3
Subsurface Environment
The subsurface environment in which steel is buried or placed impacts the rate and extent of corrosion. The subsurface is often a heterogeneous mix of solids, liquids, and gases, with variations in soil and rock composition, moisture content, groundwater flow, and the microbial activities that lead to the chemical, electrochemical, or biological process that affects corrosivity. Variations in those properties occur temporally and spatially. Several parameters have been identified that influence the corrosivity of a subsurface material, including its ability to conduct electricity (i.e., electrical conductivity), the degree to which the soil is saturated with water, pH, dissolved salts, oxidation-reduction potential (also called redox potential for reduction-oxidation)—a measure of the oxidizing power of the environment (described in Table 6.2)—and total acidity (Elias, 1990). Sagues et al. (2009) expanded this list to include temperature, oxygen concentration, scaling tendency (i.e., the deposition of a tightly adhered protective film of mineral solids), soil particle size distribution, porosity, and microbial activity. Although complete subsurface site characterization may not be possible for large infrastructure projects (see Chapter 5 for description of general methods of characterization), understanding subsurface variability has direct bearing on the ability to accurately model corrosion of buried steel. This chapter introduces both engineered and natural subsurface environments and describes how those environments may affect corrosion of buried steel.
SOIL
Soils, which are known among geoscientists as unconsolidated sediments, are surface and subsurface materials composed of three phases: inorganic mineral grains, void space occupied by gas (most commonly air), and void space occupied by liquids (most commonly water).1Box 3.1 outlines the processes by which the inorganic constituents of soil are formed. In addition to inorganic constituents, many soils contain some organic matter from decayed vegetation and active microbial communities. While some soils may be native, undisturbed in situ material, some infrastructures or environments will require excavated material used as fill (see Box 3.2 describing different types of soil). The processes of excavating, stockpiling, and filling expose soils to oxygen and can change the water content by wetting or drying. Microbial communities within the soil can be impacted by these changes,
___________________
1 The definition of “soil” differs among different technical communities. Geotechnical engineers, for example, might think of soils as consolidated or unconsolidated based on their loading histories. Soil scientists might define soils as the near-surface mineral or organic layer that has undergone some type of weathering (e.g., chemical, biological, or physical). Other experts might consider soil to be that unconsolidated material on the surface that serves as a growth medium for plants. In this report, soils are unconsolidated materials at any depth.
as can the soil geochemistry. Compaction needs to be carefully controlled to ensure uniform density of the fill as it is being placed; intimate contact at the soil–steel interface is important because pore space at the interface can increase corrosion of the steel when those pores are subsequently filled with water (Melchers and Petersen, 2018). In buried-steel applications, imported fills are chosen primarily for their engineering properties including strength and stiffness, as well as for their corrosion-resistance properties, including low electrical activity, rapid drainage, and low geochemical reactivity. While use of fine-grained soils as fill may be unavoidable in some cases, most coarse-grained soils, including gravels and sands, provide better corrosion resistance than fine-grained soils.
The abundance of groundwater is an important defining characteristic of a soil, whether an undisturbed soil or an engineered fill. A soil is considered saturated in groundwater when there is negligible air in the void space. Below the water table, all soil voids are filled with water, and hydrostatic pressure is positive. However, full or partial saturation can still occur above the water table due to capillary rise in fine-grained soils. This rise can be on
the order of meters in a clay soil. Saturation contributes to two opposing factors that affect corrosion differently. As the degree of saturation increases, the electrical conductivity increases, which tends to promote corrosion; but at the same time, lower dissolved oxygen concentrations associated with saturated conditions can inhibit corrosion by lowering the rate of the oxygen reduction (cathodic) half-cell reaction, which was introduced in Chapter 2 (see Equation 2.2). Generally, the maximum corrosion rates occur at 60–85 percent saturation (Elias, 1990). In addition to the degree of saturation, groundwater can also affect the chemistry of the soil. Dissolved constituents from the dissolution of inorganic salts, weathering of rock or soil, and the intrusion of contaminants such as road deicing salts, fertilizers, or acid mine-drainage can move into the subsurface through groundwater transport.
