12
Question 9: Insights from Terrestrial Life
What conditions and processes led to the emergence and evolution of life on Earth; what is the range of possible metabolisms in the surface, subsurface, and/or atmosphere; and how can this inform our understanding of the likelihood of life elsewhere?
Astrobiology is a holistic field of research into the origin, evolution, and distribution of life in the universe.1 As such, planetary science and astrobiology encompass a continuous spectrum spanning the multidisciplinarity of life and physical sciences and investigate the codependence and coevolution of life and the environment. Habitability refers to a set of environmental conditions capable of supporting life (see also Question 10, Chapter 13). Dynamic habitability as defined in recommendations from An Astrobiology Strategy for the Search for Life in the Universe recognizes that the combined effects of multiple parameters (e.g., T, P, salinity, pH; Table 12-1) define whether or not life can emerge and persist, and that while one or more parameters may vary outside the canonical limits to life, it is their combined effect that causes an environment to be habitable or not (NASEM 2019a).
There is an extensive literature (and debate) about the major milestones in the history of Earth and the history of life, the nature of the earliest life as discerned from fossils and chemical signatures, and the pathways and timing for the evolution of life and for key metabolisms (Betts et al. 2018; Benner et al. 2020); therefore, the committee does not attempt to summarize them here. Continued research into Earth’s physiochemical properties, geologic history, and the evolution of habitability and life through deep time—through theoretical, field, and experimental investigations—informs and inspires strategies for planetary and astrobiological exploration of the solar system and universe.
Earth represents our sole reference point, to date, for what constitutes an inhabited world (Figure 12-1). How prebiotic pathways coevolved with the environment to give rise to life on this planet remains an active area of inquiry. But research in the field and the laboratory, as well as the analysis of astromaterials—that is extraterrestrial materials collected on Earth (e.g., meteorites and cosmic dust) and from other planetary bodies via sample return missions—continue to shed light on the conditions and processes that can lead from abiotic chemical reactions to biochemistry. It is important to note that our understanding of life on Earth continues to evolve rapidly thanks to
___________________
1 A glossary of acronyms and technical terms can be found in Appendix F.
TABLE 12-1 Extremophiles Nomenclature and Ranges
| Low → Higha | |||||
|---|---|---|---|---|---|
| pH | Hyperacidophile (<pH 3) | Acidophile (<pH 5) | Neutrophile (pH 5–9) | Alkaliphile (>pH 9) | Hyperalkaliphile (>pH 11) |
| Temperature | Psychrophile (<20°C) | Mesophile (20–45°C) | Thermophile (45–80°C) | Hyperthermophile (>80°C) | |
| Salinityb | Non-halophile (<1.2%) | Halotolerant (1.2–2.9%; tolerate ≤14.6%) | Halophile (>8.8%) | Extreme halophile (>14.6%, cannot grow <8.8%) | |
| Pressure | Piezotolerant or barotolerant (0.1–10 MPa) | Piezophile or barophile (10–50 MPa) | Hyperpiezophile or hyperbarophile (>50 MPa) | ||
| Water activity | Xerophile (aw <0.7) | ||||
| Polyextremophile | Tolerance or preference for multiple parameters combined | ||||
a The distinction between an extremotolerant microbe and an extremophile is based on the location of the optimum along the specific parameter range. See main text for discussion.
b Salinity expressed as percent of NaCl (w/v). Specific resistance to more chaotropic salts has been tested for some strains, for instance in the presence of MgCl2.
SOURCE: Merino et al. (2019), https://doi.org/10.3389/fmicb.2019.00780, CC BY 4.0.
paradigm-changing discoveries of life’s ability to survive, and even thrive, in multi-extreme environments. Recent discoveries have demonstrated that life can exist in a myriad of subsurface, icy, and water-limited environments, with a previously unrecognized range of metabolic strategies, and adaptations to both rapidly changing conditions and catastrophic events, as well as to long timescales of quiescence and isolation. This evolving knowledge of Earth’s biosphere, from its origins to its current state, directly informs and influences the search for life elsewhere.
The diversity of terrestrial life—and its plasticity to respond to different levels of nutrient and energy availability, transport, and flux—informs the detectability of biospheres that might exist in habitable environments beyond Earth. Insights gained from the biochemistry, structure, and physiology of terrestrial organisms—and how they have evolved through deep time—are the basis to develop comprehensive frameworks to search for evidence of life on other worlds. Those frameworks are informed by our knowledge of terrestrial life, but not limited by the assumption that all life will necessarily follow the terran model (see also Question 10 and Question 11, Chapter 14). Major activities in the coming decade will focus on developing frameworks for robust identification of signs of life, but equally to differentiate life from abiosignatures, false negatives and false positives, and to advance detectability through validation of more comprehensive agnostic signatures of life, irrespective of its origin or molecular makeup.
Q9.1 WHAT WERE THE CONDITIONS AND PROCESSES CONDUCIVE TO THE ORIGIN AND EARLY EVOLUTION OF LIFE ON EARTH, AND WHAT DO THEY TEACH US ABOUT THE POSSIBLE EMERGENCE AND EVOLUTION OF LIFE ON OTHER WORLDS?
Understanding the boundaries between abiotic and biotic systems is an essential part of searching for life elsewhere. These boundaries depend on geochemical context and therefore need to be investigated in a range of potentially relevant environments. With few exceptions, modern Earth settings no longer reflect prebiotic conditions, because biology permeates the planet, destroying or influencing organic chemical reactions and the long-term evolution of Earth’s surface, subsurface, and atmosphere. But recent discoveries in subsurface environments wherein products of abiotic organic synthesis can be investigated provide exciting new opportunities to investigate potential prebiotic processes (McDermott et al. 2015; Lang et al. 2018; Sauvage et al. 2021; Sherwood Lollar et al. 2021). Controlled laboratory settings represent another productive strategy, where the influence of biology can be eliminated and the geologic conditions and atmospheric, crustal, and oceanic compositions of early Earth environments can be more closely replicated.
The analysis of asteroids, meteorites, comets, and other rocky bodies of the inner solar system can provide key insights into how the chemical inventories (e.g., organics and volatiles) of early Earth may have evolved (NASEM 2019a; see Table 14-1). Astromaterials also offer additional clues regarding the sources, inventories and abundances of key building block elements present during Earth’s accretion and its early evolution. The subsequent emergence of life and its evolution from the first self-replicators to the last universal common ancestor of all modern life (LUCA), represents a critical period when geochemistry and biochemistry were likely inseparable. Understanding this interdependence of the earliest forms of life and their environment helps constrain the range of geochemical conditions whence life could emerge.
Q9.1a How Was the Emergence and Evolution of Life on Earth Influenced by Volatiles, Impacts, and Planetary Evolution in Early Solar System Environments?
What were the principal components of the early solar system environment and how did controls on these components (such as available volatile inventory, volatile delivery via impacts and comets, and compositional changes in response to large impacts and planetary evolution) affect the emergence and evolution of life on Earth?
A vital aspect of understanding the physical processes of the early Earth, from its prebiotic state through to the emergence and subsequent evolution of life, is the character, evolution, and abundance of Earth’s chemical inventories, especially those deemed essential to terrestrial life (e.g., key building block elements like C, H, N, O, P, S, and redox couples whose relative biochemical importance may have varied in time and space) (also see Question 10). The earliest possible timing for sustainable prebiotic chemistry that led to life’s origins followed the moon-forming event and replacement of the magma ocean by solid crust at ~4.5 Ga (Onstott et al. 2019). Over the next half billion years, the onset of plate tectonics, formation of continents, and formation of the atmosphere and oceans, as well as ongoing bombardment, gave rise to the dynamic, diverse, and interconnected environments from which the chemistry that led to life’s origins arose (NASEM 2019a). While the Hadean is often thought to be the most dynamic period in the planet’s history, it is also the period for which proxies are the sparsest and models are the least reliable. Even constraints on the volatile delivery of organics from asteroids and comets, and whether impacts cause a net gain or loss of volatiles, remain unresolved (see Questions 3, 4, and 6; Chapters 6, 7, and 9, respectively). The next decade requires field work, modeling, and laboratory experiments to expand understanding of processes of abiotic organic synthesis and to decipher the relative role of exogenous versus indigenous building blocks for life.
Q9.1b What Can We Learn from the Moon About the Conditions, Emergence, and Evolution of Life on Early Earth?
Owing to substantial modification by plate tectonics, erosion, and life over the eons, Earth’s record of the environments and conditions during and preceding the emergence of life is less preserved than that of some other bodies in the solar system (e.g., the Moon, Mars, and Mercury). Critically, asteroidal and cometary bombardment of the inner solar system during the first billion years served as a mechanism for geologic and climate evolution and for the delivery of water and organics to early Earth and the inner solar system (NASEM 2019a; see also Question 4), likely having a significant influence on the origin and early evolution of life. Most records of ancient bombardment have been erased from Earth’s surface, limiting our capability to understand those critical events, including their possible link to the orbital evolution of giant planets in the outer solar system (see Question 7, Chapter 10). Significantly, the well-preserved surfaces, deposits, and rocks of the Moon provide one of the best chronicles of the major events that have occurred in the inner solar system (Bottke and Norman 2017; see Figure 7-1). In addition, the lunar record provides key insights into deciphering the linked dynamical evolution of the Earth–Moon system (see Questions 3 and 8, Chapter 11) that has had a prominent influence on Earth’s environment (e.g., orbital parameters, climate, and tides) throughout time. Importantly, the comparatively well-preserved ice deposits and rocks of the Moon (and to some extent on Mercury and Mars) can inform reconstructions of early Earth environments through a more complete record of the asteroidal and cometary bombardment history and delivery of volatiles and organics relevant to the origin of life.
Q9.1c What Is the Boundary Between Abiotic and Biotic Phenomena and How Does That Boundary Change with Earth’s Overall Geochemical and Biological States?
Abiotic chemical reactions provided the feedstock for prebiotic chemical evolution that ultimately gave rise to the first terrestrial organisms. Although abiotic and biotic chemistries have commonalities at the level of individual building blocks, research on the origin of life and extant biochemistry can guide system-level approaches to differentiate between biotic and abiotic chemistry. While abiotic chemistry can exhibit multiple pathways toward a particular compound, biochemistry capitalizes on a distinct subset of precursors among the abiotic possibilities. In the past decade, new approaches have emerged to understand complex networks of interacting
molecules in chemical and biological systems. These approaches, which form the basis of systems chemistry and systems biology, can enhance our knowledge of abiotic synthetic routes to the building blocks of life and to possible pathways that the earliest forms of life on Earth, or life elsewhere, might have adopted. Nonetheless, differentiating between abiotic and biotic sources and processes remains a major challenge in planetary sciences and astrobiology and an area of focus for the next decade. The recent literature regarding reports of methane in the martian atmosphere provide a case in point (see Q6.6). Even if the isotopic composition of this methane could be measured, kinetic fractionations associated with abiotic organic synthesis have been shown to be of comparable scale to those produced by kinetically controlled biological processes (Etiope and Sherwood Lollar 2013). Similarly, for both terran (Ménez et al. 2012) and meteorite studies (Steele et al. 2012; Ménez et al. 2018) it has been demonstrated that even organic carbon with “light” carbon isotope values requires careful contextual investigation of the microstructure of minerals and fracture infillings between and across those mineral boundaries to determine the abiotic versus biotic nature of macromolecular carbon (Ménez et al. 2012, 2018). Claims for the “oldest life” on Earth or for life detection beyond Earth require integration of the information from both abiotic and biotic phenomenon (see also Question 11; see Figure 12-7 below), and field, laboratory and modeling investigations provide the critical foundation to further develop those frameworks (NASEM 2019a) in preparation for return of data and/or samples from Venus (DAVINCI and VERITAS), Mars (Curiosity, Perseverance, and MSR), Europa (Clipper), Titan (Dragonfly) and other bodies (see Question 11, Chapter 14) and for interpretation of exoplanet atmospheres (see Question 12, Chapter 15). It is not sufficient that life is known to produce a certain compound on Earth; robust life detection requires sufficient investigation to eliminate abiotic sources and processes (see Question 11 for more on false positives). The significance of any potential biosignature comes from not only the probability of life having produced it, but also from the improbability of nonbiological processes producing it, underscoring the need in the coming decade to further investigate the range and breadth of abiosignatures in parallel with biosignatures (NASEM 2019a).
Q9.1d How Did Early Earth Environments and Prebiotic Pathways Coevolve and Give Rise to Life and What Major Milestones in Earth History Were Coincident (or Causative?) with Major Transitions in the Abundance, Quality, and/or Complexity of Life?
