6
Review of Studies on the Effects of UV Filters in Aquatic Environments
Effects information for use in ecological risk assessment (ERA) can include estimates based on quantitative structure-activity relationships (QSAR), laboratory toxicity tests, mesocosm studies, and field studies. Information on effects from these various sources is combined with information on exposure to evaluate environmental risk. Exposures can involve contact with the dissolved fractions of the chemical in water as well as ingestion of suspended solids, sediment, and plants and animals that have accumulated the chemicals into their tissues; Chapter 5 provides an overview of bioaccumulation of UV (ultraviolet) filters which will serve to inform exposure and effects aspects of the ERA. However, data are largely lacking on the effects associated with the dietary exposure pathways and the internal exposure concentrations of potentially bioaccumulated UV filters. As described in Chapter 5, the effects levels for bioaccumulated chemicals are known as critical body burdens and limited information is provided in Chapter 5 on such levels. The majority of toxicity data on effects are for direct exposures to UV filters in water or sediment and thus constitute most of what can be found in this chapter.
Laboratory toxicity testing data are the most widely used in the effects assessment inputs to ERAs of individual chemicals. Other studies not designed for direct use in ERAs may be informative to understanding modes of action or otherwise illuminating areas of concern for further study. While the effects of UV filters on aquatic environments is a rapidly expanding area of research (Carve et al., 2021a), there are varying degrees of data availability across UV filters, across taxa and life stages, and in their design for use in ERAs. Ideally for an ERA, enough information is available to perform a synthetic analysis of the data, such as a species sensitivity distribution (SSD; Posthuma et al., 2002), a statistical model used to predict a chemical concentration with low impact to species in the environment based on species studied in the laboratory.
This chapter reviews the information available on the effects of UV filters to aquatic organisms, with a focus on those studies informative to conducting an ERA. The chapter first provides context regarding how effects data are used as part of the overall ERA process to characterize risks to the environment. This is followed by a summary of what is currently available from laboratory toxicity tests globally on each UV filter currently approved for use in the United States. In addition to covering studies that may be used in an ERA, the chapter also describes studies that provide insights into potential modes of action. As directed in the statement of task, the committee also gave specific attention to how UV filters might affect threatened and endangered species based on potential for exposure and effects on these and related organisms. The committee also considers how UV filters may affect community and ecosystem processes. Last, the committee discusses the relevance of taking an eco-epidemiological approach to understand the interactions of UV filters and other environmental stressors, and also help clarify the contributions of UV filters to the condition of ecosystems that are under stress from a variety of sources.
HOW EFFECTS INFORMATION IS USED IN ECOLOGICAL RISK ASSESSMENTS
In an ERA, information on effects is used together with information on exposure to characterize risks (EPA, 1998; Figure 3.1). Because this coupling is essential for ERAs, the effects information must be properly aligned with exposure estimates. Alignment includes temporal influences (e.g., acute effects with short-term exposures and sublethal effects with longer-term chronic exposures) and exposures that are relevant to the biological attributes and toxicity endpoints of the ecological receptors. Additionally, alignment of units (e.g., for concentrations or dose metrics) facilitates easy comparison of whether toxicity thresholds are in range of potential exposures. As described in Chapter 1, uncertainty in lower-tier ERAs may make it less clear how well exposure and effects ranges align with each other.
Ecological risks can be characterized for various levels of biological organization including individuals, populations, communities, ecological functions, and ecosystems. With some exceptions and additions described below, ERAs by the U.S. Environmental Protection Agency (EPA) primarily focus on populations (and specifically multiple populations in an ecosystem and protection of the more sensitive species). A notable exception is assessment for species listed as threatened or endangered under the Endangered Species Act of 1973, for which there is a greater focus on individuals within the context of population recovery. Therefore, for ERAs, EPA typically relies on effects data with population relevance—individual survival, growth, and reproduction—derived from laboratory toxicity testing in a limited set of representative standard species (and life-history stages).
Acute toxicity tests are shorter in duration (typically 24–96 hours) and use relatively higher test concentrations, with results expressed following statistical analyses as an LC50 (lethal concentration for 50 percent of the test population) or as an EC50 (concentration of non-lethal effects on 50 percent of the test population that are effectively equivalent to mortality, such as immobilization in Daphnia sp.). Chronic toxicity tests measure sublethal effects over longer durations and lower concentrations, reporting statistical differences from the controls for effects related to survival, growth, development, and reproduction. For ERAs they are typically expressed as EC10s (concentrations of non-lethal effects on 10 percent of the test population) or NOECs (no observable effect concentrations [i.e., the highest concentration tested that is not statistically different from the controls]). Although not typically used in ERAs, in some cases investigators also frequently report LOECs (lowest observable effect concentration, i.e., the lowest concentration tested causing statistically significant toxic effects compared to controls and the concentration interval immediately above the NOEC), or in some cases Chronic Values (the geometric mean of the NOEC and LOEC). In the absence of chronic data, acute to chronic ratios (ACRs) have been used by hazard assessors to extrapolate chronic thresholds from acute data employing a default factor of 10 to the acute data. However, actual chronic toxicity testing results provide more reliability, especially when multiple modes of action may be involved. In a more ideal situation, an effects value for risk characterization would then be derived from an SSD (described in more detail later in the chapter), which can help identify which species will be most sensitive to a chemical, when sufficient information on a chemical is available. In cases for which an SSD cannot be developed due to insufficient information, specific acute and chronic values are chosen.
Toxicity tests for evaluating chemicals in commerce are typically conducted for standard test species and specific life stages for which there are published guidelines, such as those developed by the Organisation for Economic Co-operation and Development1 (OECD), EPA,2 and ASTM.3 Use of standardized guidelines ensures that results provide reliable data for statistical analysis and thus report accurate toxicological thresholds. This also yields results that are reproducible, can be compared between different laboratories/studies, and allow for comparison with different chemicals and species to identify chemicals of concern and/or sensitivity. However, standard methods are available only for a limited number of species and taxonomic groups; most are for freshwater taxa.
Depending on the nature of the ecological receptors that are the subject of an ERA, relevant yet nonstandard
___________________
1 See https://www.oecd.org/chemicalsafety/testing/oecd-guidelines-testing-chemicals-related-documents.htm.
2 See https://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-850-ecological-effects-test-guidelines.
(i.e., with no toxicity testing protocols) species may be evaluated. Some nonstandard species have unique biological endpoints related to their survival, growth, and reproduction. For example, reef-building corals are complex organisms, consisting of the coral animal, its symbiotic algae, and a diverse microbiome (termed a “holobiont”). A focus on the health of the coral, the symbiotic relationship or to the algal symbiont itself have been investigated with studies reporting visual bleaching of the coral, loss of symbiotic algae, changes in chlorophyll content, and an assessment of photosynthetic efficiency. While corals may recover from single and short-term bleaching events, these endpoints in addition to the resilience of the coral host may be important for the growth and survival of the coral and thus may be a population-relevant response for use in an ERA (e.g., Grottoli et al., 2006; Hughes et al., 2018b). However, corals lack standard toxicity testing methods, resulting in varying methodologies used across studies (Mitchelmore et al., 2021; Watkins and Sallach, 2021). Use of nonstandard test organisms can be challenging, requiring added effort to successfully culture and maintain such organisms under laboratory conditions and may require modifications of standard test procedures to ensure organism health (e.g., see Ågerstrand et al., 2011; Alves et al., 2017; Breuer et al., 2016; Conway et al., 2021). Documentation of methods that follow as closely as possible to the guidelines prescribed for similar standard test organisms allows for experimental reproducibility, reliability, and comparability across studies.
Effects data for use in ERAs is derived mainly for whole organisms (i.e., in vivo testing) with biological endpoints that have proven linkages to impacts at the population level. However, in vivo and in vitro tests (e.g., on cell lines or receptor-gene bioassays) on physiological (e.g., cellular, biochemical and molecular) and behavioral endpoints can be used as an indicator of exposure (e.g., biomarkers), as indicators of the potential mechanistic basis for effects, as an initial first-tier screening for potential effects, or to help interpret or support ERAs. Additionally, adverse outcome pathways (AOP) are an integrated approach that can translate these cellular/biochemical/molecular events into population-level outcomes (Ankley et al., 2010; OECD, 2017a, 2018c). At present, EPA program offices evaluating the ecological risk of chemicals do not rely upon cell lines or cellular/biochemical/molecular responses to quantify chemical risks. Future EPA risk assessments are expected to increase use of New Approach Methodologies that make use of a broader range of information including at the cellular/biochemical level (Gwinn, 2020).
These physiological and behavioral endpoints can help identify mode of action, the characterization of an adverse biological response. Mode of action is the type of adverse biological response resulting in toxic effects. Mechanisms of action are the biochemical processes underlying a given mode of action. Mode of action is especially useful in helping to explain relative sensitivities of biota to a given chemical, mixture toxicity based on concentration-addition or Toxic Unit algorithms, and as part of an AOP analysis. UV filters are chemically diverse, meaning it is likely numerous modes of action are at play. Mode of action may also vary by organism group (for example, determining a chemical is a Photosystem II inhibitor may be useful as a mode of action for algae, but be of little use in assessing fish).
ACUTE TOXICITY QSAR OVERVIEW
Determinations of QSARs are commonly employed at the earliest tiers of an ERA. This is useful to provide comparative ecotoxicity potential across a category of compounds such as the UV filters and may act as a basis to gauge the quality of measured ecotoxicity in subsequent studies (EPA, 1998). Once results from toxicity tests are available, their results generally supersede QSAR information. Table 6.1 summarizes acute aquatic toxicity QSAR results for the organic UV filters and provides a first glimpse at coverage from measured ecotoxicity studies. Model inputs were either the CAS number, if found in the ECOSAR (ECOlogical Structure-Activity Relationship Model) chemical inventory, or the SMILES notation (Table 4.1) for each filter. The focus of Table 6.1 is on freshwater standard test species, as these are the most reliable QSARs and, as will be found in the subsequent sections for each UV filter, also constitute the bulk of measured ecotoxicity. The QSARs used to provide the toxicity predictions are noted per UV filter as well as the potential broad mode of action for the categorized chemical class as
TABLE 6.1 Summary of QSAR Predictions for UV Filters for Standard Test Organisms Using ECOSAR v. 2.0
| UV Filter | ECOSAR QSAR Employed Based on Structural Features | ECOSAR Predictions | Availability of Measured Ecotoxicity Data | ||||
|---|---|---|---|---|---|---|---|
| Green algae 96-h EC50 (mg/L) | Daphnid 48-h EC50 (mg/L) | Fish 96-h LC50 (mg/L) | Algae | Daphnid | Fish | ||
| Aminobenzoic acid | Unhindered aniline (electron transport inhibitor) |
82.3 | 26.3 | 709 | N | N | N |
| Avobenzone | Diketone (reactive, neurotoxicant) |
0.262 | 0.708 | 0.636 | Y | Y | Y |
| Cinoxate | Ester (polar narcosis) |
10.32 | 26.89 | 23.7 | N | N | N |
| Dioxybenzone | Polyphenol (polar narcosis) |
0.259 | 2.40 | 2.78 | Y | Y | Y |
| Ecamsule | Vinyl/Allyl/Propagyll ketone (reactive) |
73.3b | 86.3b | 230b | Y | Y | Y |
| Ensulizole | Pyrroles/Diazoles (neurotox) |
131 | 3580 | 20100 | Y | Y | Y |
| Homosalate | Ester (polar narcosis) |
0.038 | 0.173 | 0.130 | Y | Y | Y |
| Meradimate | Ester (polar narcosis) |
0.033 | 0.154b | 0.117b | N | N | N |
| Octocrylene | Ester (polar narcosis) |
0.016 | 0.084 | 0.068 | Y | Y | Y |
| Octinoxate | Ester (polar narcosis) |
0.075 | 0.323b | 0.234b | Y | Y | Y |
| Octisalate | Ester, Phenols (polar narcosis) |
0.050 | 0.219 | 0.161 | Y | Y | Y |
| Oxybenzone | Phenols (polar narcosis) |
0.259 | 2.40 | 2.78 | Y | Y | Y |
| Padimate O | Ester (polar narcosis) |
0.076 | 0.326b | 0.235b | Y | Y | - |
| Sulisobenzone | Phenols (polar narcosis) |
462 | 1160 | 5580 | Y | Y | Y |
| Titanium dioxide | (ionoregulatory disruption) | — | — | — | Y | Y | Y |
| Trolamine salicylatea | Aliphatic amines (polar narcosis) |
411 | 217 | 2773 | N | N | N |
| Phenols (polar narcosis) |
11.4 | 61.8 | 128 | N | N | N | |
| Zinc oxide | (ionoregulatory disruption) | — | — | — | Y | Y | Y |
a Note that the UV filter is an ionic pairing of monoethanolamine and salicylate, which are modeled separately.
b QSAR predictions flagged by ECOSAR as likely exceeding solubility of the tested chemical.
discussed by Barron et al. (2015) and Kienzler et al. (2019). The publicly available modeling platform ECOSAR V 2.04 was used to develop predictions using freshwater algae, daphnid, and fish QSARs as described by Mayo-Bean et al. (2017).
As ECOSAR is built only to assess organic compounds, and QSARs for metals, metalloids, and organometals are not developed, only organic UV filters are modeled. In the end, this is not an issue as inorganic UV filters are well tested across the range of standard freshwater species. A potential mode of action for the metal-based UV filters are derived from Barron et al. (2015).
Based on ECOSAR QSARs, acute toxicities of organic UV filters ranges were 16–462,000 μg/L, 84–1,160,000 μg/L, 68–5,580,000 μg/L for algae, daphnids, and fish, respectively. Octocrylene appears to have the lowest (most toxic) acute toxicity predictions and ensulizole and sulisobenzone the highest (least toxic) predictions. The QSAR predictions are useful for prioritizing empirical data gap filling for aminobenzoic acid, cinoxate, meradimate, and trolamine salicylate. Of these four, meradimate would likely have the highest priority as being the most potentially toxic of the four UV filters that do not currently have acute toxicity data.
Several potential modes of action are indicated across all UV filters exemplifying the diversity of substitutions and functionalizations present. Most are considered polar narcotics (esters, polyphenolics, aliphatic amines). Less common are reactive compounds and potential neurotoxicants (diketones, pyrroles). Only a few compounds are completely unstudied (aminobenzoic acid, trolamine salicylate, meradimate, cinoxate) which likely reflects their in-market usage being low (see Chapter 2). The other issue that is readily identifiable in the QSAR outputs is that a number of QSARs indicate solubility of test substances may be an issue and these may also be implicated in empirical toxicity tests.
Evidence of Mode(s) of Action from Chemical Structure
ECOSAR QSARs are based on chemical specific functionalities, which are used to estimate toxicity although they can also be used to identify potential modes of action (MOA) for acute toxicity. For example, compounds with an ester link will display an ester polar narcotic mode of action (Barron et al., 2015; Mayo-Bean et al., 2017). Several potential MOAs are indicated across all UV filters, exemplifying the diversity of substitutions and functionalizations that are present. Most organic UV filters are considered polar narcotics (esters, polyphenolics, aliphatic amines) including the benzophenones (dioxybenzone, oxybenzone, sulisobenzone), which are phenol or phenol derivatives (Barron et al., 2015). While structurally distinct, the salicylate group of UV filters (homosalate, octisalate) along with meradimate, cinoxate, and octinoxate are identified as esters by ECOSAR and thus also follow a polar narcotic mode of action (Barron et al., 2015). Less common are reactive compounds (ecamsule) and those with a potential neurotoxicant mode of action (diketones, pyrroles) represented by avobenzone and ensulizole. Avobenzone is relatively unique in that it is a diketone, models as highly toxic, and is under-studied (see below). Zinc oxide (ZnO) and titanium dioxide (TiO2), as inorganic metals most likely are involved with iono-regulatory perturbations as with other metals although ECOSAR does not provide insight into inorganic compound classifications (Barron et al., 2015). It should be noted that these classifications are for acute modes of action only and it is almost certain that different and multiple chronic modes of action are present across the UV filters.
COMMITTEE APPROACH TO TOXICITY DATA RELEVANCE AND RELIABILITY FOR ERA
Although the relevance and reliability for use in ERAs of the data varies across the studies on UV filters (e.g., see discussion by Burns and Davies, 2021), the committee recognizes the value of studies that are not designed around the guidelines set for use in ERAs. Importantly, not all studies are designed with the intention of being used for an ERA in a regulatory setting or as standard toxicity tests and instead are more limited in scope to answer a specific question regarding a potential effect or effects. For example, Danovaro et al. (2008) was the first study to investigate the effects of UV filters on corals. The study was limited in scope and not suitable for inclusion in an ERA but it did draw attention to a concern that has subsequently received further research attention. In accordance
___________________
4 See https://www.epa.gov/tsca-screening-tools/ecological-structure-activity-relationships-ecosar-predictive-model.
with the statement of task, the committee identified data potentially suitable for use in an ERA based on both relevance (e.g., receptors and biological endpoints used) and some aspects of reliability (e.g., based on a few general toxicity testing principles and/or adherence to standard test methodologies). The committee also identified data that provide additional knowledge about possible effects (e.g., informative to mode of action, identifies data gaps and areas of future study).
The committee provided a focused look at the data for a subset of the UV filters: avobenzone, dioxybenzone, homosalate, octinoxate, octocrylene, oxybenzone, sulisobenzone, TiO2, and ZnO. This subset represents UV filters that are more commonly used, are on the higher end of data availability, and provide representation of a range of physico-chemical properties (inorganic and organic, varying solubilities). While homosalate has relatively fewer studies available, it was included due to its high volume of use (see Chapter 2). For this subset of UV filters, the committee categorized the laboratory toxicity test data based on the key considerations in Box 6.1. These key considerations are based on existing toxicity testing guidelines used by OECD and EPA. However, many guidelines were not included as considerations in this effort in order to be more inclusive, particularly given the number of toxicity tests conducted on nonstandard toxicity test species that were important to consider. Additional studies are also available that will unlikely be considered for use for ERAs and thus not discussed in this report. Studies on endpoints informative to mode of action are discussed later in the chapter.
