14
Question 11: Search for Life Elsewhere
Is there evidence of past or present life in the solar system beyond Earth, and how do we detect it?
Building on insights from terrestrial life and our understanding of the diversity of habitable environments elsewhere, as well as significant advancement in biosignature detection technologies, we are poised to conduct a rigorous, systematic search for life beyond Earth in the solar system.1 Four hundred years after Galileo revolutionized our understanding of our place in the universe, ours could realistically be the generation that triggers another scientific revolution, this time in biology.
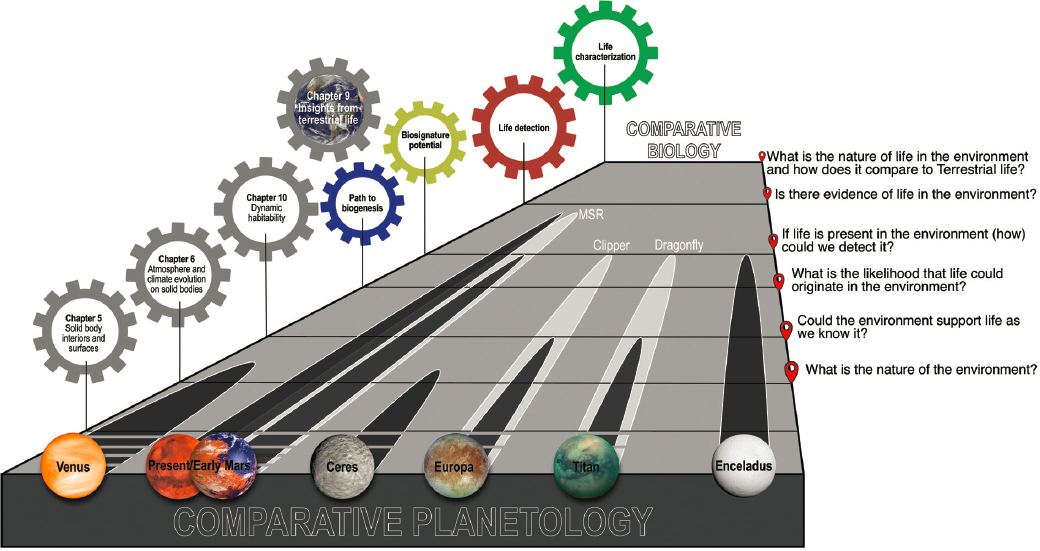
The search for evidence of life is part of the systematic progression in our understanding of planetary environments, which also encompasses detailed environmental characterization (Figure 14-1). Hence, a well-conceived arc of life detection activities serves to expand our knowledge of planetary environments, whether evidence of life is found or not. In the past decade, past and presently habitable environments beyond Earth were identified, providing a rich spectrum of worlds to explore in the context of astrobiology (Figure 14-2). Some of these environments introduce the potential to understand a biochemistry and/or emergence distinct from that of life on Earth, and thus we might begin to develop a universal theory of living systems.
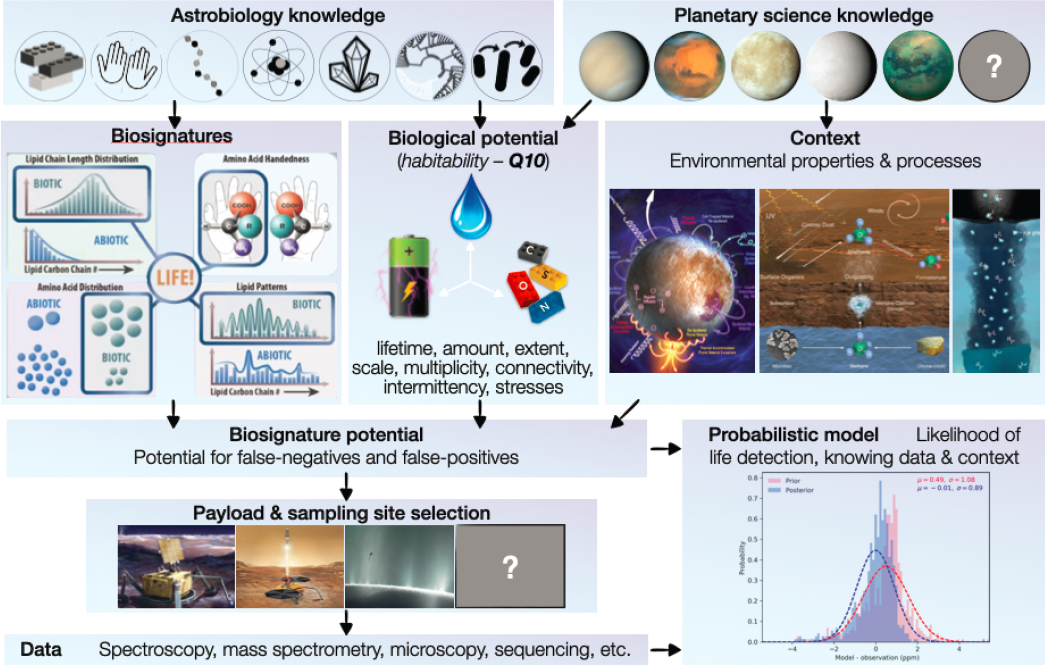
In the coming decade, biosignature searches will require evolution in how planetary systems are studied by new missions, technologies, and approaches to data analysis, shaped by our expanding knowledge of the range of habitats and biochemistries that can produce detectable biosignatures. A comprehensive framework is required to interpret potential biosignatures, abiosignatures, false positives, and false negatives, and promote confidence and consensus in interpretations. This framework needs to leverage past experiences with previous searches for signs of life in Earth and planetary environments and materials (e.g., Viking and ALH84001) to improve our approach of data interpretation and how results are communicated and evaluated, both within the planetary science community and by the general public. Our understanding of Earth life provides a starting point for investigation (Question 9, Chapter 12), contrasting the signatures of life on Earth with the prebiotic/abiotic chemistry of primitive bodies. Key areas of research also include the search for and identification of potential biosignatures that are agnostic to life’s molecular makeup or metabolism, and studies of signatures of abiotic processes and phenomena that may define the nonbiological baseline and/or mimic biosignatures, affecting biosignature reliability (NASEM 2019). Expanding the search to include the possibility of nonterran life (i.e., life “not as we know it”) (NRC 2007) requires