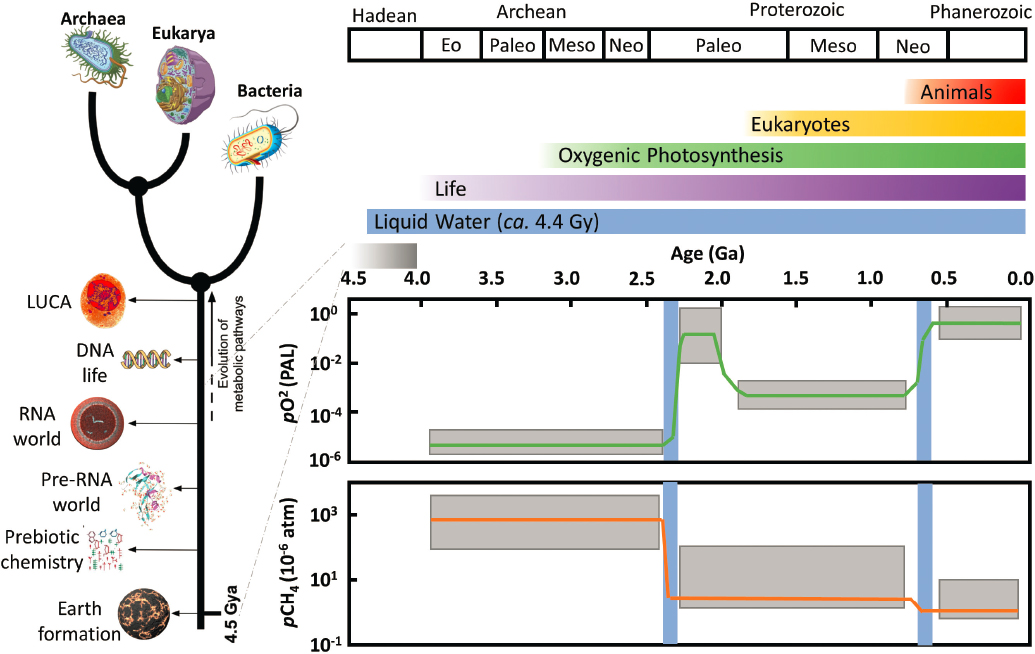
Studies of the chemical origins of life start with the hypothesis that mixtures of simple small molecules under the influence of various energy sources and early Earth environments created the building blocks of life and that interactions among these molecules eventually lead to life itself (Figure 12-2). The earliest signs of life on Earth indicate that the prebiotic chemistry that led to life’s origins most likely commenced prior to 4 Ga, which overlaps with a period when Earth experienced major transformations in its geological state, such as the transition to stable liquid water (oceans) at the surface and substantial cooling and crustal formation (Olson et al. 2018; Question 5). It is crucial not only to understand the early state of Earth’s environments but also the magnitude and timing of transitions that occurred, including catastrophic events such as large impacts, as well as the effect of lengthy quiescent periods (see also Questions 4 and 6). The specific location where life originated on early Earth (whether a surface environment in contact with the atmosphere and incoming ultraviolet radiation (Damer and Deamer 2020), or a subsurface system (Martin et al. 2008) is still vigorously debated. Smith and Morowitz (2016) suggest that the chemical potential of planetary scale disequilibrium on Earth (and hence life’s origin) is focused “to an extreme degree on the rock/water interface and in the mixing chemistry of fluids and volatiles in and near the crust.” If so, these environments are particularly pertinent for astrobiological exploration of both rocky planets and moons such as Mars (Onstott et al. 2019) and the ocean worlds (Hendrix et al. 2019; Hand et al. 2020; Sittler et al. 2020; MacKenzie et al. 2021) (see also Questions 10 and 11). For systems ranging from rocky planets to the ocean worlds and exoplanets there is strong interest in the role of water–rock reactions producing precursor molecules for potential life as well as substrates for habitability (MacKenzie et al. 2021). Both planetary-scale and local-scale disequilibria could be important for prebiotic synthesis and life’s coevolution with the planet; therefore, consideration of both planetary scale habitability and localized environments are important in designing successful strategies for searching for life beyond Earth (NASEM 2019a).
Q9.1e How Do Phylogenetic, Structural, and Biophysical Studies Inform Understanding of the Origin and Coevolution of the Genetic and Translational Machinery in Terrestrial Life, and What Was the Timeline for Their Development and Diversification?
The genetic and translational machinery were two of the earliest and most fundamental biochemical inventions of Earth’s life (Baross et al. 2020). The ancient and highly conserved nature of these two systems provide fundamental constraints on the evolutionary pathway of terrestrial biochemistry (NASA 2015; Figure 12-3), including the selection of the 20 proteinogenic amino acids and the evolution of proteins with incremental folding ability. Extant proteins utilize a small fraction of the combinatorial sequence space available for 20 proteinogenic amino acids. The ribosome (within its own proteins) contains a structural record of the earliest history of protein folding, and the special family of proteins that recognize and link cognate tRNA with the correct amino acid (i.e., aminoacyl-tRNA synthetases, or aaRS) can provide the basis for comparative evolutionary studies and reconstructing the pre-LUCA history of protein synthesis. Phylogenetic, structural, and biophysical
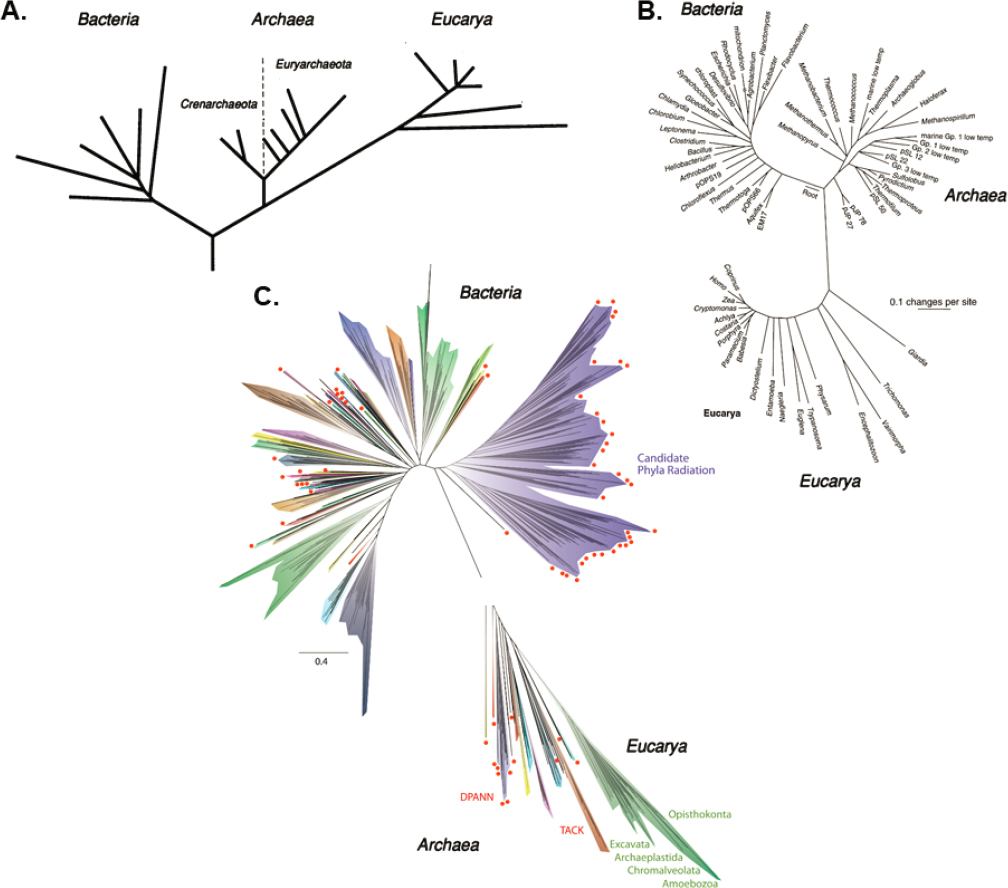
SOURCES: (A) Adapted from Woese et al. (1990). (B) From N.R. Pace, 1997, “A Molecular View of Microbial Diversity and the Biosphere,” Science 276(5313):734–740, https://doi.org/10.1126/science.276.5313.734, reprinted with permission from AAAS. (C) Hug et al. (2016), CC BY 4.0.
studies of modern organisms can shed light on the nature and functionality of the decoding-competent LUCA ribosome, and more primitive precoding ribosomes. In the next decade, with further improvements in ancestral sequence reconstruction algorithms and experimental methods, pre-LUCA aaRS ancestors and other biochemical lineages such as cytochromes, ATP synthases, and biolipids may yield even more secrets, including the fundamental drivers for the origin and evolution of the genetic code. By analogy, these investigations can offer insights into the conditions and processes necessary for the emergence and evolution of similar genetic and translation machinery on other worlds.
Q9.1f What Does the Last Universal Common Ancestor (LUCA) Represent (e.g., a Single Individual, a Species, or a Population of Species), How Does This Inform the Emergence of Core Biological Systems, and What Is the Geological and Evolutionary Context of LUCA’s Emergence?
All extant terrestrial life shares a set of genetic commonalities that indicate the existence of a hypothetical common ancestor—LUCA—linked to the core mechanisms of cellular machinery, the structure and function of biomolecules, and interactions and dependencies within cells (NASA 2015). What LUCA represents remains uncertain. Comparative genomics and cell biology suggest that the organism(s) represented by LUCA were likely cellular and contained many genes, proteins, and biological functions present within modern lineages. Further biological investigation of LUCA will require advances in paleogenomics and molecular evolutionary biology as a complement to ongoing theoretical and experimental research in geochemistry, organic chemistry, and planetary science. Reliable reconstructions of LUCA, including the nature of its cell membrane and of important membrane-related protein families, may help explain why the transition toward organismal individuality and vertical inheritance (as opposed to horizontal genetic transfer) became predominant, and whether we should expect a similar transition for other forms of life elsewhere in the universe (Figure 12-3).
Strategic Research for Q9.1
- Characterize the surface and subsurface processes (e.g., impactor flux, atmospheric conditions, volcanism, and tectonism) and the range of chemical inventories (e.g., volatiles and organics) present during the emergence of Earth’s nascent biosphere through modeling, analyses, and measurements of solar system materials (asteroids, comets, interplanetary dust particles, and meteorites), investigation of Earth’s isotopic record, and estimation of fluxes recorded and volatiles deposited on ancient, well preserved inner solar system planetary surfaces (e.g., Moon and Mercury).
- Characterize how the early, dynamic solar system environment shaped Earth’s environments and the subsequent emergence and evolution of life therein by determining a reliable absolute chronologic record of the early bombardment of the Earth-Moon system, especially prior to 3.7 Ga.
- Determine what combinations of physical and chemical processes could give rise to conditions that would promote habitability and be able to sustain life beyond Earth through theoretical modeling, field work, and laboratory experimentation informed by current knowledge of the distribution of life on Earth and Earth processes (e.g., geosphere, hydrosphere, cryosphere, and atmosphere).
- Identify and characterize chemical processes and pathways that could enable the transition from abiotic reaction networks to biochemical reaction networks through experimental, modeling and field studies of prebiotic condensation, catalysis, and self-assembly processes and the preservation/diagenesis of those signals with time.2
- Determine the evolutionary history of life’s biochemistry through deep time through genomic/proteomic and phylogenetic/metabolic analyses and reconstructions.
- Investigate the interplay between availability and biological function of essential elements and how their accessibility and biochemical efficiency changed with environmental abundances through laboratory experiments, biochemical network models, analyses of Earth’s early geologic record, and geochemical studies of other rocky worlds and astromaterials.
Q9.2 WHAT IS THE DIVERSITY, DISTRIBUTION, AND RANGE OF POSSIBLE METABOLIC STRATEGIES OF LIFE IN TERRESTRIAL ENVIRONMENTS (SURFACE, SUBSURFACE, AND ATMOSPHERE), AND HOW DID THEY EVOLVE THROUGH TIME?
Earth, with its rich diversity of habitable spaces, provides a plethora of environments to test models of biological potential—that is, a qualitative measure of the potential for life and for habitability as indicated by factors such as biogeochemical fluxes, organism/metabolic variety, and other features of the physical and chemical environment that can inform the most likely places in the solar system to find evidence of life (Figures 12-3, 12-4, and 12-5).
___________________
2 Note: The transition from abiotic to biotic conditions—including key reactions of prebiotic organic chemistry, the formation of abiotic polymers, the origin of replicating heteropolymers, the beginnings of genetics, and the dawn of Darwinian evolution—is discussed in Chapter 14 (see Q11.1).
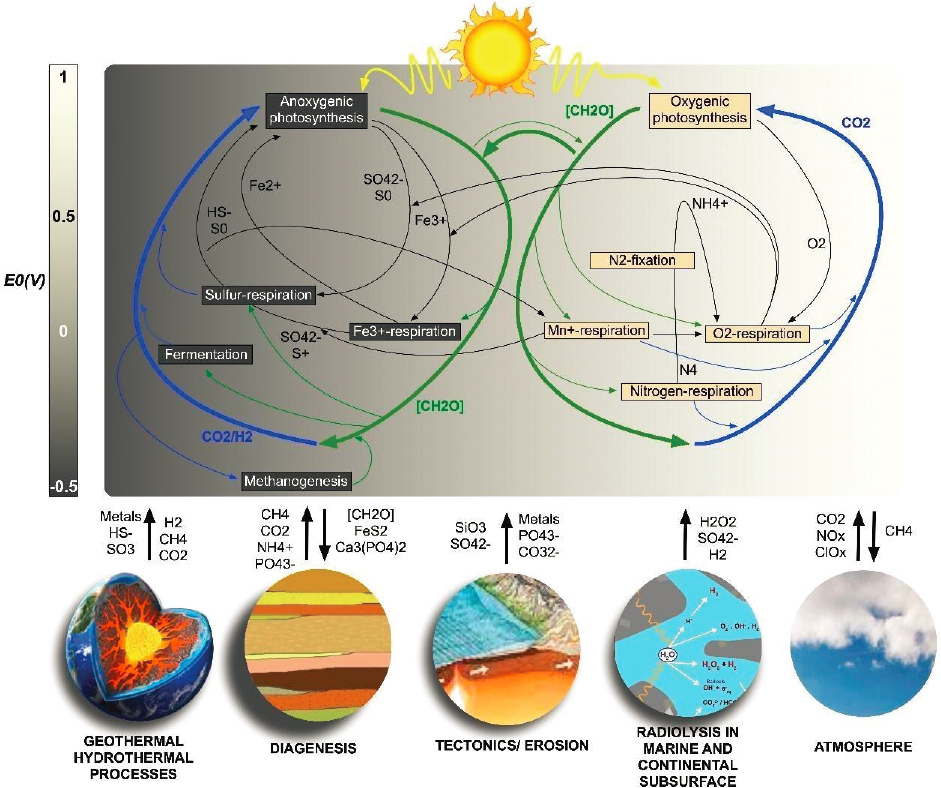
Targeted exploration on Earth is a proven, highly effective mechanism by which to validate hypotheses relevant to the search for life, from the deep ocean and subsurface to polar ice caps and deserts (Wierzchos et al. 2018). Terrestrial life has evolved a tremendous diversity of metabolic strategies that tap into an energy spectrum that spans many orders of magnitude, from the low end to the high end (see Figure 12-1).
