3
The Promise and Perils of Animal Models1
___________________
1 Nicholas Katsanis of the Duke University Medical Center spoke during this session, but due to technical problems, his presentation is not summarized here. His presentation is available at http://nas-sites.org/ilar-roundtable/roundtable-activities/precision-medicine-workshop/webcast.
MOUSE MODELS
Introduction to Model Organisms
David Valle introduced workshop participants to the five most commonly used model organisms for research: Escherichia coli (bacterium), Saccharomyces cerevisiae (brewer’s yeast), Caenorhabditis elegans (roundworm), Drosophila melanogaster (common fruit fly), and Mus musculus (house mouse). Valle stressed that each of these organisms has unique features and that researchers should choose the model that is most appropriate to answer the research question at hand.
Valle said that there are three main ways that animal models—mice in particular—can be used to advance the purposes of individualized medicine. First, animals can be used to confirm causation. When researchers perform genomic sequencing to find the genetic variant responsible for a phenotype, there is often a short list of prospective variants. Animal models can quickly confirm which variant, or combination of variants, is relevant to the phenotype. Second, animal models can serve as experimental systems for understanding pathophysiology. Finally, animal models can act as surrogates for treatment studies, particularly studies that may be difficult or time consuming to test in humans.
Confirming Causation
As an example of using mouse models to confirm causation, Valle told participants about the Centers for Mendelian Genomics. These four centers are seeking to identify all genes that have high-penetrance variants (i.e., genetic variants that often result in expression of the associated phenotype). Researchers recruit families or cohorts with identified and unexplained phenotypes, and collect genetic information. Using family relationships, allele frequency data, functional predictions, model organism results, and functional studies, researchers are able to identify the genes and variants responsible for the phenotypes. Valle said that over the last 5 years, the Centers for Mendelian Genomics have identified around 1,000 novel disease genes and have also expanded the phenotypic spectrum of known
disorders. In some instances, these genes are high-quality candidates, but in others, animal models were needed to confirm the relationship between gene variant and phenotype. However, he noted that there is a long way to go to identify every gene variant and explain every currently unexplained phenotype. Valle noted that other species can also be used to study candidate genes; a zebrafish model has been used to research bicuspid aortic valve, which is a genetically heterogeneous disorder that occurs in 1-2% of the general population.
Valle cautioned that the common approach of simply knocking out a candidate gene in a mouse model runs the risk of missing the nuances of biology and causation. He said that, while many disease genes are associated with only one phenotype, some genes have many discrete phenotypes, depending on the specific allele. He gave the example of two patients who have very different phenotypes but who have a mutation on the same gene. To fully understand this allelic heterogeneity, it is essential to insert specific allele variants into animal models and observe their effect on the phenotype.
Understanding Pathophysiology
Animal models can help contribute to understanding the pathophysiology of a disease. One example, said Valle, is in research on Marfan syndrome. The gene responsible for Marfan syndrome—FBN1—was identified in 1991. In the years since this identification, researchers have been able to determine that the mutation on FBN1 causes a disruption of the extracellular matrix with elevated levels of free TGFβ, which then affects the elasticity and function of tissues. These discoveries explain many of the phenotypic characteristics of Marfan syndrome and point toward TGFβ antagonist as an appropriate therapy.
Surrogates for Treatment Studies
Valle told workshop participants about a powerful example of using animal models as surrogates for potential treatments. A rare disease called gyrate atrophy, caused by mutations in the OAT gene, results in retinal degeneration, due to abnormal levels of ornithine accumulation caused by deficiency of the enzyme ornithine aminotransferase. Researchers speculated that an arginine-restricted diet would lower the levels of ornithine, but because patients differed significantly in terms of age, stage of degeneration, compliance with diet, and allelic variations, it was difficult to conduct a controlled study over the many years that it would take to show an effect. Using mouse models allowed researchers to have complete control over the diet and to observe retinal degeneration at a much faster pace. Despite differences in eye structure between mice and humans, researchers were able
to show that an arginine-restricted diet was therapeutically beneficial for this disorder.
International Mouse Phenotyping Consortium
Steve Brown, director of the Mammalian Genetics Unit, MRC Harwell, United Kingdom, discussed the International Mouse Phenotyping Consortium (IMPC) and its importance to precision medicine. The IMPC involves 21 research centers that are collaborating to solve a key problem in genomics: the lack of knowledge about the function of the majority of genes. Knockout models have been generated and analyzed for only a fraction of mouse genes, and data for these genes is patchy, dependent on the interests and experience of the investigator. Pleiotropy—in which one single gene produces two or more phenotypic traits—is particularly poorly understood, said Brown. Pleiotropy is manifest across a whole range of genetic phenomena, including variable expressivity and phenotypic expansion. More data about pleiotropy will be essential to understand human phenotypes and to ensure that animal models are appropriate comparators for human disease states.
To address these gaps in knowledge, IMPC is seeking to generate and comprehensively phenotype a mouse mutant for every single gene in the mouse genome. These phenotype data are uploaded from IMPC centers to the IMPC Data Coordination Centre, where they are analyzed to determine gene function, to identify disease models, and to build a deep and pleiotropic view of gene function. All of the data, as well as the mouse models themselves, are open source and available for researchers to use. For example, programs collecting data from vast numbers of patients—such as the 100,000 Genomes project—can use the IMPC data to provide information on gene function, to validate potential gene–phenotype relationships, and to perform synergistic analyses with the multidimensional genetic and phenotypic data that are collected.
Brown told workshop participants that all mice in the IMPC are coisogenic and bred on a black 6N background to reduce unwanted sources of variation. Many of the allele mutants are now generated using CRISPR/Cas-9, which is faster and cheaper. Each mutant allele is studied at each phase of life, each mutation is assessed for fertility and viability, and gross morphology and histopathology of both mutant embryos and placenta are performed. Mutant adult mice are assessed for gross pathology, whole body plethysmography, body composition, and morphology and histopathology. All of the data are validated, checked for quality, and analyzed before being placed in the core data archive.
