4
Ceramic Fiber Processing
Most CMCs are reinforced with continuous, multifilament tow ceramic fibers. Fiber tows typically consist of 500 to 1,000 filaments, with a diameter of 10 to 15 µm (0.40 to 0.59 mils) each. These fiber tows are flexible, easy to handle, and can be woven into fabrics and used to fabricate complexshaped composites. Monofilament fibers have also been used to reinforce ceramic composites, but they have several disadvantages. For example, monofilaments (especially singlecrystal fibers) are expensive, and their large diameter (> 75µm [3.0 mils]) limits their minimum bend radius, which can make fabricating complex-shaped composites difficult. This chapter, therefore, focuses on small diameter, multifilament ceramic fibers.
A wide variety of precursors and processing techniques are available for the production of both oxide and non-oxide ceramic fibers. With these techniques, microstructural development and a variety of fiber properties, such as modulus, density, dielectric constant, and tensile strength, can be controlled. In addition, the curing and pyrolysis processes can be used to control the composition of non-oxide fibers prepared by preceramic polymer routes. The key characteristic of reinforcement fibers is their ultrafine, or even nanoscale, microstructure. For example, a grain size below 0.5 µm (0.02 mils) is optimal for high strength although it has the disadvantage of increasing the rate of high-temperature creep. In the last decade, however, improvements in fiber processing have made fibers with significantly improved high-temperature creep properties possible (see Chapter 3). Fiber costs can be reduced by using improved, more efficient processing methods (see Chapter 7). The relationships between the processing, cost, and performance of ceramic fibers are discussed in this chapter.
NON-OXIDE FIBER PROCESSING
The majority of non-oxide ceramic fibers that have been studied are Si-based (tending toward SiC in composition). Their microstructures can be crystalline, amorphous, or a mixture of crystalline and amorphous material. Although other compositions have been studied, including Si3N4, B, BN, HfC, and others, efforts to develop a high-temperature fiber have focused predominantly on Si-based ceramics. Therefore, the following discussion of non-oxide fiber processing will be limited to techniques applicable to these materials, including CVD (chemical vapor deposition), chemical conversion, extrusion/sintering, and preceramic polymer processing.
The oldest commercial process for producing non-oxide fibers is CVD of a ceramic material (typically SiC) onto a heated core monofilament (e.g., carbon fiber or tungsten wire). This process is still used by Textron Company. These fibers are used primarily to reinforce metal matrix composites (MMCs) and intermetallic matrix composites (IMCs). SiC fibers prepared by CVD, however, are typically large-diameter (generally = 75 µm [0.30 mils]) monofilaments that are stiff and unsuitable for weaving or other preforming techniques. In addition, commercially available CVD SiC mono-filaments are prohibitively expensive for use in CMCs. Recent attempts to deposit SiC (via CVD) on fine-diameter, multifilament carbon fibers are described in the literature (Lackey et al., 1995; Kowbel, 1997), but high strength, multifilament fiber tows have yet to be demonstrated. A key requirement for this potentially lower-cost approach is the development of a technique for spreading the core fiber tow to achieve uniform CVD of the individual filaments without causing interfilament bonding (which would compromise the mechanical properties of the fiber tow).
Chemical conversion of carbon fiber (Cf) to SiC fiber (SiCf) is another approach to producing non-oxide ceramic fibers. Conversion typically involves reacting a carbon fiber with either silicon or silicon monoxide vapor via the following reactions:
Cf+ Si(g) ? SiCf (1)
2Cf+ SiO(g) ? SiCf+ CO(g) (2)
Attempts to produce a viable SiC fiber by Reaction 1 have not been successful because the approximately 87 percent increase in volume that occurs upon conversion disrupts the microstructure and, therefore, compromises the mechanical properties of the fiber. Reaction 2 proceeds, theoretically, with approximately 6 percent shrinkage. However, recent attempts by MER Corporation (Kowbel, 1997) to convert entire filaments via Reaction 2 have been unsuccessful. Furthermore, earlier attempts to fully convert oriented carbon fiber were also unsuccessful. The interior of these carbon fibers generally remains unconverted graphite; attempts by the New Oji Paper Company (Okada et al., 1995) using a porous, activated carbon fiber (ACF), have been successful
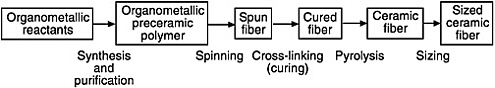
FIGURE 4-1 Typical processing scheme for producing ceramic fibers from organometallic polymer precursors. Source: Dow Corning Corporation.
in converting carbon filaments to SiC. The complete conversion to SiCf is possible because the ACF fiber has extensive surface-connected nanoporosity, which permits the diffusion of SiO vapor throughout the fiber. The ACF fiber, however, is intended for use in the purification of gases and liquids and not intended for use as a structural fiber. Because of processinduced surface flaws and extensive porosity, which remain in the converted fiber, the ACF fiber has low strength and low modulus.
A third approach to producing non-oxide fibers involves extrusion (spinning) of a ceramic powder, generally SiC, in a polymeric binder, followed by sintering. It is difficult, however, to produce fibers of less than 30 µm (1.2 mils) in diameter using this technique because the largest grain size of the powder (in the extruded fiber) must be no more than a small fraction of the fiber diameter to retain the strength and integrity of the fiber during the slurry spinning process. The tensile strength of the sintered fiber has been limited to approximately 1.4 GPa (200 ksi) because the largest grains have been critical strength-limiting flaws. Using very fine powders in extruded fiber production leads to the formation of agglomerates that also act as critical, strength-limiting flaws. The weak large-diameter fibers produced by ceramic powder extrusion have not yet been successfully woven. Two unsuccessful approaches tried by the DuPont and Carborundum companies, respectively, involved using polycarbosilane as a preceramic polymer binder (Silverman et al., 1991) or a fugitive organic binder (Frechette et al., 1994). Both efforts have been discontinued.
