5
Corrosion Protection for Buried Steel
Corrosion of buried steel can be managed through one or some combination of the following four approaches: (1) the service life of the steel can be increased with additional steel incorporated into the steel cross section; (2) the steel can be protected by physical barriers (e.g., coatings and casings); (3) cathodic protection (CP) can be used to modify the electrochemical cell; or (4) the environment itself—the buried soil and surrounding ground—can be modified to reduce the risk of corrosion. As discussed in Chapter 2, the “corrosion allowance” approach employed largely by the geo-civil industries incorporates structural redundancy into design so that if one steel infrastructure component fails through corrosion, the load will be transferred to other infrastructure components to ensure continued performance. Most geo-civil structures have a design life of less than 100 years, so a slow rate of corrosion is tolerated. Although geo-civil design can occasionally include CP (e.g., on bridges), the geo-civil design approach more commonly relies on protective physical barriers and controlled environments in conjunction with increased cross-sectional thickness to maintain a slow corrosion rate. In contrast, oil and gas pipeline industries generally favor a “corrosion protection” approach, which employs protective physical barriers as well as CP to avoid corrosion. Water utilities generally focus little on external corrosion of water pipelines, tolerating leaks and addressing breakages as they happen. Instead, the utilities focus on internal corrosion, water safety, and compliance with the Safe Drinking Water Act (42 U.S.C. § 300f). Given the large spatial distribution of pipe networks, controlling the environment is not economical. This chapter discusses the various means of protecting steel from corrosion and the ways in which each of these systems can fail.
PHYSICAL BARRIERS
Physical barriers such as coatings and casings are a first defense against corrosion. Much steel infrastructure that will be exposed to the subsurface includes a physical barrier. Coatings can range from cement to metal to polymer (see Table 5.1) and may be applied during installation or during repair. The most common physical barriers employed to protect geo-civil buried steel infrastructure are polymeric coatings (i.e., paint or epoxy). Multilayer coatings are often used, which include a primer with good adhesion properties and a topcoat with good corrosion protection properties. Coating systems are chosen based on a variety of factors including environmental and safety regulations, cost, availability, shop or field conditions during application, and effectiveness. Major factors that affect coating performance include the type of exposure and expected service life, surface preparation, adhesion of the coating to the surface, and method of application. It is important to consider that defects can be introduced
during application, handling, and installation and that coatings may develop anomalies and mechanical damage as they age.
Oil and gas pipelines generally have considerably thick coatings and can include several types, such as coal tar enamels, fusion-bond epoxy (FBE), two-layer polyethylene (PE), and three-layer PE or polypropylene, among others. Box 5.1 provides a brief description of these different coatings and when they were introduced. Like oil and gas pipelines, water pipeline coatings can include hot-applied coal tar enamel, FBE, or PE, but also commonly include liquid epoxy systems, extruded PE, wax tape, sacrificial metallic coatings, and cement coatings. Some paint systems claim to have extended lifetimes (greater than 50 years) in many underground environments, but many have service lifetimes of only 15–20 years and are permeable to a certain extent to water, oxygen, and ions (Helsel, 2018). Coatings typically lose their efficiency even before the end of their service life.
A coating defect or anomaly poses a threat because it can result in the loss of the physical barrier and allow corrosion. It is possible for all coatings to be defective, be damaged mechanically during installation (e.g., scratched), or suffer degradation in the buried environment from continuous mechanical, chemical, electrochemical, and microbial interactions with the subsurface (Li and Castaneda, 2015, 2017; Li et al., 2016). Defects may be large, such as the pores created by lack of adhesion, or they may be small intrinsic pathways between the spaghetti-like hydrocarbon strands of the polymeric structure of the paint. These defects offer pathways for the transport of water, oxygen, and ions to the steel surface. However, corrosion will only ensue if the coating detaches locally (i.e., disbondment) from the steel. Disbondment allows the formation of several monolayers of water and is the origin of the oxygen differential within the delamination zone, which is required for the corrosion process. It can allow initiation and propagation of the corrosion process, ultimately resulting in the broad failure of the protective coating system. However, if the adhesion of the coating is strong, local disbonding will not readily occur.
