Appendix F
Data Science and Machine Learning
MODELS FOR ASSESSING CHEMICAL TOXICITY
Traditional chemical toxicity testing protocols, which mainly rely on animal models, are expensive and time-consuming (Luechtefeld et al., 2018; Meigs et al., 2018; Zhu et al., 2014). Computational toxicology is a promising alternative strategy for chemical toxicity evaluations. Existing computational models for chemical toxicity evaluations include quantitative structure–activity relationship (QSAR) modeling approaches. These have been widely used to predict the toxicity of new compounds. QSAR modeling consists of chemical descriptor generation, machine learning, validation, model robustness testing, and applicability domain definition. Although QSAR modeling has existed for a long time, it has advanced to a new stage with the recent progress of machine learning to deep learning. However, critical issues exist in the current computational toxicology models, such as reliance on small training sets which cause limited coverage by the resulting models (Stouch et al., 2003), activity cliffs (Maggiora, 2006), and overfitting (Dearden et al., 2009; Tetko et al., 1995, 2008). Furthermore, the applicability of QSAR models depends on the chemical structures within the training set. Therefore, complicated toxicity phenomena, consisting of multiple mechanisms, cannot be dealt with solely by QSAR modeling (Zhu, 2020).
Data-Driven Modeling
Recent high-throughput screening (HTS) programs and their associated data-sharing efforts have revolutionized the landscape in many health fields, highlighted by the Big Data to Knowledge (BD2K) initiative by the National Institutes of Health. The BD2K initiative underscores the critical need to take advantage of the amount of data available in the health field for biomedical research (Margolis et al., 2014). For example, a significant HTS effort in toxicology is the U.S. Environment Protection Agency (EPA) research program called Toxicity Forecaster (ToxCast; Dix et al. 2007; Judson et al., 2010; Kavlock et al., 2012) and the associated Tox21 program (Hsu et al., 2017; Shukla et al., 2010; Thomas et al., 2018). ToxCast and Tox21 employ in vitro HTS tests and toxicogenomics techniques to quickly evaluate the biological activity of thousands of compounds and prioritize potentially toxic compounds for further testing. The Tox21 program has generated more than 120 million toxicity data points for approximately 8,500 chemicals (Thomas et al., 2018).
Mechanistic Modeling
An adverse outcome pathway (AOP) starts with the interaction of an exogenous chemical with one or more biomolecules such as receptors. This molecular initiating event (MIE) initiates a sequence of measurable key events at the cellular level and may lead to adverse outcomes at the tissue, organ, and eventually organism level (Ankley et al., 2010). Obtaining information on MIEs and key events in a pathway to an adverse outcome identifies the associated toxicity mechanisms of interest for risk assessment (Patlewicz et al., 2015). Currently, AOPs and relevant computational models based on AOPs are being developed for various types of toxicities, such as acute inhalation toxicity (Clippinger et al., 2018), neurotoxicity (Bal-Price and Meek, 2017; Bal-Price et al., 2017; Sachana et al., 2018), skin sensitization (Maxwell et al., 2014; OECD, 2018; Patlewicz et al., 2014), estrogen receptor binding (Benigni et al., 2017; Browne et al., 2017; Ciallella et al., 2021; Judson et al., 2015), forestomach tumors not induced by genotoxic events (Proctor et al., 2018), and drug-induced liver injury (Kim et al., 2016; Vinken et al., 2013). In addition, the toxicity data obtained from HTS programs such as ToxCast and Tox21 can be used to perform mechanistic
computational toxicology modeling, such as developing new AOPs (Ciallella and Zhu, 2019; Zhu et al., 2014). In the next 10 years, mechanistic modeling should be a major focus of computational toxicology.
IDENTIFYING INFLUENCES OF AIR QUALITY ON BIOLOGICAL AIR CONTAMINANTS
EPA, through both intramural and extramural research portfolios, has contributed to the development of high-performance computational models of atmospheric photochemistry as it relates to secondary air pollutant formation, tropospheric smog, and the oxidizing species it contains. Although not currently regulated in the United States, the chemical composition of indoor environments including indoor air has increasingly been the subject of scientific research and health-effects exposure studies. The emergence of COVID-19 as a global pandemic instigated perhaps unprecedented demand for expertise in aerosols and indoor air quality—demand that was largely addressed from the public’s perspective by the Centers for Disease Control and Prevention and indirectly by industrial and trade organizations, neither of which possessed comparable levels of expertise in atmospheric chemistry.