Preceramic Polymer Processing
Commercial and developmental fine-diameter fibers (that can be woven) have been produced by melt-spinning or solution (“dry”) spinning of organometallic polymer precursors, followed by a cross-linking (curing) step to prevent melting 1 during a subsequent pyrolysis step. This technique is known as preceramic polymer processing.
The preceramic polymer process is similar to the process used to produce carbon fiber from pitch or polyacrylonitrile (PAN) fiber. A general process scheme is shown in Figure 4-1. Several reviews of the many variations of this process are available in the open literature (Lipowitz, 1991, 1997a; Lipowitz et al., 1993; Laine et al., 1995). Although preceramic polymer processing is simple in principle, producing fibers with high tensile strength and high thermal stability requires high purity polymers, high quality spinning, and minimal impurities and mechanical damage introduced during spinning, curing, pyrolysis, and sizing (Haider and Clark, 1986; Salinger et al., 1988; Freeman et al., 1993). Multifilament spinning, generally of 200 or more filaments simultaneously, is necessary to increase throughput and reduce costs. Spinning involves two simultaneous processes: (1) extrusion of a melt or viscous solution through an orifice and (2) drawing the extruded fiber by winding at a higher velocity than the extrusion velocity.
Table 4-1 summarizes the processes used to prepare commercially available polymer-derived ceramic fibers, as well as fibers being actively developed. Although the fiber compositions vary widely, they are all prepared by a process route similar to the one shown in Figure 4-1. Advantages inherent to the polymer approach include (Lipowitz, 1997a):
-
control of fiber purity
-
control of ceramic morphology (amorphous or crystalline) and crystallite size
-
ability to produce fine diameter ( 30 µm [1.2 mils]), multifilament, continuous fibers suitable for weaving and knitting
-
ability to produce new metastable compositions unobtainable by other means
The following properties are desirable for conversion of polymers to ceramic fibers:
-
a polymer that can be pyrolyzed to the desired ceramic composition (although final composition may be modified by the cure chemistry or by using reactive gases, such as NH3 or H2 and N2 during pyrolysis)
-
a high purity polymer with minimal particulate content (particulates interfere with spinning and introduce critical flaws)
-
a highly branched polymer (which increases the ceramic char yield necessary to produce a ceramic fiber, the end product)
|
1 |
Note that melting is not required for dry-spun polymers. |
TABLE 4-1 Processes for Commercial and Developmental Polymer-Derived Ceramic Fibers
|
Company |
Fiber |
Polymer g |
Spin Method |
Cure Method |
Pyrolysis h |
Ceramic i Composition |
|
NCK a |
CG NICALON |
(Me2SiCH2)x (MeHSiCH2)y x/y=1-2M Me=CH3 polycarbosilane |
melt |
air oxidation |
N2, ~1,400°C |
Si-C-O |
|
HI-NICALON |
same |
melt |
electron beam |
N2, ~1,400°C |
SiC + C |
|
|
HI-NICALON S |
same |
melt |
electron beam |
N2 + H2, ~1,400°C |
SiC |
|
|
Ube b |
TYRANNO |
same + several % Ti |
melt |
air oxidation |
N2, ~1,400°C |
Si-C-O-Ti |
|
same + several % Ti |
melt |
electron beam |
N2, ~1,400°C |
Si-C-Ti |
||
|
TYRANNO-Z |
same + several % Zr |
melt |
air oxidation |
N2, ~1,400°C |
Si-C-O-Zr |
|
|
same + several % Zr |
melt |
electron beam |
N2, ~1,400°C |
Si-C-Zr |
||
|
MER/UM c |
— |
(MeSiH)x polymethylsilane |
solution dry |
thermal + chemical |
N2 or Ar 1,400–1,600°C |
SiC |
|
3M/UF d |
— |
higher molecular weight polycarbosilane + additives |
solution dry |
thermal + chemical |
N2 or Ar ~1,400°C |
SiC + C or SiC |
|
DCC e |
SYLRAMIC |
melt |
air oxidation |
Ar = 1,600°C |
SiC + TiB2 |
|
|
Bayer f |
— |
|
melt |
chemical |
N2/1,500°C or NH3/1,200°C |
Si-N-B-C-O or Si-N-B-O |
|
a Nippon Carbon Company, Tokyo, Japan b UBE Industries, Ltd., Tokyo, Japan c MER Corporation, Tucson, Arizona, and University of Michigan d 3M Corporation, Minneapolis, Minnesota, and University of Florida e Dow Corning Corporation, Midland, Michigan f Bayer Company, Leverkusen, Germany g Idealized, simplified polymer molecules; all am highly branched, three-dimensional structures h Pyrolysis conditions are inferred in many cases i Si is listed first, followed by other elements in order of decreasing atomic abundance |
||||||
-
a polymer displaying rheological and thermal stability in the melt (or solution) that can be readily spun into high quality fibers
-
a polymer capable of easy cross-linking to an infusible fiber after spinning
-
an air-stable and moisture-stable polymer
-
a low cost polymer
A highly-branched, low to moderate molecular weight, globular-shaped polymer molecule inherently produces a fragile, low strength preceramic polymer fiber because it is incapable of molecular orientation. This polymer can be contrasted with the commercial polymer fibers (nylons, polyesters, polyethylene, polypropylene, etc.), which are all high molecular weight, linear molecules capable of molecular orientation along the fiber axis, as well as of partial crystallization during drawing, which leads to high strength. The fragile nature of preceramic polymer fibers limits spinning and drawing speeds, imposing an economic penalty on throughput of the high capital-cost spinning fine. Limitations, such as moderate rates of cross-linking and pyrolysis, further restrict throughput rate and increase costs. Similar limitations in carbon fiber processing, however, have been overcome as the technology has matured.