This analysis is meant to inform the committee’s assessment of progress and research toward higher-tiered ERAs as requested in the statement of task and does not make use of an existing scoring method. Ultimately, as part of their ERA procedures, EPA will conduct its own relevance and reliability scoring of the data for which study inclusions can be much more nuanced (EPA, 2021c). The committee’s categorization is not meant to preempt EPA’s determinations regarding study applicability, but rather to provide a general understanding of what is currently known and where the knowledge gaps and limitations of the current dataset are in regard to the toxicity of UV filters to aquatic organisms.
Several UV filters are challenging to work with because they have low solubility, are highly sorptive, are degradable, and/or are photo-unstable. In the categorization process, the committee made an effort to be inclusive in order to acknowledge the challenges in conducting tests on these compounds. Thus, the key considerations are informed by guidelines but incorporate some variance from the guidelines. For example, the OECD (2019) guidance for use of solvents for “difficult test chemicals” recommends that the maximal amounts used be at least an order of magnitude below the NOEC for the solvent on a particular organism, not to exceed 100 μL/L (0.01% v/v). While solvents make poorly soluble and rapidly degrading UV filters easier to test, they can cause their own toxicity or may enhance toxicity of the test chemical by increasing bioavailability which would not be captured in a control and/or interfere with specific modes of action (Duis et al., 2022; Kais et al., 2013; Mitchelmore et al., 2021; Tuncer et al., 2018) and hence minimization is recommended. The committee used the recommended value to distinguish between two categories of study that are considered potentially informative for ERA up to 0.1 percent of solvent. While solvents may result in toxicity findings above solubility, limiting the environmental relevance of the results, acute results over solubility are indicative of a need for chronic studies of exposures at low concentrations.
Using reliable analytical methods to measure concentrations of exposure solutions over the duration of the test reduces the uncertainty in the derivation of toxicological thresholds, as opposed to nominal concentrations (presumed concentrations based on starting materials). Findings of toxicity based on nominal concentrations are included in the committee’s review as indicators of effects, but would require further study to derive actual dose-response relationships and reduce uncertainty that could be caused by test chemical identity, losses due to sorption to test and organism surfaces, biodegradation, photo-instability, other transformation processes, and losses during analytical processing. Testing concentrations at or below solubility levels helps avoid any complications with physical toxicity associated with chemical precipitates, micelles, crystals, or colloids.
INVESTIGATIONS ON THE TOXICITY OF ORGANIC UV FILTERS TO AQUATIC ORGANISMS
The number of studies and range of test species varies widely across the organic UV filters in regard to standard regulatory test organisms, nonstandard organisms, or in vitro tests (described in the Studies Informing Mode(s) of Action section). Toxicity endpoints are compiled from a number of sources, including publicly accessible databases (e.g., European Chemicals Agency [ECHA], EPA ECOTOX) and the peer-reviewed published literature. ECHA’s database includes toxicity data reported as part of the process for registering chemicals in the European Union. EPA ECOTOX is populated with published studies, which the committee used for reference though supplemented by additional publication searches because ECOTOX was found not to be comprehensive.
Below, the committee first describes the organic UV filters for which an assessment was made of the ERA-applicability of the existing data (as described in the previous section, “Committee Approach to Data Relevance and Reliability for ERA”). Detailed summary tables for these UV filters can be found in Appendix E, which identify the two categories of data that could be applicable for ERAs. The chapter text contains brief summaries of the information in these tables, highlighting data availability and ranges of toxicity findings. Brief summaries of the remaining organic UV filters then follow.
Avobenzone
Limited aquatic toxicity data is available for avobenzone on standard test organisms. Acute toxicity studies reported in ECHA5 report no effects under solubility (27 μg/L; Table 4.1) for freshwater algae, Raphidocelis subcapitata, (96-h EC50 > 55 μg/L for growth based on geometric mean) and Daphnia magna (48-h EC50 > 30 μg/L for immobilization). Other acute studies report results at nominal concentrations (Phaeodactylum tricornutum, Vieira Sanches et al., 2021; D. magna, Park et al., 2017; D. magna, Boyd et al., 2021). Standard chronic studies are available for D. magna, reported to ECHA (21-d NOEC ≥ 3 μg/L [the mean measured concentration achieved] for reproduction, body length, and daily immobilization) and in one published study (21-d NOEC 20 μg/L for survival; Boyd et al., 2021).
Fel et al. (2019) studied the effects of avobenzone (and other UV filters, as noted elsewhere) on dark-adapted maximum quantum yield of PSII (photosystem II), a measure of photosynthetic efficiency, in the coral Stylophora pistillata, reflecting the condition of the corals’ photosynthetic algal symbiont. A 35-d NOEC was determined as 1,000 (nominal) or 87 (measured) μg/L and interpreted as avobenzone having no effect.
As a low solubility and high log Kow (see Chapter 4) UV filter, avobenzone may be expected to partition into sediments. One chronic study has conducted toxicity testing from sediment exposure to a variety of organisms. Kaiser et al. (2012a) found no toxicity for various reproductive endpoints to infaunal and epibenthic invertebrates (Chironomus riparius, Lumbriculus variegatus, Melanoides tuberculata, and Potamopyrgus antipodarum) (50 mg/kg DW nominal). They also report no mortality to Danio rerio (zebrafish) embryos in a 48-hr sediment contact test (NOEC > 1,000 mg/kg DW nominal).
Dioxybenzone
No ERA-relevant toxicity effects are reported below solubility of dioxybenzone (13 μg/L in pure water; Table 4.1). Growth inhibition tests are reported to ECHA6 with acute and chronic interpretations for the freshwater microalgae, R. (=Pseudokirchneriella) subcapitata. Growth rate resulted in an acute 72-h ErC50 (inhibition of growth) of 1,270 μg/L and a chronic 72-h ErC10 of 1,040 μg/L, and an acute 72-h EbC50 (total biomass area under the curve) of 907 μg/L and chronic 72-h EbC10 of 549 μg/L for biomass. An acute 48-h EC50 for immobilization of D. magna has been reported as 4,270 μg/L (ECHA) and 3,550 μg/L (Liu et al., 2015). Though fish data are not
___________________
5 See https://echa.europa.eu/registration-dossier/-/registered-dossier/14835/6/1.
6 See https://echa.europa.eu/de/registration-dossier/-/registered-dossier/23375/6/2/6.
available from ECHA, a published study provides a 96-h LC50 of 395.6 μg/L for Oryzias latipes larvae (Thia et al., 2020). Meng et al. (2020) reported a 96-h LC50 of 1,147.9 μg/L (4.7 μM) in D. rerio eleutheroembryos (the embryonic stage to post hatch but before free feeding) as well as a reduced hatching rate and hatching delays (observed as low as 244.5 μg/L [1 μM]). Toxicity tests have also been conducted for nonstandard species: the planaria, Dugesia japonica (96-h LC50 33,300 μg/L, Li, 2012), and the nauplii of the marine barnacle, Balanus amphitrite (24-h EC50 2,200 μg/L for immobilization, Tsui et al., 2019).
He et al. (2019c) found varying results between two coral species, Pocillopora damicornis and Seriatopora caliendrum, in toxicity tests on both larvae (14-d) and adult nubbins (7-d). S. caliendrum was observed to be more sensitive than P. damicornis, and adult nubbins were observed to be more sensitive to mortality and bleaching than larvae. At the highest concentration tested (1,000 μg/L nominal) no mortality was observed in P. damicornis larvae, and only 30 percent mortality for S. caliendrum larvae (LOEC 500 μg/L). LOECs for adult nubbin mortality were reported at 1,000 and 100 μg/L for P. damicornis and S. caliendrum, respectively. Larval visual bleaching was only observed in S. caliendrum at LOEC of 250 μg/L. Exposure to 1,000 μg/L dioxybenzone caused 100 percent bleaching in nubbins of both species. This study also compared multiple benzophenones and found dioxybenzone to be more toxic than oxybenzone and sulisobenzone.
Homosalate
Acute toxicity information on homosalate can be found in ECHA7 for microorganisms, algae, invertebrates (daphnids), and fish. However, for fish toxicity data, octisalate is used as a surrogate because no information is available for homosalate. No acute effects were observed within the water solubility (91 μg/L; Table 4.1) and no chronic toxicity tests were conducted for registration with ECHA. Reported in ECHA are a 72-h NOEC ≥ 8.9 μg/L (the highest mean measured concentration from saturated solutions) for growth inhibition of the freshwater algae, R. (=Pseudokirchneriella) subcapitata, and a 48-h EC50 > 100,000 μg/L (nominal in a saturated solution) for immobilization of D. magna. In published literature, an acute 24-h immobilization test with nauplii from the marine barnacle, B. amphitrite, showed no effect up to concentrations of 10,000 μg/L (Tsui et al., 2019).
Octinoxate
Toxicity tests for octinoxate have been conducted for a variety of standard and nonstandard species. Acute tests on Lemna minor (duckweed), D. magna, Cyprinus caprio (carp) (all reported in ECHA8) and D. rerio (Cahova et al., 2021) have not observed effects under solubility (51 μg/L in pure water; Table 4.1). Standard chronic aquatic toxicity studies are available for D. magna, D. rerio (both reported in ECHA), and O. latipes (Lee et al., 2019). Of these, some effects are seen for D. rerio length and body weight (63-d LOEC 46.9 μg/L) and number of eggs in O. latipes (154-d LOEC 50 μg/L). Lambert et al. (2021) studied a nonstandard endpoint, number of molts, in D. magna, finding effects at 12 μg/L (21-d LOEC). Chronic sediment toxicity tests are also available from one study on a wide variety of species, finding effects on the number of embryos in the New Zealand mud snail, P. antipodarum (56-d LOEC 212.6 μg/kg dry weight geometric mean) and the tropical freshwater snail, M. tuberculata (28-d NOEC 2,000 μg/kg dry weight nominal) (Kaiser et al., 2012a).
Effects have been documented in other studies on a wider variety of species over the solubility of octinoxate. These include studies on freshwater and marine algae (Molins-Delgado et al., 2016; Paredes et al., 2014; Rodil et al., 2009; Sieratowicz et al., 2011; Tian et al., 2021; Vieira Sanches et al., 2021), D. magna (Jang et al., 2016; Molins-Delgado et al., 2016; Pablos et al., 2015; Park et al., 2017; Sieratowicz et al., 2011), the marine tube-worm Ficopomatus enigmaticus (Vieira Sanches et al., 2021), Mytilus galloprovincialis (Mediterranean mussel),
___________________
7 See https://echa.europa.eu/de/registration-dossier/-/registered-dossier/13246/6/1.
8 See https://echa.europa.eu/registration-dossier/-/registered-dossier/15876/6/1.
Paracentrotus lividus (purple sea urchin), Siriella armata (a marine mysid) (Paredes et al., 2014), Caenorhabditis elegans (Huang et al., 2018), D. rerio (Jang et al., 2016), and corals (He et al., 2019b), though other studies have found no effects. He et al. (2019b) observed partial mortality (33.3 percent) and bleaching (83.3 percent) in S. caliendrum fragments at LOEC 1,000 μg/L nominal.
Octocrylene
Acute studies on freshwater and marine algae (ECHA9; Vieira Sanches et al., 2021), fish (ECHA; Cahova et al., 2021), D. magna (2 studies in ECHA; Park et al., 2017), the barnacle B. amphitrite (Tsui et al., 2019), and the aquatic insect C. riparius (Ozáez et al., 2013) report no effects or effects are reported over solubility levels (40 μg/L in pure water; Table 4.1). Boyd et al. (2021) reported an immobilization 48-h EC50 of 30 μg/L for D. magna. In the same study, the 48-h LC50 is over two orders of magnitude higher at 3,600 μg/L. No acute toxicity (mortality and bleaching [visual and algal symbiont density] endpoints) was observed in two coral species (S. caliendrum and P. damicornis) following 7-d exposures of adult fragments up to 1,000 μg/L (He et al., 2019b).
Few chronic studies have been conducted with octocrylene. A 21-d reproductive study on D. magna, reported an EC50 of 2.66 μg/L (measured or 3.1 μg/L nominal; ECHA). The only chronic study in corals to date found no effect on survival following 35-d exposure up to 5,000 μg/L (nominal; 1,318 μg/L measured), although a decrease in photosynthetic rate at this concentration was observed (Fel et al., 2019). Other chronic studies on D. magna (ECHA; Pablos et al., 2015) as well as on algae (ECHA) and fish (2 studies in ECHA; Blüthgen et al., 2014; Zhang et al., 2016) report no chronic toxicity or report results over solubility.
As a low solubility and high log Kow UV filter, octocrylene may be expected to partition into sediments. A water-sediment toxicity test using sediments spiked with octocrylene found no effect on emergence or development time in C. riparius exposed to 2.33 mg/kg wet weight (WW) for 28 days, although a NOEC for growth was calculated at 1.27 mg/kg WW (Campos et al., 2017b). Lack of toxicity to C. riparius was also shown in the study by Kaiser et al. (2012a) following 28-day exposures to 60 mg/kg DW. In the same study, no toxicity following 28- and 56-day exposures were observed in two species of freshwater snails (> 43 and 50 mg/kg DW in M. tuberculata and Potomopyrgus antipodarum, respectively; Kaiser et al., 2012a).
Oxybenzone
Oxybenzone is the most widely studied organic UV filter, with acute and chronic toxicity reported for a number of standard and nonstandard toxicity test organisms, including corals, with a number of results reported under solubility (6,000 μg/L in pure water, Table 4.1). LC/EC50s in some standard toxicity test organisms are relatively similar across studies. Daphnia spp. 24- to 48-h EC/LC50s range from 1,090 to 3,030 μg/L (Boyd et al., 2021; Du et al., 2017, 2019a; Jang et al., 2016; Liu et al., 2015; Sieratowicz et al., 2011; ECHA). However, other invertebrate studies report very different values in the flatworm (96-h LC50 500 μg/L, Li, 2012), barnacle (96-h EC50 > 10,000 μg/L, Tsui et al., 2019), and jellyfish (72-h mortality LOEC 228 μg/L, Fitt and Hoffman, 2020). Two studies in juvenile D. rerio also present very different 96-h LC50s: 20,400 (reported in ECHA) and 3,890 μg/L (Du et al., 2017), although the latter study’s value is similar to that reported in juvenile O. latipes (96-h LC50 3,800 μg/L, reported in ECHA). These latter two fish studies are also in agreement with the QSAR predictions of 2,780 μg/L presented in Table 6.1. Algal studies present the most variable range of acute toxicity data. The majority of studies report 72- to 96-h EC50s for growth inhibition from 670 to 5,220 μg/L (Du et al., 2017; Esperanza et al., 2019; Lee et al., 2020; Sieratowicz et al., 2011; ECHA), however one study reported a 72-h EC50 for the marine algae, Isochrysis galbana, at only 13.87 μg/L (Paredes et al., 2014).
Chronic toxicity is more limited, available for 11 species (four algal, D. magna, two corals and four fish; Appendix E) providing ERA-relevant chronic toxicity data with NOECs/EC20s ranging from 10 to 1,170 μg/L
___________________
9 See https://echa.europa.eu/registration-dossier/-/registered-dossier/14858/6/2/1.
(e.g., Blüthgen et al., 2012; Boyd et al., 2021; Chen et al., 2016; Coronado et al., 2008; Kim et al., 2014; Mao et al., 2017; Pablos et al., 2015).
Acute toxicity tests have been reported for four intact hard coral species using adult fragments or coral larvae. Two studies have reported LC50s in intact corals ranging from 4-h LC50s 139 μg/L (light exposure) or 799 μg/L (dark exposure) (nominal) for S. pistillata larvae (Downs et al., 2016) to 96-h LC50s 6,150 to 7,060 μg/L (nominal although measured is provided) for adult fragments of Galaxea fascicularis (Conway et al., 2021), which may reflect the different species, life stage, and/or methodological design (e.g., exposure time), and analytical approaches used to report exposure concentrations. (i.e., nominal versus analytical). Although a third study did not report mortality and provided LOECs, the LC50s can be assumed to be less than the highest nominal concentration of 1,000 μg/L (He et al., 2019c) in the two species used (7-day exposures in S. caliendrum and P. damicornis). This study also conducted larval exposures with oxybenzone in both species. No acute mortality was observed in S. caliendrum or P. damicornis larvae following up to 1,000 μg/L (nominal) exposures for 14 days (He et al., 2019c). In contrast, acute toxicity was lower in S. pistillata larvae following shorter exposures of 24 hours (Downs et al., 2016) and so it is difficult to compare these studies with respect to differences in the concentrations, timing, and species used.
Bleaching of corals can be considered relevant to the growth and mortality of the coral holobiont, measured in various ways including reductions in algal density or the algae’s chlorophyll content. He et al. (2019c) found reductions in algal density in adult S. caliendrum only after seven days’ exposure at 1,000 μg/L. Conversely, Conway et al. (2021) found reductions in chlorophyll content concurrently occurring with tissue loss at concentrations > 2,500 μg/L, indicating that bleaching was not the cause of chlorophyll reductions in this case. Planula deformities were observed by Downs et al. (2016), reporting a 24-h EC50 in the light of 49 μg/L and 137 μg/L in the dark.
Sulisobenzone
Studies are available for standard and nonstandard species of algae, fish, and invertebrates (including corals), reporting no or low toxicity (i.e., far exceeding 1,000 μg/L though under the solubility of 300,920,000 μg/L) to sulisobenzone. For example, toxicities to freshwater algae species are only as low as 96-h EC50 38,000 μg/L (nominal) in a study in Chlamydomonas reinhardtii (Esperanza et al., 2019), with other studies reporting toxicities at higher concentrations or no effects at all (e.g., Du et al., 2017; Huang et al., 2022; ECHA10). Similarly, the acute toxicities for daphnid immobilization are only as low as 48-h EC50 30,400 μg/L (Molins-Delgado et al., 2016). Other invertebrates tested include B. amphitrite (no effects to 10,000 μg/L, Tsui et al., 2019), M. galloprovincialis, P. lividus, S. armata (with no effects at 10,000 μg/L, Paredes et al., 2014), and the planarian, D. japonica (96-h LC50 77,000 μg/L, Li, 2012).