IMPC has developed nearly 7,000 genotype-confirmed lines thus far, approximately one-third of the mouse genome. More than 50 million data
points have been collected, nearly 400,000 images have been stored, and phenotype data from nearly 5,000 phenotyped lines are available. The work of the IMPC has resulted in a number of novel insights into the mammalian genome, said Brown, including the following:
- Extensive new collection of disease models, new candidate disease genes, and new functional knowledge
- Novel insights into gene function (e.g., metabolism, deafness)
- Revelations about pervasive sexual dimorphism
- Opportunities for the identification of new gene and phenotype to elicit novel biological mechanisms
- Insights into human disease from the analysis of mouse lethal (essential) genes
The IMPC project is a substantive step toward a comprehensive catalog of mammalian gene function, Brown said. These large-scale and multidimensional data will facilitate a new era of comparative genomics that will transform the opportunities for rare disease and precision medicine initiatives, particularly with respect to the pleiotropic nature of genes. Phase II of the IMPC is now under way, said Brown, with a new focus on the identification and characterization of late-adult phenotypes, which will bring new insights to the study of late-onset disease. By early 2018, IMPC expects to have delivered mutants and phenotypes for one-third of the coding genome.
Essential Genes
Mary Dickinson, professor and Kyle and Josephine Morrow Endowed Chair and associate dean for research at Baylor College of Medicine, picked up on Brown’s presentation regarding the IMPC’s phenotyping efforts to explain that, as part of this effort, her research focuses on identifying and phenotyping embryonic lethal genes (i.e., genes that are essential). Dickinson explained that, while there are many different ways to define “essential genes,” she defines them as genes that are needed to produce a viable animal that survives to birth and weaning. By creating standardized lethal phenotyping pipelines, researchers have the opportunity to investigate development in different stages and to identify which genes are critical to survival at these stages. The pipeline is divided into four different embryonic stages, measured by embryonic days: E9.5, E12.5, E15.5, and E18.5. Each stage offers insight into different types of defects and causes of death:
E9.5: Pre-implantation, gastrulation, organization of the body plan, heart formation
E12.5: Heart function, placenta formation, circulation
E15.5 and E18.5: Organogenesis, heart failure
Dickinson has found that the largest percentage of mutated mice die prior to E9.5 (nearly half). The second largest window of lethality is after E18.5; Dickinson said that many mice are perfectly healthy at E18.5 but die shortly before or after birth. The phenotype information often helps to identify the reasons the mice die, said Dickinson, but in some cases the reasons remain completely unknown.
Fully understanding the defects of these embryos is valuable for identifying mechanistic relationships between genes and development, and understanding potential implications for human disease, said Dickinson. One tool for fuller understanding of the phenotypes of these animals is 3D high-resolution micro computed tomographic (microCT) scanning. Traditional sectioning of each embryo would be time intensive and cost prohibitive, said Dickinson, so using the microCT (using an iodine-based dye that creates variable contrast reflecting the density of different tissues) allows the researchers to capture histology-level data from entire embryos and to look for structural phenotypes that, in digital format, can be shared with the research community for the data to be interrogated in different ways. These images from the embryonic pipeline research are made available to the public through a data-coordination center. “The legacy of these data will be realized many, many years from now,” said Dickinson. An additional tool used for this research is automated phenotyping, such as automated volumetric analysis, which can facilitate identification and quantitation of phenotypes that may not be obvious to the person reading the data.
Dickinson told workshop participants about several interesting phenotypes that have been found. First, a gene called Tmem132; mutations in this gene cause embryos to be smaller, with limb abnormalities and spina bifida, and internal imaging shows abnormalities including kidney agenesis or duplications in kidney tissue. The second interesting find was a gene called SMDT1, which is a gene of unknown function. Mutant embryos showed excessive brain foliation and mysteriously died shortly after E12.5. Dickinson said there is no visible structural defect in the heart, and that they are not exactly sure what is responsible for the lethality.
In 2016, Dickinson and her colleagues published the first analysis of their research on essential genes (Dickinson et al., 2016). This report is based on the first 1,751 knockouts produced by the IMPC; 410 (23%) of these genes were found to be lethal and 198 (11%) subviable. Dickinson noted that, while the research has expanded to 4,245 knockout lines, the percentages of lethal, subviable, and viable genes have remained remarkably
stable. In looking at how these essential genes in mice may be relevant to human health, Dickinson said that, of 399 lethal murine genes, 126 have human homologs with known disease associations. At the time of the publication, 52 of these constituted mouse models were reported for the first time, demonstrating the importance of this project. Comparisons of human and mouse essential genes have shown a strong correlation, but much work is still needed to determine any overlap. Dickinson further noted that, when comparing essential genes, differences between humans and mice are quite relevant. One such difference is that mice can die at birth for a variety of reasons that would be treatable or preventable in humans; for example, mice born with a cleft palate are not treated in a neonatal intensive care unit. Another difference is that the human genome is more robust. Genes that cause lethality in mice may just cause loss-of-function in adult humans. Dickinson explained that this could be due to redundancy or compensation by other genes in the human genome. Dickinson stressed that, despite these differences, the null mouse alleles are key to understanding disease mechanisms.
One interest within Dickinson’s group is the cardiovascular development and associated required genes. To study this area more in depth, Dickinson’s team developed advanced microCT methods to image the embryo with the yolk sac and placenta intact. These techniques were used to look at embryos with mutations in Alg10B, which are lethal between E9.5 and E12.5. The mouse phenotypes showed abnormal vascular development and other defects often indicative of impaired cardiac function. In humans, this gene is associated with a reduced susceptibility to a drug-induced long QT syndrome, meaning it still affects cardiovascular function. Dickinson said that these types of scenarios—in which mutations manifest differently but within the same system—present opportunities to look at very precise disease-related alleles and compare them directly to null information.
Precision Mouse Models
Robert Burgess, principal investigator of the Jackson Center for Precision Genetics, started his presentation by asking, “What are we modeling precisely?” There are three different ways in which an animal can model a human phenotype or disease: face validity, construct validity, and predictive validity. Face validity means that the model looks right—it has the same phenotype and presentation of disease seen in humans. Construct validity means that the phenotype is appearing for the same reason that it is appearing in the human patient, for example, because of the same genetic mutation. Burgess noted that face validity and construct validity do not necessarily go hand in hand, and the relative importance of each may have to be balanced. For example, research to develop a treatment based
on pathophysiology may need better face validity, while a treatment that is based on the core mechanism of the disease might need better construct validity. Predictive validity—where results in the model translate into results in human patients—is the real goal of modeling, concluded Burgess, though he cautioned that even the best model has its limitations.
