2
Managing Acute Pain
Everyone experiences pain at some point in their lives. Pain can be mild and require no treatment or be treated easily with over-the-counter medications or nonpharmacologic approaches. Both mild and more severe pain may be acute and amenable to treatment, or the pain may be chronic and debilitating. Both acute and chronic pain may be intermittent or recurring, and acute pain may even occur on top of chronic pain resulting from a medical condition (IOM, 2011). People with acute pain need adequate pain relief, and many cases of mild acute pain do not require treatment with medications; while for more severe pain, analgesics other than opioids may be effective, so opioids are not needed. However, for severe acute pain or for acute pain that does not respond to other treatment options, opioids can often provide effective relief, and thus are sometimes needed. On the other hand, it is also important to take into account the risks of opioid prescribing to patients and to public health, including chronic opioid use, opioid use disorder, and the availability of unused pills for diversion to those for whom they were not prescribed. Finding a balance between the management of acute pain and the risks of opioid prescribing is a challenging task.
Opioids have long been prescribed to relieve acute pain. Although the widespread use of opioids1 for pain management began in the 1990s, some opioids such as morphine and opium have been used for centuries (Collier, 2018). In part, the increased use of opioids was the result of efforts in the late 1990s and early 2000s to reduce acute, chronic noncancer, and cancer pain. In 2000, The Joint Commission (2016) issued standards for pain assessment and management practices that imposed criteria for health care organization policies addressing pain that increased the use of patients’ self-reported pain to guide pain management. By 2009, in response to detrimental reports of overly aggressive treatment of pain, the standard that all patients be assessed for pain was revised to require this standard in only behavioral health care (Baker, 2017).
___________________
1 “Traditionally, the term opiates refers to substances derived from opium, such as morphine and heroin, while opioids refers to synthetic and semisynthetic opiates. However, the term opioids is now often used for the entire family of opiates, including natural, semisynthetic, and synthetic” (NASEM, 2017a, p. 23).
Overall, pain may cause physical and emotional distress and compromise a person’s ability to meet family, job, school, and other responsibilities. Acute pain also harms a person’s quality of life, including affecting sleep, physical functioning, and mental health (Sinatra, 2010). Furthermore, suboptimal pain management can contribute to increased morbidity, slow recovery, prolonged opioid use during and after hospitalization, an increased cost of care, and an increased risk of progression to chronic pain (Gan, 2017). Neonates and very young infants may be more vulnerable to the long-term effects of repeated pain on neurodevelopment and neuroendocrine and immune response (Hadjistavropoulos et al., 1997). For health care providers, alleviating pain is a primary responsibility. The Institute of Medicine2 (IOM) report Relieving Pain in America declared as its first guiding principle, “Effective pain management is a moral imperative, a professional responsibility, and the duty of people in the healing professions” (IOM, 2011, p. 3).
This chapter describes the clinical context of acute pain, including the presentation of acute pain, and the pathways by which patients seek and receive treatment for acute pain.
DEFINITIONS
Many terms are used to describe the possible adverse effects that may result from opioid use to treat acute pain, including the term “acute pain” itself. These terms are discussed briefly below.
Acute Versus Chronic Pain
The committee considered having a definition of “acute pain” to be an integral part of its task. The definition it settled on for acute pain was derived from multiple authoritative sources, some of them contradictory. The Centers for Disease Control and Prevention (CDC) emphasizes the contrasting time-dependent differences between acute and chronic pain, with acute pain often described in terms of not being chronic.
The National Pain Strategy uses physiologic, behavioral, and time-dependent criteria to define acute pain as “an expected physiologic experience to noxious stimuli that can become pathologic, is normally sudden in onset, time limited, and motivates behaviors to avoid actual or potential tissue injuries” (HHS, 2016, p. 11). This definition is also used in the pain taxonomy classification of acute pain conditions developed by the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks; the American Pain Society; and the American Academy of Pain Medicine, with a further explanation that such pain typically lasts up to 7 days but can be prolonged to 30 days (Kent et al., 2017). The 2011 IOM report Relieving Pain in America also defined acute pain as being of sudden onset and of short duration, emphasizing that acute pain is usually linked to a specific event, injury, or illness. It may also be recurrent with pain-free periods. The committee recognizes that acute and chronic pain are on a continuum and that acute pain may transition to chronic pain over time.
The 2016 guidelines for the management of postoperative pain—developed and endorsed by several professional pain societies—reference persistent acute pain but without a specific timeframe (Chou et al., 2016). Based on the integration and interpretation of existing definitions of acute pain (Chou et al., 2016), the committee considers acute pain for the purposes of this report to include a sudden onset of
___________________
2 As of March 2016, the Health and Medicine division of the National Academies of Sciences, Engineering, and Medicine continues the consensus studies and convening activities previously carried out by the Institute of Medicine (IOM). The IOM name is used to refer to publications issued prior to July 2015.
pain that lasts no longer than 90 days. Pain that lasts longer than 30 days but less than 90 days is often referred to as subacute pain and represents a transition between acute and chronic pain.
As noted in the 2017 National Academies of Sciences, Engineering, and Medicine (the National Academies) report Pain Management and the Opioid Epidemic, opioids have long been prescribed for the effective management of acute pain, such as postoperative and postprocedural pain, “and they have been found to be more effective than placebo for nociceptive and neuropathic pain of less than 16 weeks’ duration” (Furlan et al., 2011; NASEM, 2017, p. 53). However, for some types of acute pain, such as low back pain and pain after third molar extractions, the efficacy of opioids is less clear and their superiority to other medications is not established (Deyo et al., 2015; Friedman et al., 2015; NASEM, 2017). The 2017 National Academies report also stated that:
Pain diagnosis currently depends on clinical examination and testing (laboratory, imaging) to identify the etiology of the pain. The pain condition is described in terms of the pain’s location (e.g., orofacial pain, temporomandibular joint disorder, migraine, low back pain) and/or type (somatic pain is caused by injury to skin, muscles, bone, joints, or connective tissues and is nociceptive; visceral pain arises from the internal organs and is nociceptive; and neuropathic pain is presumed to be caused by a demonstrable lesion or disease of the peripheral or central somatosensory nervous system). Duration of pain is commonly defined as acute (less than 6 weeks), subacute (6–12 weeks), or chronic (more than 12 weeks). (pp. 147–148)
Chronic pain is frequently considered to be pain that lasts longer than 3 months or past the time of normal tissue healing (Dowell et al., 2016a). An extensive discussion of the causes of and treatments for chronic pain may be found in the 2011 IOM report Relieving Pain in America. Chronic pain may cause changes in the peripheral and central nervous systems such that it can become a disease in its own right. Furthermore, chronic pain has significant physiological (e.g., changes in brain anatomy), psychological (e.g., depression and anger), and cognitive effects (e.g., pain catastrophizing) that may worsen over time. Causes of chronic pain include an underlying disease or medical condition, an injury, medical treatment, inflammation, neuropathic pain, and unknown causes (IOM, 2011).
Notably, recent studies have shown that chronic opioid use may occur following surgery (Brummett et al., 2017). Bateman et al. (2016) found that approximately 1 in 300 opioid-naïve women become persistent prescription opioid users following cesarean delivery. Sun et al. (2016) found that male sex, age older than 50 years, and a preoperative history of drug abuse, alcohol abuse, depression, benzodiazepine use, or antidepressant use were all associated with chronic opioid use among adult surgical patients. Risk factors for persistent opioid use among pediatric surgical patients include older age, female sex, previous substance use disorder, and preoperative opioid use (Harbaugh and Gadepelli, 2019). Numerous studies have found also that postoperative opioid use may be correlated with patient factors beyond patient-reported pain or procedure type—such as anxiety, mental health conditions, medical comorbidities, and prolonged opioid use—that may not entirely reflect the severity of ongoing pain (Badreldin et al., 2018; Brummett et al., 2013; Committee on Practice, 2018; Hilliard et al., 2018; Kelly et al., 2018; Velanovich, 2000). For example, Hah et al. (2017) found that chronic opioid use after surgery was associated with presurgical opioid use, lower socioeconimic status, preoperative pain, and the use of antidepressants.
Opioid Use
The committee adopted the following definitions related to opioid use for this report (see Box 2-1). Unless otherwise noted, the definitions are from the report Pain Management and the Opioid Epidemic (NASEM, 2017).
Opioids relieve acute severe pain via the µ–opioid receptor in the nervous system. Opioids used for acute pain typically vary with regard to half-life and duration of action, for example, some opioids with a short half-life have a long duration of action because they have a sustained-release formulation. One advantage of using opioids to treat pain is that they come in a variety of formulations including oral, intravenous, transdermal, intranasal, epidural, and intrathecal. However, in spite of variation in the potency of various opioids (as morphine milligram equivalents [MMEs]), there is little evidence to suggest that “one opioid analgesic is superior to another in its ability to manage either acute or chronic pain” (p. 54), or that more potent opioids are associated with higher rates of adverse effects (Murphy et al., 2018).
In the primary care setting, back, neck, and joint pain; musculoskeletal injury; and headache are among the most common patient complaints (Mundkur et al., 2019), and opioids are frequently prescribed for them (Brian Bateman, Brigham and Women’s Hospital, personal communication, September 6, 2019). For example, one investigation using records from a large health insurer found that among 230,958 patients in initial pain encounters in a primary care setting for which an opioid prescription was written, the top three pain complaints were joint pain (71,735 encounters), back pain without radiculopathy (54,682 encounters), and headache (40,005 encounters) (Mundkur et al., 2018). Pain is also a common complaint in emergency departments (EDs). From 2000 to 2010, approximately 45% of ED visits were associated with a primary symptom or diagnosis of pain (Chang et al., 2014), and data from the 2016 National Hospital Ambulatory Medical Care Survey (NHAMCS) showed that in more than 20% of ED visits, the principal reason for the visit was some form of pain (the most common reason was abdominal pain at 8.6%) (Rui et al., 2016). The pain-related discharge diagnoses most likely to be associated with an opioid prescription were nephrolithiasis (62.1%), neck pain (51.6%), and dental/jaw pain (49.7%) (Kea et al., 2016).