___________________
1 A glossary of acronyms and technical terms can be found in Appendix F.
further technical and conceptual maturation, including advances in statistical methods, scaling laws, information theory, and probabilistic approaches. Understanding the relationship between the geochemical environment and the prebiotic pathways that can give rise to life requires cooperation among diverse disciplines that extends beyond the traditional platform to include geochemists, atmospheric chemists, geologists, geophysicists, astronomers, mission scientists and engineers, and astrobiologists, among others (NASEM 2019; Lyons et al. 2020).
Q11.1 PATH TO BIOGENESIS: WHAT IS THE EXTENT AND HISTORY OF ORGANIC CHEMICAL EVOLUTION, POTENTIALLY LEADING TOWARD LIFE, IN HABITABLE ENVIRONMENTS THROUGHOUT THE SOLAR SYSTEM? HOW DOES THIS INFORM THE LIKELIHOOD OF FALSE POSITIVE LIFE DETECTIONS?
Presumably, life originates from a series of abiotic reactions in an organic chemical system, in which prebiotic synthesis of building blocks increases in complexity to ultimately reach biotic synthesis in a living organism. This process of organic chemical evolution develops gradually from simple to complex in terms of both the types of chemical reactions that occur and the diversity of compounds produced. Organic chemical evolution is highly dependent on the geochemical environment: factors such as pH, salinity, temperature, pressure, and the abundance of chemical precursors influence which reactions are favored, the rates at which they proceed, and the lifetimes of reactive intermediates and products available for subsequent chemistry. By understanding the extent and history of organic chemical evolution in the various habitable environments throughout the solar system, we can identify where other worlds might lie along the path to biogenesis (Q10.4), with a spectrum of possible endmembers ranging from habitable (but uninhabited) environments to fully inhabited worlds like Earth (Q9.1d; Sutherland 2017).

A thorough understanding of abiotic organic chemical evolution across a diverse set of geochemical conditions informs the likelihood of an independent emergence of life, and helps reduce the likelihood of a false positive result in the search for life beyond Earth.