Burgess discussed the challenges in developing appropriate animal models, using his work on Charcot-Marie-Tooth (CMT) disease as an example. CMT is not a single disease but a collection of related diseases that result in peripheral neuropathy. CMT is rare, affecting around 1 in 2,500 people, and there are likely 100 different loci in the human genome that can cause CMT. As a consequence, each individual form of CMT is extremely rare. Burgess said that creating animal models for CMT is essential, as vitro models are challenging due to the complexity of this adult-onset degenerative disease. Burgess gave two examples of mouse models for CMT, though he noted that models have also been created in other organisms, including rat, zebrafish, and drosophila. Burgess discussed models developed for two versions of CMT: CMT 4D and CMT 2D.
CMT 4C is a recessive demyelinating neuropathy caused by mutations in SH3TC2. Mouse models have been developed that re-create the phenotype and loss of function seen in humans. Burgess noted that these models can recapitulate the phenotype with either a targeted knockout or a spontaneous mutation of the gene, and that because there are no treatments for this disease, predictive validity is not relevant at this point. CMT 2D is a dominant axonal neuropathy caused by mutations in the GARS gene. This pathogenesis is either related to a dominant negative or a neomorphic activity causing the mutant protein to take on a new pathological function, Burgess said. Researchers have discovered two alleles that create mice with good face and construct validity for the disease.
Based on the success of modeling the GARS mutation in mice, researchers sought to validate the pathogenicity of a de novo mutation that had been identified in a 4-year-old girl who suffered from a severe motor neuropathy and whose whole exome sequencing had identified a 12-base pair deletion in GARS. Researchers wanted to validate this mutation through mouse modeling and were also hoping to develop a gene therapy approach toward treatment. The patient mutation was introduced into the mouse genome using CRISPR/Cas-9, and a control model with the wildtype sequence was also created. Researchers found that the patient mutation did cause a dominant axonal neuropathy in the models, while the control mice were normal. Based on previous studies, researchers knew that transgenic expression of wildtype GARS did not suppress the neuropathy, so they sought to decrease the expression of the mutant form of the protein. Allele-specific knock-downs of interfering RNA (RNAi) were delivered using adeno-associated virus 9 (AAV9), an approach that showed benefit even
post-onset of the neuropathy in multiple mouse models, including the one with the human-patient mutation. Burgess noted that, while the molecular mechanism is still not entirely understood, the treatment (the only one available to date) appears to work. He added that the treatment requires testing multiple RNAi sequences for every different allele associated with the disease, and that gene therapy delivered through AAV9 is still somewhat of an experimental delivery system.
Burgess briefly discussed the rigorousness of these preclinical studies. The researchers tried to perform well-powered studies with a sufficient number of mice and used blinded analysis to the extent possible. They developed multimodal clinically relevant outcome measures including behavioral, electrophysiological, and histological measures. Key biological variables, such as age, were considered, since CMT is a degenerative disease. Finally, the therapy was tested in more than one model with more than one mutation of GARS.
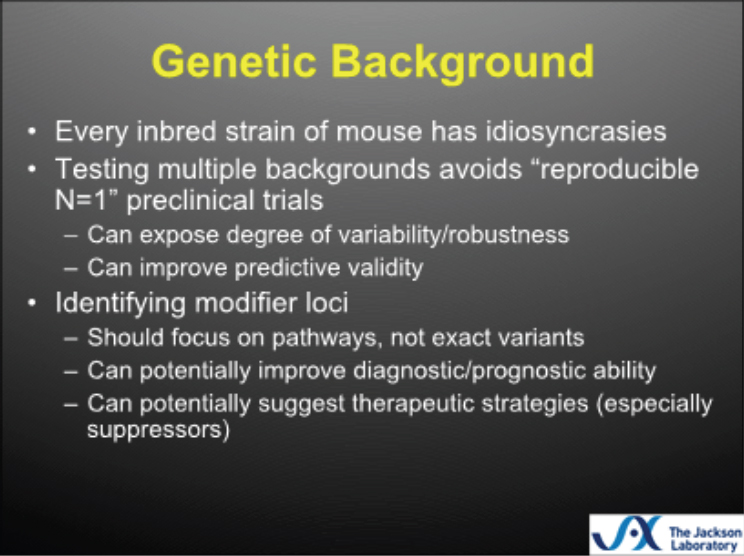
Burgess concluded with a discussion about the importance of the genetic background of mouse models (see Figure 3-1). He noted that every
SOURCE: Burgess, slide 13.
SOURCE: Burgess, slide 14.
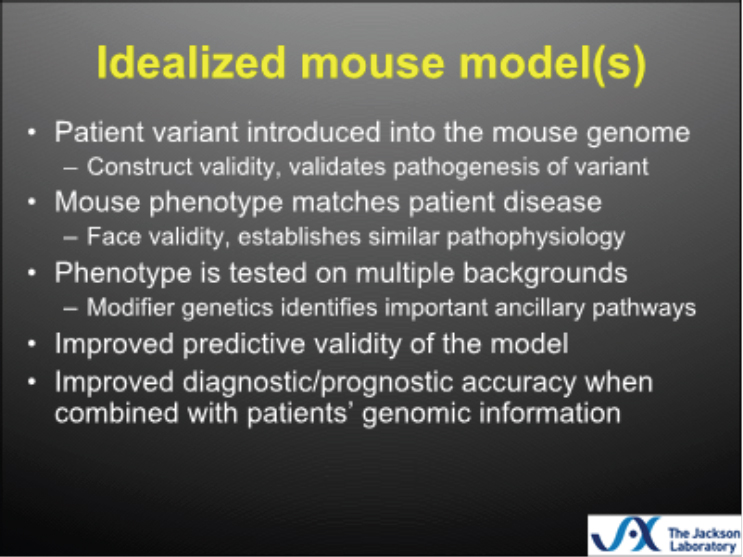
inbred murine strain has idiosyncrasies, and that testing on multiple backgrounds can help expose the potential degree of variability in response to treatment, can reveal how robust the treatment is, and can improve predictive validity. He said that testing 1,000 C57 black 6 mice is similar to testing 1 C57 black 6 mouse 1,000 times, making it difficult to extrapolate findings. He again emphasized the importance of seeking predictive validity, rather than exact face or construct validity, noting that the precise mutation in the mouse is unlikely to be the same as that in the human, but that a similar mechanistic pathway is the key to translating findings from the animal model into patient therapies. An idealized mouse model (see Figure 3-2), said Burgess, would be one in which the patient variant introduced into the mouse resulted in face validity and construct validity, the phenotype was tested on multiple backgrounds, and the model had good predictive validity and could improve patient care.
