerals, calcite-based minerals, and/or chitin based media (a waste material). Since the most promising application of these media may be a combination of media that includes oxide minerals for maximum adsorptive capacity combined with a calcite containing media such as limestone based PCC for pH control, various combinations of these media were tested. A significant amount of research progress has been made with respect to collecting equilibrium adsorption data for metal ions on oxide minerals in well-defined solution matri- ces. These data provided a substantial head start for this project as they define a baseline of adsorption properties in well-defined systems. However, the major challenge that remained was to determine how well the results obtained from these well-defined systems are able to predict adsorp- tion in the multi-component systems defined by highway runoff. The difficulty associated with predicting adsorption in multi-component systems is to capture the impacts of background organic matter and other complexing ions on adsorption behavior. Very few studies have evalu- ated the ability of SCMs to predict adsorption in systems that contain NOM from highway runoff. Consequently, the series of experiments described included some with and without organic matter and at various pH and ionic strengths. 5.2 Development of the Laboratory Testing Protocol 5.2.1 Important Factors Affecting Laboratory Evaluation of Metal Adsorption The protocol developed within this project is based on sev- eral observations that stem from the literature and experi- mental data collected within the scope of this project. These assumptions are described below. ⢠Metal cation adsorption is highly pH dependent as shown for Cu and Zn sorption onto iron oxyhydroxide in batch experiments (Figure 5-1) and for Cu adsorption in a col- umn packed with iron oxide media (Figure 5-2). Thus, control of pH in protocols for evaluating media is essential. Moreover, optimal removal may occur in systems in which multi-media systems are utilized especially if one type of media is capable of raising the pH to promote adsorption or precipitation. In such systems, batch experiments are not easily correlated to data in flow-through systems. As such, protocols that employ column experiments for eval- uating media options are preferred. ⢠Competition for sorption at the low concentrations encountered in highway runoff is often minimal as shown in Figure 5-3 and Figure 5-4 for data collected for sorp- tion of Cu(II) and Pb(II) on iron oxyhydroxide in bi-solute batch experiments. Surface complexation modeling using the DLM also verifies the minimal impact of competi- tion at low solute concentrations. These results suggest that cation-cation competition in stormwater, which has relatively low trace metal ion concentrations may be negli- gible; however, one should be aware that the more complex chemistry of actual runoff may affect this conclusion. ⢠Ionic strength effects on sorption of strongly sorbing metal ions is minimal and observed ionic strength effects can often be attributed to processes other than sorption. This C H A P T E R 5 Laboratory Testing and Modeling Methods
53 Figure 5-1. Adsorption data for single solute Cu(II) (top) and Zn(II) (bottom) onto hydrous ferric oxide using two site, mononuclear bidentate diffuse layer (DLM) surface complexation model. Dashed lines represent strong (dashed) and weak (dotted) site fractions of the total adsorption. Bulk precipitation is also included and represented by the dash-dotted lines. pH 2 3 4 5 6 7 8 Pe rc en t C u(I I) R em ov al 0 20 40 60 80 100 pH 2 3 4 5 6 7 8 pH 2 3 4 5 6 7 8 Cu(II)T= 3.0x10-5 M FeT= 6.0x10 -2 M Cu(II)T= 9.9x10-5 M FeT= 5.6x10 -3 M Cu(II)T= 1.3x10-2 M FeT= 5.6x10 -2 M pH 3 4 5 6 7 8 9 0 20 40 60 80 100 (m ol Zn /m ol Fe ) 0. 00 0. 05 0. 10 0. 15 pH 3 4 5 6 7 8 9 0 20 40 60 80 100 0. 00 0 0. 00 5 0. 01 0 0. 01 5 pH 3 4 5 6 7 8 9 Pe rc en t Z n(I I) R em ov al 0 20 40 60 80 100 0. 00 00 0. 00 05 0. 00 10 0. 00 15 ZnT= 1.1x10 -4 M FeT= 5.6x10 -2 M ZnT= 1.1x10 -3 M FeT= 5.6x10 -2 M ZnT= 1.1x10 -2 M FeT= 5.6x10 -2 M Figure 5-2. Fixed bed reactor data for Cu sorption onto granular iron hydroxide at pH îµ 6 in a synthetic stormwater solution. The column diameter was 1 cm ID, flowrate îµ 8 ml/min, and influent copper concentration of 50 mg/L. The influent ionic strength was 0.01M and the alkalinity was 2 meq/L. Two columns were connected in series with A1 being the first bed in the series. No attempt was made to control the influent pH during this experiment. 5.8 6 6.2 6.4 6.6 6.8 7 0 0.2 0.4 0.6 0.8 1 1.2 1.4 1.6 0 50 100 150 C/ C0 Elapsed Time (hrs) Granular Ferric Oxide (GFO) Media, Inï¬uent Cu = 50ug/L A1 (GFO) A2 (GFO) Inï¬uent pH
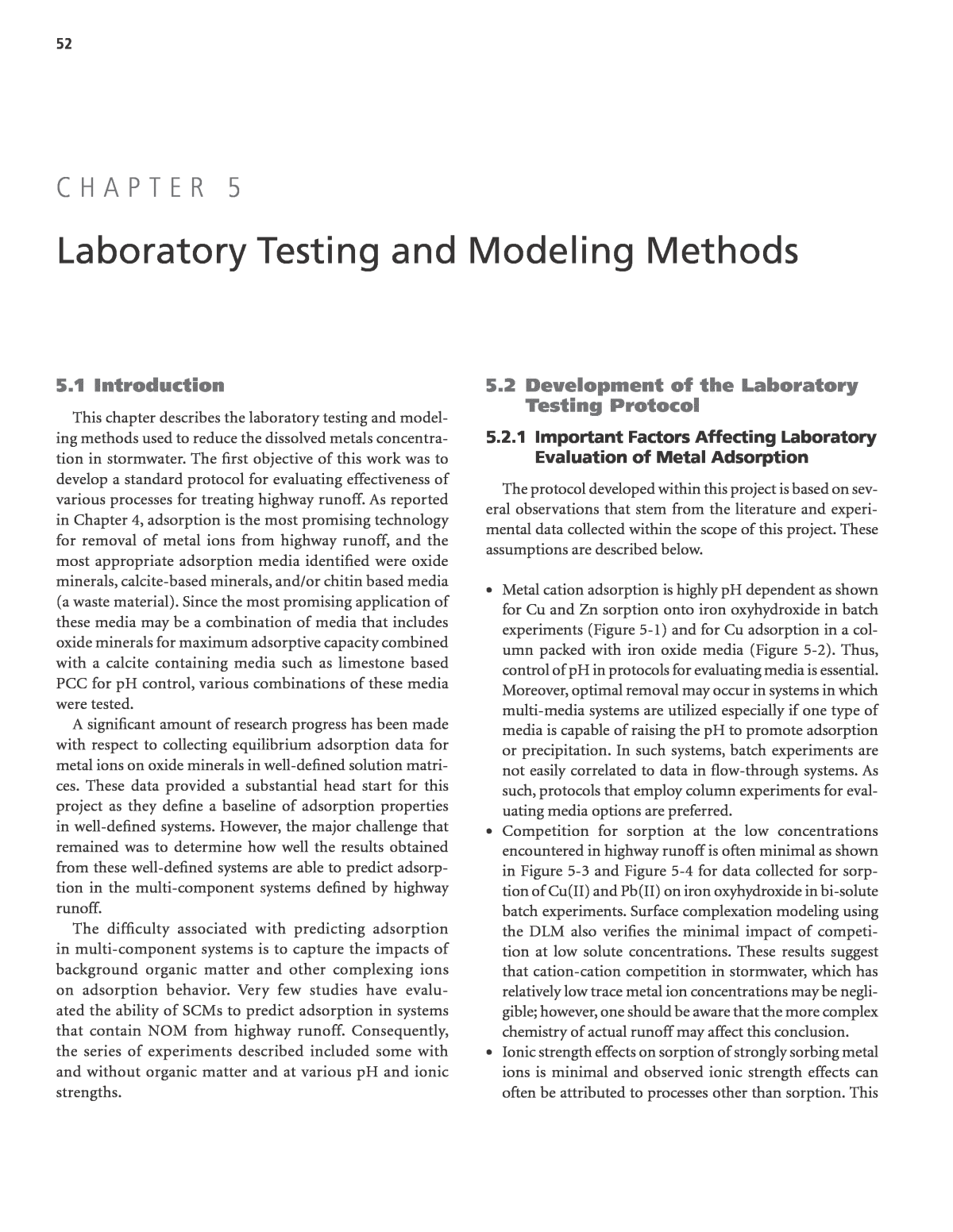
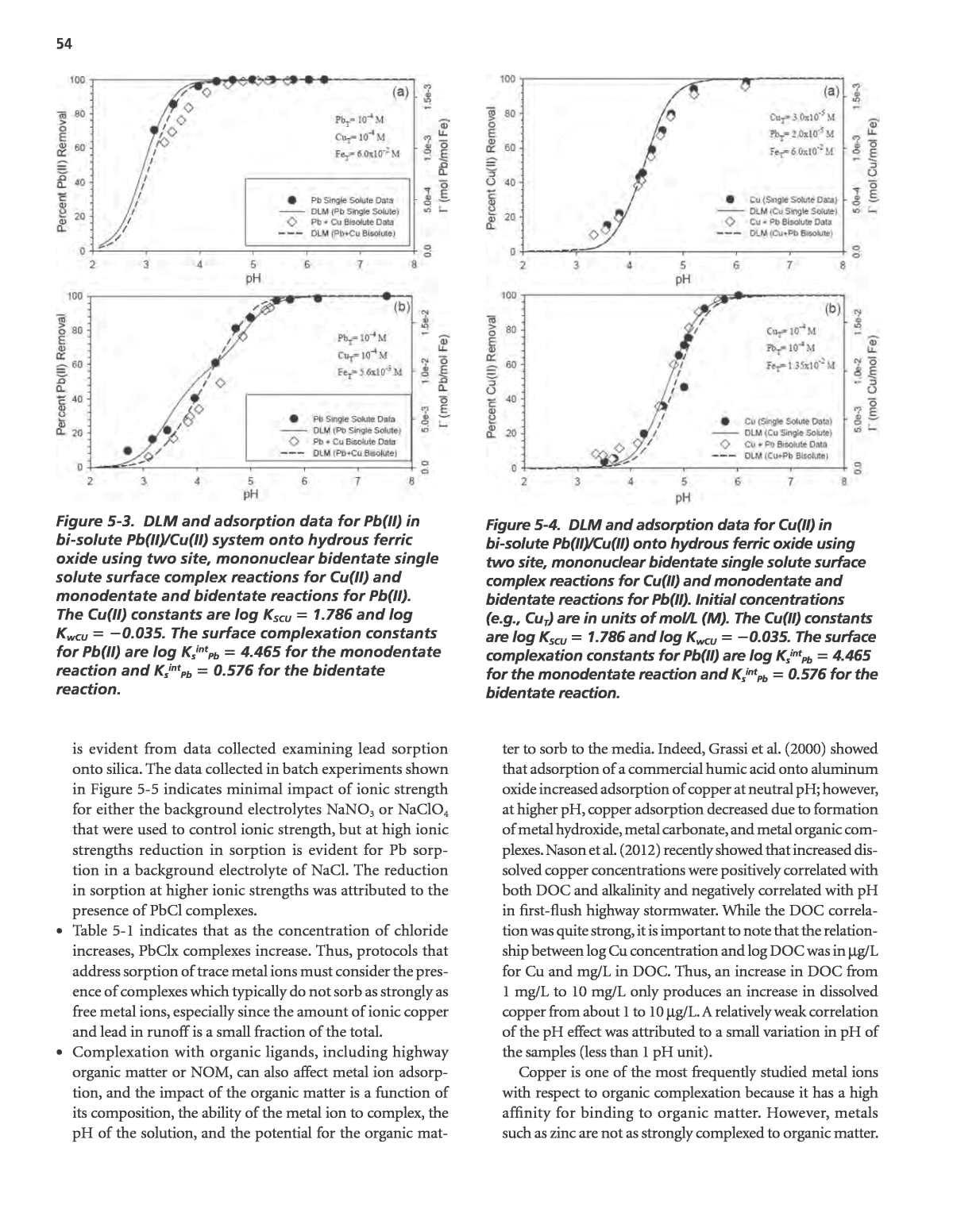
54 ter to sorb to the media. Indeed, Grassi et al. (2000) showed that adsorption of a commercial humic acid onto aluminum oxide increased adsorption of copper at neutral pH; however, at higher pH, copper adsorption decreased due to formation of metal hydroxide, metal carbonate, and metal organic com- plexes. Nason et al. (2012) recently showed that increased dis- solved copper concentrations were positively correlated with both DOC and alkalinity and negatively correlated with pH in first-flush highway stormwater. While the DOC correla- tion was quite strong, it is important to note that the relation- ship between log Cu concentration and log DOC was in mg/L for Cu and mg/L in DOC. Thus, an increase in DOC from 1 mg/L to 10 mg/L only produces an increase in dissolved copper from about 1 to 10 mg/L. A relatively weak correlation of the pH effect was attributed to a small variation in pH of the samples (less than 1 pH unit). Copper is one of the most frequently studied metal ions with respect to organic complexation because it has a high affinity for binding to organic matter. However, metals such as zinc are not as strongly complexed to organic matter. is evident from data collected examining lead sorption onto silica. The data collected in batch experiments shown in Figure 5-5 indicates minimal impact of ionic strength for either the background electrolytes NaNO3 or NaClO4 that were used to control ionic strength, but at high ionic strengths reduction in sorption is evident for Pb sorp- tion in a background electrolyte of NaCl. The reduction in sorption at higher ionic strengths was attributed to the presence of PbCl complexes. ⢠Table 5-1 indicates that as the concentration of chloride increases, PbClx complexes increase. Thus, protocols that address sorption of trace metal ions must consider the pres- ence of complexes which typically do not sorb as strongly as free metal ions, especially since the amount of ionic copper and lead in runoff is a small fraction of the total. ⢠Complexation with organic ligands, including highway organic matter or NOM, can also affect metal ion adsorp- tion, and the impact of the organic matter is a function of its composition, the ability of the metal ion to complex, the pH of the solution, and the potential for the organic mat- Figure 5-4. DLM and adsorption data for Cu(II) in bi-solute Pb(II)/Cu(II) onto hydrous ferric oxide using two site, mononuclear bidentate single solute surface complex reactions for Cu(II) and monodentate and bidentate reactions for Pb(II). Initial concentrations (e.g., CuT) are in units of mol/L (M). The Cu(II) constants are log KSCU îµ 1.786 and log KwCU îµ î²0.035. The surface complexation constants for Pb(II) are log KsintPb îµ 4.465 for the monodentate reaction and KsintPb îµ 0.576 for the bidentate reaction. Figure 5-3. DLM and adsorption data for Pb(II) in bi-solute Pb(II)/Cu(II) system onto hydrous ferric oxide using two site, mononuclear bidentate single solute surface complex reactions for Cu(II) and monodentate and bidentate reactions for Pb(II). The Cu(II) constants are log KSCU îµ 1.786 and log KwCU îµ î²0.035. The surface complexation constants for Pb(II) are log KsintPb îµ 4.465 for the monodentate reaction and KsintPb îµ 0.576 for the bidentate reaction.
