Appendix B
Commissioned Paper
THE FUNCTIONAL IMPACT OF ORTHOSTATIC INTOLERANCE IN EHLERS-DANLOS SYNDROME
Peter C. Rowe, M.D.
Professor of Pediatrics; Sunshine Natural Wellbeing Foundation Professor of Chronic Fatigue and Related Disorders; Director, Chronic Fatigue Clinic, Johns Hopkins Children’s Center
Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Prepared for the National Academies of Sciences, Engineering, and Medicine’s Committee on Selected Heritable Disorders of Connective Tissue and Disability
Correspondence: Dr. Peter C. Rowe, Division of Adolescent/Young Adult Medicine, 200 N Wolfe St, Room 2077, Baltimore, MD, USA 21287; TEL: 410-955-9229, FAX: 410-614-1178. prowe@jhmi.edu
INTRODUCTION
This manuscript addresses the impact of orthostatic intolerance on overall function in those with heritable connective tissue disorders, primarily Ehlers-Danlos syndrome (EDS) and hypermobility spectrum disorders. After discussing the symptoms, physiology, and diagnostic testing for the common forms of orthostatic intolerance, we describe the association
between orthostatic intolerance and other disabling conditions, including heritable disorders of connective tissues. We then review the therapeutic measures to attenuate the impact of circulatory dysfunction on daily activities for affected children and adults, moving on to discuss the determinants of the ability to work or attend school. We present some of the techniques for measuring work- and school-related function in those with orthostatic intolerance, and interventions, procedures, and accommodations to mitigate the impact of orthostatic intolerance. Research has identified a strong relationship between joint hypermobility/EDS, orthostatic intolerance, and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) (Barron et al., 2022; De Wandele et al., 2014a,b; Roma et al., 2018b; Rowe et al., 1999). As a result, we will draw on observations from the ME/CFS literature to inform the discussion of treatment and accommodations in the heritable disorders of connective tissue.
ORTHOSTATIC INTOLERANCE: SYMPTOMS, PHYSIOLOGY, DIAGNOSIS
Orthostatic intolerance refers to a heterogeneous group of circulatory disorders in which individuals develop symptoms upon assuming and maintaining upright posture, improving (although not necessarily resolving completely) after they return to a recumbent posture (Gerrity et al., 2002–2003; Low et al., 2009). Hemodynamic abnormalities in orthostatic intolerance can include classical or delayed orthostatic hypotension, neurally mediated hypotension (NMH), and postural tachycardia syndrome (POTS) (Freeman et al., 2011; Goldstein et al., 2002; Low et al., 2009; Rosen and Cryer, 1982; Schondorf and Low, 1993; Sheldon et al., 2015; Stewart et al., 2018). More recently it has become evident that a relatively large proportion of individuals with orthostatic intolerance lack these abnormal heart rate and blood pressure responses to upright posture, but have substantial reductions in brain blood flow when upright (van Campen et al., 2020a).
The most common symptoms of chronic orthostatic intolerance are shown in Table B-1. These clinical features are largely due to two principal physiological changes in response to orthostatic stress: (1) reduced cerebral blood flow and (2) the compensatory adrenergic response to reduced cerebral blood flow (Low et al., 2009). Many of the symptoms in Table B-1 overlap with features seen in EDS, including fatigue, cognitive problems, headaches, exercise intolerance, and anxiety. Patients with orthostatic intolerance can experience lengthy delays before diagnosis, and thus a similarly lengthy delay in initiating therapy. For example, one large survey of POTS patients identified a median diagnostic delay of 24 months (Shaw et al., 2019). Orthostatic intolerance syndromes are relevant for those with connective tissue laxity because treatment of their circulatory conditions
TABLE B-1
Symptoms of Orthostatic Intolerance
| Due to ↓ CBF | Due (largely) to the Hyperadrenergic Response to ↓ CBF |
|---|---|
| Lightheadedness | Dyspnea |
| Syncope | Chest discomfort |
| Diminished concentration | Palpitations |
| Headache | Tremulousness |
| Blurred vision | Anxiety |
| Fatigue | Diaphoresis |
| Exercise intolerance | Nausea |
NOTE: CBF = cerebral blood flow.
is often associated with amelioration of symptoms and improved overall function.
Some symptoms of orthostatic intolerance, such as lightheadedness, improve promptly on lying down. Once provoked, other symptoms, such as fatigue and brain fog, can persist for hours to days. The initial papers by Sir Thomas Lewis on vasovagal syncope in the 1920s described a soldier who had fainted, had a clear vasovagal reaction to venipuncture, and was fatigued and tremulous for the next 36 hours (Lewis, 1932), indicating a debt of fatigue persisting after the individual had assumed a recumbent posture. While the mechanism for the protracted symptoms is unclear, such an episode establishes the potential for recurrent orthostatic stress in daily life to be associated with chronic symptoms.
Physiologic Responses to Upright Posture
For adults, the assumption of an upright posture is associated with a gravitational redistribution of approximately 500–1000 mL of blood to the dependent vasculature (Smit et al., 1999; Smith et al., 1994). Adolescents are thought to experience a similar change. In response to the gravitational pooling, less blood returns to the heart, which leads to a decrease in cardiac output, reduced stretching of baroreceptors, and ultimately a reduction in blood flow to the brain. The vasomotor center in the brain stem responds by increasing sympathetic neural outflow and by reducing vagal tone. These changes lead to improved vasoconstriction and as much as a 30–40 beats-per-minute (bpm) increase in heart rate, returning sufficient venous blood
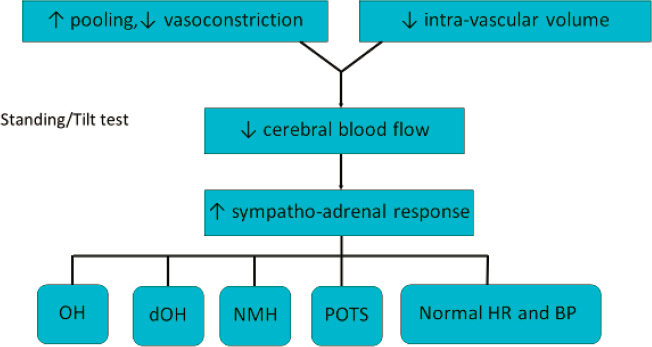
NOTE: BP = blood pressure; dOH = delayed orthostatic hypotension; HR = heart rate; NMH = neurally mediated hypotension; OH = orthostatic hypotension; POTS = postural tachycardia syndrome.
to maintain blood pressure and cerebral perfusion (Medow et al., 2008; Rowell, 1992; Wieling and Shepherd, 1992).
Figure B-1 illustrates the principal physiological contributors to orthostatic intolerance—namely, excessive gravitational pooling of blood, often related to a defect in vasoconstriction; low blood volume; and an increased sympathetic nervous system and adrenal catecholamine response to the orthostatic reduction in cerebral blood flow. Pathophysiologic contributors to decreased vasoconstriction and increased peripheral pooling of blood include the duration of quiet upright posture, increased compliance of the blood vessel wall in response to hydrostatic pressure, the presence of venous varicosities, obstruction to venous return, and vasodilating substances.
Low blood volume has been observed in a variety of conditions of orthostatic intolerance, including POTS and ME/CFS (Hurwitz et al., 2010; Okamoto et al., 2012; Streeten and Bell, 1998); although, because of its high cost, the measurement of blood volume using nuclear medicine techniques is not readily available outside of research settings. Low blood volume has been associated with lower renin-to-aldosterone ratios and with reductions in antidiuretic hormone (Okamoto et al., 2012; Wyller et al., 2010). Decreased plasma volume, which can result from physical inactivity, can exacerbate symptoms of orthostatic intolerance and interfere further with daily function (Fortney et al., 1996; Takenaka et al., 2002). When individuals who have increased peripheral pooling of blood and/or low blood volume assume an upright posture, they experience a greater decrease in
the return of blood to the heart and a markedly increased catecholamine response (Benditt et al., 2003; Rosen and Cryer, 1982).
Goldstein and colleagues (2003) have proposed that the relative balance of epinephrine to norepinephrine can influence the pattern of circulatory response. Individuals with POTS have higher norepinephrine levels, likely related to norepinephrine-mediated vasoconstriction, thereby helping to maintain blood pressure longer, while contributing to the heart rate stimulation that defines POTS. In contrast, those with recurrent neurally mediated syncope have higher epinephrine levels during tilt, in part related to epinephrine’s vasodilatory effect on the skeletal muscle vasculature, and resultant hypotension. These two conditions, however, are not mutually exclusive, as patients who develop POTS during the first 10 minutes upright can develop NMH as the orthostatic stress becomes more prolonged, usually 5–59 minutes after the initial orthostatic tachycardia (Kanjwal et al., 2011; Rowe et al., 2001; Sandroni et al., 1996).
Forms of Orthostatic Intolerance
Orthostatic Hypotension
Classical orthostatic hypotension (cOH) requires a sustained reduction in blood pressure of at least 20 mm Hg systolic or 10 mm Hg diastolic during the first 3 minutes after assuming an upright posture (Freeman et al., 2011). This condition is more common in older adults, especially those with comorbid diabetes mellitus or Parkinson’s disease (Freeman et al., 2018). While less common in children, cOH can be identified in pediatric patients during febrile illnesses, with voluntary or involuntary fluid and caloric restriction, excessive histamine release, adrenal insufficiency, hemorrhage, or as a response to certain medications. Delayed orthostatic hypotension (dOH) is defined by the same changes in blood pressure as cOH, but occurs after the first 3 minutes upright (Freeman et al., 2011).
Postural Tachycardia Syndrome
Increasingly recognized as a common form of orthostatic intolerance in those with EDS, POTS is more common in females than males; is more common after than before the onset of puberty; and often follows an apparent infectious illness, immunization, trauma, or surgery (Fedorowski, 2019; Grubb, 2008; Vernino et al., 2021). POTS should only be diagnosed (1) in the presence of an increase in heart rate of ≥ 30 bpm in adults (≥ 40 bpm in those under age 20) from lowest supine to peak standing over the first 10 minutes upright, and (2) in the absence of orthostatic hypotension in the first 3 minutes upright. Some have suggested a heart rate of >120 bpm
during the first 10 minutes upright as an additional criterion (Fedorowski, 2019). Approximately 40 percent of healthy adolescents would be misclassified as having POTS using the adult heart rate increment of 30 bpm (Singer et al., 2012). At all ages, the diagnosis of POTS is based on more than just the heart rate and also requires the individual to have chronic orthostatic symptoms. Heart rate elevation alone is insufficient for a diagnosis of POTS in individuals who are dehydrated or are being treated with vasodilating medications. Conversely, at the time of orthostatic testing, concurrent consumption of substances that increase plasma volume, such as salt tablets, or agents used to treat orthostatic intolerance (e.g., serotonin reuptake inhibitors, oral contraceptives, stimulant medications, beta adrenergic antagonists, or other medications) can obscure the diagnosis of POTS.
Inappropriate Sinus Tachycardia
As the name suggests, this condition is diagnosed when the patient is in sinus rhythm, but has a heart rate higher than 100 bpm at rest. Symptoms of inappropriate sinus tachycardia are similar to those in POTS (Sheldon et al., 2015).
Neurally Mediated Hypotension
NMH is a reflex form of hypotension, and while its physiology is synonymous with vasovagal syncope or neurocardiogenic syncope (Bou-Holaigah et al., 1995; Grubb, 2005; Rowe et al., 1995), NMH is a more accurate description of the phenotype. Many with NMH experience the same characteristically abrupt drop in blood pressure during tilt testing as those with vasovagal syncope, and they can experience chronic daily orthostatic symptoms without having completely lost consciousness. In both children and adults, NMH occurs after the 3-minute cutoff for orthostatic hypotension and is characterized by at least a 25 mm Hg reduction in systolic blood pressure. At the time of presyncope or hypotension, affected individuals can develop a relative slowing of the heart rate that can progress to junctional bradycardia or even asystole. This disorder can be identified during standing, but more commonly requires prolonged period (> 10 minutes) of upright tilt-table testing, which typically provokes characteristic orthostatic symptoms (Wieling et al., 2004). When orthostatic testing lasts less than 10 minutes, this form of hypotension can be missed (Bou-Holaigah et al., 1995). NMH is more common in females than in males and in younger adults and adolescents than in older people; often, the family history is positive (Grubb, 2005).