Research in the emergence of life on Earth and knowledge of extant biochemistry can guide system-level analyses to differentiate between extraterrestrial biotic and abiotic chemistry (Q9.1c). While abiotic chemistry can utilize multiple pathways to generate a particular compound, biotic chemistry is characterized by evolved chemical systems that capitalize on a distinct subset of precursors among the abiotic possibilities. In the past decade, new approaches to understanding complex networks of interacting molecules in chemical and biological systems have emerged. These approaches, which form the basis of systems chemistry and systems biology, need to be incorporated into astrobiology-focused missions by including analyses of potential precursors associated with any molecular target. It is not enough to simply search for a biosignature; an understanding of environmental processes that might result in false positives, such as high concentrations of abiotic organic compounds, is needed to interpret potential biosignatures as a robust biological signal. To assist in these analyses, significant support for research in prebiotic and general organic chemistry will be needed to enhance our knowledge of abiotic synthetic routes to the building blocks of life and to uncover possible pathways that other forms of life might have adopted. In concert with such efforts, contextual environmental and/or geological information needs to be gathered in order to understand what other features in the environment might provide added confidence to the assessment of the biogenicity of a potential biosignature.
A complete picture of the organic chemical evolution in habitable environments requires a thorough understanding of the organics present in and on planetary bodies as they formed (Questions 2 and 3, Chapters 5 and 6), and processes that might have modified them over time (Questions 4–6 and 10, Chapters 7–9 and 13). The organic inventories of meteorites, interplanetary dust particles (IDPs), asteroids, and comets are also important, in that these exogenous sources of organic matter can act as “chemical seeds” for the organic evolution of habitable environments, and also represent important abiotic chemical backgrounds against which signs of life need to be
identified. Laboratory experiments designed to simulate the conditions and processes that occur in extraterrestrial material environments, such as cosmic ice irradiation and low-temperature photochemistry and geochemistry, are an important complement to the characterization of astromaterials (Hoehler et al. 2020). These experiments are advantageous in that they can help create a system-level understanding of the dynamic processes that give rise to organic inventories in astromaterials (Ditzler et al. 2020).
Q11.1a What Is the Organic Molecule Inventory of Habitable Environments Throughout the Solar System, Including Complex Organic Molecules That Can Serve as Prebiotic Building Blocks of Life?
The first step to assessing the extent of organic chemical evolution in a habitable environment is understanding what organic molecules exist there now, and in what geochemical context. Such work is under way (Table 14-1)
TABLE 14-1 Organic Inventory of Worlds Throughout the Solar System to Date
| Body | Organicsa Detected or Predicted to Date | Complexity | Reference(s) |
|---|---|---|---|
| Mercury | Possibly cold-trapped volatiles (C, H, O, and N-bearing species), aldehydes, amines, alcohols, cyanates, ketones and organic acids, refractory (tholin-like) organic materials | Low | Zhang and Paige, 2009; Delitsky et al. 2017; Hamill et al. 2020 |
| Venus | Possibly HCN, methane, ethane, ethene, and benzene | Low | Johnson and de Oliveira 2019, Mogul et al. 2021 |
| The Moon | Possibly methane and other cold-trapped volatiles (C, H, O, and N-bearing species) | Low | Zhang and Paige, 2009; Colaprete et al. 2010 |
| Mars | Methane, chlorobenzene, dichloroalkanes; thiophenic, aromatic, and aliphatic compounds | Low/Moderate | Freissinet et al. 2015; Eigenbrode et al. 2018 |
| Ceres | Aliphatic organic, possibly amines | Low | De Sanctis et al. 2018; Raponi et al. 2021 |
| Europa | C≡N, C-H functional groups, refractory (tholin-like) organic materials | Low | McCord et al. 1998; Chyba and Phillips 2002 |
| Ganymede and Callisto | C≡N, C-H functional groups, possibly refractory (tholin-like) organic materials | Low | McCord et al. 1997, 1998 |
| Enceladus | Methane, other hydrocarbons, aromatics, amino acids, small and large (macromolecular) O-, N-bearing organics with ethoxy, hydroxyl, and carbonyl functional groups | Moderate | Postberg et al. 2018; Waite et al. 2006; Steel et al. 2017 |
| Titan | Hydrocarbons, nitriles, aromatics, heterocyclic species, acetylene, ethylene, cyanoacetylene, other N- and O-bearing species, nucleobases, amino acids, heteropolymeric species up to 10,000 Da, refractory (tholin-like) organic materials | High | Brown et al. 2010; Lunine et al. 2020 |
| Uranian satellites | Possibly refractory (tholin-like) organic materials (could also be amorphous pyroxene) | Low | Cartwright et al. 2018 |
| Triton | Ethane, hydrocarbons, acetylene, nitriles, heteropolymers, refractory (tholin-like) organic materials | Low | Thompson et al. 1989; Quirico et al. 1999 |
| Pluto | Methane, acetylene, ethylene, HCN, cyanoacetylene, amino acids, nucleobases, refractory (tholin-like) organic materials | Low/Moderate | Cruikshank et al. 2019 |
| Comets | Acetylene, methane, methanol, formate, methylamine, ethylamine, PAHs, aromatic nitriles, amino acids, refractory (tholin-like) organic materials | Low/Moderate | Wickramasinghe and Allen, 1986; Clemett et al. 2010; Altwegg et al. 2016 |
| Asteroids | Hydrocarbons, polyaromatic carbon, refractory (tholin-like) organic materials | Low | Cruikshank et al. 1987; Chan et al. 2021; Fink et al. 1992 |
a An organic molecule is one that contains at least one carbon atom bonded to hydrogen.