UNIQUE ANIMAL-BASED APPROACHES
Opossum
Jennifer Maier, postdoctoral research associate at the University of California, Los Angeles, introduced workshop participants to a novel species for animal-based research. Monodelphis domestica—otherwise known as the opossum—is a small, pouchless marsupial native to South America. Maier noted that this opossum is a different species than Didelphis virginiana, the opossum common to the United States. The monodelphis opossum breeds year-round, with litters of up to 13 pups. It is a genetically diverse, U.S. Department of Agriculture (USDA)-regulated species that is easy to care for, requiring a standard rat cage with nesting and bedding material, and commercially available opossum chow. However, being a nonsocial species, these animals cannot be group-housed after a certain age.
Opossums have some advantages over mice, including a shorter gestation period (14 days versus 21), slow development, and the fact that there are some human orthologs present in opossums but absent in mice. The pups are born at a stage that is equivalent to a 10.5-12.5-day-old mouse embryo, take 2 months to wean, and because the opossum does not have a pouch, the pups are exposed during this time and can be observed and manipulated, said Maier. Compared to mice, opossums take 8 months to reach full maturity and live about 3 years in captivity. The unique biological characteristics of opossums make them a relevant model for studying certain diseases; for example, they have a slower metabolic rate and a lower body temperature than placental mammals.
Maier said that the opossum research community is growing, and that many of the techniques that are used in mice can be adapted to opossums. There are a number of resources available for monodelphis research, including a well-annotated genome, husbandry guides, transcriptome libraries, a well-established embryology guide, and the OpossumBase website, which contains genetic and genomic data for the species.
The research that Maier presented focused on the development and evolution of the limbs. She noted that mammalian limbs have a huge morphological diversity, from the wings of a bat to the pectoral fin of a dolphin to the arm of a human. Research on the limb system is well established, particularly in animals such as the mouse and the chicken, and many of the required genes are known and conserved in the opossum. Congenital limb malformations are quite common in humans, caused by genetic mutations or environmental triggers.
One area of research in Maier’s lab focuses on the effects of retinoic acid on opossum limb and craniofacial development. Retinoic acid is a derivative of vitamin A that is contained in some pharmaceuticals, including
acne treatments. Pregnant opossums were treated orally with retinoic acid and the embryos were collected just before birth. All of the animals that were treated with retinoic acid had missing digits (oligodactyly), and some sort of craniofacial defect. Researchers determined that these malformations were associated with disruption of the expression of fibroblast growth factor in the two major limb-signaling centers (the apical ectodermal ridge and the zone of polarizing activity).
Another area of opossum research involves thalidomide, a well-known teratogen that caused thousands of children to be born with limb, craniofacial, and vascular deformities in the mid-1950s. Mice and rats are not susceptible to thalidomide, making it difficult to study in traditional animal models. Research in which pregnant opossums were injected with thalidomide revealed that the animal is susceptible to the teratogen, with high penetrance of mild deformation and recapitulation of several more severe human thalidomide phenotypes. Opossums exposed to thalidomide gave birth to pups with defects of the heart, blood vessels, limbs, amniotic sac, and craniofacial area. Research is under way for molecular characterization of these phenotypes, said Maier.
In addition to these areas of research on limb formation, monodelphis is also currently being used to study the formation of the mammalian middle ear and intervertebral discs, spinal regeneration, ultraviolet-induced melanoma, and diet-induced hyperlipidemia. Despite the benefits of using monodelphis as a model organism, there are also some drawbacks, Maier said. One major challenge is the absence of techniques to create knockout or other genetically modified opossums, which complicates functional testing. Maier’s lab has received a grant to develop a method for making a transgenic opossum, and the lab is currently studying a method in which spermatogonial stem cells are cultured, expanded, and modified in vitro before being transplanted into testes. In conclusion, Maier said that opossums are an excellent model for biomedical and evolutionary questions, and that advances in genetic modification of the species will make the opossum an even more useful species for research.
Precision Pathology
Keith Mansfield, director of Discovery and Investigative Pathology at Novartis Institutes for Biomedical Research, spoke to workshop participants about using molecular pathology to evaluate animal models for precision disease modeling. He started with a basic definition of molecular pathology, describing it as focused on the study and diagnosis of disease through the examination of molecules (generally DNA, RNA, and protein) within organs, tissues, or bodily fluids. Molecular pathology is multidisciplinary in nature, integrating genomics, genetics, proteomics, and
physiology with morphology, and it utilizes a number of tools, including immunohistochemistry and in situ hybridization. Mansfield said that molecular pathology is used to delineate the molecular basis of morphological or structural changes in tissues and how those changes are related to functional alterations. Molecular pathology is frequently used in the clinical diagnosis and management of cancer patients and is one of the foundations of precision medicine. Mansfield’s presentation focused on describing a few of the main molecular pathology techniques and tools and how they may relate to precision medicine.
One key technique in molecular pathology is molecular localization, the ability to define the spatial distribution of molecules, such as proteins or nucleic acids, in tissue sections. Molecular localization allows the pathologist to understand how alterations in the spatial orientation of these molecules relate to functional deficits in the organism. Molecular localization can be performed using tools such as immunohistochemistry or in situ hybridization.
Immunohistochemistry is a technique that uses antibodies—usually linked to an enzyme or a dye—to test for disease markers in a tissue sample. Mansfield said that immunohistochemistry has advanced enormously over the past decade; automated staining platforms have increased the efficiency and reproducibility of the assay, reducing the length of the process from 12 to 1-2 hours. However, he noted that a shortcoming of these assays is the quality of commercial antibodies, and the resulting difficulty in validating an assay before use in the clinic or an experiment. There is no single gold standard to validate an immunohistochemistry assay, said Mansfield. In his experience, most commercially available antibodies are not specific or sensitive for the target. Traditional immunohistochemistry assays can be used to simultaneously analyze 5-6 antibodies. However, new platforms—such as image-amassed cytometry, or laser ablation inductively coupled plasma amassed imaging—are highly multiplexed assays, allowing the analysis of up to 20 or 30 markers simultaneously. Mansfield noted that these technologies are not yet ready for routine use, but they have a lot of potential utility.