As has been reviewed elsewhere (Grubb, 2005; Jhanjee et al., 2009; van Dijk et al., 2021; van Lieshout et al., 1991; Wieling et al., 2004), the
pathophysiology of NMH involves reduced venous return, which then initiates a series of neural responses that result in withdrawal of sympathetic tone and a relatively unopposed vagal response. These responses lead to slowing of the heart rate and vasodilation, which can result ultimately in a profound drop in blood pressure or syncope if the individual remains upright. Markedly elevated epinephrine levels are present prior to syncope (Benditt et al., 2003; Jardine et al., 1997). Precipitating factors for NHM can include prolonged standing; warm environments (such as hot weather, hot showers, or hot tubs); pain; venipuncture; the sight of blood; or sudden stretching of mechanoreceptors in the gastrointestinal tract, bladder, and lungs. These phenomena can occur individually or in combination (Grubb, 2005; Jhanjee et al., 2009; van Lieshout et al., 1991; Wieling et al., 2004). Although many patients with NMH experience only isolated episodes of syncope that are separated by long periods of normal function, NMH physiology can be associated with chronic, daily orthostatic symptoms. Patients referred for evaluation of recurrent syncope report chronic fatigue, as well as impaired quality of life (Kenney and Graham, 2001; Legge et al., 2008; Rose et al., 2000). Among patients evaluated for chronic orthostatic intolerance, approximately 25 percent have neurocardiogenic syncope without POTS (Goldstein et al., 2005). Thus, POTS cannot be regarded as the only form of chronic orthostatic intolerance.
Low Orthostatic Tolerance
This condition is characterized by the presence of frequent orthostatic symptoms without the heart rate and blood pressure changes that characterize OH, NMH, or POTS (IOM, 2015). Recent research has shown that many of these patients have reductions in cerebral blood flow as measured either by transcranial Doppler ultrasound (which measures cerebral blood flow velocity) or by Doppler ultrasound of the internal carotids and vertebral arteries, which measures total blood flow to the brain (Novak, 2018; van Campen et al., 2020a).
Testing for Orthostatic Intolerance
There is no gold standard test for orthostatic intolerance, but head-up tilt table tests and standing tests are the primary types of testing used in clinical practice. The methods for conducting each type of test vary. We do not know all the pathophysiological contributors to such symptoms as lightheadedness, and some classical “orthostatic” symptoms can be reproduced by postural maneuvers, such as passive straight leg raising, that do not involve upright posture (Rowe et al., 2016).
Head-Up Tilt Table Testing
Modern head-up tilt table testing is conducted using a motorized table with a footboard for weight-bearing (Benditt et al., 1996). The test begins with the patient lying supine and loosely restrained by belts that prevent injury if loss of consciousness ensues. In our center, the period of supine rest lasts 15 minutes, but some investigators have positioned patients supine for up to 60 minutes. Next, the tilt table is gradually raised to a 60–70 degree angle. Higher tilt table angles during testing can provoke syncope more readily in healthy controls. Depending on which form of orthostatic intolerance is being evaluated, the duration of upright tilt can vary between institutions. Ten minutes of upright posture is sufficient to detect OH and POTS, but upright tilt for 45–60 minutes is often required for detection of dOH and NMH.
A variety of methodological factors can affect tilt response (Benditt et al., 1996; Moya et al., 2009; Sheldon, 2005), including the pretest sodium intake, the duration of the pretest fast, the ambient temperature in the laboratory, the time of day for the study, and whether the patient is permitted to remain on medications that have hemodynamic effects. Test factors can include the degree of patient movement permitted, whether there is invasive instrumentation (higher rates of syncope can be seen if arterial catheters are placed), the use of pharmacologic agents to provoke hypotension (e.g., isoproterenol [Natale et al., 1995], nitroglycerine), and the way an abnormal test is defined. Past studies often required reproduction of syncope as the end-point of testing. However, OH, POTS, and NMH can be identified before syncope occurs, and the table can be returned to the horizontal position.
In most laboratories, heart rate and blood pressure are monitored using beat-to-beat measurements, although in some centers only intermittent blood pressure measurements are made, for example, every 1–2 minutes for 5–10 minutes, then every 5 minutes unless presyncopal symptoms are identified. Measurement of end-tidal CO2 for detecting orthostatic hypocapnia is a helpful adjunctive technique (Naschitz et al., 2000; Natelson et al., 2007; Novak, 2018; Razumovsky et al., 2003; van Campen et al., 2020a). During both supine and upright phases, at approximately 5-minute intervals, patients are asked to rate changes in symptoms on a 0–10 scale (lightheadedness, fatigue, headache, mental fogginess, warmth, shortness of breath, nausea, and pain).
Head-up tilt testing is not necessary in all individuals with orthostatic symptoms. Among those in whom the clinical history is consistent with reflex or neurally mediated syncope, and the heart is structurally normal, head-up tilt testing is no longer thought to be necessary for diagnosis (Benditt et al., 1996; Moya et al., 2009; Sheldon, 2005; Strickberger et al.,
2006). Among the most impaired individuals with ME/CFS or EDS, upright tilt can provoke postexertional exacerbation in symptoms during and for at least a week after the tilt test (van Campen et al., 2021b). In the most impaired individuals, brief orthostatic vital signs obtained in supine, seated, and standing positions over 1–3 minutes in some instances, or a 20-degree tilt angle, are sufficient as an indication of orthostatic intolerance. In those who have symptoms provoked during the tilt testing, and who might be at risk for postexertional malaise in the following days, our experience is that this symptom flare can be ameliorated by administration of 1–2 L of warmed normal saline immediately after the end of the period upright (IOM, 2015).
Standing Tests
Two closely related forms of standing tests are used in the orthostatic intolerance literature: active standing (Plash et al., 2013; Streeten et al., 2000) and passive standing, in which the individual leans against a wall (Hyatt et al., 1975; Roma et al., 2018a). Standing tests can be conducted in a clinical office, and thus are cheaper and more readily available than tilt table tests. No specialist consultation or specialized equipment is required. The period of supine rest before standing varies between studies, but in most instances a consistent baseline heart rate and blood pressure can be obtained within 5–15 minutes. Although the duration of upright posture can be as long as 60 minutes (Streeten et al., 2000), most centers perform a 10-minute standing test to identify POTS and to ascertain worsening symptoms during orthostasis. As with 10-minute tilt testing, this duration of standing will miss many instances of dOH and NMH. Symptoms are recorded immediately before standing and at intervals of 1–2 minutes when upright. Some studies during the COVID-19 pandemic have been conducted at home, but it is important that the test be witnessed given the potential for injury to occur if individuals have a rapid onset of syncope. We discourage the postural counter-maneuvers that can improve venous return to the heart during quiet standing (e.g., fidgeting, shifting weight, and contracting the leg muscles). While helpful in day-to-day life, these constitute partial treatment during the diagnostic test. Box B-1 provides the instructions and reporting form for conducting the passive standing test used in the Johns Hopkins Chronic Fatigue Clinic.
Few investigations have compared the available orthostatic testing methods. Among the studies that have compared tilt testing and active standing, none have examined prolonged upright posture (Hyatt et al., 1975; Plash et al., 2013). During the first 5 minutes upright, similar heart rate changes occur during active standing and tilt testing (Hyatt et al., 1975). Beyond the first 5 minutes, however, passive tilt provokes a larger
change in heart rate in those with POTS than does active standing (Plash et al., 2013). While application of lower-body negative pressure with the patients in a supine position can approximate the physiology of upright testing, it is usually only used in research settings (Wyller et al., 2008). While perhaps desirable in clinical settings for patient flow, shorter periods of passive standing or head-up tilt will miss diagnosing a proportion of those who meet POTS criteria after 10 minutes of testing; therefore, a full 10-minute period upright is recommended (Roma et al., 2018a).
ASSOCIATION OF ORTHOSTATIC INTOLERANCE WITH OTHER DISABLING CONDITIONS
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
Among those with ME/CFS or the related condition fibromyalgia, orthostatic stress consistently provokes fatigue and other symptoms (IOM, 2015; Martinez-Lavin, 2006; van Campen et al., 2020a). In pediatric ME/CFS, rates of orthostatic intolerance exceed 96 percent of affected participants (IOM, 2015; Stewart et al., 1999). Differences in cardiovascular responses between ME/CFS patients and healthy controls can be detected
with as little as 20 degrees of upright stress (van Campen 2020c; Wyller et al., 2007). In our experience, approximately 50–60 percent of those meeting criteria for pediatric ME/CFS also meet criteria for joint hypermobility, defined in those studies as a Beighton score of at least 4 (Barron et al., 2002; Roma et al., 2019). In adults with ME/CFS, past studies reported a variable prevalence of orthostatic intolerance.
Combining 14 controlled studies evaluated in a 2015 review of the evidence by the Institute of Medicine (IOM, 2015), 484 adults with ME/CFS had been evaluated with orthostatic stress tests lasting more than 10 minutes. Of these, 202 (42 percent) developed hypotension during the test, compared with 15 percent among healthy controls. The percentage of abnormalities across studies varied markedly, from 0 to 96 percent, suggesting wide variability in testing methods and patient selection (IOM, 2015).
More recently, van Campen and colleagues (2018) demonstrated that total cerebral blood inflow could be measured reliably using a Doppler technique that captures flow through each internal carotid and each vertebral artery, with image acquisition for the four vessels taking approximately 3 minutes. Adding the flows through each of the four vessels (necessary in part because of unilateral vessel dominance in some patients) provides a measure of total cerebral blood flow (van Campen et al., 2018). Based on reductions of cerebral blood flow >2 standard deviations from the mean reduction of 7 percent seen in controls between supine and 30 minutes upright, a > 13 percent reduction of cerebral blood flow is defined as abnormal.
Applying the same Doppler technology to measure cerebral blood flow during tilt testing in adults with ME/CFS, van Campen and colleagues (2020a) studied 429 adults with ME/CFS and 44 healthy controls. In response to 30 minutes of head-up tilt table testing, 247 (58 percent) of the ME/CFS participants had a normal heart rate and blood pressure response, as did all of the healthy controls, while 62 (14 percent) with ME/CFS developed dOH and 120 (28 percent) met criteria for POTS (van Campen et al., 2020a). This study was unable to evaluate those with neurally mediated syncope because the blood pressure drop was too rapid to allow for 3 minutes of image acquisition. Figure B-2 shows the percent reduction in cerebral blood flow from the supine values at mid-tilt and end-tilt: healthy individuals experienced a 7 percent reduction in brain blood flow compared with a 26 percent reduction in the ME/CFS patients overall. In subgroups of ME/CFS, those with dOH had a 28 percent reduction, and those with POTS had a 29 percent reduction. Importantly, among the 58 percent with a normal hemodynamic response (no dOH or POTS)—who in the absence of the Doppler measures might have been diagnosed as having nothing wrong—there was a 24 percent reduction in cerebral blood flow, representing over a three-fold greater reduction in brain blood flow than the

NOTE: BP = blood pressure; CBF = cerebral blood flow; dOH = delayed orthostatic hypotension; HC = healthy controls; HR = heart rate; ME/CFS = myalgic encephalomyelitis/chronic fatigue syndrome; POTS = postural tachycardia syndrome.
SOURCE: van Campen et al., 2020a. This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0.
healthy controls. In total, 90 percent of adults with ME/CFS experienced a significant reduction in cerebral blood flow (van Campen et al., 2020a). There was a significant correlation between summed orthostatic symptoms and the degree of cerebral blood flow reduction at mid-tilt. The implication from this study is that limiting the diagnosis of orthostatic intolerance to heart rate and blood pressure abnormalities has the potential to misdiagnose the majority of people who have a clinically significant drop in cerebral blood flow. This novel method of confirming orthostatic intolerance by measuring cerebral blood flow has yet to be adopted widely, but it appears to be a more sensitive measure for diagnosis in a variety of patient groups.