Some polymer systems do not require a separate crosslinking step because the polymer undergoes thermal crosslinking in the early pyrolysis stage (Sacks et al., 1995; Zhang et al., 1994; Kowbel, 1997), which could reduce processing costs. However, these polymers are dry (solution) spun, which requires controlled evaporation and the recovery of the organic solvent, which would detract from the cost reduction achieved by eliminating the separate cross-linking step.
Microstructural Development
During the curing step, polymer fiber is converted to a highly cross-linked, infusible gel (with infinite molecular weight). Cross-linking can be accomplished by chemical, thermal, or radiation methods. Cure chemistry may modify the composition of the polymer and, thus, the ceramic product during the introduction of chemical cross-links (e.g., adding oxygen during oxidative air cure).
During the pyrolysis step (Figure 4-2), loss of volatile polymer components followed by thermal degradation and further cross-linking of the polymer occurs, ultimately producing an inorganic, ceramic composition. This process involves the simultaneous loss of a large volume of gas and a two-fold or greater shrinkage in volume, largely because of the increase in density from the polymer stage (p ~ 1g/cm3 [0.04 lb/in3]) to the ceramic stage (p = 2g/cm3 [0.07 lb/in3]). The loss of a large volume of pyrolysis gases and the simultaneous densification of the solid phase leads to nanometer-size channels for gas evolution in region B. In
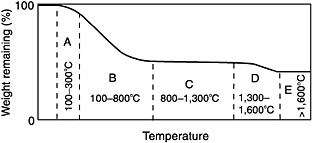
FIGURE 4-2 Schematic illustration of the pyrolysis process. Region A (~100 to 300°C [212 to 572°F]): loss of volatile oligomers. Region B (~300 to 800°C [572 to 1,472°F]): loss of pyrolysis products. Region C (~800 to 1,200°C [1,472 to 2,192°F]): continued loss of some hydrogen and methane. Region D (~1,300 to 1,600°C [2,372 to 2,912°F]): thermal degradation-loss of carbon monoxide, some silicon monoxide, and nitrogen (if present); loss of strength. Region E (~1,600 °C [2,912°F]): little or no further weight loss. Source: Lipowitz, 1991.
region C, viscous flow of the predominantly glassy ceramic product leads to collapse of the nanochannels, which closes the nanopores (Lipowitz et al., 1990; Suzuya et al., 1996). Subsequent heating above the pyrolysis temperature, even without further gas evolution or crystallization, causes fiber shrinkage as pore volume decreases (Lipowitz, 1991).
Generally, pyrolysis takes place in an inert atmosphere, such as argon or nitrogen, with a maximum temperature of 1,100 to 1,400° C (2,012 to 2,552°F). In some instances, however, reactive atmospheres are used to modify the composition of the ceramic fiber. For example, an ammonia atmosphere will remove carbon from the polymer and replace it with nitrogen, (Okamura et al., 1987) thus permitting preparation of Si-N or Si-N-O compositions from organosilicon polymers containing carbon or carbon + oxygen, respectively. A hydrogen atmosphere removes carbon from polycarbosilane without substituting another element into the ceramic product. Hydrogen atmospheres have been used to prepare a near-stoichiometric SiC fiber from polycarbosilane, which otherwise produces a carbon-rich ceramic fiber (Ichikawa et al., 1995).
The initial ceramic produced during pyrolysis (region C) tends to be primarily, if not completely, a metastable, amorphous structure. Crystallization tends to occur more readily, and at a lower temperature, as the fiber approaches the composition of a stoichiometric crystalline material (e.g., SiC, Si3N4, Si2N2O, BN, . . . ). Thus, a nonstoichiometric Si-N-B-C-O composition (Baldus, 1997; Baldus and Jansen, 1997)—which is significantly different in composition from any simple crystalline material—does not crystallize until the temperature exceeds ~1,800°C (3,272°F) in a nitrogen atmosphere. In contrast, ceramic grade Nicalon™ (an Si-C-O composition) begins to form nanocrystallites of ß-SiC at approximately 1,200 to 1,300 °C (2,192 to 2,372°F) in argon.
Si-C-O (made by Nippon Carbon Company) and Si-C-O-Ti (or Zr) compositions (made by UBE Industries) undergo
carbothermal reduction above approximately 1,300°C (1,372°F) to form large grains of crystalline SiC, eventually resulting in a significant loss of strength:
4C + 2SiO2? SiC + 3CO + SiO (3)
Both UBE Industries and Nippon Carbon Company have improved the thermal stability of recently introduced products by minimizing the oxygen content in the fiber. This was accomplished by using an electron beam rather than an oxidative air cure process (Hasegawa, 1997; Takeda, 1996). In addition, UBE Industries has recently developed a series of fibers containing several weight percent Zr instead of Ti, which UBE claims reduces salt corrosion and oxidative strength loss.
Dow Corning Corporation has introduced a polycrystalline SiC fiber (Sylramic™) that incorporates up to several weight percent boron in a Si-C-O or Si-C-O-(Ti) fiber prior to a high temperature (= 1,500°C [2,732°F]) carbothermal reduction process (i.e., reaction 3), producing a stoichiometric ß-SiC (or ß-SiC + TiB2) fiber (Lipowitz, 1997a). It is well known that boron suppresses exaggerated grain growth and aids in sintering of SiC. The incorporation of boron, which can be added to the polymer or added during the cure or pyrolysis steps, permits formation of a high strength, high modulus polycrystalline SiC fiber after sintering (or densification) at high temperature. Increasing the sintering temperature leads to increasing grain size, density, elastic modulus, strength, and creep resistance over a wide temperature range.