In situ hybridization allows pathologists to identify specific chromosomes in tissue through the hybridization (attachment) of probes that have been linked to a dye. Like immunohistochemistry, automated staining platforms have made in situ hybridization more efficient and reproducible. A new generation of probes has improved sensitivity and specificity, said Mansfield, because they can detect splice variants and single-nucleotide polymorphisms (SNPs).
Biobanks are a sometimes forgotten but critical resource in molecular pathology, said Mansfield. Biobanks that contain well-curated collections of normal and diseased human biological samples, in combination with
samples from animal models, can help researchers determine the similarities and differences in morphology and molecular alterations between humans and animal models. This side-by-side comparison can determine the comparative relevance as well as the potential limitations of animal models and inform the selection of models that most closely parallel the aspects of the disease under investigation. Such careful selection of animal models can reduce the number of animals needed to complete an experiment. Mansfield gave an example of the importance of choosing the right animal model: in comparative molecular pathology research of pancreatic adenocarcinoma, researchers found that there were significant differences in the morphology between human and mouse tumor cells. Mansfield noted that, in this case, genetically engineered organoids were better at reproducing the morphology than mouse models. Mansfield stressed that these differences between human and animal models do not mean that animal models are not useful, but that we need to understand how differences in morphology may impact the relevance of the disease in modeling human cancer.
Digital pathology has advanced the field of molecular pathology in significant ways, said Mansfield. In traditional pathology, tissue samples are collected, processed, stained, and delivered to the pathologist who looks at them through a microscope and writes a report. The slides are archived, but there is little ability to access information contained in the slide or in the report at a later date. In contrast, in digital pathology slides are scanned and stored in a database along with all associated metadata. This allows pathologists to review slides and data at any time and to analyze morphological diagnoses along with patient metadata in order to discover novel patterns associated with diseases. In addition, digital pathology facilitates whole-slide image analysis, allowing a pathologist to quantitatively measure things such as staining intensities, cell number, cell morphology, and the spatial relationship between cells.
Tissue microarrays—in which one slide contains samples from many cases—is another advance in molecular pathology. Tissue microarrays can hold 100 or more samples on a single slide, and custom arrays can be created that focus on a single species, organ, or disease process. Mansfield noted that the commercially available tissue microarrays are inconsistent in quality, so he finds internal creation of the assays to be beneficial. The automated platforms for immunohistochemistry and in situ hybridization can be used for these tissue microarrays, and thousands of samples can be analyzed in a matter of hours. Image analysis software can be used to assess and measure the images that are generated with this high-throughput analysis.
Other new technologies in molecular pathology include spatial transcriptomics and genomic expression profiling. Spatial transcriptomics allows the pathologist to profile several thousand genes within a single slide,
and to resolve the spatial orientation of changes within that gene expression. Genomic expression profiling can be coupled with morphological interpretation of tissue to assist in elucidating the pathogenesis of disease. Paired samples are routinely taken for genomic profiling and processing for histological analysis, said Mansfield. The analysis involved in genomic expression profiling is complex because changes in overall gene expression may result from alterations in individual cells as well as from changes in the cellular composition of tissue. Molecular localization studies can be used to confirm expression changes and cellular source.
Mansfield concluded that molecular pathology advances our understanding of comparative pathogenesis of human disease in relevant animal models. He said that molecular pathology is a rapidly advancing field that integrates traditionally anatomical pathology skills with molecular biology and bioinformatic approaches. Mansfield stressed that the interrogation of tissues from precision animal models should be made in conjunction with the evaluation of human disease tissues in order to better understand the models’ relevance and limitations. Future advances in the field of molecular pathology will build on the use of bioinformatics, particularly highly multiplex localization assays and computational interrogation of digital slide databases.
Mouse Hospital Co-Clinical Trials
John Clohessy, director of the Mouse Hospital/Preclinical Murine Pharmacogenetics Facility at Beth Israel Deaconess Medical Center and Harvard Medical School, presented a unique and promising approach in animal-based research for precision medicine. The “mouse hospital” model allows clinical trials to be held simultaneously with mice and humans, enabling rapid, real-time transfer of information from mouse experiments to human trials. For these trials, appropriate genetically engineered mouse models (GEMMs) are identified based on tumor genetics to act as surrogates for the human patient, or patient tumors are engrafted in immunodeficient mice to create patient-derived xenograft (PDX) models. The mouse models are used to test sensitivity of tumors to treatments, and real-time integration of these data is used to inform patient treatment protocols and improve patient outcome.
Clohessy explained that the genesis of the mouse hospital model came out of an acknowledgment that every patient has a unique disease in terms of its molecular characterization and its progression and response to treatments. The traditional drug development process does not reflect this, while also suffering from a number of shortcomings. Traditionally, diseases and cancer types are classified based on antiquated nomenclature and histopathology, said Clohessy, rather than the tumors’ molecular profiles. Drugs
are evaluated based on statistical trends in patient response, with response rates as low as 20% often deemed adequate for approval. A low response rate does not necessarily mean that the drugs are not useful, said Clohessy, but that perhaps the patient populations are not as well defined as they could be. Clohessy noted that, although there are many promising agents, and much has been invested in the quest to find new cancer drugs, the number of new drugs approved by the U.S. Food and Drug Administration (FDA) has decreased in recent years. In addition, cancer is revealing itself to be a very sophisticated disease capable of modifying itself and developing resistance to treatments.
For these reasons—the unique and complex nature of cancer and the inadequate current drug development system—Clohessy said there is a need for improved disease models and a new system of disease classification and drug development. This ideal new system would provide disease models, including patient surrogates, to account for the complexity of biologic systems and the inherent inter- and intra-patient heterogeneity of human cancers. The system would link agents to their molecular targets using predictive modeling. Finally, there would be improved clinical trial designs based on predictive biomarker validation strategies, which could be tested and validated with a single trial.
The mouse hospital’s mission is to provide expertise in the design and implementation of preclinical trials to test new drugs in mouse models of human disease. By performing pre- and co-clinical trials with GEMMs and PDX models, researchers can determine which patients are more likely to respond to novel therapeutic agents, on the basis of their genetic makeup. In addition, the progress from bench to bedside for promising new agents, or combinations of already approved drugs, can be streamlined. The mouse hospital, said Clohessy, is a state-of-the-art animal facility, with personnel who are trained in diseased mouse care, husbandry, and therapeutic treatments, as well as dedicated areas in the vivarium for in vivo imaging, behavioral testing, and surgical procedures.