In subsequent work, van Campen and colleagues (2020b) have shown that sitting can provoke a clinically important reduction in cerebral blood flow. This has implications for workplace accommodations for those with heritable disorders of connective tissue. In a study of 100 adults with ME/CFS who had severe functional impairment (mostly bed-bound or dependent on others for daily care), cerebral blood flow was similar to that of the 15 healthy controls when tested in a supine position. However, when tested in a seated position, these patients with ME/CFS developed a 24.5 percent reduction in cerebral blood flow compared with a 0.4 percent reduction in healthy controls (van Campen et al., 2020b). Similarly, a separate study of 19 patients severely affected by ME/CFS, all of whom met the criteria for an abnormal cerebral blood flow reduction, a shorter 15-minute tilt test with a reduced 20-degree head-up tilt angle was capable of provoking a significant (27 percent) decline in cerebral blood flow (van Campen et al., 2020c).
Ehlers-Danlos Syndrome
Chronic fatigue is a prevalent symptom and an important determinant of impaired health-related quality of life in EDS (Rombaut et al., 2010). The association between EDS, chronic fatigue syndrome, and orthostatic intolerance was first described in 1999 (Rowe et al., 1999). Over a 1-year period, 12 of 100 consecutively tested adolescents with chronic fatigue syndrome also met criteria for EDS. The median Beighton score was 7 (range, 5–9), and all 12 patients reported joint dislocations, 3 of whom had undergone joint surgery. Genetic and ophthalmologic consultations confirmed that 6 patients satisfied the criteria at the time for classical EDS and 6 satisfied the criteria for hypermobile EDS. All 12 experienced an increase in their usual symptoms during the first 10 minutes of orthostatic testing, and 10 met the criteria for POTS. Of the 12 patients with chronic fatigue syndrome, 9 developed NMH either alone or in combination with POTS. In these 12, their EDS had not been recognized despite a median of 37 months (range, 12–62 months) of fatigue beforehand (Rowe et al., 1999).
Given the overlap among EDS, orthostatic intolerance, and ME/CFS, Barron and colleagues (2002) compared the prevalence of nonsyndromic joint hypermobility in adolescents with ME/CFS and in healthy controls. The ME/CFS group had a 60 percent prevalence of Beighton scores ≥ 4, compared with 24 percent of healthy adolescents (P < .001), a rate similar to that reported in other studies of healthy individuals at the same age. The odds ratio for having joint hypermobility if participants had ME/CFS was 3.5 (95% confidence interval [CI], 1.6–7.5; P < .001) (Barron et al., 2002). This study provided further corroboration of an association between orthostatic intolerance and joint hypermobility.
De Wandele and colleagues (2014a) performed the largest and most comprehensive study of the association between autonomic dysfunction and heritable disorders of connective tissue. In a convenience sample of 80 patients with hypermobile EDS (hEDS), 11 patients with classical EDS, 7 patients with vascular EDS, and 43 healthy controls (n = 43), 94 percent of those with hEDS reported symptoms of orthostatic intolerance (De Wandele et al., 2014a). Their burden of autonomic symptoms was also higher than that of the other EDS groups and the controls.
De Wandele and colleagues (2014b) also conducted comprehensive autonomic testing on 39 female adults with hEDS and a sex-matched group of 35 similar-aged controls. During head-up tilt testing, the hEDS group developed orthostatic symptoms at an earlier point than the control group. POTS and other forms of orthostatic intolerance were more common in those with hEDS than in controls (41 percent vs. 11 percent for POTS, and 74 percent vs. 34 percent for any form of orthostatic intolerance). Joint hypermobility correlated with a larger heart rate increment and lower blood pressure when upright.
Subsequent work by van Campen and colleagues (2021a) has confirmed that adults who have ME/CFS differ in the degree of cerebral blood flow reduction during orthostatic stress depending on their degree of joint hypermobility. In a case-control study of females matched by age and disease duration, 100 hypermobile ME/CFS patients were compared with 100 patients who had ME/CFS without joint hypermobility. Joint hypermobility was considered to be present if a rheumatologist, geneticist, or rehabilitation specialist had made the diagnosis of either hEDS or joint hypermobility. If patients had not been formally diagnosed with hypermobility, participants were asked whether they were hypermobile or highly flexible, which was then confirmed with a Beighton score. Participants were included in the hypermobility group if the Beighton score was 6 or higher. During tilt testing, those with joint hypermobility were significantly more likely to develop POTS than the nonhypermobile patients and had a significantly larger reduction in cerebral blood flow. As shown in Figure B-3, cerebral blood flow fell by 32 percent in the hypermobile patients compared with 23 percent in those without hypermobility (P < .0001), regardless of whether the hemodynamic phenotype involved POTS or was characterized by a normal heart rate and blood pressure response (van Campen et al., 2021a). The larger cerebral blood flow reduction is consistent with the hypothesis that increased blood vessel laxity contributes to increased gravitational pooling of blood in the presence of higher hydrostatic pressure below the heart during upright posture (Barron et al., 2002; Rowe et al., 1999).
Accurate prevalence estimates for orthostatic intolerance among those with EDS are not available, as there have been no population-based studies that have employed measures to evaluate for joint hypermobility or EDS
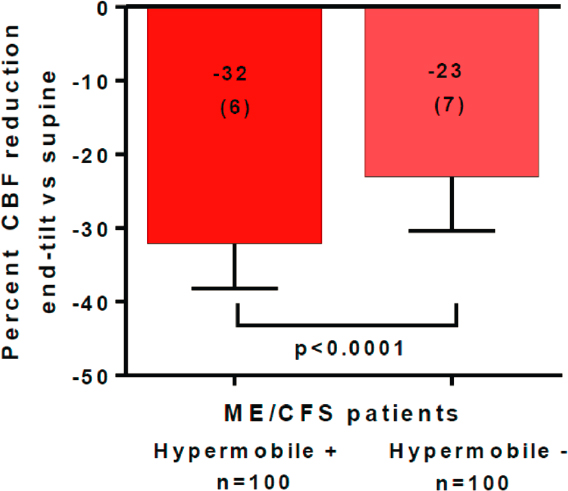
SOURCE: Reproduced from van Campen et al., 2021a.
in which all participants have undergone consistent orthostatic testing. In clinic-based reports, which have varying degrees of referral bias, between 41 and 100 percent of people with joint hypermobility or EDS endorse symptoms of orthostatic intolerance on a regular basis (Roma et al., 2018b). The studies with the highest prevalence involve more complete ascertainment of orthostatic symptoms (see, e.g., De Wandele et al, 2014a). Similarly, heart rate and blood pressure abnormalities can be identified in 56–80 percent of joint hypermobility patients; the higher prevalence rates are reported in studies with more prolonged orthostatic testing (Roma et al., 2018b).
TREATMENT OF ORTHOSTATIC INTOLERANCE
While the treatment of orthostatic intolerance begins with nonpharmacologic interventions, in our experience, most individuals with more than mild functional impairments will require medications. Pharmacologic treatment has the potential to ameliorate the impact of orthostatic intolerance, but improvements are not automatic or universally effective.
Nonpharmacologic Measures
Nonpharmacologic treatment for orthostatic intolerance is focused in four main areas. Individuals need to (1) avoid conditions that increase dependent pooling of blood, (2) avoid depletion of salt and water and other causes of low blood volume, (3) use techniques to improve venous return to the heart, and (4) avoid situations that further increase catecholamines.
Avoid conditions that increase dependent pooling of blood
Patients are advised to avoid prolonged sitting by obtaining permission to move around during classroom lectures or longer meetings, and by standing and stretching periodically when seated at a desk. Similarly, they are advised to avoid long lines (e.g., by shopping at nonpeak times, obtaining permission to preboard when traveling). In the home setting, patients can take short, cooler baths and showers; avoid hot tubs and saunas; and avoid sunbathing. Many prefer to study in a horizontal position that reduces gravitational pooling of blood in the legs, and improves brain blood flow. Because large meals and high carbohydrate intake can contribute to a shift of blood volume to the splanchnic circulation, patients often fare better with frequent, smaller meals.
Avoid depletion of salt and water
Adolescents and adults with orthostatic intolerance need to drink 2–3 L of fluid daily, drinking fluids every 1–2 hours during the day. Adequate sodium intake helps retain fluids in the intravascular space, but no specific amount of sodium works for each individual. We recommend salting food according to taste, adding buffered salt tablets if needed, and supplementing with oral rehydration fluids. Among those with orthostatic intolerance, higher-sodium food options include olives, dill pickles, soups, tomato juice, salted nuts, soy sauce, and salsa. An important accommodation needed at school or work is access to salty snacks and fluids throughout the day.
Improve venous return to the heart
Without necessarily understanding why, many individuals with orthostatic intolerance have already adopted postural countermaneuvers that utilize the muscle pump function of the lower limbs to improve venous return. Examples include standing with the legs crossed, shifting weight while standing from one leg to the other, sitting with the knees to the chest, or performing leg muscle contraction exercises before standing (Smit et al., 1997; van Lieshout et al., 1992). Wearing heeled shoes or boots can
promote increased calf muscle contraction. We advise against sitting on a high stool with the legs dangling freely, as that position provides no resistance to blood pooling in the legs. Positioning the knees higher than the hips improves afterload and tolerance of sitting. Examples include sitting in a low chair (Smit et al., 1997), resting the feet on a low foot rest, or sitting with one or both legs folded under the buttocks.
Compression garments of various types can be helpful. Waist-high stockings are more effective for compression than are thigh-high, which in turn are more effective than knee-high (Bourne et al., 2021; Heyer, 2014; van Campen et al., 2022). We usually recommend support hose with 20–30 mm Hg compression. While stockings with 30–40 mm Hg compression provide a greater effect, they can be difficult and somewhat impractical to get off and on. For those with joint hypermobility, the effort required to pull up 30–40 mm Hg compression stockings has the potential to cause hand and wrist pain or subluxation; therefore, we usually recommend avoiding this level of compression in the hypermobile population. Some prefer wearing abdominal binders or body shaper garments, both of which can reduce excessive pooling in the splanchnic beds. An older method of improving blood volume is to elevate the bed frame by 10–15 degrees so that the head is higher than the feet. While this is not comfortable for everyone, and theoretically could reduce brain blood flow in severely impaired patients, in those who tolerate the head-up position, it is thought to help by reducing urine formation and retaining more vascular volume fluid at night (MacLean and Allen, 1940; van Lieshout et al., 2000).
Many medications and supplements have the potential to increase vasodilation, including niacin, narcotic analgesics, phenothiazine antiemetics, and antipsychotic medications. Their use may need to be minimized or avoided. Low doses of tricyclic antidepressants can occasionally aggravate hypotension. This class of medications is not absolutely contraindicated, as tricyclic antidepressants are often tolerated and are useful in this population for management of headaches, pain, mast cell activation, and insomnia.
Avoid increasing catecholamines
Catecholamine levels are increased in those with orthostatic intolerance and worsen with upright posture. Because physiological stressors, including pain and emotional distress, can elevate catecholamine levels even higher, stress avoidance can help with symptom management.
It is important to screen for medications that have the potential to increase catecholamines and thereby aggravate orthostatic symptoms. For example, in patients with comorbid asthma, beta-adrenergic agonists (e.g., albuterol) can mimic the effects of epinephrine and contribute to lightheadedness and tremulousness. Caffeine intake (including in coffee or soft
drinks) can help symptoms by acting as a vasoconstrictor, but some patients experience adverse effects, such as excessive stimulation or diuretic effects.
Treatment of Other ME/CFS Symptoms and Comorbid Conditions
The second step in managing orthostatic intolerance is to treat other comorbid conditions. This includes managing migraine headaches, allergies, mast cell activation syndrome, anxiety, depression, menstrual dysfunction, and areas of biomechanical dysfunction with physical therapy or osteopathic manual therapy (Rowe, 2016). Temporomandibular joint dysfunction and neurogenic thoracic outlet syndrome are common conditions among those with joint hypermobility and EDS, and are important comorbidities to treat. Patients therefore need permission to miss school or work without penalty in order to incorporate appointments and treatments into their weekly schedule.
Pharmacologic Interventions
The third step in managing orthostatic intolerance is to initiate pharmacologic therapy. While ideally we attempt to identify a single effective medication, rational polytherapy (using medications that have different mechanisms of action) is often required for optimal symptom control. Medications should be introduced one at a time in most instances, starting at low doses and increasing gradually.