Decades ago, researchers examined the role that air pollution played in accelerating the loss of viability of pathogens. Declassified, posthumous published British Defence Ministry research (Hood, 2009) revealed the effects of chemical oxidants in air on pathogens: Escherichia coli bacteria exhibited 10 to 100 times faster loss of viability in polluted outdoor air than in pristine laboratory air (Benbough and Hood, 1971; May et al., 1969), an effect called the open air factor and later associated with the oxidants nitric oxide (NO), nitrogen dioxide (NO2), and ozone (O3), all components of photochemical smog (de Mik and de Groot, 1977). Such contaminants in outdoor air have relevance to infectious disease transmission among animals and livestock (e.g., avian influenza, porcine reproductive and respiratory virus syndrome) and are reflective of the elevated oxidant (O3) concentrations that can arise in rural and urban areas alike: in 2017, EPA data show that wilderness areas in the northern Rockies and urban areas in the industrialized Ohio Valley both reported ~60 ppbv as their fourth annual maximum ambient O3 concentration. Existing models of atmospheric photochemistry could help begin an examination of how airborne infectious disease transmission risk in animal agriculture may be moderated by climate, meteorology, and environmental contamination that are localized in scale.
With respect to the indoor environment and the lasting impact of the COVID-19 pandemic on perceptions of risk, the absence of regulatory drivers and the high degree of complexity and heterogeneity among indoor environments all contribute to the availability of few—if any—chemical models of indoor air with which to study indoor transmission risks of infectious diseases. Indoors, O3 concentrations of up to 360 ppb have been measured in the vicinity of laser printers during operation (Tuomi et al., 2000). World Health Organization data on the indoor environment indicate that residential natural gas stoves are responsible for NO/NO2 concentrations indoors in the range of 78-1,000 ppb (WHO, 2010). Nazaroff, Weschler and co-workers have thoroughly explored reactions between oxidants and typical chemical compounds found in indoor air (Nazaroff, 2016; Nazaroff and Weschler, 2004; Singer et al., 2006).
MODELS FOR ASSESSING POLLUTION CONTROL SCENARIOS
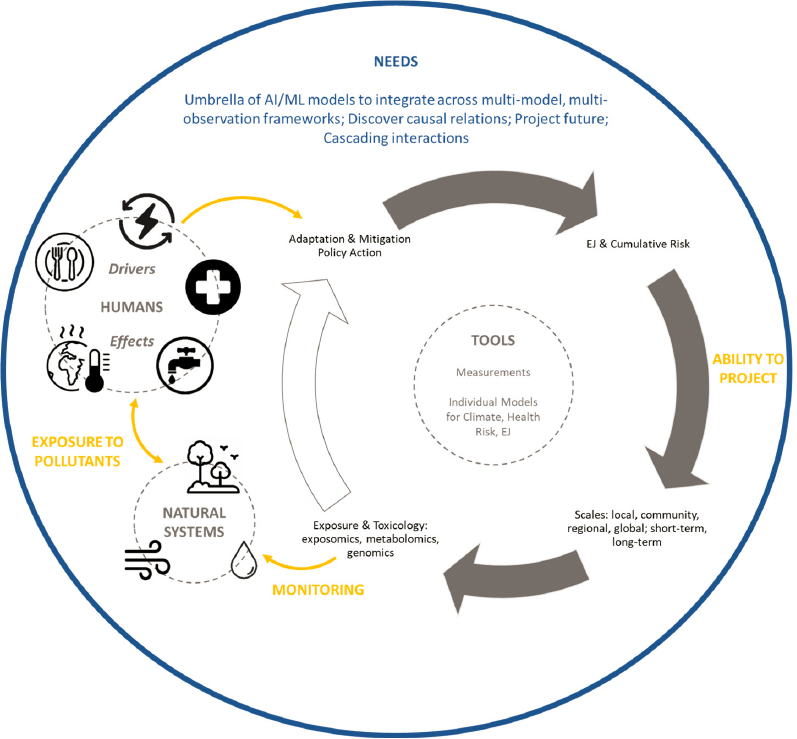
Even the most advanced integrated assessment models do not capture the complex nonlinear interactions across human and natural systems and across different spatial and temporal scales. Advances in artificial intelligence and machine learning can lead to the development of universal integrating tools for assessing multiple and unknown interactions across complex systems, with increased predictability and computational efficiency. Figure F-1 presents a schematic illustrating a grand vision for developing an umbrella of machine learning approaches for integrating across multi-dimensional, multi-observational, and multi-process modeling frameworks to estimate how adaptation and mitigation approaches affect coupled human–natural systems across local, community, regional, and global scales. Different measurements and process-based modeling approaches analyze one or more components of coupled systems spanning human health, ecosystems, food, energy, and water, among other. Exposure and toxicology studies including exposomics, genomics, and metabolomics provide a wealth of information about how human decisions
and climate change might affect human health and ecosystems. Air and water quality monitoring guided by these toxicology studies can detect the consequences of policy actions on the built and natural environments. Current predictive tools, such as climate, health, and economic models, could help in assessing the effects of these human actions (Xing et al., 2022).