Soil particle or grain size is another important defining characteristic of soil. This report uses the Unified Soil Classification System (ASTM D2487-17e1, 2020) (see Table 3.1), which classifies soil according to grain size, grain size distribution, and plasticity (the nonrecoverable deformation of the soil with no cracking) (ASTM D2487-17e1, 2020). Gravels and sands are considered coarse-grained soils that display engineering behaviors governed by gravitational forces. Silts and clays are considered fine-grained soils that display engineering behaviors governed by both gravitational and electrostatic forces. Note that these definitions differ from classification schemes used in geology and agriculture. Grain size has important impacts on parameters such as electrical conductivity, because the dominant soil mineralogy changes as grain sizes decrease. The electrical conductivity of coarse-grained soils (gravels and sands) is generally low, resulting in limited corrosion; however, fine-grained soils, especially high-plasticity clays (see Table 3.1) with high cation exchange capacity (CEC), have relatively low electrical resistivity and contribute to corrosion of buried steel. The CEC of a soil is a measure of its ability to exchange or sorb positively charged ions; as CEC increases, resistivity decreases. Additionally, the organic matter present in a soil has the ability to form complexes with dissolved ions, which can increase conductivity by increasing ion solubility, binding corrosive ions, or decreasing the activity of dissolved ions through hydration. The drainage ability of a soil is also dependent on grain size and contributes to the corrosion of buried steel through the retention of water at the soil–steel interface. Coarse-grained soils can be free draining, have high hydraulic conductivity (10−3–10−2 cm/s), and may not easily retain water at the steel surface. In contrast, fine-grained soils with low hydraulic conductivity (10−7–10−5 cm/s) retain water at the steel–soil interface. Drainage in soils with a range of grain sizes (e.g., silty sand or clayey sands) is dominated by the smaller grain fraction that fills the space between larger grains and intermediate water retention. Additionally, the smaller particles may produce small-diameter pore networks, which tend to retain moisture through capillary action.
Two additional parameters that contribute to the advancement of corrosion are the oxidative power of the environment and pH. The corrosion cell reactions introduced in Chapter 2 include at least one oxidation reaction (metal dissolution) and one reduction reaction (usually the oxygen reduction reaction or hydrogen evolution reaction) in electrolytes such as wet soil. Other reduction reactions are also possible depending on the presence and concentration of oxidizing agents in the environment, including nitrate (NO3–), manganate (MnO4−2), ferric (Fe+3), and sulfate (SO2−4) ions, as well as dissolved carbon dioxide gas (CO2) (Borch et al., 2010). The reduction reactions do not consume metal like the oxidation reactions do, but they are critical in determining the rate
TABLE 3.1 Unified Soil Classifications
| Unified Soil Classification | Grain Size (mm) | Secondary Descriptors |
|---|---|---|
| Gravel size | >4.75 | Well graded, poorly graded, with silt, or with claya |
| Sand size | 0.075–4.75 | Well graded, poorly graded, with silt, or with clay |
| Silt size | 0.002–0.075 | High plasticity or low plasticityb |
| Clay size | <0.002 | High plasticity or low plasticity |
a A well-graded soil has a wide range of particle sizes, with no concentration of particles in a single size and no gaps in the grain size distribution. A poorly graded soil consists of particles that are predominantly one size, as defined in ASTM D422-63 (2016). Note: In the geosciences, a well-graded soil is defined as “poorly sorted” and a poorly graded soil is defined as “well sorted.”
b Plasticity is the ability of a soil to deform without cracking, and is quantified as the range of water content between the soil’s liquid limit (water content at which the soil flows like a liquid) and plastic limit (water content at which the soil exhibits cracking under applied stress) as defined in ASTM D4318-17e1 (2018)
SOURCE: Adapted from ASTM D2487-17e1 (2020).
of metal corrosion, which is often limited by the rate of transport of the cathodic reactant or electron acceptor to the steel surface. In many environments, dissolved oxygen is the energetically preferred electron acceptor. This is often the case in high hydraulic conductivity environments, where dissolved oxygen can easily be replenished via groundwater transport. In other environments (e.g., low hydraulic conductivity environments), oxygen cannot easily be replenished. Unless some of the other electron acceptors mentioned above are present, these environments will have limited oxidizing power (i.e., redox potential). The rate, or kinetics, of an electrochemical reaction often depends exponentially on the electrochemical potential, which is influenced by the oxidizing power of the environment, whereas the thermodynamic tendency for the reaction to proceed depends on both potential and concentration of the reactants and products.