Once disbondment is initiated, the coating defect can grow into a blister. This occurs by one of two mechanisms. Anodic undermining or oxide lifting results from the action of voluminous iron oxides. The volume of these corrosion products is greater than that of the metal from which they formed, and that increased volume from reaction can lift the coating, allowing the defect to propagate across the surface. The second mechanism is cathodic delamination in which the oxide corrosion product blocks oxygen, which moves the cathodic oxygen reduction reaction to the blister edge because oxygen and water can be transported through the coating. The reduction of oxygen at the blister edge results in an increase in the pH (see Equation 2.2), which destabilizes the bonds between the polymer coating and the metal surface, promoting an extension of the delaminated region. Cement and metallic coatings are not susceptible to these types of disbonding mechanisms.
Casings (made of PE, concrete, or steel and which are isolated from the steel pipe with a dielectric coating) are physical barriers that are often used on oil pipelines when increased strength or protection is required, such as under rivers, roads, or rail crossings. Casings also have their own set of risks—such as shorted casings where the carrier pipe and casing come in contact with and shield the carrier from CP—but there are installation methods to mitigate this risk, and regular maintenance, corrosion monitoring, and observation of the casings can validate their integrity (Rankin and Al Mahrous, 2005).
TABLE 5.1 Common Types of Protective Coatings
| Type of Coatinga | Example | Comment |
|---|---|---|
| Cement coatings | Grout around subsurface anchoring devices | Can lose ability to protect against corrosion after cracking. Difficult to apply at pipe joints. Susceptible to degradation in soils with high sulfate and chloride ion content. |
| Metallic coatings | Zinc galvanization and aluminum coatings | Once the surface layer is consumed, the protection no longer exists, and the underlying steel will then corrode. |
| Polymeric | Epoxy coating/paint | Somewhat permeable to water, oxygen, and ions, which often leads to coating failure. The protection decreases with time even before the end of service life. |
a In some cases, these coatings can be combined.
Since metallic coatings (e.g., zinc and aluminum) provide sacrificial CP to the substrate steel, these coatings are discussed in the section on Sacrificial (Galvanic) Cathodic Protection, below.
MICROBIALLY INFLUENCED CORROSION AND COATINGS
Microbially influenced corrosion (MIC) has been identified as a problem for legacy pipeline coatings (i.e., coatings that have been superseded but are still in service) including coal tars, asphalts, greases, tapes, polyvinyl chloride (PVC), PE, and polyester polyurethanes (Little and Wagner, 2002). Soil bacteria, archaea, and fungi can derive nutrients from the water-soluble components of some pipeline coatings. For example, biodegradation of low-molecular-weight components from asphalt coatings by microorganisms results in permeable, embrittled coatings (Little and Lee, 2018). Similarly, plasticizers (e.g., phthalates) can be selectively removed from PVC coatings through water dissolution and biodeterioration. Some polymeric coatings (e.g., polyester polyurethanes) can be directly degraded by extracellular enzymes, acids, or peroxides. Howard (2011) indicated that many soil microorganisms can use polyester polyurethanes as the sole carbon and energy source. Breaches in coatings allow ingress of water and the possibility of biotic or abiotic corrosion.
CATHODIC PROTECTION
CP is an electrochemical technique designed to provide corrosion protection by polarizing a structure in the cathodic direction (i.e., cathodic polarization) using an electrical current. Kuhn (1928) established that polarization to −0.850 V versus a copper–copper sulfate reference electrode (CSE) was required to ensure corrosion protection of a soil-buried cast iron pipe. That criterion, a protection potential, is used today and incorporated into standard practices such as NACE SP0169 (2013) and DNV (2010). The current required to maintain the protection potential (−0.850 V versus CSE) can be provided by sacrificial anodes or impressed current, as described below. The steel structure does not need to be polarized into the immune region of the Pourbaix diagram (see Figure 3.1) for CP to be effective. In fact, polarization to a potential that is too low can result in excessive hydrogen production, which can damage the steel or coatings on the steel. Effective CP need only reduce the corrosion rate of the steel to a value that is approximately 10 times less than that of unprotected steel.
