4
New Control Approaches to Enable Quality Assurance and Process Capability
Innovations in pharmaceutical manufacturing will require modern process-control approaches to support quality assurance and process capability, particularly for complex processes and products. In the pharmaceutical industry, control strategy is defined as a “planned set of controls, derived from current product and process understanding, that assures process performance and product quality” (ICH 2009, p. 15). Although that definition is broad and encompasses much more than just engineering controls, the main goal of any control strategy (engineering or administrative)1 is to maintain a system in a state of control to minimize the chances of producing a product with poor quality characteristics (that is, to ensure quality) and to segregate, if appropriate, such materials effectively if departures from quality expectations are encountered. In this chapter, the committee discusses novel technologies and engineering applications that can be used to ensure process-outcome quality and thus increase manufacturing-process capability. New approaches for process and product sensing, data analysis and modeling, artificial intelligence (AI) and machine learning (ML) methods, and advanced process control are highlighted, and technical and regulatory challenges associated with the technologies and some recommendations for overcoming them are also provided.
SYSTEMS
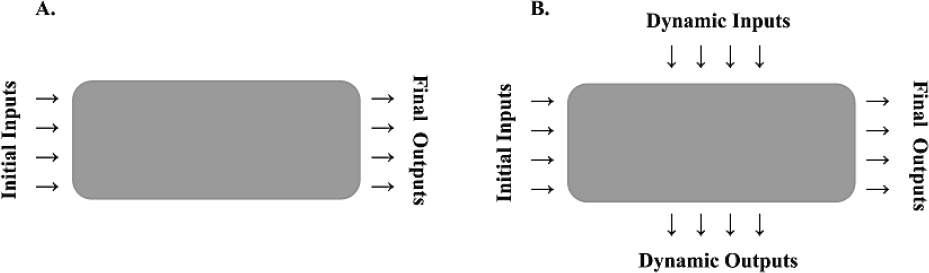
Before discussing the various components of control strategy, it is important to clarify the concept of systems and to make a distinction between simple and complex systems (see Figure 4-1). System has been defined as “groups or combinations of interrelated, interdependent, or interacting elements forming collective entities” (Arnold and Wade 2015, p. 675). Pharmaceutical-operation systems can exhibit different degrees of complexity. Complex systems tend to have higher degrees of freedom (variables), behave nonlinearly, and exhibit multiple variable interdependences. An example of a complex system is the cell-based synthesis of monoclonal antibodies using bioreactors. During their production, the system will exhibit nonlinear relationships between variables and dynamic outputs that affect each other (for example, the interrelationship between ammonia concentrations and cell
___________________
1 Administrative controls are the use of training, policies, and procedures to dictate how humans work, whereas engineering controls are the controls built into systems, equipment, and facilities by using technology.
density) and thus do not depend solely on process inputs. Capture of the interdependences inherent in complex systems requires much deeper process understanding; thus, the predictability of such systems might not be as high as that of simpler systems. Consequently, complex systems impose greater demands on the control strategy.
In simple systems, the final outputs of the process depend solely on measurable inputs. An example of a simple system is the process of compressing granules into tablets in which the granules have been preprocessed to provide the desired composition and structure for tablet formation. During the compression process, tablet weight and hardness depend on tablet-press inputs and granule attributes, but the process has no dynamic inputs or dynamic outputs beyond the control of humidity, which can affect plasticity. Thus, outputs, such as tablet weight and hardness, can be predicted and controlled more easily than, for example, glycosylation with mannose-type N-glycans in the production of monoclonal antibodies.
As pharmaceutical manufacturing processes become more integrated, their complexity as systems will increase; this is the case for advanced manufacturing applications, such as continuous manufacturing and intensified operations (Huang et al. 2020). The complexity of pharmaceutical processes has implications for the measurement, modeling, and control technologies used in their design and operation. The measurement of critical quality attributes and process parameters might require a broader and more sophisticated portfolio of sensor technologies. The models, although based on equations rooted in fundamental knowledge, will typically need to be supplemented with data-derived relationships, perhaps involving ML, that span the knowledge gap. The control systems might require a portfolio of hierarchical, model-based and adaptive control technologies. AI and specifically ML methods might need to play substantial roles in predicting and controlling the performance of complex pharmaceutical-manufacturing systems.
SENSORS
Sensors or analyzers are devices used to detect or measure a system characteristic or property. From a process perspective, sensing can be accomplished in three main configurations: in-line, at-line, and off-line. In-line measurements are taken directly from the process (for example, a pH measurement inside a reactor). At-line measurements are taken next to the process (for example, a tablet-weighing station near a tableting machine), typically with automatic sampling. Off-line measurements are taken outside the manufacturing suite (for example, an impurity measurement in a quality-assurance laboratory). Although all the sensors provide useful information about the manufacturing process, only in-line and some at-line sensors can be considered process analyzers because only they can provide timely information on the health of the process to support process-control decisions. Offline sensors, typically laboratory analytic instruments, are commonly used to measure the final quality of a product, to ensure thorough product characterization during development, or to develop calibrations for in-line and at-line sensors.
Innovative Off-Line Analytic Methods and Sensors to Support Product Development
During the pharmaceutical-development phase, information is obtained through process studies that establish scientific understanding of the product and processes. Off-line sensors tend to provide the more detailed information about the chemical and physical characteristics of materials that helps to build that understanding. However, these analytic tools do not provide real-time results and so are deployed in off-line configurations to obtain data that require high resolution, such as data on molecular structure, glycosylation, impurities, and crystal structure. Several innovations in such analytic methods have advanced to the stage where they will support filings within the next 5 or more years.