OXIDE FIBER PROCESSING
Commercial polycrystalline oxide fibers used to reinforce ceramic composites are produced by spinning and pyrolyzing chemically-derived precursors. These chemical processes are commonly referred to as sol-gel or metal-organic processing. Chemical processing produces fibers with high temperature properties not attainable by traditional processing methods, such as melt-spinning (e.g., spinning molten glasses). Although alternative melt-spinning approaches are being investigated, a high SiO2 content is characteristic of melt-spun fibers, which reduces the high-temperature properties of the fibers.
One novel process, which allows fiber drawing from refractory oxide melts, is called containerless processing. Amorphous mullite and YAG (yttria-alumina-garnet) fibers with strengths as high as 5 GPa (725 ksi) have been produced from levitated, undercooled melt droplets. However, because these fibers crystallize at elevated temperatures, resulting in significant strength degradation, they will not be considered further in this report. The preparation of single-crystal fibers by drawing from a melt are also excluded because the slow production rates inherent in this process will preclude commercialization of these fibers for the foreseeable future. 2
Extreme care must be taken at all stages of fiber processing to prevent the formation of strength-limiting defects and flaws, such as particulate inclusions, pores, bubbles, blisters, cracks, and surface damage. For example, current Nextel 610 fibers have a significantly higher tensile strength (3.0 GPa [435 ksi]) than other commercially available oxide fibers, and 50 percent higher than the originally introduced Nextel 610 fiber. This improvement is the result of an intensive program to eliminate flaws by improving process cleanliness during precursor preparation and by improving fiber processing techniques (Wilson, 1997).
In the last decade, many new oxide fibers with improved high-temperature performance have been commercialized. The keys to these improvements has been (1) the design of fiber microstructures to reduce the volume of amorphous phases and (2) the development of multiphase polycrystalline fibers. Eliminating amorphous phases prevents rapid, viscous deformation under load at high temperatures. Multiphase polycrystalline microstructures appear to inhibit creep, particularly at elevated temperatures. Examples of developmental fibers with improved high-temperature properties include polycrystalline Al2O3, YAG, and mullite fibers.
Chemical Processing
Chemical processing was first used to produce ceramic oxide fibers in the 1960s (Walner et.al., 1965; Lockhart and Walner, 1966). Initially, most work focused on fibers with high alumina content, although several other compositions, including those based on ZrO2 and TiO2, were also produced. A number of chemically-derived alumina-silica fibers, notably Saffil (noncontinuous 95 percent Al2O3- 5 percent SiO2 fibers) and Nextel 312 (mullite in a borosilicate glass), were commercialized in the late 1970s. However, these fibers had poor load-bearing capability above 800°C (1,472°F) and, therefore, were not optimal for reinforcing high-temperature ceramic composites. They were (and continue to be), however, used for thermal insulation, where load-bearing capability is not a major concern. The first commercial oxide fibers designed specifically to reinforce composites was DuPont's Fiber FP, a polycrystalline Al2O3 fiber with significantly better high-temperature capability than Al2O3-SiO2 fibers. However, Fiber FP, and the improved PRD-166 Al2O3- ZrO2 fiber, had poor tensile strength and handleablity, which
|
2 |
The drawing rate of single-crystal fibers from a melt is fundamentally limited by the rate of heat transfer from the growing tip of the fiber. For Al2O3, the maximum rate is ~25 mm/min. (1 in./min.) for a 5 rail fiber. At this rate, it would take ~5,000 hours to make a single pound of fiber. Drawing multiple filaments (e.g., 25) from a single melt has been demonstrated but is still too slow, even for high value applications. |
eventually prevented full commercialization. Neither fiber has been produced since 1985.
Figure 4-3 is a flow diagram showing the chemical processing of ceramic oxide fibers. Because all commercially available oxide fibers are based on alumina, these fibers are the focus of this discussion. As indicated in Figure 4-3, an alumina precursor is mixed with inorganic additives (e.g., a silica precursor) and organic additives to modify the precursor chemistry and allow the preparation of a viscous liquid suitable for fiber spinning. The fiber precursor is typically synthesized as a dilute solution so it can be filtered to remove particulate contamination. After filtration, the precursor solution is concentrated in a vacuum to remove excess solvent and form a viscous spin dope (the material from which fibers are drawn). Most oxide fibers are extruded into continuous filaments using dry-spinning; the spin dope contains a solvent, usually water, which is evaporated during spinning to produce a rigid fiber. The “green” (i.e., unfired) filaments are then pyrolyzed at temperatures of 300 to 500°C (572 to 932°F) to remove volatile components of the precursor, producing ceramic fibers. Heat treatment above 800 °C (1,472°F) results in crystallization of the fiber into alumina or other ceramic compounds, depending on the composition of the

FIGURE 4-3 Flow chart of chemical processing of ceramic oxide fibers. Source: 3M Company
precursors. Sintering (densification) may occur before or after crystallization.
Chemistry of Oxide Fiber Precursors
One distinct advantage of oxide fibers in terms of processing is that several alumina precursors suitable for forming fibers are available. The aqueous chemistry of aluminum allows for the formation of viscous basic aluminum salt solutions that can be made into fibers by dry-spinning. Polymeric aluminoxane precursors have also been used to produce alumina-based fibers. Recently, aluminoxanes that can be melt-spun into fibers have been produced. Details of both types of chemical precursors are given below.