An analysis of data from the NHAMCS and the National Ambulatory Medical Care Survey for Adolescents and Young Adults showed that opioid prescribing rates were highest for adolescents and young adults presenting to the ED with dental disorders, followed by clavicle and ankle fractures (Hudgins et al., 2019). Among patients who undergo inpatient or outpatient surgery, more than 80%
report pain at discharge, and of these patients about 75–86% reported their pain as severe or extreme (Apfelbaum et al., 2003; Gan, 2017; Gan et al., 2014; IOM, 2011). Data show that medical opioid use among opioid-naïve high school seniors is independently associated with a 33% increase in risk of future opioid misuse after high school (Miech et al., 2015). Adolescents who take opioids, whether prescription or illicit, may be particularly vulnerable to subsequent misuse and substance use disorder (Cerda et al., 2015; Kelley-Quon et al., 2019; Miech et al., 2015). As a result of the increase in opioid misuse and deaths in the United States, a number of professional societies, government agencies, state legislatures, health care organizations, and health insurers have taken a variety of steps to reduce the number of opioid prescriptions, pills prescribed, and total dispensed MMEs (Davis et al., 2019; Dowell et al., 2016b; Schuchat et al., 2017).
PRESENTATION AND TREATMENT OF ACUTE PAIN
There are many effective treatments for acute pain. The 2011 IOM report Relieving Pain in America found that “Pain care must be tailored to each person’s experience” (p. 8) because people vary in their pain tolerance and in their need for pain management. Appropriate and timely treatment of the underlying cause of pain is often a crucial aspect of pain relief. For example, pain management for an ankle sprain or fracture may include immobilization, rest, ice, compression, and elevation of the damaged area, whereas for a back sprain, bed rest and heat may offer effective pain relief. CDC recommends a stepwise approach to treating pain, using nonopioid modalities first and as adjuncts before using opioids (Dowell et al., 2016a; WHO, 1990). The 2017 National Academies report Pain Management and the Opioid Epidemic stated:
there are some circumstances in which nonopioid analgesics (e.g., nonsteroidal anti-inflammatory drugs) are likely to be as effective as opioids, or more so, for reducing pain associated with the conditions for which they are indicated, and when used appropriately, these analgesics carry a lower risk of adverse outcomes relative to opioids. (p. 4)
Interventional, regional anesthetic approaches are also effective for some indications (e.g., nerve blockades for total knee replacement). Nonpharmacologic interventions, such as acupuncture, physical therapy, exercise, cognitive-behavioral therapy, and mindfulness meditation may also be effective for pain control (NASEM, 2017).
The committee recognizes that there are major injuries, diseases, operations, and treatments with known severe pain and that patients with these indications may require immediate access to opioids. For example, patients with severe sickle cell vaso-occlusive crisis should not be subjected to first-line treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) or other nonopioids when they present with severe pain. Patients recovering from an extensive scoliosis spine fusion should also have immediate access to opioids because of the severity of their pain. However, medical innovations may change a clinician’s approach to the management of a patient’s acute pain. For example, the use of regional anesthetic techniques such as liposomal bupivacaine or collagen mesh-embedded bupivacaine may supplant the need for opioids as these analgesic treatments become more widely used. And the use of indwelling catheters for specific nerve blocks, especially for orthopedic procedures, may obviate the need for opioids for postoperative pain.
A patient’s pain presentation may also be influenced by ethnic, racial, physiological, cultural, and religious factors (Green et al., 2004; Meints et al., 2019; Mossey, 2011). Some people from racial and ethnic groups, such as African Americans, Hispanics, and Native Americans, report a higher prevalence
of pain symptoms for certain medical indications than the white population (Campbell and Edwards, 2012). Given the patient-specific factors that can influence pain management, it follows that special considerations may influence the approach clinicians take when prescribing opioids. These factors include
- patients who have not had appropriate pain treatment;
- patients who are unable to communicate their pain, such as infants or those with cognitive impairments;
- patients with chronic pain who are already using opioids and might be opioid-tolerant;
- patients in whom the pharmacology of opioids may differ from the typical, such as children or the elderly;
- patients for whom the understanding of or adherence to a treatment plan of care may be challenging;
- patients who may be at risk for substance use disorder; and
- patients who have genomic or other medical factors that may affect their response to opioid treatment.
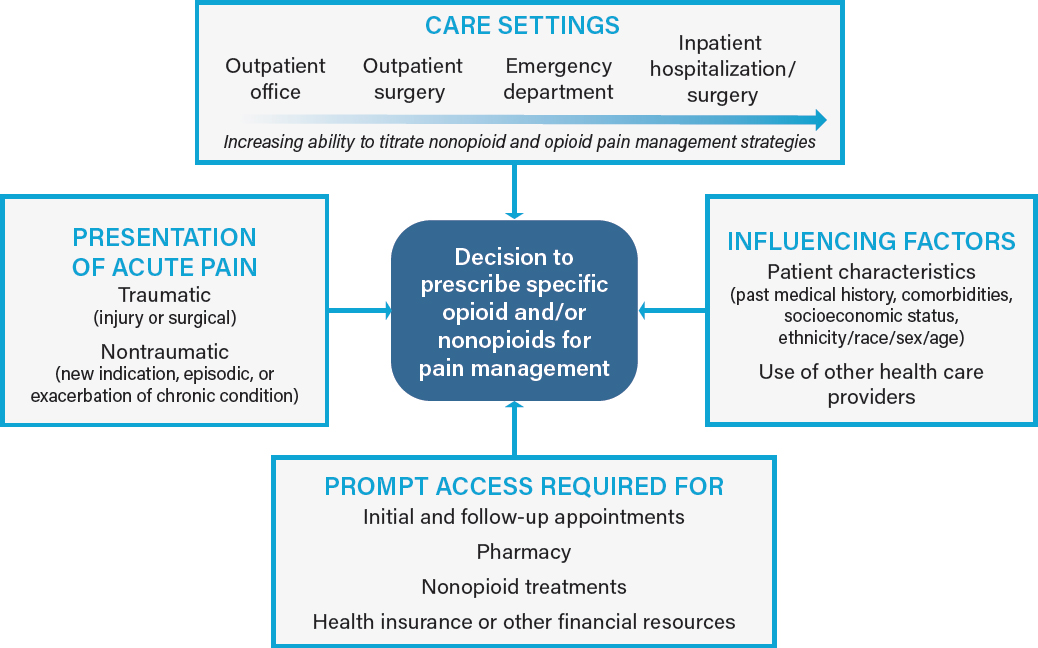
Optimal postoperative pain management requires an understanding of each patient-specific factor. In the sections below, the committee considers patient, population, and clinician factors that influence both the presentation and the treatment of acute pain (see Figure 2-1). All of the factors in the boxes may influence a clinician’s decision to prescribe opioids for a patient’s acute pain. Health care settings and access to care are discussed in later sections.
Age
The presentation of acute pain may vary by age, with, for example, such groups as the elderly, infants, and neonates presenting differently from typical adults (Bartley and Fillingim, 2013; Campbell and Edwards, 2012; Edwards et al., 2001; Fillingim et al., 2009; Green et al., 2003; Pieretti et al., 2016). Furthermore, several studies have found that a person’s pain threshold may change as he or she ages (Kaye et al., 2010). Acute postoperative pain may be intensified by certain factors, such as fear, anxiety, coping style, and by a lack of social support in both children (Verghese and Hannallah, 2010) and adults (Kennedy et al., 2019).
Infants and young children rely on caregivers to assess their pain intensity, and such pain assessments often involve behavioral and physiological parameters, since self-reported measures may not be possible in preverbal children or accurate in hospitalized young children (Berde and Greco, 2011). Similarly, some older adult patients who experience acute pain may be unable to clearly communicate their symptoms because of aging-related cognitive issues, including advanced dementia (Morrison and Siu, 2000; Schuler et al., 2004). Elderly patients, especially those with dementia, and young children are also more likely to have their pain undertreated (Birnie et al., 2014; Krauss et al., 2016; McAuliffe et al., 2012).
There are several changes in pharmacokinetics and pharmacodynamics that occur with age. Smith (2009) found that reduced clearance of morphine, codeine, fentanyl, and oxymorphone in older patients suggested that these patients begin with lower initial doses. Pain management must also account for patients in whom the pharmacodynamics of an opioid are different, such as children, the elderly, pregnant or nursing women, and burn or trauma patients (Finley et al., 2014; Keene et al., 2011; Malcolm, 2015; Raymond et al., 2018).
Sex
Some research suggests sex differences may also exist in the processing of pain; these differences can inform clinical pain management (Paller et al., 2009). However, results are mixed. While some studies show that women may demonstrate higher levels of pain sensitivity and have greater prevalence of many commonly observed clinical pain signs and symptoms than men, which appears to be due in part to differences in genetics, sex hormones, and attitudes to pain (Bartley and Fillingim, 2013; Fillingim, 2018; Fillingim et al., 2009; Otto et al., 2019; Pieretti et al., 2016), others show little difference in pain perception between the sexes (Gadkaree et al., 2019). Cattaneo et al. (2017) found that following major abdominal surgery, there was a statistically significant daily periodicity (p<0.001) in morphine consumption with consumption higher around 2 AM (rate 0.4 mg/min) and lower around 12 PM (rate 0.05 mg/min). The daily periodicity of morphine consumption was different between men and women (p=0.004), with males consuming more morphine during the night; there were no differences in daily periodicity for the categories of age and body mass index. Romano et al. (2019) also found that among men and women (median age 73) with worsening cognition, women reported significantly less unpleasantness with the percept of moderate pain and men reported significantly higher unpleasantness with moderate pain perception (p=0.033).