One innovative analytic method that has been gaining attention is the liquid chromatography–mass spectrometry (LC–MS) multi-attribute method (MAM). MAM is being studied to monitor and quantify molecular product-quality attributes and product-related or process-related impurities of post-translational modifications of biologics (Rogers et al. 2015). MAM can be used “not only during product characterization, formulation development, stability testing, and development of the manufacturing process, but also as a platform quality control method in dispositioning clinical materials for both innovative biotherapeutics and biosimilars” (Rogers et al. 2017, p. 1). MAM can be used to set specifications in a more targeted manner and to perform new peak detection, which can be used as a sensitive impurity test (Schiel 2020; Starkey 2020). Although MAM is an off-line sensing approach, it is also being investigated for use in in-line sensing (Swann 2020).
Another tool that should see increasing use in the future for the evaluation of therapeutic proteins is two-dimensional nuclear magnetic resonance spectroscopy, which has the potential to be used to compare structural attributes of proteins (Schiel 2020). That potential capa-
bility is important because structural similarity is hypothesized to be indicative of functional similarity and thus could inform decisions about safety and efficacy. Additional tools noted by Schiel (2020) that could soon find their way into biopharmaceutical development and quality-assurance laboratories include
- Electron microscopy to evaluate the dynamics of the conformational ensembles of therapeutic monoclonal antibodies (Castellanos et al. 2018; Lei et al. 2019; Xu et al. 2019). Protein dynamics might affect mechanisms of action, side effects, adverse immune responses, viscosity, and stability.
- Hydrogen–deuterium exchange mass spectrometry (HDX–MS) to study protein structural dynamics to shed light on how it influences, for example, stability, interactions, or adverse immune responses (Hudgens et al. 2019). HDX–MS is sensitive to post-translational modifications.
Innovative In-Line Sensing Approaches and Sensors to Support Process Control
When designing strategies for pharmaceutical-process monitoring and control, engineers have gravitated toward simple, robust, low-maintenance sensors. The output of such sensors typically is only one process measurement per device (univariate sensors). Examples are pH meters, mass flow meters, thermocouples, scales, and humidity sensors. Although such sensors do support quality assurance, their primary role is as part of the rudimentary automation system. Specifically, the process variable measurement that the sensor provides is typically used as part of a low-level feedback control strategy centered on a single unit operation. Because they typically do not measure quality attributes, such sensors alone cannot enable active process control of product quality and cannot provide enough observability to support more advanced control strategies.
In response to the process analytic technology (PAT) initiative, the industry has taken steps to adopt sensors that monitor multiple process variables and, most important, quality attributes (outcomes). Some of the most promising process sensors are based on vibrational spectroscopy (Romero-Torres et al. 2009). They offer multiple benefits, such as in situ measurements, no need for sample preparation, and rapid scanning. However, they do require tailored calibrations, which are normally constructed by using multivariate statistical approaches.2 Thus, companies need to expand (and even redefine) their analytic-chemistry (and supporting) competences with chemometric skills that are not part of traditional academic curricula. Such novel and sophisticated sensors are also more expensive and less rugged than the classic sensors. Thus, the adoption of these spectroscopy-based sensors for process monitoring has been slower than might be expected. Nevertheless, the major companies have invested in the development of measurement and control strategies that use spectroscopic sensing devices and have actively shared their experiences throughout the industry (Futran 2020). In the next 5 years, the Food and Drug Administration (FDA) will need to continue developing workforce competences in spectroscopic methods and their deployment constraints. Although the technologies are not new to the pharmaceutical industry, they are not yet standard (Futran 2020).
Other novel process sensing approaches that are receiving attention are based on electric capacitance volume-tomography measurement of mass flow of particulate streams (Li et al. 2015) and dielectric spectroscopy for viable-cell density, cell size, intracellular conductivity, and membrane capacitance (Opel et al. 2010). The in-line measurement of mass flow in continuous solid oral-dosage lines offers the benefits of enabling direct monitoring of intermediate process streams to establish the state of control and of enabling decoupling of control structures.
An approach to increase the observability obtainable with individual sensors is to combine information from multiple sensors to monitor the state of a process or infer unmeasured (or unmeasurable) process variables. Combining information from multiple sensors is typically achieved by using models, which can be data-driven, hybrid, or mechanistic. A soft sensor is one such application; it consists of a model that draws on multiple sensor measurements as inputs to predict an unmeasurable process variable. In the next 5–10 years, the committee expects pharmaceutical companies to use more model-based monitoring that integrates the information from multiple sensors (established and advanced) and to use models to infer process state and process outcome, including quality. Depending on the scope of a model and whether sensor information is taken at a specific time or over a time window, several approaches—soft sensors, model-based data-reconciliation methods, or state estimation—are available (Moreno et al. 2019).
Technical Challenges
The challenges in adopting novel sensing approaches are closely tied to the maturity of the sensing technology
___________________
2 See http://ftp.uspbpep.com/v29240/usp29nf24s0_c1119.html#usp29nf24s0_c1119.
and the level of customization and rigor needed for its intended use. As discussed above, advanced and multipurpose sensing technologies typically require tailored multivariate chemometric models for monitoring or quantifying chemicals or properties in complex mixtures. The custom models need to be developed, validated (including design of new validation protocols), maintained, and updated by experts who understand the science behind the sensing mechanism, the complex-mixture properties (and dynamics), and the fundamentals behind the multivariate algorithm used. Given that the competences needed are not part of any academic curricula but rather a specialization, it is challenging to recruit a critical mass of talent to develop and support these applications.