55 Binding constants for metal-ligand complexes are frequently an order of magnitude smaller for both inorganic species and one to three orders of magnitude smaller for organic species. Thus, the presence of organic matter may have little effect on sorption of zinc, unless the organic matter sorbs to the media and changes the affinity of zinc for the surface. The variable impact of organic matter on sorption further suggests that protocols for evaluating media for metal ion removal of highway runoff should employ organic matter and background solution chemistry that closely mimics the composition of highway runoff. Moreover, given the widely different concentrations and affinities of the organic matter and the potential for the organic matter to coat media and block access to sorption sites over time warrants studies of sorption that are conducted in dynamic flow-through sys- tems rather than batch experimental systems. In batch reac- tors, all constituents are added at the start of the experiment and changes in water and surface chemistry are minimized. ⢠Finally, precipitation of metal ions in highway runoff is also highly pH dependent and varies with metal ions [Ksp for Cu(OH)2(s) = 10-20.4 vs. Ksp for Zn(OH)2(s) = 10-16.8]. The types of precipitates that are expected in these systems include metal hydroxide solids, metal carbonate solids (although alkalinity values in many of these systems are low), and mixed metal precipitates such as metal-hydroxy- carbonates. The potential for dissolution of metal ions from the sorbent media also provides potential for formation of mixed metal precipitates. There is ample evidence in the literature, for example, that iron, aluminum, silica, and car- bonates can form mixed precipitates with metal cations. Moreover, the formation of these mixed precipitates occurs well below the solubility limit for the pure phase precipitates [i.e., (Cu)x(Al)x(OH)x is less soluble that Cu(OH)2)] as has been shown for cobalt mixed precipitates (Thompson et al., 1999). This mechanism has been suggested by Karthikeyan et al. (1999) for both iron and aluminum oxides. As dis- solution of the media is affected by pH, ionic strength, and solution composition, it is necessary to evaluate the poten- tial for both bulk precipitation and surface precipitation. However, in batch solutions, it is not possible to distinguish between sorption and precipitation. In contrast, precipita- tion can lead to breakthrough profiles that plateau at less than complete breakthrough (i.e., C/Co values less than 1) in column systems in which dissolution occurs dynamically. The protocol developed for evaluating adsorptive media for metal ion sorption captures the important outcomes from these observations by primarily relying on performance of the media in column experiments and utilizing background solutions that are tailored to the expected composition of highway runoff. The test procedure was modified through the Figure 5-5. Pb(II) adsorption onto quartz as a function of background electrolyte and ionic strength at an initial Pb(II) concentration of 10-5M added to each of a series of batch reactors containing 100 g/L quartz. pH 4 5 6 7 8 Pe rc en t P b( II ) R em ov al 0 20 40 60 80 100 Pb S ur fa ce C ov er ag e ( m ol / m 2 ) 0.000 0.005 0.010 0.015 0.020 0.025 0.01M NaCl 0.01M NaClO4 0.01M NaNO3 0.1M NaCl 0.1M NaClO4 0.1M NaNO3 pH 4 5 6 7 8 Pe rc en t P b( II ) R em ov al 0 20 40 60 80 100 Pb S ur fa ce C ov er ag e ( m ol /m 2 ) 0.000 0.005 0.010 0.015 0.020 0.025 0.01M NaCl 0.1M NaCl 0.5M NaCl 1.0M NaCl NaCl Concentration Pb2+ Pb(OH)+ PbCl+ PbCl2 PbCl3- PbCl42- total Pb-Cl 0.01 68.9 5.5 24.5 1.1 0.0 0.0 25.6 0.10 16.1 0.0 57.2 25.6 1.0 0.0 83.8 0.50 1.5 0.0 26.1 58.2 11.6 2.6 98.5 1.00 0.3 0.0 12.4 55.2 22.0 10.1 99.7 Table 5-1. Percentage of total Pb(II) in various complexes as a function of NaCl concentration.
56 course of experimentation to ensure that the protocol could handle different media and solution compositions. Pre- filtering and pH control were key modifications to the design to ensure that the tests could be conducted over suitable time frames and still capture the contaminant breakthrough pro- files. Nonetheless, even with the in series short bed approach used in the column set up, it was not possible to capture the complete breakthrough profiles for all media/solution char- acteristics. Thus, a procedure for estimating capacity with- out complete breakthrough was developed to accommodate less than 100% contaminant breakthrough over the course of the experiments. The final protocol provides an efficient method for comparison of media under varying stormwater compositions which can be conducted within several days of experimentation. The protocol includes isolation and concentration of the organic matter from actual highway runoff; developing a synthetic inorganic water that mimics the inorganic compo- sition, ionic strength, and pH of highway runoff; and con- ducting experiments with various potential adsorbents using the background water in the presence and absence of organic matter and at varying pH and ionic strengths. The protocol was tested on two commercially available iron oxides and a manganese oxide. In addition, experimental columns were operated in series in which either crushed waste concrete or crab shell waste was evaluated for its ability to increase the pH prior to contact with the oxide media. Finally, a simplis- tic modeling approach, which can be applied to site-specific conditions, was used to model the effluent behavior from the columns. The model, CXTFIT/EXCEL (Tang et al. 2010), runs in Microsoft EXCEL and includes options to account for equilibrium and non-equilibrium transport in columns. Visual MINTEQ was used to assist in the evaluation of the effects of complexation with carbonate. 5.3 Development of a Synthetic Runoff Cocktail The following sections describe how the synthetic runoff cocktail was developed. Descriptions are provided on the col- lection and processing of organic matter in highway runoff and the composition of the inorganic components in the syn- thetic cocktail. 5.3.1 Collection and Preparation of Highway Runoff Organic Matter One of the goals of this project was to identify a means of conducting experiments with organic matter from actual highway runoff events while overcoming concerns of vari- ability in water quality from different stormwater events and changes in stormwater composition during the course of the experimental system. To overcome these challenges, this research utilized an organic matter isolation approach devel- oped by Pressman et al. (2010) in which runoff is pretreated to remove suspended solids and cations, and organic matter is then concentrated in a reverse osmosis system. This section describes this approach to concentrate organic matter. Concentration processes employing reverse osmosis require significant pretreatment to prevent fouling of the reverse osmo- sis membrane. As a result, prior to concentration, pretreatment must remove suspended solids and excess ions from the water. In some cases, if the material is to be freeze-dried and the con- centrate contains sulfate, sulfate precipitation may be required as well (Serkiz and Perdue 1990; Maurice et al. 2002; Pressman et al. 2010). The pretreatment process consists of filtration followed by ion exchange. A schematic of the process is shown in Fig- ure 5-6. This can be accomplished using in-line water filters. Figure 5-6. Schematic diagram of the organic matter pretreatment process using filtration and ion exchange. Specific brands of equipment are provided as examples.