Body Weight
Body weight, which is related in part to sex, age, and other factors such as comorbidities, as well as the growing problem of obesity in the U.S. population, may also affect opioid prescribing requirements
and the presentation of adverse effects. Most research on the use of opioids in obese individuals has focused on administration during anesthesia and in the immediate postsurgical period (Lloret-Linares et al., 2013; Schug and Raymann, 2011), rather than prescribing at discharge. Patanwala et al. (2014) found that body mass index did not affect a patient’s pain response to a fixed dose of intravenous morphine administered in the ED. Similar results were found by Xia et al. (2014) for intravenous hydromorphone administered to patients with body weights ranging from 45 to 157 kg. The authors of both studies suggest that there is no advantage to weight-based opioid dosing versus fixed opioid dosing for pain response. As such, weight-based dosing is not typically considered in adult opioid dosing; however, extremes in weight should be considered as they may increase the risk for adverse effects, including respiratory depression in patients who are obese (Lloret Linares et al., 2009). Moreover, multiple factors including unique pharmacokinetics, developmental characteristics, and extreme variations in weight (0.4–150 kgs) require weight-based dosing in neonates, infants, and children (Kopecky, 2019).
Drug Interactions
Opioids are often taken concurrently with other pharmaceuticals—prescribed, over-the-counter, and illicit—and this use can result in drug interactions. Between 2016 and 2017, an estimated 267,000 ED visits were associated with prescription opioid harms (Lovegrove et al., 2019). Data from the National Electronic Injury Surveillance System–Cooperative Adverse Drug Event Surveillance project showed that almost half of the visits (47.6%) were associated with nonmedical opioid use, 38.9% with therapeutic use, and 13.5% with self-harm. Use of other pharmaceuticals, particularly benzodiazepines, were co-implicated in ED visits across all three groups. Concurrent use of illicit drugs, particularly marijuana, was most common among nontherapeutic visits, whereas alcohol was the most commonly associated with opioids taken for self-harm.
Drug interactions fall into two broad categories: pharmacodynamic and pharmacokinetic. In pharmacodynamic interactions, drugs directly influence the effect of each other (Cascorbi, 2012), as is the case with opioids and benzodiazepines. Both drugs are sedatives and suppress breathing. In 2016, it was estimated that as many as 30 million people in the United States may have used benzodiazepines, although misuse appears to be relatively uncommon (only 2.1% reported misuse) (NIDA, 2018). The National Institute on Drug Abuse (NIDA) reports that more than 30% of overdoses involving opioids also involve benzodiazepines (NIDA, 2018). Indeed, the combination of opioids and benzodiazepines has been shown to significantly increase the risk for overdose (odds ratio [OR]=5.05, 95% confidence interval [CI] 3.68–6.93) during the first 90 days of co-prescribing in patients older than 65 years of age; however, the risk decreased to 1.87 (95% CI 1.25–2.80) at 91 to 180 days of concurrent use (Hernandez et al., 2018). Other important pharmacodynamic interactions may occur between opioids and other central nervous system (CNS) depressants such as muscle relaxants, barbiturates, anxiolytics, benzodiazepine-like and nonbenzodiazepine hypnotics and sedatives (e.g., Zolpidem), gabapentinoids, antihistamines, antipsychotics, and alcohol (Dowell et al., 2016). The Centers for Medicare & Medicaid Services has issued a memo warning Medicare sponsors about those drugs because when used in conjunction with opioids they can potentiate the effect of the latter drugs (Majestic, 2018).
In pharmacokinetic drug interactions, one medication impacts the absorption, distribution, metabolism, or elimination of another medication (Cascorbi, 2012). Pharmacokinetic enhancers for opioids are strong inhibitors of the cytochrome P450 enzymes, specifically CYP3A4. Inhibition of CYP3A4 leads to a subsequent increase in the serum concentration of the opioid due to its decreased metabolism. Examples of cytochrome P450 enzyme inhibitors are drugs for the human immunodeficiency virus, antifungals, and some antibiotics (Majestic, 2018). Opioids metabolized by the cytochrome P450
system (e.g., codeine, oxycodone, hydrocodone, fentanyl, tramadol, and methadone) are associated with numerous drug–drug interactions that can result in either a reduction in opioid effect or excess opioid effects. Conversely, opioids that are not metabolized by that system (e.g., morphine, oxymorphone, and hydromorphone) tend to be involved in fewer CYP450-associated pharmacokinetic drug–drug interactions (Overholser and Foster, 2011). Some opioids (e.g., tramadol, codeine) can be considered pro-drugs in that their metabolism results in compounds with greater activity; if this metabolic activity is inhibited, a decreased analgesic effect would be expected. Conversely, when the administered opioid is active and metabolized to inactive metabolites (e.g., fentanyl), inhibition interactions are expected to prolong or enhance opioid effects (Overholser and Foster, 2011). However, the issue may be further complicated in that some opioids are metabolized to both inactive and active metabolites by multiple enzymes. An example of this is oxycodone, for which the enzyme CYP3A converts oxycodone to the less active compound noroxycodone and the enzyme CYP2D6 converts oxycodone to the more active compound oxymorphone.
Drug–drug interactions may also influence whether clinicians should prescribe opioids to patients. For example, taking opioids with benzodiazepines, alcohol, or medications that depress the CNS have resulted in serious side effects such as difficulty breathing and even death (Hwang et al., 2016; Jones et al., 2014; Park et al., 2015). Even tobacco use may affect opioid use. Radcliff et al. (2017) found that ED patients with active tobacco use had a poorer response to the administration of intravenous opioids for severe pain than did patients with inactive tobacco history. U.S. Food and Drug Administration (FDA)approved drug labels for opioids formulations contain “black box” warnings that specifically call out the risks and mitigation strategies for drug interactions between opioids and other common drugs such as benzodiazepines, other CNS depressants, and alcohol. Interactions with other drugs such as monoamine oxidase inhibitors may also be indicated (NLM, n.d.).
Comorbidities
When considering the appropriate treatment modality for a patient with acute pain, organ function and other medical comorbidities need to be evaluated. Comorbidities may be the result of aging (e.g., osteoarthritis), injury, or have other known or unknown causes (e.g., cardiovascular disease, diabetes, cancer).
Opioid metabolism and excretion can be impaired by liver and kidney disease, and special consideration needs to be taken in the choice of analgesia treatment for patients with these conditions (Davison, 2019; Soleimanpour et al., 2016). The use of opioids to treat acute pain in the elderly or people with kidney disease may be complicated by the fact that the use of alternative analgesics such as NSAIDs are contraindicated for those populations (Horl, 2010).
As the use of prescription opioids has increased in recent years, so too has the number of individuals receiving medications for substance use disorder and addiction treatment with methadone or buprenorphine (Huxtable et al., 2011). Both factors have, in turn, increased the number of opioid-tolerant patients, making the treatment of acute and chronic pain more difficult. Studies have demonstrated that past opioid use or dependence is likely to result in increased mortality, postoperative complications, and longer hospital stays (Best et al., 2015; Cooney and Broglio, 2017). As a benchmark, FDA defines opioid-tolerant individuals as having received the equivalent of at least 60 mg/day of oral morphine, 25 mcg/hour of transdermal fentanyl, 30 mg/day oral oxycodone, 8 mg/day of oral hydromorphone, 25 mg/day of oral oxymorphone, or an equivalent analgesic dose of another opioid for at least 1 or more weeks (FDA, 2016).
Risk factors for developing substance use disorder include, but are not limited to, being a young adult (aged 18–34 years), being male, having a history of psychiatric outpatient visits or psychiatric
diagnosis, and having been diagnosed with nonopioid substance use disorder, depression, posttraumatic stress disorder, or hepatitis (Edlund et al., 2010; White et al., 2009).
For adolescents, environmental factors such as family life and peer relationships as well as major life events are important factors to consider when prescribing opioids (Swadi, 1999; Thatcher and Clark, 2008; Whitesell et al., 2013; Zimmerman and Farrell, 2017). Adolescents and adults who experienced adverse childhood events may be at increased risk for substance misuse (Hailes et al., 2019; Quinn et al., 2019). Other risk factors for opioid misuse include a history of medical use of a prescription opioid (Miech et al., 2015) and psychosocial factors such as depressive episodes (Edlund et al., 2015) and anxiety (Boyd et al., 2014).
Genetics
Certain risk factors for opioid use disorder have been traced to genetics, and early research suggests it may be possible to identify this risk by examining an individual’s genotype (Koolen and Van der Rijt, 2017). Certain genetic variants in sensitivity to pain and to the rewarding properties of opioids, along with differences in how people metabolize opioids, will likely affect their response to treatment. Genetic factors can also interact with psychosocial factors such as stress and pain catastrophizing to influence pain (Fillingim, 2019).
In the future, genetic screening may enable clinicians to tailor opioid doses for acute pain for individual patients (Berrettini, 2017; Madadi et al., 2013). Also, differences in opioid metabolism due to variations in metabolic phenotypes have been demonstrated in children. In particular, postoperative deaths were reported among children who were prescribed codeine, with the deaths attributed to atypical cytochrome CYP2D6 pharmacogenetics (Kelly et al., 2012). Children who have CYP2D6 ultra-rapid metabolizer genotypes are at increased risk for serious adverse effects due to the excessive conversion of codeine into morphine, whereas in children who have significantly reduced levels of this enzyme, codeine has poor efficacy. Safety concerns regarding the use of codeine in children led FDA to restrict its use for this population (FDA, 2018). Balyan et al. (2017) examined plasma levels of oxycodone and oxymorphone in 30 children who were administered oral oxycodone postoperatively. Children with an extensive metabolizer phenotype were found to have a higher conversion of oxycodone to oxymorphone than children who were poor or intermediate metabolizers. Similar studies conducted among adults using a randomized controlled trial design suggest the risk of overdose or death from opioid treatment can be decreased through an understanding of a patient’s CPY2D6 phenotype (Linares et al., 2014).