Regulatory Challenges
A perceived regulatory challenge in adopting novel sensors, particularly those usually characterized as PAT, is the notion that the intended use of any advanced sensor is always real-time-release testing. That notion has created confusion in the pharmaceutical industry and potentially led to missing an opportunity inasmuch as new sensing technology is commonly scrutinized with the same rigor as methods used for quality control and product release. For example, using Raman spectroscopy as part of a glucose-feedback controller should not be seen differently from using a classic pH meter as part of a pH-control strategy. The confusion might be caused by the practice in the pharmaceutical industry of using regulatory language when describing technology (for example, equating Design of Experiments with Quality by Design, a spectrometer with PAT, or near infrared spectroscopy with real-time-release testing). The use of new technologies to improve process capability (not necessarily to replace final testing) can be focused on improving process reliability (for example, saving batches, improving process predictability, and reducing the cost of quality) and on increasing performance. Better performance and capability can then allow for increasing plant throughput capacity (increasing productivity and minimizing product shortages) and making a case for reduced testing (after high capabilities are demonstrated). Real-time-release testing can also be implemented in cases in which it is possible to measure or estimate a quality attribute with high fidelity (low risk) by using information obtained before completion of the manufacturing process.
Another regulatory challenge (or perceived regulatory challenge) in the use of spectroscopy-based methods that require tailored models is that any change in a model as part of lifecycle-management activities requires a prior approval supplement. Development and Submission of Near Infrared Analytical Procedures Guidance for the Industry3 indicates that post-approval changes will be risk-based. Also, the International Council for Harmonisation (ICH) has recognized the need for more guidance and clarity related to these new measurement approaches and has issued a final concept paper (ICH Q14).4 The purpose of ICH Q14 is “harmonising the scientific approaches of Analytical Procedure Development, and providing the principles relating to the description of Analytical Procedure Development process. Applying this guideline will improve regulatory communication between industry and regulators and facilitate more efficient, sound scientific and risk-based approval as well as post-approval change management of analytical procedures.” The work plan for the new ICH guideline has May 2022 as the date for adoption.
DATA ANALYTICS AND SYSTEM MODELING
In this report, the term data analytics is used to describe the process of gaining knowledge from data related to the manufacturing process. Typically, that knowledge is captured via statistical, AI-based or mathematical models. As discussed in ICH (2012), models can be used to increase scientific understanding, estimate state variables of a process, predict process behavior, and drive control strategies. Models can be created by data-mining, by using first principles, or by combining data-driven and mechanistic models (hybrid models) (Romero-Torres et al. 2018). In contrast with the more mechanistically based models that are required for product and process design, models that are used to support real-time manufacturing decisions are generally hybrid models that include the use of reduced-order forms of mechanistic models. Models can be used at any stage of the process lifecycle, and the level of oversight should be “commensurate with the level of risk (to the patient) associated with the use of the specific model” (ICH 2012, p. 10). Table 4-1 explains the three categories in which models can fall regarding submissions.
In this section, the committee discusses the combination of data analytics and various types of models to improve quality assurance and process control and capability. In some cases, the combination could potentially lead to a reduction in or elimination of some tests.
___________________
3 See https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-and-submission-near-infrared-analytical-procedures. See Types of Changes and Reporting Categories, p. 17.
4 See https://database.ich.org/sites/default/files/Q2R2-Q14_EWG_Business_Plan.pdf, p. 1.
TABLE 4-1 ICH Model Categories
| Model Category | Intended Use | Examples |
|---|---|---|
| Low-impact model | Typically used to support product or process development | MLR model to evaluate DOE data for formulation optimization |
| Medium-impact model | Can be useful in ensuring product quality but is not the sole predictor of product quality | Most design-space models and models used for process control, such as PLS model of a Raman-based application to control glucose and lactate concentrations in a bioreactor |
| High-impact model | Can be used as a predictor or surrogate of product quality | A chemometric model for product assay, a surrogate model for dissolution, and real-time-release testing models |
Abbreviations: MLR, multilinear regression; DOE, design of experiments; and PLS, partial least squares.
Source: ICH (2012).
Models for System Design and Process Understanding
Models used for design are typically mechanistically based, for example, consisting of sets of ordinary or partial differential equations. The developer of the model understands the fundamental principles appropriate for describing the system and assembles the equations into a system that can be solved numerically. An example is a computational fluid-dynamics (CFD) model that can be used to simulate mixing of an active pharmaceutical ingredient (API) and provide much more spatially detailed information than conventional sensors. CFD models can often be used for primary mixing validation (Prior 2020). Furthermore, that type of simulation can predict behavior and allow computational exploration of different scenarios during the development phase. Such simulations, however, are often too computationally time-consuming to provide answers in real time and might not be able to capture all the underlying complex phenomena or to account for stochastic behavior in a system. For those reasons, data-driven or hybrid models that incorporate data and mechanistic understanding of a process are important alternatives.
In the next 5 years, the committee expects to see the increased use of a combination of first-principles models, data-driven models (such as deep neural networks), and hybrid models. Such combinations could improve process development while reducing the number of experiments needed to establish better process conditions (von Stosch and Willis 2016; Kaschif 2019; Narayanan et al. 2019).
Advanced Analytics for Process Monitoring and Continued Verification
A key application of advanced analytics is in continued process verification (CPV) and in process monitoring. CPV is the ongoing program of verifying the state of the process during routine production (FDA 2011). It encompasses data on relevant process trends and quality attributes of incoming materials or components, in-process material, and finished products. The data-analytics techniques commonly used for CPV are univariate statistical process control methods, which are now well established. However, there is increased use of multivariate statistical process control (MSPC) methods that use more sophisticated techniques, such as principal component analysis and partial least squares, to characterize the ideal multivariate fingerprint of a validated state. That fingerprint can then be used to measure how far newly manufactured batches are from the validated state (or how close). According to ICH (2012), MSPC models that are used for CPV with a traditional method for release testing would probably be classified as medium-impact models.