Commonly used medications for orthostatic intolerance are listed in Table B-2 by their general mechanism of action (Rowe et al., 2017). Some physicians recommend either a low-dose beta blocker or midodrine as the first-line agent in those with POTS (Johnson et al., 2010). Often, however, the specifics of the patient’s condition and existing comorbidities allow an individualized approach. For example, fludrocortisone might be a good first choice if the patient has a relatively low resting blood pressure for age or an increased desire for salt. Beta blockers would need to be used with caution in those with asthma, but would be a reasonable first choice for those with elevated resting heart rates or headaches. Midodrine is an effective treatment for recurrent syncope, but dosing in 4-hour increments makes it less convenient for students to take when in school. Stimulants such as methylphenidate or dextroamphetamine and others can be helpful in as vasoconstrictors, and can help with fatigue and cognitive symptoms. Pyridostigmine bromide can help with gastrointestinal motility as well as with orthostatic intolerance.
Adolescent and young adult females with acne, excessive menstrual blood loss, dysmenorrhea, or perimenstrual exacerbation of orthostatic intolerance symptoms can benefit from hormonal contraceptive therapy
TABLE B-2
Medications for Orthostatic Intolerance
| Medication | Usual Adolescent Dose | Comments/Indications |
|---|---|---|
| Vasoconstrictors | ||
| Midodrine | 2.5 mg every 4 hours while awake. Increase every 3–7 days by 2.5 mg until an optimal dose is achieved. Max 10 mg every 4 hours while awake. | Suggested as first-line therapy for those with baseline hypotension. |
| Stimulants | ||
| Methylphenidate | Immediate-release form: 5–10 mg BID, increasing gradually to 15–40 mg/day. Sustained-release form: start with 10 mg once daily and increase gradually until an optimal effect is found. | Suggested as first-line therapy for those with prominent cognitive dysfunction or a personal or family history of attention deficit hyperactivity disorder. Common adverse effects can include reduced appetite, insomnia, and agitation. |
| Dextroamphetamine | Sustained-release form: 5–10 mg in the morning. Increase by 5–10 mg weekly. Max 15–40 mg daily. | |
| Volume Expanders | ||
| Sodium chloride | Oral: 1 g tablets with meals. IV: 1–2 L over 1–2 hours. | Oral supplements not always sufficient as the only therapy. IV normal saline is impractical over the longer term but can help restore baseline function after acute infections or as rescue therapy. |
| Fludrocortisone | 0.05 mg daily for 1 week, then 0.1 mg daily. Increase gradually to max of 0.2 mg daily. | Suggested as first-line therapy for those with baseline hypotension or increased salt appetite. Potassium supplementation is recommended to prevent hypokalemia, as fludrocortisone increases urinary potassium losses. Can aggravate acne. |
| Hormonal contraceptives | Most are fine. Conventional dosage or continuous pills for 84 days (one period every 3 months). | Indicated for females with dysmenorrhea or when fatigue and lightheadedness worsen with menses. |
| Medication | Usual Adolescent Dose | Comments/Indications |
|---|---|---|
| Desmopressin acetate | 0.1 mg at bedtime, increasing to 0.2 mg daily. | Suggested for those with nocturia. Hyponatremia can occur. |
| Sympathetic Tone and Heart Rate Modifiers | ||
| Pyridostigmine bromide | Rapid release: 30 mg daily, increase by 30 mg every 3–7 days to 60 mg BID or TID. Sustained-release: 180 mg daily. | Effective in POTS and neurally mediated hypotension. Also helpful for gastrointestinal motility problems. |
| Clonidine | 0.05 mg at bedtime. Increase after 1 week to 0.1 mg nightly. Occasionally higher doses are tolerated. | Suggested for those with anxiety, problems with attention, hyperhidrosis, or insomnia. |
| Ivabradine | Start with 2.5 mg BID. Max 10 mg BID. | Suggested for those with elevated baseline heart rate. |
| Beta adrenergic antagonists | ||
| Atenolol | 12.5–25 mg daily, increase by 12.5 mg increments until optimal effect. Usual dose is 25–50 mg. Doses above 1 mg/kg often aggravate fatigue and lightheadedness, so lower doses are usually better tolerated. | Suggested as first-line therapy for those with a relatively elevated resting heart rate, anxiety, or headache. Can exacerbate asthma. Contraindicated for diabetics. |
| Propranolol | 0.5–1 mg/kg body weight 10–20 mg 3–4 times daily. | |
| SSRI/SNRI | ||
| Sertraline | 25–100 mg daily. | Indicated for dysthymia, depression, or anxiety. |
| Escitalopram | 5 mg daily for 2–4 weeks, increase to 10 mg daily up to max of 40 mg daily. | |
| Duloxetine | 20–30 mg daily for 2 weeks, increase to max of 60–90 mg daily. | Useful if myalgias are prominent. |
NOTE: BID = twice daily; POTS = postural tachycardia syndrome; TID = three times daily.
SOURCE: Modified from Rowe et al., 2017. This work is licensed under the Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0.
(Boehm et al., 1997). Women whose orthostatic symptoms are aggravated in the perimenstrual period often fare better when treated with continuously active hormonal regimens that induce one menstrual period every 90 days or more. The mechanism by which hormonal contraceptives improve orthostatic symptoms is not entirely clear.
Even in nondepressed patients with NMH refractory to other therapies, selective serotonin reuptake inhibitors (SSRIs) can lead to improvements in orthostatic symptoms (Grubb et al., 1994). Duloxetine (a serotonin and norepinephrine reuptake inhibitor [SNRI]) has been shown in randomized trials to be effective in fibromyalgia (Arnold et al., 2004) and has the potential to be effective for pain, independent of its effect on mood. The SSRI and SNRI medications might also be attractive initial options when symptoms such as anxiety, pain, dysthymia, or premenstrual syndrome are present.
Concurrent use of medications with different mechanisms of action (e.g., a mineralocorticoid, a beta blocker, and a vasoconstrictor) is sometimes necessary in managing severe orthostatic intolerance. Among patients whose orthostatic symptoms are relatively refractory to treatment, it is important to examine whether a comorbid condition is contributing to difficulty in obtaining relief. One example would be instability of the cervical spine, which can be associated with frequent syncope and presyncope that improves after surgical stabilization (Henderson et al., 2021), although the prevalence of this abnormality and the optimal methods of selecting patients for surgery need to be studied.
Intravenous Saline
In some individuals with orthostatic intolerance for whom medications have provided insufficient relief, periodic intravenous infusions of normal saline have helped (Burklow et al., 1999; Moak et al., 2016; Ruzieh et al., 2017). IV saline infusions can be used as “rescue therapy” when orthostatic symptoms become more intense (such as after an infection) or to allow tolerance of an important personal or family event. Because most adolescents and adults with orthostatic intolerance have a lower blood volume, our experience has been that they can withstand a rapid infusion of 2 L of normal saline over 1–2 hours. Infusions improve autonomic tone, tolerance of upright tilt (Burklow et al., 1999), and a more rapid restoration of intravascular volume than is possible orally. We recommend peripheral IV catheters where possible, as these can be removed at the end of the infusion. Placement of peripherally inserted central catheters (PICCs) or central lines increases the risk of thrombosis, local infection, or bacteremia (Moak et al., 2016), so placing an indwelling catheter must be undertaken only when more conservative and safer measures have been exhausted, and after demonstration that the IV fluids improve quality of life and function.
Ivabradine
A variety of medications can be used to combat the vasoconstriction defect, improve blood volume, or affect the release or impact of catecholamines. Ivabradine is a newer medication introduced for the treatment of tachycardia in the setting of congestive heart failure (DiFrancesco, 2010). It slows heart rate by selectively blocking specific channels in the sinoatrial node. Selectively blocking these channels can lower heart rate without other important effects on blood pressure and cardiac or autonomic function.
Ivabradine has been used in the treatment of idiopathic sinus tachycardia and postural tachycardia syndrome, as has been demonstrated by several groups. McDonald and colleagues (2011) reported improvement in fatigue and tachycardia in 55 percent of POTS patients treated with ivabradine. Barzilai and Jacob (2015) reported that ivabradine attenuated orthostatic symptoms and heart rate at rest and during tilt table testing. Ivabradine has also been shown to help with sinus tachycardia–related syncope and inappropriate sinus tachycardia (Sutton et al., 2014).
To illustrate the potential use of ivabradine in this context, Table B-3 shows the resting heart rate and the response to exercise in a young adult patient who had mast cell activation, joint hypermobility, and orthostatic intolerance as comorbid features of her ME/CFS. Her resting heart rate was 115 bpm, consistent with inappropriate sinus tachycardia—a form of circulatory dysfunction that overlaps substantially with POTS. When she tried to exercise, as many advise as a primary treatment of orthostatic intolerance, just 2 minutes of activity on an elliptical machine caused an elevation in her heart rate to 180 bpm, associated with provocation of a migraine that lasted 2 days. She was provoking postexertional malaise and harming her
| Dose | Resting HR | Exercise HR |
|---|---|---|
| 0 mg | 115 | 170-180 in 2 min, w/HA |
| 2.5 mg | 110 | 170-180 in 2 min, w/HA |
| 5 mg BID | 90 | 155, no HA |
| 7.5 mg | 80 | 140, no HA |
| 10 mg BID | 72 | 130s with 30-40 minutes on elliptical; no HA |
NOTE: BID = twice daily; HA = headache; HR = heart rate.
overall function by trying to perform too much exercise before her circulatory dysfunction had been treated.
For this patient, increases in ivabradine were associated with gradual lowering of her resting heart rate to 72 bpm. With improvement in circulation, she could exercise for 40 minutes on the elliptical with a rise in heart rate to a more normal 130 bpm, no longer associated with provoking a migraine. She is not cured, but her symptoms are well-managed with ivabradine. Each year, she has a 2-week period during which ivabradine treatment is interrupted by her insurance company’s insistence that she have the drug reauthorized. Even though this individual is quite fit, she experiences an immediate resumption of tachycardia and exercise intolerance when the ivabradine is withheld. Parenthetically, this young woman is an occupational therapist who has worked for the past 2 years on a hospital COVID-19 ward, helping on the proning team. She is an example of a hypermobile patient who has responded to treatment and is not inexorably consigned to disability.
Some have argued that the main treatment of orthostatic intolerance and ME/CFS needs to rely on graded increases in exercise, and this position often leads physicians to insist that the patient complete a course of graded exercise therapy before medications will be prescribed (Fu and Levine, 2018). This patient’s course illustrates the harm that could arise if there is an excessive emphasis of physical exercise alone to treat orthostatic intolerance. In her situation, exercise was possible only after the control of her circulatory dysfunction, not the other way around.
DETERMINANTS OF THE ABILITY TO WORK
In examining determinants of the ability to work in those with orthostatic intolerance, important contributors include the overall severity of an individual’s self-reported symptoms, the degree to which those symptoms interfere with activities, and the degree to which the individual is able to be upright without symptoms. Often, symptoms of orthostatic intolerance and ME/CFS can be unpredictable from day to day, influenced by the level of activity or the degree of orthostatic and other physiologic stressors in the preceding days, as these can provoke postexertional malaise (PEM). PEM involves an increase in a variety of symptoms (not just fatigue) after people have increased their usually tolerated physical, cognitive, or orthostatic stress. PEM symptoms can include lightheadedness, cognitive dysfunction, headache, sensitivity to sensory stimuli, and generalized pain. Another provocation of PEM is excessive neuromuscular strain (often termed “adverse neural tension”) that involves application of an elongation strain to the spinal cord and peripheral nerves, although this is less well-studied than the other stressors (Rowe et al., 2016).
In earlier work among adults, VanNess and colleagues (2010) evaluated 25 females with ME/CFS and 23 sedentary controls after a maximal cardiopulmonary exercise test (duration 5–15 minutes). At 24 hours, 87 percent of healthy controls—but no patients with ME/CFS—reported a full recovery; by 48 hours, the rate of full recovery had reached 100 percent of the controls and only 4 percent of the patients. A full 60 percent of the patients with ME/CFS reported that it took at least 5 days to recover. Common PEM symptoms included fatigue, lightheadedness, pain, and cognitive dysfunction (VanNess et al., 2010).