Because many of the important oxidation and reduction reactions involve hydrogen (H+) or hydroxyl (OH−) ions, pH is important. The effects of potential and pH are captured by Pourbaix diagrams, which are plots in potential (E)-pH space of thermodynamic equilibrium conditions for reactions in a corroding metal system (Pourbaix, 1974). Figure 3.1 is a Pourbaix diagram for iron (Grundl et al., 2011). The text shows the E-pH field of predominance for each species. The lines represent the conditions for equilibrium between the two phases that the line separates. For example, the horizontal line at the bottom of the plot is for the equilibrium between solid metallic
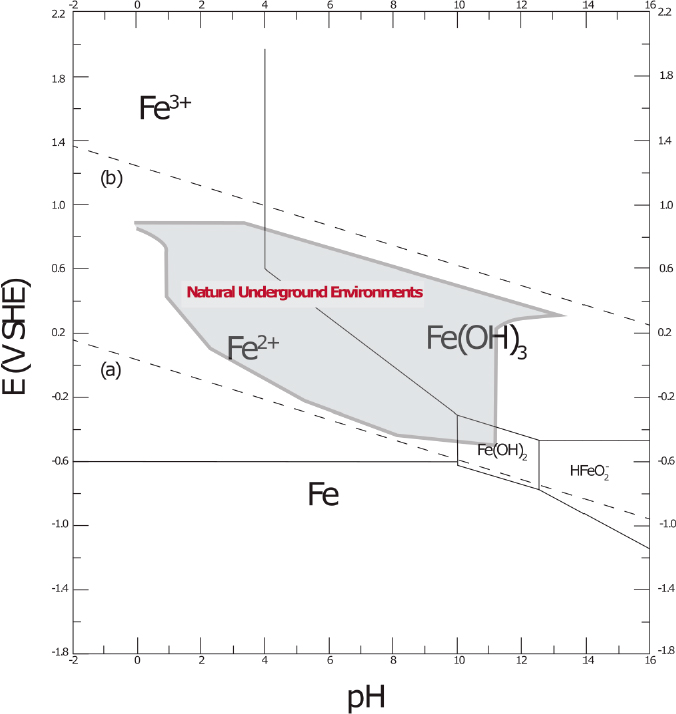
SOURCE: Modified from Grundl et al. (2011).
iron (Fe) and ferrous (Fe2+) ions. Thermodynamic considerations indicate that metallic iron will either corrode to form Fe2+ or react to form an oxide at regions of potential and pH above these lines. The gray area surrounded by the bold line represents the E-pH range of natural underground environments. This region is located above the region where metallic iron is stable, and so iron and steel will be oxidized in these environments.
Corrosion will be slow at relatively high pH values where a solid oxide such as iron(II) hydroxide (Fe(OH)2) or iron(III) hydroxide (Fe(OH)3) is stable, because the spontaneous formation of the oxides can provide protection (this probability of oxide formation is called “scaling tendency”). However, steel will corrode extensively in neutral and acidic environments (e.g., pH <4.5; Roberge, 2000; Shreir et al., 1994), which is common in subsurface conditions. While the pH of water in equilibrium with the atmosphere is approximately 5.7 because of the effects of carbon dioxide in air, the pH of the subsurface is often controlled by the minerals in the surrounding soil and rock (e.g., pH of clay and iron-bearing soils tends to be less than 7; pH of carbonate-containing soils tends to be greater than 7). Additionally, respiration, especially aerobic respiration, will add carbon dioxide. Natural organic matter, which consists of highly complex chemical compounds of carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur resulting from the degradation of organic materials (e.g., vegetation, microbes), may release H+ ions that decrease the pH.
ROCK ENVIRONMENTS
Rocks are solid masses or cemented aggregates of minerals and glassy noncrystalline materials (e.g., volcanic glasses). They are broadly classified as igneous (forming through cooling and solidifying of magma), metamorphic (alterations of preexisting rock through changes in temperature and pressure), or sedimentary (cementation of rock and mineral fragments after deposition). Table 3.2 lists some commonly encountered rock types. Grain size in rocks refers to the size of the individual mineral grains or crystals that make up the rock mass, with sizes that vary from microscopic to centimeter or larger in scale. Rocks may also include void space at as much as 40 percent of total volume, which is a function of the formation environment. Voids in rocks can be occluded and inaccessible to fluid flow, or open to flow and permeation by gases and liquids. Joints, faults, bedding planes, and other discontinuities in a rock mass typically impact the engineering behavior of the rock mass and control the transport of fluids (water, gases, and other liquids) through the rock mass, creating zones of differential moisture and chemical and oxygen contents and affecting corrosivity within those zones. Rock and steel are seldom in direct permanent contact, but the tip of a steel foundation pile, for example, may be driven through soil to be end-bearing on rock. In all other instances, except some temporary supports, steel buried within a rock mass is installed in an oversized hole that is then backfilled with cementitious grout or concrete, a chemical resin, or soil.