As a result, biological potential in different environments can also vary significantly. At the high end of the spectrum, light-dependent ecosystems can support large amounts of biomass recycled in short periods of time. At the low end of the spectrum, dark ecosystems subsist in conditions that provide only marginal energy for cell growth and division and seem to barely support the maintenance of basic cellular functions (Hoehler and Jorgenson 2013; Hoehler et al. 2020). Ecosystems elsewhere in the solar system, if they exist, will likely lie closer to the low-energy end of the spectrum (see Figure 12-1), and therefore might be difficult to detect. Understanding the biochemical underpinnings of the different metabolic strategies realized on Earth, their evolutionary history, and their dependence on sources of electron donors and electron acceptors (e.g., H2, CH4, SO42−), is key to assessing the likelihood that similar strategies might have evolved elsewhere, as well as the sizes of the biospheres those metabolisms might support (see Figure 12-1). Each metabolic strategy is optimized for a specific range of physical and chemical parameters, such as salinity, pH, or temperature. For some parameters such as high temperature, pH, or salinity, there appear to be boundaries beyond which the metabolic activity of all but the most specialized organisms is severely impaired, or entirely disrupted. For other parameters, such as low temperature, the boundaries are more diffuse. An area of focus is understanding the cellular and metabolic impacts of poly-extremes (e.g., an organism experiencing multiple extreme conditions at once is called a polyextremophile), particularly those that might act synergistically, to inform the most likely environments beyond Earth where life might be detectable.
Q9.2a What Are the Different Energy Sources That Life Can Exploit on Earth and Other Planets?
Understanding what limits the wavelengths and intensities of light that can be utilized by life, finding possible alternatives to light energy, and characterizing the spectrum of redox pairs that terrestrial life can exploit, informs the quantity and quality of energy sources that could potentially be utilized by life elsewhere (Hoehler et al. 2020). While thermodynamic disequilibrium can arise in the absence of life, all life that we know on Earth exploits the chemical
energy released through organisms’ metabolic functions, as chemicals react back toward equilibrium (see Figure 12-1; NASEM 2019a). Indeed, in the past decade it has even been hypothesized that life should be envisaged as a “fourth geosphere”—a natural consequence of the processes and fluxes that drive thermodynamic disequilibria (Smith and Morowitz 2016). While some organisms carry out oxygenic photosynthesis—the biological process in which water is split for cellular energy, generating O2—other organisms extract energy from redox reactions between a broad range of oxidants (e.g., O2, NO3−, SO42−, CO2) and reductants (e.g., Fe2+, H2, H2S, CH4, and more complex organics) (chemotrophy) (see Figure 12-1). In the case of earliest Earth, we have evidence of ancient microbial life associated with geothermal settings, akin to modern day hot springs. There, thermally driven convection gives rise to water–rock reactions that, in turn, can act as a source for, and bring together, reactants that can participate in a variety of redox chemical reactions that release energy in a form that can be exploited by a diversity of microbial metabolisms (see Figure 12-1). In addition to calculating how much energy might be available for life to exploit for new growth, studies are needed that constrain the amount of energy that life expends to repair damaged cellular components and biomolecules, or synthesize new ones (maintenance energy, Hoehler et al. 2020). Coupling the quantity and quality of known energy sources for life with the amount of energy per unit time (power) required by organisms to perform basic biological functions, also informs the biological potential of a habitable environment.
Q9.2b How Does the Biological Potential (i.e., Abundance, Productivity, and Diversity) of Light-Dependent Ecosystems Compare to That of Light-Independent Ones?
The amount of energy delivered by visible and near-infrared light far exceeds the amount of energy delivered by the chemical reactions that life can exploit in dark environments (Hoehler et al. 2020). The detectability of light-dependent and light-independent ecosystems varies accordingly, both in terms of the types and abundances of biosignatures they can generate. Quantitative estimates are needed that compare the biological potential of ecosystems that rely on sunlight as an energy source (directly or indirectly), from those that are truly light independent. The former are especially pertinent to discussions of the potential for life in (near)surface environments and atmospheres of other planetary bodies (e.g., Mars, Venus, and Titan). The latter are relevant to the discussion of the potential for life in dark, subsurface environments in multiple solar system targets (e.g., Mars, Europa, Titan, and Enceladus). Understanding the size of a biosphere that can be sustained by light-dependent and light-independent primary production pathways will provide a baseline to inform science and instrument requirements for life-detection missions that target atmospheric/surface or subsurface environments, respectively. It will be equally important to characterize additional light-independent chemotrophic pathways that also rely on products of radiolysis or water–rock reactions. These pathways can coexist with and complement light-independent primary production, enabling the recycling of products and reactants, and increasing the overall productivity and diversity of a subsurface biosphere whether capable of direct investigation on Earth (Lin et al. 2006; D’Hondt et al. 2009, 2019; Sauvage et al. 2021; Sherwood Lollar et al. 2021) or through habitability models and designing exploration strategies for Mars (Onstott et al. 2019), Europa (Hand et al. 2017), Enceladus (Vance et al. 2007; Waite et al. 2017), TitanTitan (Cable et al. 2018), or the small bodies such as Ceres, for example.
Q9.2c How Do Environmental Factors and Fluxes Control or Limit the Different Metabolic Strategies, Growth Rates, or Productivity in Different Planetary Analogs on Earth, and How Do They Co-Vary?
Together, physical and chemical conditions—including salinity, temperature, pressure, pH, ultraviolet, and ionizing radiation—define a multi-dimensional space that represents the habitability envelope of life on Earth (see also Question 10). Individually, each environmental parameter affects one or more aspects of cellular function, from osmotic regulation and transport across the cell membrane, to the stability and functionality of biomolecules and the overall structure of the cell (see Figure 12-5). The magnitudes of these physical and chemical parameters, and their synergistic interactions, affect overall productivity, cellular abundance, and phylogenetic diversity in a given environment. At the high- or low-end values of those parameters, cellular countermeasures are required to mitigate negative effects, which impose higher energetic demands on the individual cells and the overall ecosystem. Because the extent of that effect is likely to depend on the types and diversity of metabolic strategies realized in the environment, the flux of nutrients and availability of energy also determine the ability of individual organisms and groups of organisms
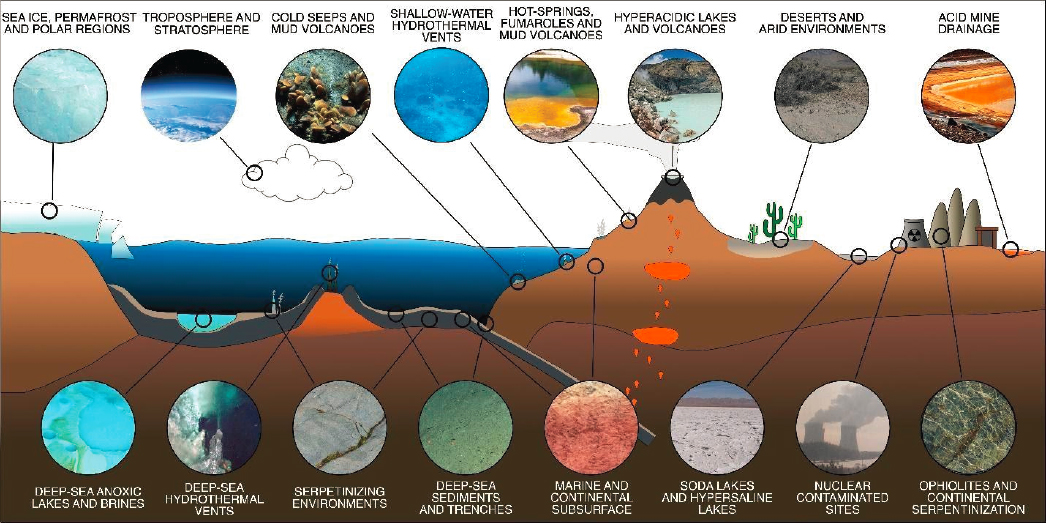
to adapt to poly-extremes. Laboratory and modeling studies, along with research in Earth environments, allow us to probe the fringes of this multi-dimensional space, and the effect on biological potential. Field studies of nutrient and energy resources and fluxes in so-called extreme environments (subterranean, oceanic and/or atmospheric; see Figure 12-4) over different timescales, complemented with theoretical and laboratory studies on the tolerance of terrestrial microorganisms to multiple environments stressors (e.g., temperatures, desiccation, ultraviolet radiation, extreme pH, and salinity) are essential to determine the requirements for the survival of life in similar extraterrestrial settings. Results from these studies can be used to assess whether physicochemical conditions in other solar system environments could support Earth-like life, and to constrain the sizes of the biospheres they might contain.
Q9.2d How and When Did Viruses Originate and What Role Have They Played and Continue to Play in the Evolution of Life on Earth?
Viruses are key contributors to Earth’s ecosystems, but there is much yet unknown regarding their influence on cellular life, their role in evolutionary history, their physical interactions with the Earth system, and their persistence and decay under various environmental conditions (see Figure 12-5). Viruses and virus-like replicators are the only known biological entities that contain all types of genomes, including single-stranded and double-stranded RNA and DNA. RNA viruses may serve as models for how RNA and ribonucleoproteins could have propagated via simple self-replicating RNA structures and ribozyme activity; thus, elucidating the history of viruses could provide important clues regarding the emergence and evolution of life on Earth (Forterre 2006; Koonin et al. 2021). In addition, viruses seem to be ubiquitous in terrestrial ecosystems, where they act as agents of microbial evolution and contribute to microbial fitness by moving ecologically important genes from host to host.
Strategic Research for Q9.2
- Characterize light-dependent and light-independent life and its metabolisms and assess its contribution to biological diversity, productivity, and abundance in Earth environments using sequence-based molecular studies, field observations, and metabolic models.
- Investigate how multiple environmental factors (e.g., pH, temperature, pressure, salinity, and redox potential) and fluxes (e.g., energy and nutrients), affect different metabolic strategies in Earth environments through field and laboratory observations and metabolic models of microbial community responses to environmental stress, including multi-dimensional factors.
- Elucidate the evolutionary history (origin and divergence) of metabolic pathways as a guide for the interpretation of biosignatures in terms of coevolution of life and its host world through sequence-based molecular clock studies and modeling of life’s biochemistry.
- Investigate the roles of viruses in Earth environments through sequence-based molecular studies, long-term environmental monitoring of viruses, and laboratory studies of virus-host interactions.
Q9.3 HOW DO INVESTIGATIONS OF EARTH’S SUBSURFACE ENVIRONMENTS INFORM WHAT HABITABILITY AND/OR LIFE ON OTHER WORLDS MIGHT LOOK LIKE?
On Earth, the discoveries of active microbial communities existing in the subsurface of the ocean floor and continental lithosphere, often far from the influence of the Sun’s energy, provide new models for understanding rock-hosted, chemosynthetic life that may exist on other worlds (see Figure 12-4). Many outstanding scientific issues remain unexplored in the subsurface, including quantifying the total biomass and its distribution, the degree to which subsurface ecosystems on Earth exist independently of surface energy sources, the stability of these ecosystems over time and space, and how life adapts when subject to its environmental and energetic limits (see recommendations from NASEM 2019a). Growing sophistication in our understanding of subsurface life and its trajectory on this planet could reveal much about how life could persist on other worlds. We know that subsurface communities on Earth range from energy-rich environments where “fast” life predominates (e.g., hydrothermal vents) to energy-limited environments where “slow” life is barely able to survive and can be difficult to detect (e.g., continental rocks, Magnabosco et al. 2018; deep-sea sediments and rocks, Trembath-Reichert et al. 2017; Suzuki et al. 2020) with the
latter providing critical endmembers likely to be applicable to the search for life off-Earth (Onstott et al. 2019). A range of strategies for accessing subsurface samples using both in situ and remote capabilities is needed to investigate subsurface processes and inform our understanding of the controls on interactions between a planet’s surface and subsurface. The investigation of subsurface habitats on Earth is one of the most readily actionable strategies to address questions about the processes governing habitability and the nature, diversity, and preservation of both extant and extinct subsurface communities on Earth and other worlds (see also Question 10).
Q9.3a What Are the Physical and Chemical Processes and Spatial and Temporal Controls Sustaining Subsurface Life on Earth and How Does This Expand Concepts of Habitability Elsewhere in the Universe?
Research into the physical and chemical subsurface environment is critical to understanding how subsurface life and its host environment coevolve. Investigations of rock-hosted and other subsurface environments on Earth make up the critical test bed for informing the search for life on multiple targets in the solar system, including sub-ocean silicate crusts of Europa, Enceladus, or other ocean worlds (Hand et al. 2020), and Mars. On Mars, where the loss of its dynamo-driven magnetic field between 4.1 to 3.9 Ga and subsequent loss of atmosphere (Ehlmann et al. 2016) led to surface conditions less hospitable for life, subsurface environments may have provided a widespread stable refuge for life (and signatures of life) to persist (Ehlmann et al. 2011; Onstott et al. 2019; Stamenkovic et al. 2019). Studies of geophysical, geochemical, geological, and hydrogeological processes are vital to understanding subsurface habitability on Earth and beyond at both local and global scales. This involves determining the spatial and temporal distribution of subsurface water—the inventories, sources, and sinks of energy—that could generate habitability in the subsurface, and the processes that sustain these inventories over time and space (Schrenk et al. 2013; Li et al. 2016; D’Hondt et al. 2019; LaRowe and Amend 2019; Onstott et al. 2019; Sauvage et al. 2021; Sherwood Lollar et al. 2021). For instance, shallow subsurface life ranges from cryptoendolithic communities existing only millimeters below rock surfaces and typically consisting of cyanobacteria and algae (Wierzchos et al. 2018) to complex aerobic and anaerobic communities in near-surface groundwaters and extending to kilometers depth in sedimentary basin systems (Head et al. 2014). Deeper yet, in the oceans, studies have focused both on marine sediments (Inagaki et al. 2015; Heuer et al. 2020), and on microbial ecosystems permeating the ocean lithosphere and hydrothermal vents and seeps, reflecting a broad range of microbial metabolisms ranging from heterotrophs degrading residual photosynthate to chemolithoautotrophic communities deriving their energy from water–rock reactions such as serpentinization and radiolysis. In continental settings, the past decade has seen a shift from investigations of sedimentary systems to studies of crystalline rocks and fracture waters ranging from thin ophiolite wedges (Schrenk et al. 2013) to kilometers deep in more than billion-year-old rocks of the crystalline cratons of, for example, the Canadian Shield, South Africa, and Fennoscandian Shield (Sherwood Lollar et al. 2014). These studies have expanded our understanding of the range of water–rock reactions and energy sources (magmatic, serpentinization, and radiolysis) capable of sustaining life in isolation from the surface photosphere, and as such, are particularly relevant to developing a more comprehensive understanding of planetary habitability (see Question 10) and the search for life elsewhere (see Questions 11 and 12, Chapter 15).