57 Most in-line filters have a maximum operating pressure of about 100 psi and a maximum differential pressure of about 35 psi. Due to the variation in particle size in stormwater, a step-wise filtration process is justified. Previous research has shown that triple filtration accomplished using a series of three progressively smaller in-line filters is sufficient to remove suspended solids, maintain flow, and reduce system pressure (Serkiz and Perdue 1990; Maurice et al., 2002). The resulting water after passing through the 0.45 micron filter should be free of all suspended solids (TSS) and consist only of TDS. It is important to note that the TDS includes both organic and inorganic compounds. Inorganic compounds adsorbed onto suspended solids may have been lost in this process, but the loss of organic anions is likely minimal. Following the removal of TSS from the water sample via fil- tration, the water sample is passed through an ion exchange resin to remove inorganic cations and isolate the organic anions. Since the objective is to isolate the organic anions in the water sample a hydrogen ion (H+) form macroporous resin is used to remove inorganic cations. During this phase, two reactions occur. Inorganic cations sorbed onto anionic com- pounds in the water desorb and then adsorb onto the H+ form resin, removing the cations from the water and releasing free hydrogen atoms. The free hydrogen ions then lower the pH of the effluent to a value of approximately 2 and react with the organic anions to form organic acids (Serkiz and Perdue 1990; Maurice et al. 2002; Speth et al. 2008; Pressman et al. 2010). Selecting the appropriate ion exchange resin is critical to the successful removal of inorganic cations and, therefore, to the successful concentration of DOM through reverse osmo- sis. Failure to remove the cations completely will have two impacts on the outcome of the concentration process. First, inorganic compounds that remain in the water will con- centrate alongside the organic matrix and could impact the results of the sorption studies. Secondly, higher influent TDS concentrations will result in higher osmotic pressure during reverse osmosis. It is because of this factor that a hydrogen ion form resin is used. Many types of resin are available on the market. For this application, it is generally agreed that macro- porous resins are more effective and pose less of a threat to the integrity of the organic matrix than gel type resins (Serkiz and Perdue 1990; Speth et al. 2008; Pressman et al. 2010). Once the resin is specified, it is critical to determine how much resin is required. The mass required is a function of the water sample volume, total inorganic cation concentration, and the exchange capacity of the resin. Typical exchange capacity values range from 1.8 to 2.25 eq/L. Constituent concentrations in stormwater and highway runoff differ significantly from those reported in typical natural waters and vary with each storm based upon location, environmental factors, human traf- fic factors, and storm frequency (Beck 1974; Weber 1974; Serkiz and Perdue 1990; Barrett 2008; Pressman et al. 2010; Sansalone 2011). Thus, analysis of the water composition prior to con- centration is recommended to ensure that the ion exchange system is appropriately sized. One of the key design parameters for successful ion exchange is empty bed contact time (EBCT), which is calculated by dividing the resin container volume by the flow rate. EBCTs between 5 and 30 minutes are typical for ion exchange processes. A photograph of the complete filtration and ion exchange system is shown in Figure 5-7. Concentration of the DOM is accomplished using reverse osmosis operated in a recycle mode as shown schematically and visually in Figure 5-8 and Figure 5-9, respectively. This membrane process is typically used to remove ions from water and provide ion free water for consumption or use. The con- centrate stream containing the ions is then disposed. However, in this case, the goal is to utilize the concentrate flow. As the feed water passes through the reverse osmosis membrane, the organic constituents are retained in the concentrate flow and constituent free water flows in the permeate stream. Thus, the permeate stream is wasted in the system and the concentrate flow is recycled back to the feed and the system run in recycle mode until the desired concentration factor is achieved. Dur- ing this process, the concentration of organic matter in the recycled feed water steadily increases as the constituent mass is retained and the âcleanâ water is wasted as permeate. In the practical sense, several design and operation param- eters can be manipulated to maximize permeate flow and constituent rejection to achieve optimal results depending on the operation. The primary parameters that are easily modi- fied to meet the system requirements are the number, size, type, and alignment of membranes and the influent, perme- ate, and concentrate flow rates. The type and size of the reverse osmosis membrane con- trol the rejection properties and membrane SA of the system. Figure 5-7. Photograph of a pretreatment process consisting of filtration and ion exchange.
58 For this application, a brackish water membrane provides the tightness and pressure rating required to concentrate the DOM. Having a greater membrane SA enables higher flow rates at lower osmotic pressures (Pressman et al. 2010). However, at low influent flow rates, larger membranes experience less salt recovery, or more loss of constituents to the permeate flow than the same membrane with less SA. Filmtech brackish water membranes are designed to achieve approximately 99.5% NaCl rejection, which has a molar weight of 58.44 g/mol, at 15% recovery. Typical organic compounds range in size from 16 g/mol for the simple n-alkane group to 390 g/mol for the highly complex phthalate group chemical. Thus, large, complex organic compounds should not permeate through the resin regardless of the operating parameters. This conjecture is supported by Speth et al. (2008) and Namjesnik- Dejanovic (2004). It should be noted that several simple organic compounds found in the branched and n-alkane, aldehyde, ali- cylic hydrocarbon, and aliphatic amine groups are smaller than sodium chloride and may permeate the membrane at a rate proportional to the operating conditions as observed above. All of these groups have been found to be present in stormwater runoff but, as discussed previously, compositions vary widely over both the spatial and time domains. The reverse osmosis system is capable of achieving con- centration factors for the organic matter of approximately 150X. This material may be used in this form, further purified through electro-dialysis, or freeze-dried and stored for future use. If the material is to be freeze-dried, then sulfate must be Figure 5-8. Schematic view of the concentration process using one Filmtech BW30-4040 membranes. Figure 5-9. Photograph of a reverse osmosis process.
59 removed from the system. Because sulfate is an anion, it is not removed during the cation ion exchange process and is concentrated through reverse osmosis proportionally to the DOM (Serkiz and Perdue 1990; Pressman et al. 2010). The preferred method for removing sulfate from the concentrated sample is precipitation using barium chloride. The reaction that takes place is as follows: + +( ) ( )â âSO BaCl BaSO 2Cl42 aq 2 4 s Precipitation works better at higher concentrations. It is for this reason that the sulfate is not removed until after it has been concentrated. The goal of the concentration process is to minimize losses of organic matter throughout the pretreatment, concentra- tion, sulfate precipitation, and freeze drying process. Previ- ous research has shown that this is achievable with recoveries of organic matter of over 85% (Pressman 2012). In tests with highway runoff collected in Austin, TX the initial TOC concentration of the raw water was 8.86 mg/L and the aver- age concentration of our reconstituted organic matter was 8.60 mg/L. This is similar to the median value of 12.6 mg/L in the FHWA database. Thus, it appears that the process was successful in recovering the organic matter. However, recon- stitution of the organic matter presented several challenges including relatively high concentrations of Ba in the recon- stituted water (approximately 30 mg/L), and it was difficult to re-dissolve the organic matter to create a concentrated solution for use in the column experiments. While the high barium concentration did not appear to interfere with the experiments, it was a significantly higher concentration than was observed in the raw water (9 mg/L). The challenge of re- dissolving the organic matter was resolved by implementing a sonicating step into the reconstitution procedure. The process for reconstituting the organic matter is a topic discussed in depth by McCurry et al. (2011), where they rec- ommend continuously mixing a concentrated solution con- taining the dried (NOM) for 72 hours at a pH of 10 (achieved by adding NaOH). However, original efforts to implement this procedure did not dissolve all of the organic matter. Addition of a 1 hour sonication step after adding the solid material to water was more successful. Moreover, in the trials the pH of reconstitution had no effect on the TOC as dem- onstrated when equal portions of freeze-dried organic matter were added to a series of five separate beakers. After 1 hour of sonication, 200 ml samples were drawn from the beakers and passed through 0.45 mm filters into precleaned/treated TOC vials at dilutions of 1:10 (30 mL total). Figure 5-10 shows the results of two separate TOC measure- ments from the same experiment, and these results suggest that the pH of the solution into which NOM was dissolved had little to no effect on the reconstitution of the organic material. Since determining that pH of the dissolution water does not influence the reconstitution of the organic matter, the pH adjustment step was removed from the procedure; however, to aid in solids separation post sonication, the reconstituted super-concentrated solution was centrifuged at 10,000 RPM for 15 minutes. The centrifugation helped to separate out any of the inorganics and insoluble components of the freeze- dried NOM material. 5.3.2 Composition of the Inorganic Constituents in the Synthetic Runoff The organic matter represents only one component of the highway runoff composition. The inorganic composition of the water to be used in experimental evaluation must also be selected to match conditions anticipated in the field. Ideally, the composition is based on measured concentrations of the regional highway runoff collected over a range of conditions and sampling events. Alternatively, literature values can be used. For this set of experiments the concentrations provided in Table 5-2 were used to create the synthetic runoff. Values for chloride and hydrogen ion were varied to match the ionic strength. The four main cations identified were magnesium (Mg), cal- cium (Ca), potassium (K), and sodium (Na). None of these ions is expected to substantially interfere with heavy metal adsorp- tion onto most media except with respect to their impact on ionic strength and precipitation. In addition to matching the ion concentrations, the overall ionic strength was considered as well. This was done by calculating the ionic strength of the water from the composition in Table 5-3 and matching it to the ionic strength obtained through addition of a series of chemicals that match both the ionic content and ionic strength. Geochemical codes such as Visual MINTEQ can be useful in evaluating the composition. The stormwater recipe created to match the com- position and ionic strength of literature values is detailed in Figure 5-10. Effect of pH on TOC concentrations achieved by dissolving freeze-dried highway runoff organic matter into 200 ml vials.