Health Disparities
Ideally, patients who have similar presentations of acute pain should be treated in a similar manner, but this is not always the case and can result in health disparities. Health disparities can result from a number of factors such as socioeconomic differences, ethnicity and race, treatment setting, access to care, and implicit or explicit clinician bias.
Staton et al. (2007) found that physicians in primary care centers were twice as likely to underestimate pain in black patients as in all other ethnicities combined. A meta-analysis of 14 studies published from 1990 to 2018 comparing racial and ethnic differences in the administration of analgesia for acute pain in EDs showed that black and Hispanic patients were less likely than white patients to receive analgesia for acute pain (OR=0.60, 95% CI 0.43–0.83 and OR=0.75, 95% CI 0.52–1.09, respectively) (Lee et al., 2019). Using data from the National Ambulatory Medical Care Survey, Ly et al. (2019) found that
among patients presenting with abdominal pain in an outpatient setting, black patients were 6.0% less likely and Hispanic patients 6.3% less likely than white patients to receive opioids; similarly, black and Hispanic patients presenting with back pain were 7.1% and 14.8% less likely, respectively, than white patients to receive opioids for back pain. These disparities may also be seen in children of parents with limited English proficiency. Jimenez et al. (2014) found that among 237 hospitalized children of parents with limited English proficiency there were fewer postsurgical pain assessments and higher levels of recorded pain before they received opioids than among 237 children of parents who were proficient in English.
Institutional and structural racism as well as other forms of discrimination in the United States influence the provision of medical care (IOM, 2003). Populations that have historically been discriminated against in the United States, such as people of color, are more likely to have their pain undertreated than other groups. One review of 34 studies of pain treatment found that blacks experienced opioid prescription disparities for both traumatic/surgical pain and nontraumatic/nonsurgical pain, whereas these disparities were ameliorated for Hispanics with traumatic/surgical but remained for nontraumatic/nonsurgical pain (Meghani et al., 2012). Both Hispanics and blacks experienced opioid treatment disparities with regard to nontraumatic or nonsurgical pain and opioid prescriptions. For blacks, opioid treatment disparities remained consistent across pain types, settings, study quality, and data collection periods. One study found that black pediatric patients with appendicitis were less likely to receive opioid analgesia for moderate and severe pain than white patients (12.2% versus 33.9%) (Goyal et al., 2015). A study by Pletcher et al. (2008) found that between 2001 and 2005, whites were more likely to receive an opioid prescription in the ED (31%) compared with blacks (23%), Hispanics (24%), and Asians (28%, p<0.001 for trend). These prescribing differences did not decrease over time and were evident for all types of pain visits, were more pronounced with increasing pain severity, and were detectable for long-bone fracture and nephrolithiasis as well as among children.
Another, later study found that non-Hispanic blacks were less likely than non-Hispanic whites to receive an opioid prescription at discharge from an ED for “non-definitive” conditions such as back pain and abdominal pain (OR=0.56–0.67, p value<0.05), but not for toothache (Singhal et al., 2016). However, there were no significant differences in the prescribing of opioids between the two groups for the definitive diagnoses of long-bone fracture and kidney stone and no significant differences for Hispanics and any diagnosis.
Clinician factors, such as implicit or explicit bias in patient treatment, may contribute to disparities in the management of acute care and are related to differences in pain assessment and treatment. For example, some evidence suggests that clinicians tend to underestimate the pain of patients of color (Anderson et al., 2000). Furthermore, the authors found that patients of color report that clinicians often do not believe they have pain or do not understand their pain. Individuals with mental illness or a substance use disorder have historically been undertreated when experiencing pain. This is primarily due to the clinicians’ misperception of patients with substance use disorders—and those with mental illness—as drug-seeking and noncompliant (Haller and Acosta, 2010; Iocolano, 2000).
Clinical uncertainty on the part of clinicians (e.g., in interpreting disease symptoms in minority patients) can itself be a source of disparate treatment (Balsa et al., 2003). Clinician biases, implicit or explicit, about patients of color can also contribute to disparities (IOM, 2003). In a study that examined physician bias, investigators found that stereotypes about various racial groups were likely to influence provider communications about health recommendations (van Ryn, 2002). When cognitive capacity is taxed, memory is biased toward information that is consistent with stereotypes, which then leads to the underestimation and undertreatment of pain (Mathur et al., 2014; Staton et al., 2007; Trawalter et al., 2012).
Health Literacy
Health literacy, that is “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions” (HHS, n.d.) and efforts taken to account for differences in health literacy can impact the effective management of acute pain. Pain management plans are most effective when they address pain, account for comorbid conditions, and have the patient’s understanding and agreement. Comorbidities, such as schizophrenia and depression or cognitive impairment, may impair an individual’s ability to understand and follow a care plan. Health literacy, numeracy, and language barriers may have a similar impact on care plans. For example, a 2018 study found that among patients with musculoskeletal pain who received primary care, 6-month physical function scores were lower and pain intensity scores were higher among those with poorer health literacy; however, a limitation of the study was that health literacy was assessed with only a single-question literacy screen (Lacey et al., 2018). Health literacy correlated with older age, less education, comorbidities, and mental health but not with gender. Thus, clinicians should consider what supportive factors a patient may require to implement the care plan (e.g., the presence of a caregiver or nurse to assist with a treatment regime), should ensure that appropriate follow-up is scheduled, and should determine whether the patient has interim access to care for urgent issues, if needed.
ACCESS TO APPROPRIATE ACUTE PAIN MANAGEMENT
Implementing opioid treatment requires patients to have access to appropriate, timely management of their acute pain. Such access, as noted in Figure 2-1, requires a multistep process involving different aspects of the health care system, which can be affected by the health care setting in which the presentation, diagnosis, and treatment of the acute pain occurs. Appropriate treatment for pain will be possible only if each step in the process is achieved. In this section, the committee considers the factors that affect a patient’s clinical evaluation, pain management strategy, and access to those strategies.
Health Care Settings
Patients seek and receive treatment for acute pain in diverse health care settings, including hospitals, EDs, primary care offices, urgent care centers, long-term care facilities, pharmacies, and specialty clinics such as pain management, surgery, pediatrics, internal medicine, chiropractic, obstetrics and gynecology, and osteopathy. The health care setting in which pain is treated, including follow-up care, plays an influential role on the clinician’s ability and decision to prescribe an opioid after discharge and how to determine the proper dose. Specifically, there is variability in a clinician’s ability to prescribe and titrate nonopioid and opioid pain management strategies during the health care encounter prior to writing a prescription for outpatient pain management. During a 15- to 30-minute general outpatient office visit, a clinician will not typically have the opportunity to test pain management strategies. This might lead to prescribing a default amount of opioids to avoid undertreatment of pain at home once the anesthesia wears off (e.g., after a third molar extraction), which in turn might result in overprescribing.
In contrast, acute pain in the ED can be treated initially with an array of nonopioid and nonpharmacologic alternatives including NSAIDs, acetaminophen, and topical anesthetics. The clinician can then observe the patient’s response to these treatments and provide opioids if pain control is insufficient over the course of several hours. Finally, inpatient hospitalization for acute medical pain or after inpatient surgery can provide an extended opportunity to titrate pain control over a period of more than 1 day. Patients who do not require opioids during the final 24 hours of hospitalization often do not require opioid prescriptions at discharge (Hill et al., 2018).
Other setting-specific considerations include the ability to establish follow-up encounters with patients. For example, primary care clinicians may be able to schedule a follow-up visit, telemedicine visit, or phone call to determine the need for a refill, whereas ED clinicians typically do not have ongoing relationships with patients once they are discharged and usually recommended that patients see other providers. Furthermore, some patients receiving care in the ED may not have regular providers or may be unable to schedule a timely visit with a provider before a prescription runs out, and thus may be lost to follow-up.
Postoperative pain requirements may be different for similar procedures performed as inpatient versus outpatient. For example, patients undergoing knee arthroplasty with a planned inpatient stay, during which both intravenous and oral opioid regimens are given for postoperative pain control, may experience different pain management than patients undergoing knee arthroplasty as an outpatient procedure, for which postoperative prescribing must anticipate the potential pain a patient might experience at home when intravenous opioids are no longer available (Kelly et al., 2018).
Clinical Evaluation
Effective acute pain management requires first that a patient have timely access to a clinical evaluation for his or her pain. Prompt treatment of acute pain may help prevent additional morbidity or the development of chronic pain (Sinatra, 2010). However, patients may face a number of barriers to getting an evaluation, including a lack of health insurance coverage, few local providers being willing to accept a patient’s insurance, delays for an appointment with a clinician, and logistical difficulties with keeping the appointment, such as a lack of transportation, difficulty in taking time off from work or school, and child care responsibilities.
A thorough clinical evaluation should include a review of a patient’s medical record, including current and past medical illnesses; comorbidities; past medication history (particularly any history of substance misuse); and an assessment of the cause, site, severity, and impact of the pain. An assessment of comorbidities should also include an account of any psychological components, such as anxiety or depression, that may affect the symptoms of acute pain or that may influence a management plan (Michaelides and Zis, 2019). Historical patient information can be more easily accessed if a patient is returning to a clinician who he or she has seen previously for prior episodes of acute pain or other medical conditions, or if the clinician providing the evaluation of the acute pain incident has coordinated care with the patient’s primary care and/or other health care providers to gather all relevant past and current medical information.