Sacrificial (Galvanic) Cathodic Protection
Sacrificial CP, otherwise known as galvanic CP, uses the differences in the nobilities of two metals to form an electrochemical cell, such that when the two are connected, the less noble metal (anode) will corrode faster than if not connected and the more noble metal (cathode) will corrode slower (see Chapter 4). Buried steel can be cathodically protected with external magnesium or zinc anodes connected (or “bonded”) to the steel so that corrosion is primarily displaced from the protected structure to the galvanic anodes. The current flowing through the environment from the sacrificial anode to the structure is accompanied by anodic dissolution of the sacrificial anode material and its irreversible loss. Once the zinc or magnesium anodes are consumed, they need to be replaced for corrosion protection to continue. The service life of the anodes is considered in the design phase of steel structures, but future maintenance and replacement of anodes must continue throughout the life cycle of the steel structures. Sacrificial CP is typically used where the current required for protection is small and the contact resistance between the anodes and soil is limited (i.e., in low-resistivity soil). Remote sacrificial anodes are also used to supplement CP with impressed current in zones of insufficient protection.
Zinc coating (i.e., galvanization) can also provide a type of sacrificial CP for buried steel (see Table 5.1), where the zinc coating is providing protection for the underlying steel. As with bonded remote anodes, when the zinc coating is fully consumed, the protection against corrosion is lost. Several processes can be used to apply galvanization to steel surfaces including hot dipping (ASTM A123/A123M-17, 2017), spin dipping (ASTM A153/A153M-16a, 2016), electroplating (ASTM B633-19, 2019), thermally spraying (AWS C2.23M/C2.23:2018, 2018), or painting (ASTM D6386-16, 2016; ASTM D7396-14(2020), 2020). The coating thickness and the adherence of the galvanization to the steel vary depending on the technique used to coat the steel. In general, hot-dip galvanization results in a thicker coating and a metallic bond with the steel. Steel that includes threaded parts or other pieces that fit together is galvanized through spin dipping or electroplating. The thermally sprayed process is sometimes used for steel piles while painting is only used as a repair for nicks that may be observed prior to installation of buried galvanized steel. Galvanizing is common for less-critical pipelines (e.g., culvert piping for which the consequence of failure is not catastrophic; see Box 5.2), due to the limited amount of zinc typically applied to the steel (e.g., 3- to 5-mil zinc coating thickness). Galvanization is not used for critical pipelines such as oil, gas, or transmission water pipelines (with high consequence of failure), which rely on impressed current CP (ICCP). Installation of external anodes is more practical than galvanizing since these anodes provide a larger amount of sacrificial zinc or magnesium, can be monitored for their efficiencies, and are replaceable once consumed.
Aluminum coating on steel (referred to as aluminized steel) is commonly used for culvert pipe application. The aluminization process results in a dual coating with an inner intermetallic brittle layer approximately 15 µm thick (composed of Fe2Al5) and a nearly pure outer soft aluminum matrix layer approximately 30 µm thick (Caseres and Sagues, 2005). Aluminized pipes are commonly ribbed or corrugated for structural strength (corrugation process to occur after aluminized coating is applied to the steel). Ribbed pipes have better hydraulic efficiency and are often preferred.
While the zinc coating (on galvanized steel application) is subject to continuous corrosion to provide protection, in the case of aluminized steel, a thermodynamically stable thin passive film of aluminum oxide is expected to form rapidly and prevent further corrosion. While the presence of passive film can increase the durability of aluminized steel by several folds compared to galvanized steel (Cerlanek and Powers, 1993), it may also cause localized corrosion and premature failure in areas of coating defects and blemishes. Coating blemishes may occur due to improper storage, handling, installation, or manufacturing defects in corrugation process. Additionally, aluminized passive film is only stable in the pH range of 4 to 8.5. In the pH ranges below 4 and beyond 8.5, the passive film will dissolve and no longer provide protection, and the corrosion rate of aluminized steel will be higher than that of galvanized steel.