Models for Advanced Process Control
Models are at the heart of advanced process control (APC) strategies, and applications include model predictive control (MPC) (Lee 2011), fault detection and diagnosis (Venkatasubramanian et al. 2003), condition-based monitoring (Ganesh et al. 2020), and real-time process optimization (Huang et al. 2020). Those capabilities, which are becoming part of the Industry 4.0 paradigm (Deloitte 2015; Romero-Torres et al. 2017), move process knowledge and understanding to true real-time process optimization and operations management. In APC, analytics and computational modeling can be incorporated to recognize that an event has happened. Depending on the time scale and magnitude of an event, different actions need to be taken, including the following:
- Exercise immediate feedback-control action.
- Identify and diagnose a process fault that requires timely intervention.
- Identify a performance degradation that requires scheduled maintenance action.
- Identify a discrepancy between model prediction and process performance that requires real-time optimization to update process set points.
The BioPhorum Operations Group describes a digital-plant maturity model with five levels,5 and real-time process optimization and operations management are characteristic of the highest level in the maturity model, which is referred to as the adaptive plant. By using advanced and soft sensors and data-driven modeling, some companies have realized integrated real-time optimization of operations, such as wet granulation and fluid-bed drying (Huang 2020).
The Digital Twin
Digital twins could have many applications in pharmaceutical manufacturing, such as process flowsheet simulation, real-time process corrections, and reduction in timelines for technology transfer (Futran 2020; Huang 2020). If a physical asset is replicated as a digital twin, real-time corrections can be tested to evaluate potential implications before changes are applied to the “real” system. Similarly, a change in the technology-transfer process can be examined by using a digital twin to analyze how it could affect the process before it is made in the “real” system or process.
The initial version of the mathematical model that underpins the digital twin might not capture the stochastic behavior of the system because it uses mean or most likely model parameter values. However, the digital twin can be used with Monte Carlo or established Bayesian inference methods to capture the effects of uncertainty in the model parameters and system outputs. Specifically, the combination of the mathematical model with real-time process data available from sensors at a particular time or over a time window can be used to assess the effect of parameter uncertainty on predicted system performance and quantitative risk associated with system outputs. Note that the level of remaining uncertainty depends on multiple factors, including the number of variables that affect the solution that can be collected from sensors (process degrees of freedom) and the ability to collect important variables through sensing (process observability). One potential innovation that will change process development in the next 5–10 years is the use of digital twins that are developed with hybrid modeling approaches, including AI methods.
Potential of Artificial Intelligence
AI refers broadly to computer simulation of intelligent behavior, which includes model training or learning from experiences quantified through data. As the use of automation increases, for example, in the digital-plant maturity model, the application of AI to APC increases. ML is a subset of AI that uses large amounts of data and statistical methods of fitting data to facilitate classification (such as the type of fault that occurred) or regression (such as the amount of error between a first-principles model and reality). Statistical methods that are used in ML (such as principal component analysis) can vary widely in their complexity and interpretability. As computational power has increased, more-complex fitting methods have been implemented for better matching of large amounts of data (Greengard 2016). Deep neural networks, for example, use many layers of neurons and connections to represent highly nonlinear correlations and can provide accurate predictions when appropriately trained. In 2015, a Microsoft research team demonstrated that a deep neural network could outperform human classification of images (He et al. 2015). With successes like those, neural networks continue to increase in complexity and accuracy.
General advances in AI and ML can be found in voice recognition, targeted advertising, and self-driving cars; all are driven by vast data collection and advances in algorithms. Although the committee did not identify many direct uses of ML in its investigations, innovators clearly are recognizing its potential, and the amount of data that are and will be collected through sensors will enable increased use of these techniques in the coming years. The identification of trends in large pharmaceutical process datasets and the generation of the data-driven component of hybrid models, as described earlier in this chapter, are natural targets for the application of ML methods. In those cases, assuming that the datasets used to train the models adequately cover the operating range of the system variables and encompass all the variables that must be measured for the system to be observable, ML methods can produce models of sufficient accuracy to enable increased automation and progress toward an adaptive plant. Those advances can lead to more autonomous robotics that contribute to a reduction in human in-
___________________
5 See https://www.biophorum.com/download/digital-plant-maturity-model-v-2/.
tervention, as was described for aseptic filling in Chapter 3. The use of ML can also lead to more innovation by uncovering previously unknown correlations in the data.
Multilevel Control Structures
The sensors, process analytics, and modeling techniques described in the previous sections constitute the core components that are required for the implementation of fully integrated manufacturing systems. In batch operations traditionally used in pharmaceutical manufacturing, each unit operation might be equipped with its own process-control system that consists of its controlled variables, manipulated variables, sensors that are used to measure the controlled variables, and specific control logic for adjusting the manipulated variables. As the industry progresses from traditional batch operation to integrated process trains, as is the case in continuous manufacturing, the dynamics of the successive unit operations need to be closely linked. Moreover, to replace the quality-assurance checks, critical process parameters and critical quality attributes (CQAs) have to be monitored and controlled in real time by incorporating them into the control-system design. If a performance-based control approach is used (ICH Q12), the control logic to maintain a CQA within a target might span more than one unit operation (for example, ratio control of multiple powder feeders to maintain the API concentration measured at the outlet of the powder blender). However, those two control levels—control of basic equipment operation and CQAs—do not suffice to ensure that the entire production line is maintained in a state of control. A third level of coordination is needed among the unit operations. Thus, a plantwide control strategy that might include both feedback and feedforward elements or might involve more sophisticated model-predictive control systems discussed in the previous sections is needed (Su et al. 2019).