The ultimate goal, said Clohessy, is to have mouse hospitals throughout the country and the world. These hospitals would be credentialed and accredited, and the data from the pre- and co-clinical trials would be centrally deposited. Clohessy explained that the mouse hospital is currently in the proof-of-concept phase, working in concert with the cancer clinical center and the cancer research institute at Beth Israel Deaconess Medical Center. Clohessy said that the collaboration between the patient care teams and the researchers is an iterative process that allows patient treatments and patient care to be tailored to improve outcome.
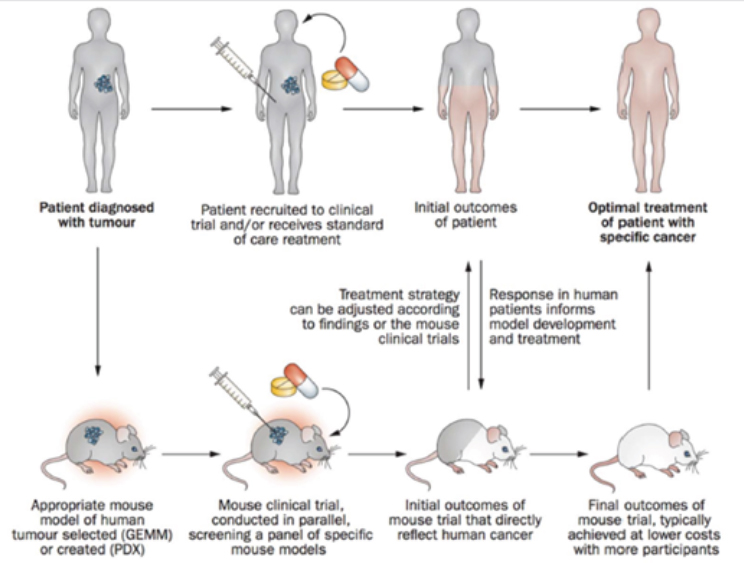
Clohessy explained the ideal structure of these co-clinical trials (see Figure 3-3). First, when a patient is diagnosed with a tumor, a mouse model is either selected (GEMM) or created (PDX). While the patient is undergo-
SOURCE: Clohessy and Pandolfi, 2015.
ing standard-of-care treatment, a mouse clinical trial is conducted to screen treatments. The findings from the initial mouse trials are used to adjust the treatment strategy for the human patient, and at the same time, the initial outcomes of the human patient are used to inform the direction of research in the mouse models. Final outcomes from the mouse trial, along with information about how the human patient has responded to treatments, are used to optimize the treatment of the human patient.
In order to fully realize the potential of mouse co-clinical trials, a number of challenges need to be overcome, said Clohessy.
1. Reevaluation and Repurposing of Existing Models
There may be improved ways to utilize the multitude of available cancer models, Clohessy said, and described ongoing research that uses GEMMs to study lung cancer at a single-cell level. Researchers focus on subpopulations of tumor cells to understand how these respond to treat-
ment, to predict the emergence of resistance or to identify populations of cells that may be resistant at an early stage of the disease. These data from the animal models are used in concert with data from primary human tumors to more fully understand cancer at the cellular level and develop more precise treatments.
2. New Approaches for Model Development
Using GEMMs in a co-clinical trial approach is lengthy and costly, said Clohessy, as it takes a significant amount of time to develop and breed mouse cohorts that are appropriate models for a single patient. To address this challenge, the mouse hospital has developed a platform called orthotopic graft of GEMM-derived organoids (OGGO). This platform has allowed researchers to isolate prostate cells, grow organoids in vitro, and manipulate their genetic makeup. These organoids can be placed into genetically homogeneous mice to allow researchers to track the development of disease and study the diversity of human cancers.
3. Co-clinical Implementation of Models Toward Therapy
Leukemia mouse models are used to develop and evaluate novel therapeutic approaches, said Clohessy. Mutations in the IDH2 gene occur in about 20% of patients with acute myeloid leukemia. A recently developed mouse model allows the study of the dependence of the disease on IDH2 expression. Through a serial transplantation protocol, researchers have developed a number of models with variable sensitivity to the de-induction of the mutation. These models are compared using metabolomics, genomics, and transcriptomics, and through this comparison, researchers have identified both novel resistance mechanisms and novel vulnerabilities. Clohessy said that clinical trials for therapeutic approaches based on these findings are in development.
Pets with Naturally Occurring Tumors
Amy LeBlanc, director of the National Cancer Institute’s Comparative Oncology Program, told workshop participants about how the Comparative Oncology Program (COP) may present an opportunity to further the field of precision medicine. Through the Comparative Oncology Trials Consortium, COP facilitates clinical trials for pets with cancer at 22 veterinary teaching schools in North America. These trials offer pets (and their owners) the chance to receive treatments, while also offering researchers an opportunity to assess new treatments with naturally occurring cancer models and to collect relevant data on toxicity, response, drug dosing, bio-
markers, and histology. In addition, COP has a laboratory that is currently focused on the biology and metabolism of metastasis in osteosarcoma in dogs, and the development of molecular imaging tools to help interrogate laboratory findings and translate them into the clinical trial setting. About 4.2 million dogs are diagnosed with cancer every year, and cancers in dogs and humans are remarkably similar, said LeBlanc. Dogs develop cancer in many of the same areas of the body as humans. While dogs and humans are not always a perfect match, there are many types of cancer in dogs that are appropriate models for humans, said LeBlanc.