The committee recognizes that individual patients with an acute pain diagnosis will respond differently to treatment and there is variability in time to recovery. For example, Komatsu et al. (2017) found in a study of mothers after cesarean childbirth that it took 50 days, 24 days, and 43 days, respectively, for 95% of the women to achieve pain resolution, opioid cessation, and other analgesic cessation; these women had used opioids for a median duration of only 8 days (range 0–39). The median time to “pain and opioid-free functional recovery” was 27 days, but there was a broad range of 19–40 days. This study demonstrates that while pain is an important factor in functional recovery, there is a highly variable trajectory for each outcome (e.g., pain, functional recovery, opioid use, and other analgesic use) and opioid use resolution precedes analgesic resolution.
Patients with ongoing pain that lasts beyond the expected recovery period will require re-evaluation for adequate pain control. They need timely access to a clinician who can assess their pain, determine whether additional medication is necessary or an alternative treatment strategy is warranted, and determine whether further evaluation is indicated for the persistent pain. The goals for patients with ongoing
acute pain is to manage the pain and prevent both chronic pain and long-term treatment with opioids. Therefore, continuous monitoring of the patient’s treatment and recovery is essential to ensure that the appropriate amounts of opioids are prescribed in conjunction with other treatment modalities.
Pain Management Strategies
Patients with acute pain may be prescribed a variety of treatments, depending on the cause of the pain and the patient’s history. Initial treatments may include nonpharmaceutical interventions (e.g., physical therapy, ice, and immobilization), nonopioid analgesics, or a combination of nonopioid treatments. If these approaches are effective in relieving the acute pain within the projected healing period for that condition, opioids may not be necessary.
Since the 1990s anesthesiologists and surgeons have collaborated to enhance recovery from surgical procedures, but their initial efforts were not always focused on reducing opioid use per se. Instead, programs were developed to expedite discharge from the hospital or to convert previous overnight or multiday hospital stays into ambulatory surgical experiences. More recently it has been recognized that the overall opioid burden after surgery can be reduced by programs such as enhanced recovery and implementing the wider use of nonopioid and multimodal analgesia (Jandali et al., 2019; Simpson et al., 2019). Furthermore, chronic relapses of painful diseases, such as sickle cell anemia, can be more effectively managed with opioid sparing techniques. These initiatives have resulted in reducing, but not necessarily eliminating, the need for outpatient opioid prescribing.
Prompt Access to Pain Management Interventions
After the patient receives his or her treatment recommendations, referrals, and prescriptions, other factors will affect the patient’s ability to implement pain management. For instance, various factors that may affect a patient’s access to care must be considered, including pharmacy access, health insurance guidelines and restrictions, and state laws limiting opioid prescriptions.
Health insurance coverage of nonopioid treatments is not always consistent with clinical standards, whereas opioids are commonly covered (Becker et al., 2017; Simmonds et al., 2015; Weeks, 2016). For example, a 2018 review of insurance coverage for nonpharmacologic treatments for low back pain found that physical and occupational therapy and chiropractic care were covered by about 90% of all of the insurance plans examined but that other nonpharmacologic treatments, such as psychological interventions, transcutaneous electrical nerve stimulation, and acupuncture, were not covered or were only partially covered (Heyward et al., 2018). Huskamp et al. (2018) studied 100 plans offered on the 2017 Health Insurance Marketplaces, with a randomly selected plan from a rural county and an urban county in each state; 100% of these plans covered at least short-acting opioid pain medication.
People without health coverage are less likely to obtain recommended treatments than those with coverage (Garfield et al., 2016). Some health insurers require that clinicians adhere to opioid prescribing rules. For example, Medicare requires that prescribers conduct opioid pain medication safety checks, get prior authorization, limit quantities, and use step therapy. Also, Medicare might not cover some drugs provided to patients in hospital outpatient settings, such as EDs (CMS, 2019). Prescribers may request exemptions for their patients as necessary, but this may prevent prompt access to opioids for acute pain.
Other barriers that may impede access to acute pain treatment include the lack of access to a conveniently located pharmacy or other treatment facilities (e.g., physical therapy clinic); a patient’s inability to pay for his or her prescriptions, including copays; and a patient’s inability to attend timely follow-up appointments. The latter barrier may occur for a variety of reasons such as difficulty in taking time off
work or school, poor access to transportation, living in a long distance from a health care facility, and the need for child care during the patient’s appointment. Health care providers may prescribe for their patients under the assumption that patients have equal access to care at the point of prescribing and that patients in pain have sufficient opportunity to fill the prescription (e.g., prescription drug coverage, access to a pharmacy that stocks opioids).
Patients also need access to a pharmacy that is appropriately stocked in order to fill an opioid prescription. A review of community pharmacies between 2007 and 2015, found that there was significant variation in the number of pharmacies per capita at the county level, that pharmacies are not distributed equally based on population, and that other factors also varied, including hours of operation, and the availability of home delivery service, multilingual staff, a drive-up window, and e-prescription options (Qato et al., 2017). Jefferson et al. (2019) found that 50% of patients who identified as black and who had a cancer diagnosis had difficulty obtaining opioids from a neighborhood pharmacy, primarily because the drugs were not in stock. An earlier study found that only 25% of pharmacies in predominantly nonwhite neighborhoods had sufficient opioid supplies to treat patients in severe pain, as compared with 72% of pharmacies in predominantly white neighborhoods (p<0.001) (Morrison et al., 2000). Some patients may live a considerable distance from a pharmacy (e.g., a rural area), requiring lengthy travel to fill a prescription. All of these factors can affect a patient’s ability to maintain a pain management regime. Bissonnette et al. (2016) found that home delivery and drive-up options may be especially important for elderly populations and that multilingual staff are essential for ensuring that non-English speaking patients are able to receive proper instructions on how to take prescribed opioid or nonopioid analgesics.
REFERENCES
Anderson, K.O., T.R. Mendoza, V. Valero, S.P. Richman, C. Russell, J. Hurley, C. DeLeon, P. Washington, G. Palos, R. Payne, and C.S. Cleeland. 2000. Minority cancer patients and their providers. Cancer 88(8):1929–1938.
APA (American Psychiatric Association). 2013. Diagnostic and statistical manual of mental disorders, 5th edition (DSM-5). Washington, DC: American Psychiatric Association Publishing.
Apfelbaum, J.L., C. Chen, S.S. Mehta, and T.J. Gan. 2003. Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesthesia & Analgesia 97(2):534–540.
Badreldin, N., W.A. Grobman, K.T. Chang, and L.M. Yee. 2018. Opioid prescribing patterns among postpartum women. American Journal of Obstetrics and Gynecology 219(1):e101–e103.
Baker, D.W. 2017. History of The Joint Commission’s pain standards: Lessons for today’s prescription opioid epidemic. JAMA 317(11):1117–1118.
Balsa, A.I., N. Seiler, T.G. McGuire, and M.G. Bloche. 2003. Clinical uncertainty and healthcare disparities. American Journal of Law & Medicine 29(2–3):203–219.
Balyan, R., M. Mecoli, R. Venkatasubramanian, V. Chidambaran, N. Kamos, S. Clay, D.L. Moore, J. Mavi, C.D. Glover, P. Szmuk, A. Vinks, and S. Sadhasivam. 2017. CYP2D6 pharmacogenetic and oxycodone pharmacokinetic association study in pediatric surgical patients. Pharmacogenomics 18(4):337–348.
Bartley, E.J., and R.B. Fillingim. 2013. Sex differences in pain: A brief review of clinical and experimental findings. British Journal of Anaesthiology 111(1):52–58.
Bateman, B.T., J.M. Franklin, K. Bykov, J. Avorn, W.H. Shrank, T.A. Brennan, J.E. Landon, J.P. Rathmell, K.F. Huybrechts, M.A. Fsicher, and N.K. Choudhry. 2016. Persistent opioid use following cesarean delivery: Patterns and predictors among opioid-naïve women. American Journal of Obstetrics and Gynecology 215:353.
Becker, W.C., L. Dorflinger, S.N. Edmond, L. Islam, A.A. Heapy, and L. Fraenkel. 2017. Barriers and facilitators to use of non-pharmacological treatments in chronic pain. BMC Family Practice 18(1):41.
Berde, C., and C. Greco. 2012. Pediatric regional anesthesia: Drawing inferences on safety from prospective registries and case reports. Anesthesia & Analgesia 115(6):1259–1262.
Berrettini, W. 2017. A brief review of the genetics and pharmacogenetics of opioid use disorders. Dialogues in Clinical Neuroscience 19(3):229–236.
Best, M.J., L.T. Buller, A.K. Klika, and W.K. Barsoum. 2015. Outcomes following primary total hip or knee arthroplasty in substance misusers. The Journal of Arthroplasty 30(7):1137–1141.
Birnie, K.A., C.T. Chambers, C.V. Fernandez, P.A. Forgeron, M.A. Latimer, P.J. McGrath, E.A. Cummings, and G.A. Finley. 2014. Hospitalized children continue to report undertreated and preventable pain. Pain Research & Management 19(4):198–204.
Bissonnette, S., L.M. Goeres, and D.S. Lee. 2016. Pharmacy density in rural and urban communities in the State of Oregon and the association with hospital readmission rates. Journal of the American Pharmecutical Association 56(5):533–537.
Brummett, C.M., A.M. Janda, C.M. Schueller, A. Tsodikov, M. Morris, D.A. Williams, and D.J. Clauw. 2013. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty: A prospective, observational cohort study. Anesthesiology 119(6):1434–1443.