ENGINEERED FILLS
It is often the case that native soils or rock are unable to provide the conditions necessary to support the performance of infrastructure. In such cases, engineered fills may be used. Engineered fills are soils or granular materials that are treated or created with specified particle size, chemistry, moisture content, or plasticity. They are often mechanically placed in layers and compacted to specified density to achieve performance goals specified by an engineer. They can be designed or selected to be less corrosive than the native subsurface. However, corrosion can still occur in engineered fill due to its inherent properties or a change in environment.
Engineered fills may be in contact with steel as backfill (engineered fill placed in an excavation) around pipes and storage tanks, or as part of the material penetrated by a steel pile for a foundation or to retain an excavation. A common occurrence of steel buried in engineered fill is in mechanically stabilized earth construction.
TABLE 3.2 Commonly Encountered Rock Types
| Igneous | Metamorphic | Sedimentary |
|---|---|---|
| Diabase, diorite, granite, basalt, obsidian, tuff | Gneiss, schist, slate, marble | Conglomerates, sandstones, shales, limestone |
GROUT, CONCRETE, AND FLOWABLE FILL
The subsurface environment around buried steel may include grout, concrete, or flowable fill. Grouts solidify after application but upon application can be categorized as suspensions, solid particles suspended in a liquid phase (e.g., cementitious grouts); emulsions, liquid phase suspended in a liquid phase (e.g., bitumen in water); foams (e.g., gas bubbles in a liquid); and solutions, molecular mixtures of two substances (e.g., chemical grouts). These are discussed the next section. The ability of grout to flow is determined by its viscosity, which is controlled by variables such as ratio of water to cement, the addition of superplasticizers, and additives that affect viscosity and slow the cure time.
Cementitious grout is a grout with various proportions of cement and water. It may include other constituents, such as clay (e.g., montmorillonite), to achieve desired properties. Cementitious grout is used to permeate soils and to fill rock fractures, and its most common use with respect to buried steel is to surround steel inserted in a drill hole (e.g., ground anchors). The grout suspension is pumped into the annular space of a drill hole, between the steel and soil or rock, where it cures to provide a bond between the steel and the earth material. Concrete is similar to cementitious grout but with sand and gravel additives of various sizes, called aggregates. Corrosion of steel bar in reinforced concrete is an important process that has received considerable study (e.g., Broomfield, 2003), but it is not considered in this report. Similar to concrete, flowable fills also use cement and aggregates but in different proportions such that they are less viscous and often more permeable. The cured strength is typically closer to that of soil than concrete.
During hardening of cement mixtures, water is generally lost to the environment, leaving voids that can transmit fluids. The permeability created by these newly formed voids provides an environment in which corrosion mechanisms might be initiated. Subsequent applied loads, thermal cycles, and wetting and drying cycles will further increase permeability via microcracking. Mehta and Monteiro (2014) outline several subsurface processes and reactions that are particularly damaging to cement mixtures and thus affect permeability. These are often the result of or triggered by the presence of water. For example, gels form when acidic siliceous soils react with alkaline concrete pore fluids. The gels expand in the presence of water to cause cracking (alkali-silica reactivity). Water in pore spaces may simply expand when frozen and contract when thawed (freeze-thaw cycles), causing cracking. Cracking in cements may also be caused by a series of chemical reactions that occur between cement paste and sulfate ions (sulfate attack).
Noncementitious grouts may be made of silicates, acrylics, and polyurethanes. Epoxy resin grouts are often used to grout steel into drill holes. The epoxy resin is similar to that used explicitly for corrosion protection on steel (see Chapter 5), so the steel is well protected where coverage is complete. However, since mixing of the resin and its distribution throughout a drill hole occurs in the field, the coverage is often imperfect and corrosion can occur at gaps in coverage.