Model Maturity and Limitations
Despite the tremendous progress in developing computational models, there is still a need for traditional experiments both to assess the validity of individual models and to allow modifications in an iterative fashion whereby the modeling informs the types of experiments that need to be done, and the experimental data refine the model. For example, geographical information system (GIS)-based models have been used to comprehensively map the rivers and streams of the United States and to provide a range of flow rates from drought to flood conditions. Watersheds receive inputs from multiple sources, including point sources, such as wastewater treatment plants and industrial operations, as well as nonpoint sources, such as agriculture and hard-surface runoff. Modeling environmental concentrations based on incomplete monitoring data is aided by GIS-based models that have comprehensive information about river flows at high and low seasonal volumes. These models can be used as the basis for estimating the concentrations of environmental chemicals released from wastewater treatment plants, with the models being validated by environmental sampling and analytical chemistry (McDonough et al., 2016). Kapo et al. (2008) compared various modeling approaches for estimating environmental concentrations, all of which are useful when combined with expert judgment. These global models have been used to estimate environmental concentrations of
consumer product chemicals (e.g., surfactants, perfumes), which were then verified by empirical measurement (McDonough et al., 2016, 2017).
REFERENCES
Ankley, G. T., R. S. Bennett, R. J. Erickson, D. J. Hoff, M.W. Hornung, R. D. Johnson, D. R. Mount, J. W. Nichols, C. L. Russom, P. K. Schmieder, J. A. Serrrano, J. E. Tietge, and D. L. Villeneuve. 2010. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry 29(3):730-741. https://doi.org/10.1002/etc.34.
Bal-Price, A., and M. E. Meek. 2017. Adverse outcome pathways: Application to enhance mechanistic understanding of neurotoxicity. Pharmacology & Therapeutics 179:84–95. https://doi.org/10.1016/j.pharmthera.2017.05.006.
Bal-Price, A., P.J. Lein, K.P. Keil, S. Sethi, T. Shafer, M. Barenys, E. Fritsche, M. Sachana, M.E. Meek. 2017. Developing and applying the adverse outcome pathway concept for understanding and predicting neurotoxicity. Neurotoxicology 59:240–255. https://doi.org/10.1016/j.neuro.2016.05.010.
Benbough, J. E., and A. M. Hood. 1971. Viricidal activity of open air. Journal of Hygiene 69(4):619-626. https://doi.org/10.1017/S0022172400021896.
Benigni, R., C. L. Battistelli, C. Bossa, A. Giuliani, and O. Tcheremenskaia. 2017. Endocrine disruptors: Data-based survey of in vivo tests, predictive models and the adverse outcome pathway. Regulatory Toxicology and Pharmacology 86:18-24. https://doi.org/10.1016/j.yrtph.2017.02.013.
Browne, P., P. D. Noyes, W. M. Casey, and D. J. Dix. 2017. Application of adverse outcome pathways to U.S. EPA’s endocrine disruptor screening program. Environmental Health Perspectives 125(9):096001. https://doi.org/10.1289/EHP1304.
Ciallella, H. L., and H. Zhu. 2019. Advancing computational toxicology in the big data era by artificial intelligence: Data-driven and mechanism-driven modeling for chemical toxicity. Chemical Research in Toxicology 32(4):536-547. https://doi.org/10.1021/acs.chemrestox.8b00393.
Ciallella, H. L., D. P. Russo, L. M. Aleksunes, F. A. Grimm, and H. Zhu. 2021. Revealing adverse outcome pathways from public high-throughput screening data to evaluate new toxicants by a knowledge-based deep neural network approach. Environmental Science & Technology 55(15):10875-10887. https://doi.org/10.1021/acs.est.1c02656.