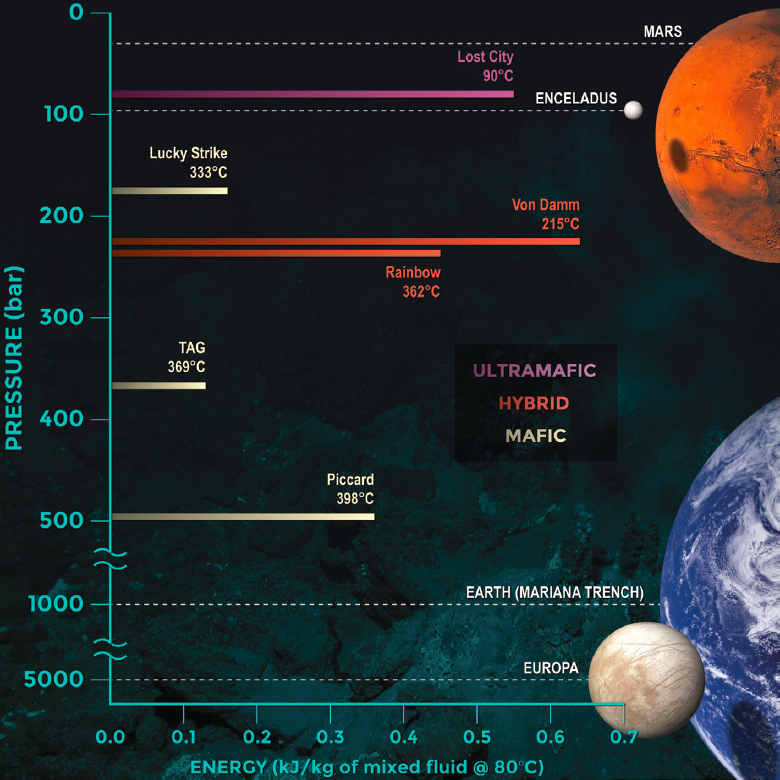
Modeling of anticipated water–rock reaction conditions and the available energy for life offers great potential for exploring the past and present habitability of other planetary bodies, but with two important caveats. First, theoretical modeling of water–rock reactions is most robust for situations in which equilibrium is anticipated to have been obtained, whereas in a dynamic setting, reaction kinetics might dominate. Second, the maximum chemical energy that might be available from any set of reaction conditions does not necessarily imply that life is able to exploit it. Figure 12-6 uses deep-ocean hydrothermal systems to show how the cumulative catabolic energy available from subsurface water–rock reactions, at any given location, exhibits multi-parameter co-variability. In this illustration, a broad array of parameters combine in multiple ways, providing diverse and alternate pathways to high energy yields or, conversely, to low energy yields. Pressure and hydrogeology each play a role in controlling the maximum temperatures that fluids can reach within such a water-rock reaction-path system. Furthermore, the rates of fluid flow through the system determines the amount of time available for chemical reactions to proceed, as well as the rate at which reactants are mined from the system. In parallel, the bulk composition and mineralogy of the host rock influences which key chemical species are removed from or enriched in the circulating fluid relative to the original source fluid. Note that the composition of that source fluid would also be expected to vary among other (ocean) worlds.
Q9.3b Can Subsurface Life Exist in the Absence of Surface Life and Can We Test This on Earth to Inform the Search for Life Elsewhere?
While many subsurface ecosystems exist without direct influence of the Sun, life in these environments may still use the products of photosynthetic life as oxidants, reductants, or carbon sources. Studies of subsurface environments on Earth where chemolithoautotrophic life predominates will help inform what a subsurface biosphere might look like on another rocky planet or ocean world without energy from the Sun, while also helping to distinguish whether the surface biosphere on Earth is an outgrowth of the subsurface biosphere, or if the colonization of the subsurface is facilitated by surface phototrophy. There are a range of rock-hosted environments in both the continental and marine subsurface on Earth where water-rock reactions such as serpentinization or radiolysis
create energy sources (e.g., methane and molecular hydrogen) to support life (Schrenk et al. 2013; Sherwood Lollar et al. 2014). However, observations of pertinent biomarkers and direct evidence for microbial ecosystems are insufficient to establish the indigenicity of life in the subsurface or its relationship to the surface biosphere (Onstott et al. 2019). Signs of extant life and indicators of past life may have penetrated the subsurface long after the host rock formed, and the permeation of the geologic setting by younger groundwaters needs to be addressed to constrain the timing and history of subsurface life (Lin et al. 2006; Becraft et al. 2021). “Follow the rock, and the water” is a necessary strategy that needs to be as deeply embedded in terran studies as it is in the search for life elsewhere (Lollar et al. 2019). Exploration of the deep seafloor and continental lithosphere, together with parallel investigation of the rock record and hydrogeologic cycle through deep time, are a key strategy for the next decade to expand our picture of the coevolution of the subsurface biosphere and Earth and the degree of interconnection and/or isolation between the surface and subsurface habitable zones.
Q9.3c How Does Subsurface Life Adapt to “Extreme” Environmental Parameters and Variation in Available Energy Sources and/or Supply?
Subsurface life on Earth thrives in nominally “extreme” environments—a term that is less in vogue now that we recognize it embeds observational bias in the sense that we reset the “limits” based on what we have observed or chosen to investigate. An environment “extreme” to our perspective is habitable for the organisms adapted to live therein (see Figure 12-4). The tremendous variation in temperature, pressure, pH, salinity, radiation, and energy/nutrient supply that terran life has evolved to successfully exploit, means that the current “observed limits” for each parameter (see Table 12-1) remain an important feature of on-going research.
Importantly, there is an urgent requirement to understand life’s response and ability to adapt to the multiple parameters that organisms encounter in Earth’s subsurface (e.g., combined effects of extreme temperature and pressure, or T-P and low water activity combined). A key focus for future work involves investigation of life and habitability both where energy available for life is abundant and where it is limited by paucity of source or by transport (NASEM 2019a). This includes organisms living in deeply buried, energy-starved sediments, with estimated metabolic turnover rates as slow as one cell division every thousand years (Trembath-Reichert et al. 2017; Hoehler et al. 2020; Sauvage et al. 2021), as well as life in other subsurface environments such as the continental rocks, marine ocean crust, hypersaline habitats, and subglacial ice. Such observations of life in its natural habitat are critical, as are mechanistic studies to understand the biological responses to different stressors and how organisms adapt to survive and thrive in these habitats.
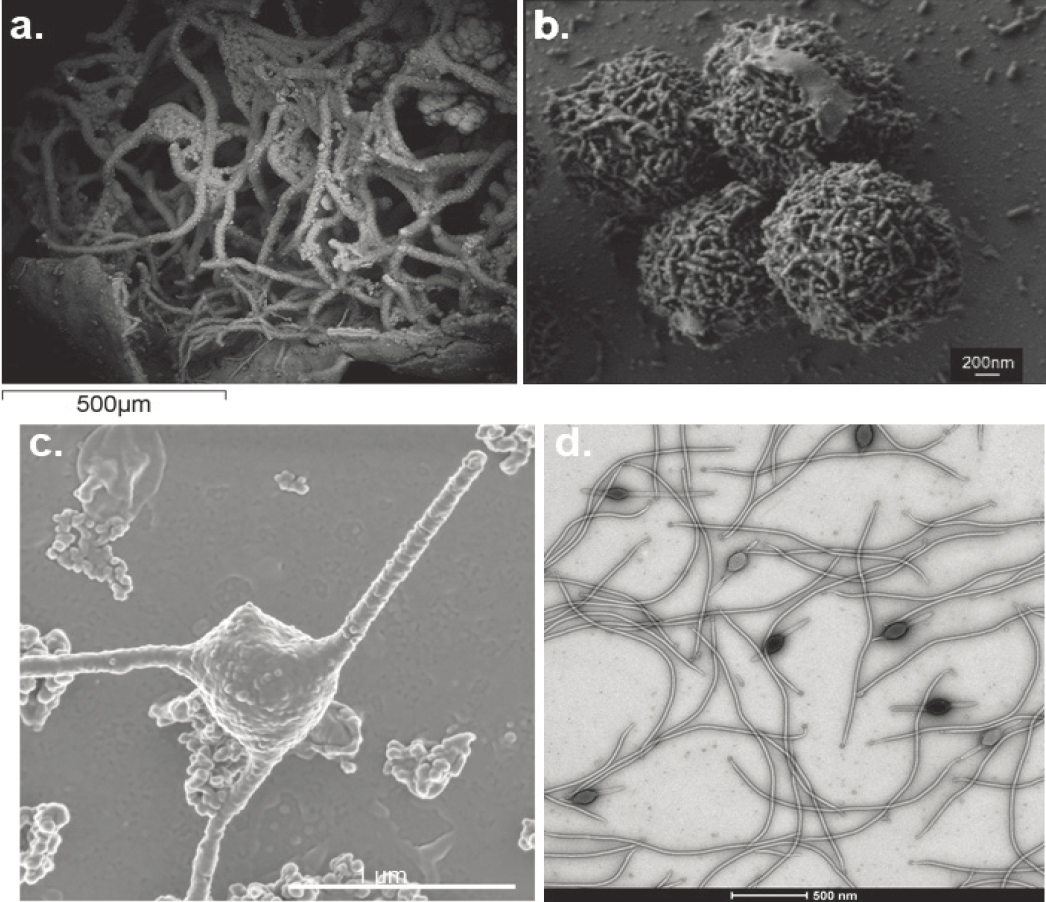
It is important, also, to remember that our predictions of where life could exist beyond Earth need not be restricted to environments that we have already identified on Earth but also to where theory and experiments predict that life might also be present but awaiting discovery. In many cases, the planetary science and astrobiology community’s priority for exploration of such predicted environments might converge with those of scientists studying terrestrial systems, but that need not necessarily be the case. One example of successful convergence that emerged in the past decade was ultramafic-hosted submarine hydrothermal systems and mafic and ultramafic hosted systems in the continental crust. These systems are relevant to plumes detected on Enceladus (Waite et al. 2017) and suggested for Europa (Sparks et al. 2017). Such environments were among those originally postulated to have the capacity to host abiotic organic synthesis on early Earth, Mars, and Europa (Shock and Schulte 1998). Subsequently, targeted field exploration has led to the demonstration of de novo subsurface abiotic organic synthesis (McDermott et al. 2015; Sherwood Lollar et al. 2021), but—in the same locations—the concomitant discovery of novel subsurface chemolithoautotrophic life forms (Reveillaud et al. 2016; Onstott et al. 2019; see Figure 12-6).
Q9.3d How Does the Biological Potential of Life Vary in the Subsurface, How Much of the Total Planetary Biomass Is Represented by Subsurface Communities, and What Are the Mechanisms for Life’s Dispersal and Transport Within and Out of the Subsurface?
The total volume of subsurface habitats on Earth is immense, but life is heterogeneously distributed, and the biological potential (abundance, productivity, diversity, and others) can vary by orders of magnitude as a function of environmental factors (Magnabosco et al. 2018; Onstott et al. 2019; Trembath-Reichert et al. 2021).
Processes such as transport, flux, preservation, degradation, concentration, and dilution can modify and impact the distribution and activity of life in the subsurface, and habitable niches may be ephemeral or isolated (Lin et al. 2006; Lollar et al. 2019). Three-dimensional global assessments of the biological potential of the subsurface on Earth are only roughly quantified and severely lacking in many volumetrically large environments, particularly in marine and continental basement rock, where studies have only begun in the past two decades (NASEM 2019a).
Strategic Research for Q9.3
- Assess controls on the distribution of subsurface water, the inventories, sources, and sinks of energy, and the processes that sustain these inventories through time and space through field and laboratory studies of subsurface waters (including saline and hypersaline), and targeting both continental subsurface, and marine subsurface habitats on Earth.
- Determine to what degree subsurface ecosystems exist independently of surface energy sources through field work, modeling, and laboratory studies of chemolithoautotrophic life in subsurface environments on Earth.
- Examine how subsurface life adapts when subject to environmental and energetic limits, as well as life’s response and ability to adapt to multiple parameters at the same time with theoretical, field, and laboratory studies of subsurface environments on Earth.
- Determine the total planetary biomass in continental and marine subsurface environments and how biomass is maintained and preserved in habitability conditions that are both heterogeneous in space and time as well as stable on long spatial and temporal scales through theoretical and field studies of Earth’s subsurface environments.
- Determine the technical limits to the collection, preservation, and detection of cells and/or biological/organic compounds and their delivery to the surface by developing new technologies and strategies to enable remote, automated, and in situ tools for accessing, sampling, and measuring subsurface processes and life through laboratory studies, and field deployments in subsurface environments on Earth, including examining known nonhabitable environments on Earth.
Q9.4 HOW CAN OUR KNOWLEDGE OF LIFE AND WHERE AND HOW IT ARISES AND IS SUSTAINED ON EARTH ILLUMINATE THE SEARCH FOR LIFE BEYOND EARTH?
