1
Introduction
BACKGROUND FOR THE STUDY
From many perspectives, human milk is the biologic norm for feeding the human infant during the first 6 months of life, and it is a preferred food from 6 to 12 months. Human milk is a complex food, and its biologic effects extend well beyond its known nutritional value; however, human milk composition and the complexity of its composition is not wholly known or understood. Thus, describing the composition of human milk, as well as both the individual and combined effects of milk components on infant growth and development, is central to optimizing infant health and development (Neville et al., 2012).
Human milk has a multitude of nutritive and nonnutritive functions and components. These include supporting healthy growth, protecting against infection, supporting immune and intestinal health and cognitive development, and establishing the unique gastrointestinal microbiome (Neville et al., 2012). New analytical tools are now available that provide more quantitative and sensitive analytical methods than were available in recent decades, and these tools are transforming scientific understanding of the diversity and uniqueness of the composition of human milk. For example, human milk has relatively high concentrations of molecules that foster brain development including choline, sialic acid and long-chain polyunsaturated fatty acids (LC-PUFA) (Koletzko et al., 2008; Ojo-Okunola et al., 2020; Salem and Van Dael, 2020). Prenatal exposure to choline and its long-term effects on hippocampal development, learning, memory, and emotional behavior have been studied widely in animal models; however, similarly relevant data for humans are lacking (Blusztajn and Mellott, 2013; Mun et al., 2019). More recently, an important role for LC-PUFA in brain maturation has been recognized. These lipids, particularly docosahexaenoic acid (DHA) and arachidonic acid (AA), are present in human milk at far higher concentrations than in bovine milk. Additionally, they can be increased in human milk by maternal diets containing large amounts of fish oil (Martin et al., 2016; Middleton et al., 2018).
Numerous substances, such as oligosaccharides (e.g., small-chain galactooligosaccharides and long-chain fructo-oligosaccharides) have been identified in human milk as potential dietary compounds (Donovan and Comstock, 2016; Plaza-Diaz et al., 2018; Smilowitz et al., 2013). Taken together, new and emerging evidence about the composition of human milk will contribute to better understanding nutrient requirements during infancy and the value of human milk to human health. Furthermore, defining human milk composition, volume, and the myriad factors that influence milk components is needed for developing future Dietary Reference Intake (DRI) values for nutrient intake during the first 12 months of life and beyond.
The request from the U.S. Department of Agriculture (USDA) and the U.S. Department of Health and Human Services (HHS) to scan the new and emerging evidence describing the nutrient content of human milk arose in anticipation of future DRI reviews that would require updated information about nutritional requirements for infants from birth to 5.9 months of age, and from 6 to 12 months of age that would contribute to the totality of data needed to develop
DRI recommendations. The primary data source for infant nutritional requirements is the nutrient content of human milk. However, while knowledge of the range of nutritional compounds in human milk has expanded, only those nutrients with existing DRI values were to be considered in the evidence scan.
COMMITTEE’S TASK AND APPROACH
USDA’s Agricultural Research Service (USDA-ARS), HHS, and the U.S. Food and Drug Administration’s (FDA’s) Center for Food Safety and Applied Nutrition (FDA-CFSAN) requested the National Academies of Sciences, Engineering, and Medicine (the National Academies) to carry out an evidence scan on new and emerging evidence describing the nutrient content of human milk as well as the volume of milk consumed by healthy breastfed infants (see Box 1-1).