Clippinger, A. J., D. Allen, H. Behrsing, K. A. BéruBé, M. B. Bolger, W. Casey, M. DeLorme, M. Gaça, S. C. Gehen, K. Glover, P. Hayden, P. Hinderliter, J. A. Hotchkiss, A. Iskandar, B. Keyser, K. Luettich, L. Ma-Hock, A. G. Maione, P. Makena, J. Melbourne, L. Milchak, S. P. Ng, A. Paini, K. Page, G. Patlewicz, P. Prieto, H. Raabe, E. N. Reinke, C. Roper, J. Rose, M. Sharma, W. Spoo, P. S. Thorne, D. M. Wilson, and A. M. Jarabek. 2018. Pathway-based predictive approaches for non-animal assessment of acute inhalation toxicity. Toxicology in Vitro 52:131-145. https://doi.org/10.1016/j.tiv.2018.06.009.
de Mik, G., and I. de Groot. 1977. The germicidal effect of the open air in different parts of The Netherlands. Journal of Hygiene 78(2):175-187. http://www.jstor.org/stable/3861871.
Dearden, J. C., M. T. D. Cronin, and K. L. E. Kaiser. 2009. How not to develop a quantitative structure–activity or structure–property relationship (QSAR/QSPR). SAR and QSAR in Environmental Research 20(3-4):241-266. https://doi.org/10.1080/10629360902949567.
Dix, D. J., K. A. Houck, M. T. Martin, A. M. Richard, R. W. Setzer, and R. J. Kavlock. 2007. The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicological Sciences 95(1):5-12. https://doi.org/10.1093/toxsci/kfl103.
Hood, A. M. 2009. The effect of open-air factors on the virulence and viability of airborne Francisella tularensis. Epidemiology and Infection 137(6):753-761. https://doi.org/10.1017/S0950268809002076.
Hsu, C.-W., R. Huang, M. S. Attene-Ramos, C. P. Austin, A. Simeonov, and M. Xia. 2017. Advances in high-throughput screening technology for toxicology. International Journal of Risk Assessment and Management 20(1-3):109-135. https://www.inderscienceonline.com/doi/abs/10.1504/IJRAM.2017.082562.
Judson, R. S., K. A. Houck, R. J. Kavlock, T. B. Knudsen, M. T. Martin, H. M. Mortensen, D. M. Reif, D. M. Rotroff, I. Shah, A. M. Richard, and D. J. Dix. 2010. In vitro screening of environmental chemicals for targeted pesting prioritization: The ToxCast project. Environmental Health Perspectives 118(4):485-492. https://doi.org/10.1289/ehp.0901392.
Judson, R. S., F. M. Magpantay, V. Chickarmane, C. Haskell, N. Tania, J. Taylor, M. Xia, R. Huang, D. M. Rotroff, D. K. Filer, K. A. Houck, M. T. Martin, N. Sipes, A. M. Richard, K. Mansouri, R. W. Setzer, T. B. Knudsen, K. M. Crofton, and R. S. Thomas. 2015. Integrated model of chemical perturbations of a biological pathway using 18 in vitro high-throughout screening assays for the estrogen receptor. Toxicological Science 148(1):137-154. https://doi.org/10.1093/toxsci/kfv168.
Kapo, K. E., G. A. Burton, D. de Zwart, L. Posthuma, and S. D. Dyer. 2008. Quantitative lines of evidence for screening-level diagnostic assessment of regional fish community impacts: A comparison of spatial database evaluation methods. Environmental Science & Technology 42:9412-9418.
Kavlock, R., K. Chandler, K. Houck, S. Hunter, R. Judson, N. Kleinstreuer, T. Knudsen, M. Martin, S. Padilla, D. Reif, A. Richard, D. Rotroff, N. Sipes, and D. Dix. 2012. Update on EPA’s ToxCast program: Providing high throughput decision support tools for chemical risk management. Chemical Research in Toxicology 25(7):1287-1302. https://doi.org/10.1021/tx3000939.
Kim, M. T., R. Huang, A. Sedykh, W. Wang, M. Xia, H. Zhu. 2016. Mechanism profiling of hepatotoxicity caused by oxidative stress using antioxidant response element reporter gene assay models and big data. Environmental Health Perspectives. 124(5):634–641. https://doi.org/10.1289/ehp.1509763.