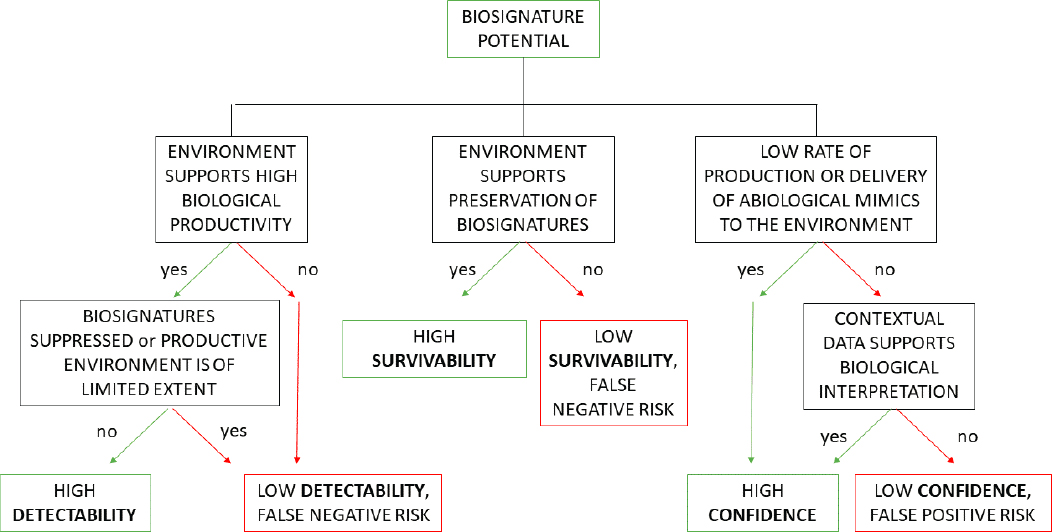
Life on Earth currently provides the only reference for a habitable and inhabited world. Challenges include an incomplete understanding of how the essential traits of life (e.g., metabolism, Darwinian evolution, and bioenergetics) arose from the geochemical environment and uncertainty concerning the factors essential for the most basic living system (Figures 12-7 and 12-8). A canonical biosignature is defined as an object, substance, and/or pattern whose origin specifically requires a biological agent. To qualify as biosignatures, these “features must be sufficiently complex and/or abundant so that they retain a diagnostic expression of some of life’s universal attributes” (Des Marais et al. 2003, 2008). Universal attributes are defined under the broad discipline of “Universal Biology,” which leverages universal laws of physics and chemistry to understand the processes that might lead to the development of life beyond Earth, without Earth-centric biases (Des Marais et al. 2008). Further, the significance of any potential biosignature comes not only from the probability of life having produced it but also from the improbability of nonbiological processes producing it (NASEM 2019a). Recent and continuing Earth-based research into characterizing universal or agnostic chemical, morphologic, or physiologic/metabolic biosignatures and abiosignatures, and characterizing reliability, survivability and preservation,3 and detectability have been recommended as essential in the next decade to advance the search for life beyond Earth (NASA 2015; Meadows 2017; Meadows et al. 2018; NASEM 2019a) (see also Questions 10 and 11).
___________________
3 Note: The report treats survivability and preservation as synonyms, as is common in the literature.
Q9.4a How Did the Essential Traits of Terrestrial Life (Such as Metabolism and Bioenergetics) Arise from Geochemical Environments, and What Factors Can We Consider Essential for the Most Basic Living System?
All extant life on Earth shares a common biochemistry based on a relatively small set of organic molecules, the same mechanisms of information storage and inheritance (RNA, DNA, and protein), ATP energy currency, a dependence on water, a related cellular organization, and a handful of core metabolic pathways with reactions performed by proteins with a shared ancestry. Fundamental questions remain concerning how these essential traits arose, the factors that are essential to the most basic living system, whether there are alternatives in a planetary context, and whether/why there is not (apparently) any life on Earth that does not use this common scaffolding.
Q9.4b What Chemical, Morphologic, or Physiologic/Metabolic Biosignatures Are Likely to Be Prevalent in Earth Life, Irrespective of Its Origin, Molecular Makeup, or Metabolism? What Is the Range of Agnostic Biosignatures on Earth?
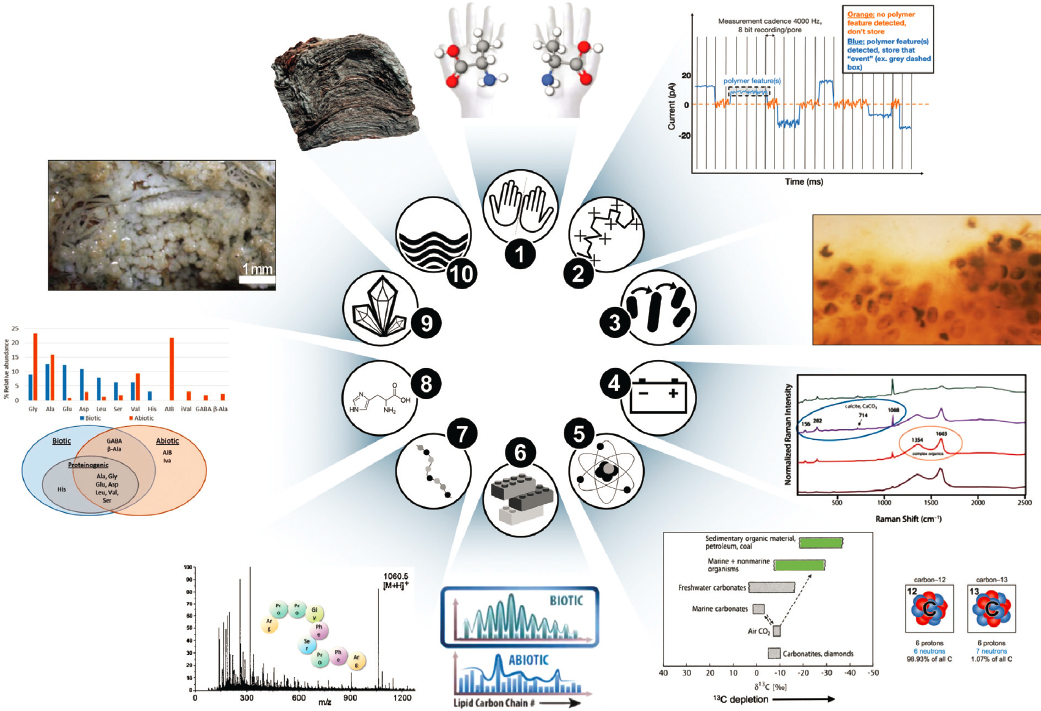
Examples of terrestrial biosignatures include but are not limited to cellular and extracellular morphologies, biogenic fabrics in rocks, bio-organic molecular structures and biomarkers, chirality, biogenic minerals, biogenic stable isotope patterns in minerals and organic compounds, atmospheric gases, remotely detectable features on planetary surfaces, and temporal changes in global planetary properties (NASA 2015; Meadows 2017; Meadows et al. 2018; NASEM 2019a). Molecular biosignatures alone may include enantiomeric excess, diastereoisomeric and structural isomer preference, repeating constitutional sub-units or atomic ratios, systematic isotopic ordering at molecular and intramolecular levels, and uneven distribution patterns (e.g., carbon number, concentration, and δ13C) of structurally related compounds (Summons et al. 2008; see Figure 12-7).
In the coming decade, biosignature research needs to include a concerted effort to better understand abiosignatures (i.e., a signature of abiotic processes and phenomena), in particular those that may mimic biosignatures (see also Question 11). A continued focus on characterizing agnostic (or universal) chemical, morphologic, or physiologic/metabolic biosignatures, and a decoupling of expectations based on terran life is warranted to improve the capability to search for life beyond Earth (Kempes et al. 2021; Marshall et al. 2021). Agnostic biosignatures are those that manifest themselves as unexpected complexity, and can present themselves at multiple scales, for example, from sustained redox disequilibria in a planetary atmosphere to patterns in the abundance of organic molecules that would not occur by chance (NASEM 2019a). An example of an agnostic atmospheric biosignature on Earth is provided by the high concentrations of CH4 in Earth’s O2-rich atmosphere (see also Questions 6 and 12). This redox disequilibrium cannot be sustained by known abiological fluxes of methane and is therefore the product of a biosphere that supplies CH4 at a rate that exceeds its rapid photochemical and oxidative destruction rates (Question 11). Research in the next decade on universal biosignatures will seek to identify attributes that are highly prevalent or even universally present across biological systems and not unique to Earth. There are two aspects of universal biosignatures that need to be considered: Is the proposed universal signature, or combination of signatures, robust (i.e., can it be used to distinguish life from nonlife) and is it truly universal (i.e., can it be generalized to life beyond Earth)? Universal principles of biology, physical laws of biology, complexity, and emergence of life (Kempes et al. 2019; Kim et al. 2019; Walker 2019) can guide theoretical, field, and experimental approaches to search for distinguishable features of terrestrial life and abiotic chemistry by examining data from various types of biotic and abiotic samples to reveal key differences stemming from biology versus abiotic baseline processes (Chan et al. 2019).
Q9.4c What Is the Spectrum of Abiotic Processes for Which Abiosignatures Exist and What Subset of Those Abiosignatures Can Mimic Biosignatures on Earth?
The literature related to claims regarding the discovery of the earliest signs of life on Earth is replete with examples of false positive detections. For example, initial claims regarding the biological origin of isotopically light graphite in ~3.8 Ga rocks of the Akilia and Isua terranes of Greenland (Schidlowsk et al. 1979; Mojzsis et al. 1996), were based in part on a lack of recognition that metamorphic decomposition of iron carbonate to form graphite and magnetite imparted an isotopic fractionation between graphite and carbonate that mimics that of photosynthetic metabolisms. Initial claims regarding the biogenicity of these graphite occurrences have largely been overturned in favor of an abiotic origin (van Zuilen et al. 2003). This interpretation is supported by the development and refinement of a geological context indicating a metamorphic origin for carbonate rocks and associated graphite (Rosing et al. 1996). Such context is critical in cases where biogenicity is in question, and in which plausible abiotic processes could mimic a purported biological feature (e.g., stromatolite morphologies; Grotzinger and Knoll 1999). Research in the somewhat younger Pilbara region of Western Australia provides excellent examples of how confidence in the assessment of biogenicity is improved through a complete understanding of the context in which a biosignature is found (NASEM 2019a).
Q9.4d How Do Nutrient and Energy Flux Affect Metabolic and Biosynthetic Rates as Well as Rates of Abiotic Destruction and Attrition of Biosignatures for Earth Life, and What Is the Impact of Resource Limitation on Biosignature Detectability?
Potential biosignatures are assessed by how well they fulfill three criteria: reliability, survivability, and detectability (Meadows 2017; Meadows et al. 2018; NASEM 2019a). Resource limitation, including nutrient and energy flux, can affect these latter two criteria of survivability (metabolic and biosynthetic rates) and detectability (rates of destruction and attrition of biosignatures) (see also Question 11). Life that may grow/replicate quickly in a nutrient- and energy-rich environment may be detectable because its biosignature signal is elevated (e.g., in soils). Resource-limited life may grow/replicate slowly, and may either be detectable because the noise is diminished or may not be detectable without appropriately sensitive instruments (e.g., in crustal rocks). Assessing the relative signal-to-noise of each type of population in its given environmental context would help to identify corresponding biosignatures that are most relevant and distinctive (Hoehler and Jorgenson 2013; NASEM 2019a).
Strategic Research for Q9.4
- Assess how the essential traits of Earth life, such as metabolism or bioenergetics, arose from the geochemical environment through theoretical, field, and laboratory studies of the connectivity and stoichiometry of metabolic networks, and the geological availability of exploitable redox gradients.
- Develop a comprehensive framework for biosignature categories of Earth life to guide the understanding of what biosignatures will be prevalent in life through community-level dialog and consensus, supported by laboratory/experimental and modeling/theoretical research as well as field work on environmentally relevant biosignature classes as well as abiosignatures.
- Elucidate the survivability and detectability of biosignatures and abiosignatures on Earth with theoretical, field, and laboratory studies of the impact of resource limitation, and relative signal-to-noise ratios of biological versus abiotic processes in environmental context.
Q9.5 HOW DO RECORD BIAS, PRESERVATIONAL BIAS, FALSE NEGATIVES, AND FALSE POSITIVES PLAY A ROLE IN BIOSIGNATURE DETECTABILITY AND RELIABILITY ON EARTH AND WHAT ARE THE IMPLICATIONS FOR TARGETS BEYOND?
The history of Earth’s earliest life and environments is written in the regions of the planet where rocks have managed to survive the ravages of time, tectonism, and weathering, leaving behind a fragmental record of life that starts ~3.5–3.8 Ga (see Figure 12-2). This record provides numerous examples of how biases, and false negatives and false positives, can act to confound efforts at biosignature detection, with important lessons for the search for life beyond Earth. Overcoming these biases requires a thorough understanding of the physical and chemical properties operating in the system in question that act to enhance, preserve, mask, or destroy biosignatures produced in them, and the limits of how far lessons from modern systems or more recent, better preserved geological terrains can be extrapolated in time and space (see Figure 12-8; see also Question 11).
Q9.5a How Do Different Physical Environments Modulate the Detectability of Potential Biosignatures on Earth (i.e., Their Nature, Abundance, Diversity, and Survivability)?
There is a broad range of factors to contend with in considering how an environment might modulate the detectability of a biosignature within it, with a major concern being the possibility that the biosignature will get “lost in the noise” of the environment in which it resides, rendering it undetectable and generating a “false negative” for the presence of life in the environment. The history of atmospheric O2 is part of the coevolution of the planet and life (Question 6) and discussed in the context of its potential as a biosignature for exoplanets (Question 12). Yet the delayed rise in the concentration of O2 in Earth’s atmosphere following the advent of cyanobacterial oxygenic photosynthesis (the biological process in which water is split for cellular energy, generating O2), spanning up to a billion years of Earth history (Lyons et al. 2014), is perhaps the most well-known example of an environment suppressing the rise of a biosignature. The exact timing of oxygenic photosynthesis and the detection of O2 in Earth’s atmosphere remain controversial (Lyons et al. 2014). In-depth study across a range of inhabited environmental settings, on both the modern and ancient Earth, will yield important insight into the search for life beyond Earth, especially low-energy terrestrial biospheres that can only support a low total biomass that may be difficult to detect against the backdrop of abiological physical and chemical processes. Biomass distribution in these settings is highly heterogeneous; key is the use of preserved environmental guideposts—that is, mineralogic or physical interfaces, to first identify the energetically favorable locales for life and then search for the biosignatures at microscopic scales (Onstott et al. 2019; Table 12-2). Earth’s subsurface provides important opportunities to test and develop understanding of biosignature detectability—where the goal is to “find the biologic needle in the abiotic haystack”—the inverse problem to the norm of our “life-saturated” planet where abiotic analogs of prebiotic processes are the proverbial needles in a biological haystack (Sherwood Lollar et al. 2021; Smith et al. 2021).