The spinning solution, or spin dope, must be stable with respect to the crystallization and precipitation of insoluble salts and organic-containing complexes, and with respect to rapid increases in viscosity (gelation) resulting from the progression or cross-linking of precursor species. Spin dopes prepared exclusively from colloidal sols (e.g., boehmite, AlOOH) are not suitable for forming continuous fibers because they are shear thinning. Colloidal SiO2, however, is commonly used as a component in oxide fiber spin dopes (Table 4-2). Other SiO2 precursors, such as partially hydrolyzed alkoxides and polysiloxanes, have also been used as a method of introducing SiO2 into fiber compositions. Other inorganic modifiers to oxide fiber compositions can be readily added using soluble salts (e.g., nitrates).
Basic Aluminum Salts
The aqueous chemistry of aluminum has been extensively studied (Bertsch, 1989; Singhal and Keefer, 1994). Fully hydrolyzing aluminum salts, such as AlCl3, produce insoluble aluminum hydroxides upon reaction with certain additives (e.g., 3 moles of base, such as NaOH). However, soluble aluminum complexes can be formed by partial hydrolysis with less than the stoichiometric 3 moles of anion per Al. These are commonly called basic aluminum salts with the nominal formula AlXn(OH)3-n, where X is either an inorganic ligand (e.g., Cl-, NO3-, etc.) or an organic ligand (e.g., HCOOH-). Basic aluminum salts can be formed using a number of methods, including dissolution of Al metals in salt solutions, dissolution of Al hydroxides in acid, hydrolysis of alkoxides in the presence of complexing ligands, ion exchange, and neutralization of acidic salt solutions.
The aluminum species present in solution are typically octahedrally coordinated, hydroxy bridged species. A number of polynuclear aluminum species, including monomers, dimers, oligomers of intermediate molecular weight, and an Al13 complex (a cage of 12 octahedrally coordinated aluminums surrounding a single tetrahedrally coordinated aluminum),
TABLE 4-2 Composition and Precursors of Commercially Available Oxide Fibers a
|
Composition (%) |
||||||
|
Fiber |
Manufacturer |
Al2O3 |
SiO2 |
B2O3 |
Al2O3 precursor |
SiO2 precursor |
|
Nextel 312 |
3M |
62 |
24 |
14 |
aluminum carboxylates |
colloidal silica |
|
Nextel 440 |
3M |
80 |
28 |
2 |
aluminum carboxylates |
colloidal silica |
|
Nextel 550 |
3M |
73 |
27 |
— |
aluminum carboxylates |
colloidal silica |
|
Nextel 610 |
3M |
100 |
— |
— |
aluminum carboxylates |
— |
|
Nextel 720 |
3M |
85 |
15 |
— |
aluminum carboxylates |
proprietary |
|
Altex |
Sumitomo |
85 |
15 |
— |
tri-isopropyl aluminoxane |
polysilicate ester |
|
Alcen |
Nitivy |
70,60,80 |
30,40,20 |
— |
AlClx(OH)y |
colloidal silica |
|
Rubilon |
Nichias |
68 |
27 |
5 |
aluminum carboxylates |
colloidal silica |
|
Almax |
Mitsui |
100 |
— |
— |
AlClx(OH)y + Al2O3 particles |
— |
|
Fiber FP |
DuPont b |
100 |
— |
— |
AlClx(OH)y+ Al2O3 particles |
— |
|
PRD-166 |
DuPont b |
80 c |
— |
— |
AlClx(OH)y+ Al2O3 particles |
— |
|
a Fiber composition taken from vendor specifications, precursor composition from the patent literature b No longer commercially available c 20 percent ZrO2 |
||||||
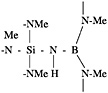
have been identified in aqueous solutions by Al27 nuclear magnetic resonance (NMR). Recently, Al27 NMR data suggesting 5-coordinated Al species have been produced (Wood et al., 1990). These polynuclear complexes are stabilized against further cross-linking and hydrolysis by complexing ligands. An aluminum dimer complexed with two carboxylate ligands, acetic acid, and lactic acid is shown below (Everitt, 1988):
Ligands can be monodentate, such as acetic acid (COCH3, upper right above), or bidentate, such as lactic acid (C2OCH3, left). In many cases, mixtures of ligands are used. The relative amounts of each type of Al complex in solution vary with the type and amount of anion ligand present, concentration, heat treatment time and temperature, and preparation route. In general, lower anion levels result in higher levels of polymerized or clustered aluminum species. Viscosity in basic aluminum salts is not derived from the presence of linear polymers but is believed to result from hydrogen bonding between adjacent Al complexes.
In practice, 0.5 to 2.0 moles of anion ligand are used per mole of Al for fiber spinning precursors. A variety of ligands have been used to produce spinnable alumina precursors, including chloride, nitrate, and carboxylates. Small amounts of ligand can allow gelation via the cross-linking of polynuclear clusters; larger amounts, however, cause problems during fiber heat treatment because of the excessive amounts of fugitives that must be removed during pyrolysis. Large ligands, such as lactic acid or acetyl acetonate, can be used to produce spinnable precursors, but ceramic yield on firing is lower than when smaller ligands, such as Cl, are used.
Aluminum chlorohydrates (AlClx(OH)y) are used to prepare a number of commercial fibers, including Fiber FP and PRD-166, Almax, and Alcen (Table 4-2). Rubilon and the Nextel series of fibers are produced using aluminum carboxylates.
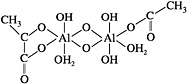
Polymeric Aluminoxane
Alumina fibers based on polymeric aluminoxanes were initially developed in the late 1970s (Horikiri et al., 1978). Aluminoxanes consist of an Al-O backbone polymer coordinated by chelating ligands, such as carboxylates and acetoacetonates, as shown below (R1 and R2 are organic ligands, and m is the degree of polymerization):
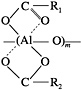
Ligands that have been used as components in spinnable aluminoxanes include ethyl acetylacetonate (Yogo et al., 1993), propionic acid (Glaubitt et al., 1994), 3-ethoxypropionic acid (Kimuta et al., 1989), acetic acid (Yogo and Iwahara, 1992) and isobutyric acid (Glaubitt, et al., 1994).