A relatively low result for mortality in 7-dph larval O. latipes (medaka) of 96-h LC50 1,803.6 μg/L has been reported (Thia et al., 2020). This is in contrast to other fish results: a 96-h LC50 in Leuciscus idus reported as being between 215,000 μg/L (NOEC) and 416,000 μg/L (LC100), and a 96-h LC50 of 633,000 μg/L reported for zebrafish (Du et al., 2017).
Chronic studies are available (reported in ECHA) for reproduction in daphnids (21-d NOEC 5,000 μg/L) and mortality (14-d NOEC > 4,897 μg/L) and growth (14-d NOEC 1,048 μg/L) in juvenile fathead minnow (Pimephales promelas), and have found no toxicity at concentrations tested.
Other Organic UV Filters
A summary of the available information on ecamsule, ensulizole, octisalate, and padimate O is found below. The committee found no toxicity studies for aminobenzoic acid, cinoxate, meradimate, and trolamine salicylate
___________________
10 See https://echa.europa.eu/registration-dossier/-/registered-dossier/10063/6/2/1.
(though some studies informative to mode of action have been conducted), which appear to presently have low or no use in sunscreens.
Ecamsule
Toxicity information for ecamsule (solubility > 600,000,000 μg/L; Table 4.1) has been reported to ECHA11 and also published in a single peer-reviewed study, none of which report toxicity up to the concentrations measured. Reported in ECHA are no effects up to 100,000 μg/L (nominal) at 72 hours for growth inhibition of the freshwater algae, Desmodesmus subspicatus; 96-h LC50 > 100,000 μg/L (nominal) for the freshwater fish bluegill, Lepomis macrochirus; and 48-h EC50 > 100,000 μg/L (nominal) for immobilization of D. magna. Fel et al. (2019) measured photosynthetic efficiency (Fv/Fm) in a chronic toxicity test on nubbins of the coral species, S. pistillata reporting a NOEC of 5,000 μg/L (nominal, 5,025 μg/L measured), the highest concentration tested.
Ensulizole
Acute toxicity tests have been reported to ECHA12 for exposure to ensulizole for algae, fish, and invertebrates, none of which report adverse effects up to or above solubility (109,000 μg/L). Reported in ECHA are a 72-h NOEC ≥ 100,000 μg/L (the maximum nominal concentration used confirmed through analytical verification) for growth inhibition of the freshwater algae, R. (=Pseudokirchneriella) subcapitata; 96-h LC0 ≥ 10,000,000 μg/L (nominal) for zebrafish; and 24-h EC0 > 1,000,000 μg/L (nominal) for immobilization of D. magna. In an additional published study, Cahova et al. (2021) found no statistically significant difference between groups of D. rerio embryos exposed to ensulizole and control groups for mortality, hatching rate, and malformations at concentrations up to 100 μg/L and durations up to 96 hpf (hours post fertilization).
Octisalate
Only 5 percent immobilization occurred in D. magna at saturation, and thus the EC50 is reported in ECHA13 as above the solubility of octisalate in water (74 μg/L). A 72-h NOEC ≥ 11 μg/L and a LOEC > 11 μg/L (the maximum concentration that could be maintained under test conditions) for growth inhibition of the freshwater algae, R. (=Pseudokirchneriella) subcapitata is reported in ECHA. Three zebrafish acute toxicity tests with octisalate reported no mortality within water solubility in ECHA, reporting LC50 values ranging from > 82,000 μg/L to 613,000 μg/L (nominal). Any observed mortality was attributed to physical effects. A study on one marine species, B. amphitrite, reported no effects at the concentrations tested (Tsui et al., 2019).
A chronic study in ECHA on D. magna reported a 21-d EC10 of 8.4 μg/L for reproduction and 14.0 μg/L for mortality.
Padimate O
Toxicity testing for padimate O has been conducted on algae, daphnids, as well as two marine invertebrates and an aquatic insect. Toxicity studies are absent for fish other than a QSAR modeling study that indicated reduced toxicity of padimate O’s degradation products (Studziński et al., 2021). No effects have been observed in acute immobilization assays on daphnids (ECHA;14Molins-Delgado et al., 2016).
Acute toxicity tests on algae report varying results. The acute algal growth inhibition test for padimate O submitted to ECHA using the freshwater algae R. subcapitata reported no effects up to solubility. In the same species, Molins-Delgado et al. (2016) reported progressively lower EC50s as padimate O with time (70 μg/L for 24-h;
___________________
11 See https://echa.europa.eu/de/registration-dossier/-/registered-dossier/31837/6/1.
12 See https://echa.europa.eu/de/registration-dossier/-/registered-dossier/5464/6/1.
13 See https://echa.europa.eu/de/registration-dossier/-/registered-dossier/14203/6/1.
14 See https://echa.europa.eu/de/registration-dossier/-/registered-dossier/24214/6/1.
50 μg/L for 48-h; 30 μg/L for 72-h), attributed to reduced concentrations of padimate O. However, in a different freshwater algal species, Scenedesmus vacuolatus, Rodil et al. (2009) reported reduced toxicity of degradation products. Based on measured concentrations, a 24-h EC50 of 170 μg/L for phytotoxicity was determined.
Two studies conducted toxicity testing in the absence of light to reduce the influence of degradation products. Giraldo et al. (2017) reported a 48-h EC50 of 130 μg/L for embryo development in M. galloprovincialis (Mediterranean mussel), a 48-h EC50 of 279 μg/L for embryo development of P. lividus (purple sea urchin), and a 72-h EC50 of 59 μg/L for growth inhibition for the marine microalgae, I. galbana.
INVESTIGATIONS ON THE TOXICITY OF INORGANIC UV FILTERS TO AQUATIC ORGANISMS
The two inorganic UV filters, TiO2 and ZnO, share common characteristics that influence their toxicity to aquatic organisms, described here before subsequent sections reviewing the toxicity testing conducted for each UV filter individually. Determining their toxicity is complicated by their particulate nature, for which there is significant variation in crystalline structure, particle size, and surface area. These features can impact the interaction of organisms in the environment and their potential non-target impacts (Klaper, 2020). TiO2 for example can be present as rutile, anatase, brookite, or amorphous form (Reyes-Coronado et al., 2008). The anatase form has the highest photocatalytic activity and thus greater toxicity (described further below, Menard et al., 2011). Whether or not they have coatings, their degree of aggregation, and their interactions with other materials in the water column will also affect their toxicity. Therefore, results of toxicity testing on these UV filters can vary significantly, requiring consideration of these influential factors. Most toxicity studies have been conducted on nanoparticle sizes (< 100 nm), and thus some of the findings described here are only known based on studies of nano forms. However, a few toxicity studies described in the following sections provide comparative data between nano and micro/macro sized forms.
Reactive oxygen species (ROS; e.g., superoxide anion radicals, hydroxyl radicals, hydrogen peroxide) generation by the inorganic UV filters and by the cells in an organism is the most heavily cited mechanism of action for toxicity as they can readily damage lipids, proteins, and DNA if antioxidant and other protective mechanisms are overwhelmed. ROS are produced upon exposure of these particles to light, particularly in the UV wavelengths (Auffan et al., 2010; Clechet et al., 1979). Theoretically, physico-chemical characteristics that change the structure of the material (e.g., size, chemical composition, surface chemistry) and therefore the bandgap (the difference between energy bands created by overlapping electron orbitals, across which electrons are excited such as by UV radiation) can influence the production of ROS although the exact relationships have not been clearly defined (He et al., 2015). Nano-sized TiO2 produce significantly greater amounts of ROS than their macrosized counterparts, however size to ROS generation is not a linear function. For example, Jiang et al. (2008) found that over a range of 4–195 nm of anatase nano-TiO2, 30 nm and larger generated the highest ROS levels per unit surface area.
Several studies have found increased toxicity of nano-TiO2 in the presence of UV light (Jovanović, 2015; Mansfield et al., 2015; Xiong et al., 2013; Zhang et al., 2012). However, other studies indicate that generation of ROS and biocidal properties are possible with no UV or minimal UV present (Armelao et al., 2007; Fox and Dulay, 1993; Jones et al., 2008; Reeves et al., 2008; Sayes et al., 2006). Additionally, latitude of the environment, depth organisms are found, and organic matter and phytoplankton found in the water can influence attenuation of UV light and thus presence of UV light may not always be an exposure factor (Babin and Stramski, 2004; Belzile et al., 2002; Booth and Morrow, 1997; Overmans and Agustí, 2019). When natural bacteria communities from freshwater lakes in Sweden with high dissolved organic carbon were exposed to TiO2 nanoparticles, the particles alone reduced bacteria abundance at 100 μg/L but UV light at surface level and 1 m depth did not enhance toxicity (Farkas et al., 2015).
Toxicity of ZnO may be more complicated as particle dissolution into zinc ions also increases with a decrease in particle size and it is difficult to separate the relevance of ROS generation in toxicity versus this ion generation (Ma et al., 2013a; Raghupathi et al., 2011). In fact, ZnO may impart toxicity due to three mechanisms: dissolved ion release either in the media or within the organism, ROS generation in the media or in the organism, or the
particles themselves disrupting membranes or interacting with internal membranes, organelles or DNA (Brunner et al., 2006; Franklin et al., 2007; Kasemets et al., 2009; Kocbek et al., 2010). Zinc is an important trace element for many organisms, important as a co-factor for many enzymes including those involved in oxidative stress response (i.e., antioxidant enzymes), DNA repair and apoptosis so when consumed by an organism or absorbed by cells ZnO nanoparticles release an excess of ions and cause disequilibrium and greater ROS generation internally causing cytotoxicity (Ali et al., 2018).
An additional suggested cause of toxicity is accumulation within an organism, causing interference with consumption of prey items, decreasing energy availability and thereby impacting reproduction and survival (Glazier and Calow, 1992; Rosenkranz et al., 2009; Zhu et al., 2010).
Surface chemistry and co-occurrence of other metals or organics may also have an impact on interactions in biological systems. Commercially available sunscreens often contain particles coated with or doped with aluminum hydroxide, magnesium, silica, zirconium, or organic polymers such as polydimethylsiloxane, which can decrease photocatalytic effects to limit UV biological reactivity (EPA, 2009; Pan et al., 2009; Smijs and Pavel, 2011; Wakefield et al., 2004a). These coatings prevent the formation of ROS as well as the dissolution or breakdown of the particles. They also increase the ability for particles to be suspended in solution (Labille et al., 2010). For example, manganese doping reduced ROS by 90 percent (Wakefield et al., 2004b). Aluminum hydroxide-coated nano-TiO2 significantly decreased ROS production and toxicity to Hyalella azteca (a sediment living marine invertebrate) (Wallis et al., 2014). Chitosan and polyethylene glycol–coated ZnO nanomaterials were significantly less toxic to zebrafish than native particles (Girigoswami et al., 2015). Even after aging, the remaining surface coating may still be protective (Auffan et al., 2010). However, in one case, silica coating actually increased ROS generation, hypothesized to be due to the formation of a Ti-O-Si chemical bond (Baek et al., 2017).
Alternatively, organic coatings may alter the hydrodynamic diameter of particles. Silane coatings on ZnO nanoparticles create a more hydrophilic particle and greater acute toxicity to the marine copepod, Tigriopus japonicus, that is more due to the size-dependent uptake and therefore zinc exposure rather than ROS generation differences. A study with Escherichia coli found that carboxymethyl cellulose (CMC) and polyvinyl pyrrolidone (PVP) coating of nano-TiO2 decreased ROS, but increased toxicity by decreasing the sizes of aggregates (Virkutyte et al., 2012).
The presence of other compounds can decrease the toxicity of nanoparticle formulations. Natural organic matter (NOM, see Chapter 4 and Figure 4.1) alone can also decrease toxicity significantly (Li et al., 2016b). Commercially available sunscreens can contain various organic compounds as surfactants, emulsifiers, and even EDTA (ethylenediaminetetraacetic acid) as a chelating agent to sequester and reduce the impact of metal ions released from the formula (Virkutyte et al., 2012). However, chemical breakdown upon entering the environment (or hypothetically in an organism) returns ROS reactivity (Auffan et al., 2010; Carlotti et al., 2009; Labille et al., 2010; Wallis et al., 2014).
Transformations in the environment can affect exposure and toxicity to marine organisms. Coatings on ZnO and TiO2 are susceptible to chlorine, calcium sulfate, and other salts in swimming pools where degradation of the protective coating can occur, allowing for increased ROS production (Virkutyte et al., 2012). Silicon dioxide coatings (a common coating) can be very unstable and dissolve rapidly in fresh and seawater, leaving reactivity similar to the original nanoparticle (Slomberg et al., 2021). Coated TiO2 can transform rapidly when hydrophobic coatings dissolve in aquatic systems, leaving a relatively uncoated material; this can increase the dispersion of the remaining nanoparticle that otherwise would have settled into sediments. This in turn increases bioavailability to microorganisms and filter-feeders in the water column (Labille et al., 2010). In chronic assays, coatings did not make a difference because in the timeframe of the study the coatings degrade and most particles dissolved into ionic form, which caused the toxicity (Lai et al., 2021).
The degree of aggregation also affects reactivity and the potential for environmental impacts (Baveye and Laba, 2008). In a colloidal suspension (suspended insoluble particles), as nanoparticles age and particle and aggregate sizes increase, the band gap energy decreases which decreases the potential for creation of ROS (Kolá et al., 2006). The ultimate impact of aging depends on the starting particulates, as some are able to better withstand low
pH and ionic strengths. Alternatively, NOM in the environment can quench ROS and decrease toxicity of metal oxides like TiO2 (Li et al., 2016b) and change exposure of organisms to the particle and ROS (Yang et al., 2013).
Many test organisms do not reflect the actual locations where inorganic sunscreen components may ultimately settle and accumulate. The organisms exposed and experiencing chronic exposures may vary solely due to the surface coating and its stability in aquatic media. Data on sediment dwelling organisms is limited and may be the more important exposure for particulates that have the tendency to aggregate and settle.
The committee reviewed the literature on TiO2 and ZnO toxicity, including what could be found in the EPA ECOTOX database. EPA did conduct an initial case study on TiO2 in 2010 (Varner et al., 2010). The additional studies that have occurred since this initial analysis have been included in Appendix E. Summarized here are typical or ranges of toxicity endpoints and other highlights from the published studies. The data present in ECOTOX does not distinguish between studies related to their crystalline form or their particle size and some of the studies do not have complete characterization data of the particulates, particularly the size and surface area of the particles.
TiO2
The EPA ECOTOX database contains 896 records for aquatic toxicity from 47 total studies for TiO2, which generally are studies on nanosized TiO2 with uncoated particles. Most data are related to standard freshwater toxicity test species D. magna, rainbow trout (Oncorhynchus mykiss), and zebrafish (D. rerio). The data across species indicate that most endpoints across all species tested show effects above 10 μg/L, across all endpoints. Although several investigators do note the ability to conduct dose-response assays in a traditional manner, nanoparticle toxicity experiments suffer some issues of reproducibility due to factors such as concentration-dependent aggregation and sedimentation (EPA, 2010; Hartmann et al., 2010).
For R. (=Pseudokirchneriella) subcapitata, acute 72-h EC50 values for growth inhibition can range from 5,830 to 241,000 μg/L (Aruoja et al., 2009; Hartmann et al., 2010; Ji et al., 2011). In chronic 7-d assays, R. subcapitata was more sensitive with an IC25 of only 1,000–2,000 μg/L (Hall et al., 2009). Using a nonstandard assay (soil media and an extended time period to capture the growth phase of algae), Kulacki and Cardinale (2012) found that across 10 species of freshwater algae, nano-TiO2 did not impact growth but did impact biomass for some species at high concentrations (> 100,000 μg/L).
Lovern and Klaper (2006) did not find that TiO2 (mixture of anatase and rutile) was acutely toxic to daphnids at any concentration up to 100,000 μg/L after 48 hours. Wiench et al. (2009) tested several commercially available TiO2 nanomaterials and larger that were both coated and uncoated and found that all had similar impact in acute 48-hour assays to daphnids (EC50 > 100,000 μg/L). However, Hall et al. (2009) found that changing water constituents could cause significant variation; by including 1,500 μg/L organic carbon or clay caused a change in acute 48-hour toxicity of an LC50 from 760 μg/L in Ceriodaphnia dubia to > 100,000 μg/L. Additionally, Amiano et al. (2012) found that the 48-h EC50 for immobilization dropped from 29,700 to 1,200 μg/L TiO2 after exposure to UVA radiation. Exposures of ZnO up to 100,000 μg/L are not toxic to daphnids, but in the presence of UV, the LC50 is 29.8 μg/L (Ma et al., 2012).
Toxic effects increased to daphnids over time. Zhu et al. (2010) found that increasing the duration of an acute assay from 48 hours to 72 hours increased mortality (LC50 from > 100,000 μg/L to 2,020 μg/L) and immobilization (EC50 from > 100,000 μg/L to 1,620 μg/L), which they hypothesized to be due to the increasing accumulation of particles in the gut of the organism preventing normal function. Under normal test conditions for chronic reproduction in daphnids, the anatase form of TiO2 (A-100) caused significant declines at 60 μg/L (21-d LOEC; Seitz et al., 2013).