60 Table 5-3. The ionic strength of the water was calculated to be 0.009 M. Moreover, use of a program such as Visual MINTEQ can also provide saturation indices that assess whether any solid phases are likely to form in the system. An example of a saturation index for this water is shown in Table 5-4. Note that the saturation indices are all negative, which indicates that all potential precipitates are below saturation. 5.3.3 Media Selection and Preparation Media selection is a key step in developing a treatment sys- tem for removing metal ions from highway runoff or other waters. However, the protocol described in this report is meant to be generic for a range of media. For this study, two iron oxides and two MO were obtained commercially and the two waste products, crab shell and concrete, were obtained locally. The basis for selecting the oxide minerals included previous use as an adsorbent for metal and oxyanion sorption, com- mercial availability, and availability in particle diameters suit- able for the experimental column and field conditions. The mineralogy of each of the iron and MO were similar; however, they were purchased through two different sources. Granular iron oxides are produced from several differ- ent forms of iron oxide including: granular ferric hydroxide (GFH), which is a poorly crystallized akaganeite (b-FeOOH) distributed by companies including US Filter, a goethite based Bayoxide (E33) distributed by AdEdge or Severn Trent, and hematite (a-Fe2O3) based iron oxide (e.g., ARM 200 distrib- uted by Engelhard Inc.). The reported SAs of 250 m2/g and 142 m2/g for GFH and E33, respectively, suggest that GFH provides more surface sites for adsorption; however, previ- ous research has demonstrated that for arsenic removal both of these granular materials, GFH and E33, perform similarly in batch and column experiments. Two sources of goethite based oxide mineral [granular ferric oxide (GFO) and E33] used in the work were obtained from Bulk Reef Supply and AdEdge Inc., respectively. X-ray diffraction confirmed that that Bulk Reef Supply Material was primarily goethite. The manganese oxide minerals were MTM (Northern Media Company), which contains a granular core with a coat- ing of manganese dioxide and a similar product provided by Pureflow PM-200 manganese dioxide. Analysis of the mate- rial indicated that the manganese dioxide was amorphous in nature and as such did not exhibit an x-ray diffraction signal. The SA of the MnO2 was determined to be 23.6 m2/g. The PCC used in the experiments was obtained from a recycling facility in the Austin area and the crab shell was purchased from DirtWorks (www.dirtworks.net), where it is sold as a soil amendment/fertilizer. The crab shell waste was chosen because crab shell is known to contain calcite and chi- tin. Calcite can be used to increase the pH of the stormwater and chitin exhibits adsorptive properties for metal ions. The Constituents g/mol mg/L {Req'd} mol/L Na 23 50 0.002174 K 39 50 0.001282 Ca 40 50 0.00125 Mg 24 10 0.000417 NO3 62 110 0.001774 Cl 35.5 110 0.003099 SO4 96 10 0.000104 HCO3 61 100 0.001639 HCl 36.5 30 0.000822 Table 5-2. Literature values for ions/anion concentrations. Lab Salts g/mol {Conc} mg/L Measured Mass (mg) / 40L Batch Solution MgSO4·7H2O 246 55 2,200 KCl 74.5 25 1,000 KNO3 101 90 3,600 NaNO3 85 90 3,600 CaCl2 111 200 8,000 CuSO4·5H2O 249.5 0.196 (0.050 Cu) From Concentrate ZnSO4·7H2O 287.6 0.440 (0.100 Zn) From Concentrate Table 5-3. Synthetic stormwater composition.
61 SA of the crab-shell waste was determined to be 13.3 m2/g and the XRD spectra verified the presence of calcite. All media must be sized to meet the hydrodynamic consider- ations of the experimental column system. Specifically, in order to prevent channeling and inhibit wall effects in columns, the column diameter should be at least 30 times that of the particle diameter (Chellam and Wiesner 1993). All filtration media used were sieved to particle diameters between 0.25 mm to 0.40 mm (sieve sizes 60 and 40), which meet the criteria that the diameter of the particles be at least 30 particle diameters smaller than the column internal diameter. Media diameter can be controlled either by crushing the media to the appropriate size or sieving the material to the appropriate diameter. 5.4 Batch Reactor Studies Preliminary assessment of the capacity of the iron oxide media was conducted to estimate the equilibration times for sorption of copper and zinc and obtain an estimate of the sorption capacity of the media to ensure that the column Table 5-4. MINTEQ saturation indices output @ pH = 6.3. Mineral log IAP Sat. index Stoichiometry Antlerite 2.124 -6.664 3 Cu+2 4 H2O -4 H+1 1 SO4-2 Aragonite -10.238 -1.902 1 Ca+2 1 CO3-2 Artinite -2.379 -11.979 -2 H+1 2 Mg+2 1 CO3-2 5 H2O Atacamite 3.67 -3.721 2 Cu+2 3 H2O -3 H+1 1 Cl-1 Azurite -21.181 -3.781 3 Cu+2 2 H2O -2 H+1 2 CO3-2 Brochantite 8.334 -6.888 4 Cu+2 6 H2O -6 H+1 1 SO4-2 Brucite 8.763 -8.337 1 Mg+2 2 H2O -2 H+1 CaCO3xH2O(s) -10.238 -3.094 1 Ca+2 1 CO3-2 1 H2O Calcite -10.238 -1.758 1 Ca+2 1 CO3-2 Chalcanthite -10.296 -7.656 1 Cu+2 1 SO4-2 5 H2O Cu(OH)2(s) 6.21 -3.08 1 Cu+2 2 H2O -2 H+1 Cu2(OH)3NO3(s) 3.365 -5.886 2 Cu+2 3 H2O -3 H+1 1 NO3-1 CuCO3(s) -13.695 -2.195 1 Cu+2 1 CO3-2 CuOCuSO4(s) -4.086 -14.39 -2 H+1 2 Cu+2 1 H2O 1 SO4-2 CuSO4(s) -10.296 -13.236 1 Cu+2 1 SO4-2 Dolomite (ordered) -21.38 -4.29 1 Ca+2 1 Mg+2 2 CO3-2 Epsomite -7.743 -5.617 1 Mg+2 1 SO4-2 7 H2O Gypsum -6.839 -2.229 1 Ca+2 1 SO4-2 2 H2O Halite -5.143 -6.693 1 Na+1 1 Cl-1 Huntite -43.665 -13.697 3 Mg+2 1 Ca+2 4 CO3-2 Hydromagnesite -35.806 -27.04 5 Mg+2 4 CO3-2 -2 H+1 6 H2O KCl(s) -5.406 -6.306 1 K+1 1 Cl-1 Langite 8.334 -9.155 -6 H+1 4 Cu+2 7 H2O 1 SO4-2 Lime 9.667 -23.032 -2 H+1 1 Ca+2 1 H2O Magnesite -11.142 -3.682 1 Mg+2 1 CO3-2 Malachite -7.485 -2.016 2 Cu+2 2 H2O -2 H+1 1 CO3-2 Melanothallite -11.29 -17.547 1 Cu+2 2 Cl-1 Mg(OH)2 (active) 8.763 -10.031 1 Mg+2 2 H2O -2 H+1 Mg2(OH)3Cl:4H2O(s) 8.776 -17.224 2 Mg+2 1 Cl-1 -3 H+1 7 H2O MgCO3:5H2O(s) -11.142 -6.602 1 Mg+2 1 CO3-2 5 H2O Mirabilite -9.292 -8.178 2 Na+1 1 SO4-2 10 H2O Natron -12.691 -11.38 2 Na+1 1 CO3-2 10 H2O Nesquehonite -11.142 -6.472 1 Mg+2 1 CO3-2 3 H2O Periclase 8.763 -12.821 -2 H+1 1 Mg+2 1 H2O Portlandite 9.667 -13.037 1 Ca+2 2 H2O -2 H+1 Tenorite(am) 6.21 -2.28 1 Cu+2 1 H2O -2 H+1 Tenorite(c) 6.21 -1.43 1 Cu+2 -2 H+1 1 H2O Thenardite -9.292 -9.614 2 Na+1 1 SO4-2 Thermonatrite -12.691 -13.328 2 Na+1 1 CO3-2 1 H2O Vaterite -10.238 -2.325 1 Ca+2 1 CO3-2 The ratio of the ion activity product (IAP) to the reaction equilibrium constant is the saturation index.
62 design was appropriate and that experimental runs would be conducted within a reasonable time frame. A preliminary rate study was performed to verify that 48 hours of equilibration time was sufficient. VWR Polypropyl- ene 15 mL centrifuge tubes were used as batch reactors. Inside a CO2-free glove box (Labcono Catalog No. 50700009147), a solids slurry stock solution was prepared using 1000 mg/L of the iron oxide fines in CO2-free Millipore water. An inorganic stormwater stock solution containing an ionic composition similar to the feed used in the column experiments (with MES buffer added to control pH) was also prepared in the glove box using CO2-free Millipore water. Both solutions were adjusted to a pH of approximately three. To assemble the reactors, first 5 mL of Fe oxide slurry and 5 mL of inorganic stormwater salts stock solution were added to the reactor. The reactor was then spiked with Cu and Zn stock solutions to achieve the desired metal concentrations of 1250 mg Cu/L and 2500 mg Zn/L. Visual MINTEQ was used to confirm that the resulting reactors would not be over- saturated and lead to precipitation. The reactors were mixed well before the pH was adjusted using CO2-free 0.1M NaOH to a pH of 6 ± 0.02. To verify the final solids concentration, gravimetric analysis of three 2.5 mL aliquots of solids stock solution, spaced evenly throughout reactor preparation, was performed. In addition, control reactors with either Fe oxide or Cu and Zn excluded were prepared to check for other metals sources and losses. The sealed caps were wrapped in Parafilm before the reactors were moved from the glove box to tumble end-over-end at ambient temperature (20â25°C). The equilibration times evaluated ranged from 0 to 98.5 hours, as presented in Figure 5-11. At the time of sampling, the final pH of each reactor was measured in the glove box. After pH measurement, the reac- tors were centrifuged at 3000 rpm for 10 minutes. A 4-inch Air-Tight disposable hypodermic syringe needle was used to remove the supernatant, which was then filtered through a disposable VWR 0.2 mm polyethersulfone membrane fil- ter. The first 1 mL that passed through the filter was wasted. The filtered supernatant was then acidified to 1.5% HNO3 to avoid sorption to container walls or precipitation. The prepared supernatant was analyzed by inductively coupled plasma (ICP) optical spectroscopy on the Varian 710-ES ICP Optical Spectrometer to determine the final amount of metal remaining in solution. The amount of metal adsorbed was determined from a mass balance by subtracting the final con- centration in solution from the initial solute concentration. Batch equilibrium reactors were also used to generate sin- gle points on adsorption isotherms (fixed pH and varying adsorbate concentration). VWR Polypropylene 15 mL cen- trifuge tubes were used as the batch reactors. Inside a CO2- free glove box (Labcono Catalog No. 50700009147), a solids slurry stock solution was prepared using 1000 mg/L of iron oxide in CO2-free Millipore H2O. The inorganic stormwater salts stock solution with MES buffer was also prepared as described for the rate study. All three of these solutions were adjusted to a pH of approximately 3. To assemble the reactors, first 2.5 mL of pH 3 CO2-free Mil- lipore water was added to a 15 mL centrifuge tube. The volume of Cu and Zn stock solution to be added was then removed to minimize dilution of the metals. Next, 2.5 mL of Fe oxide slurry and 5 mL of inorganic stormwater salts stock solution were added. The reactor was then spiked with varying Cu and Zn stock solutions to achieve the desired metal concentrations. 5.5 Experimental Column System The experimental column system can be constructed using a variety of materials and in a variety of configurations. The essential components include an influent storage tank; a pump or system configuration that provides constant flow; a means of maintaining constant pH and water chemistry of the influent; and influent and effluent sampling ports for analysis of flow rate, pH, metal ion concentrations, and TOC as required. It is often desirable to run columns in parallel so as to allow evalu- ation of different media using the same influent water compo- sition. In addition, it is important to use inert materials that do not adsorb or leach metals or background organic matter from the system during the experiment. For these reasons, the experimental columns used in this research were constructed from Teflon components. Specific details of the system used in this research serve as an example of the type of experimental reactor system that can be used to evaluate media. Experimental Flow Path The apparatus constructed for this experiment was made up of three series (A,B,C) of fixed bed reactors (FBR), each containing three individual columns connected with tubes and fittings made from Teflon®. The columns were aligned vertically, with the feed solution entering the lowest column in each series (Column 1) from the bottom, and flowing through the top into the bottom of Column 2. This flow path continued through the top of Column 2 and into the bottom of Column 3. After passing through the final column (Col- umn 3), the solution passed a ball valve used for sampling, and was then discharged. Cocktail Feed Tank Setup The feed solution was kept in a 50L Nalgene tank (Tank 1) with a constant volume of 40L, providing a consistent posi- tive head. A tube connected to the bottom of Tank 1 had a ball valve port attached which was used for sampling. Past the ball valve, the line was split into three separate streams leading
63 into a peristaltic pump. The three separate lines were fed to the bottoms of each of the column series (A,B,C). See Figure 5-12 for a detailed diagram of the apparatus. System Rinse In order to rinse out tubing and newly packed columns, Tank 1 was filled with Millipore water, which was pumped through the tubes for at least 24 hours prior to starting the trial. Once the system had been flushed for at least a day, the synthetic stormwater solution was pumped into an empty Tank 1, brought to the constant 40L head mark, and the pumps were initiated. Nitrogen Blanket In most experiments, the alkalinity was negligible in order to eliminate potential complexation by copper. A N2 tank was used to prevent CO2 exchange and maintain a constant influ- ent pH throughout the run. A regulator was connected to an N2 gas cylinder, and two gas lines delivered a low flow of N2 into the headspace of both the filling tank (Tank 2) and Tank 1. The Pe rc en t A ds or be d (% ) Pe rc en t A ds or be d (% ) Time (hr) Time (hr) Figure 5-11. Equilibration of copper (top) and zinc (bottom) onto GFO at pH 6.0.