Aluminoxanes are synthesized by adding the ligand to aluminum alkyls or alkoxides to partially chelate the aluminum, followed by the addition of 0.5 to 1 mole of water per mole or Al to polymerize the aluminoxane. Additional ligands are usually added to modify precursor rheology to improve spinning and pyrolysis behavior. Depending on the ligands used, the precursors can be either melt-spun (Kadokura et al., 1990) or dry-spun. Melt-spun fibers are cured (to make them infusible) by completing hydrolysis on exposure to humid atmospheres. The ceramic yield of aluminoxane polymers can be as high as 50 weight percent. Sumitomo Altex fibers are produced using this technique.
Dry Spinning
Figure 4-4 is a schematic drawing of the dry-spinning process. The spin dope is pumped by a metering pump through a spinneret. Spinning is performed by extruding the fiber into a spinning tower under carefully controlled humidity, temperature, and air flow conditions. Spin dope viscosity is typically 100 to 1,000 Pa-s for fiber spinning. It is desirable for the spin dope to exhibit Newtonian (i.e., nonshear thinning) behavior so its viscosity remains consistent as it is extruded through the orifice of the spinneret. Fiber diameter is controlled by varying the volumetric pumping rate relative to the speed of the draw wheels. The diameter is reduced substantially during spinning; the draw ratio (speed of the draw wheels relative to the extrusion rate) is typically between 4 and 25. In order to stabilize spinning, the forces acting on the fiber must be in balance:
Fdraw+ Fgrav= Fvisc+ Finertia+ Fsurf (4)
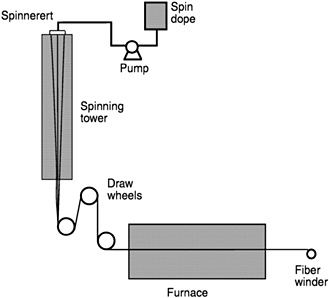
FIGURE 4-4 Schematic illustration of the dry-spinning process. Source: 3M Company
The downward forces from the draw wheel and gravitation must, therefore, equal the restraining forces generated by the viscosity and surface tension of the spin dope plus the inertial drag of the fiber as it is accelerated. Low viscosity leads to insufficient rheological force to resist gravitation; fiber diameter is reduced to the point where fracture occurs by necking. High viscosity can lead to capillary fracture, which occurs when the draw force exceeds the stretching capability of the fiber. Maintaining a balance of forces is very difficult because all of these factors are continuously changing. As the fiber progresses down the spin tower, the fibers are drawn down to smaller diameters, fiber velocities are increased, and the viscosity of the spin dope increases as the solvent evaporates, resulting in the fiber becoming rigid.
Pyrolysis
After spinning, the green fibers are conveyed into a furnace for heat treatment. Pyrolysis (the conversion of the chemical precursor into a ceramic) typically involves the loss of 50 to 80 percent of the initial weight of the green fiber. In addition, volumetric shrinkage can be 80 percent or higher. Volatile components (including CO, CO 2, H2O, produced by oxidation, and low molecular weight organics, depending on precursor chemistry) must diffuse out of the fiber during pyrolysis. Thus, pyrolysis must be performed with care to decompose the green fiber to the oxide form gently so as not to introduce defects or flaws. Removing fugitive components becomes increasingly difficult as fiber diameter increases. Typically, a fiber diameter of 20 µ m (0.80 mils) is the upper
limit for chemically-derived fibers. Defects that can occur during pyrolysis include the partial melting of fusible fiber components, the evolution of gases causing pinholes, the formation of bubbles or voids or even bloating, the entrapment of carbon, phase separation, and cracking. Adding colloidal or particulate materials to fiber precursors increases ceramic yield, thereby reducing shrinkage and facilitating pyrolysis. Colloidal additives may, however, have a detrimental effect on fiber properties.
The results of simultaneous thermal gravimetric analysis (TGA), differential TGA (DTGA), and differential thermal analysis (DTA) of a chemically-derived alumina fiber produced from aluminum carboxylates are given in Figure 4-5. The TGA curve shows a weight loss of 10 to 15 percent at 100°C (212°F). This corresponds to an endotherm on the DTA plot and is indicative of water loss. A series of weight loss peaks appear on the DTGA curve in the temperature range of 300 to 450°C (572 to 842°F). Two DTA exotherms at 420 °C (788°F) and 455°C (851°F) are also present. These peaks correspond to the exothermic decomposition of the organic components in the green fiber. A weight loss of ~50 percent occurs during this process. By the time a temperature of 650°C (1,202°F) is reached, weight loss ceases. At this point, the fiber is alumina but is x-ray amorphous. At 860°C
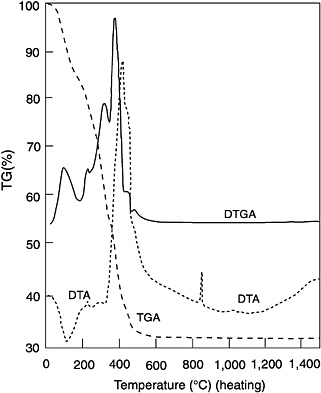
FIGURE 4-5 Differential thermal analysis (DTA), differential thermogravimetric analysis (DTGA), and thermogravimetric analysis (TGA) of chemically-derived alumina fiber. Source: 3M Company
(1,580°F), an exotherm occurs as the fiber crystallizes to cubic alumina spinel (?- Al2O3). An exotherm at 1,030°C (1,886°F) indicates the temperature at which the transformation to a-Al2O3 occurs.