Objective correlates of these postexertional symptoms have come from a series of studies by Light and colleagues (2012). After patients with ME/CFS and healthy controls completed a 25-minute period of exercise to 70 percent of their maximal predicted heart rate, symptoms were recorded after 8, 24, and 48 hours. Gene expression changes in peripheral blood mononuclear cells were measured at the same time intervals for a series of pain pathway, adrenergic, and immune genes. The healthy controls had minimal symptom and gene-expression changes after exercise. In contrast, patients with ME/CFS had a distinctive increase in gene expression peaking at 24 hours, coinciding with increases in self-reported symptoms. The adrenergic gene expression was eight- to nine-fold higher by 24 hours after exercise. The patterns of gene expression in the adults with ME/CFS were distinct from those of individuals with other fatiguing illnesses, including multiple sclerosis.
Neuropsychological testing after exercise has also shown a significant increase in symptoms for patients with ME/CFS compared with healthy controls. Healthy controls experienced a pre- to postexercise improvement in cognitive performance, contrasting with worsening performance for patients with ME/CFS (LaManca et al., 1998). Functional magnetic resonance imaging (fMRI) has confirmed greater increase in brain activity during a challenging working memory task from pre- to postexercise in several regions for those with ME/CFS compared with controls (Cook et al., 2007).
These postexercise studies are relevant to the understanding of variability in the ability to work among those with EDS and orthostatic intolerance. They provide objective evidence of changes that correlate with self-reported exacerbations in symptoms, helping to explain why individuals might be able to perform a given activity on one day, only to be impaired for days afterwards.
Patients with joint hypermobility and EDS can have other associated comorbid conditions that also contribute to orthostatic intolerance and reduced cerebral blood flow, including pelvic venous insufficiency (pelvic congestion syndrome, ovarian varices, May-Thurner anomaly, and others) (Knuttinen et al., 2021; Sandman et al., 2021), mast cell activation syndrome (Seneviratne et al., 2017), Chiari malformation and various forms
of ligamentous instability at the skull base (including atlantoaxial and craniocervical instability) (Henderson et al., 2021; Milhorat et al., 2007), cervical spinal stenosis (Rowe et al., 2018), and obstruction to cerebral venous drainage (Arun et al., 2022), among other conditions. Variability in the symptoms related to these problems contributes to the variability of overall function and ability to work.
MEASURING WORK- AND SCHOOL-RELATED FUNCTION IN THOSE WITH ORTHOSTATIC INTOLERANCE
Overall function in chronic illness is most readily measured by self-reported, health-related quality-of-life questionnaires, such as the SF-36 (36-item Short Form Survey), EuroQoL (European Quality of Life), or PROMIS (Patient-Reported Outcomes Measurement Information System) measures in adults (Cook et al., 2012; EuroQol, 1990; Ware and Sherbourne, 1992). For pediatric patients, the Functional Disability Inventory or Pediatric Quality of Life (PedsQL) instruments are age specific, reach young-adult age ranges, and are capable of distinguishing healthy from chronically ill individuals (Claar and Walker, 2006; Varni et al., 2001; Walker and Greene, 1991). Brief self-reported measures of general or cognitive fatigue include the PedsQL Mutidimensional Fatigue Inventory (MFI), the Wood Mental Fatigue Inventory, and the Fatigue Severity Scale, among others (Krupp et al., 1989; Varni and Limbers, 2008; Wood et al., 1991).
Few studies have compared objective with self-reported measures of physical function. Van Campen and colleagues (2020d) examined the correlation of the physical functioning scale (PFS) of the SF-36, the percent peak VO2 (volume oxygen) of a cardiopulmonary stress test, and the number of steps per day using an actometer in 99 female ME/CFS patients in whom the three different measures were completed within 3 months (van Campen et al., 2020d). These measures were significantly and positively correlated (PFS vs. % peak VO2, PFS vs. steps/day, and steps/day vs. % peak VO2: all P < .001). Despite the close correlation, van Campen and colleagues (2020d) emphasize the limitations of relying completely on these measures given a large variation within individual patients on the three measures. For example, when examining individual variability among those who scored at the 30 percent level of the PFS, individual patients had taken between 1,558 and 4,266 steps per day. The number of steps per day varied between 6,277 and 9,641 for those at a PFS of 60 percent, reflecting substantially different levels of function (van Campen and colleagues, 2020d). The same held true in this study for the peak VO2 on a cardiopulmonary exercise test versus the actigraphy data. The number of steps per day for individual patients ranged between 1,135 and 4,683 at a peak VO2 between 50 and 60 percent of normal. The researchers concluded that while the SF-36 PFS can
distinguish between diseased and nondiseased individuals, it is less useful to define the disability level for individual patients, especially in light of the variation of the number of steps per day for a certain value of the PFS (van Campen et al., 2020d). This is likely to be the case among those with EDS, as function can be affected not only by orthostatic intolerance and fatigue, but also by joint instability and pain.
On neurocognitive tests, while those with ME/CFS usually demonstrate scores at baseline similar to those of healthy controls (Lange et al., 2005), fMRI studies show that they activate more regions of the brain to obtain the same scores (Cockshell and Mathias, 2010). Abnormalities might appear if the testing is repeated after exercise (Cook et al., 2007) or after the addition of orthostatic stress. For example, among pediatric patients with POTS and ME/CFS, the speed and accuracy of responses is similar when assessed in a supine position, but patients with ME/CFS begin to make more errors and have slower response time as the degree of orthostatic stress increases (Ocon et al., 2012). Similarly, after a period of orthostatic stress, adults with ME/CFS can have impairment of cognitive function. When tested 5 minutes after a period of 30 minutes of head-up tilt, compared with pretilt responses, the proportion of correct responses on an N-back test (which measures attention and working memory) drops from 57 to 41 percent (P < .0001). The raw reaction time on the test increased from 950 to 1102 msec (P < .0001) (van Campen et al., 2020e). One-week after the tilt test, cognitive scores in adults with ME/CFS had not returned to normal (van Campen et al., 2021b).
These studies illustrate the challenges of objective confirmation of physical and cognitive function in those with ME/CFS. Test responses are likely to vary depending on the activity level on a given day and the extent to which PEM is provoked by orthostatic stress on the days before testing. Given the overlaps in physiology and symptoms, these challenges also are likely to apply to those with heritable disorders of connective tissue. As a corollary to the observation that orthostatic intolerance can be present and correlate with self-reported symptoms, even in the absence of objective heart rate and blood pressure responses to upright tilt, caution is needed in relying exclusively on neuropsychological tests, and self-reported symptoms cannot be ignored.
MITIGATING THE IMPACT OF ORTHOSTATIC INTOLERANCE: MODIFICATIONS AND ACCOMMODATIONS AT WORK AND IN SCHOLASTIC SETTINGS
Accommodations are unlikely to allow those with severe orthostatic intolerance to be able to work or attend school. For example, in a study by Henderson and colleagues (2021) on atlantoaxial instability in 20
individuals with EDS or hypermobility spectrum disorder, 9 had multiple episodes of syncope and presyncope weekly, which would create a risk of injury and be incompatible with steady classroom attendance or employment. Surgical stabilization, however, significantly decreased the frequency of syncope and presyncope. If individuals have orthostatic intolerance and are at risk for syncope, it would be unsafe for them to climb, bend, or stand for long periods of time. A cashier or store clerk, for example, would need to perform activities in a seated position.
Similarly, in those satisfying the definition of severe ME/CFS, a 20 degree upright tilt angle lowered cerebral blood flow by 27 percent, almost a four-fold greater reduction than is seen in healthy individuals (van Campen et al., 2020c). It is reasonable to assume that in such individuals, upright sitting at a desk would provoke clinically significant cognitive dysfunction and other symptoms, making work and study impossible.
Because orthostatic symptoms are worse in the morning when blood volume is at its lowest, those with mild to moderate orthostatic intolerance might benefit from a later start to their day. Individuals with moderate orthostatic intolerance, fatigue, and PEM might benefit from a reduction in their workload. For example, some students can attend school on Mondays and Tuesdays, but adding a third consecutive day provokes enough PEM that they miss the next two school days. They often benefit from a planned day off midweek, which allows enough rest and recovery to permit attendance on Thursdays and Fridays, increasing their attendance from 3 to 4 days weekly.
In those with milder orthostatic intolerance, the ability to perform tasks in an upright position can be improved by frequent breaks to provide an ability to get up and move around, which in turn uses the muscles of the limbs to pump pooled blood back to the heart and brain. A variety of postural countermaneuvers when standing or sitting also improves venous return and blood pressure, including standing with the legs crossed, walking rather than standing still, sitting with the knees up to the chest, bending forward, or sitting in lower chairs to allow the knees to be higher than the hips. Hot environments are detrimental to those with orthostatic intolerance, as heat will shift blood from the central circulation to the skin. Patients with heat intolerance usually need to be in an air-conditioned environment.
Because PEM and postinfectious exacerbations in symptoms can be unpredictable, those with orthostatic intolerance need flexible hours and attendance. Because increased physical activity can provoke further PEM, lifting objects too frequently might aggravate fatigue and PEM, especially as the weight of the items increases. These individuals often need elevator access, and some benefit from a place to lie down at work.
CONCLUDING COMMENTS
Orthostatic intolerance is common in individuals with EDS and hypermobility spectrum disorders and can be associated with heterogeneous blood pressure and heart rate responses to orthostatic stress, including POTS, orthostatic hypotension, and NMH. Importantly, as measured by Doppler techniques, orthostatic reductions in cerebral blood flow can be present even in those with a normal heart rate and blood pressure response to upright posture. Prominent orthostatic intolerance symptoms include lightheadedness or syncope, fatigue, exercise intolerance, cognitive dysfunction, and headaches. Management of orthostatic intolerance requires close attention to the factors that provoke symptoms, a willingness to try several medications before achieving a good fit, and a realization that medications often can help manage symptoms but do not necessarily cure orthostatic intolerance. The response to therapy remains less predictable than is desired. Complicating the management of orthostatic intolerance in EDS is the presence of multiple comorbidities. Measuring the ability to work in those with orthostatic intolerance is challenging, given the potential for prolonged upright posture to be followed by unpredictable postexertional increases in symptoms for days afterwards. Orthostatic intolerance can be a substantial contributor to disability, and those with orthostatic intolerance can have comorbid ME/CFS that further limits function. Practitioners’ ability to measure the severity of impairments in daily function is limited. Assessment of disability in those with orthostatic intolerance and comorbid hypermobility spectrum disorders cannot ignore self-reported severity of symptoms.
ACKNOWLEDGMENTS
Some of the text for this manuscript was modified using the Creative Commons practices from the following open access publication:
Rowe, P. C., R. A. Underhill, K. J. Friedman, A. Gurwitt, M. S. Medo, M. S. Schwartz, N. Speight, J. Stewart, R. Vallings, and K. Rowe. 2017. Myalgic encephalomyelitis/chronic fatigue syndrome diagnosis and management in young people: A primer. Frontiers in Pediatrics 5:121. https://doi.org/10.3389/fped.2017.00121.
The figures and tables were either created by the author (Figure B-1, Box B-1, and Tables B-1 and B-3) or taken from the open access publication above (Table B-2) or from open access publications by the author and his colleagues Linda van Campen and Frans Visser (Figures B-2 and B-3).
REFERENCES
Arnold, L. M., Y. Lu, L. J. Crofford, M. Wohlreich, M. J. Detke, S. Iyengar, and D. J. Goldstein for the Duloxetine Fibromyalgia Trial Group. 2004. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis & Rheumatism 50(9):2974-2984. https://doi.org/10.1002/art.20485.
Arun, A., M. Amans, N. Higgins, W. Brinjikji, M. Sattur, S. Satti, P. Nakaji, M. Luciano, T. Huisman, A. Moghekar, V. Pereira, R. Meng, K. Fargen, and F. Hui. 2022. A proposed framework for cerebral venous congestion. Neuroradiology Journal 35(1):94-111. https://doi.org/10.1177/19714009211029261.