64 tops of the tanks were covered with Parafilm® to minimize gas exchange between the tanks and the atmosphere. Solution Refill Setup A filling tank (Tank 2) was the main storage basin which fed influent water directly into the constant head tank (Tank 1). Tank 1 was set up with an overflow line to feed back into Tank 2 when the volume reached 40L. Tank 2 was filled on a daily basis by syphoning stormwater solution from a mixing tank (Tank 3, not shown). The influent water was prepared in Tank 3, and like the other two tanks, it was acid washed prior to use in the experiments. Column Assembly The columns consisted of an 8cm long Teflon tube with an ID of 0.9cm and OD of 1.3cm, with Teflon joints on both ends to allow connection to the next column in series. All individual parts on the column were made of Teflon, and acid washed prior to use. A ball valve was installed at the effluent end of each of the columns so that the efflu- ent from each column could be sampled immediately after passing through the media. The columns were filled with Teflon® beads (Polytetrafluoroethylene with > 40mm particle size) and a known mass of media as shown in Figure 5-13. Stormwater Cocktail Stormwater Cocktail Figure 5-12. Experimental column setup (not to scale). Figure 5-13. Example of a packed column surrounded by Teflon® beads.
65 5.6 Experimental Results 5.6.1 Batch Reactor Studies of Cu Adsorption Capacity and Equilibration Times The evaluation of zinc and copper adsorption to GFO suggests that equilibration is achieved within several hours at pH 6 as shown in Figure 5-11. The data also suggests that adsorption of copper is significantly greater than zinc as nearly all of the copper is removed from solution and only approximately 20% of the zinc added to the system is removed from the solution. The capacity of the GFO for cop- per and zinc at the influent concentrations of 1,250 mg/L and 2,500 mg/L, respectively, were 2.45 mg/mg and 1 mg/mg. Assuming a linear isotherm equation for the low end of the isotherm: =q K Ce D e in which qe represents the mass adsorbed per mass of adsor- bent in mg/mg, Ce is the equilibrium concentration of the metal ion in mg/L, and KD is the linear partitioning coeffi- cient in L/kg, the value of log KD for each metal ion is 4.99 and 2.7, respectively. A complete isotherm developed for adsorption of copper onto GFO is presented in Figure 5-14 and suggests that the adsorption of copper onto GFO is non-linear, which is con- sistent with previous research examining sorption of metal ions onto oxide minerals at fixed pH. Indeed, the data can be described by a Langmuir isotherm: = + q Q KC 1 KC e max e e where Qmax is the maximum adsorption capacity and K is a constant that is related to the energy of adsorption. Isotherm parameters determined from the data are Qmax = 20 mg/mg, K = 0.01 L/mg. It is also evident that the concentrations of interest for stormwater appear within the linear portion of the isotherm below 20 mg/L. In fact, within a flow-through treatment system the equilibrium concentration is equal to the desired effluent concentration which is below 3 mg/L for copper. Using a value of 3 mg/L and 0.01 for Ce yields a value of 0.03 for the second term in the denominator, which is sig- nificantly less than 1. Thus, the denominator is approximately one and the isotherm becomes linear in which KD is the prod- uct of Qmax and K or 0.2 L/mg (200,000 L/kg) corresponding to a log KD of 5.3. Linear regression of the qe versus Ce data yields a slope (or KD value) of 0.18 L/mg. These results are consistent with the value of KD estimated from the rate study. Estimates of breakthrough in the flow-through columns sys- tems can be made using these values of KD. Figure 5-14. Adsorption isotherm data (symbols) and Langmuir model fit (solid line) for copper sorption onto GFO at pH 6 with an initial zinc concentration of 3,300 îg/L.
66 5.6.2 Column Protocol Verification Selected results from column experiments conducted in this research are presented to highlight several different key issues including the ability to distinguish performance of dif- ferent media for different metal ions, sensitivity of removal to changes in pH, the role of organic matter, the role of ionic strength, and the potential benefits of dual media systems. A summary of the results from all of the experiments is pre- sented in Table 5-5. The table contains the adsorption capac- ity values and log KD values calculated from the column data. The capacity of each type of media to adsorb copper or zinc was determined by measuring the difference in influent and effluent concentrations over the course of each run (until breakthrough or a plateau was observed). By the principle of mass balance, the metal not accounted for in the effluent stream was attributed to sorption by the media or precipita- tion within the column. In addition to influent and effluent metal concentrations, the masses of media packed into each column at setup and the flow rates through each column over time were measured. Measurement of the flow rates allowed for an accurate determination of the cumulative volume out- put from each column. Essentially, a numerical integration utilizing the trapezoid rule was performed on the difference between influent and effluent concentration as a function of cumulative volume output, yielding the total mass of metal adsorbed onto each column. This value was then divided by the recorded mass of media for each column, resulting in the adsorptive capacity in mg metal per mg media. Modifications to this procedure were required for breakthrough profiles that did not reach complete breakthrough (C/Co = 1). In those cases, the mass removed was only determined up to the point that a plateau in the breakthrough profile was achieved. As there were three beds per column, measurements of effluent taken at each bed incorporated the adsorptive capac- ity of that bed and any bed upstream. That is, while the influ- ent of Column 1 was indeed the measured influent to the column, the influent to Column 2 was the effluent from Col- umn 1 and would differ from the original effluent. Hence, in calculating capacity the bed masses were combined in order to characterize the cumulative adsorptive capacity of one, two, or three columns in series. In most cases Columns 1, 2, and 3 were the same type of media, so this approach was appropriate. In cases where the beds were mixed (i.e., utilizing crab shell or concrete to boost influent pH prior to a metal oxide bed), the calculated capacities apply to the overall behavior of the combination of media within the column and not only to the oxide media in question. This distinction is significant because the crab media, and to a lesser extent the concrete, were observed to adsorb metal from solution, hence altering the composition of the influent to the metal oxide media bed. The coefficients of adsorption, KD, were calculated by divid- ing the mass capacity of each bed (calculated as described earlier) by the concentration of solution in equilibrium with the media. In cases where the column achieved breakthrough, this calculation was straightforward since the equilibrium concentration was simply the influent concentration to the column. However, in cases where a plateau was observed at lower-than-influent concentrations, the equilibrium concen- tration of the solution was assumed to be the average between the true influent concentration and the effluent concentra- tion of the observed plateau. This assumption stems from the hypothesis that the observed plateaus are a result of precipita- tion processes occurring within the column, hence lowering the apparent effluent concentration while potentially main- taining higher metal concentrations within the column itself. That this impact was only seen with the iron oxide media and Cu may represent an advantage for iron oxide media and cop- per removal in real systems. Values of log KD for sorption of copper on GFO are lower than the log KD value estimated from the batch reactor data, demonstrating the importance of conducting experiments in flow-through systems which more closely capture the dynamics of the field systems. The results also verify that the values of log KD are dependent on pH. To determine whether there is a linear relationship between log KD and pH as suggested by Langmuir (1997) for sorption on hydrous ferric oxide, log KD values from Trials 1 through 4 and Trial 11 without organic matter were grouped according to the influent pH. A plot of log KD versus pH is shown in Fig- ure 5-15 along with the linear regression of the data that shows that the log KD increases over an order of magnitude for each unit change in pH. This is consistent with the results obtained by Langmuir (1997) in which he derived the equa- tion log KD = -5.48 + 1.77pH for sorption of zinc to hydrous ferric oxide. The summary table also shows that zinc log KD values are approximately an order of magnitude lower than the values for copper for all of the media. This is consistent with the batch reactor experiments; however, the batch reactor experi- ments showed an even greater reduction in zinc sorption. The estimate of the log KD for zinc sorption from the batch reactor studies was not representative of the column system because it was obtained from a single isotherm point at an equilib- rium zinc concentration of approximately 2,000 mg/L; a con- centration that is well above the linear range of the isotherm. Using a log KD derived from data collected in the non-linear region of the isotherm will underestimate the value at lower equilibrium concentrations. 5.6.3 Contrasting Performance of Different Media and Different Metal Ions Figure 5-16 and Figure 5-17 show the results for sorption of copper and zinc in column trains A1-A3 and B1-B3 using