Brummett, C.M., J.F. Waljee, J. Goesling, S. Moser, P. Lin, M.J. Englesbe, A.S.B. Bohnert, S. Kheterpal, and B.K. Nallamothu. 2017. New persistent opioid use after minor and major surgical procedures in U.S. adults. JAMA Surgery 152(6):e170504.
Campbell, C.M., and R.R. Edwards. 2012. Ethnic differences in pain and pain management. Pain Management 2(3):219–230.
Cascorbi, I. 2012. Drug interactions—Principles, examples and clinical consequences. Deutsches Arzteblatt International 109(33–34):546–556.
Cattaneo, S., P. Ingelmo, L. Scudeller, M. Gregori, D. Bugada, M. Baciarello, M. Marchesini, G. Alberio, M. Normanno, G.S. Jotti, T. Meschi, G. Fanelli, and A. Massimo. 2017. Sex differences in the daily rhythmicity of morphine consumption after major abdominal surgery. Journal of Opioid Management 13(2):85–94.
Cerda, M., J. Santaella, B.D. Marshall, J.H. Kim, and S.S. Martins. 2015. Nonmedical prescription opioid use in childhood and early adolescence predicts transitions to heroin use in young adulthood: A national study. Journal of Pediatrics 167(3):605–612.
Chang, H.Y., M. Daubresse, S.P. Kruszewski, and G.C. Alexander. 2014. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. American Journal of Emergency Medicine 32(5):421–431.
Chou, R., D.B. Gordon, O.A. de Leon-Casasola, J.M. Rosenberg, S. Bickler, T. Brennan, T. Carter, C.L. Cassidy, E.H. Chittenden, E. Degenhardt, S. Griffith, R. Manworren, B. McCarberg, R. Montgomery, J. Murphy, M.F. Perkal, S. Suresh, K. Sluka, S. Strassels, R. Thirlby, E. Viscusi, G. A. Walco, L. Warner, S.J. Weisman, and C.L. Wu. 2016. Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. The Journal of Pain 17(2):131–157.
CMS (Centers for Medicare & Medicaid Services). 2019. Drug plan coverage rules. https://www.medicare.gov/drug-coverage-part-d/what-drug-plans-cover/drug-plan-coverage-rules (accessed August 13, 2019).
Collier, R. 2018. A short history of pain management. CMAJ 190:e26–e27.
Committee on Practice Bulletins—Obstetrics. 2018. ACOG practice bulletin no. 198: Prevention and management of obstetric lacerations at vaginal delivery. Obstetrics & Gynecology 132(3):e87–e102.
Cooney, M.F., and K. Broglio. 2017. Acute pain management in opioid-tolerant individuals. The Journal for Nurse Practitioners 13(6):394–399.
Davis, C.S., A.J. Lieberman, H. Hernandez-Delgado, and C. Suba. 2019. Laws limiting the prescribing or dispensing of opioids for acute pain in the United States: A national systematic legal review. Drug and Alcohol Dependence 194:166–172.
Davison, S.N. 2019. Clinical pharmacology considerations in pain management in patients with advanced kidney failure. Clinical Journal of the American Society of Nephrology 14(6):917–931.
Deyo, R.A., M. Von Korff, and D. Duhrkoop. 2015. Opioids for low back pain. British Medical Journal 350:g6380.
Dowell, D., T.M. Haegerich, and R. Chou. 2016a. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 15(15):1624–1645.
Dowell, D., K. Zhang, R.K. Noonan, and J.M. Hockenberry. 2016b. Mandatory provider review and pain clinic laws reduce the amounts of opioids prescribed and overdose death rates. Health Affairs (Millwood) 35(10):1876–1883.
Edlund, M.J., B.C. Martin, M.-Y. Fan, A. Devries, J.B. Braden, and M.D. Sullivan. 2010. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: Results from the Troup study. Drug and Alcohol Dependence 112(1–2):90–98.
Edwards, C.L., R.B. Fillingim, and F. Keefe. 2001. Race, ethnicity and pain. Pain 94(2):133–137.
FDA (U.S. Food and Drug Administration). 2016. Highlights of prescribing information: Hydromorphone HCl extended-release tablets, for oral use, CII. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/202144s006lbl.pdf (accessed June 7, 2019).
FDA. 2018. FDA Drug Safety Communication: FDA requires labeling changes for prescription opioid cough and cold medicines to limit their use to adults 18 years and older. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-requires-labeling-changes-prescription-opioid-cough-and-cold (accessed July 11, 2019).
Fillingim, R.B., C.D. King, M.C. Ribeiro-Dasilva, B. Rahim-Williams, and J.L. Riley, 3rd. 2009. Sex, gender, and pain: A review of recent clinical and experimental findings. Journal of Pain 10(5):447–485.
Finley, G.A., J.M. Chorney, and L. Campbell. 2014. Not small adults: The emerging role of pediatric pain services. Canadian Journal of Anesthesia 61(2):180–187.
Friedman, B.W., A.A. Dym, M. Davitt, L. Holden, C. Solorzano, D. Esses, P.E. Bijur, and E.J. Gallagher. 2015. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: A randomized clinical trial. JAMA 314(15):1572–1580.
Furlan, A., L.E. Chaparro, E. Irvin, and A. Mailis-Gagnon. 2011. A comparison between enriched and nonenriched enrollment randomized withdrawal trials of opioids for chronic noncancer pain. Pain Research & Management 16(5):337–351.
Gadkaree, S.K., D.A. Shaye, J. Occhiogrosso, and L.N. Lee. 2019. Association between pain and patient satisfaction after rhinoplasty. JAMA Facial Plastic Surgery. doi: 10.1001/jamafacial.2019.0808. [Epub ahead of print].
Gan, T.J. 2017. Poorly controlled postoperative pain: Prevalence, consequences, and prevention. Journal of Pain Research 10:2287–2298.
Gan, T.J., A.S. Habib, T.E. Miller, W. White, and J.L. Apfelbaum. 2014. Incidence, patient satisfaction, and perceptions of post-surgical pain: Results from a U.S. national survey. Current Medical Research Opinion 30(1):149–160.
Garfield, R., A. Damico, and K. Orgera. 2016. The coverage gap: Uninsured poor adults in states that do not expand Medicaid. Kaiser Family Foundation. https://www.kff.org/medicaid/issue-brief/the-coverage-gap-uninsured-poor-adults-in-states-that-do-not-expand-medicaid (accessed September 27, 2019).
Goyal, M.K., N. Kuppermann, S.D. Cleary, S.J. Teach, and J.M. Chamberlain. 2015. Racial disparities in pain management of children with appendicitis in emergency departments. JAMA Pediatrics 169(11):996–1002.
Green, C.R., K.O. Anderson, T.A. Baker, L.C. Campbell, S. Decker, R.B. Fillingim, D.A. Kalauokalani, K.E. Lasch, C. Myers, R.C. Tait, K.H. Todd, and A.H. Vallerand. 2003. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Medicine 4(3):277–294.
Green, C.R., S.K. Ndao-Brumblay, A.M. Nagrant, T.A. Baker, and E. Rothman. 2004. Race, age, and gender influences among clusters of African American and white patients with chronic pain. The Journal of Pain 5(3):171–182.
Hadjistavropoulos, H.D., K.D. Craig, R.E. Grunau, and M.F. Whitfield. 1997. Judging pain in infants: Behavioural, contextual, and developmental determinants. Pain 73(3):319–324.
Hah, J.M., B.T. Bateman, J. Ratliff, C. Curtin, and E. Sun. 2017. Chronic opioid use after surgery: Implications for perioperative management in the face of the opioid epidemic. Anesthesia & Analgesia 125(5):1733–1740.
Hailes, H.P., R. Yu, A. Danese, and S. Faze. 2019. Long-term outcomes of childhood sexual abuse: An umbrella review. The Lancet Psychiatry 6(10):830–839.
Haller, D.L., and M.C. Acosta. 2010. Characteristics of pain patients with opioid-use disorder. Psychosomatics 51(3):257–266.
Harbaugh, C.M., and S.K. Gadepalli. 2019. Pediatric postoperative opioid prescribing and the opioid crisis. Current Opinion in Pediatrics 31:378–385.
Hernandez, I., M. He, M.M. Brooks, and Y. Zhang. 2018. Exposure-response association between concurrent opioid and benzodiazepine use and risk of opioid-related overdose in Medicare Part D beneficiaries. JAMA Network Open 1(2):e180919.
Heyward, J., C.M. Jones, W.M. Compton, D.H. Lin, J.L. Losby, I.B. Murimi, G.T. Baldwin, J.M. Ballreich, D.A. Thomas, M.C. Bicket, L. Porter, J.C. Tierce, and G.C. Alexander. 2018. Coverage of nonpharmacologic treatments for low back pain among U.S. public and private insurers. JAMA New Open 1(6):e183044.
HHS (U.S. Department of Health and Human Services). 2016. National pain strategy: A comprehensive population health-level strategy for pain. https://www.iprcc.nih.gov/sites/default/files/HHSNational_Pain_Strategy_508C.pdf (accessed June 6, 2019).
HHS. n.d. Quick guide to health literacy: Fact sheet. https://health.gov/communication/literacy/quickguide/factsbasic.htm (accessed June 17, 2019).
Hill, M.V., R.S. Stucke, S.E. Billmeier, J.L. Kelly, and R.J. Barth. 2018. Guideline for discharge opioid prescriptions after inpatient general surgical procedures. Journal of the American College of Surgeons 226(6):996–1003.
Hilliard, P.E., J. Waljee, S. Moser, L. Metz, M. Mathis, J. Goesling, D. Cron, D.J. Clauw, M. Englesbe, G. Abecasis, and C.M. Brummett. 2018. Prevalence of preoperative opioid use and characteristics associated with opioid use among patients presenting for surgery. JAMA Surgery 153(10):929–937.