Such hierarchical control-system design offers multiple additional possibilities. It can accommodate implementation of modular systems (see Chapter 5) in which each module has its native local control system, and a plantwide control level is configured on the basis of the specific arrangement of the modules. The design can accommodate hybrid production lines in which some of the unit operations are operated in batch mode and others in continuous mode. A hybrid production system might be appropriate if a continuous unit operation is too difficult to control, is subject to performance degradation, or has a long residence time. To benefit from process integration, however, the batch steps must also have control systems in place for critical process variables and CQAs. Moreover, to achieve acceptable plant dynamics, the batch steps will need to be downsized and have automated loading and unloading to achieve overall continuous material flow on a system scale. To balance batch size and cycle times, the batch stages might need to be operated in parallel. However, to control complexity, the number of transitions from batch to continuous or from continuous to batch in the overall process train might need to be restricted. Finally, in this hierarchical control structure, specific processing stages that involve robotic operations can readily be accommodated: the robotic stage is only an electromechanical unit that is locally controlled and can operate in batch or continuous mode as part of a hybrid production or continuous process train.
Technical Challenges
The innovations described above entail many technical challenges. The main challenges in adopting models for system design and process understanding are due to system complexity, knowledge and data availability, and workforce competence. For simpler systems, it is easier to identify the physical and chemical phenomena that govern their behavior; for complex systems, this level of mechanistic representation is difficult to assemble.
In adopting advanced analytics for CPV, there are several challenges. First, the use of analytics requires information systems through which process information (in-line, at-line, and off-line) is aggregated automatically and reliably, but such centralized data repositories require investment and some specialized training. Second, products made in campaigns might not reflect random behavior because of systematic changes in equipment, staff, and raw materials, and this is especially true for products that are manufactured with low frequency (many products are made with frequencies of fewer than 25 batches/year). Third, alarms or investigation actions are usually based not on statistical control limits but rather on action limits and registered specifications, and systematic variation is usually not investigated unless process performance falls outside action limits or registered specifications. Fourth, in many companies, there is no formal governance or business process for continuous improvement based on CPV activities. Fifth, if effective knowledge-management programs are lacking, the right information is often not available to the right people at the right time.
Increased application specifically of AI and ML tools poses several challenges. Some of the most accurate ML models, such as deep neural networks that use many complex layers, can become difficult to interpret. Although the structure of a neural network is well defined, the weights
that are associated with the connections in the network and the bias are determined during model training in an iterative fashion by using numerical algorithms. During training, the model predicts output on the basis of training-data input, the error between the model’s prediction and the training-data output is assessed, and the model weights and bias are modified by the algorithm logic to decrease the error in the model’s prediction. Three major technical challenges arise from these types of models:
- As the scope of the ML model and dataset are expanded to increase model prediction accuracy, the model also increases in complexity and decreases in interpretability. Although a less interpretable model might capture correlations better, the ability of a human to use that information to attribute causation will decrease. For example, deep neural networks can easily contain tens of thousands of learned parameters that are associated with abstract correlations in the data. Associating the model structure and learned weights with physical reality to understand why a prediction was made remains an open field of research.6
- By design, ML approaches, including neural networks, are intended to change as they are given new data. Although accumulation of new data typically increases accuracy, the continuous nature of the evolution of the model makes it difficult to assess why a given input can result in a different prediction from one version of the model to another. To facilitate interpretation, model training can be performed in discrete events that create new model versions. However, that approach inherently introduces delays in model improvement and adds software engineering complexity.
- In the training of complex models, especially nonlinear ones, the risk of overfitting a model can be substantial. An overfitted model might not capture actual system behavior and might thus lead to faulty predictions. Research is continuing in this field.
The committee emphasizes that data analytics and modeling are at the heart of APC and that FDA will need to prepare for advances in them. There are, however, challenges that the pharmaceutical-manufacturing industry will need to address for successful implementation of these technologies. First, few experts in data analytics and system modeling are also knowledgeable in pharmaceutical manufacturing. Data analytics and system modeling constitute a specialty in themselves that requires advanced knowledge of statistics and mathematics. Experts in this field are in high demand outside the pharmaceutical industry, so efforts need to be made to grow expertise and to retain it. To achieve reliable results robustly, it is important that data analysts or modelers can work closely with domain experts during the model-identification phase, that they can communicate effectively with FDA regulators, and that the FDA staff have the background to engage in the discussion.
A second major challenge is to build an effective infrastructure for knowledge management. ICH Q10 addresses the need for knowledge management as an enabling capability for product quality, control, and continual improvement, but there are many subtleties and complications in doing so effectively (ICH 2009). Collecting sufficient curated and contextualized data, which are needed to create ML models, takes time and expertise, which are scarce.
A third major challenge, which is related to the second, is the issue of observability. Not all important variables that enable system predictability are measured or measurable. As discussed in the section on digital twins, the incorporation of more variables can decrease uncertainty but is not always possible. When it is not, some variables might be inferred from variables that are measured directly by using models.
A fourth challenge is the availability of representative data. Many products are manufactured with low frequencies (for example, three to five batches per year). That translates into a lack of representative data that can be used to characterize the long-term behavior of a system and to design robust model-maintenance programs.
Finally, the technical challenges in the implementation of APC reside mainly in the establishment of reliable data flow from sensors and process equipment and the development of robust models for control. However, important issues are associated with design of the control-system logic. Specifically, there are challenges in the design of flexibly configurable process-control systems for modular processes. The hierarchical architecture can readily accommodate alternative configurations of module-level and plantwide control elements, but the design of platforms that enable flexible configuration of those control elements as modules are being reconfigured for different products requires further development. Ensuring system integrity will also be a key requirement. Similarly, the robust operation of highly intensified unit operations or sequences of operations can be achieved only through active process control inasmuch as intensification by its very nature exploits higher degrees of interaction between process variables. Such intensified operations thus might
___________________
6 See https://www.darpa.mil/program/explainable-artificial-intelligence.
require customized control-system designs, including the use of more advanced methods, such as adaptive and nonlinear model predictive control.