Impressed Current Cathodic Protection
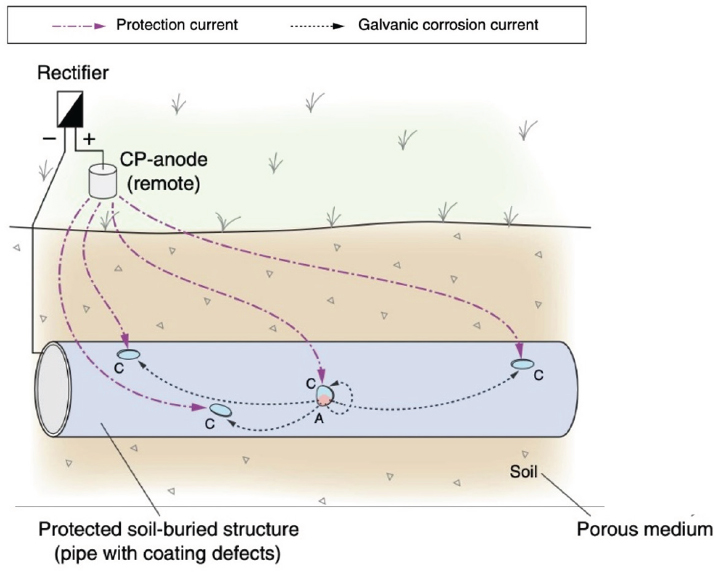
In some applications, the potential difference between sacrificial anode(s) and the steel structure cannot generate sufficient current for protection to occur. In other situations, repeated replacement of consumable anodes is not possible. To overcome the limitations inherent in the use of galvanic CP, direct current (DC) from any power source can be used for ICCP systems (Beavers, 2001). Typically, alternating current (AC) power sources are used in conjunction with rectifiers to convert AC to low-voltage DC (see Figure 5.1). The anodes for ICCP are made
from nonconsumable materials such as chromium-bearing high-silicon cast irons or conductive oxides. ICCP is used extensively in oil, gas, and large water transmission pipelines. The advantage of ICCP is that the anode does not need to be replaced, thus reducing maintenance costs. However, installation costs are higher due to the cost of required hardware and electrical work. Additionally, aboveground ICCP hardware must be safe and secure. ICCP also requires an AC power supply over the life of the CP system, which adds to maintenance costs. Finally, ICCP has a greater likelihood of creating stray-current interferences on other steel infrastructure in the vicinity.
Cathodic polarization of a buried steel surface immediately reduces the kinetically controlled iron dissolution rate and increases the cathodic reduction rate on the steel surface. Two cathodic reactions at the metal surface are responsible for a progressive change in the corrosivity of the area around a cathodically protected surface: reduction of oxygen and reduction of water (see Equations 2.2 and 2.4). Both reactions produce an accumulation of OH− at the surface, contributing to increased local pH (Leeds and Leeds, 2015). The magnitude of the pH change depends on the extent of cathodic polarization and the local environmental conditions, including soil chemistry and microstructure, hydrodynamics (stagnant or moving water), and the presence and activities of microorganisms (Angst, 2019). The alkaline zones generated by cathodic polarization can extend from the steel surface into the soil for centimeters to decimeters and can persist for hours after polarization ceases (Angst, 2019; Brenna et al., 2017). Alteration of the local chemical environment is an essential component for corrosion control of buried steel by CP because steel corrodes slower in high pH environments even in the absence of CP (Angst, 2019).
In both galvanic CP and ICCP, the anodic reaction is shifted primarily from the protected metal structure to the remote anode. Both are used in combination with coatings to reduce the spatial area requiring CP. In such applications, the role of CP is primarily the protection of exposed metal where coatings have been damaged or degraded. The need and design for CP are determined through field and laboratory tests (see Chapter 6). Certain geotechnical conditions (e.g., the presence of coarse-grained soils) limit the installation of CP due to both high resistivity and the difficulty of anode trenching.
SOURCE: Modified from Angst (2019).