Barron, D. F., B. A. Cohen, M. T. Geraghty, R. Violand, and P. C. Rowe. 2002. Join hypermobility is more common in children with chronic fatigue syndrome than in healthy controls. Journal of Pediatrics 141:421-425. https://doi.org/10.1067/mpd.2002.127496.
Barzilai, M., and G. Jacob. 2015. The effect of ivabradine on the heart rate and sympathovagal balance in postural tachycardia syndrome patients. Rambam Maimonides Medical Journal 6:e0028. https://doi.org/10.5041/RMMJ.10213.
Benditt, D., D. Ferguson, B. Grubb, W. Kapoor, J. Kugler, B. Lerman, J. Maloney, A. Ravielle, B. Ross, R. Sutton, M. Wolk, and D. Wood. 1996. Journal of the American College of Cardiology 28(1):263-275. https://doi.org/10.1016/0735-1097(96)00236-7.
Benditt, D., C. Ermis, B. Padanilam, N. Samniah, and S. Sakagushi. 2003. Catecholamine response during haemodynamically stable upright posture in individuals with and without tilt-table induced vasovagal syncope. Europace 5(1):65-70. https://doi.org/10.1053/eupc.2002.0271.
Boehme, K., K. Kip, B. Grubb, and D. Kosinski. 1997. Neurocardiogenic syncope: Response to hormonal therapy. Pediatrics 99(44):623-625. https://doi.org/10.1542/peds.99.4.623.
Bou-Holaigah, I., P. Rowe, J. Kan, and H. Calkins. 1995. The relationship between neutrally mediated hypotension and the chronic fatigue syndrome. Journal of the American Medical Association 274(12):961-967. https//doi.org/10.1001/jama.1995.03530120053041.
Bourne, K. M., R. S. Sheldon, J. Hall, M. Lloyd, K. Kogut, N. Sheikh, J. Jorge, J. Ng, D. V. Exner, J. V. Tyberg, and S. R. Raj. 2021. Compression garment reduces orthostatic tachycardia and symptoms in patients with postural orthostatic tachycardia syndrome. Journal of the American College of Cardiology 77(3):285-296. https://doi.org/10.1016/j.jacc.2020.11.040.
Burklow, T. R., J. P. Moak, J. J. Bailey, and F. T. Makhlouf. 1999. Cardiac syncope: Autonomic modulation after normal saline infusion. Journal of the American College of Cardiology 33(7):2059-2066 https://doi.org/10.1016/S0735-1097(99)00133-3.
Claar, R. L., and L. S. Walker. 2006. Functional properties of pediatric pain patients: Psychometric properties of the Functional Disability Inventory. Pain 121(1-2):77-84 https://doi.org/10.1016/j.pain.2005.12.002.
Cockshell, S. J., and J. L. Mathias. 2010. Cognitive functioning in chronic fatigue syndrome: A meta-analysis. Psychological Medicine 40(8):1253-1567. https://doi.org/10.1017/S0033291709992054.
Cook, D. B., P. J. O’Connor, G. Lange, and J. Steffener. 2007. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. Neuroimage 36(1):108-122. https://doi.org/10.1016/j.neuroimage.2007.02.033.
Cook, K. F., A. M. Bamer, D. Amtmann, I. R. Molton, and M. P. Jensen. 2012. Six patient-reported outcome measurement information system short form measures have negligible age or diagnosis-related differential item functioning in individuals with disabilities. Archives of Physical Medicine and Rehabilitation 93(7):1289-1291 https://doi.org/10.1016/j.apmr.2011.11.022.
De Wandele, I., P. Calders, W. Peersman, S. Rimbaut, T. DeBaker, F. Malfait, A. De Paepe, and L. Rombaut. 2014a. Autonomic symptom burden in the hypermobility type of Ehlers-Danlos syndrome: A comparative study with two other EDS types, fibromyalgia, and healthy controls. Seminars in Arthritis and Rheumatism 44(3):353-361. https:///doi.org/10.1016/j.semarthrit.2014.05.013.
De Wandele, I., L. Rombaut, L. Leybaert, P. Van de Borne, T. De Backer, F. Malfait, A. De Paepe, and P. Calders. 2014b. Dysautonomia and its underlying mechanisms in the hypermobility type of Ehlers-Danlos syndrome. Seminars in Arthritis and Rheumatism 44(1):93-100. https://doi.org/10.1016/j.semarthrit.2013.12.006.
DiFrancesco, D. 2010. The role of the funny current in pacemaker activity. Circulation Research 106(3):434-446. https://doi.org/10.1161/CIRCRESAHA.109.208041.
EuroQol Group. 1990. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 16(3):199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
Fedorowski, A. 2019. Postural orthostatic tachycardia syndrome: Clinical presentation, aetiology and management. Journal of Internal Medicine 285(4):352-366. https://doi.org/10.1111/joim.12852.
Fortney, S., V. Schneider, and J. Greenleaf. 1996. The physiology of bed rest. In Handbook of physiology, vol. 2, edited by M. Fregley, and C. Blatters. New York: Oxford University Press. Pp. 889-939.
Freeman, R., W. Wieling, F. B. Axelrod, D. G. Benditt, E. Benarroch, I., Biaggioni, W. Cheshire, T. Chelimsky, P. Cortelli, C. Gibbons, D. Goldstein, R. Hainsworth, M. Hilz, G. Jacob, H. Kaufmann, J. Jordan, L. Lipsitz, B. Levin, P. Low, C. Mathias, S. Raj, D. Robertson, P. Sandroni, I. Schatz, R. Schondorff, J. Stewart, and J. Gert van Dijk. 2011. Consensus statement on the definition of orthostatic hypotension, neutrally mediated syncope and the postural tachycardia syndrome. Clinical Autonomic Research Society 21(2):69-72. https://doi.org/10.1007/s10286-011-0119-5.
Freeman, R., A. R. Abuzinadah, C. Gibbons, P. Jones, M. G. Miglis, D. I. Sinn. 2018. Orthostatic hypotension: JACC state-of-the-art review. Journal of the American College of Cardiology 72(11):1294-1309. https://doi.org/10.1016/j.jacc.2018.05.079.
Fu, W., and B. Levine. 2018. Exercise and non-pharmacological treatment of POTS. Autonomic Neuroscience 215:20-27. https://doi.org/10.1016/j.autneu.2018.07.001.
Gerrity T. R., J. Bates, D. S. Bell, G. Chrousos, G. Furst, T. Hedrick, B. Hurwitz, R. W. Kula, S. M. Levine, R. C. Moore, and R. Schondorf. 2002–2003. Chronic fatigue syndrome: What role does the autonomic nervous system play in the pathophysiology of this complex illness? Neuroimmunomodulation 10(3):134-141. https://doi.org/10.1159/000067176.
Goldstein, D., C. Holmes, S. Frank, R. Dendi, R. Cannon, Y. Sharabi, M. Esler, and G. Eisenhofer. 2002. Cardiac sympathetic dysautonomia in chronic orthostatic intolerance syndromes. Circulation 106(18):2358-2365. https://doi.org/10.1161/01.CIR.0000036015.54619.B6.
Goldstein, D., C. Holmes, S. Frank, M. Naqibuddin, R. Dendi, S. Snader, and H. Calkins. 2003. Sympathoadrenal imabalance before neurocardiogenic syncope. American Journal of Cardiology 91(1):53-58. https://doi.org/10.1016/s0002-9149(02)02997-1.
Goldstein, D. S., B. Eldadah, C. Holmes, S. Pechnik, J. Moak, and Y. Sharabi. 2005. Neurocirculatory abnormalities in chronic orthostatic intolerance. Circulation 111(7):839-845. https://doi.org/10.1161/01.CIR.0000155613.20376.CA.
Grubb, B. P. 2005. Neurocardiogenic syncope. New England Journal of Medicine 352(10):1004-1010. https://doi.org/10.1056/nejmcp042601.
Grubb, B. P. 2008. Postural tachycardia syndrome. Circulation 117(21):2814-2817. https://doi.org/10.1161/circulationaha.107.761643.
Grubb, B. P., D. Samoil, D. Kosinski, K. Kip, and P. Brewster. 1994. Use of sertraline chloride in the treatment of refractory neurocardiogenic syncope in children and adolescents. Journal of the American College of Cardiology 24(2):480-484. https://doi.org/10.1016/0735-1097(94)90308-5.
Henderson, F. C., P. C. Rowe, M. Narayanan, R. Rosenbaum, M. Koby, K. Tuchmann, and C. A. Francomano. 2021. Refractory syncope and pre-syncope associated with atlanto-axial instability: Preliminary evidence of improvement following surgical stabilization. World Neurosurgery. 149:e854–e865. https://doi.org/10.1016/j.wneu.2021.01.084.
Heyer, G. L. 2014. Abdominal and lower-extremity compression decreases symptoms of postural tachycardia syndrome in youth during tilt table testing. Journal of Pediatrics 165(2):395-397. https://doi.org/10.1016/j.jpeds.2014.04.014.
Hurwitz, B. E., V. T. Coryell, M. Parker, P. Martin, A. LaPerriere, N. G. Klimas, G. N. Sfakianakis, and M. S. Bilsker. 2010. Chronic fatigue syndrome: Illness severity, sedentary lifestyle, blood volume and evidence of diminished cardiac function. Clinical Science (London) 118(2):125-135. https://doi.org/10.1042/CS20090055.
Hyatt, K. H., L. B. Jacobson, and V. S. Schneider. 1975. Comparison of 70 degrees tilt, LBNP, and passive standing as measures of orthostatic tolerance. Aviation, Space, and Environmental Medicine 46(6):801-808.
IOM (Institute of Medicine). 2015. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: Redefining an illness. Washington, DC: The National Academies Press.
Jardine, D. L., I. C. Melton, I. G. Crozier, S. I. Bennett, R. A. Donald, and H. Ikram. 1997. Neurohormonal response to head-up tilt and its role in vasovagal syncope. American Journal of Cardiology 79(9):1302-1306. https://doi.org/10.1016/S0002-9149(9X)00084-9.
Jhanjee, R. I., Can, and D. Benditt. 2009. Syncope. Disease-a-Month Series 55(9):532-585. https://doi.org/j.disamonth.2009.04.004.
Johnson, J. N., K. J. Mack, N. L. Kuntz, C. K. Brands, C. J. Porter, and P. R. Fischer. 2010. Postural orthostatic tachycardia syndrome—A clinical review. Pediatric Neurology 42(2):77-85.
Kanjwal, K., M. Sheikh, B. Karabin, Y. Kanjwal, and B. Grubb. 2011. Neurocardiogenic syncope coexisting with postural orthostatic tachycardia syndrome in patients suffering from orthostatic intolerance: A combined form of autonomic dysfunction. Pacing and Clinical Electrophysiology 34(5):549-554. https://doi.org/10.1111/j.1540-8159.2010.02994.x.
Kenney, R. A., and L. A. Graham. 2001. Chronic fatigue syndrome symptoms common in patients with vasovagal syncope. American Journal of Medicine 110(3):242-243. https://doi.org/10.1016/S0002-9343(00)00704-X.
Knuttinen M-G., K. S. Zurcher, N. Khurana, I. Patel, A. Foxx-Orenstein, L. A. Harris, A. Lawrence, F. Aguilar, M. Sichlau, B. H. Smith, and S. J. Smith. 2021. Imaging findings of pelvic venous insufficiency in patients with postural orthostatic tachycardia syndrome. Phlebology 36(1):32-37. https://doi.org/10.1177/0268355520947610
Krupp, L. B., N. G. LaRocca, J. Muir-Nash, and A. D. Steinberg. 1989. The Fatigue Severity Scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology 46(10):1121-1123. https://doi.org/10.1001/archneur.1989.00520460115022.
LaManca, J., S. Sisto, J. DeLuca, S. Johnson, G. Lange, J. Pareja, S. Cook, and B. Natelson. 1998. Influence of exhaustive treadmill exercise on cognitive functioning in chronic fatigue syndrome. American Journal of Medicine 105(3A):59S-65S. https://doi.org/10.1016/s0002-9343(98)00171-5.