Acute toxicity to fish is low, ranging from 96-h LC50 155,000 μg/L (medaka; Ma et al., 2012) to 96-h LC50 500,000 μg/L (fathead minnow; Hall et al., 2009) to findings of no effects up to 1,000,000 μg/L (fathead minnow [P. promelas], reported in ECHA; the estuarine sheepshead minnow (Cyprinodon variegatus), reported in ECHA). TiO2 was also found to have low chronic toxicity to fathead minnows (7-d IC25 > 340,000 μg/L for growth; Hall et al., 2009). Although not a traditional toxicological endpoint, hatching time decreased in medaka at LOEC 30 μg/L (Paterson et al., 2011). D. rerio embryos that were exposed simultaneously to UV and TiO2 in nanoparticle form for 21 days (at which point they had transitioned to juvenile hood) experienced mortality at 1 μg/L (LOEC) (Bar-Ilan et al., 2013a).
Even high doses directly injected cause little impact on the kidney and other tissues (Scown et al., 2009). In addition, little impact was found in D. rerio physiology except during reproduction, during which viable embryo production was reduced after exposure to 1,000 μg/L bulk or nano form of TiO2 (Ramsden et al., 2013).
Overall there are fewer studies conducted on marine organisms. Artemia salina readily take up particles from sediment and were shown unable to excrete them; however, no mortality was recorded after 24 hours and increased to 18 percent only at 100,000 μg/L (LC50 > 100,000 μg/L; Ates et al., 2013a). In fact, there are more studies that find no impact up to 10,000 μg/L and above including acute toxicity in mature marine abalone (Haliotis diversicolor supertexta) (Zhu et al., 2011), and development of the sea urchin (P. lividus) (Catalano et al., 2020). The only impact seen was a decline in cyanobacterial populations when nanoparticles aggregated out of solution and trapped bacteria populations at 100,000 μg/L (Dedman et al., 2021).
There are minimal studies on the impacts of inorganic sunscreen components on corals. Caribbean mountainous star coral (Orbicella [=Montastraea] faveolata) exposed to 100 μg/L and 10,000 μg/L nano-TiO2 suspensions for 17 days caused zooxanthellae expulsion but did not cause mortality. The decrease in zooxanthellae only reached 14 percent and 25 percent of the control, which is only considered slight bleaching (Jovanović and Guzmán, 2014). Corinaldesi et al. (2018) found little impact on bleaching of Acropora spp. From 6,300 μg/L exposure to a rutile, coated nano form of TiO2 commonly used in sunscreen.
ZnO
The ECOTOX database for ZnO nanoparticle studies contains 349 records (313 aquatic records from 38 studies) over 27 species evaluated for many endpoints. The data show that ZnO has significant acute ecotoxicity impacts. Algae and crustacea are the most sensitive species as shown previously in a review of the literature (Bondarenko et al., 2013). Comparative studies have found ZnO toxicity to be higher than other nanomaterials (Adams et al., 2006; Aruoja et al., 2009; Brunner et al., 2006; Ji et al., 2011).
Toxicity to algae can vary with species, which may be due to algal morphology and physiology as well as culture media used. The 72-h EC50 range is about 40–60 μg/L for exposure to bulk and nano-ZnO in R. (=Pseudokirchneriella) subcapitata (Aruoja et al., 2009; Franklin et al., 2007). In contrast, after a 6-day exposure, the NOEC ranged between 1,000 μg/L for zinc ions (Zn2+) to 50,000 μg/L for bulk sizes for Chlorella sp. (Ji et al., 2011).
The fairy shrimp Thamnocephalus platyurus, was shown to have the most sensitive response among invertebrates, with a 48-h EC50 of 180 μg/L for the nano form and 240 μg/L for bulk particles (Heinlaan et al., 2008). For other invertebrates, ZnO is also toxic but at much higher concentrations. In daphnids, the 24-h LC50 is 3,200 μg/L for the nano form and 8,800 μg/L for bulk form (Heinlaan et al., 2008) and in C. elegans the 24-h LC50 is 2,300 μg/L for both the nanomaterial forms and bulk particles (Wang et al., 2009).
Zhu et al. (2008) found that in D. rerio, the 96-h LC50s for nano and bulk ZnO are 1,793 μg/L and 1,550 μg/L respectively, and the EC50s for hatching is 2,065 μg/L and 2,066 μg/L. At 1,000 μg/L, ZnO nanoparticles cause developmental defects in zebrafish embryos (Choi et al., 2016).
Fewer studies are available on the effects of ZnO on marine organisms. As is the case in freshwater, ZnO dissolves in seawater and causes toxicity that is not dependent on the morphology or size of the ZnO particle. The toxicity of ZnO has been attributed to dissolved Zn2+ ions in acute tests with marine diatoms Skeletonema costatum and Thalassiosia pseudonana, crustaceans T. japonicus and Elasmopus rapax, and the medaka fish Oryzias melastigma (Wong et al., 2010). In the marine diatoms, Thalassiosira pseudonana, Chaetoceros gracilis, and P. tricornutum, nanoparticles at all concentrations 10,000 μg/L and above decreased growth, which they related to bioaccumulation of Zn ions released from nanoparticles (Peng et al., 2011). ZnO particles can aggregate in seawater, which indicates some distribution to sediment dwelling organisms (Wong et al., 2010). Exposure to nano and bulk forms of zinc delayed growth and reproduction in the 500–1,000 μg/L range (NOECs from various chronic time points) in the sediment dweller amphipod Corophium volutator (Fabrega et al., 2012). The documented impacts of ZnO on corals is minimal. Acropora spp. of corals exposed to 6,300 μg/L of uncoated ZnO nanoparticles (20–200 nm) caused significant coral bleaching by release of zooxanthellae after 48 hours of exposure and those that were released were damaged (Corinaldesi et al., 2018).
SYNTHESES OF UV FILTER TOXICITY DATA
Due to the limited availability of chronic toxicity data, acute toxicity provides the best means for taking a summary view of the data for UV filters. For the purposes of categorizing acute toxicity thresholds, EPA classifies toxicity occurring at concentrations between 100 μg/L and 1,000 μg/L as “highly toxic” to aquatic organisms (EPA, 2022b; see application for example in Hemmer et al., 2011). Although this does not represent a determination of hazard for risk assessment, the committee references this as a comparison of laboratory toxicity test results across UV filters. Notably, the solubility (measured in pure water) of many of the UV filters is below 1,000 μg/L (Table 4.1), so aquatic exposure for these UV filters would typically be expected to be below this number (though physical toxicity from undissolved particulates can be identified in properly designed studies). As noted earlier, exposures in the environment can also occur through ingestion of UV filters adsorbed to organic particulates or from dietary sources; effects associated with such exposures are not well captured with standard aquatic toxicity tests unless specifically designed for this route of uptake. Further, measured and modeled exposure concentrations of UV filters in water are typically less than 1,000 μg/L and most of the measurements fall in the range of 0.01 to 10 μg/L with notable higher concentrations for oxybenzone, ZnO, and TiO2 in a few studies (see Chapter 4); thus, the possible convergence and overlap of effects and exposure ranges for UV filters may occur at concentrations less than 1,000 μg/L.
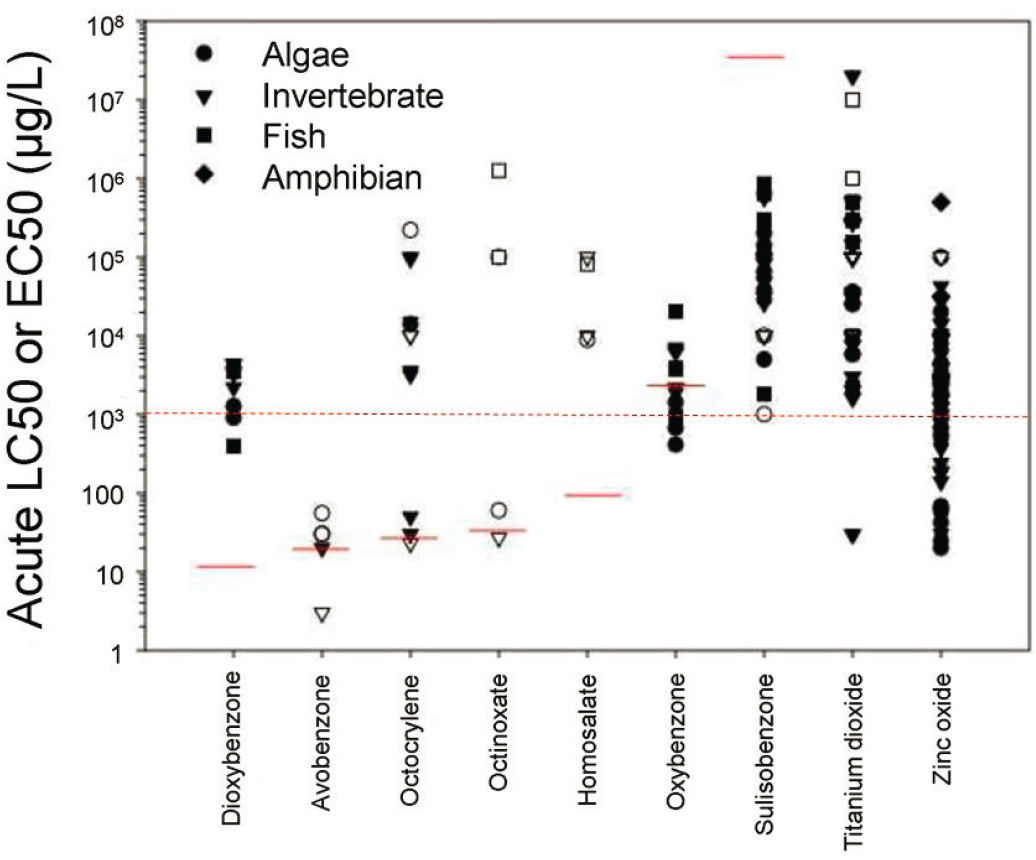
Results of acute toxicity tests assessed as potentially ERA-applicable by the committee are plotted in Figure 6.1 (limited to category 1 studies for ease of graphing). The numerical results depicted in Figure 6.1 are those found in Appendix E; this graphical illustration gives a sense of general trends and data availability. The toxicity threshold value of 1,000 μg/L is indicated as a red dashed line. As is evident, the majority of results are above the solubility limit and should therefore be interpreted with caution with regard to exposures to dissolved phases. For organic UV filters, the general order of toxicity is in accordance with solubility and log Kow (low solubility, high log Kow have the lowest toxicity values in general). Large variability across and even within taxonomic groups are often observed and most UV filters had toxicities that spanned 2–5 orders of magnitude. Across the depicted studies, algae were more sensitive to oxybenzone, sulisobenzone, and ZnO. Invertebrates were more sensitive to avobenzone, octocrylene, octinoxate, and TiO2 although with the exception of TiO2, all others in this group were bounded values. Fish were most sensitive to dioxybenzone; all results for dioxybenzone were about 1.5 orders of magnitude or more above solubility. Toxicity results for TiO2 under 1,000 μg/L occurred in the presence of UV light (category 2 studies are included in the graph to make this point). Based on acute data, organic and inorganic UV filters completely overlap with respect to acute toxicity.
Comparative Toxicity of UV Filters to Standard Test Organisms
Standard test species provide an opportunity for comparison of the relative toxicity of UV filters. When studied using established standard methods with a narrower range of test conditions, they allow study results to be compared to each other. Additionally, the existence of standard methods encourages the production of a higher volume of studies, providing a more robust dataset for analysis. For UV filters, a sufficient number of studies of acute and chronic toxicity to D. magna and acute toxicity to standard fish species are available for comparison. Comparisons for other taxonomic groups including algae and corals would require a structured research program to be possible. Algal studies are extremely varied with respect to species, endpoints measured, durations of studies, and statistics applied. Coral studies are diverse and may require further investigation prior to national or international standardization with different durations, life stages, endpoints, replication, and water qualities used. These wide differences in methodologies make comparisons difficult at best and could be misleading without substantially more research. That said, individual studies do have value and can be interpreted within a limited context (e.g., for a specific UV filter).
Daphnia magna
Figure 6.2 summarizes available 48-h EC50 survival/immobility data for D. magna assessed across all UV filters. If greater than (bounded) EC50s were found in the database, but measured EC50s were available, only measured data were used (found in Appendix E). If only 24-h data were available, these data were used. When multiple studies were available, geometric means were calculated to represent the central tendency of the responses. In the case of meradimate, cinoxate, trolamine salicylate, and aminobenzoic acid, no measured D. magna acute toxicity data were available. For these UV filters, ECOSAR v. 2.0 (Mayo-Bean et al., 2017) was used to estimate acute D. magna toxicity.
UV filters in Figure 6.2 are organized by organic filters (arranged in order of solubility) and then inorganic filters. Several trends are readily apparent. For the most hydrophobic filters, the aquatic toxicity measured is routinely above the limit of solubility. One should keep in mind that solubility here is based on measurements at
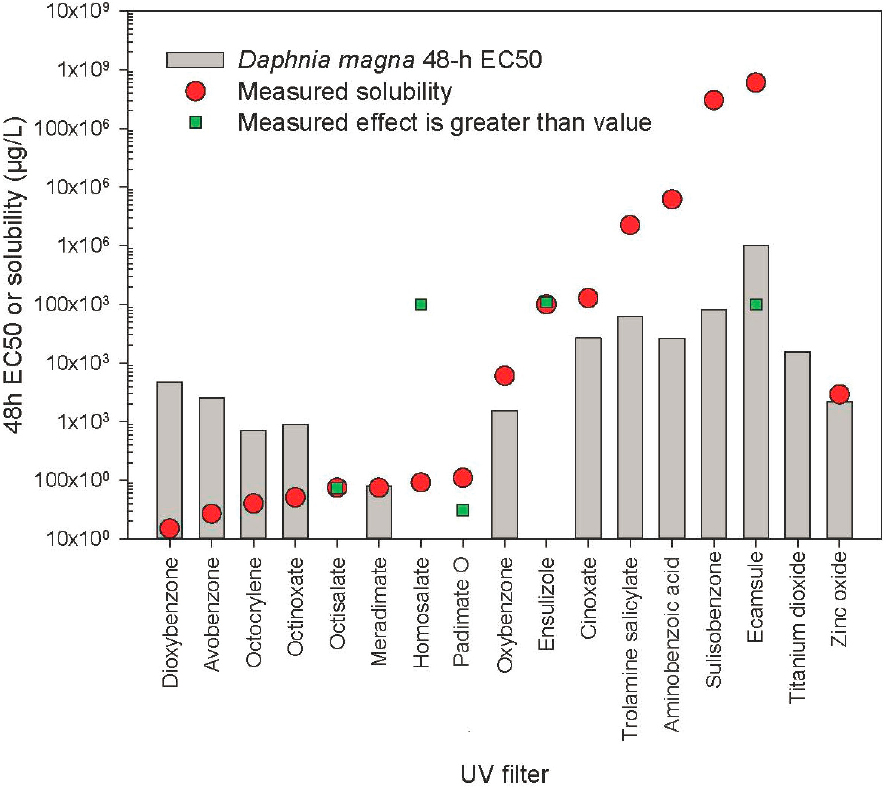
25oC in pure water without anions and cations present. Typical test temperature of D. magna studies is 20–22oC and in water with ions present; therefore, solubility of UV filters will certainly be lower (and unknown) than in standard pure water. Studies on several UV filters (octisalate, homosalate, padimate O, ensulizole, and ecamsule) were conducted with solubility limits in mind and the 48-h EC50s were quoted as above the limit of solubility or, as in the case of padimate O, no response was observed at slightly below the solubility limit. TiO2 is generally suggested to be highly insoluble in water and reliable solubility estimates are not available. Organic UV filters that are less hydrophobic (oxybenzone and to the right) have toxicities that mirror their overall solubility. Filters to the left of oxybenzone would be regarded as not toxic at their limits of solubility. Insoluble TiO2 is not toxic within the range of solubility and ZnO toxicity is close, but slightly below, the limit of solubility.
Chronic toxicity to D. magna (measured as the 21-d NOEC for survival or reproduction) was explored similar to the acute comparison above (Figure 6.3). One UV filter (octinoxate) had four available studies and three derived a greater than value which were not used in the plot. Octinoxate provides a good example of the complexity of assessing UV filters, even at low concentrations. The one measured value with an unbounded NOEC was 40 μg/L. Chronic effects to D. magna were also estimated as bounded values (i.e., “greater than” values), underscoring the difficulty of identifying responses for highly hydrophobic and low solubility compounds. For the three highly hydrophobic UV filters (avobenzone, octocrylene, and octinoxate), no effects were found near the limit of solubility.
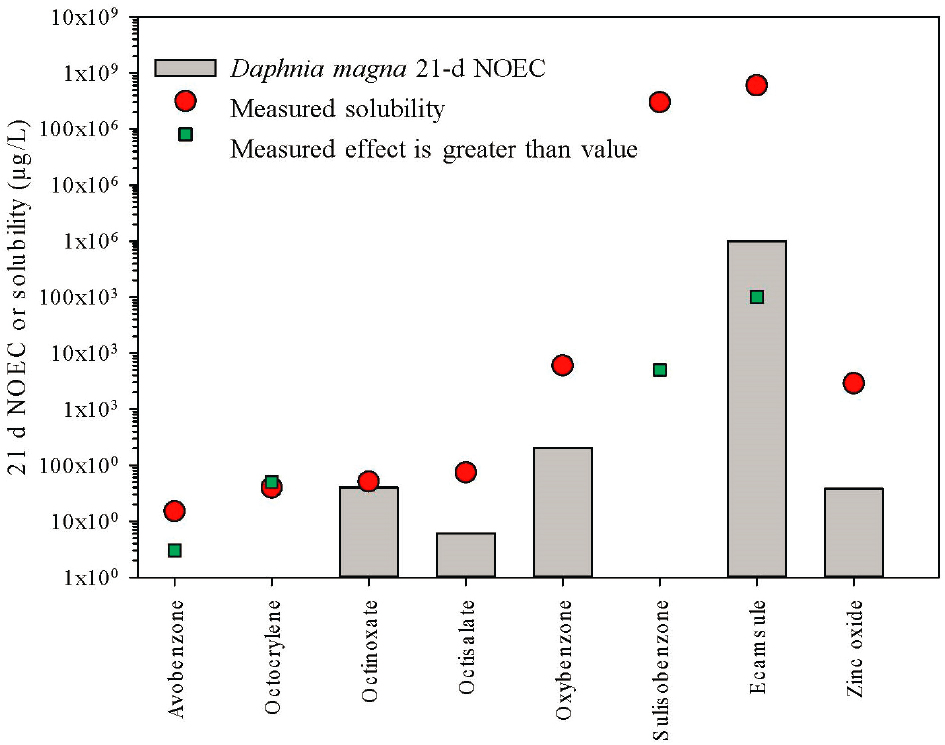
Oxybenzone and ZnO chronic toxicity was below measured solubilities; therefore, one can judge these results as reliable for input into risk assessment. Four of the UV filters had measured NOECs given as bounded or greater than values (avobenzone, ecamsule, octocrylene, and sulisobenzone) below solubility. The true NOEC may be above these values, but could be considered as useful, conservative estimates of toxicity for use in subsequent ERAs.