Regulatory Challenges
Several important regulatory challenges are associated with the technologies described above. The regulatory challenges for increased automation and AI align closely with their technical challenges. The lack of interpretability in some of the most accurate models and the continuous nature of the evolution of the models might lead to difficulty in regulatory applications. Nonetheless, the committee concludes that many applications of increased automation and AI pose low impact, as defined in Table 4-1, and provide value to process improvements. Therefore, such advances should be acceptable to regulators. At the same time, higher-impact uses of increased automation and AI can be complemented with first principles to lower the risk posed by the applications and meet regulatory expectations.
In the case of APC, many advanced control strategies require a high degree of at-scale process understanding to allow for system modeling in the presence of common disturbances. That degree of at-scale process understanding is not usually available at the time of filing, especially for such complex processes as bioreactions. Thus, for companies to be able to adopt more advanced control mechanisms, such as MPC and hierarchical control system designs, they probably will have to require regulatory post-approval changes. ICH Q12 is expected to facilitate such changes and encourage the continuous adoption of innovation.7
The committee notes that the hybrid production mode of operation potentially raises a regulatory issue associated with the definition of the batch. As noted earlier, in such hybrid lines, batches are generated and processed in one or more internal processing units that then feed continuous units, but the final process output stream is continuous. Flexibility in the interpretation of what constitutes a lot or batch in the context of continuous manufacturing has been allowed, and the committee finds that similar flexibility should be allowed in the interpretation of a batch with hybrid production systems that would be independent of the operational batches that are internal to the hybrid process.
OVERCOMING REGULATORY CHALLENGES
FDA has been active in creating an ecosystem that will enable the adoption of more sophisticated control mechanisms. Its efforts include issuing the PAT guidance and other advanced guidelines and creating the Emerging Technology Team. The committee applauds those efforts but finds that the agency can help to foster innovation further and provides suggestions below.
- Terminology alignment and clarification. There is a great opportunity for terminology alignment and clarification. Differences in definitions throughout the industry have caused substantial confusion. From a regulatory perspective, it might be beneficial for the agency to work with the industry to distinguish regulatory language from descriptions of scientific or engineering principles and practices. Doing so will be key in helping the pharmaceutical industry to share best practices and adopt a more fit-for-purpose approach in evaluating the adoption of novel sensors and control strategies for various applications. An updated PAT guideline might also be beneficial; it should incorporate standard control-theory terms, such as process observability, fault detection, fault classification, and process-condition monitoring. An example of confusing terminology is the use of the term control when referring to specifications.
- Expectation-setting and management. One of the main reasons that the pharmaceutical industry has been slower to adopt more advanced control strategies is unrealistic expectations. As discussed, the most-cited value proposition for new control approaches is usually real-time-release testing or at least reduction in the time for post-manufacture quality assessment. Reduction or elimination of quality testing, especially for complex systems, should be the result of good engineering design and reserved for processes that have high process capability, observability, and predictability. Nevertheless, processes with low capabilities and predictabilities can benefit tremendously from better control mechanisms to increase the process reliability that directly affects “supply-ability.” Depending on the manufacturing frequency, cost of goods, process complexity, and available infrastructure, a company can make business decisions about what level of observability and control should be built into its processes. A recommendation is to communicate innovation value proposition in the context of the pharmaceutical supply chain, financials, and operations.
- People, process, and technology. Another im
___________________
7 See https://www.fda.gov/files/drugs/published/Q12-Technical-and-Regulatory-Considerations-for-Pharmaceutical-Product-Lifecycle-Management-Core-Guideline-Guidance-for-Industry.pdf.
- portant concept that surfaced during the committee workshops (NASEM 2020a,b) and has been extensively discussed by change-management leaders, such as McKinsey8 and Boston Consulting,9 is “people, process, and technology.” Although the concept is not new and is related to expectation-setting and management, the committee finds that it should have greater presence in FDA’s discussions of new control approaches and innovations. The implementation of technology alone will not lead to improved process capabilities, supply-chain reliability, and agility. Technology adoption should go through business processes, such as stage gating, and should be mapped through the lens of change management. If that is done, it will become evident that key branches of the typical pharmaceutical organization are not part of the innovation conversations or even adoption of business workflows.
- Impact of manufacturing-equipment health. Condition-based monitoring of manufacturing equipment and processes enables timely identification of performance degradation and reduction in unplanned down-times and thus improves process capability and provides higher assurance of product quality. The committee recommends that the agency become familiar with condition-based monitoring approaches and provide incentives for their use.
- Inspector competences. The increased reliance on advanced control strategies—including fault detection and mitigation strategies and condition-based monitoring—requires that inspection staff have the expertise to understand the technologies and best practices in their application. FDA needs to have the additional resources to hire and continue training and retention of these essential human resources.
- Modularization replication. The trend toward modularization of process systems, plug-and-play unit operations, and even miniaturized portable production systems provides opportunities to incorporate sensing and control technologies. The trend is described in detail in the next chapter. Given the many modular concepts, system definition and standardization might be more challenging than control integration. Here, the influence of regulators can have a beneficial effect on driving standards for modularization that have integrated sensing and control technologies. Such standards could substantially reduce timelines for the startup of pharmaceutical manufacturing in new facilities and in retrofits of conventional facilities.
- Digitized work instructions. As more observability and new alarms are implemented to alert personnel about possible process and equipment upsets, there will be a need to rely on digitized work instructions that can walk personnel through a set of decision and action workflows (logic) that might be too complicated to be captured in paper format (or on a single visual workflow). The committee expects these expert-system digitized instructions to become more common, and FDA should become aware of the trend.