TABLE 12-2 Biosignature Reliability, Detectability, and Preservation Vary with Time in the Geologic Record
| Age (Ma) | Biosignature Type Reported | Forms | Formation Type (location) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Isotope | Geochemistry/Mineralogy | Morphology | Organic Carbon | Lipid | ||||
| 1 | X | X | filaments | Ries Impact Crater (Germany) | a, b | |||
| 2 | X | X | X | concretions | Navajo Sandstone (U.S.) | c | ||
| 15 | X | X | microcolonies | Columbia River Basalt (U.S.) | d | |||
| 60 | X | X | concretions | Moeraki Formation (New Zealand) | e | |||
| 84–95 | X | X | X | concretions | Gammon Shale, Mancos Shale Formation, Fronter Formation (U.S.) | f, g | ||
| 115–400 | X | X | X | X | X | microcolonies | Fennoscandian shield granite (N. Europe) | h–j |
| 120 | X | X | X | X | X | microcolonies | Southern Iberia Abyssal Plain (Atlantic Ocean) | k |
| 152 | X | X | concretions | Kimmeridge Clay (England) | l | |||
| 315 | X | X | concretions | Lower Westphalian coal (Scotland) | o | |||
| 355 | X | X | X | filaments | Fennoscandian shield granite (Europe) | m | ||
| 385 | X | X | X | filaments | Arnstein pillow basalt (Germany) | p, q | ||
| 388 | X | filaments | Tynet Burn limestone (Scotland) | r | ||||
| 443 | X | X | ichnofossils | Caledonian ophiolite (New Caledonia) | s | |||
| 458 | X | X | filaments | Lockne Impact Structure (Sweden) | t | |||
| 551 | X | X | concretions | Doushantuo Formation (China) | u | |||
| 1,175 | X | reduction spheroids | Bay of Stoer Formation (Scotland) | n | ||||
| 1,950 | X | X | ichnofossils | Jormua ophiolite complex (Finland) | v | |||
| 2,900–3,350 | X | X | X | ichnofossils | Euro Basalt (Australia) | w, y | ||
| 3,240 | X | filaments | Sulphur Springs Group (U.S.) | y | ||||
| 3,460 | X | Dresser Formation (Australia) | z | |||||
| 3,465 | X | X | X | microcolonies | Apex Chert (Australia) | za–zc | ||
| 3,465 | X | X | ichnofossils | Hooggenoeg Formation (Africa) | zd, ze | |||
| 3,770–4,280 | X | filaments | Nuvvuagittuq belt (Canada) | zf | ||||
a Sapers et al. (2014), b Sapers et al. (2015), c Loope et al. (2010), d McKinley et al. (2000), e Thyne and Boles (1989), f Coleman (1993), g McBride et al. (2003), h Pedersen et al. (1997), i Drake et al. (2015a), j Drake et al. (2015b), k Klein et al. (2015), l Irwin (1980), m Drake et al. (2017), n Spinks et al. (2010), o Curtis et al. (1986), p Drake et al. (2018) and Eickmann et al. (2009), q Peckmann et al. (2007), r Trewin and Knoll (1999), s Furnes et al. (2002), t Ivarsson et al. (2013), u Dong et al. (2008), v Furnes et al. (2005), w Banerjee et al. (2007), x McLoughlin et al. (2012), y Rasmussen (2000), z Shen and Buick (2004), za Schopf et al. (2018), zb Pinti et al. (2009), zc Ueno et al. (2006), zd Banerjee et al. (2006), ze Furnes et al. (2004), zf Dodd et al. (2017).
SOURCE: Modified from Onstott et al. (2019), CC BY 4.0.
Q9.5b Which Biosignatures Are Most Likely to Survive in the Environment, and at What Timescales of Preservation?
The timescales and mechanisms of survivability make up a core research area that includes the survivability of a modern biosignature against processes that would act to destroy it as it forms; the survivability of a fossil biosignature against geological forces such as weathering, diagenesis, influx of brines or groundwaters; and metamorphism, and/or the survivability of abiosignatures (inherently the study of the relative rates of abiotic and biological, and potentially cryptic, processes) (Hoehler and Jorgenson 2013; NASEM 2019a). At one end of the spectrum, informational biomolecules, such as DNA, are highly reliable biosignatures, but they are susceptible to rapid chemical and enzymatic degradation, making their detection in geological samples older than 1 million years highly problematic (NASEM 2019a). Similarly, fossilized biological materials are biased toward multicellular life, on Earth preserved primarily from the Phanerozoic. Table 12-2 demonstrates the categories of biomarkers and the shift in the balance of detectability and reliability as a function of preservation time (Onstott et al. 2019). In older materials, light stable isotopic biosignatures or organic biomarker compounds as examples, have greater survivability, although perhaps less specificity with respect to the nature of the processes that produced them. However, emerging techniques for determining isotopic fractionation of specific atoms within organic compounds offer an approach to overcoming these shortcomings (Hofmann et al. 2020). Morphological and mineralogical biosignatures (e.g., stromatolite morphology and biogenicity) can survive many of the geological forces that act to remove less robust biosignatures from the rock record but are considered much less reliable (Allwood et al. 2018). Continued field, modeling and laboratory-based research is required to inform our understanding of how to reliably probe Earth’s geobiological record and apply those lessons to other habitable worlds (see also Question 11).
Q9.5c What Taphonomic Processes and Environmental Conditions Are Particularly Favorable for Biosignature Preservation on Earth?
Taphonomy is the field of study concerned with processes that result in the formation, preservation, alteration, and destruction of biosignatures. Generally speaking, the taphonomic conditions that favor biosignature preservation are those that promote fossilization through rapid authigenic mineralization and occlusion of porosity during burial, which act to isolate the biosignature from the forces (e.g., oxidation, erosion, and dissolution) that act to destroy them (NASEM 2019a). The taphonomic processes that serve to enhance the preservation of a biosignature, however, also impart biases in Earth’s geobiological record. Record bias can occur when a particular environment and geologic repository are targeted for investigation to the exclusion of other lithologies (NASEM 2019a). Preservation bias is the tendency for environmental and geological forces to favor one “taphonomic window” over another. Together, these two tendencies can create an overarching sense that Earth’s fossil record is written almost entirely in the form of sedimentary rock because of its inherently higher preservation and taphonomic potential (i.e., a preservation bias). This results in a strong tendency to look for the remains of organisms that thrive in surface and near-surface sedimentary environments (i.e., a record bias). Attempts to search for ancient forms of life outside of sedimentary systems (e.g., hydrothermal or deep subsurface settings; ancient continental systems versus younger marine crust and sediments) are faced with distinct challenges, although there are (sometimes controversial) examples that provide useful guideposts (e.g., Staudigel et al. 2015; Djokic et al. 2021) (see also Questions 5 and 6).
Q9.5d How Do “Unknown Unknowns” Impact the Search for Life Beyond Earth?
Identifying life in isolated refugia or ephemeral habitats on Earth (e.g., in the Atacama Desert; ice-free polar regions; hydrothermal vents, arctic and sea ice, and subsurface fracture fluids) has demonstrated that habitability, rather than being a binary state, is a continuum defined over varying time and spatial scales (see also Questions 5 and 10). Increased understanding of life’s limits in so-called extreme environments (see Figure 12-3) has led to a resurgence of interest in adaptations of life to saline fluids and multi-parameter space (e.g., T-P-pH-Eh). The recent discovery of communities existing in the subsurface of the ocean floor and continental lithosphere, away from the influence of the Sun’s energy, has provided new models for rock-hosted, chemosynthetic life that may exist on other worlds (Lin et al. 2006; Onstott et al. 2019; Dunham et al. 2021; Sauvage et al. 2021; Sherwood Lollar
et al. 2021). Expanded understanding of habitability of subsurface environments, brine stability of chemosynthetic organisms, and adaptations of life to saline fluids, through continued field, laboratory, and modeling studies have widespread implications for the search for life in the solar system (NASEM 2019a).
Strategic Research for Q9.5
- Assess the ways in which physical and chemical processes in Earth’s habitable environments affect biosignature and abiosignature detectability and whether those processes favor the detection of certain biosignatures over others using field studies, laboratory, theoretical, and remote sensing approaches designed to investigate the rates of biosignature production and destruction and rates of physical and chemical processes.
- Investigate the processes and environmental conditions on Earth that are most likely to result in the preservation of biosignatures with field studies of biosignature preservation in relevant planetary environments and laboratory studies of the alteration of proposed biosignatures under planetary environmental conditions.
- Characterize on Earth the range of abiotic processes that define the abiotic (nonliving) baseline (abiosignatures), as well as those that are capable of generating false positives from mimics (e.g., inorganically generated morphological biosignatures) on Earth with field/geological studies, laboratory studies focused on generating and characterizing abiosignatures, and analysis of meteorite falls and finds to assess their inventory of chemical compounds that could be mistaken for false positives by a planetary life-detection mission.
- Explore the full range of habitable environments known on Earth and their preserved rock record to develop a more complete understanding of what it means to be “habitable,” reduce record bias in our understanding of how the record of life is preserved, and mitigate against “unknown unknowns” that might confound the search for life outside of Earth by conducting exploratory field studies of the biology, biomass, and processes of biosignature generation and preservation in isolated refugia, low-energy systems, and ephemeral environments and investigations of environments where the biotic signal to abiotic noise or baseline may be particularly low (e.g., so-called extreme, oligotrophic, or subsurface environments).
REFERENCES
Aksyonov, S.A., and P. Williams. 2001. “Impact Desolvation of Electrosprayed Microdroplets—A New Ionization Method for Mass Spectrometry of Large Biomolecules.” Rapid Communications in Mass Spectrometry 15(21):2001–2006.
Allwood, A.C., M.T. Rosing, D.T. Flannery, J.A. Hurowitz, C.M. Heirwegh. 2018. “Reassessing Evidence of Life in 3,700-Mil-lion-Year-Old Rocks of Greenland.” Nature 563:241–244.
Banerjee, N., H. Furnes, K. Muehlenbachs, H. Staudigel, and M. de Wit. 2006. “Preservation of ca. 3.4-3.5 Ga Microbial Biomarkers in Pillow Lavas and Hyaloclastites from the Barberton Greenstone Belt, South Africa.” Earth and Planetary Science Letters 241:707–722.
Banerjee, N., A. Simonetti, H. Furnes, K. Muehlenbachs, H. Staudigel, L. Heaman, and M.J. Van Kranendonk. 2007. “Direct Dating of Archean Microbial Ichnofossils.” Geology 35:487–490.
Baross, J.A., R.E. Anderson, and E.E. Stüeken. 2020. “The Environmental Roots of the Origin of Life.” Pp. 71–92 in Planetary Astrobiology, V.S. Meadows, G.N. Arney, B. Schmidt, and D.J. Des Marais, eds. Tucson: University of Arizona Press.
Becraft, E.D., M.C.Y. Lau Vetter, O.K.I. Bezuidt, J.M. Brown, J.M. Labonté, K. Kauneckaite-Griguole, et al. 2021. “Evolutionary Stasis of a Deep Subsurface Microbial Lineage.” ISME Journal 15:2830–2842.
Benner, S.A., E.A. Bell, E. Biondi, R. Brasser, T. Carell, H.-J. Kim, S.J. Mojzsis, A. Omran, M.A. Pasek, and D. Trail. 2020. “When Did Life Likely Emerge on Earth in an RNA-First Process?” ChemSystemsChem 2:e2000010.
Betts, H.C., M.N. Puttick, J.W. Clark, et al. 2018. “Integrated Genomic and Fossil Evidence Illuminates Life’s Early Evolution and Eukaryote Origin.” Nature Ecology and Evolution 2:1556–1562.
Bottke, W.F., and M.D. Norman. 2017. “The Late Heavy Bombardment.” Annual Review of Earth and Planetary Sciences 45:619–647. https://doi.org/10.1146/annurev-earth-063016-020131.
Cable, M.L., H.V. Tuan, H.E. Maynard-Casely, M. Choukroun, and R. Hodyss. 2018. “The Acetylene-Ammonia Co-Crystal on Titan.” ACS Earth and Space Chemistry 2:366–375.
Chan, M.A., N.W. Hinman, S.L. Potter-McIntyre, K.E. Schubert, R.J. Gillams, S.M. Awramik, P.J. Boston, et al. 2019. “Deciphering Biosignatures in Planetary Contexts.” Astrobiology 19(9):1075–1102. https://doi.org/10.1089/ast.2018.1903.
Coleman, M.L. 1993. “Microbial Processes: Controls on the Shape and Composition of Carbonate Concretions.” Marine Geology 113:127–140.
Creamer, J.S., M.F. Mora, and P.A. Willis. 2017. “Enhanced Resolution of Chiral Amino Acids with Capillary Electrophoresis for Biosignature Detection in Extraterrestrial Samples.” Analytical Chemistry 89(2):1329–1337.
Curtis, C.D., M.L. Coleman, and L.G. Love. 1986. “Pore Water Evolution During Sediment Burial from Isotopic and Mineral Chemistry of Calcite, Dolomite and Siderite Concretions.” Geochimica et Cosmochimica Acta 50:2321–2334.
Damer, B., and D. Deamer. 2020. “The Hot Spring Hypothesis for an Origin of Life.” Astrobiology 20:429–452.
Des Marais, D.J., L.J. Allamandola, S.A. Benner, A.P. Boss, D. Deamer, P.G. Falkowski, J.D. Farmer, et al. 2003. “The NASA Astrobiology Roadmap.” Astrobiology 3(2):219–235.
Des Marais, D.J., J.A. Nuth, L.J. Allamandola, A.P. Boss, J.D. Farmer, T.M. Hoehler, B.M. Jakosky, et al. 2008. “The NASA Astrobiology Roadmap.” Astrobiology 8:715–730. https://doi.org/10.1089/ast.2008.0819.
D’Hondt, S., A.J. Spivack, R. Pockalny, T.G. Ferdelman, J.P. Fischer, J. Kallmeyer, L.J. Abrams, et al. 2009. “Subseafloor Sedimentary Life in the South Pacific Gyre.” Proceedings of the National Academy of Sciences 106:11651–11656.
D’Hondt, S., R. Pockalny, V.M. Fulfer, and A.J. Spivack. 2019. “Subseafloor Life and Its Biogeochemical Impacts.” Nature Communications 10:3519.