Experimental Conditions Experimental Results Trial Influent Conc. Media pH Ionic Strength Matrix Flow Rate (mL/min) Bed pH (average) Avg. Capacity ( g/mg) log Avg. KD (KD in L/kg) Cu / Zn g/mg (average) Bed 1 Bed 2 Bed 3 Cu Zn Cu Zn 1 25.3 Cu (No Zinc) GFO GFO GFO 6.26 0.009 M Inorganic Only 6.8 6.35 2.00 N/A 5.11 N/A MnO2 MnO2 MnO2 6.9 6.66 0.26 N/A 4.01 N/A Crab Crab GFO 6.9 6.73 0.36 N/A 4.33 N/A 2 38.8 / 101 GFO GFO GFO 6.30 0.009 M Inorganic incl. NaHCO3 7.7 6.39 1.80 0.486 4.76 3.62 MnO2 MnO2 MnO2 7.7 6.5 0.142 0.0029 3.57 1.02 Crab Crab E33 8.5 6.54 0.909 0.419 4.37 3.59 3 39.7 / 95.3 GFO GFO GFO 6.02 0.009 M Inorganic only 7.8 6.07 0.788 0.417 4.38 3.64 MnO2 MnO2 MnO2 7.9 6.03 0.098 0.0179 3.38 2.25 Crab Crab MnO2 7.8 6.52 1.00 1.05 4.42 4.01 4 40.2 / 118.5 E33 E33 E33 6.14 0.011 M Inorganic only 8 6.01 2.01 0.754 4.87 3.8 GFO GFO GFO 8 5.94 2.15 0.616 4.93 3.72 Crab Crab E33 8 6.2 1.67 1.39 4.76 4.03 5 47.2 / 113.6 E33 E33 E33 5.03 0.011 M Inorganic only 8 5.22 0.0286 N/A 2.78 N/A GFO GFO GFO 8 5.24 0.120 N/A 3.37 N/A Crab Crab E33 8 5.79 0.472 N/A 3.79 N/A 6 40.7 / 114.7 E33 E33 E33 5.91 0.011 M Inorganic only 8 5.73 1.54 0.258 4.70 3.24 GFO GFO GFO 8 5.68 1.65 0.304 4.72 3.38 Crab Crab E33 8 6.09 0.345 N/A 3.63 N/A 7 43.1 / 109.7 E33 E33 E33 5.90 0.009 M Inorganic only 8 5.82 0.875 N/A 4.43 N/A GFO GFO GFO 8 5.84 1.04 N/A 4.53 N/A Concrete Concrete E33 8 6.85 1.50 1.28 4.61 4.03 8 37.3 Cu (No Zinc) GFO GFO GFO 6.14 Low Inorganic and NOM (8.9) 7.2 6.29 0.98 N/A 4.61 N/A MnO2 MnO2 MnO2 6.8 6.217 0.07 N/A 3.25 N/A Crab Crab GFO 6.5 6.496 1.26 N/A 4.65 N/A 9 49.8 / 150.4 GFO GFO GFO 5.88 0.009 M Inorganic and NOM (16.2) 7.6 5.95 1.13 0.516 4.40 3.53 MnO2 MnO2 MnO2 7.8 5.83 0.063 0.012 3.10 1.87 Crab Crab MnO2 8.5 6.39 2.03 2.44 4.56 4.16 10 58.9 / 183.4 GFO GFO GFO 6.01 0.009 M Inorganic and NOM (19.7) 9.9 6.04 1.28 0.736 4.27 3.73 E33 E33 E33 9.9 6.08 2.06 1.70 4.67 3.98 Concrete Concrete E33 10 6.69 0.371 0.697 3.60 3.78 11 32.8 / 105.1 GFO GFO GFO 6.91 0.009 M Inorganic Only 8 6.86 5.71 2.16 5.31 4.31 MnO2 MnO2 MnO2 8 6.86 0.305 0.100 4.00 2.97 Crab Crab GFO 8 7.06 2.38 2.27 4.92 4.31 12 42.8 / 102.6 E33 E33 N/A 5.92 0.100 M Inorganic Only 7.7 5.90 1.50 0.447 4.88 3.64 Concrete E33 N/A 7.7 6.61 1.36 1.29 4.59 4.10 Bone Meal E33 N/A 7.7 6.08 0.561 0.273 4.17 3.42 13 36.7 / 43.6 GFO Bone Meal GFO 2cmGFO 2cm 7.41 N/A Actual Stormwater 8 7.5 0.25 0.59 3.89 4.29 14 8.89 / 28.5 GFO Concrete GFO 2cmGFO 2cm 7.52 0.010 M Inorganic and High CO3 7.8 7.6 0.235 1.31 4.51 4.79 GFO is granular ferric oxide, E33 is an iron oxide, MnO 2 is manganese oxide, Crab is crushed crab shell waste, Concrete is crushed concrete, NOM is natural organic matter. Trials 4-7 refer to experiments run in series conducted without changing media. Table 5-5. Summary of results from column experiments including average sorption capacity and estimated log KD values.
68 Figure 5-15. Relationship between pH and log KD for sorption of copper onto GFO (Trials 2, 3, and 11). GFO obtained from Bulk Reef Supply in Column Train A and MTM granular manganese dioxide from Northern Media Company (Muscatine, IA) in Column Train B. It is readily apparent that the iron oxide media performs signifi- cantly better than the manganese dioxide media. Complete breakthrough of copper occurs in the manganese dioxide columns at approximately 20,000 bed volumes. In contrast, copper breakthrough profiles plateau at 80, 60, and 40% in the three sequential columns, A1-A3. This behavior sug- gests that adsorption is not the only removal mechanism in the iron columns and suggests that copper precipitation is occurring within the bed. In contrast, zinc adsorption reaches complete breakthrough for both media. Since copper is sig- nificantly more insoluble than Zn, the dramatic differences in the results are not surprising. Figure 5-16. FBR breakthrough profiles for Cu (top) and Zn (bottom) from a synthetic stormwater solution onto granular iron hydroxide (Columns A1-A3 in series). The column diameter was 1 cm ID, flowrate îµ 8 ml/min, the influent copper concentration was 50 mg/L, and the influent Zn(II) concentration was 100 mg/L. The influent ionic strength was 0.01M. Three columns were connected in series with A1 being the first bed in the series.
69 5.6.4 Impact of Highway Organic Matter In a separate experiment (Trial 9), reconstituted organic matter was added to the synthetic stormwater solution con- taining salts and adjusted to a pH of approximately 6. The average influent pH to the columns containing organic mat- ter was 5.9, within 0.1 pH unit of the experiments without organic matter. The column experiments captured the impact of the organic matter for both oxide minerals. Results from these experiments for the iron oxide media are shown in Fig- ure 5-18 for the three columns, A1-A3. For all three columns in series the initial breakthrough in columns with and without organic matter appears similar; however, the plateau obtained as breakthrough occurs in each case is at a higher value of C/Co indicating lower Cu removals. In fact, in the presence of organic matter, almost complete breakthrough is achieved for column A1, the first column in the treatment train. The reduced removal of copper could be due to the presence of organic matter complexation with the copper that reduces the extent of copper precipitation. Reductions in copper removal were also observed for the manganese oxide media as seen for Column B2 in Figure 5-19. In both cases, complete copper breakthrough occurred; however, the presence of the organic matter led to more rapid breakthrough again suggesting that Cu-organic complexation reduced the extent of adsorption. These results serve to emphasize that organic matter is an essential component of a representative synthetic storm water solution. While synthetic inorganic stormwater solutions allow Figure 5-17. FBR breakthrough profiles for Cu (top) and Zn (bottom) from a synthetic stormwater solution onto granular manganese oxide (Columns B1-B3 in series). The column diameter was 1 cm ID, flowrate îµ 8 ml/min, the influent copper concentration was 50 mg/L, and the influent Zn(II) concentration was 100 mg/L. The influent ionic strength was 0.01M. Three columns were connected in series with B1 being the first bed in the series.
70 Figure 5-18. Comparison of FBR breakthrough profiles for Cu from synthetic stormwater solution with and without reconstituted NOM. The columns were packed with granular iron oxide (Columns A1âA3 in series). The column diameter was 1 cm ID, flowrate îµ 8 ml/min, the influent copper concentration was 50 mg/L, and the influent Zn(II) concentration was 100 mg/L. The influent ionic strength was 0.01M and the TOC of the experiments with NOM was 17 mg/L. Figure 5-19. Comparison of FBR breakthrough profiles for Cu from synthetic stormwater solution with and without reconstituted NOM. The columns were packed with granular manganese oxide (Columns B1âB3 in series) but only data from Column B2 is shown for clarity. The column diameter was 1 cm ID, flowrate îµ 8 ml/min, the influent copper concentration was 50 mg/L, and the influent Zn(II) concentration was 100 mg/L. The influent ionic strength was 0.01M and the TOC of the experiments with NOM was 17 mg/L.
71 for the isolation and study of mechanisms affecting adsorptive behavior (i.e., pH, ionic strength, alkalinity), to accurately cap- ture the performance of the media systems under real condi- tions organic matter is indispensable. A plot of log KD vs. TOC for Trials 4, 8â11, and 13 is shown in Figure 5-20. Despite scat- tering due to differences in pH between runs, the relationship between log KD and TOC is clear. 5.6.5 Effects of pH and Ionic Strength on Cu(II) and Zn(II) Sorption The effects of pH were investigated in an iron oxide col- umn experiment in which the ionic strength was increased slightly to 0.011M through addition of 500 mg/L NaCl to the synthetic stormwater solution at the start of the experi- ment, and the pH was set to approximately pH 6 (stage 1). In stage 2, the influent pH was reduced from approximately pH 6 to pH 5 after the columns had achieved breakthrough or steady-state operation. The pH was then increased again to pH 6 in Stage 3, and then the influent ionic strength was reduced to 0.009M in Stage 4 of the experiment by omitting the additional 500 mg/L NaCl. The results from this experi- ment for the first column (A1) are presented in Figure 5-21, which shows the four different operating stages. As expected, the reduction in pH led to a decrease in copper removal. Moreover, as the pH was decreased, desorption of copper (and to a lesser extent Zn) led to C/Co values in excess of one. This dramatic increase in copper concentrations above Figure 5-20. Relationship between log KD and TOC for sorption of copper onto GFO (Trials 4, 8â11, and 13). Figure 5-21. Effect of changes in pH and chloride concentration in FBR breakthrough profiles for removal of Cu and Zn in the first column in series (A1). The columns were packed with granular iron oxide (Columns A1âA3 in series). The column diameter was 1 cm ID, flowrate îµ 8 ml/min, the influent copper concentration was 50 mg/L, and the influent Zn(II) concentration was 100 mg/L.