- Guidance. To reduce the perceived uncertainty in FDA acceptance of innovations in sensing, modeling, ML applications, and advanced control, it would be desirable for FDA to issue focused guidance on their current good manufacturing practices (cGMP) implementation and expectations for their management, analogous with the recent near-infrared analytic-procedures guidance (FDA 2015). Such guidance could draw on recent initial industry efforts led by the Pharmaceutical Discovery, Development and Manufacturing Forum of the American Institute of Chemical Engineers (PD2M-AIChE), which has resulted in implementation guidelines that draw on recent industry experiences with soft-sensor method validation, APC, and ML methods (Huang et al. 2020).
REFERENCES
Arnold, R. D., and J. P. Wade. 2015. A Definition of Systems Thinking: A Systems Approach. Procedia Computer Science 44:669-678.
Castellanos M. M., S. C. Howell, D. T. Gallagher, and J. E. Curtis. 2018. Characterization of the NISTmAb Reference Material using small-angle scattering and molecular simulation: Part I: Dilute protein solutions. Analytical and Bioanalytical Chemistry 410(8):2141-2159.
Deloitte A. G. 2015. Industry 4.0 Challenges and solutions for the digital transformation and use of exponential technologies. https://www2.deloitte.com/content/dam/Deloitte/ch/Documents/manufacturing/ch-en-manufacturing-industry-4-0-24102014.pdf (accessed August 19, 2020).
FDA (U.S. Food and Drug Administration). 2011. Guidance for Industry. Process Validation: General Principles and Practices. https://www.fda.gov/media/71021/download (accessed August 15, 2020).
FDA. 2015. Development and Submission of Near Infrared Analytical Procedures: Guidance for Industry. https://www.fda.gov/media/91343/download (accessed November 19, 2020).
Futran, M. 2020. Our journey towards engineering control in Janssen Manufacturing. Presentation at Virtual Workshop on Technical and Regulatory Barriers to In-
___________________
8 See https://www.mckinsey.com/business-functions/mckinsey-digital/our-insights/the-next-generation-operating-model-for-the-digital-world.
9 See https://image-src.bcg.com/Images/BCG-Take-Control-of-Your-Digital-Future-May-2018_tcm30-191195.pdf.
novations in Pharmaceutical Manufacturing, June 2. https://www.nationalacademies.org/event/06-02-2020/docs/DED6547CFED25273902E2EEA0A0A5046BB18BAC24C23 (accessed July 19, 2020).
Ganesh, S., Q. Su, L. B. D. Vo, N. Pepka, B. Rentz, L. Vann, N. Yazdanpanah, T. O’Connor, Z. K. Nagy, and G. V. Reklaitis, 2020. Design of Condition-based Maintenance Framework for Process Operations Management in Pharmaceutical Continuous Manufacturing. International Journal of Pharmaceutics 587:119621.
Greengard, S. 2016. GPUs Reshape Computing. Communications of the ACM 59(9):14-16.
He, K., X. Zhang, S. Ren, and J. Sun. 2015. Delving Deep into Rectifiers: Surpassing Human-Level Performance on ImageNet Classification. Proceedings of the 2015 IEEE International Conference on Computer Vision (ICCV) pp. 1026-1034.
Huang, J. 2020. Value-focused Analytics and Digital Technology Roadmap for Advancing Biomanufacturing. Presentation at Workshop on Innovations in Pharmaceutical Manufacturing, February 27. https://www.nationalacademies.org/event/02-27-2020/docs/DD05BA407A4983D5B14DA119269A5CD-D9177C1471A27 (accessed June 17, 2020).
Huang, J., T. O’Connor, K. Ahmed, S. Chatterjee, C. Garvin, K. Ghosh, M. Ierapetritou, M. Jeffers, D. L. Pla, S. L. Lee, S. Lovett, O. Lyngberg, J. Mack, E. McManus, S. Romero-Torres, C. Undey, V. Venkatasubramanian, and M. Warman. 2020. AIChE PD2M Advanced Process Control workshop-moving APC forward in the pharmaceutical industry. Journal of Advanced Manufacturing and Processing e10071.
Hudgens, J. W., E. S. Gallagher, I. Karageorgos, K. W. Anderson, J. J. Filliben, R. Y. Huang, G. Chen, G. M. Bou-Assaf, A. Espada, M. J. Chalmers, E. Harguindey, H. M. Zhang, B. T. Walters, J. Zhang, J. Venable, C. Steckler, I. Park, A. Brock, X. Lu, R. Pandey, A. Chandramohan, G. S. Anand, S. N. Nirudodhi, J. B. Sperry, J. C. Rouse, J. A. Carroll, K. D. Rand, U. Leurs, D. D. Weis, M. A. Al-Naqshabandi, T. S. Hageman, D. Deredge, P. L. Wintrode, M. Papanstasiou, J. D. Lambris, S. Li, and S. Urata. 2019. Interlaboratory Comparison of Hydrogen-Deuterium Exchange Mass Spectrometry Measurements of the Fab Fragment of NISTmAb. Analytical Chemistry 91(11):7336-7345.
ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use). 2009. Guidance for Industry: Q10 Pharmaceutical Quality System. https://www.fda.gov/media/71553/download (accessed August 2, 2020).
ICH. 2012. Guidance for Industry: Q8, Q9, Q10 Questions and Answers. 2012. https://www.fda.gov/media/83904/download (accessed August 2, 2020).
Kaschif, A. S., C. Antoniou, R. Guenard, and S. Romero-Torres. 2019. Hybrid Model Identification for Monoclonal Antibody Production Bioreactor – A Digital Twin. American Pharmaceutical Review 22(5):36-47.
Lee, J. H. 2011. Model Predictive Control: Review of the Three Decades of Development. International Journal of Control Automation and Systems 9(3):415-424.