Fish
A similar analysis was conducted for acute toxicity values collected for standard fish species including fathead minnow, zebrafish, rainbow trout, and medaka. Similar to D. magna described above, if greater than (bounded) EC50s were found in the database, but measured EC50s were available, only measured data was used (found in Appendix E). Studies were generally 96 h, however out of 30 acute studies, two others of duration 48 h and 144 h were included for completeness as they were the only information available. When multiple studies were available, geometric means were calculated to represent the central tendency of the responses. Chronic toxicity data for fish exposed to UV filters is too sparse to allow meaningful analysis.
Ten of the UV filters had data and of these, five had unbounded values (not greater than) (Figure 6.4). The data set was dominated by zebrafish (D. rerio) and Japanese medaka (O. latipes) at 63 percent and 13 percent of the available fish toxicity data, respectively. Some trends are present but not as robust as for D. magna. Dioxybenzone,
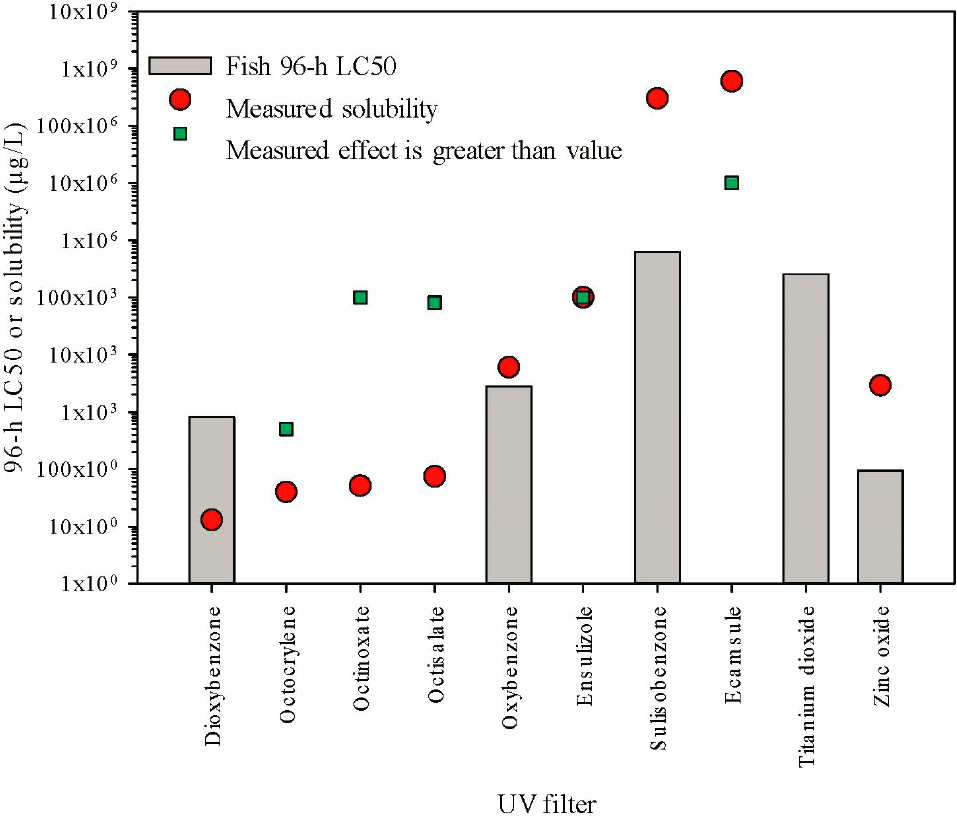
the most hydrophobic filter with fish acute data, had an LC50 that was about 60-fold above the solubility limit. Fish tested against other highly hydrophobic filters were assessed well above the limit of solubility and still had LC50s that were expressed as greater than values.
There are no clear trends among the standard species with respect to sensitivity. D. magna were more sensitive to oxybenzone, sulisobenzone, and TiO2 whereas fish were more sensitive to ZnO and dioxybenzone (although all results were above the solubility limit for this compound).
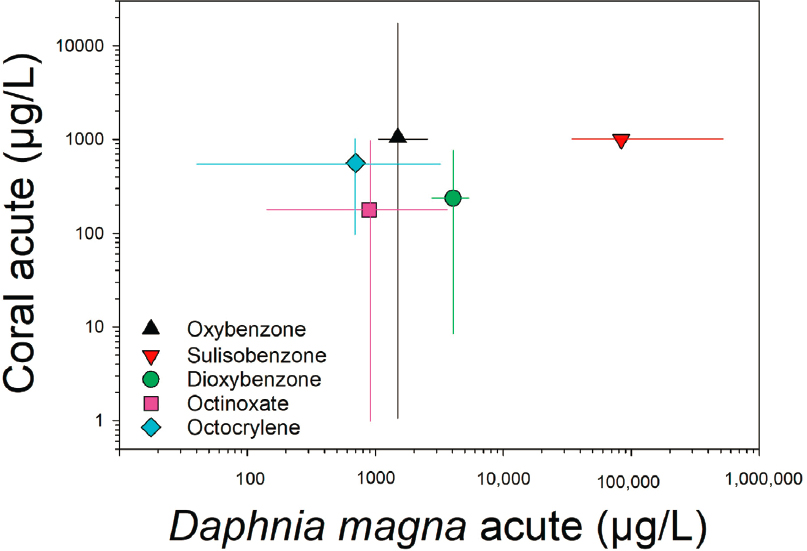
Comparison of Acute Toxicity Data for Daphnia magna and Corals
D. magna acute toxicity data were compared to data on acute toxicity to corals as a means to ascertain corals’ sensitivity to UV filters relative to that of standard freshwater invertebrates. Data for oxybenzone, sulisobenzone, dioxybenzone, octinoxate, and octocrylene were gathered from the literature after evaluation for data quality (i.e., data provided in the tables in Appendix E). All D. magna data were 48-h LC/EC50 studies. Corals included S. pistillata, S. caliendrum, P. damicornis, and G. fascicularis. Coral endpoints, a nonstandard assay organism,
included larval deformity, mortality, chlorophyll-a fluorescence, polyp retraction,15 bleaching rate, algae density, PSII yield, and visual color. Durations ranged from 24 h to 7 d. Statistics used to summarize response profiles included 19 that were LC50 or EC50 and 40 entries were expressed as NOECs. The comparisons should be judged cautiously because the most frequent comparisons are for coral NOECs versus D. magna 48-h EC50s, which are always higher than their associated NOECs.
Figure 6.5 displays the geometric mean of D. magna and coral toxicity using symbols with lines spanning the range of observations in the data. Large amounts of overlap are seen regardless of the UV filter. Coral data were much more variable, likely a reflection of the variety of endpoints, statistics, species employed, life stage tested and nonstandardized assay conditions (or in the case of sulisobenzone the single data point at 1,000 μg/L is due to limited studies). Corals and D. magna both project wide ranges of response variability in spite of one being nonstandard (coral) and one standard (D. magna). Wide ranges of variability are not atypical for even standard assays (Belanger et al., 2013). Both corals and D. magna were similarly sensitive (within a factor five) to oxybenzone, octinoxate and octocrylene and most disparate for sulisobenzone (D. magna being apparently more tolerant). Caution should be exercised for the latter comparison as the sulisobenzone coral data are 7-d NOECs being compared to 48-h EC50s for D. magna). Not enough data exists for either D. magna or corals to make meaningful comparisons for chronic toxicity to UV filters.
Species Sensitivity Distributions
SSDs have been used in ecotoxicology and ERAs since the 1980s to summarize the entirety of available ecotoxicological data for a chemical and predict a concentration at a low level of probability where it is unlikely for environmental organisms to be harmed (OECD, 1992a; Stephan et al., 1985). This concentration is universally
___________________
15 Polyp retraction was included due to the potential that this may be an endpoint with impacts on coral viability, although this link has not yet been made.
codified as the fifth percentile of the modeled distribution of values known as the Hazardous Concentration (HC5). The predicted HC5 from an SSD can be stated as follows: the concentration predicted to affect 5 percent of untested laboratory taxa (or not affect 95 percent of tested taxa) with an assumption that the species chosen are a random sample of environmental organisms and exposure to the stressor is similar between laboratory and field. Thus, SSDs are laboratory-generated predictions of potential responses to organisms in the field.
Belanger et al. (2017) summarized de minimis requirements for robust SSDs, either acute or chronic, to be interpretable and useful for a regulatory purpose (Belanger et al., 2017; Carr and Belanger, 2019). Quality criteria have been discussed in great detail at the international level (ECETOC, 2014) and these are generally followed when applied in the United States (Belanger and Carr, 2019; Belanger et al., 2017; Carr and Belanger, 2019). Concepts cover minimum input values, diversity of test species, experimental robustness of individual studies, and statistical criteria. SSDs built from acute data (survival endpoint) are used in EPA ambient water quality criteria and in pesticide registrations. SSDs built from chronic data are used in other relevant contexts where exposures may be of a longer duration (e.g., from wastewater discharge). Chronic SSDs differ from acute SSDs as the prediction is for a concentration of non-effects (no-observed-effect concentrations from individual studies).
A final consideration is how SSDs and the resulting HC5 may be used in risk assessment. HC5s are probabilistic assessments, accompanied by confidence intervals, that allow for consideration of uncertainty in a hazard assessment. In contrast, hazard extrapolation factors (also called Application Factors, AFs) are deterministic and applied to the data derived from the most sensitive taxon to make a prediction of the predicted no effect concentration (or PNEC). Ecotoxicologists recognized long ago that this discourages the development of new data by the private sector, as the only outcome possible was to continually lower the PNEC (OECD, 1992a). It also does not recognize the importance of accumulated toxicological understanding (Belanger et al., 2017; van de Plassche et al., 1999). AFs can also be applied to HC5s, and the factor used will depend on the strength of the SSD and reflect the particular regulatory actions needed (ECHA, 2014). For extremely robust SSDs, an AF of 1 has been applied whereas marginally robust SSDs may receive AFs of 5–10 depending on the country and situation (Belanger et al., 2017).
Example SSDs were possible based on data sets gathered by the committee, which cover four different UV filters (oxybenzone, octinoxate, TiO2, ZnO) in acute and in some cases (oxybenzone, ZnO) chronic contexts. Until now, SSDs have rarely been used to summarize UV filter effects data as the data are only recently accumulated to the level needed to conduct these assessments. SSDs were built for UV filters possessing sufficient information (acute and chronic) using methods and software outlined by Belanger and Carr (2019) (freely available on Github16 using the open-source statistical language R, documentation available from the National Academies upon request). The program flexibly determines an HCx where X is of the user’s choosing, provides plots (both cumulative frequency distribution and the parent distribution), and tabulates data. Tests for groupings of interest are made to determine if taxonomic groups (algae, invertebrates, fish, macrophytes) are measurably different as a group with respect to sensitivity or if sensitivity differences with respect to habitat (e.g., fresh versus saltwater species designations) exist. Statistical simulations are run to identify the relative importance of each individual data point in driving the outcome and predictions of the necessary additional data needed to drive the HC5 downward. Both are useful in assessing the robustness of the HC5 itself. Studies were included for use in an SSD when the toxicity data point was not bounded by a “less than” or “greater than” value and the statistical analysis was defensible. Endpoints of regulatory value were used (survival for acute tests; survival, growth, and reproduction for chronic tests). Chronic studies had to be of long enough duration for the taxon (substantial portions of a lifetime or to a critical life stage) to be considered a long-term exposure. Standard and nonstandard taxa were utilized whenever possible and SSDs were postulated only when algae, invertebrate, and fish toxicity data were available.
SSD models for the four UV filters indicate aquatic hazard is greatest for ZnO and least for TiO2. Octinoxate had an acute HC5 that was an order of magnitude lower than oxybenzone, with both being between the extremes of the inorganic UV filters. The chronic oxybenzone HC5 and underlying data suggest a non-specific (either polar or non-polar narcosis) mode of action for the UV filter; however, data are lacking for all other organic UV filters to broaden the conclusion. Other UV filters will require substantial expansion of their individual underlying data sets to develop quality SSD models. In any case, the SSDs for oxybenzone, octinoxate, TiO2, and ZnO would
___________________
16 See https://github.com.
be useful to assist aquatic hazard assessments of these chemicals by lowering uncertainties with extrapolation of individual toxicity studies.
Oxybenzone
Sufficient high-quality data were available for this committee to develop acute and chronic SSDs for oxybenzone (see Figure 6.6). The effect data used are given in Appendix E. Eighteen freshwater and marine taxa were used in establishing the acute oxybenzone SSD. In spite of likely physical chemical differences between fresh and saltwater, there are no significant differences in taxonomic sensitivity with respect to source environment or for taxonomic groups (i.e., algae, invertebrates, fish). The HC5 was 353 μg/L (95 percent confidence interval of 121–679 μg/L) using a log-normal fitted distribution (Table 6.2). Corals were not uniquely sensitive (i.e., similar sensitivities are seen in other taxa); while S. pistillata is the driving taxon, in its absence the HC5 is not greatly higher. The acute SSD appears robust as it would require a taxon that is 15.4 times more sensitive than the present most sensitive taxon to reduce the HC5 by a factor of 3, with a chance of 1 in 2.6 million that such a taxon would be found. The oxybenzone chronic HC5 was 48.5 μg/L (95 percent confidence interval of 5.3–145.3 μg/L). The data set is relatively small (10 taxa) with no discrimination of most sensitive taxonomic groups or the environments from which they were derived. In order to shift the HC5 lower by a factor of three would require finding an additional more sensitive taxon that is 2.4 times as sensitive as the current most sensitive species. Thus, it is possible that the SSD would be lowered by gathering additional chronic ecotoxicity data. If the present cumulative probability distribution is true, the chance of finding this taxon would be 1 in 812. It is interesting to note that the ratio of the acute and chronic HC5, which is akin to an acute:chronic ratio (ACR) as practiced in ecotoxicology (Raimondo et al., 2007), is 7.3 and suggests a narcotic mode of action. The ACR is calculated as the ratio of the median acutely lethal concentration (LC50 or EC50) and a chronic no-observed-effect concentration (NOEC) and can be used to extrapolate chronic toxicity from acute data (the ACR has yet to be robustly determined for any UV filters). In contrast to the committee’s SSDs, Carve et al. (2021b) determined chronic HC5s for oxybenzone of 10.6 and 0.07 μg/L for freshwater and marine, respectively. However, the SSDs were constructed using several species and using data from acute cell line toxicity extrapolated to chronic endpoints (whereas robust SSDs utilize only bona fide chronic data for constructing chronic SSDs; ECETOC 2014; Belanger et al., 2017). Studies in Carve et al.’s (2021) SSDs were of more varying quality than the ones used here and it has not been demonstrated that acute cell line data can be so extrapolated.
Octinoxate
Based on the acute toxicity values for 11 species, sufficient information was available to develop an SSD for octinoxate, with some caveats. Octinoxate has similar ecotoxicity compared to many other UV filters. The HC5, based on a logistic fitted distribution, was 26.7 μg/L (95 percent confidence interval of 0.6–187.7 μg/L) (Figure 6.6). The fit, although judged adequate, is undermined by one highly insensitive taxon (by more than an order of magnitude to the next most insensitive species), the zebrafish D. rerio. If the taxon is deleted, and the SSD re-run, the HC5 increases to 61.4 μg/L. Algae and invertebrates largely overlapped in sensitivity. Development of additional fish and coral invertebrate data would be key to improving the SSD for octinoxate.