72 the influent concentration highlights the need to control pH in metal ion sorption treatment systems. In order to assess the effect of high ionic strength, an addi- tional test was conducted with synthetic stormwater of ionic strength on an order of magnitude higher (0.1M) than typical highway runoff (0.009M). To obtain the higher ionic strength, 5340 mg/L of NaCl were added to the synthetic stormwater solution in order to raise the ionic strength to 0.1M while the concentrations of other cations and anions were maintained consistent with previous tests. The test results, as shown in Figures 5-22 and 5-23, show that metal removal capacity appears to be somewhat lower in high ionic strength water. However, this small difference also can be attributed to the higher overall pH during the low ionic strength test as com- pared to the high ionic strength test (see Figure 5-24), and hence the difference is not necessarily a direct result of ionic strength differences. Considering the fact that the high ionic strength used in this test is highly unlikely in real conditions, it can be concluded that the impact of ionic strength is not as significant as the impact of pH or other variables in the design of heavy metal removal systems for stormwater. 5.6.6 Evaluation of pH Stabilizing Media The strong effect of pH on column performance suggests that a media that both increases and stabilizes column pH would yield improved performance in the field. Two differ- ent media were tested in this research to assess whether per- formance could be improved by adding pH stabilizing media to the front of the treatment system. Experiments were con- ducted by adding either crushed crab shell or concrete to the first two columns in the treatment train (Columns C1 and Figure 5-22. Effect of changes in ionic strength in FBR breakthrough profiles for removal of copper on granular iron hydroxide media. High I represents an ionic strength of 0.1M. Low I represents an ionic strength of 0.009M. The column diameter was 1 cm ID, flowrate îµ 8 ml/min, and the influent copper concentration was 50 mg/L.
73 C2). In the first set of experiments shown in Figures 5-25 and 5-26, crushed crab shell was placed in Columns C1 and C2, and granular manganese oxide was placed in Column C3 in the series. The addition of the crab shell particles ini- tially had a dramatic impact on increasing pH (Figure 5-25) and removal of zinc (Figure 5-26). In fact, the crab shell itself appears to outperform the manganese dioxide as no additional removal is observed in Column 3. Similar results were observed in experiments conducted in the absence of organic matter as shown by comparing the log KD values in Table 5-5. The addition of crab shell waste had less impact on copper removal in the column experiments. In the four stage experi- ment, described previously, in which the pH was varied during the course of the experiment, the presence of crushed crab shell in Phase I showed comparable removal of copper (see C2 in Fig- ure 5-27) compared to the single iron oxide column (A1). These results are evident in Table 5-5, by comparison of the log KD values of 4.91 for the column packed only with GFO to the log KD of 4.75 for the series of columns packed with crushed crab shell followed by GFO. Moreover, when the pH was decreased to pH 5, the impact of desorption was minimized in the C col- umn train compared to column trains packed entirely with iron oxide. Indeed, the average pH in the columns over the dura- tion of the pH 5 stage remained over pH 6. In the final stage of this experiment, the crab shell waste was replaced with concrete waste. While this led to an initial increase in pH (Figure 5-28) in all of the columns, which was accompanied by increased removal, over time the pH dropped and removal decreased to levels consistent with the crab shell packed columns. The spike in pH was likely due to the fact that the material was not washed prior to use. Regardless, it appears that the presence of a pH stabilizing material is beneficial in the design of a passive treat- ment system for metal ion removal. Another important point of interest is that the pH of the system increased for a short period of time after the flow was interrupted for more than 24 hours. This type of behavior may have significant impacts in field sce- narios and should be evaluated further. Figure 5-23. Effect of changes in ionic strength in FBR breakthrough profiles for removal of zinc on granular iron hydroxide media. High I represents an ionic strength of 0.1M. Low I represents an ionic strength of 0.009M. The column diameter was 1 cm ID, flowrate îµ 8 ml/min, and the influent Zn(II) concentration was 100 µg/L.
74 Figure 5-24. FBR pH data for Cu and Zn sorption onto granular iron hydroxide in two tests varying in the ionic strength of the synthetic stormwater solution. High I represents an ionic strength of 0.1M. Low I represents an ionic strength of 0.009M. A nitrogen blanket was used to control the influent pH during this experiment. Figure 5-25. pH profiles from FBR experiments with crushed crab shell placed in the first two columns (C1 and C2) and in final column (C3) packed with manganese dioxide. The column diameter was 1 cm ID, flowrate îµ 8 ml/min, influent copper concentration was 50 mg/L, and the influent Zn(II) concentration was 100 mg/L. The influent ionic strength was 0.01M and the TOC was 17 mg/L. 5.6.7 Experiments Conducted at pH Representative of Stormwater Experiments conducted at higher pH (between 6.8 and 7.0) demonstrate the potential of the iron oxide media for achieving the effluent criteria of 3.0 mg/L copper for a sus- tained period of time. The columns were operated in this pH range for 130,000 bed volumes. However, after approximately 40,000 bed volumes the influent pH began dropping due to the loss of the nitrogen blanket used to maintain the stor- age tank CO2 free. Nevertheless, the data in Figure 5-29 show that the effluent concentration from GFO Column C remains consistently low, suggesting that this media is capable of achieving effluent Cu concentrations that meet the water quality criteria under the low alkalinity conditions used in this research. While removal of Cu from the column packed
75 Figure 5-26. Zn(II) breakthrough profiles from FBR experiments with crushed crab shell placed in the first two columns (C1 and C2) in the final column in series (C3) packed with MnO2. The column diameter was 1 cm ID, flowrate îµ 8 ml/min, the influent copper concentration was 50 mg/L, and the influent Zn(II) concentration was 100 mg/L. The influent ionic strength was 0.01M and the TOC was 17 mg/L. Figure 5-27. Effect of changes in pH and ionic strength in FBR breakthrough profiles for removal of Cu in a series of columns packed with crushed crab shell (C1 and C2) and granular iron oxide (C3). The column diameter was 1 cm ID, flowrate îµ 8 ml/min, the influent copper concentration was 50 mg/L, and the influent Zn(II) concentration was 100 mg/L. The crab shell was replaced with unwashed concrete in the last stage of the experiment. Two periods of flow interruption are displayed in the plot.
76 Figure 5-28. pH profile in FBR breakthrough profiles for removal of Cu in a series of columns packed with crushed crab shell (C1 and C2) and granular iron oxide (C3). The column diameter was 1 cm ID, flowrate îµ 8 ml/ min, the influent copper concentration was 50 mg/L, and the influent Zn(II) concentration was 100 mg/L. The crab shell was replaced with unwashed concrete in the last stage of the experiment. with manganese dioxide also shows dramatic improvement at the higher pH, the results are still not as promising as the iron oxide media. In fact, the waste crab material out- performs this particular manganese dioxide in part because the average pH in the column is higher in the crab-shell/GFO columns. Performance of the media for zinc removal also improves at pH 7. Log KD values for zinc removal from the GFO column series and the waste crab/GFO column series were similar at pH 7 whereas at lower pH, zinc removal was significantly better in the columns packed with crab/GFO or crab/MnO2. Thus, the presence of the crab shell mainly contributes to zinc removal in lower pH systems. 5.6.8 Experiments Conducted with Real Stormwater Experiments were conducted utilizing actual stormwater in place of synthetic stormwater in order to evaluate perfor- mance under conditions closer to those that would be seen in the field. Highway runoff was collected immediately after a rain event from a site on Loop 360 in Austin, TX and was pre-filtered in order to remove particulate matter that would clog the FBR. The filtration process consisted of circulating the runoff through a nominal 0.45 mm cartridge filter (Pen- tek model 155403-75) for 12 hours. The pH, alkalinity, TSS, TOC, and heavy metal content of the highway runoff were characterized after filtration (Table 5-6). In this test, GFO was used as the sole sorbent media in conjunction with crab shell and bone meal as pH stabilizers. No attempt to control the influent pH was made so to more closely simulate field conditions. The results indicate that cop- per removal was not as successful when compared to the tests conducted previously with synthetic stormwater (Figure 5-30). This is likely attributable to the high alkalinity and pH of the runoff, since in this environment much of the copper is present in a relatively inert form. By simulation with Visual MINTEQ, it was determined that under the conditions found in the actual stormwater, over 80% of total copper exists as dissolved copper carbonate, which does not sorb to iron oxide to the same as free copper, and less than 3% of total copper is present in the form of cupric ion. Conversely, zinc removal was relatively more successful as compared to the tests utilizing synthetic storm- water (Figure 5-30). This can be explained by the high pH of the stormwater, which increases the tendency of zinc ion to sorb onto the surface of the oxide media. Figure 5-31 shows the relative effects of crab shell and bone meal on Cu and Zn adsorption from real stormwater onto GFO media. As can be seen in the plots, the breakthrough profiles are almost identical in both cases, suggesting that there is no significant difference between them in terms of improving heavy metal removal.
77 Figure 5-29. FBR breakthrough profiles for Cu from a synthetic stormwater solution onto granular iron hydroxide (Columns A1âA3 in series), MnO2 (Columns B1âB3 in series), and waste crab shell/waste crab shell/GFO columns in series (Columns C1âC3 in series, respectively). The column diameter was 1 cm ID, flowrate îµ 8 ml/min, the influent copper concentration was 50 mg/L, and the influent Zn(II) concentration was 100 mg/L. The influent ionic strength was 0.01M. Three columns were connected in series with A1 being the first bed in the series.
78 5.6.9 Experiments Conducted at Alkalinity Representative of Stormwater Experiments were conducted with a synthetic runoff solu- tion formulated to have an alkalinity similar to that observed in real stormwater in order to understand the breakthrough behavior observed with real stormwater. The alkalinity of the solution was raised to 2.15 meq/L by addition of NaHCO3 so to be comparable to the alkalinity of the real stormwater (2.4 meq/L). Otherwise, the solution was identical in composi- tion to the synthetic inorganic runoff solutions used in previ- ous experiments. The amounts of copper and zinc added in order to achieve 50 and 100 mg/L, respectively, in the influent were also the same as those used previously. In this test, as in the real stormwater test, GFO was used as the sole sorbent media in conjunction with crushed concrete. Since attempts to set the solution to a lower pH via addition of acid would simply have titrated away the desired alkalinity, the pH was left uncontrolled and rose steadily from 7.25 at the beginning of the run to about 7.65 at completion. Analysis revealed that influent copper and zinc concentra- tions were about five times lower than expected: 8.9 mg/L Cu and 28.5 mg/L Zn. A possible explanation for the apparent disappearance of copper and zinc from the influent solu- tion is that mixed calcium carbonate precipitates of copper and zinc formed due to elevated levels of calcium in the sys- tem, removing the metals from solution before they could Figure 5-30. Comparing FBR breakthrough profiles for copper and zinc removal between tests utilizing synthetic stormwater and actual stormwater. pH 7.78 Alkalinity (meq/L) 2.4 TSS (mg/L) 0.8 TOC (mg/L) 10.1 Cu conc. (µg/L) 36.0 Zn conc. (µg/L) 49.8 Fe conc. (µg/L) 129.4 Cr conc. (µg/L) 2.0 Table 5-6. Characterization of collected stormwater used in FBR study (post-filtration).