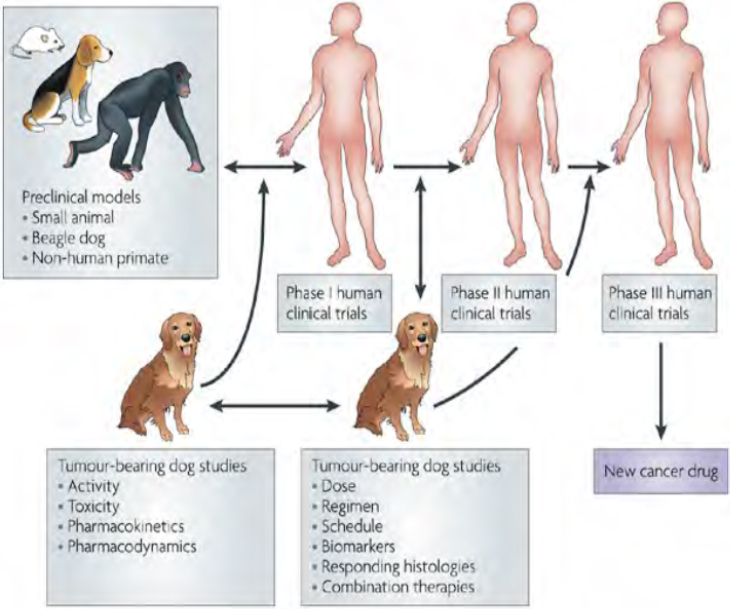
Comparative oncology trials provide an iterative process where studies on dogs can inform studies on humans, and vice versa, with the overall gain in knowledge advancing the understanding of cancer and potential treatments, said LeBlanc (see Figure 3-4). For example, initial studies on dogs with tumors can produce data about potential treatments, including activity, toxicity, pharmacokinetics, and pharmacodynamics. These data
SOURCE: Paoloni and Khanna, 2008.
can then inform phase I human clinical trials, and data from both of these trials can inform further research on the dog and human patients on dose, regimen, and biomarkers.
LeBlanc gave two examples of successful comparative oncology trials. One trial focused on a novel immunocytokine (NHS-IL12), which the manufacturing company was ready to give up on. Dogs receiving the immunocytokine responded favorably. The efficacy data resulted in enough enthusiasm to generate an Investigational New Drug application to the FDA, and eventually phase I clinical trials. The data also helped in biomarker selection. The second example was a trial to compare three TOPO-1 inhibitors in dogs with lymphoma. The data from dogs suggested that one of the drugs was more biologically active than the other two, a result that was not obvious from preclinical mouse research.
The initiation of a new clinical trial by COP is being done to meet a specific need that is unmet by human drug development and fill a gap, said LeBlanc. She said that data on drug efficacy may not always be the top priority; trials may focus on target modulation or pharmacodynamic and pharmacokinetic relationships. The FDA views data from comparative oncology trials as important but supplementary; according to LeBlanc, the agency has stated that data from comparative oncology trials will not derail the clinical development of a drug or result in a clinical hold.
Regarding precision medicine, LeBlanc said that the biggest area yet to be addressed is the genetic landscape of canine cancers. Most comparative oncology research to this point has focused on clinical observations, rather than on collecting and analyzing genetic data about potential mutations associated with cancers. In order to move precision medicine forward, comparative oncology researchers will need analytic tools and expertise to collect genetic information; LeBlanc noted that because they are not bound by privacy rules regarding human data collection, there is an opportunity to build a robust set of data in this area.
LeBlanc discussed new funding initiatives from the National Cancer Institute (NCI) that pertain to precision medicine. In 2016, the NCI distributed eight awards (totaling about $4 million) for cancer centers to characterize the molecular landscape of selected canine cancers. The goals of these awards are to
- sequence (by whole exome sequencing and RNAseq) at least 25 canine tumors, including B-cell lymphoma, glioma, osteosarcoma, melanoma, bladder cancer, and mammary cancer;
- determine the mutational load in the cancers chosen for study;
- use appropriate computational tools to characterize neoantigens that can strongly bind canine MHC antigens; and
- describe and characterize the T lymphocyte numbers and subsets, as well as other relevant aspects of the tumor microenvironment, within the canine tumors.
The second step of this initiative was a Request for Application (RFA) for studies investigating the utility of dogs with cancer as models for immunotherapy development. The short-term goals of this RFA are to establish a network of laboratory scientists and canine clinical trialists to study the anti-tumor effects of immunotherapy agents and novel combinations of immunotherapy and other modalities, and also to establish a coordinating center to implement the clinical protocols and manage data from these trials. In the long-term, the NCI will use these data to establish the suitability of canine models for studying immunomodulating agents, and to eventually translate the findings to human studies. The awards from this RFA were announced in mid-2017, with five institutions receiving about $15 million in total.
LeBlanc concluded that finding success in precision medicine in this area depends on the advancement of our knowledge regarding cancers in dogs. She said, however, that there has been significant progress in recent years in terms of awareness, advocacy, funding, and research, and that comparative oncology presents a great opportunity to learn more and contribute to the care and treatment of both humans and dogs.
CHALLENGES OF USING ANIMAL MODELS FOR PRECISION MEDICINE
Big Data
Clinical care and research on humans and animals generate a huge amount of data, said Melissa Haendel, associate professor of medical informatics and clinical epidemiology at Oregon Health & Science University. There are data from individual patients regarding clinical phenotypes, -omics, socioeconomic factors, environmental exposure, and other factors; there are population-level data on population frequencies, disease correlations, risk statistics, and exposure data; and there are data stemming from model organism research. Despite this plethora of data, most clinical diagnostic pipelines leverage only a tiny fraction of it. Haendel said that the challenge lies not in producing more data but in improving the utilization of data.
One of the problems, said Haendel, is that different communities—including researchers, clinicians, patients, and animal scientists—use different terms to describe signs and symptoms of the same clinical entity.” A disorder characterized by thickening of the skin on the palms and soles may
be called by the clinician “palmoplantar hyperkeratosis,” by the patient “thick hand skin,” and by an animal scientist “ulcerated paws.” Haendel noted that human patients are often described in terms of billing or electronic health record codes, while model organisms are described fairly specifically in terms of anatomy, morphology, and other characteristics. The issue, said Haendel, is that these data are not relatable to one another, and the language barriers between fields become very problematic. Because of this wide variety in terms, it is quite challenging for researchers to work across multiple research or clinical datasets.
Haendel said that there are hundreds, if not thousands, of standards used to describe phenotypic features. The Human Phenotype Ontology project attempts to bridge this phenotypic semantic confusion by using a logic structure to classify phenotypes according to standardized terms. The project uses species-neutral ontologies and homologous concepts, drawing from evidence from the literature, database resources such as OMIM and Orphanet, and other existing clinical and research ontology standards. The logic model can deduce that palmoplantar hyperkeratosis and ulcerated paws are the same phenotype, based on the underlying phenotypic profile and the terms used to describe the phenotype. This approach makes it possible to integrate data from both clinical and model organism sources. Each source has its own ontology, but the application of species-neutral ontology allows the data to be harmonized. Haendel noted that between—or even within—data sources, the same phenotype is sometimes associated with a different component of the genotype, and that harmonizing these sources creates an opportunity to find novel associations.