Assessment of Cathodic Protection
Practical application of CP for buried steel is complex. Both types of CP require electrical and ionic connection between the anodes and all surfaces to be protected. Ionic connectivity means that ionic current can flow from the anodes through the soil electrolyte to reach the steel surface. Spatial variations in the underground environment can limit transport and cause local variations in moisture near the steel structure that disrupt the overall path of current to the anode. Environmental pH, temperature, oxygen content, ionic concentrations, biological activity, and resistivity influence the current in the electrochemical system and ionic transport at the steel–subsurface interface. Temperature can accelerate chemical and electrochemical reactions, increasing the current needed for protection. Fundamental mechanisms for CP effectiveness are somewhat controversial. For example, it is unclear whether CP results in a reduction in the number and size of corroding sites or a reduction in the corrosion rate of those sites (Angst, 2019).
Another matter of debate involves the relationship between cathodically polarized steel and soil microorganisms. For example, Miyanaga et al. (2007) suggested that bacterial cells within an artificial preexisting biofilm were killed or damaged by the pH increase during CP. However, several researchers have demonstrated that CP cannot prevent biofilm formation and may attract microorganisms (Guan et al., 2016; Jansen et al., 2017). Specific influences are difficult to tease out because there are often differences in electrolytes, microorganisms, and experimental conditions. The results of polarization experiments conducted in liquid media cannot be interpreted as significant to cathodically polarized steel in soil.
Much of the confusion related to microorganisms and the effectiveness of CP is specific for sulfate-reducing bacteria (SRB). In anaerobic environments, SRB generate hydrogen sulfide (H2S) that dissociates to H+, bisulfide (HS−), and sulfide (S2−). Most CP standards recommend protection potentials that are at least 0.100 V more negative than the normal reference condition (i.e., −0.950 VCSE) if there are insoluble ferrous sulfides (NACE SP0169, 2013; DNV, 2010). Barlo and Berry (1984) concluded that even more negative potentials were required to prevent corrosion of steel when SRB were active, but Jansen et al. (2017) demonstrated that once biofilms were established, it was difficult or impossible to prevent MIC irrespective of applied potential. Furthermore, increased pH that typically accompanies cathodic polarization can be significantly reduced by the presence of biofilms that provide a chemical pH buffering capacity. Overall, there is general agreement that more negative protection potentials are needed for CP in the presence of iron sulfides, but there are unwanted consequences of extreme cathodic polarization. Electrical fields and high pH generated by cathodic polarization can cause coating disbondment (Pope and Morris, 1995). The additional negative potential applied to protect against MIC also increases the generation of atomic hydrogen, which can enter and degrade the steel (Kim, 2002).
CONTROL OF ENVIRONMENT
Site conditions, location, climate, and properties of the fill, native soils, or rock are factors that can affect corrosion. The climate, and often the location, cannot be altered, but the site and ground conditions may be controlled. Mitigation strategies often use lime to increase the pH, compounds that inhibit corrosion, or methods to control the amounts of dissolved solids by removing soluble salts. These mitigation strategies are described in more detail in Table 5.2.
TABLE 5.2 Mitigation Strategies to Control Corrosivity of the Environment
| Method | Comment |
|---|---|
| Mix lime with fill sources prior to or during placement | Results in a desirable, homogeneous distribution of the lime or corrosion inhibitor. |
| Inject lime into fills during service | Spacing and dosage per injection site need to be properly selected such that the compounds permeate uniformly throughout the fill. |
| Install barriers that intercept and treat groundwater | Barriers may include chimney drains or blankets (Berg et al., 2009). These barriers can protect fills from contaminants (e.g., deicing salts applied to pavements, phosphates from applications of fertilizers). |
| Leach and drain soils to remove salts | Remove soluble salts by continuously spraying the surface with clean water until the leachate no longer includes impurities. This approach requires that an internal drainage system is incorporated within the fill to effectively collect and remove leachate from the system. |
| Separate soils by grain size (i.e., scalping) to remove fractions containing soluble salts | Finer fractions (i.e., those passing the #40 sieve) may be more corrosive due to lower pH and resistivity and higher sulfate concentration. |
SOURCES: Thapalia et al. (2011); Timmerman (1992).