Djokic, T., M.J. Van Kranendonk, K.A. Campbell, J.R. Havig, M.R. Walter, and D.M. Guido. 2021. “A Reconstructed Subaerial Hot Spring Field in the ~3.5 Billion-Year-Old Dresser Formation, North Pole Dome, Pilbara Craton, Western Australia.” Astrobiology 21(1):1–38.
Dodd, M.S., D. Papineau, T. Grenne, J.F. Slack, M. Rittner, F. Pirajno, J. O’Neil, and C.T.S. Little. 2017. “Evidence for Early Life in Earth’s Oldest Hydrothermal Vent Precipitates.” Nature 543:60–64.
Dong, J., S. Zhang, G. Jiang, Q. Zhao, H. Li, X. Shi, and J. Liu. 2008. “Early Diagenetic Growth of Carbonate Concretions in the Upper Doushantuo Formation in South China and Their Significance for the Assessment of Hydrocarbon Source Rock.” Science in China Series D: Earth Science 51:1330–1339.
Drake, H., M.E. Åström, C. Heim, C. Broman, J. Åström, M. Whitehouse, M. Ivarsson, S. Siljeström, and P. Sjövall. 2015a. “Extreme 13C Depletion of Carbonates Formed During Oxidation of Biogenic Methane in Fractured Granite.” Nature Communications 6:7020. https://doi.org/10.1038/ncomms8020.
Drake, H., E.L. Tullborg, M. Whitehouse, B. Sandberg, T. Blomfeldt, and M.E. Åström. 2015b. “Extreme Fractionation and the Microscale Variation of Sulphur Isotopes During Bacterial Sulphate Reduction in Deep Groundwater Systems.” Geochimica et Cosmochimica Acta 161:1–18.
Drake, H., C. Heim, N.M.W. Roberts, T. Zack, M. Tillberg, C. Bromane, M. Ivarsson, M.J. Whitehouse, and M.E. Åström. 2017. “Isotopic Evidence for Microbial Production and Consumption of Methane in the Upper Continental Crust Throughout the Phanerozoic Eon.” Earth and Planetary Science Letters 470:108–118.
Drake, H., M.J. Whitehouse, C. Heim, P.W. Reiners, M. Tillberg, K.J. Hogmalm, M. Dopson, C. Broman, and M.E. Åström. 2018. “Unprecedented 34S-Enrichment of Pyrite Formed Following Microbial Sulfate Reduction in Fractured Crystalline Rocks.” Geobiology 16:556–574.
Dunham, E.C., J.E. Dore, M.L. Skidmore, E.E. Roden, and E.S. Boyd. 2021. “Lithogenic Hydrogen Supports Microbial Primary Production in Subglacial and Proglacial Environments.” Proceedings of the National Academy of Sciences 118(2):e2007051117.
Ehlmann, B.L., J.F. Mustard, S.L. Murchie, J.P. Bibring, A. Meunier, A.A. Fraeman, and Y. Langevin. 2011. “Subsurface Water and Clay Mineral Formation During the Early History of Mars.” Nature 479:53–60.
Ehlmann, B.L., F.S. Anderson, J. Andrews-Hanna, D.C. Catling, P.R. Christensen, B.A. Cohen, C.D. Dressing, et al. 2016. “The Sustainability of Habitability on Terrestrial Planets: Insights, Questions, and Needed Measurements from Mars for Understanding the Evolution of Earth-Like Worlds.” Journal of Geophysical Research: Planets 121. https://doi.org/10.1002/2016JE005134.
Eickmann, B., W. Bach, S. Kiel, J. Reitner, and J. Peckmann. 2009. “Evidence for Cryptoendolithlc Life in Devonian Pillow Basalts of Variscan Orogens, Germany.” Palaeogeography Palaeoclimatology Palaeoecology 283:120–125.
Etiope, G., and B. Sherwood Lollar. 2013. “Abiotic Methane on Earth.” Review of Geophysics 51:276–299.
Falkowski, P.G., T. Fenchel, and E.F. Delong. 2008. “The Microbial Engines That Drive Earth’s Biogeochemical Cycles.” Science 320:1034–1039. https://doi.org/10.1126/science.1153213.
Fani, R. 2012. “The Origin and Evolution of Metabolic Pathways: Why and How Did Primordial Cells Construct Metabolic Routes?” Evolution: Education and Outreach 5:367–381.
Forterre, P. 2006. “Three RNA Cells for Ribosomal Lineages and Three DNA Viruses to Replicate Their Genomes: A Hypothesis for the Origin of Cellular Domain.” Proceedings of the National Academy of Sciences 103:3669–3674.
Furnes, H., K. Muehlenbachs, T. Torsvik, O. Tumyr, and L. Shi. 2002. “Biosignatures in Metabasaltic Glass of a Caledonian Ophiolite, West Norway.” Geological Magazine 139:601–608.
Furnes, H., N.R. Banerjee, K. Muehlenbachs, H. Staudigel, and M. de Wit. 2004. “Early Life Recorded in Archean Pillow Lavas.” Science 304:578–581.
Furnes, H., N.R. Banerjee, K. Muehlenbachs, and A. Kontinen. 2005. “Preservation of Biosignatures in Metaglassy Volcanic Rocks from the Jormua Ophiolite Complex, Finland.” Precambrian Research 136:125–137.
Grotzinger, J.P., and A.H. Knoll. 1999. “Stromatolites in Precambrian Carbonates: Evolutionary Mileposts or Environmental Dipsticks?” Annual Review of Earth and Planetary Sciences 27:313–358.
Hand, K.P., A.E. Murray, J.B. Garvin, W.B. Brinckerhoff, B.C. Christner, K.S. Edgett, B.L. Ehlmann, et al. 2017. Report of the Europa Lander Science Definition Team. https://solarsystem.nasa.gov/docs/Europa_Lander_SDT_Report_2016.pdf.
Hand, K.P., C. Sotin, A. Hayes, and A. Coustenis. 2020. “On the Habitability and Future Exploration of Ocean Worlds.” Space Science Reviews 216(5):95. https://doi.org/10.1007/s11214-020-00713-7.
Head, I.M., N.D. Gray, and S.R. Larter. 2014. “Life in the Slow Lane: Biogeochemistry of Biodegraded Petroleum Containing Reservoirs and Implications for Energy Recovery and Carbon Management.” Frontiers in Microbiology 5. https://doi.org/10.3389/fmicb.2014.00566.
Hendrix, A.R., T.A. Hurford, L.M. Barge, M.T. Bland, J.S. Bowman, W. Brinckerhoff, B.K. Buratti, et al. 2019. “The NASA Roadmap to Ocean Worlds.” Astrobiology 19. https://doi.org/10.1089/ast.2018.1955.
Heuer, V.B., F. Inagaki, Y. Morono, Y. Kubo, A.J. Spivack, B. Viehweger, T. Treude, et al. 2020. “Temperature Limits to Deep Subseafloor Life in the Nankai Trough Subduction Zone.” Science 370:1230–1234.
Hoefs, J. 2004. Stable Isotope Geochemistry. Berlin: Springer-Verlag.
Hoehler, T.M., and B.B. Jørgensen. 2013. “Microbial Life Under Extreme Energy Limitation.” Nature Reviews in Microbiology 11:83–94.
Hoehler, T.M., W. Bains, A. Davila, M.N. Parenteau, and A. Pohorille. 2020. “Life’s Requirements, Habitability, and Biological Potential.” Pp. 37–70 in Planetary Astrobiology, V.S. Meadows, G.N. Arney, B. Schmidt, and D.J. Des Marais, eds. Tucson: University of Arizona Press.
Hofmann, A.E., L. Chimiak, B. Dallas, J. Griep-Raming, D. Juchelka, A. Makarov, J. Schwieters, et al. 2020. “Using Orbitrap Mass Spectrometry to Assess the Isotopic Compositions of Individual Compounds in Mixtures.” International Journal of Mass Spectrometry 457:116410. https://doi.org/10.1016/j.ijms.2020.116410.
Hug, L., B. Baker, K. Anantharaman, C.T. Brown, A.J. Probst, C.J. Castelle, C.N. Butterfield, et al. 2016. “A New View of the Tree of Life.” Nature Microbiology 1:16048. https://doi.org/10.1038/nmicrobiol.2016.48.
Imachi, H., M.K. Nobu, N. Nakahara, Y. Morono, M. Ogawara, Y. Takaki, Y. Takano, et al. 2020. “Isolation of an Archaeon at the Prokaryote–Eukaryote Interface.” Nature 577:519–525.
Inagaki, F., K.-U. Hinrichs, Y. Kubo, M.W. Bowles, V.B. Heuer, W.-L. Hong, T. Hoshino, et al. 2015. “Exploring Deep Microbial Life in Coal-Bearing Sediment Down to 2.5 km Below the Ocean Floor.” Science 349:420–424.
Irwin, H. 1980. “Early Carbonate Precipitation and Pore Fluid Migration in the Kimmeridge Clay of Dorset, England.” Sedimentology 27:577–591.
Ivarsson, M., C. Broman, E. Sturkell, J. Ormö, S. Siljeström, M. van Zuilen, and S. Bengtson. 2013. “Fungal Colonization of an Ordovician Impact-Induced Hydrothermal System.” Science Reports 3. https://doi.org/10.1038/srep03487.
Kempes, C.P., M.A.R. Koehl, and G.B. West. 2019. “The Scales That Limit: The Physical Boundaries of Evolution.” Frontiers in Ecology and Evolution 7. https://doi.org/10.3389/fevo.2019.00242.
Kempes, C.P., S.I. Walker, H.V. Graham, C.H. House, and H.L. Smith. 2021. “Generalized Stoichiometry and Biogeochemistry for Astrobiological Applications.” Bulletin of Mathematical Biology 83:73. https://doi.org/10.1007/s11538-021-00877-5.
Kim, H., H.B. Smith, C. Mathis, J. Raymond, and S.I. Walker. 2019. “Universal Scaling Across Biochemical Networks on Earth.” Science Advances 5(1):eaau0149.
Klein, F., S.E. Humphris, W. Guo, F. Schubotz, E.M. Schwarzenbach, and W.D. Orsi. 2015. “Fluid Mixing and the Deep Biosphere of a Fossil Lost City-Type Hydrothermal System at the Iberia Margin.” Proceedings of the National Academy of Sciences 112:12036–12041.
Koonin, E.V., V.V. Doija, M. Krupovic, and J.H. Kuhn. 2021. “Viruses Defined by the Position of the Virosphere Within the Replicator Space.” Microbiology and Molecular Biology Reviews 85(4):e00193-20.
Lang, S.Q., G.L. Früh-Green, S.M. Bernasconi, W.J. Brazelton, M.O. Schrenk, and J.M. McGonigle. 2018. “Deeply-Sourced Formate Fuels Sulfate Reducers But Not Methanogens at Lost City Hydrothermal Field.” Scientific Reports 8:755. https://doi.org/10.1038/s41598-017-19002-5.
LaRowe, D., and J. Amend. 2019. “Energy Limits for Life in the Subsurface.” Pp. 585–619 in Deep Carbon: Past to Present, B. Orcutt, I. Daniel, and R. Dasgupta, eds. Cambridge, UK: Cambridge University Press.
Li, L., B.A. Wing, T.H. Bui, J.M. McDermott, G.F. Slater, S. Wei, G. Lacrampe-Couloume, et al. 2016. “Sulfur Mass-Independent Fractionation in Subsurface Fracture Waters Indicates a Long-Standing Sulfur Cycle in Precambrian Rocks.” Nature Communications 7:13252.
Lin, L.-H., P. Wang, D. Rubmle, J. Lippmann-Pipke, E. Boice, L.M. Pratt, B. Sherwood Lollar, et al. 2006. “Long-Term Sustainability of a High-Energy, Low-Diversity Crustal Biome.” Science 314:479–482.
Lollar, G.S., O. Warr, J. Telling, M.R. Osburn, and B. Sherwood Lollar. 2019. “‘Follow the Water’: Hydrogeochemical Constraints on Microbial Investigations 2.4 km Below Surface at the Kidd Creek Deep Fluid and Deep Life Observatory.” Geomicrobiology Journal 36(10):859–872.
Loope, D.B., R.M. Kettler, and K.A. Weber. 2010. “Follow the Water: Connecting a CO2 Reservoir and Bleached Sandstone to Iron-Rich Concretions in the Navajo Sandstone of South-Central Utah, USA.” Geology 38:999–1002.
Lu, R., C. Cheng, T. Nagel, H. Milsch, H. Yasuhara, O. Kolditz, and H. Shao. 2021. “What Process Causes the Slowdown of Pressure Solution Creep.” Geomechanics and Geophysics for Geo-Energy and Geo-Resources 7:57. https://doi.org/10.1007/s40948-021-00247-4.
Lyons, T., C. Reinhard, and N. Planavsky. 2014. “The Rise of Oxygen in Earth’s Early Ocean and Atmosphere.” Nature 506:307–315. https://doi.org/10.1038/nature13068.
MacKenzie, S.M., S.P.D. Birch, S. Horst, C. Sotin, E. Barth, J.M. Lora, M.G. Trainer, et al. 2021. “Titan: Earth-Like on the Outside, Ocean World on the Inside.” arXiv 2102.08472v1.
Magnabosco, C., L.-H. Lin, H. Dong, M. Bomberg, W. Ghiorse, H. Stan-Lotter, K. Pedersen, et al. 2018. “The Biomass and Biodiversity of the Continental Subsurface.” Nature Geosciences 11:707–717.
Magnus, I., and S. Holmström. 2012. “The Use of ESEM in Geobiology.” In Scanning Electron Microscopy, K. Viacheslav, ed. https://www.intechopen.com/chapters/30964.
Marshall, S.M., C. Mathis, E. Carrick, P.S. Gromski, G.J.T. Cooper, G. Keenan, H.V. Graham, et al. 2021. “Identifying Molecules as Biosignatures with Assembly Theory and Mass Spectrometry.” Nature Communications 12:3033.
Martin, W., J. Baross, D. Kelley, and M.J. Russell. 2008. “Hydrothermal Vents and the Origin of Life.” Nature Reviews Microbiology 6:805–814.