In response to the USDA-ARS and FDA-CFSAN request, the Health and Medicine Division of the National Academies established a committee with expertise in epidemiology, public health, physiology of lactation, nutrient composition of human milk, infant growth and development, nutrient requirements during pregnancy and in infancy, DRIs, and systematic evidence reviews. The committee held an open meeting with subject-matter experts (see Appendix B) and worked in closed session and by conference calls to deliberate on its task. To guide its work, the committee constructed a model for the task (see Figure 1-1).
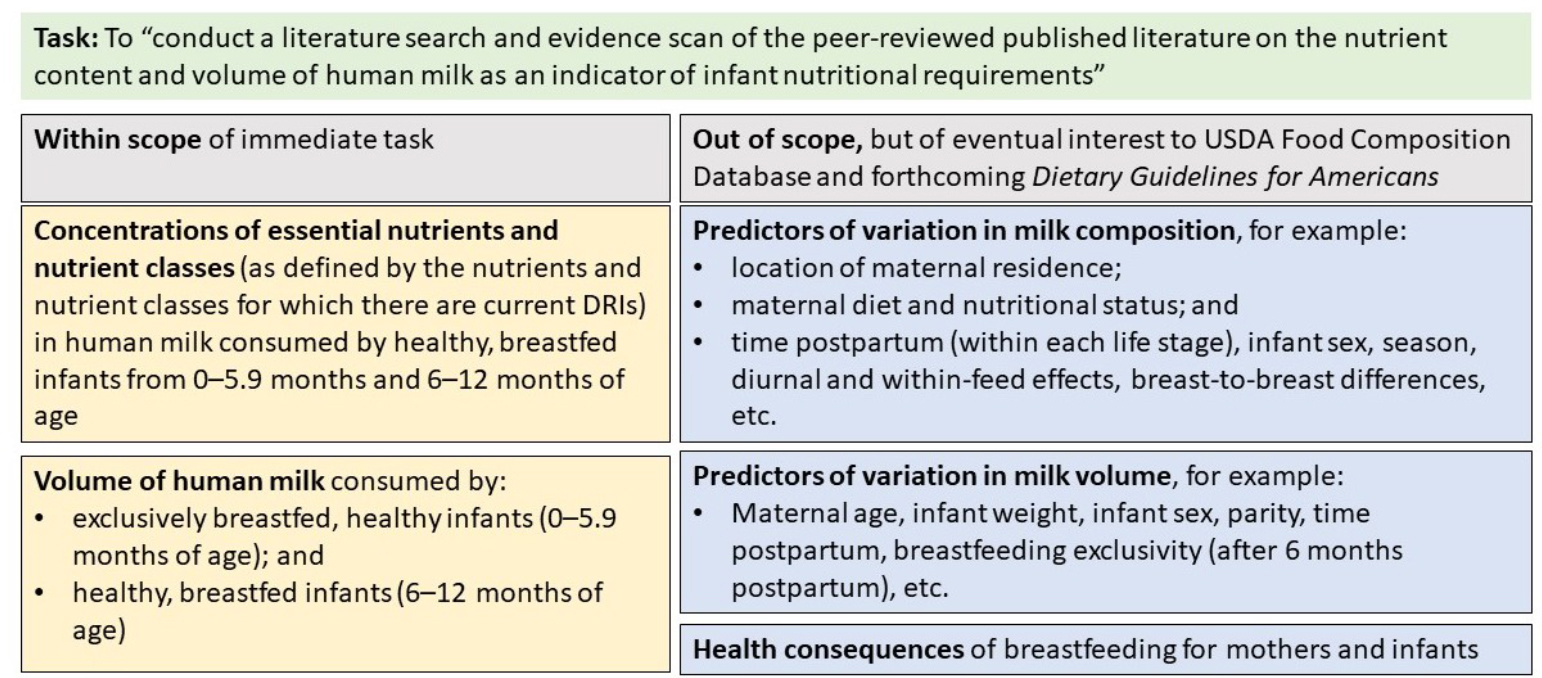
NOTE: DRI = Dietary Reference Intake; USDA = U.S. Department of Agriculture.
ORGANIZATION OF THE REPORT
This report is organized into four chapters. In this chapter, the background for the study, the Statement of Task, and the study strategy are described. In Chapter 2, the committee describes its search strategy, including the prespecified criteria for assessing relevant evidence, and presents a flow diagram to illustrate the evidence-screening and review process. Chapter 3 presents a table of the evidence-scanning results and the committee’s assessment of relevant evidence. Chapter 4 provides the committee’s discussion of the findings and perspective on future directions.
REFERENCES
Blusztajn, J. K., and T. J. Mellott. 2013. Neuroprotective actions of perinatal choline nutrition. Clinical Chemistry and Laboratory Medicine 51(3):591-599.
Donovan, S. M., and S. S. Comstock. 2016. Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Annals of Nutrition and Metabolism 69(Suppl 2):42-51.
Koletzko, B., E. Lien, C. Agostoni, H. Bohles, C. Campoy, I. Cetin, T. Decsi, J. W. Dudenhausen, C. Dupont, S. Forsyth, I. Hoesli, W. Holzgreve, A. Lapillonne, G. Putet, N. J. Secher, M. Symonds, H. Szajewska, P. Willatts, R. Uauy, and World Association of Perinatal Medicine Dietary Guidelines Working Group. 2008. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: Review of current knowledge and consensus recommendations. Journal of Perinatal Medicine 36(1):5-14.
Martin, C. R., P. R. Ling, and G. L. Blackburn. 2016. Review of infant feeding: Key features of breast milk and infant formula. Nutrients 8(5):279.
Middleton, P., J. C. Gomersall, J. F. Gould, E. Shepherd, S. F. Olsen, and M. Makrides. 2018. Omega-3 fatty acid addition during pregnancy. Cochrane Database Systemic Reviews 11:CD003402.
Mun, J. G., L. L. Legette, C. J. Ikonte, and S. H. Mitmesser. 2019. Choline and DHA in maternal and infant nutrition: Synergistic implications in brain and eye health. Nutrients 11(5):1125.
Neville, M. C., S. M. Anderson, J. L. McManaman, T. M. Badger, M. Bunik, N. Contractor, T. Crume, D. Dabelea, S. M. Donovan, N. Forman, D. N. Frank, J. E. Friedman, J. B. German, A. Goldman, D. Hadsell, M. Hambidge, K. Hinde, N. D. Horseman, R. C. Hovey, E. Janoff, N. F. Krebs, C. B. Lebrilla, D. G. Lemay, P. S. MacLean, P. Meier, A. L. Morrow, J. Neu, L. A. Nommsen-Rivers, D. J. Raiten, M. Rijnkels, V. Seewaldt, B. D. Shur, J. VanHouten, and P. Williamson. 2012. Lactation and neonatal nutrition: Defining and refining the critical questions. Journal of Mammary Gland Biology and Neoplasia 17(2):167-188.
Ojo-Okunola, A., S. Cacciatore, M. P. Nicol, and E. du Toit. 2020. The determinants of the human milk metabolome and its role in infant health. Metabolites 10(2):77.
Plaza-Diaz, J., L. Fontana, and A. Gil. 2018. Human milk oligosaccharides and immune system development. Nutrients 10(8):1038.
Salem, N., Jr., and P. Van Dael. 2020. Arachidonic acid in human milk. Nutrients 12(3):626.
Smilowitz, J. T., A. O’Sullivan, D. Barile, J. B. German, B. Lonnerdal, and C. M. Slupsky. 2013. The human milk metabolome reveals diverse oligosaccharide profiles. Journal of Nutrition 143(11):1709-1718.