Heat Treatment and Fiber Microstructure
The goal of heat treatment is to develop a ceramic microstructure with both good strength and good high-temperature properties (i.e., low creep rate, high creep rupture strength, and resistance to thermal degradation via grain growth). Fiber microstructure is determined by both the chemistry and composition of the fiber precursor. High fiber strength at room temperature requires a small grain size ( = 0.5 µm [0.02 mils]). The Griffith equation, sf = KIc/vpc, indicates that for Al2O3, (fracture toughness, KIc = 4), a fracture strength (sf) of 3 GPa (435 ksi) can only be achieved if the flaw size (c) is = 0.6 µm (0.02 mils). Thus, not only must defect concentration be kept low by careful fiber processing, but grain size must not exceed this limit. Unfortunately, creep rates increase with small grain size. Thus, optimizing overall performance requires making a trade-off between strength and creep resistance.
Microstructure development in alumina-based fibers is strongly influenced by transformation sequences that occur as alumina precursors crystallize. The stable phase of alumina at all temperatures is a-Al2O3. However, a series of cubic alumina spinels, commonly called transition aluminas, form during heat treatment of alumina precursors. The nature of the transformation (i.e., temperature, porosity, grain size) depends on the precursor. However, for amorphous precursors, like basic aluminum salts and aluminoxanes, ?-Al2O3 (cubic) or ?-Al2O3 (tetragonal) crystallize in the range 800 to 900°C (1,472 to 1,652°F) (Figure 4-5). In many systems, d-Al2O3 and T-Al2O3 are formed with additional heating in the range 1,000 to 1,150°C (1,832 to 2,102°F). These polymorphs are similar to ?-Al2O3 but have a higher degree of ordering on the cation lattice. The transformation to a-Al2O3 occurs between 1,000°C (1,832°F) and 1,200°C (2,102°F). These transformation sequences are determined by the chemistry of the fiber precursor (Wood et al., 1990). In transition aluminas, the Al3+ cations are present on both tetrahedral and octahedral sites.
The chemistry of the alumina precursor used for fiber spinning varies with synthesis technique. This chemistry is maintained throughout the pyrolysis process and affects the nature of the crystals formed at higher temperatures. For instance, the presence of the Al13 complex leads to highly ordered spinel structures, whereas the presence of 5-coordinated Al species in solution produces poorly ordered crystals (Wood et al., 1990).
Adding inorganic components to alumina-based precursors also has a strong affect on crystallization behavior and the fiber microstructure. For instance, small grain size can be achieved by stabilizing transition alumina by adding SiO2
(Stacey, 1988). Most commercial oxide fibers have a major phase of transition alumina stabilized by silica (Table 4-2). The grain size of alumina spinels is very small, typically 100 nm (0.004 mils) or less. Adding SiO2 increases the temperature of the a-Al2O3 transformation by as much as several hundred degrees (to 1,400°C [2,552°F]) if the Al and Si precursors are intimately mixed. SiO2-stabilized alumina spinels are very resistant to grain growth at temperatures up to 1,200°C (2,192°F) for extended periods. The crystallization of spinels can be eliminated, leading to the direct formation of mullite at 980°C (1,796°F) through the synthesis of atomically-mixed Al2O3-SiO2 precursors (e.g., Al alkoxides + tetraeth-oxysilane) (Schneider et al., 1992). Similar effects have been observed with other aluminates, such as YAG (King et al., 1993) and MgAl2O4. If colloidal silica or aluminas are used (i.e., “diphasic” precursors), the crystallization of mullite occurs at 1,280°C (2,536°F). Dopants can also affect crystallization. For instance, B2O3 reduces the transformation temperature of mullite (Richards et al., 1991).
Microstructural stability and creep performance can be improved by crystallizing the fiber to eliminate amorphous phases and form creep-resistant phases, such as mullite or YAG. However, control over the nucleation process during the crystallization of high-temperature phases is essential to attaining the fine grain sizes (= 0.5 µm) required for high strength. In alumina-silica fibers, large grain sizes often result from the crystallization of new phases, such as mullite and a-Al2O3. A low volumetric nucleation density, which leads to large grain sizes, is typical of both alumina and mullite.
In addition to large grains, crystallization often leads to high levels of porosity, which inhibits sintering. Figure 4-6 shows a crystalline a-Al2O3 fiber heated to 1,400°C (2,552°F). The grain size is more than 2 µm (0.08 mils), which leads to very poor strength. Enhancing the nucleation rate to improve microstructures can be done using seeding or nucleating agents. Figure 4-7 shows Nextel 610 fiber, which has a nucleation agent added, producing a uniform, high density microstructure with a grain size of ~100 nm (0.004 mils). Fiber FP/PRD-166 and Almax fibers, which are made by adding crystalline a- or ?-Al2O3 particles, respectively, are other examples of seed or nucleation sites reducing grain size.
Grain growth is another factor to be considered in the design of fiber microstructures for high-temperature applications. The degradation of fiber strength at high temperatures occurs via grain growth, which generates critical flaws in the microstructure. The presence of second phases on grain boundaries can reduce grain boundary migration rates and limit degradation associated with grain growth. For instance, PRD-166, which contains 20 percent ZrO2, retains good strength to higher temperatures than Fiber FP, a similar fiber with only a single phase of a-Al2O3. Grain growth of alumina is also inhibited in Nextel 720, which consists of a two-phase mixture of a-Al2O3 and mullite.
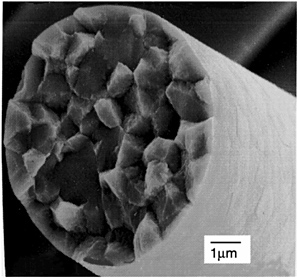
FIGURE 4-6 a-Al2O3 fiber with large grain size resulting from low nucleation density. Source: 3M Company.