TiO2
An SSD was developed by the committee for acute exposure to nano-TiO2 using the methods described above (Figure 6.6). The acute SSD was built from standard and nonstandard studies and, using a logistic distribution model, was determined to be 3,961μg/L with a 95 percent confidence limit of 467–12,630 μg/L). In order to devise the SSD, data for nano-sized materials compiled by Garner et al. (2015) were used as the starting point
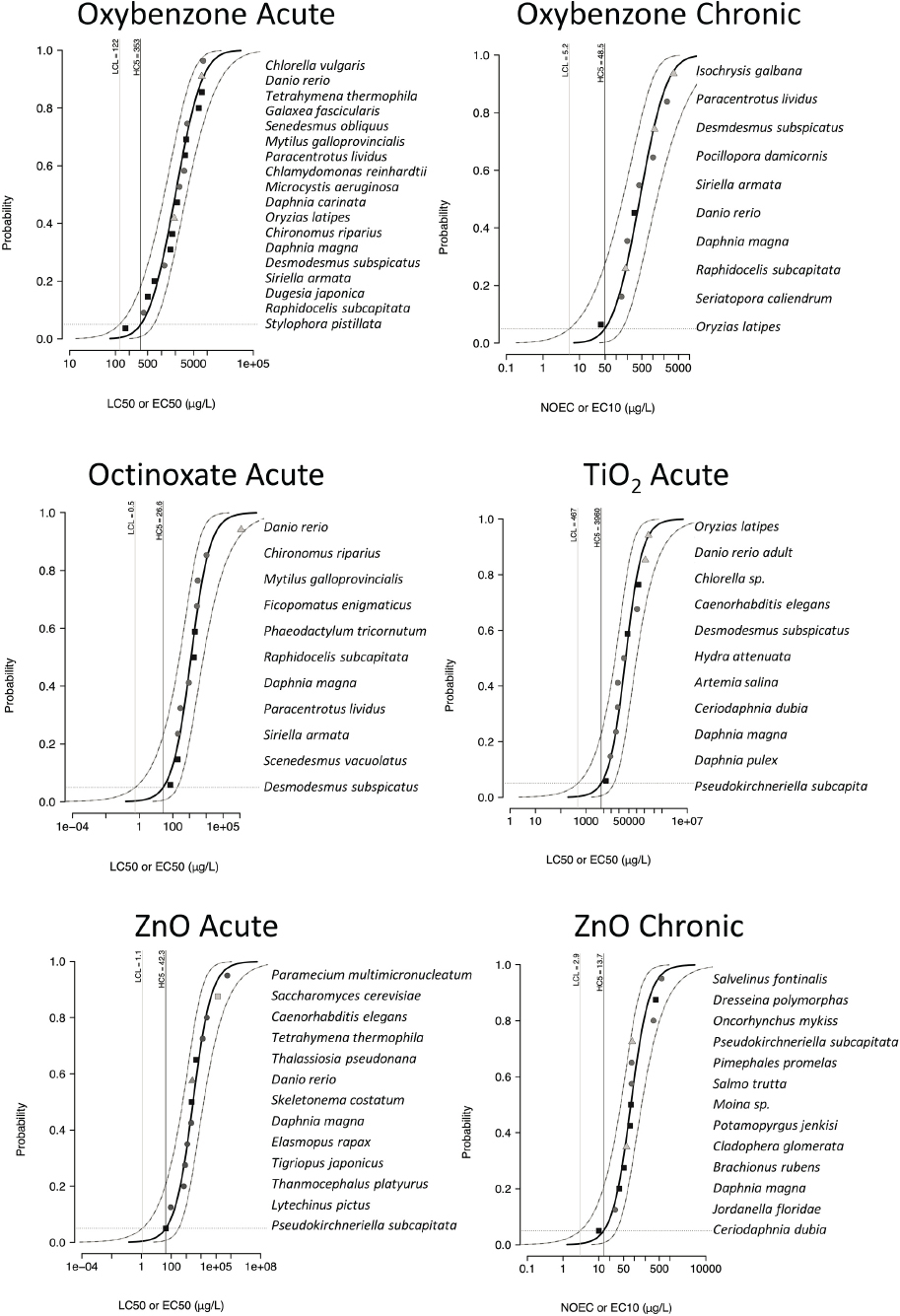
TABLE 6.2 Summary of HC5 Predictions for Several UV Filters
| UV Filter | Model | Number of Species | HC5 (μg/L) | 95% Confidence Limits (μg/L) |
|---|---|---|---|---|
| Oxybenzone; acute | Log-normal | 18 | 353 | 121–679 |
| Oxybenzone; chronic | Log-normal | 10 | 48.5 | 5.3–145.3 |
| Octinoxate; acute | Log-logistic | 11 | 26.7 | 0.6–187.7 |
| TiO2; acute | Log logistic | 11 | 3,961 | 467–12,630 |
| ZnO; acute | Log-logistic | 13 | 42.3 | 1.116–309.0 |
| ZnO; chronic | Log-logistic | 13 | 13.6 | 3.0–31.0 |
and combined with additional data (e.g., from ECHA).17 The SSD was devised by combining fresh and saltwater organisms, although saltwater data are clearly lacking overall. There were no statistically significant differences with respect to overall sensitivity of the different represented trophic groups (algae, invertebrates, and fish), but the data set is admittedly small. A new taxon would need to have an acute LC50 4.5 times as low as the present most sensitive organism (the green alga, R. subcapitata) to cause a shift in the distribution. The probability of finding such a result is 1 in 2,710 assuming the distribution is correct. Insufficient data was present to develop a chronic SSD using true chronic studies without further extrapolation (e.g., using ACRs).
Other SSD models for TiO2 have been published in the literature and vary markedly from that in Figure 6.6 and Table 6.2. Literature models of SSDs derive similar estimates of protective concentrations but are entirely based on freshwater rather than marine species, as there are few data for marine organisms. The acute and chronic SSDs of Garner et al. (2015) had only six species, were constructed by splitting out and using different life stages of the same species to result in n = 10 data points, and applied ACRs when needed to achieve a “chronic” data set. These largely impair the utility and predictive power of the extrapolation and do not follow international standards for SSD quality (summarized by Belanger et al., 2017). Sørensen et al. (2020) concluded that despite the extensive data set for TiO2 there is currently insufficient data to meet ECHAs minimum input data requirements for SSD construction (n = 10 and preferably 15 species) as there are insufficient data across a number of taxonomic groups available, specifically for long-term chronic NOEC measurements. Although many point to the concern that characteristics such as coating, size, shape, and experimental conditions could impact these estimates, Chen et al. (2018) found that although these led to some differences in SSDs, the HC5s did not differ significantly. Based on their SSD, Gottschalk and Nowack (2013) determined that there was no clear taxa that would be impacted by nano-TiO2 in freshwater systems based on expected concentrations in the environment.
ZnO
Sufficient information was available to develop acute and chronic SSDs for ZnO with certain caveats (Figure 6.6, Table 6.2). Freshwater and marine data were combined and all sizes of nano-ZnO were used for acute SSD development. Data compiled by Garner et al. (2015) were used as the starting point and combined with additional data (e.g., from ECHA). The HC5 using a logistic model was 42.3 μg/L with a 95 percent confidence interval of 1.116–309.0 μg/L. A total of 13 taxa were used in the SSD and there was no evidence of either exposure medium (freshwater/saltwater) or trophic group (algae, invertebrates, fish, fungi) being uniquely sensitive. A new test of a species previously undocumented in the SSD would need to be 3 times as sensitive as the most sensitive taxon (an LC50 of 14.1 μg/L) to lower the HC5 by 3. The chance of encountering this result, if the distribution is correct, is 1 in 122. A chronic SSD on Zn2+ previously published by Versteeg et al. (1999) and Belanger and Carr (2019) was used by the committee as a surrogate for ZnO. This assumption is reasonable given the relatively fast dissolution of ZnO in water. One advantage of this chronic data set is that the water hardness has been universally
___________________
17 Text was modified here and elsewhere in the report, after release of the prepublication report, in order to clarify the data source for the species sensitivity distributions for TiO2 and ZnO (acute).
adjusted to the same value, thereby accounting for the single largest environmental variable that drives observed differences in zinc toxicity. The chronic Zn2+ HC5 was 13.6 μg/L with a 95 percent confidence interval of 3.0–31.0 μg/L. Only freshwater organisms were used in the SSD. A new chronic result would need to be 2.2 times lower than the present most sensitive taxon to lower the HC5 by a factor of 3. Given the large data set and good fit of the distribution, the probability of encountering such a taxon would be 1 in 65,800.
SSD models for ZnO based on acute information were developed and similar patterns of sensitivity for ZnO and Zn2+ appear likely (Sørenson et al., 2020). Most of the ZnO studies demonstrate that much of the toxicity occurs in the acute time frame of exposures. Adam et al. (2015) estimated that based on acute toxicity information the HC5 was 60 μg/L with a 90 percent confidence range of 30–150 μg/L. Similar estimates for nano and bulk forms of ZnO were confirmed via many individual toxicology studies. By these analyses, ZnO is more toxic to aquatic organisms than TiO2. In addition, SSD calculations indicate the HC5 and the shape of the sensitivity distribution curve support the hypothesis that the ionic form of zinc and ZnO particles are not largely different. The SSD identified in the ECHA REACH dossier for Zn2+ is consistent with the chronic SSD in Figure 6.6 (HC5 of 20.6 μg/L adjusted for water hardness in freshwater and 6.1 μg/L in seawater). EPA acute ambient water quality criteria for Zn2+ are 0.98 and 0.94 μg/L, respectively.18 These values are based on an SSD methodology; however, the calculation method for these values differs greatly from other conventional SSD methods (Belanger et al., 2017; Stephan et al., 1985).
STUDIES INFORMING MODE(S) OF ACTION
A review of available studies involving UV filter exposure and aquatic organism responses using in vivo and in vitro genomic, biochemical/biomarker, physiology, and cell-/receptor-based assay systems reveals a diverse array of information is available (see Appendix F). This overview is not fully comprehensive as this is a dynamic area of current research with new information published almost daily. Studies span multiple taxonomic groups of algae, invertebrate, fish, and mammalian species. The diversity of studies, most of which are not directly relatable to each other as a consequence of differing methodological approaches (e.g., dosing regimens, solvent use, durations, test media) leads to a patchy and incomplete view of acute or chronic modes of action. The committee did not conduct a review of study quality similar to the cursory review completed for the in vivo toxicity studies. Methodological approaches for testing these endpoints vary considerably from those specified for toxicity testing. All conclusions regarding MOA would be speculative and outside the construct of AOP analysis (Ankley et al., 2010), which would be used to link observed changes at the suborganismal level to population responses. Hints as to mechanisms of toxic action that underpin these MOAs may be seen in these studies and a broad synthesis of the available information is described below.
Several UV filters appear associated with the induction of oxidative stress in a number of taxa including algae, protozoans, coral, fish, and marine turtles. This mechanism of action has been highlighted in recent studies for aminobenzoic acid (Huang et al., 2020; Liu et al., 2020), avobenzone (Liu et al., 2021), dioxybenzone (Thia et al., 2020), oxybenzone (Cocci et al., 2020), sulisobenzone (Liu et al., 2015), octinoxate (Gao et al., 2016), ensulizole and octocrylene (Falfushynska et al., 2021), TiO2 (Canesi et al., 2010; Federici et al., 2007; Wang et al., 2008), and ZnO (Meng et al., 2018; Wong et al., 2010).
Influences of UV filters relevant to potential neurotoxicity were identified in a few studies. Avobenzone, identified as a potential neurotoxicant/reactive compound (Barron et al., 2015; Mayo-Bean et al., 2017), disrupted the genetic pathways affecting swimming performance and induced upregulation of associated genes in zebrafish (Liu et al., 2021). Disruption in swimming behavior has also been observed in fish after exposure to oxybenzone and dioxybenzone (Thia et al., 2020) and nano but not bulk TiO2 (Boyle et al., 2013). Polyp retraction in corals has been observed as a sensitive endpoint in some studies after exposure to dioxybenzone, oxybenzone, and octinoxate. The impact of this behavior on coral viability, growth or reproduction is unknown; it may be a defensive mechanism that results in protection from stressors or it may be indicative of a neurological response to an irritant that ultimately results in harm to the organism (Conway et al., 2021; He et al., 2019a,b; Mitchelmore et al., 2021;
___________________
18 See https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table#table.
Stien et al., 2019, 2020). Studies with octinoxate suggested inhibition of acetylcholinesterase activity, a neural toxicological response in zebrafish (Nataraj et al., 2020) and crucian carp (Ma et al., 2017).
An extremely wide array of studies has attempted to detect and understand potential endocrine modulation of UV filters in aquatic biota. These include investigations with dioxybenzone (Lee et al., 2018; Thia et al., 2020), octinoxate (Christen et al., 2011; Inui et al., 2013; Kunz and Fent, 2006b; Zhou et al., 2019a; Zucchi et al., 2011b), octisalate (Kunz and Fent, 2006b; Soto and Rodríguez-Fuentes, 2014; Stien et al., 2020), octocrylene (Blüthgen et al., 2014; Yan et al., 2020; Zhang et al., 2016), oxybenzone (Blüthgen et al., 2012; Chen et al., 2016; Kim et al., 2014; Kinnberg et al., 2015; Kopp et al., 2017; Kunz and Fent, 2006b; Reyes-Coronado et al., 2008; Wang et al., 2016) and sulisobenzone (Kunz and Fent, 2006b; Zucchi et al., 2011a). Establishing a consequential endocrine modulation or endocrine disruptive activity that eventually impacts population level responses of growth, reproduction and appropriate sex ratios is largely absent in the suite of studies described here. Octinoxate has been studied in two separate and internationally accepted tests which are used to confirm endocrine disruptive properties: the Xenopus laevis (African clawed frog) Amphibian Metamorphosis Assay (OECD TG 201) and the D. rerio (zebrafish) Fish Sexual Reproduction Test (OECD TG 234) (reported to ECHA19). Both assay systems indicated octinoxate did not possess endocrine disrupting properties and endocrine-mediated changes. Endpoints included gonadal histology, fish length and weight, sex ratios, number of hatched eggs, vitellogenin, and genetic phenotypic sex ratios. A study on oxybenzone with TG 234 has also been performed (Kinnberg et al., 2015). In this assay, D. rerio was exposed for 60 d post hatch. Monotonic dose-dependent skewing of the phenotypic sex ratio and reduced gonadal maturation in both female and male fish were observed (NOECs of 139–388 μg/L). Exposure to oxybenzone did not affect the vitellogenin concentration in TG 234. Oxybenzone could be a weak endocrine disruptor and definitive studies, such as OECD TG 240, the Medaka Extended One Generation Reproduction Test (OECD, 2015a) or the androgenized stickleback screen (OECD, 2017b), would be helpful to establish the mode of action.
Other UV filters with a higher suspected endocrine modulation (dioxybenzone, octisalate, octocrylene) may benefit from similar formal test guideline investigations to more definitively relate in vitro observations to in vivo responses. Based on present evidence though, dioxybenzone, octisalate, and octocrylene appear to be weaker candidates for endocrine modulation.
Stien et al. (2020) studied metabolomic responses to avobenzone, octocrylene, and octisalate and found several steroid markers that could be associated with inflammatory or other pathways were expressed in coral. Another study saw induction of heat-shock protein 70 upon exposure to nano-TiO2 that caused slight bleaching, which recovered to control levels by measurements after 7 days during a 17-day exposure, indicating some level of acclimation (Jovanović and Guzmán, 2014).
Though they have not been the focus of studies on effects of UV filters, aquatic mammals and near-aquatic animals that spend part of their time in aquatic environments (typically in search of food) such as aquatic birds and mammals would also be considered in the report’s scope. No studies were found to address these organisms directly, but for the mammals, other mammalian laboratory evidence (e.g., from rats and mice) could be considered relevant. It should be noted however that dosing (dose preparation and route of administration), dosimetry, and assay durations would require deeper inspection to assess their relevance for aquatic and near-aquatic species and their potential environmental exposures. A large number of studies on mammalian cell lines, reporter gene assays, and other in vitro constructs such as receptor binding assays are available. Endocrine modulation of mammalian systems has been hypothesized for cinoxate (Shimoi et al., 1989); dioxybenzone (Kerdivel et al., 2013; Nakagawa and Suzuki, 2002; Zhan et al., 2021), homosalate (Cizmas et al., 2004; Gomez et al., 2005; Jiménez-Díaz et al., 2013; Ma et al., 2003; Schlumpf et al., 2001; Schreurs et al., 2002, 2005; Yazar and Oztas, 2018), meradimate (Rehfeld et al., 2018), octinoxate (Alamer and Darbre, 2018; Balázs et al., 2016; Heneweer et al., 2005; Schlumpf et al., 2001, 2004; Schreurs et al., 2002, 2005), oxybenzone (Balázs et al., 2016; Carve et al., 2021b; Krause et al., 2012; Lee et al., 2018; Wang et al., 2016); padimate O (Gomez et al., 2005; Morohoshi et al., 2005; Schlumpf et al., 2001; Schreurs et al., 2002), and sulisobenzone (Ma et al., 2003; Morohoshi et al., 2005). Some studies are in conflict (e.g., Jiménez-Díaz, 2013; Morohoshi et al., 2005) where octisalate was found to be endocrine active, weakly endocrine active, and not endocrine active.
___________________
19 See https://echa.europa.eu/registration-dossier/-/registered-dossier/15876.
Other potentially important modes of action have been addressed, albeit incompletely, including potential for mutagenicity and ultimately genotoxicity (Bonin et al., 1982; Jeon, 2017; Kotnik et al., 2016; Zhao et al., 2013) with dioxybenzone and octinoxate. These also should be cautiously interpreted, as significant elements of genotoxicity weight of evidence are lacking in these studies (see OECD, 2015a, 2020).
As part of the ToxCast program,20 EPA (or contractors or federal partners) has conducted high-throughput assays for suborganismal endpoints (including many receptors involved in stress and endocrine responses) for avobenzone, dioxybenzone, cinoxate, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, oxybenzone, padimate O, and trolamine salicylate. While these are in vitro studies and are not designed to indicate population-level relevance of the results, these data do identify potential interactions with key pathways involved with important pathways related to endocrine disruption and stress. Results for these UV filters indicate that for select organic UV filters there is some indication they have the potential to interact as either an agonist or antagonist with the ER receptor (octisalate, octocrylene, meradimate, benzophenone, avobenzone); the AHR receptor (padimate); the CAR constitutive androstane receptor signaling pathway (padimate O, cinoxate, octocrylene, meradimate, cinoxate); and the AR androgen receptor (meradimate, homosalate, benzophenone, oxybenzone) that could be explored further for phenotypic, apical endpoint impacts.
Acute MOA for UV filters can be hypothesized based on structure and a broader knowledge of mode of action assignment based on structural features (Barron et al., 2015; Mayo-Bean, 2017). Data needed to establish chronic MOAs to downstream cellular, tissue, organ, individual, and population responses are limited. Given the diversity of chemical structural features it is certain that multiple MOAs exist for the UV filters. However, evidence is accumulating that oxidative stress, genotoxicity, and neurotoxic MOAs are present. Limited data for endocrine modulation are presently available for the UV filters used in the United States with perhaps the exception of oxybenzone and octinoxate, which lacks any evidence based on test guideline studies. For the inorganic UV filters (TiO2 and ZnO) ionoregulatory disruption is the most likely relevant MOA. The relevance of non-aquatic mammalian studies to aquatic mammals is also a source of uncertainty which would require a separate research effort.