Luechtefeld, T., C. Rowlands, and T. Hartung. 2018. Big-data and machine learning to revamp computational toxicology and its use in risk assessment. Toxicology Research 7(5):732-744. https://doi.org/10.1039/c8tx00051d.
Maggiora, G. M. 2006. On outliers and activity cliffs—Why QSAR often disappoints. Journal of Chemical Information and Modeling 46(4):1535. https://doi.org/10.1021/ci060117s.
Margolis, R., L. Derr, M. Dunn, M. Huerta, J. Larkin, J. Sheehan, M. Guyer, and E. D. Green. 2014. The National Institutes of Health’s Big Data to Knowledge (BD2K) initiative: Capitalizing on biomedical big data. Journal of the American Medical Informatics Association 21(6):957-958. https://doi.org/10.1136/amiajnl-2014-002974.
Maxwell, G., C. MacKay, R. Cubberley, M. Davies, N. Gellatly, S. Glavin, T. Gouin, S. Jacquoilleot, C. Moore, R. Pendlington, R. O. Saib, D. Sheffield, R. Stark, and V. Summerfield. 2014. Applying the skin sensitisation adverse outcome pathway (AOP) to quantitative risk assessment. Toxicology in Vitro 28(1):8-12. https://doi.org/10.1016/j.tiv.2013.10.013.
May, K. R., H. A. Druett, and L.P. Packman. 1969. Toxicity of open air to a variety of microorganisms. Nature 221:1146-1147. https://doi.org/10.1038/2211146a0.
McDonough, K., K. Casteel, N. Itrich, J. Menzies, S. Belanger, K. Wehmeyer, and T. Federle. 2016. Evaluation of anionic surfactant concentrations in US effluents and probabilistic determination of their combined ecological risk in mixing zones. Science of the Total Environment 572:434-441. https://doi.org/10.1016/j.scitotenv.2016.08.084.
McDonough, K., K. Casteel, A. Zoller, K. Wehmeyer, E. Hulzebos, J.-P. Rila, D. Salvito, and T. Federle. 2017. Probabilistic determination of the ecological risk from OTNE in aquatic and terrestrial compartments based on US-wide monitoring data. Chemosphere 167:255-261. https://doi.org/10.1016/j.chemosphere.2016.10.006.
Meigs, L., L. Smirnova, C. Rovida, M. Leist, and T. Hartung. 2018. Animal testing and its alternatives—The most important omics is economics. ALTEX: Alternatives to Animal Experimentation 35(3): 275-305. https://doi.org/10.14573/altex.1807041.
Nazaroff, W. W. 2016. Indoor bioaerosol dynamics. Indoor Air 26(1):61-78. https://doi.org/10.1111/ina.12174.
Nazaroff, W. W., and C. J. Weschler. 2004. Cleaning products and air fresheners: Exposure to primary and secondary air pollutants. Atmospheric Environment 38(18):2841-2865. https://doi.org/10.1016/j.atmosenv.2004.02.040.
OECD (Organisation for Economic Co-operation and Development). 2018. Guidance Document on Good In Vitro Method Practices (GIVIMP). OECD Series on Testing and Assessment No. 286. Paris: OECD Publishing. https://doi.org/10.1787/9789264304796-en.
Patlewicz, G., C. Kuseva, A. Kesova, I. Popova, T. Zhechev, T. Pavlov, D. W. Roberts, and O. Mekenyan. 2014. Towards AOP application—Implementation of an integrated approach to testing and assessment (IATA) into a pipeline tool for skin sensitization. Regulatory Toxicology and Pharmacology 69(3):529-545. https://doi.org/10.1016/j.yrtph.2014.06.001.
Patlewicz, G., T. W. Simon, J. C. Rowlands, R. A. Budinsky, and R. A Becker. 2015. Proposing a scientific confidence framework to help support the application of adverse outcome pathways for regulatory purposes. Regulatory Toxicology and Pharmacology 71(3):463-477. https://doi.org/10.1016/j.yrtph.2015.02.011.
Proctor, D. M., M. Suh, G. Chappell, S. J. Borghoff, C. M. Thompson, K. Wiench, L. Finch, and R. Ellis-Hutchings. 2018. An adverse outcome pathway (AOP) for forestomach tumors induced by non-genotoxic initiating events. Regulatory Toxicology and Pharmacology 96:30-40. https://doi.org/10.1016/j.yrtph.2018.04.016.