McBride, E.F., M.D. Picard, and K.L. Milliken. 2003. “Calcite Cemented Concretions in Cretaceous Sandstone, Wyoming and Utah, U.S.A.” Journal of Sedimentary Research 73:462–483.
McDermott, J.M., J.S. Seewald, C.R. German, and S.P. Sylva. 2015. “Pathways for Abiotic Organic Synthesis at Submarine Hydrothermal Fields.” Proceedings of the National Academy of Sciences 112:7668–7672.
McKinley, J.P., T.O. Stevens, and F. Westall. 2000. “Microfossils and Paleoenvironments in Deep Subsurface Basalt Samples.” Geomicrobiology Journal 17:43–54.
McLoughlin, N., E.G. Grosch, M.R. Kilburn, and D. Wacey. 2012. “Sulfur Isotope Evidence for a Paleoarchean Subseafloor Biosphere, Barberton, South Africa.” Geology 40:1031–1034.
Meadows, V.S. 2017. “Reflections on O2 as a Biosignature in Exoplanetary Atmospheres.” Astrobiology 17:1022–1052.
Meadows, V.S., C.T. Reinhard, G.N. Arney, M.N. Parenteau, E.W. Schwieterman, S.D. Domagal-Goldman, A. Lincowski, et al. 2018. “Exoplanet Biosignatures: Understanding Oxygen as a Biosignature in the Context of Its Environment. Astrobiology 18:630–662.
Ménez, B., V. Pasini, and D. Brunelli. 2012. “Life in the Hydrated Suboceanic Mantle.” Nature Geoscience 5:133–137.
Ménez, B., C. Pisapia, M. Andreani, F. Jamme, Q.P. Vanbellingen, A. Brunelle, L. Richard, et al. 2018. “Abiotic Synthesis of Amino Acids in the Recesses of the Oceanic Lithosphere.” Nature 564:59–63.
Merino, N., H.S. Aronson, D.P. Bojanova, J. Feyhl-Buska, M.L. Wong, S. Zhang, and D. Giovannelli. 2019. “Living at the Extremes: Extremophiles and the Limits of Life in a Planetary Context.” Frontiers in Microbiology 10:780. https://doi.org/10.3389/fmicb.2019.00780.
Mojzsis, S.J., G. Arrhenius, K.D. McKeegan, T.M. Harrison, A.P. Nutman, and C.R.L. Friend. 1996. “Evidence for Life on Earth Before 3,800 Million Years Ago.” Nature 384:55–59.
Mustard, J.F., M. Adler, A. Allwood, D.S. Bass, D.W. Beaty, J.F. Bell III, W.B. Brinckerhoff, et al. 2013. Report of the Mars 2020 Science Definition Team. Mars Exploration Program Analysis Group (MEPAG). http://mepag.jpl.nasa.gov/reports/MEP/Mars_2020_SDT_Report_Final.pdf.
Mykytczuk, N.C.S., S.J. Foote, C.R. Omelon, G. Southam, C.W. Greer, and L.G. Whyte. 2013. “Bacterial Growth at −15°C; Molecular Insights from the Permafrost Bacterium Planococcus halocryophilus Or1.” The ISME Journal 7:1211–1226.
NASA (National Aeronautics and Space Administration). 2015. NASA Astrobiology Strategy 2015. Washington, DC: NASA Astrobiology Program. https://astrobiology.nasa.gov/nai/media/medialibrary/2015/10/NASA_Astrobiology_Strategy_2015_151008.pdf.
NASA. 2020. Enceladus Orbilander: A Flagship Mission Concept for Astrobiology. Planetary Mission Concept Study for the 2023–2032 Decadal Survey. Johns Hopkins University Applied Physics Laboratory. https://science.nasa.gov/files/atoms/files/Enceladus%20Orbilander.pdf.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2019a. An Astrobiology Strategy for the Search for Life in the Universe. Washington, DC: The National Academies Press.
Neveu, M., L.E. Hays, M.A. Voytek, M.H. New, and M.D. Schulte. 2018. “The Ladder of Life Detection.” Astrobiology 18:1375–1402.
Olson, S.L., E.W. Schwieterman, C.T. Reinhard, and T.W. Lyons. 2018. “Earth: Atmospheric Evolution of a Habitable Planet.” Pp. 2817–2853 in Handbook of Exoplanets, H. Deeg and J. Belmonte, eds. Cham, Switzerland: Springer. https://doi.org/10.1007/978-3-319-55333-7_189.
Onstott, T.C., B.L. Ehlmann, H. Sapers, M. Coleman, M. Ivarsson, J.J. Marlow, A. Neubeck, and P. Niles. 2019. “Paleo-RockHosted Life on Earth and the Search on Mars: A Review and Strategy for Exploration.” Astrobiology 19(10):1230–1262. https://doi.org/10.1089/ast.2018.1960.
Pace, N.R. 1997. “A Molecular View of Microbial Diversity and the Biosphere.” Science 276:734–740.
Peckmann, J., W. Bach, K. Behrens, and J. Reitner. 2007. “Putative Cryptoendolithic Life in Devonian Pillow Basalt, Rheinisches Schiefergebirge, Germany.” Geobiology 6:125–135.
Pedersen, K., S. Ekendahl, E.-L. Tullborg, H. Furnes, I. Thorseth, and O. Tumyr. 1997. “Evidence of Ancient Life at 207 m Depth in a Granitic Aquifer.” Geology 25:827–830.
Pinti, D.L., R. Mineau, and V. Clement. 2009. “Hydrothermal Alteration and Microfossil Artefacts of the 3,465-Million-Year-Old Apex Chert.” Nature Geosciences 2:640–643.
Rasmussen, B. 2000. “Filamentous Microfossils in a 3,235-Million-Year-Old Volcanogenic Massive Sulphide Deposit.” Nature 405:676–679.
Reveillaud, J., E. Reddington, J. McDermott, C. Algar, J.L. Meyer, S. Sylva, J. Seewald, et al. 2016. “Subseafloor Microbial Communities in Hydrogen-Rich Vent Fluids from Hydrothermal Systems Along the Mid-Cayman Rise.” Environmental Microbiology 8:1970–1987.
Rosing, M.T., N.M. Rose, D. Brigwater, and H.S. Thomsen. 1996. “Earliest Part of Earth’s Stratigraphic Record: A Reappraisal of the 3.7 Ga Isua (Greenland) Supracrustal Sequence.” Geology 24(1):43–46.
Sapers, H.M., G.R. Osinski, N.R. Banerjee, and L.J. Preston. 2014. “Enigmatic Tubular Features in Impact Glass.” Geology 42:471–474.
Sapers, H.M., N.R. Banerjee, and G.R. Osinski. 2015. “Potential for Impact Glass to Preserve Microbial Metabolism.” Earth and Planet Science Letters 430:95–104.
Sauvage, J.F., A. Flinders, A.J. Spivack, R. Pockalny, A.G. Dunlea, C.H. Anderson, D.C. Smith, et al. 2021. “The Contribution of Water Radiolysis to Marine Sedimentary Life.” Nature Communications 12(1):1297. https://doi.org/10.1038/s41467-021-21218-z.
Schidlowski, M., P.W.U. Appel, R. Eichman, and C.E. Junge. 1979. “Carbon Isotope Geochemistry of the 3.7×109-Yr-Old Isua Sediments, West Greenland: Implications for the Archaean Carbon and Oxygen Cycles.” Geochimica et Cosmochimica Acta 43:189–199.
Schopf, J.W., K. Kitajima, M.J. Spicuzza, A.B. Kudryavtsev, and J.W. Valley. 2018. “SIMS Analyses of the Oldest Known Assemblage of Microfossils Document Their Taxon-Correlated Carbon Isotope Compositions.” Proceedings of the National Academy of Sciences 115:53–58.
Schrenk, M.O., W.J. Brazelton, and S.Q. Lang. 2013. “Serpentinization, Carbon, and Deep Life.” Reviews in Mineralogy and Geochemistry 75:575–606.
Shen, Y., and R. Buick. 2004. “The Antiquity of Microbial Sulfate Reduction.” Earth-Science Reviews 64:243–272.
Sherwood Lollar, B., T.C. Onstott, G. Lacrampe-Couloume, and C.J. Ballentine. 2014. “The Contribution of the Precambrian Continental Lithosphere to Global H2 Production.” Nature 516:379–382.
Sherwood Lollar, B., V.B. Heuer, J. McDermott, S. Tille, O. Warr, J.J. Moran, J. Telling, and K.-U. Hinrichs. 2021. “A Window into the Abiotic Carbon Cycle—Acetate and Formate in Fracture Waters in 2.7 Billion Year Old Host-Rocks of the Canadian Shield.” Geochimica et Cosmochimica Acta 294:295–314.
Shock, E.L., and M.D. Schulte. 1998. “Organic Synthesis During Fluid Mixing in Hydrothermal Systems.” Journal of Geophysical Research 103(E12):28513–28527.
Sittler, E.C., J.F. Cooper, S.J. Sturner, and A. Asgraf. 2020. “Titan’s Ionospheric Chemistry, Fullerenes, Oxygen, Galactic Cosmic Rays and the Formation of Exobiological Molecules on and Within Its Surfaces and Lakes.” Icarus 344:113246.
Smith, E., and H.J. Morowitz. 2016. “The Origin and Nature of Life on Earth: The Emergence of the Fourth Geosphere.” Contemporary Physics 58:115–116.
Smith, H.L., A.K. Hyde, D.N. Simkus, H.V. Graham, and C.H. House. 2021. “The Grayness of the Origin of Life.” Life 11:498. https://doi.org/10.3390/life11060498.
Sparks, W.B., B.E. Schmidt, M.A. McGrath, K.P. Hand, J.E. Spencer, M. Cracraft, and S.E. Deustua. 2017. “Active Cryovolcanism on Europa?” Astrophysical Journal Letters 839(2):L18. https://doi.org/10.3847/2041-8213/aa67f8.
Spinks, S.C., J. Parnell, and S.A. Bowden. 2010. “Reduction Spots in the Mesoproterozoic Age: Implications for Life in the Early Terrestrial Record.” Astrobiology 9:209–216.
Stamenkovic, V., L.W. Beegle, K. Zacny, D.D. Arumugam, P. Baglioni, N. Barba, J. Baross, et al. 2019. “The Next Frontier for Planetary and Human Exploration.” Nature Astronomy 3:116–120. https://doi.org/10.1038/s41550-018-0676-9.
Staudigel, H., H. Furnes, and M. DeWit. 2015. “Paleoarchean Trace Fossils in Altered Volcanic Glass.” Proceedings of the National Academy of Sciences 112:6892–6897.
Steele, A., F.M. McCubbin, M. Fries, L. Kater, N.Z. Boctor, M.L. Fogel, P.G. Conrad, et al. 2012. “A Reduced Organic Carbon Component in Martian Basalts.” Science 337:212–215.
Stevens, E.W.N., J.V. Bailey, B.E. Flood, et al. 2015. “Barite Encrustation of Benthic Sulfur-Oxidizing Bacteria at a Marine Cold Seep.” Geobiology 13:588–603.
Summons, R.E., P. Albrecht, G. Mcdonald, and J.M. Moldowan. 2008. “Molecular Biosignatures.” Space Science Reviews 135:133–159. https://doi.org/10.1007/s11214-007-9256-5.
Suzuki, Y., S. Yamashita, M. Kouduka, Y. Ao, H. Mukai, S. Mitsunobu, H. Kagi, et al. 2020. “Deep Microbial Proliferation at the Basalt Interface in 33.5–104 Million-Year-Old Oceanic Crust.” Communications Biology 3:136. https://doi.org/10.1038/s42003-020-0860-1.
Thyne, G.D., and J.R. Boles. 1989. “Isotopic Evidence for Origin of Moeraki Septarian Concretions, New Zealand.” Journal of Sedimentary Petrology 59:272–279.
Trembath-Reichert, E., Y. Morono, A. Ijiri, T. Hoshino, K.S. Dawson, F. Inagaki, and V.J. Orphan. 2017. “Methyl-Compound Use and Slow Growth Characterize Microbial Life in 2 km-Deep Subseafloor Coal and Shale Beds.” Proceedings of the National Academy of Sciences 114:44.
Trewin, N.H., and A.H. Knoll. 1999. “Preservation of Devonian Chemotrophic Filamentous Bacteria in Calcite Veins.” Palaios 14:288–294.
Ueno, Y., K. Yamada, N. Yoshida, S. Maruyama, and Y. Isozaki, Y. 2006. “Evidence from Fluid Inclusions for Microbial Methanogenesis in the Early Archaean Era.” Nature 440:526–519.
Van Zuilen, M.A., A. Lepland, J. Teranes, J. Finarelli, M. Wahlen, and G. Arrhenius. 2003. “Graphite and Carbonates in the 3.8 Ga Old Isua Supracrustal Belt, Southern West Greenland.” Precambrian Research 126:331–348.
Vance, S., J. Harnmeijer, J. Kimura, H. Hussmann, B. Demartin, and J.M. Brown. 2007. “Hydrothermal Systems in Small Ocean Planets.” Astrobiology 7:987–1005.
Waite, J.H., C.R. Glein, R.S. Perryman, B.D. Teolis, B.A. Magee, G. Miller, J. Grimes, et al. 2017. “Cassini Finds Molecular Hydrogen in the Enceladus Plume: Evidence for Hydrothermal Processes.” Science 356(6334):155–159.
Walker, S.I. 2019. “The New Physics Needed to Probe the Origins of Life.” Nature 569:36–38.
Wierzchos, J., M.C. Casero, O. Artieda, and C. Ascaso. 2018. “Endolithic Microbial Habitats as Refuges for Life in Poly-Extreme Environment of the Atacama Desert.” Current Opinion in Microbiology 43:124–131.
Woese, C.R., O. Kandler, and M.L. Wheelis. 1990. “Towards a Natural System of Organisms: Proposal for the Domains Archaea, Bacteria, and Eucarya.” Proceedings of the National Academy of Sciences 87(12):4576–4579. https://doi.org/10.1073/pnas.87.12.4576.
This page intentionally left blank.

SOURCE: Composed by P.K. Byrne. Images: Courtesy of NASA/JPL-Caltech/Space Science Institute.






