Lei, D., J. Liu, H. Cleveland, T. E. Marino, M. Lei, and G. Ren. 2019. Single-Molecule 3D Images of “Hole-Hole” IgG1 Homodimers by Individual-Particle Electron Tomography. Scientific Reports 9(1):8864.
Li, J., M. Kong, C. Xu, S. Wang, and Y. Fan. 2015. An Integrated Instrumentation System for Velocity, Concentration and Mass Flow Rate Measurement of Solid Particles Based on Electrostatic and Capacitance Sensors. Sensors 15(12):31023-31035.
Moreno, M., J. Liu, Q. Su, C. Leach, A. Giridhar, N. Yazdanpanah, T. O’Connor, Z. K. Nagy, and G. V. Reklaitis. 2019. Steady-State Data Reconciliation of a Direct Continuous Tableting Line. Journal of Pharmaceutical Innovation 14:221-238.
Narayanan, H., M. Sokolov, M. Morbidelli, and A. Butté. 2019. A new generation of predictive models: The added value of hybrid models for manufacturing processes of therapeutic proteins. Biotechnology and Bioengineering 116(10):2540-2549.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2020a. Innovations in Pharmaceutical Manufacturing: Proceedings of a Workshop—in Brief. Washington, DC: The National Academies Press. https://doi.org/10.17226/25814.
NASEM. 2020b. Barriers to Innovations in Pharmaceutical Manufacturing: Proceedings of a Workshop—in Brief. Washington, DC: The National Academies Press. https://doi.org/10.17226/25907.
Opel, C. F., J. Li, and A. Amanullah. 2010. Quantitative modeling of viable cell density, cell size, intracellular conductivity, and membrane capacitance in batch and fed‐batch CHO processes using dielectric spectroscopy. Biotechnology Progress 26(4):1187-1199.
Prior, J. 2020. Opportunities and Challenges in Biologics Manufacturing Process Data Analytics Innovation. Presentation at Workshop on Innovations in Pharmaceutical Manufacturing, February 27. https://www.nationalacademies.org/event/02-27-2020/docs/D5F77E6D80511C72245E7F47FD-351D6889EC227CD15Cdocs/D5F77E6D80511C72245E7F47FD-351D6889EC227CD15C (accessed June 15, 2020).
Romero-Torres, S., J. Huang, and P. E. Hernandez-Abad. 2009. Practical Considerations on PAT Analyzer Selection - Raman vs. NIR Spectroscopy. American Pharmaceutical Review 12(7):12-19.
Romero-Torres, S., J. Mayne, and M. Kidambi. 2017. Towards Pharma 4.0; Leveraging Lessons and Innovation from Silicon Valley. American Pharmaceutical Review 20(1):1-9.
Romero-Torres, S., K. Wolfram, J. Armando, S. K. Ahmed, J. Ren, C. Shi, D. Hill, and R. Guenard. 2018. Biopharmaceutical Process Model Evolution-Enabling Process Knowledge Continuum from an Advanced Process Control Perspective. American Pharmaceutical Review 21(4)1-10.
Rogers, R. S., N. S. Nightlinger, B. Livingston, P. Campbell, R. Bailey, and A. Balland. 2015. Development of a quantitative mass spectrometry multi-attribute method for characterization, quality control testing and disposition of biologics. mAbs 7(5):881-890.
Rogers, R. S., M. Abernathy, D. D. Richardson, J. C. Rouse, J. B. Sperry, P. Swann, J. Wypych, C. Yu, L. Zang, and R. Deshpande. 2017. A View on the Importance of “Multi-Attribute Method” for Measuring Purity of Biopharmaceuticals and Improving Overall Control Strategy. AAPS Journal 20(1):7.
Schiel, J. 2020. Innovative Analytical Technologies and Biopharmaceutical Reference Materials. Presentation at Workshop on Innovations in Pharmaceutical Manufacturing, February 27. https://www.nationalacademies.org/event/02-27-2020/docs/D9025231365D2BD92F63812F495D442B65F83EA751D7 (accessed May 30, 2020).
Starkey, J. 2020. Control Strategy as a Critical as a Critical Aspect of Manufacturing Innovation. Presentation at Virtual Workshop on Technical and Regulatory Barriers to Innovations in Pharmaceutical Manufacturing, June 2. https://www.nationalacademies.org/event/06-02-2020/docs/D6844C91F035D812C8A191DE35B439B2B6C0480E7D97 (accessed August 22, 2020).
Su, Q., S. Ganesh, M. Moreno, Y. Bommireddy, M. Gonzalez, G. V. Reklaitis, and Z. K. Nagy. 2019. A perspective on Quality-by-Control (QbC) in pharmaceutical continuous manufacturing. Computers & Chemical Engineering 125:216-231.
Swann, P. 2020. Innovative Strategies to Control Product Quality Attributes and Reduce Commercialization Timelines. Presentation at Virtual Workshop on Technical and Regulatory Barriers to Innovations in Pharmaceutical Manufacturing, June 2. https://www.nationalacademies.org/event/06-02-2020/docs/DC6EB341890ACCA4EDEBA8C74BC916508F716B98A560 (accessed August 7, 2020).
Venkatasubramanian, V., R. Rengaswamy, K. Yin, and S. N. Kavuri. 2003. A review of process fault detection and diagnosis. Part I: Quantitative model-based methods. Computers & Chemical Engineering 27(3):293-311.
von Stosch, M., and M. J. Willis. 2016. Intensified design of experiments for upstream bioreactors. Engineering in Life Sciences 17(11):1173-1184.
Xu, A.Y., M. M. Castellanos, K. Mattison, S. Krueger, and J. E. Curtis. 2019. Studying Excipient Modulated Physical Stability and Viscosity of Monoclonal Antibody Formulations Using Small-Angle Scattering. Molecular Pharmaceutics 16(10):4319-4338.