79 even enter the columns. Indeed, precipitates were visually observable in the feed tank. This explanation is supported by a column experiment performed subsequently in which magnesium chloride was substituted for calcium chloride in the synthetic runoff cocktail, allowing the same alkalinity and ionic strength to be achieved without the presence of calcium. In this case, the expected influent concentrations of 50 mg/L Cu and 100 mg/L Zn were measured, and no precipitates were visually observable in the solution. Despite low influent concentrations, sufficient data were col- lected in order to evaluate removal efficacy and capacity under these conditions. Comparing log KD values for copper between Trials 11, 13, and 14 (low CO3 at pH 7, real stormwater, and high CO3 at pH 7.5, respectively) and Trials 8-10 (with organic mat- ter) suggests a relationship between copper removal, organic matter, and carbonate concentration. Namely, log KD values for copper decrease both in the presence of organic matter and in the presence of high carbonate concentrations as compared to the inorganic, low carbonate case (Trial 11 vs. Trials 8â10 and Trial 14). Moreover, log KD values for copper are lower than any of the above trials in the case of real highway runoff, which contained elevated concentrations of both organic matter and carbonate. Hence, the inhibitory effects that NOM and carbon- ate each exert on adsorptive copper removal are additive. The effect of NOM can be attributed to complexation of copper ions from solution, and the effect of carbonate can be attributed to the complexation and potential formation of mixed copper precipitates, as seen in this experiment. Indeed, the copper breakthrough profile (Figure 5-32) shows essentially immedi- ate development of plateaus, suggesting that precipitation was a significant controlling process in this system. For zinc, slightly higher log KD values were observed with high carbonate solutions as compared to the low carbonate case (Trial 11). This relationship, where zinc removal out- performs copper removal, mirrors the behavior observed in the real highway runoff experiment, suggesting that high carbonate concentrations may not be inhibitory to zinc removal. As noted before, this may be explained simply by the fact that high pH increases the tendency of zinc to sorb to the media surface (Trial 14 pH was 7.5 while Trial 11 pH was 6.9) and the formation of aqueous zinc carbonate complexes is not as thermodynamically favorable as copper carbonate complexes. In conjunction, the results of Trials 8â10 and 13â14 indi- cate that synthetic stormwater experiments incorporating both NOM and carbonate most accurately represent the behavior of real stormwater. While an experiment incorporating all of these factors simultaneously was not performed, based on the data at hand this extrapolation is not unreasonable. A linear relationship exists between log KD and TOC, and although slightly weaker, relationship exists between log KD and alkalin- ity (Figure 5-33). The additive manner by which organic matter Figure 5-31. Comparing the effects of crab shell and bone meal on copper and zinc removal in a FBR.
80 Figure 5-32. FBR breakthrough profiles for copper and zinc from a synthetic stormwater solution high in carbonate concentration (Trial 14). 0.00 0.20 0.40 0.60 0.80 1.00 1.20 0 50 100 150 200 C/ C0 Time (hours) Copper Removal with High Carbonate C0 Column 1 (GFO) Column 2 (Concrete) Column 3 (GFOx2cm) Column 4 (GFOx2cm) 0.00 0.20 0.40 0.60 0.80 1.00 1.20 0 50 100 150 200 C/ C0 Time (hours) Zinc Removal with High Carbonate C0 Column 1 (GFO) Column 2 (Concrete) Column 3 (GFOx2cm) Column 4 (GFOx2cm) Figure 5-33. Relationship between log KD and alkalinity for sorption of copper onto GFO (Trials 2, 11, 13, and 14).
81 and carbonate were observed to affect copper sorption onto GFO (as in Trial 13) follows from the demonstrated linearity of the individual relationships. Based on these results, it is our recommendation that both NOM and carbonate be included as components in future synthetic stormwater solutions to accurately simulate dissolved metal sorption in the laboratory. 5.7 Application of Results for Field Scale Design Most highway runoff waters are characterized by rela- tively low alkalinity and pH between 6.5 and 8.0. The results of the experimental testing suggest that iron oxide media is capable of achieving copper effluent concentrations of 3 mg/L or less for extended periods of time in the column reactors. The results also indicate that the use of a mixed oxide system containing concrete or crab shell can achieve similar, if not better, results for copper without raising the pH of the efflu- ent. The mixed media system with iron oxide also provided better removal of zinc. The data from experiments conducted using the protocol described earlier can be used to guide the design of field scale treatment systems by assisting in select- ing appropriate media and by estimating the capacity of the media. In addition, modeling approaches can be used to assist in scaling from the bench scale system to the field. In this research, iron oxide was identified as the most prom- ising media. The adsorption capacity of the iron oxides tested in this work was evaluated from batch and column data. The batch reactor experiments suggested that at the low effluent concentrations desired for the field system, the adsorption iso- therms were linear. For copper and zinc, log KD values obtained from the column experiments for GFO at circumneutral pH were 5.2 and 4.3, respectively. At this pH, the use of a mixed media system in which the first two columns in the series con- tained crab shell waste (containing calcite or chitosan) did not improve removal of either copper or manganese. The impact of organic matter may reduce the capacity of the system, but as shown in this research the impact may be minimal if pH is controlled near circumneutral values. Finally, the results also suggest that adsorption to the oxide media is rapid, and at flow rates typical of a highway runoff filtration system rates of adsorption should not limit the adsorption process. The KD values obtained at pH 7 can be used to provide an estimate of the capacity of the full-scale system for cop- per removal from highway runoff of similar composition and to assess the economic feasibility of the material. KD repre- sents the mass of contaminant removed per mass of adsor- bent. Therefore, for a given amount of copper to be removed from the stormwater, the required mass of media can be calcu- lated. This process is described for copper removal by the GFO iron oxide media used in this research. At pH 7, the estimated log KD for this system is conserva- tively assumed to be 5. In addition, the assumption of 30 in./yr of rainfall over a one acre drainage area yields a volume of stormwater runoff of approximately 3,000 m3 per year. For an influent copper concentration in the stormwater runoff of 10 mg/L and desired effluent concentration of 3 mg/L, the required mass of copper adsorbed per year is approximately 22 g. Using the linear isotherm equation: =q K Ce D e and a value of 3 mg/L for Ce and a KD of 105 L/kg, yields a required adsorption capacity of 300 mg removed/kg of GFO. Dividing the mg of copper adsorbed per year by 300 mg removed/kg of GFO yields a GFO demand of 72 kg/yr (150 lbs/yr) or 1,600 lbs over a 10-year design period. The reported bulk density of GFO and E33 are 0.45 g/cm3, which means that 720 kg of media 1.5 m3 of volume. Assuming a design depth of 45 cm indicates that 1.5 m3 of media are required for the 10-year design period. The cost for oxide media ranges from $4 to $10/lb with GFH at the lower end of the price range compared to E33 or GFO media. Thus, the media costs for the 10-year design period range between $6,400 to $16,000/acre. This research also highlighted the benefits of adding a media to help control pH and increase removal of zinc. Both of the materials used in this research are waste materials. Either waste concrete or crab shell added to the system would provide pH control as well as adsorptive capacity. For example, the design of a system containing crushed crab shell could be conducted by using one-third of the bed for the waste material. As shown in Figure 5-29 this would not impact copper removal signifi- cantly. The bulk density of the crushed concrete (0.54 g/cm3) is comparable to the bulk density of the E33, so the bed design would be similar. In addition, the cost of crushed concrete is significantly lower than the cost of iron oxide media and would represent a significant savings. More detailed design of the full-scale system can be deter- mined through modeling exercises. Based on the results of this research, the application of site-specific models that incorporate the retardation factors observed in the experi- ments conducted using the protocol described in this report may be most appropriate. More complex models such as SCMs and ligand binding models can be incorporated into transport codes and are useful for evaluating the impact of various parameters on adsorption; however, the results of this research suggested that pH control and assessment of organic matter and carbonate complexation are the most important operating parameter for stable operation of a treatment system. One model that is relatively simplistic and user-friendly is CXTFIT/EXCEL (Tang et al. 2010). The original form of the
82 model was developed for tracer experimental data analy- sis to investigate transport of solutes in the groundwater systems. The model optimizes parameters for equilibrium or non-equilibrium advection-dispersion-reaction models. It uses an ordinary non-linear least squares method based on the assumption of normally distributed error and equal variance. Figure 5-34 provides an example of the model output for the removal of zinc from column A3 in which Columns A1, A2, and A3 were packed with iron oxide and the influent zinc concentration was 100 mg/L. The disper- sion coefficient and retardation factor were the only fitting parameters in the model. The same dispersion coefficient was used for copper removal and then data for copper were fit by optimizing the value of the retardation coefficient as Figure 5-34. Example CXTFIT/EXCEL equilibrium advection-dispersion- retardation model calibration of zinc breakthrough profile of column A3 in a bed packed with granular iron oxide (Columns A1âA3 in series). The column diameter was 1 cm ID, flowrate îµ 8 ml/ min, the influent copper concentration was 50 mg/L, and the influent Zn(II) concentration was 100 mg/L. The influent ionic strength was 0.01M. Figure 5-35. Example CXTFIT/EXCEL equilibrium advection-dispersion- retardation model calibration of copper breakthrough profile of column A3 in a bed packed with granular iron oxide (Columns A1âA3 in series). The column diameter was 1 cm ID, flowrate îµ 8 ml/ min, the influent copper concentration was 50 mg/L, and the influent Zn(II) concentration was 100 mg/L. The influent ionic strength was 0.01M. shown in Figure 5-35. More sophisticated analysis would include a reaction term to account for precipitation in the column. This model can be used in a predictive mode by adjusting the hydrodynamic parameters associated with the field scale conditions which will be evaluated in future phases of research. SCMs can also be used to adapt the design sorption capac- ities to changing water quality conditions. Visual MINTEQ is a geochemistry code that incorporates ligand binding, sur- face complexation, and aqueous complexation using stan- dard models. Moreover, Visual MINTEQ includes databases for surface complexation using the DLM for hydrous ferric oxide and hydrous manganese oxide. Thus, water quality conditions can be input to the model and the inherent data- base can be used to assess changes in sorption capacities for the specific metal ions.