Recently, several laboratories have produced polycrystalline YAG fibers. Successful processes included using colloidal sols (King and Halloran, 1995), carboxylates (King and Halloran, 1995; Budd and Wilson, 1993; Pak and Kimel, 1994), alkoxides (King et al., 1993), and mixed yttrium-aluminoxane polymers (Chen and Mazkiyasni, 1995). BSR creep experiments on polycrystalline YAG fibers have demonstrated creep resistance at 100 to 200°C (212 to 392°F) higher than the useful operating temperatures for commercially available oxide

FIGURE 4-7 Nextel 610 fiber showing small grain size resulting from the addition of nucleation agents. Source: 3M Company.
fibers (Morscher et al., 1994). In this last instance, second phase particles of ZrO2 were reported to reduce grain growth and improve creep resistance in YAG fibers (Morscher et al., 1994).
Unfortunately, the strength of these experimental YAG fibers is generally below 1.0 GPa (145 ksi). In many cases, these low fiber strengths resulted from process-related flaws, such as large pores and cracks created by imperfect pyrolysis of the precursor. However, crystal growth during crystallization of the YAG phase produced fibers with relatively large grain sizes (> 0.5 µm [0.02 mils]), which limited potential fiber strength. The production of high strength YAG fibers will require development of novel precursor chemistries that can be spun and pyrolyzed to form flaw-free fibers on a commercial scale, as well as a better understanding of factors that affect crystallization and grain growth.
RECOMMENDATIONS AND FUTURE DIRECTIONS
Although the oxidation resistance of oxide fibers is attractive, their poor creep resistance is a limiting factor. A number of the recommendations below suggest promising processing improvements that could improve this situation. Recommendations regarding property improvements of non-oxide fibers are less of a priority because, for many applications, adequate properties have already been attained. Therefore, the committee concluded that directing resources toward improving the properties of oxide fibers was more important. Note, however, that fiber processing costs for both oxide and non-oxide fibers can be reduced.
Non-Oxide Fibers
The extraordinary structural and oxidative stability claimed for the amorphous Bayer Si-N-B-C(O) fiber and the formation of an in situ BN layer on oxidation suggest directions for further investigation. Non-oxide, multi-element glasses may be a fertile area for the discovery of compositional variants that have unexpectedly useful thermomechanical and thermochemical properties. Amorphous fibers composed of combinations of elements that form strong covalent bonds (e.g., Si, C, N, B, O, and possibly Al, Ge, P, and S) may produce stable, amorphous compositions that are resistant to crystallization because of their high activation energies for bond rearrangement and a lower probability (entropy factor) of rearrangement to simple crystalline structures.
Methods for reducing the costs of processing steps in preceramic polymer processing routes for non-oxide fibers should be pursued. For example, spinnable, low-cost polymer precursors that cross-link (cure) rapidly on line or thermally cross-link in the early stage of pyrolysis have the potential to increase throughput and lower costs. These fibers should preferably be melt-spun rather than dry-spun to eliminate the need for the extra steps of solvent evaporation and recovery. Low-cost polymers with molecular structures suitable for rapid spinning and rapid on-line curing are also promising. Faster on-line pyrolysis, using polymer structures that maximize ceramic yield and minimize pyrolysis gas formation, is also a desirable goal. Non-polymer precursor processing routes should also be investigated.
New ceramic compositions and microstructures with thermomechanical and thermochemical properties beyond the capability of SiC will be required for the next generation of high-temperature materials. Developing and commercializing new non-oxide fibers that cost less and/or have improved properties are likely to be lengthy and expensive processes. Because non-oxide ceramic fibers have not been profitable so far, industry alone is unlikely to support further development.
Oxide Fibers
Although the creep resistance of oxide fibers is inferior to Si-based non-oxide fibers, recently developed oxide fibers, such as Nextel 720 and YAG fibers, have demonstrated adequate creep resistance for use in structural composites in the 1,000 to 1,200°C (1,832 to 2,192 °F) range. A fertile area for research is the development of chemical precursors that can be used to produce fine-grained, fully crystalline fibers of creep-resistant complex oxides, such as YAG and mullite. A thorough understanding of the crystallization of creepresistant phases with the proper microstructures will be required to improve creep properties without decreasing strength. In many cases, intermediate phases form prior to the crystallization of the desired phase. The demonstration of reduced rates of thermal degradation in multiphase oxide fibers complicates matters but also suggests directions for future research. The use of dopants that reduce diffusion rates to minimize grain growth kinetics should be investigated as a way of improving creep resistance. Other opportunities for improving the creep-resistant properties of oxide fibers are discussed in Chapter 5.
Si-based non-oxide fibers are covalently bonded, which leads to very high fiber strength compared to oxide fibers. However, the high strength of Nextel™ 610 fibers, attained via a targeted flaw reduction program, suggests that new types of creep-resistant oxide fibers may benefit from similar programs, which will probably be necessary for the development of high strength, creep-resistant oxide fibers, which may have relatively large grain sizes compared to most commercially available oxide fibers. The committee recommends that efforts be made to determine the effect of grain size, microstructure, and flaw population on the strength and creep resistance of oxide fibers.
The utility of oxide fibers in composites can also be enhanced by using heat treatments to produce in-situ interface coatings. For instance, Nextel 312 (which contains 14 percent
B2O3) develops a BN-coating up to 10 nm (4 x 10-4 mils) thick when it is heated in NH3 (Khasgiwale et.al., 1995). This process has led to the development of low cost Blackglass™ ceramic composites. Other fiber surface modifications have also been demonstrated, for example, the formation of fibers with metal-modified surfaces by exposing spinel fibers to reducing environments (Sowman, 1988). The committee recommends that research on ceramic oxide fiber processing include fiber surface modifications and the development of in-situ coatings.