Lange, G., J. Steffener, D. B. Cook, B. M. Bly, C. Christodoulou, W-C. Liu, J. Deluca, and B. H. Natelson. 2005. Objective evidence of cognitive complaints in chronic fatigue syndrome: A BOLD fMRI study of verbal working memory. Neuroimage 26(2):513-524. https://doi.org/10.1016/j.neuroimage.2005.02.011.
Legge, H., M. Norton, and J. L. Newton. 2008. Fatigue is significant in vasovagal syncope and is associated with autonomic symptoms. Eurospace 10(9):1095-1101. https://doi.org/10.1093/europace/eun164.
Lewis, T. 1932. A lecture on vasovagal syncope and the carotid sinus mechanism. British Medical Journal 1(3723):873-876. https://doi.org/10.1136/bmj.1.3723.873.
Light, A. R., L. Bateman, D. Jo, R. W. Hughen, T. A. VanHaitsma, A. T. White, and K. C. Light. 2012. Gene expression alterations at baseline and following moderate exercise in patients with chronic fatigue syndrome and fibromyalgia syndrome. Journal of Internal Medicine 271(1):64-81. https://doi.org/10.1111/j.1365-2796.2011.02405.x.
Low, P. A., P. Sandroni, M. Joyner, and W. K. Shen. 2009. Postural tachycardia syndrome (POTS). Journal of Cardiovascular Electrophysiology 20(3):352-358. https://doi.org/10.1111/j.1540-8167.2009.01407.x.
MacLean, A. R., and E. V. Allen. 1940. Orthostatic hypotension and orthostatic tachycardia: Treatment with the “head-up” bed. Journal of the American Medical Association 115(25):2162-2167. https://doi.org/10.1001/jama.1940.02810510038010.
Martinez-Lavin, M. 2006. Biology and therapy of fibromyalgia: Stress, the stress response system, and fibromyalgia. Arthritis Research and Therapy 9(4):216. https://doi.org/10.1186/ar2146.
McDonald, C., J. Frith, and J. L. Newton. 2011. Single centre experience of ivabradine in postural orthostatic tachycardia syndrome. Europace 13(3):427-430. https://doi.org/10.1093/europace/euq390.
Medow, M. S., J. M. Stewart, S. Sanyal, A. Mumtaz, D. Sica, and W. H. Frishman. 2008. Pathophysiology, diagnosis, and treatment of orthostatic hypotension and vasovagal syncope. Cardiology in Review 16(1):4-20. https://doi.org/10.1097/CRD.0b013e31815c8032.
Milhorat, T. H., P. A. Bolognese, M. Nishikawa, N. B. McDonnell, and C. A. Francomano. 2007. Syndrome of occipitoatlantoaxial hypermobility, cranial settling, and Chiari malformation Type I in patients with hereditary disorders of connective tissue. Journal of Neurosurgery: Spine 7(6):601-609. https://doi.org/10.3171/SPI-07/12/601.
Moak, J. P., D. Leong, R. Fabian, V. Freedenberg, E. Jarosz, C. Toney, S. Hanumanthaiah, and A. Darbari. 2016. Intravenous hydration for management of medication-resistant orthostatic intolerance in the adolescent and young adult. Pediatric Cardiology 37(2):278-282. https://doi.org/10.1007/s00246-015-1274-6.
Moya, A., R. Sutton, F. Ammirati, J. Blanc, M. Brignole, J. Dahm, J-C. Deharo, J. Gajek, K. Gjesdal, A. Krahm, M. Massin, M. Pepi, T. Pezawas, R. Ruiz Grannell, F. Sarasin, A. Ungar, J. Gert van Dijk, E. Walma, and W. Wieling. 2009. Guidelines for the diagnosis and management of syncope. European Heart Journal 30(21):2631-2671. https://doi.org/10.1093/eurheartj/ehp298.
Naschitz, J., I. Rosner, M. Rozebaum, L. Gaitini, I. Bistritzki, E. Zuckerman, E. Sabo, and D. Yeshurun. 2000. The capnography head-up tilt test for evaluation of chronic fatigue syndrome. Seminars in Arthritis and Rheumatism 30(2):79-86. https://doi.org/10.1053/sarh.2000.9201.
Natale, A., M. Akhtar, M. Jazayeri, A. Dhala, Z. Blanck, S. Deshpande, A. Krebs, and J. Sra. 1995. Provocation of hypotension during head-up tilt testing in subjects with no history of syncope or presyncope. Circulation 92(1):54-58. https://doi.org/10.1161/01.CIR.92.1.54.
Natelson, B., R. Intriligator, N. Cherniack, H. Chandler, and J. Stewart. 2007. Hypocapnia is a biological marker for orthostatic intolerance in some patients with chronic fatigue syndrome. Dynamic Medicine 6, Article 2. https://doi.org/10.1186/1476-5918-6-2.
Novak, P. 2018. Hypocapnic cerebral hypoperfusion: A biomarker of orthostatic intolerance. PLoS ONE 13(9):e0204419. https://doi.org/10.1371/journal.pone.0204419.
Ocon, A. J., Z. R. Messer, M. S. Medow, and J. M. Stewart. 2012. Increasing orthostatic stress impairs neurocognitive functioning in chronic fatigue syndrome with postural tachycardia syndrome. Clinical Science (London, England: 1979) 122(5):227-228. https://doi.org/10.1042/CS20110241.
Okamoto, L. E., S. R. Raj, A. Peltier, A. Gamboa, C. Shibao, A. Diedrich, B. K. Black, D. Robertson, and I. Biaggioni. 2012. Neurohumoral and haemodynamic profile in postural tachycardia and chronic fatigue syndromes. Clinical Science (London, England: 1979) 122(4):183-192. https://doi.org/10.1042/CS20110200.
Plash, W. B., A. Diedrich, I. Biaggioni, E. M. Garland, S. Y. Paranjape, B. D. Black, W. Dupont, and S. R. Raj. 2013. Diagnosing postural tachycardia syndrome: Comparison of tilt testing compared with standing haemodynamics. Clinical Science (London, England: 1979) 124(2):109-114. https://doi.org/10.1042/CS20120276.
Razumovsky, A., K. DeBusk, H. Calkins, S. Snader, K. Lucas, P. Vyas, D. Hanley, and P. C. Rowe. 2003. Cerebral and systemic hemodynamics changes during upright tilt in chronic fatigue syndrome. Journal of Neuroimaging 13(1):57-67. https://doi.org/10.1111/j.1552-6569.2003.tb00158.x.
Roma, M., C. Marden, and P. C. Rowe. 2018a. Passive standing tests for the office diagnosis of postural tachycardia syndrome: New methodological considerations. Fatigue: Biomedicine, Health and Behavior 6(4):179-192. https://doi.org/10.1080/21641846.2018.1512836.
Roma, M., C. L. Marden L., I. De Wandele, C. A. Francomano, and P. C. Rowe. 2018b. Postural tachycardia syndrome and other forms of orthostatic intolerance in Ehlers-Danlos syndrome. Autonomic Neuroscience 215(December):89-96. https://doi.org/10.1016/j.autneu.2018.02.006.
Roma, M., C. L. Marden, M. Flaherty, S. E. Jasion, E. M. Cranston, and P. C. Rowe. 2019. Impaired health-related quality of life in adolescent myalgic encephalomyelitis/chronic fatigue syndrome: The impact of core symptoms. Frontiers in Pediatrics 7(February):26. https://doi.org/10.3389/fped.2019.00026.
Rombaut, L., F. Malfait, A. Cools, A. De Paepe, and P. Calders. 2010. Musculoskeletal complaints, physical activity and health-related quality of life among patients with Ehlers-Danlos syndrome hypermobility type. Disability and Rehabilitation 32(16):1339-1345. https://doi.org/10.3109/09638280903514739.
Rose, M. S., M. L. Koshman, S. Spreng, and R. Sheldon. 2000. The relationship between health-related quality of life and frequency of spells in patients with syncope. Journal of Clinical Epidemiology 53(12):1209-1216. https://doi.org/10.1016/S0895-4356(00)00257-2.
Rosen, S. G., and P. E. Creyer. 1982. Postural tachycardia syndrome. Reversal of sympathetic hyperresponsiveness and clinical improvement during sodium loading. American Journal of Medicine 72(5):847-850. https://doi.org/10.1016/0002-9343(82)90559-9.
Rowe, P. C. 2016. Fatigue and the chronic fatigue syndrome. In Neinstein’s adolescent and young adult health care, 6th ed., edited by L. Neinstein. Philadelphia, PA: Wolters Kluwer.
Rowe, P. C., I. Bou-Holaigah, J. S. Kan, and H. Calkins. 1995. Is neutrally mediated hypotension an unrecognized cause of chronic fatigue? Lancet 346(8950):623-624. https://doi.org/10.1016/s0140-6736(95)90525-1.
Rowe, P. C., D. F. Barron, H. Calkins, I. H. Maumenee, P. Y. Tong, and M. T. Geraghty. 1999. Orthostatic intolerance and chronic fatigue syndrome associated with Ehlers-Danlos syndrome. Journal of Pediatrics 135(4):494-499. https://doi.org/10.1016/S00223476(99)70173-3.
Rowe, P. C., H. Calkins, K. DeBusk, R. McKenzie, R. Anand, G. Sharma, B. Cuccherini, N. Soto, P. Hohman, S. Snader, K. Lucas, M. Wolff, and S. Straus. 2001. Journal of the American Medical Association 285(1):52-59. https://doi.org/10.1001/jama.285.1.52.
Rowe, P. C., K. R. Fontaine, M. Lauver, S. E. Jasion, C. L. Marden, M. Moni, C. B. Thompson, and R. L. Violand. 2016. Neuromuscular strain increases symptom intensity in chronic fatigue syndrome. PLOS One 11(7):e0159386. https://doi.org/10.1371/journal.pone.0159386.
Rowe, P. C., R. A. Underhill, K. J. Friedman, A. Gurwitt, M. S. Medow, M. S. Schwartz, N. Speight, J. M. Stewart, R. Vallings, and K. S. Rowe. 2017. Myalgic encephalomyelitis/chronic fatigue syndrome diagnosis and management in young people: A primer. Frontiers in Pediatrics 5(June):121. https://doi.org/10.3389/fped.2017.00121.
Rowe, P. C., C. L. Marden, S. Heinlein, and C. C. Edwards, II. 2018. Improvement of severe myalgic encephalomyelitis/chronic fatigue syndrome symptoms following surgical treatment of cervical spinal stenosis. Journal of Translational Medicine 16(1):21. https://doi.org/10.1186/s12967-018-1397-7.
Rowell, L. 1992. Human cardiovascular control. New York: Oxford University Press.
Ruzieh, M., A. Baugh, O. Dasa, R. Parker, J. Perrault, A. Renno, B. Karabin, and B. Grubb. 2017. Effects of intermittent intravenous saline infusions in patients with medication-refractory postural tachycardia syndrome. Journal of Interventional Cardiac Electrophysiology 48(3):255-260. https://doi.org/10.1007/s10840-017-0225-y.
Sandman, W., T. Scholbach, and K. Verginis. 2021. Surgical treatment of abdominal compression syndromes: The significance of hypermobility-related disorders. American Journal of Medical Genetics Part C: Seminars in Medical Genetics 187(4):570-578. https://doi.org/10.1002/ajmg.c.31949.
Sandroni, P., T. L. Opfer-Gehrking, E. E. Benarroch, W-K. Shen, and P. A. Low. 1996. Certain cardiovascular indices predict syncope in the postural tachycardia syndrome. Clinical Autonomic Research 6(4):225-231. https://doi.org/10.1007/BF02291138.
Schondorf, R., and P. Low. 1993. Idiopathic postural orthostatic tachycardia syndrome: An attenuated form of acute pandysautonomia? Neurology 43(1):132-137. https://doi.org/10.1212/wnl.43.1_part_1.132.
Seneviratne, S., A. Maitland, and L. Afrin. 2017. Mast cell disorders in Ehlers–Danlos syndrome. American Journal of Medicine Part C: Seminars in Medical Genetics 175(1):226-236. https://doi.org/10.1002/ajmg.c.31555.