POTENTIAL FOR EFFECTS ON THREATENED AND ENDANGERED SPECIES
To the committee’s knowledge, there are few studies on the effects of UV filters on species listed as threatened or endangered under the Endangered Species Act (ESA). In the absence of these studies, other factors that can be used to approximate which listed species may merit further assessment are (1) extrapolation from studies on similar species (i.e., surrogate species), with the awareness that sensitivities may still vary among species and life stages, and (2) the potential for exposure to UV filters. As part of the consultation process between EPA and the National Oceanic and Atmospheric Administration (NOAA) and the U.S. Fish and Wildlife Service (USFWS), EPA conducts a two-step biological evaluation to assess potential risk from a chemical to listed species. In the first step, EPA considers whether a chemical “may affect” a listed species based on spatial overlap between its range and the area a chemical is expected to occur (presentation by Sandy Raimondo, July 13, 2021; EPA, 2020a). This determination identifies which species might undergo a more detailed assessment to determine if a chemical is “likely to adversely affect” listed species. This second step will consider chemical attributes that influence how they may interact with receptors, such as to which environmental compartment they will partition. Site-specific, quantitative estimates of risk are not developed until the third step involving consultation with NOAA and USFWS that results in a biological opinion. An additional step that EPA may take when data is available is to use Interspecies Correlation Estimation (WebICE21) where effects known for tested (surrogate) species are used to predict effects for an untested species. Taxonomic distance is a key factor in this extrapolation; however, information is infrequent for marine species (Raimondo et al., 2010). For WebICE extrapolations for endangered species, predictions are made at the genus or family taxonomic levels.
In this section, the committee considers potential exposure of groups of listed species and toxicity information about members of these groups, though the species of study themselves may not be listed. Most of this
___________________
20 See https://www.epa.gov/chemical-research/toxicity-forecasting.
information draws from data already included elsewhere in the report but is presented here to describe potential utility to managing listed species. Because UV filter exposure information is limited, generalizations are made about the potential presence of UV filters. Additionally, the committee did not review the range or critical habitat of every listed species, but rather, made assumptions based on general life history traits for each taxonomic group or observations made of tissue measurements in surrogate species. These summaries can therefore be considered preliminary assessments to guide and prioritize further research and detailed assessment focused on protection of listed species.
Corals
Currently, 24 reef-building corals are listed under the ESA (NOAA, 2022). There is not yet any critical habitat designation for the 12 species that occur in U.S. waters (seven in the Indo-Pacific, five in the Caribbean). However, their range is typically in shallow water, close to land, and they are frequent attractions for recreational activity. The overlap between coral reef habitat and recreational activity is already a management concern related to coral reef protection (e.g., Hannak et al., 2011; Marion and Rogers, 1994). This overlap suggests that the release of UV filters from recreation as identified in Chapter 4 could also be an exposure concern. Numerous UV filters have been measured near coral reefs globally, as discussed in the data reviewed in Chapter 4 including in locations containing ESA listed coral and other coral species (Bargar et al., 2015; Downs et al., 2016; Mitchelmore et al., 2019; Tsui et al., 2017, 2019). The potential impact of sunscreens to corals, including an ESA listed species; Acropora cervicornis and other Acropora sp.) was first suggested by Danovaro et al. (2008), which subsequently has led to numerous laboratory studies with various coral species and life-stages exposed to a wide range of UV filters as summarized in recent reviews (Miller et al., 2021; Mitchelmore et al., 2021; Moeller et al., 2021; Watkins and Sallach, 2021).
The only effects data available for a listed coral species was conducted on calicoblast cells (not containing symbiotic algae, which is the calcifying cell type in a coral) for two threatened Caribbean coral species, A. cervicornis and O. (=Montastraea) annularis in an in vitro cell culture exposure to oxybenzone (Downs et al., 2016). As described in earlier sections, laboratory toxicity tests have been conducted with various species of coral, (none ESA listed) with wide variations in species responses. Coral species are absent from current WebICE extrapolation databases.
Fish
In the United States, there are 124 species of fish that are listed by the USFWS as either threatened or endangered, and 43 marine and anadromous species are listed by NOAA (2022; USFWS, 2021). A large fraction of these fish are freshwater species. Exposure and toxicity studies have not been conducted for listed fish species, thus assumptions are made based on surrogates. Freshwater fish may encounter UV filters from treated and untreated wastewater or recreational release, as well as potentially via the food web. Toxicity tests have been conducted on several standard freshwater species for 10 of the UV filters (described in Comparative Potency of UV Filters to Standard Test Organisms), with several UV filters having measured 96-h LC50s under solubility limits. Information on exposure and effects on marine fish is more sparse. WebICE models for fish are more abundant, although marine taxa are represented by only two species of marine mullet with highly limited cross-taxonomic coverage.
Mollusks
In freshwater, about 25 mussel species have been declared extinct and the USFWS has listed 91 species as either threatened or endangered (USFWS, 2021). Freshwater mussels are sessile, often long-lived animals with complex life histories. Their biology—particularly their siphoning of the water column and their life stages occurring in both the sediment and the water column—make them vulnerable to numerous changes in water quality and habitat alterations that have occurred in the last 150 years. Additionally, because most mussels rely on a specific species of fish as a host for larval development, changes in water quality and habitat that impact their host fish species may result in indirect effects on mussel population viability. Informal input from USFWS representatives
shared with the committee indicated that mussels can often be found downstream of recreational areas where UV filters could be present, but otherwise their exposure to UV filters has not been measured in the United States (presentation by Tom Augspurger, July 13, 2021). Freshwater mussels are fairly well represented in WebICE with additional marine mussels and oysters included.
No exposure or toxicity data currently exist for ESA listed species although data is available for the marine species M. galloprovincialis (Mediterranean mussel), as discussed earlier. It should be noted that freshwater and marine mussels are not closely related and are in different subclasses (Palaeoheterodonta and Pteriomorphia, respectively). Some organic UV filters have been found in mussel tissues (Bachelot et al., 2012; Castro et al., 2018; Falfushynska et al., 2021; Gomez et al., 2012; Picot-Groz et al., 2014, 2018; Vidal-Liñán et al., 2018) and oysters in the Chesapeake Bay (He et al., 2019c). Studies informative to mode of action are also available for mussels (Bordalo et al., 2020; Falfushynska et al., 2021; Giraldo et al., 2017; Paredes et al., 2014). Exposure and mode of action studies for toxicity of the UV filter, TiO2, particularly nanoparticles, have been conducted in aquatic mollusks. Multiple studies have measured TiO2 in tissues (Johnson et al., 2015; Saidani et al., 2019; Sendra et al., 2017). Some studies informative to mode of action have been conducted for mussels (Barmo et al., 2013; Bordalo et al., 2020; Monteiro et al., 2019; Saidani et al., 2019; Sureda et al., 2018; Wang et al., 2019), oysters (Johnson et al., 2015), and clams (Guan et al., 2018).
In the marine environment, one mollusk species is threatened, the chambered nautilus (Nautilus pompilius) and two are endangered throughout their range, black abalone (Haliotis cracherodii) and white abalone (Haliotis sorenseni) (NOAA, 2022). As a possible surrogate, one toxicity study on TiO2 exposure is available on the marine abalone, Haliotis diversicolor supertexta (Zhu et al., 2011).
Birds
Although no marine birds are ESA-listed, USFWS identifies 97 bird species that are threatened or endangered (USFWS, 2021), and many bird species rely on prey from aquatic ecosystems such as fishes and invertebrates. Studies have found UV filters in the tissues (González-Rubio et al., 2020, study of benzophenones) and eggs (Molins-Delgado et al., 2017, study of oxybenzone and metabolites) of birds that feed on aquatic organisms, indicating the potential for exposure through trophic transfer and maternal transfer (see “Exposure Beyond Aquatic Ecosystems” in Chapter 5). No toxicity studies for birds on the UV filters available in the United States were found. WebICE extrapolations for threatened and endangered birds appear limited at this time given the size of the database and are mostly songbirds or fowl. Species whose habitats are coastal estuarine/marine or are raptorial are absent.
Mammals
Eight species of marine mammals are listed by the USFWS as threatened or endangered in the United States (including polar bears, sea otters, dugong, manatee), and 28 marine mammal species (including dolphin, porpoises, seals, sea lions, and several whale species) that are managed by NOAA. There are presently only two studies involving UV filter exposure to marine mammals. Gago-Ferrero et al. (2013a) found octocrylene in Franciscana dolphin (Pontoporia blainvillei) livers (89–782 ng/g lipid weight) in 21 of 56 samples. The second study measured UV filters (including octinoxate, padimate O, and octocrylene) in paired mother-fetus dolphins from Franciscana and Guiana dolphins (Sotalia guianensis) (Alonso et al., 2015). The findings showed maternal transfer of UV filters to the fetus occurred in both species, with higher concentrations in the fetus. Studies informative to MOA have been conducted on mammalian cell lines, with unknown applicability to aquatic mammals, and are described in the MOA section above. Mammalian entries for WebICE are highly limited, restricting the potential for toxicity extrapolation.
Amphibians and Reptiles
Thirty-five amphibian species are listed as threatened or endangered by the USFWS, as are 39 reptiles, a number of which are associated with aquatic environments (e.g., American crocodile, American alligator,
Copperbelly water snake, multiple turtle species). Additionally, six threatened and endangered sea turtle species are managed jointly by USFWS and NOAA. Few studies address exposure or effects of UV filters on amphibians or reptiles. Only one study was found involving a listed species—the loggerhead turtle, Caretta caretta (Cocci et al., 2020). Three of the UV filters under consideration for this report (ensulizole, oxybenzone, homosalate) were measured in plasma samples, ranging from below limits of detection levels to 28.43 μg/mL, with oxybenzone detected most often (37 percent of individuals). Toxicity studies have been conducted on African clawed frog tadpoles (Xenopus laevis), finding slight toxicity to nano-TiO2 (under 32 nm) but none to larger particles (Zhang et al., 2012) and on the marsh frog (Pelophylax ridibundus), finding minimal physiological effects from nano-ZnO and zinc ions (Falfushynska et al., 2017).
Aquatic Plants
There are 938 species of plants that are listed under ESA by the USFWS as threatened or endangered (USFWS, 2021). There are a number of listed plants that live in freshwater habitats, including riparian and wetland areas; however, the committee did not parse out how many listed species are aquatic versus nonaquatic. The first and only marine plant species listed (current status is proposed for delisting) under the ESA is Johnson’s seagrass (Halophila johnsonii). Only a few studies exist investigating the occurrence and toxicological effects of UV filters on any aquatic vascular plants.
Studies have shown that the organic UV filters oxybenzone (Agawin et al., 2022; Chen et al., 2017, 2018a,b; Cuoto et al., 2019), avobenzone (Agawin et al., 2022; Seyer et al., 2021), octisalate (Seyer et al., 2021), octocrylene (Ribeiro et al., 2017; Seyer et al., 2021) and sulisobenzone (Agawin et al., 2022) can be taken up and undergo biotransformation through root systems of the aquatic plants Cyperus alternifolius (freshwater pond plant) and Lemna gibba (duckweed) and salt marsh plants, Spartina maritima and Halimione portulacoides, and the sea grass, Posidonia oceanica, and translocated to shoots and leaves.
The three main types of impairment reported from organic UV filters on plants have been growth inhibition, oxidative stress, and dysfunction of photosynthesis (Chen et al., 2018b; Zhong et al., 2019, 2020a,b).
Inorganic UV filters also can be taken up and translocated systemically in aquatic plants. TiO2-nanoparticles (8 nm, anatase) were shown to enter through the roots of the aquatic plant, Spirodela polyrrhiza (type of duckweed) (Movafeghi et al., 2018). Among commonly reported effects for nano-TiO2 exposures in plants have been impacts to growth (Movafeghi et al., 2018; Song et al., 2012), genotoxicity (Foltête et al., 2011; Ghosh et al., 2010), and oxidative stress (Movafeghi et al., 2018; Song et al., 2012). Similarly, ZnO nanoparticles have been shown to accumulate in the aquatic plants, Hydrilla verticillate and Phragmites australis (Song and Lee, 2016). Reported effects, particularly to nano-ZnO, include reduced growth (Song and Lee, 2016), oxidative damage (Janani et al., 2021), and genotoxicity (Janani et al., 2021).
COMMUNITY AND ECOSYSTEM EFFECTS
Organisms in nature are embedded within communities and ecosystems, with many interactions with other species and ecosystem processes. UV filters could indirectly influence organisms if they modify species interactions (e.g., predator–prey dynamics, competition, mutualism) and/or ecosystem processes (e.g., primary production, nutrient cycling, energy transfer). Moreover, some species rely on others for habitat. Ecosystem-level effects can be difficult to assess because experiments and toxicity studies that traditionally use single species cannot simply be summed to predict ecosystem effects due to complex interactions among species. Studies on species interactions and ecosystem processes are frequently lacking in the literature on the effects of chemical contaminants (Richmond et al., 2017). Although limited information is available on community and ecosystem effects of UV filters on aquatic ecosystems, the studies described in this section shed light on the potential effects of these compounds.
Community Effects
The consequences for species interactions in an ecosystem have not been addressed in research on UV filters to date. Effects of UV filters may propagate through ecological communities through effects on the prey, competitors, and symbionts of individual species. The committee highlights three broad categories of key communities that have significant roles in the functioning of ecosystems through their interactions with other species groups.
- Microbial communities: Understanding the influence of UV filters on microbial communities is challenging because they are diverse assemblages that are not well described or understood (e.g., Bent and Forney, 2008). They play a critical role in several ecosystem processes (described in the next section), but can also influence ecosystem processes indirectly by affecting the health of other important taxa (see Bundschuh et al., 2016, for the example of nanoparticles). Microbial communities exist on the surfaces of organisms like corals and fishes and are important in the gut flora of many organisms. Microbial communities are also important in providing essential settlement cues for the larvae of numerous benthic marine invertebrate species, including corals, and when altered, can interfere with population replenishment (Dobretsov and Rittschof, 2020; Jorissen et al., 2021; Kegler et al., 2017). These surface and gut microbiomes of organisms are often distinct from each other and the surrounding microbial communities (Chiarello et al., 2020), and any changes in the composition of surficial microbiomes associated with exposure to chemical pollutants may have unanticipated effects on organism health, although large temporal variability has impeded linkages to population level consequences. Two studies have found that exposure to the UV filter oxybenzone altered the microbiota in the intestine of fish, Carassius auratus (Zhang et al., 2020), and the microbiome of the coral, S. pistillata (Wijgerde et al., 2020). These studies measured single timepoints and did not measure long-term effects or establish the relevance of these changes or population-level consequences. A study screening 27 species from five phyla of marine bacteria isolated from coastal bacterio-planktonic communities showed that homosalate inhibited growth for two species and octinoxate inhibited growth for five species (Lozano et al., 2020).
- Macroinvertebrate communities: Macroinvertebrates, which include a wide variety of taxa, play key roles in aquatic ecosystem functioning and are important food resources for higher trophic levels such as fish. Many freshwater insects have aerial adults, so they are also a food source for riparian spiders, birds, lizards, and bats. Studies on the aquatic midge, C. riparius, have found reproductive and developmental/growth effects to larvae and F1 generations resulting from sediment exposure to oxybenzone and octocrylene (Campos et al., 2017b, 2019).
- Coral reefs: Coral reefs are home to an estimated 25 percent of the ocean’s biodiversity, including fishes, invertebrates, algae, and a rich microbiome (NOAA, 2019). As previously stated, corals are holobiont organisms, usually composed of a coral host and associated symbiotic algae and microbiomes. Thus, corals can be impacted by stressors that target the corals, algae, and/or the microbes. Coral losses then cascade to other members of the coral reef ecosystem. Bleaching (typically a temporary or permanent loss or harm of the algal symbiont) or photosynthetic output of the symbiont are common sublethal endpoints of concern in studies of UV filter effects on corals (Conway et al., 2021; Corinaldesi et al., 2018; Danovaro et al., 2008; He et al., 2019b,c; Jovanović and Guzmán, 2014; Wijgerde et al., 2020). The implications of these studies at a population or ecosystem level are also complicated by the ability for corals to recover from these impacts to the algal symbionts, which will vary by species and by the full range of stresses on an organism (NASEM, 2019).
Ecosystem Processes
Effects on Nutrient Cycling
Nutrient transformations that occur in microbial communities can have large effects on ecosystem health, yet little is known about the influence of UV filters on these microbially mediated processes. The effect of octinoxate
on denitrification and anaerobic ammonium oxidation (anammox) processes in river sediment slurries was examined in only one study, conducted by Xu et al. (2020). These processes remove excess nitrate, which can cause eutrophication in rivers, lakes, and estuaries. They reported decreased anammox rates at all concentrations measured (0.01, 0.1, 1, 10, and 100 μg/L at incubation times up to 48 h), though with no concentration dependence. Inhibition of denitrification occurred at the two lowest and the highest concentrations. In addition, genes encoding nitrite reductase (nirS) and nitrous oxide reductase (nosZ) were downregulated compared to controls in the presence of octinoxate in all treatments.
Effects on Organic Matter Decomposition Rates
Organic matter decomposition is an essential ecosystem function that is indicative of numerous ecological processes including bacterial and fungal organic matter processing and animal consumption of organic matter. Macroinvertebrates are one important driver of organic matter decomposition in freshwater ecosystems (Wallace and Webster, 1996). One study found that oxybenzone at a concentration of 3.55 mg/kg in sediment reduced the feeding rate of the shredding caddisfly (Sericostoma vittatum) on leaves by approximately 50 percent after exposure for 6 days (Campos et al., 2017a).