Horl, W.H. 2010. Nonsteroidal anti-inflammatory drugs and the kidney. Pharmaecuticals 3(3):2291–2321.
Hudgins, J.D., J.J. Porter, M.C. Monuteaux, and F.T. Bourgeois. 2019. Trends in opioid prescribing for adolescents and young adults in ambulatory care settings. Pediatrics 143(6):e20181578.
Huskamp, H.A., L.E. Riedel, C.L. Barry, and A.B. Busch. 2018. Coverage of medications that treat opioid use disorder and opioids for pain management in marketplace plans, 2017. Medical Care 56(6):505–509.
Huxtable, C.A., L.J. Roberts, A.A. Somogyi, and P.E. Macintyre. 2011. Acute pain management in opioid-tolerant patients: A growing challenge. Anaesthesia and Intensive Care 39(5):804–823.
Hwang, C.S., E.M. Kang, C.J. Kornegay, J.A. Staffa, C.M. Jones, and J.K. McAninch. 2016. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002−2014. American Journal of Preventive Medicine 51(2):151–160.
Iocolano, C.F. 2000. Perioperative pain management in the chemically dependent patient. Journal of PeriAnesthesia Nursing 15(5):329–347.
IOM (Institute of Medicine). 2003. Unequal treatment: Confronting racial and ethnic disparities in health care. Washington, DC: The National Academies Press.
IOM. 2011. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC: The National Academies Press.
Jandali, D.B., D. Vaughan, M. Eggerstedt, A. Ganti, H. Scheltens, E.A. Ramirez, P.C. Revenaugh, S. Al-Khudari, R.M. Smith, and K.M. Stenson. 2019. Enhanced recovery after surgery in head and neck surgery: Reduced opioid use and length of stay. The Laryngoscope. July 17. [Epub ahead of print].
Jefferson, K., T. Quest, and K.A. Yeager. 2019. Factors associated with black cancer patients’ ability to obtain their opioid prescriptions at the pharmacy. Journal of Palliative Medicine 22(9):1143–1148.
Jimenez, N., D.L. Jackson, C. Zhou, N.C. Ayala, and B.E. Ebel. 2019. Postoperative pain management in children, parental English proficiency, and access to interpretation. Hospital Pediatrics 4(1):23–30.
Jones, C.M., L.J. Paulozzi, and K.A. Mack. 2014. Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths—United States, 2010. Morbidity and Mortality Weekly Report 63(40):881–885.
Kaye, A.D., A. Baluch, and J.T. Scott. 2010. Pain management in the elderly population: A review. The Ochsner Journal 10(3):179–187.
Kea, B., R. Fu, R.A. Lowe, and B.C. Sun. 2016. Interpreting the National Hospital Ambulatory Medical Care Survey: United States emergency department opioid prescribing, 2006–2010. Academic Emergency Medicine 23(2):159–165.
Keene, D.D., W.E. Rea, and D. Aldington. 2011. Acute pain management in trauma. Trauma 13(3):167–179.
Kelly, L.E., M. Rieder, J. van den Anker, B. Malkin, C. Ross, M.N. Neely, B. Carleton, M.R. Hayden, P. Madadi, and G. Koren. 2012. More codeine fatalities after tonsillectomy in North American children. Pediatrics 129(5):e1343–e1347.
Kelly, M.P., T.E. Calkins, C. Culvern, M. Kogan, and C.J. Della Valle. 2018. Inpatient versus outpatient hip and knee arthroplasty: Which has higher patient satisfaction? Journal of Arthroplasty 33(11):3402–3406.
Kelley-Quon, L.I., J. Cho, D.R. Strong, R.A. Miech, J.L. Barrington-Trimis, A. Kechter, and A.M. Leventhal. 2019. Association of nonmedical prescription opioid use with subsequent heroin use initiation in adolescents. JAMA Pediatrics July 8:e191750. [Epub ahead of print].
Kennedy, P., R. Joshi, and A. Dhawan. 2019. The effect of psychosocial factors on outcomes in patients with rotator cuff tears: A systematic review. Arthroscopy 35(9):2698–2706.
Kent, M.L., P.J. Tighe, I. Belfer, T.J. Brennan, S. Bruehl, C.M. Brummett, C.C. Buckenmaier, A. Buvanendran, R.I. Cohen, P. Desjardins, D. Edwards, R. Fillingim, J. Gewandter, D.B. Gordon, R.W. Hurley, H. Kehlet, J.D. Loeser, S. Mackey, S.A. McLean, R. Polomano, S. Rahman, S. Raja, M. Rowbotham, S. Suresh, B. Schachtel, K. Schreiber, M. Schumacher, B. Stacey, S. Stanos, K. Todd, D.C. Turk, S.J. Weisman, C. Wu, D.B. Carr, R.H. Dworkin, and G. Terman. 2017. The ACTTION–APS–AAPM pain taxonomy (AAAPT) multidimensional approach to classifying acute pain conditions. The Journal of Pain 18(5):479–489.
Komatsu, R., B. Carvalho, and P.D. Flood. 2017. Recovery after nulliparous birth: A detailed analysis of pain analgesia and recovery of function. Anesthesiology 127(4):684–694.
Koolen, S.L.W., and C.C.D. Van der Rijt. 2017. Is there a role for pharmacogenetics in the dosing of fentanyl? Pharmacogenomics 18(5):417–419.
Kopecky, E.A. 2019. Opioid pharmcology: Developmental effects on opioid metabolism. Clnical Journal of Pain 35(6):481–486.
Krauss, B.S., L. Calligaris, S.M. Green, and E. Barbi. 2016. Current concepts in management of pain in children in the emergency department. The Lancet 387(10013):83–92.
Lacey, R.J., P. Campbell, M. Lewis, and J. Protheroe. 2018. The impact of inadequate health literacy in a population with musculoskeletal pain. Health Literacy Research and Practice 2(4):e215–e220.
Lee, P., M. Le Saux, R. Siegel, M. Goyal, C. Chen, Y. Ma, and A.C. Meltzer. 2019. Racial and ethnic disparities in the management of acute pain in U.S. emergency departments: Meta-analysis and systematic review. American Journal of Emergency Medicine 37(9):1770–1777.
Linares, O.A., D. Daly, A.D. Linares, D. Stefanovski, and R.C. Boston. 2014. Personalized oxycodone dosing: Using pharmacogenetic testing and clinical pharmacokinetics to reduce toxicity risk and increase effectiveness. Pain Medicine 15(5):791–806.
Lloret Linares, C., X. Decleves, J.M. Oppert, A. Basdevant, K. Clement, C. Bardin, J.M. Scherrmann, J.P. Lepine, J.F. Bergmann, and S. Mouly. 2009. Pharmacology of morphine in obese patients: Clinical implications. Clinical Pharmacokinetics 48(1):635–651.
Lloret-Linares, C., A. Lopes, X. Declèves, A. Serrie, S. Mouly, J.F. Bergmann, and S. Perrot. 2013. Challenges in the optimisation of post-operative pain management with opioids in obese patients: A literature review. Obesity Surgery 23(9):1458–1475.
Lovegrove, M.C., D. Dowell, A.I. Geller, S.K. Goring, K.O. Rose, N.J. Weidle, and D.S. Budnitz, 2019. U.S. emergency department visits for acute harms from prescription opioid use, 2016–2017. American Journal of Public Health 109:784–791.
Ly, D.P. 2019. Racial and ethnic disparities in the evaluation and management of pain in the outpatient setting, 2006–2015. Pain Medicine 20(2):223–232.
Madadi, P., J. Sistonen, G. Silverman, R. Gladdy, C.J. Ross, B.C. Carleton, J.C. Carvalho, M.R. Hayden, and G. Koren. 2013. Life-threatening adverse events following therapeutic opioid administration in adults: Is pharmacogenetic analysis useful? Pain Research and Management 18(3):133–136.
Majestic, M. 2018. Opioid Potentiators Memo. U.S. Department of Health and Human Services, Centers for Medicare & Medicaid Services, Center for Program Integrity. https://mmp.michigancompletehealth.com/content/dam/centene/michigan-complete-health/pdfs/Opioid_Potentiators_Memo.pdf (accessed November 15, 2019).
Malcolm, C. 2015. Acute pain management in the older person. Journal of Perioperative Practice 25(7–8):134–139.
Mathur, V.A., J.A. Richeson, J.A. Paice, M. Muzyka, and J.Y. Chiao. 2014. Racial bias in pain perception and response: Experimental examination of automatic and deliberate processes. The Journal of Pain 15(5):476–484.
McAuliffe, L., D. Brown, and D. Fetherstonhaugh. 2012. Pain and dementia: An overview of the literature. International Journal of Older People Nursing 7(3):219–226.
Meghani, S.H., E. Byun, and R.M. Gallagher. 2012. Time to take stock: A meta-analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Medicine 13(2):150–174.
Meints, S.M., C. Mosher, K.L. Rand, L. Ashburn-Nardo L, and A.T. Hirsh. An experimental investigation of the relationships among race, prayer, and pain. Scandanivan Journal of Pain 18(3):545–553.
Mercadante, S. 2015. Opioid metabolism and clinical aspects. European Journal of Pharmacology 769:71–78.
Michaelides, A., and P. Zis. 2019. Depression, anxiety and acute pain: Links and management challenges. Postgraduate Medicine 12:1–7.
Miech, R., L. Johnston, P.M. O’Malley, K.M. Keyes, and K. Heard. 2015. Prescription opioids in adolescence and future opioid misuse. Pediatrics 136(5):e1169–e1177.
Morrison, R.S., and A.L. Siu. 2000. A comparison of pain and its treatment in advanced dementia and cognitively intact patients with hip fracture. Journal of Pain and Symptom Management 19(4):240–248.