Shaw, B., L. Stiles, K. Bourne, E. Green, C. Shibao, L. Okamoto, E. Garland, A. Gamboa, A. Diedrich, V. Raj, R. Sheldon, I. Biaggioni, D. Robertson, and S. Raj. 2019. The face of postural tachycardia syndrome—Insights from a large cross-sectional online community-based survey. Journal of Internal Medicine 286(4):438-448. https://doi.org/10.1111/joim.12895.
Sheldon, R. 2005. Tilt testing for syncope: A reappraisal. Current Opinion in Cardiology 20(1):38-41.
Sheldon, R. S., B. P. Grubb, B. Olshansky, W. K. Shen, H. Calkins, M. Brignole, S. R. Raj, A. D. Krahm, C. A. Morillo, J. M. Stewart, R. Sutton, P. Sandroni, K. J. Friday, D. Tessariol Hachul, M. I. Cohen, D. H. Lau, K. A. Mayuga, J. P. Moak, R. K. Sandhu, and K. Kanjwal. 2015. Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 12(6):e41-e63. https://doi.org/10.1016/j.hrthm.2015.03.029.
Singer, W., D. M. Sletten, T. L. Opfer-Gehrking, C. K. Brands, P. R. Fischer, and P. A. Low. 2012. Postural tachycardia syndrome in children and adolescents: What is abnormal? Journal of Pediatrics 160(2):222-226. https://doi.org.10.1016/j.jpeds.2011.08.054.
Smit, A. A., M. A. Hardjowijono, and W. Wieling. 1997. Are portable folding chairs useful to combat orthostatic hypotension? Annals of Neurology 42(6):975-978. https://doi.org/10.1002/ana.410420620.
Smit, A. A., J. R. Halliwell, P. A. Low, and W. Wieling. 1999. Pathophysiologic basis of orthostatic hypotension in autonomic failure. Journal of Physiology 519(1):1-10.
Smith, J. J., C. M. Porth, and M. Erickson. 1994. Hemodynamic response to the upright posture. Journal of Clinical Pharmacology 34(5):375-386. https://doi.org/10.1002/j.1552-4604.1994.tb04977.x.
Stewart, J. M., M. H. Gewitz, A. Weldon, and J. Munzo. 1999. Patterns of orthostatic intolerance: The orthostatic tachycardia syndrome and adolescent chronic fatigue. Journal of Pediatrics 135:(2 Pt 1):218-225. https://doi.org/10.1016/S0022-3476(99)70025-9.
Stewart, J. M., J. R. Boris, G. Chelimsky, P. R. Fischer, J. E. Fortunato, B. P. Grubb, G. L. Heyer, I. T. Jarjour, M. S. Medow, M. T. Numan, P. T. Pianosi, W. Singer, S. Tarbell, T. C. Chelimsky, and Pediatric Writing Group of the American Autonomic Society. 2018. Pediatric disorders of orthostatic intolerance. Pediatrics 141(1):e20171673. https://doi.org/10.1542/peds.2017-1673.
Streeten, D. H. P., and D. S. Bell. 1998. Circulating blood volume in chronic fatigue syndrome. Journal of Chronic Fatigue Syndromes 4(1):3-11. https://doi.org/10.1300/J092v04n01_02.
Streeten, D. H., D. Thomas, and D. S. Bell. 2000. The roles of orthostatic hypotension, orthostatic tachycardia, and subnormal erythrocyte volume in the pathogenesis of the chronic fatigue syndrome. American Journal of Medical Science 320(1):1-8. https://doi.org/10.1097/00000441-200007000-0000.
Strickberger, S., D. Benson, I. Biaggioni, D. Callans, M. Cohen, K. Ellenbogen, A. Epstein, P. Friedman, J. Goldberger, P. Heidenreich, G. Klein, B. Knight, C. Morillo, R. Myerburg, and C. Sila. 2006. AHA/ACCF scientific statement on the evaluation of syncope: From the American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke; Quality of Care and Outcomes Research Interdisciplinary Working Group; American College of Cardiology Foundation; and Heart Rhythm Society. Journal of the American College of Cardiology 47(2):473-484. https://doi.org/10.1016/j.jacc.2005.12.019.
Sutton, R., T. V. Salukhe, A-C. Franzen-Mcmanus, A. Collins, P. B. Lim, and D. P. Francis. 2014. Ivabradine in treatment of sinus tachycardia mediated vasovagal syncope. Europace 16(2):284-288. https://doi.org/10.1093/europace/eut226.
Takenaka, K., Y. Suzuki, K. Uno, M. Sato, T. Komuro, Y. Haruna, H. Kobayashi, K. Kawakubo, M. Sonoda, M. Asakawa, K. Nakahara, and A. Gunji. 2002. Effects of rapid saline infusion on orthostatic intolerance and autonomic tone after 20 days bed rest. American Journal of Cardiology 89(5):557-561. https://doi.org/10.1016/s0002-9149(01)02296-2.
van Campen, C., F. Verheugt, and F. C. Visser. 2018. Cerebral blood flow changes during tilt table testing in healthy volunteers, as assessed by Doppler imaging of the carotid and vertebral arteries. Clinical Neurophysiology Practice 3(March):91-95. https://doi.org/10.1016/j.cnp.2018.02.004.
van Campen, C. M. C., F. W. A. Verheugt, P. C. Rowe, and F. C. Visser, 2020a. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: A quantitative, controlled study using Doppler echography. Clinical Neurophysiology Practice 5(February):50-58. https://doi.org/10.1016/j.cnp.2020.01.003.
van Campen, C., P. C. Rowe, and F. C. Visser. 2020b. Reductions in cerebral blood flow can be provoked by sitting in severe myalgic encephalomyelitis/chronic fatigue syndrome patients. Healthcare 8(4):394. https://doi.org/10.3390/healthcare8040394.
van Campen, C., P. C. Rowe, and F. C. Visser, 2020c. Cerebral blood flow is reduced in severe ME/CFS patients during mild orthostatic stress testing: An exploratory study at 20 degrees of head-up tilt testing. Healthcare 8(2):169. https://doi.org/10.3390/healthcare8020169.
van Campen, C., P. C. Rowe, F. Verheugt, and F. C. Visser. 2020d. Physical activity measures in patients with myalgic encephalomyelitis/chronic fatigue syndrome: Correlations between peak oxygen consumption, the physical functioning scale of the SF-36 questionnaire, and the number of steps from an activity meter. Journal of Translational Medicine 18(1):228. https://doi.org/10.1186/s12967-020-02397-7.
van Campen, C., P. C. Rowe, F. Verheugt, and F. C. Visser. 2020e. Cognitive function declines following orthostatic stress in adults with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Frontiers in Neuroscience: Autonomic Neuroscience 14(June):688. https://doi.org/10.3389/fnins.2020.00688.
van Campen, C., P. C. Rowe, and F. C. Visser. 2021a. Myalgic encephalomyelitis/chronic fatigue syndrome patients with joint hypermobility show larger cerebral blood flow reductions during orthostatic stress testing than patients without hypermobility: A case control study. Medical Research Archives 9(6). https://doi.org/10.18103/mra.v9i6.2494.
van Campen, C., P. C. Rowe, F. Verheugt, and F. C. Visser. 2021b. Numeric rating scales show prolonged post-exertional symptoms after orthostatic testing of adults with myalgic encephalomyelitis/chronic fatigue syndrome. Frontiers in Medicine 7(January):602894. https://doi.org/10.3389/fmed.2020.602894.
van Campen, C., P. C. Rowe, and F. C. Visser. 2022. Compression stockings improve cardiac output and cerebral blood flow during tilt testing in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) patients: A randomized crossover trial. Medicina 58(1):51. https://doi.org/10.3390/medicina58010051.
van Dijk, J.G, I. A. van Rossum, and R. D. Thijs. 2021. The pathophysiology of vasovagal syncope: Novel insights. Autonomic Neuroscience: Basic and Clinical 236:1-11.
van Lieshout, J. J., J. W. Wieling, J. M. Karemaker, and D. L. Eckberg. 1991. The vasovagal response. Clinical Science (London, England: 1979) 81(5):575-586. https://doi.org/10.1042/cs0810575.
van Lieshout, J J., A. D. ten Harkel, and W. Wieling. 1992. Physical manoeuvres for combating orthostatic dizziness in autonomic failure. Lancet 339(8798):897-898. https://doi.org/10.1016/0140-6736(92)90932-s..
van Lieshout, J. J., A. D. ten Harkel, and W. Wieling. 2000. Fludrocortisone and sleeping in the head-up position limit the postural decrease in cardiac output in autonomic failure. Clinical Autonomic Research 10(1):35-42. https://doi.org/10.1007/BF02291388.
VanNess, J. M., S. R. Stevens, L. Bateman, T. L. Stiles, and C. R. Snell. 2010. Postexertional malaise in women with chronic fatigue syndrome. Journal of Women’s Health 19(2):239-244. https://doi.org/10.1089/jwh.2009.1507.
Varni, J. W., and C. A. Limbers. 2008. The PedsQL™ Multidimensional Fatigue Scale in young adults: Feasibility, reliability and validity in a University student population. Quality of Life Research 17(1):105-114.
Varni, J. W., M. Seid, and P. S. Kurtin. 2001. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical Care 39(8):800-812. https://doi.org/10.1097/00005650-200108000-00006.
Vernino, S., K. M. Bourne, L. E. Stiles, B. P. Grubb, A. Fedorowski, J. M. Stewart, A. C. Arnold, L. A. Pace, J. Axelsson, J. R. Boris, J. P. Moak, B. P. Goodman, K. R. Chémali, T. H. Chung, D. S. Goldstein, A. Diedrich, M. G. Miglis, M. M. Cortez, A. J. Miller, R. Freeman, I. Biaggioni, P. C. Rowe, R. S. Sheldon, C. A. Shibao, D. M. Systrom, G. A. Cook, T. A. Doherty, H. I. Abdallah, A. Darbari, and S. R. Raj. 2021. Postural orthostatic tachycardia syndrome (POTS): State of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting—Part 1. Autonomic Neuroscience 235(November):102828. https://doi.org/10.1016/j.autneu.2021.102828.
Walker, L. S., and J. W. Greene. 1991. The functional disability inventory: Measuring a neglected dimension of child health status. Journal of Pediatric Psychology 16(1):39-58. https://doi.org/10.1093/jpepsy/16.1.39.
Ware, J. E., Jr., and C. D. Sherbourne. 1992. The MOS 36-item short-form health survey (SF-36) I. Conceptual framework and item selection. Medical Care 30(6):473-483.
Wieling, W., and J. T. Shepherd. 1992. Initial and delayed circulatory responses to orthostatic stress in normal humans and in subjects with orthostatic intolerance. International Angiology 11(1):69-82.
Wieling, W., K. S. Ganzeboom, and J. P. Saul. 2004. Reflex syncope in children and adolescents. Heart 90(9):1094-1100. https://doi.org/10.1136/hrt.2003.022996.
Wood, G. C., R. P. Bentall, M. Göpfert, and R. H. Edwards. 1991. A comparative assessment of patients with chronic fatigue syndrome and muscle disease. Psychological Medicine 21(3):619-628. https://doi.org/10.1017/s003329170002225x.
Wyller, V. B., J. P. Saul, J. P. Amlie, and E. Thaulow. 2007. Sympathetic predominance of cardiovascular regulation during mild orthostatic stress in adolescents with chronic fatigue. Clinical Physiology and Functional Imaging 27(4):231-238. https://doi.org/10.1111/j.1475-097X.2007.00743.x.
Wyller, V. B., J. P. Saul, L. Walloe, and E. Thaulow. 2008. Sympathetic cardiovascular control during orthostatic stress and isometric exercise in adolescent chronic fatigue syndrome. European Journal of Applied Physiology 102(6):623-632. https://doi.org/10.1007/s00421-007-0634-1.
Wyller, V. B., J. A. Evang, K. Godang, K. K. Solhjell, and J. Bollerslev. 2010. Hormonal alterations in adolescent chronic fatigue syndrome. Acta Paediatrica (Oslo, Norway: 1992) 99(5):770-773. https://doi.org/10.1111/j.1651-2227.2010.01701.x.








