Two additional studies are available for inorganic UV filters. In a microcosm experiment, Jain et al. (2019) examined the effects of nano forms of TiO2 and ZnO on organic matter decomposition. Leaf mass loss (an indicator of decomposition rates) was suppressed by both TiO2 and ZnO. Fungal sporulation was higher in the lowest concentration of TiO2 relative to the controls and was suppressed relative to the controls in the higher concentrations of TiO2 and all concentrations of ZnO. There were no significant effects on bacterial abundances in the experiment. The authors also observed stunted growth of fungal hyphae exposed to nanoparticles. In freshwater mesocosm (Du et al., 2019b) and microcosm (Du et al., 2020) experiments, ZnO nanoparticles reduced decomposition rates, microbial activity, microbial metabolic activity, and fungal community structure.
Effects on Primary Production
Primary production by benthic or water column primary producers provides the base of the aquatic food web in most aquatic ecosystems. Studies are available for algae, typically freshwater algae, for all UV filters other than those with no toxicity information at all. Specific to photosynthesis, a toxicological study investigating the influence of oxybenzone on a green alga and cyanobacteria suggests that this UV filter can significantly decrease the production of photosynthetic pigments in primary producers, finding cyanobacteria to be more sensitive than green algae (Mao et al., 2017).
EFFECTS OF UV FILTERS IN THE CONTEXT OF MULTIPLE STRESSORS
Aquatic ecosystems experience myriad stressors that contribute to impaired health of organisms and reduced condition and resiliency of the ecosystem as a whole. This section of the report considers the effects of UV filters in the context of co-occurring stressors. Specifically, it addresses two related concepts: (1) attributing observed ecosystem degradation to one or more specific causes (i.e., whether UV filters contributed to the decline of a population or inhibit recovery), and (2) identifying potential cumulative or interactive effects from multiple UV filters and/or co-occurring physical, biological and/or chemical stressors. Understanding how causes act together through a retrospective causal analysis helps guide ERAs where effects may arise from the co-occurrence of stressors.
When biological impairments are observed within aquatic ecosystems of the United States, eco-epidemiological methods may be used to investigate potential causes (Bro-Rasmussen and Løkke, 1984; Posthuma et al., 2016). Eco-epidemiological approaches include retrospective environmental causal assessments (Norton et al., 2018), and these can be used to guide cumulative impact and risk assessments (EPA, 1999a, 2003a). The causal analysis methodology used for eco-epidemiological investigations is outlined in the Causal Analysis/Diagnosis
Decision Information System (CADDIS22) causal assessment framework. Causal assessment links to other assessments related to prediction, evaluation of conditions, causal relationships, and solutions (Cormier and Suter, 2008).
Causal Analysis and UV Filters
In this section, UV filters are examined in the context of other known stressors by first considering the stressors that are identified by the scientific community as prominent causes of impaired aquatic ecosystems, with consideration of how to assess the degree to which UV filters specifically are a contributing factor. UV filters may contribute their own effects independently or by interacting with other stressors, an idea explored in the next section on cumulative and interacting effects. Coral reefs are provided as an example for analysis due to the attention that has been given to them in regard to both climate change and local stressors, including UV filters.
Following the CADDIS framework, causal analysis starts with identifying impairment of an ecosystem. This is followed by assessment of the candidate stressors and their plausibility (such as identifying likelihood of exposure). EPA conducts National Aquatic Resource Surveys to provide an assessment of the condition of the nation’s waters. From the most recent assessment, 26 to 30 percent of river and stream miles (surveyed 2013–2014; EPA, 2020b) and 48 percent of wetlands (surveyed in 2011; EPA, 2016c) in the United States were rated as good quality as measured by biological indicators, and 33 to 53 percent of lakes rated as “least disturbed” based on biological indicators (surveyed in 2012; EPA, 2016d). The surveys do not comprehensively document all possible causes of poor condition, though they measure some indicators related to key concerns like eutrophication, physical habitat quality, and select contaminants. For example, nearly half of the stream miles in the United States were rated poor due to nutrient pollution (58 and 43 percent for phosphorus and nitrogen, respectively). Based on biological indicators, 71 percent of surveyed estuaries were rated as good; however, only 15 percent were rated as good based on chemical contamination of fish (surveyed in 2015; EPA, 2021d).
Aquatic ecosystems are vulnerable to both impacts from climate changes as well as local stressors. Freshwater ecosystems are threatened by contaminants and nutrients, hydrology alterations, invasive species, and climate change (e.g., Craig et al., 2017). Stressors in marine waters include land-based runoff of nutrients and other contaminants in coastal areas, overfishing or destructive fishing techniques, coastal development projects, and climate change (warming, acidification, and sea level rise) (e.g., Halpern et al., 2019). The Sixth Assessment Report of the Intergovernmental Panel on Climate Change documents what is known about changing climate conditions and resulting impacts to affected organisms. There is high or very high confidence that increasing water temperatures and changes in flow regimes in freshwater ecosystems have led to population extinctions and increased risk to species extinction (Parmesan et al., 2022). Similarly, there is high or very high confidence that an increasing frequency and intensity of prolonged marine heatwaves in marine waters are driving shifts in community composition, biodiversity loss, and collapse of fisheries and aquaculture (Cooley et al., 2022).
While UV filters have not been specifically included in assessments of ecological conditions, what is clear is that typically, multiple stressors have led to degraded conditions and chemical exposure is a likely occurrence in fresh and marine waters.
Corals
Coral ecosystems exhibit a wide range of conditions depending on location. Within U.S. jurisdictions, remote reefs (e.g., American Samoa, Flower Garden Banks) scored as very good to good condition while reefs closer to human populations ranged from fair to impaired (e.g., Puerto Rico, U.S. Virgin Islands, Florida, Northwest Hawaiian Islands, Guam, and main Hawaiian Islands) based on benthic coral data (2012–2018) assessments. Overall, they are considered in fair condition in the Pacific and Atlantic regions of the United States, but projected to become impaired without efforts to reverse this course (Towle et al., 2022). A combination of climate change factors, disease, and local human activity contribute to the observed degradation of coral reefs. Climate change has led to a dramatic increase in coral loss through prolonged ocean warming and multiple global bleaching events
___________________
(Hughes et al., 2017, 2018a). While increasing ocean temperatures have affected even remote reefs, the greatest degree of impairment is found near human habitation and activity (NOAA, 2020; Towle et al., 2022). Local and regional stressors that degrade the condition of coral reefs include land-based sources of pollution and sedimentation, disease outbreaks, fishing impacts, freshwater influx, habitat destruction associated with coastal development, marine debris, and overtourism (Caparrós-Martínez et al., 2022; Knowlton, 2021; NOAA, 2020; Peterson, 2020).
An approach to understanding the main drivers of coral condition has been proposed in the work of Dyer and Green (in review; described in a presentation to the committee by Scott Dyer on September 16, 2021). They used a principal component analysis and correlation analysis to find associations between coral cover and diversity and a range of environmental variables and stressors (as well as correlations among these variables and stressors), finding strong associations with temperature and wave energy as well a range of other variables, including UV filters. Other endpoints in addition to coral cover and diversity may also serve as indicators of their health, including those that reflect longer-term health like recovery from disturbance or reproductive output.
A critical challenge to assessing UV filters in the context of other stressors is the limited measurement data set. For a causal analysis it is desirable to have an equitable representation of the stressors so that their relative importance can be adequately compared. However, as described in Chapter 4, the spatial and temporal variability and longer-term mean exposure concentrations for UV filters are not well characterized.
Possible Cumulative and Interacting Effects
The suite of stressors on aquatic ecosystems described above collectively contribute to observed impairments and could include independent effects but also effects involving interactions (additive, synergistic and/or antagonistic) with mechanistic underpinnings. As noted previously, multiple UV filters can be expected to enter the water at any given location due to their presence in mixtures in individual sunscreen products as well as different combinations in use across products. For this reason, mixture interactions among UV filters may need consideration in assessing risks. This is especially the case for direct introductions of UV filters during recreational activities where a number of UV filters are introduced together to localized areas. Additionally, there could be a greater suite of chemicals present for which possible interactions might be considered, such as other chemicals present in discharges from wastewater treatment plants, and in rinse-off from other personal care products and even sunscreens imported by tourists containing UV filters not marketed in the United States (studies on such mixtures have been conducted by, e.g., Cahova et al., 2021; Ozáez et al., 2016). One study found a combination of PFOS (perfluorooctane sulfonate) and octinoxate increased octinoxate’s toxicity in an acute immobilization test for D. magna (Pablos et al., 2015). Methods for evaluating cumulative chemical risks typically involve groupings by toxicological endpoint and ideally mode of action (EPA, 1999b, 2008). Specific questions can be addressed through laboratory studies with mixtures or with a few predominant compounds with respect to toxicological properties and exposure profiles.
There is limited EPA guidance on conducting an ecological cumulative risk or impact analysis for chemicals in concert with other environmental co-stressors such as temperature. Therefore, information on other stressors may be brought into a chemical risk assessment to provide context for management decisions. This may especially be the case for threatened and endangered species or protected habitats and ecosystems such as marine protected areas or designated critical habitats for endangered species. In the case of pesticides, the EPA Office of Pesticides Program (EPA, 2020a) has a protocol for considering cumulative risks for endangered and threatened species when it is determined that a pesticide may affect listed species or may destroy or adversely modify critical habitat. The potential for effects on habitat represents possible co-occurring stressors on the species that are additional to direct exposure.
Hooper et al. (2013) provide an example AOP framework that presents an option for linking the traditional chemical toxicity AOP with a climate-related AOP (including temperature as well as other environmental variables) and acclimation pathway. Figure 6.7 highlights two interactions: toxicant-induced climate sensitivity and climate-induced toxicant sensitivity, where one type of stress makes an organism more sensitive to the other stress. Studies on the interaction between temperature (and other climate impacts) and UV filter toxicity could inform an AOP consistent with the design proposed by Hooper et al. (2013).
Mixtures of UV Filters and/or Sunscreen Formulations
A few studies have evaluated the effects of sunscreen mixtures, either as combinations of UV filters alone or as fully formulated sunscreens. Molins-Delgado et al. (2016) investigated the effect of binary mixtures of organic UV filters on D. magna. They found reduced toxicity from the mixture of oxybenzone and octinoxate compared to that expected based on individual toxicity. Similarly, Du et al. (2017b) reported antagonistic effects (reduced effects compared to what might be expected from the additive individual toxicities) of the mixture of oxybenzone and sulisobenzone on Chlorella vulgaris, D. magna, and D. rerio. Both studies used the toxic unit approach, which expresses the relative contribution of each compound to the joint toxicity (Altenburger et al., 2003). Muñiz-González and Martínez-Guitarte (2018) investigated the effect on transcription of genes related to the endocrine system of the mixture of padimate O and octocrylene on C. riparius and found no interaction between the two UV filters. A study on the combination of benzophenone-1 and 4,4'-dihydroxybenzophenone (both potential metabolites of oxybenzone, see Figure 5.3), found antagonistic effects on estrogenic activity, while other combinations of chemicals (excluding benzophenone-1) presumed to be full or partial human estrogen receptor alpha agonists (UV filters not used in the United States) showed synergistic effects (Kunz and Fent, 2006a).
He et al. (2019b) exposed two coral species to dilutions of a “wash-off” of a sunscreen product (containing 7 percent octinoxate, 3.6 percent octocrylene) and compared results to single chemical and binary mixture exposures. Sunscreen was rinsed off hands and then diluted in filtered sea water. The test solution containing 5 percent of sunscreen “wash-off” (422.34 μg/L octinoxate and 33.50 μg/L octocrylene) was significantly more toxic than individual chemical exposures; mortality occurred in both species within 24-h (S. caliendrum 66.7–83.3 percent; P. damicornis 33.3-50 percent). A binary mixture of octinoxate (1,500 μg/L) and octocrylene (100 μg/L)
(concentrations expected to be similar to 20 percent sunscreen dilution) resulted in 100 percent mortality for S. caliendrum and 50 percent for P. damicornis. The LOECs for mortality, visual bleaching, and (for S. caliendrum only) zooxanthellae density, were all lower (i.e., more toxic) than individual and binary mixtures (LOEC 400 μg/L for octinoxate, LOEC 30 μg/L for octocrylene). Tissue levels had tenfold greater concentrations of UV filters from exposure from sunscreen products than in single compound exposures.
Other studies have measured effects of sunscreen formulations containing one or more organic and inorganic UV filters but have not been designed to distinguish between the UV filters and the inactive ingredients (Corinaldesi et al., 2017; McCoshum et al., 2016; Romero et al., 2020).
Interactions with Environmental Co-Stressors
In the case of UV filters, interactions have been observed with increasing temperature and salinity. Changes in these environmental conditions can be considered stressors themselves as well as influence the toxicity of UV filters. UV radiation has been shown in multiple studies to increase the toxicity of TiO2 (Amiano et al., 2012; Clemente et al., 2104; Johnson et al., 2015; Ma et al., 2012; results described further in the TiO2 toxicity section). Some studies have shown increased toxicity of oxybenzone in light conditions compared to dark in coral planula (Downs et al., 2016) and in zebrafish embryos (Zhang et al., 2021). Additionally, Vuckovic et al. (2022) investigated observed toxicity from oxybenzone to an anemone (Aiptasia sp.) and coral species (Discosoma sp.) under simulated sunlight from a mechanistic perspective, identifying the formation of glucoside conjugates as a potential phototoxicity mechanism. Yung et al. (2017) found that increasing temperature and salinity increased aggregation of nano and bulk ZnO and decreased dissolution to Zn2+, while also influencing gene expression related to an oxidative stress response. The diatom T. pseudonana was found to be more tolerant to nano-ZnO when experiencing its preferred temperature range rather than higher or lower extremes, and also more tolerant at higher salinities. Muñiz-González and Martínez-Guitarte (2020) observed an influence of temperature on gene expression responses to oxybenzone in the larvae of C. riparius.
Interactions between stressors have been observed in corals (reviewed by Ban et al., 2014; Negri et al., 2020; Nordborg et al., 2020, 2021) and more recently some preliminary studies on UV filters have shown interacting effects with temperature. Bleaching is a known response of corals to multiple forms of stress. It is commonly associated with increasing temperatures but the presence of additional stressors may lead to increased likelihood of coral bleaching. Additionally, corals impaired by local stresses have a reduced resilience to the impacts of climate change (i.e., an inability to recover from a bleaching event) such as through impairment in their reproductive capabilities or disruption of the relationship between the coral and its symbiotic algae (NASEM, 2019; NOAA, 2020). Two studies have investigated the interplay between temperature and UV filter toxicity and indicate the potential for interactions warranting further study (Danovaro et al., 2008; Wijgerde et al., 2020).
FINDINGS AND KNOWLEDGE GAPS
Despite the array of studies conducted on UV filter toxicity thus far, many gaps remain. Some UV filters are more well studied than others, and the applicability of the data for conducting risk assessments or inferring causality varies. The committee summarizes here the findings and knowledge gaps related to data availability as well what is known about toxicity from the data that is available. Filling knowledge gaps in toxicity data will inform higher tiered risk assessments, including the creation of SSDs when deemed necessary, as well as information about potential effects on threatened and endangered species via data for surrogate species. Importantly, findings about toxicity, when possible, must be put in perspective with environmental exposure in order to ultimately determine risk. The committee does not provide findings related to the characterization of risk.
Finding: Acute toxicity has been observed under 1,000 μg/L for dioxybenzone, octinoxate, oxybenzone, padimate O, TiO2 (in the presence of UV radiation), and ZnO and in a few studies for avobenzone and octocrylene. In addition, QSAR estimates for aminobenzoic acid, cinoxate, meradimate, homosalate, and octisalate suggest possible toxicity below 1,000 μg/L. Toxicity values typically exceed solubility for the poorly soluble UV filters (solubility under 100 μg/L).
Finding: Acute and chronic toxicity studies vary in their relevance and the reliability of methods, particularly due to the importance of studying nonstandard test species for which there are not standardized methods, thus limiting identification of appropriate toxicity thresholds.
Finding: Sufficient data are available for the development of acute species sensitivity distribution (SSDs) for oxybenzone, octinoxate, TiO2, and ZnO and chronic SSDs for oxybenzone and ZnO, which can be used as toxicity thresholds in risk assessments.
Finding: Limited data indicate that interacting effects are possible from the combination of multiple UV filters or UV filters and other environment stressors. Increasing temperatures are of particular concern due to the potential for increasing cumulative and interacting impacts from climate change.
Knowledge Gap: Data are limited on chronic toxicity of UV filters. Chronic studies are especially important for the poorly soluble organic UV filters and for the inorganic UV filters.
Knowledge Gap: There is limited toxicity information overall on nonstandard organisms, particularly marine organisms. The lack of standard toxicity test methodologies on species of interest like corals, which have unique biology and therefore toxicological endpoints, challenges conducting tests that are comparable to each other and studies on other taxa.
Knowledge Gap: Toxicity tests on benthic organisms and sediment exposure are very limited. Sediment exposure toxicity tests are most critical for hydrophobic organic UV filters and for the inorganic UV filters, a portion of which will aggregate and settle into sediments.
Knowledge Gap: A number of studies on inorganic UV filters distinguish between particle size but other factors are not well studied, particularly the effect of surface coatings.
Knowledge Gap: There are very few studies on the toxicity of UV filter degradates, despite observations of degradation of avobenzone, padimate O, octinoxate, and oxybenzone during toxicity tests.
Knowledge Gap: There are few studies on community and ecosystem dynamics, the need for which is supported by the few that indicate potential effects on ecosystem processes like nutrient cycling, organic matter decomposition, and effects on primary producers and other key species.
Knowledge Gap: Studies that link downstream cellular, tissue, organ, individual, and population responses to population endpoints are generally lacking. These would be informative to understanding mode of action, particular chronic modes of action that influence endpoints such as growth and reproduction.
This page intentionally left blank.








