Morrison, R.S., S. Wallenstein, D.K. Natale, R.S. Senzel, and L.-L. Huang. 2000. “We don’t carry that”—Failure of pharmacies in predominantly nonwhite neighborhoods to stock opioid analgesics. New England Journal of Medicine 342(14):1023–1026.
Mossey, J.M. 2011. Defining racial and ethnic disparities in pain management. Clinical Orthopaedics and Related Research 469(7):1859–1870.
Mundkur, M.L., K. Rough, K.F. Huybrechts, R. Levin, J.J. Gagne, R.J. Desai, E. Patorno, N.K. Choudhry, and B.T. Bateman. 2018. Patterns of opioid initiation at first visits for pain in United States primary care settings. Pharmacoepidemiology and Drug Safety 27(5):495–503.
Mundkur, M.L., J.M. Franklin, Y. Abdia, K.F. Huybrechts, E. Patorno, J.J. Gagne, T.E. Meyer, J. Staffa, and B.T. Bateman. 2019. Days’ supply of initial opioid analgesic prescriptions and additional fills for acute pain conditions treated in the primary care setting—United States, 2014. Morbidity and Mortality Weekly Report 68(6):140–143.
Murphy, D.L., J.A. Lebin, S.G. Severtson, H.A. Olsen, N. Dasgupta, and R.C. Dart. 2018. Comparative rates of mortality and serious adverse effects among commonly prescribed opioid analgesics. Drug Safety 41:787–795.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2017. Pain management and the opioid epidemic: Balancing societal and individual benefits and risks of prescription opioid use. Washington, DC: The National Academies Press.
NIDA (National Institute on Drug Abuse). 2018. Opioid prescribers can play a key role in stopping the opioid overdose epidemic. https://www.drugabuse.gov/publications/improving-opioid-prescribing/improving-opioid-prescribing (accessed November 1, 2019).
NLM (National Library of Medicine). n.d. DailyMed. https://dailymed.nlm.nih.gov/dailymed/index.cfm (accessed November 14, 2019).
Otto, A., K. Emery, and J.N. Côté. 2019. Sex differences in perceptual responses to experimental pain before and after an experimental fatiguing arm task. Biological Sex Differences 10(1):39.
Overholser, B.R., and D.R. Foster. 2011. Opioid pharmacokinetic drug–drug interactions. American Journal of Managed Care 17:S276–S287.
Paller, C.J., C.M. Campbell, R.R. Edwards, and A.S. Dobs. 2009. Sex-based differences in pain perception and treatment. Pain Medicine 10(2):289–299.
Park, T.W., R. Saitz, D. Ganoczy, M.A. Ilgen, and A.S. Bohnert. 2015. Benzodiazepine prescribing patterns and deaths from drug overdose among U.S. veterans receiving opioid analgesics: Case-cohort study. British Medical Journal 350:h2698.
Patanwala, A.E., K.L. Holmes, and B.L. Erstad. 2014. Analgesic response to morphine in obese and morbidly obese patients in the emergency department. Emergency Medicine Journal 31:13.
Pieretti, S., A. Di Giannuario, R. Di Giovannandrea, F. Marzoli, G. Piccaro, P. Minosi, and A.M. Aloisi. 2016. Gender differences in pain and its relief. Annali dell’Istituto Superiore di Sanità 52(2):184–189.
Pletcher, M.J., S.G. Kertesz, M.A. Kohn, and R. Gonzales. 2008. Trends in opioid prescribing by race/ethnicity for patients seeking care in U.S. emergency departments. JAMA 299(1):70–78.
Qato, D.M., S. Zenk, J. Wilder, R. Harrington, D. Gaskin, and G.C. Alexander. 2017. The availability of pharmacies in the United States: 2007–2015. PLOS ONE 12(8):e0183172.
Quinn, K., B.C. Frueh, J. Scheidell, D. Schatz, F. Scanlon, and M.R. Khan. 2019. Internalizing and externalizing factors on the pathway from adverse experiences in childhood to non-medical prescription opioid use in adulthood. Drug and Alcohol Dependence 197:212–219.
Rabbitts, J.A., E. Fisher, B.N. Rosenbloom, and T.M. Palermo. 2017. Prevalence and predictors of chronic postsurgical pain in children: A systematic review and meta-analysis. The Journal of Pain 18(6):605–614.
Radcliff, J.A., R.M. Rafeq, J.F. Bowen, L. Pontiggia, and S. Sen. 2017. Predictors of response in emergency department patients receiving intravenous opioids for severe pain. Pharmacotherapy 37(7):799–805.
Raymond, B.L., B.T. Kook, and M.G. Richardson. 2018. The opioid epidemic and pregnancy: Implications for anesthetic care. Current Opinion in Anesthesiology 31(3):243–250.
Romano, R.R., A.R. Anderson, M.D. Failla, M.S. Dietrich, S. Atalla, M.A. Carter, and T.B. Monroe. 2018. Sex differences in associations of cognitive function with perceptions of pain in older adults. Journal of Alzheimers Disease 70(3):715–722.
Rui, P., K. Kang, and J. Ashman. 2016. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf (accessed June 5, 2019).
Schuchat, A., D. Houry, and G.P. Guy, Jr. 2017. New data on opioid use and prescribing in the United States. JAMA 318(5):425–426.
Schug, S.A., and A. Raymann. 2011. Postoperative pain management of the obese patient. Best Practice & Research: Clinical Anesthesiology 25(1):73–81.
Schuler, M., N. Njoo, M. Hestermann, P. Oster, and K. Hauer. 2004. Acute and chronic pain in geriatrics: Clinical characteristics of pain and the influence of cognition. Pain Medicine 5(3):253–262.
Simmonds, M.J., E.P. Finley, S. Vale, M.J. Pugh, and B.J. Turner. 2015. A qualitative study of veterans on long-term opioid analgesics: Barriers and facilitators to multimodality pain management. Pain Medicine 16(4):726–732.
Simpson, J.C., X. Bao, and A. Agarwala. 2019. Pain management in enhanced recovery after surgery (ERAS) protocols. Clinics in Colon and Rectal Surgery 32(2):121–128.
Sinatra, R. 2010. Causes and consequences of inadequate management of acute pain. Pain Medicine 11(12):1859–1871.
Singhal, A., Y.-Y. Tien, and R.Y. Hsia. 2016. Racial-ethnic disparities in opioid prescriptions at emergency department visits for conditions commonly associated with prescription drug abuse. PLOS ONE 11(8):e0159224.
Smith, H.S. 2009. Opioid metabolism. Mayo Clinic Proceedings 84(7):613–624.
Soleimanpour, H., S. Safari, K. Shahsavari Nia, S. Sanaie, and S.M. Alavian. 2016. Opioid drugs in patients with liver disease: A systematic review. Hepatitis Monthly 16(4):e32636.
Staton, L.J., M. Panda, I. Chen, I. Genao, J. Kurz, M. Pasanen, A.J. Mechaber, M. Menon, J. O’Rorke, J. Wood, E. Rosenberg, C. Faeslis, T. Carey, D. Calleson, and S. Cykert. 2007. When race matters: Disagreement in pain perception between patients and their physicians in primary care. Journal of the National Medical Association 99(5):532–538.
Sun, E.C., B.D. Darnall, L.C. Baker, and S. Mackey. 2016. Incidence of and risk factors for chronic opioid use among opioid-naïve patients in the postoperative period. JAMA Internal Medicine 176(9):1286–1293.
Swadi, H. 1999. Individual risk factors for adolescent substance use. Drug and Alcohol Dependence 55(3):209–224.
Thatcher, D.L., and D.B. Clark. 2008. Adolescents at risk for substance use disorders: Role of psychological dysregulation, endophenotypes, and environmental influences. Alcohol Research & Health 31(2):168–176.
The Joint Commission. 2016. Joint Commission statement on pain management. https://www.jointcommission.org/joint_commission_statement_on_pain_management (accessed April 15, 2019).
Trawalter, S., K.M. Hoffman, and A. Waytz. 2012. Racial bias in perceptions of others’ pain. PLOS ONE 7(11):e48546.
van Ryn, M. 2002. Research on the provider contribution to race/ethnicity disparities in medical care. Medical Care—Philadelphia 40(Part 1):I-140–I-151.
Velanovich, V. 2000. Laparoscopic vs. open surgery. Surgical Endoscopy 14(1):16–21.
Verghese, S.T., and R.S. Hannallah. 2010. Acute pain management in children. Journal of Pain Research 3:105–123.
Weeks, J. 2016. Influential U.S. medical organizations call for insurance coverage of non-pharmacologic approaches to pain. Journal of Alternative and Complementary Medicine 22(12):947–949.
White, A.G., H.G. Birnbaum, M. Schiller, J. Tang, and N.P. Katz. 2009. Analytic models to identify patients at risk for prescription opioid abuse. American Journal of Managed Care 15(12):897–906.
Whitesell, M., A. Bachand, J. Peel, and M. Brown. 2013. Familial, social, and individual factors contributing to risk for adolescent substance use. Journal of Addiction 2013:9.
WHO (World Health Organization). 1990. Cancer pain relief and palliative care: Report of a WHO expert committee. Geneva, Switzerland: WHO.
Xia, S., D. Choe, L. Hernandez, and A. Birnbaum. 2014. Does initial hydromorphone relieve pain best if dosing is fixed or weight based? Annals of Emergency Medicine 63(6):692–698.
Zimmerman, G.M., and C. Farrell. 2017. Parents, peers, perceived risk of harm, and the neighborhood: Contextualizing key influences on adolescent substance use. Youth Adolescence 46(1):228–247.
This page intentionally left blank.






