Using these data for diagnosis depends on the use of a fuzzy matching algorithm that compares patient genotypic and phenotypic data against similar data from human and model organism sources. Through this process, the algorithm may, for example, find that an ortholog of “Gene M” from a mouse with a similar phenotype might be a candidate for the disease of “Patient A.” In addition to the new insight into a potential gene–phenotype association, this process also may result in collaboration between a clinician and a researcher, each of whom have expertise to share, said Haendel.
The use of such data-harmonizing technology, Haendel said, has improved the diagnostic rate of the NIH’s Undiagnosed Diseases Program by 10 to 20%. The technology also facilitates the matching of patients across the globe through a site called Matchmaker Exchange. By matching undiagnosed patients using both clinical and public data sources, candidate variants can potentially be validated, and patients and providers from around the world can collaborate to find diagnoses.
Haendel closed by stressing the importance of developing the right model for a disease. She said that researchers sometimes settle on models
that generally recapitulate some phenotypic aspects of the disease, rather than using precise phenotypic data to find which aspects of the phenotype are actually being recapitulated. While a model organism is unlikely to recapitulate every aspect of the phenotype, having more information about similarities and dissimilarities between the organism and humans will enable researchers to learn more about the disease. She added that, in some cases, nontraditional organisms may be the best choice for a model—for example, aged cats are good models for Alzheimer’s disease, armadillos are a natural host of the myobacterium that causes leprosy, and pig eyes most closely resemble human eyes. Haendel said that she and her colleagues are currently working on new strategies to assess the suitability of different organisms for modeling specific phenotypes, so that organisms are not chosen simply because they are the ones “we happen to have in our basement.”
The Animal Rule and Appropriate Modeling
Jens Kuhn, virology lead at the NIH/National Institute of Allergy and Infectious Diseases Integrated Research Facility (IRF) at Fort Detrick, began with a brief description of the work performed by the IRF. The IRF seeks to develop prevention and treatment options for infectious human diseases, whether naturally emerging or deliberately introduced (e.g., in a bioterror attack). He noted that the infectious agents that IRF focuses on tend to be quite rare, with outbreaks few and far between. The one notable exception, he said, is Ebola, which has received more money and attention since the 2014 epidemic, and as a result is better understood than the other agents. Kuhn said that the goal of IRF is the rapid development and the licensure of efficacious countermeasures against diseases caused by these agents. The infectious agents that they focus on have the ability to migrate quickly, with a short incubation period, and tend to appear in areas that lack the resources for proper testing, treatment, and supportive care. He noted that, while most natural outbreaks of these diseases are small and isolated, the potential for bioterrorism implies the need to have something ready to treat a lot of patients. Due to the deadly nature of the agents being investigated, animal models are essential for understanding the mechanisms of disease and testing the safety and efficacy of potential countermeasures.
The FDA’s Animal Rule was designed with this type of research in mind, where human efficacy trials are not feasible or ethical due to the nature of the agent being studied. The rule, which was implemented in 2002 amidst concerns about bioterrorism, allows the FDA to rely on evidence from animal studies when considering approval of a medical countermeasure. In order to fall under the auspices of the animal rule, there are a number of criteria that must be met: There must be a “reasonably well understood pathophysiological mechanism,” the effect of the countermea-
sure must be “demonstrated in more than one animal species . . . predictive for humans” or in a single species that is “sufficiently well-characterized” for predicting the response in humans, the animal study end point must be “clearly related to the desired benefit in humans,” and the pharmacokinetic and pharmacodynamic modeling must allow “selection of an effective dose in humans” (21 CFR Sec. 314.610).
Kuhn said that it would be extremely challenging for research on rare infectious agents to meet these criteria for a number of reasons. The details of the human clinical disease for most of these agents are not well-defined. Because outbreaks tend to be rare and in isolated, resource-poor areas, the phenotypic descriptions of these diseases are limited to a few observations that doctors have made, for example, noting that the patient has rash, pale skin, and tremor. With such a lack of information, how can researchers develop a reasonable model that faithfully recapitulates the human disease? Kuhn said the emphasis has long been on developing models with face validity (i.e., models that recapitulate the symptoms), but that this approach is problematic and leads to using animal models that may not, in fact, be particularly relevant. For example, mice infected with Ebola do not develop a rash, whereas nonhuman primates (NHPs) do. This phenotypic difference, combined with the fact that NHPs are closely related to humans, has sometimes led researchers to conclude that NHPs are the gold standard in research and to dismiss the use of other animal models. However, there are two issues with this conclusion. First, said Kuhn, the gold standard animal model becomes reified despite significant differences between the model and humans. For example, even though gorillas share 99.6% of human genes, they are vegetarians and thus would be a terrible model for studying the effects of meat consumption on the human body. Second, the non-expression of a specific phenotype does not necessarily mean that the disease is not recapitulated. That is, the pathway of the disease may be the same in an animal model and in humans, but the animal model may not express the phenotype due to some other factor, for example, a stop codon.
Kuhn concluded with a quote from Thomas Hartung: “If there was an animal model good enough to substitute for people we would not have a 92% failure rate in clinical trials” (Dolgin, 2013, p. 118). Kuhn noted that the issue is not whether animal models are useful, but rather that the research community has not thought in depth about how to best develop and utilize the models. He suggested that inertia has led researchers to continue to use the same models they have always used, and to look for face validity rather than at deeper pathways and more relevant mechanisms. Kuhn imagined a future scenario in which animal models would be highly relevant and useful for all types of clinical trials: “we would have tons of different animal models at our fingertips that are very well characterized all the way to the genomic and mutational and allele level, and we would
have . . . 1 million human genomes available and they are also fully characterized and span the entire spectrum of ethnicities.” Kuhn said that in order to reach this future, researchers must start not with the practical and regulatory concerns about what is possible or acceptable, but instead by choosing the best animal model and the best approach for answering the scientific question at hand.

