Sachana, M., A. Rolaki, and A. Bal-Price. 2018. Development of the adverse outcome pathway (AOP): Chronic binding of antagonist to N-methyl-D-aspartate receptors (NMDARs) during brain development induces impairment of learning and memory abilities of children. Toxicology and Applied Pharmacology 354:153-175. https://doi.org/10.1016/j.taap.2018.02.024.
Shukla, S. J., R. Huang, C. P. Austin, and M. Xia. 2010. The future of toxicity testing: A focus on in vitro methods using a quantitative high-throughput screening platform. Drug Discovery 15(23-24):997-1007. https://doi.org/10.1016/j.drudis.2010.07.007.
Singer, B. C., B. K. Coleman, H. Destaillats, A. T. Hodgson, M. M. Lundin, C. J. Weschler, and W. W. Nazaroff. 2006. Indoor secondary pollutants from cleaning product and air freshener use in the presence of ozone. Atmospheric Environment 40(35):6696-6710. https://doi.org/10.1016/j.atmosenv.2006.06.005.
Stouch, T. R., J. R. Kenyon, S. R. Johnson, X. Q. Chen, A. Doweyko, and Y. Li. 2003. In silico ADME/Tox: Why models fail. Journal of Computer-Aided Molecular Design 17(2-4):83-92. https://doi.org/10.1023/a:1025358319677.
Tetko, I. V., D. J. Livingstone, and A. I. Luik. 1995. Neural network studies, 1: Comparison of overfitting and overtraining. Journal of Chemical Information and Computer Science 35(5):826-833. https://doi.org/10.1021/ci00027a006.
Tetko, I. V., I. Sushko, A. K. Pandey, H. Zhu, A. Tropsha, E. Papa, T. Öberg, R. Todeschini, D. Fourches, and A. Varnek, 2008. A critical assessment of QSAR models of environmental toxicity against Tetrahymena pyriformis: Focusing on applicability domain and overfitting by variable selection. Journal of Chemical Information and Modeling 48(9):1733-1746. https://doi.org/10.1021/ci800151m.
Thomas, R. S., R. S. Paules, A. Simeonov, S. C. Fitzpatrick, K. M. Crofton, W. M. Casey, and D. L. Mendrick. 2018. The US federal Tox21 program: A strategic and operational plan for continued leadership. ALTEX: Alternatives to Animal Experimentation 35(2):163-168. https://doi.org/10.14573/altex.1803011.
Tuomi, T., B. Engström, R. Niemelä, J. Svinhufvud, and K. Reijula. 2000. Emission of ozone and organic volatiles from a selection of laser printers and photocopiers. Applied Occupational and Environmental Hygiene 15(8):629-634. https://doi.org/10.1080/10473220050075635.
Vinken, M., B. Landesmann, M. Goumenou, S. Vinken, I. Shah, H. Jaeschke, C. Willett, M. Whelan, and V. Rogiers. 2013. Development of an adverse outcome pathway from drug-mediated bile salt export pump inhibition to cholestatic liver injury. Toxicological Sciences 136(1):97-106. https://doi.org/10.1093/toxsci/kft177.
WHO (World Health Organization). 2010. WHO Guidelines for Indoor Air Quality: Selected Pollutants. https://www.ncbi.nlm.nih.gov/books/NBK138705.
Xing, J., S. Zheng, S. Li, L. Huang, X. Wang, J. T. Kelly, S. Wang, C. Liu, C. Jang, Y. Zhu, J. Zhang, J. Bian, T.-Y. Liu, and J. Hao. 2022. Mimicking atmospheric photochemical modeling with a deep neural network. Atmospheric Research 265:105919. https://doi.org/10.1016/j.atmosres.2021.105919.
Zhu, H. 2020. Big data and artificial intelligence modeling for drug discovery. Annual Review of Pharmacology and Toxicology 60(1):573-589. https://doi.org/10.1146/annurev-pharmtox-010919-023324.
Zhu, H., J. Zhang, M. T. Kim, A. Boison, A. Sedykh, and K. Moran. 2014. Big data in chemical toxicity research: The use of high-throughput screening assays to identify potential toxicants. Chemical Research in Toxicology 27(10):1643-1651. https://doi.org/10.1021/tx500145h.