NOTE: Black text represents organics detected via remote sensing or in situ measurements; blue text represents compounds predicted by laboratory experiments/modeling. Complexity is compared to Earth as baseline (see Chapter 12).
but incomplete (Q10.4). Of great value would be a comprehensive inventory of organic molecules that includes both volatile and condensed phases, as well as soluble and insoluble (e.g., kerogen) fractions and abundant and trace species. Knowledge of the chemical structures (i.e., functional groups and binding motifs) of these organic compounds is important to ascertain synthesis mechanisms and reactivity. The distribution of organics within the habitable environment, particularly with respect to key habitability parameters such as energy sources, pressure, or temperature, can provide important clues regarding their origin.
The co-location of certain organic molecules with others, or depletion of one molecule with respect to another, may be diagnostic of the chemical pathways that dominate in that region of the environment, although such interpretations are necessarily convolved with careful assessment of the physicochemical properties of that region (see Q11.1c), which can affect the sorption and volatility/fugacity/solubility (and hence detectability) of organic molecules. This also applies to inorganic species, which may catalyze or otherwise facilitate certain organic chemical reactions.
Q11.1b What Is the Extent of Molecular Complexity (e.g., Size, Heteroatom Diversity, Structure, Pathway Assembly Index) and Degree of Organization (e.g., Isomeric Preference, Polymerization) That Can Be Generated Abiotically Under Habitable Conditions? How Does This Compare to Prebiotic Experiments to Date?
The degree of molecular complexity reflects the extent of organic chemical evolution in an environment and can possibly serve to discriminate biotic from abiotic systems. Key indicators of molecular complexity include, but are not limited to, the size and composition (arrangement, type, and ratio of atoms, including diversity of heteroatoms) of molecules, as well as their chemical structure (e.g., molecular geometry, presence of functional groups, intramolecular interactions such as folding conformations, and binding motifs). The pathway assembly index—a probabilistic measure of the number of steps needed to construct a molecule (Marshall et al. 2017)—can also be useful in distinguishing biotic from abiotic molecules, although more work is needed to determine if all complex abiotic molecules will pass this test. Abiotic generation of organic molecules in unhabitable conditions also needs to be considered, as such materials could be inherited by habitable environments at a later stage.
Biology is the dominant source of organic matter on Earth, where it has typically masked the signal from prebiotic/abiotic chemistry in all surface and near-surface environments (Q9.1). Therefore, the extent of molecular complexity that can be achieved through purely abiotic means, under widespread habitable conditions, is a challenge to assess through the study of natural terrestrial samples (Barge et al. 2020), although in the subsurface of both marine and continental systems, environments exist where low rates of biotic processes are such that abiotic signatures (particularly for abiotic H2, CH4, ethane, acetate, formate, and other simple organic compounds) have been identified (Sherwood Lollar et al. 2014; McDermott et al. 2015; Lang et al. 2018). Work in the laboratory—where the influence of biology can be controlled, reduced, or eliminated—can serve to inform this question, notwithstanding the challenge of mimicking a broad diversity of geochemical conditions in a laboratory setting. Other habitable environments in the solar system, particularly those with no detectable evidence of biology, can serve as prebiotic laboratories on a planetary scale to understand the extent of molecular complexity and degree of organization that can be achieved without the influence of life (Q10.4). Analyses by the Perseverance Mars rover in situ, and of samples to be returned by the Mars Sample Return campaign, may prove key in this regard. Methods to evaluate the degree of organization of abiotic systems include, but are not limited to, isomeric preference—whether molecules will react with or bind to another molecule of only a certain stereoisomeric conformation—as well as the type and number of monomeric units in any macromolecules (i.e., polymerization) and any intermolecular interactions (i.e., quaternary structure).
If endmember cases of abiotic organic complexity and degree of organization can be established, they would serve as a metric against which habitable environments could be compared in the chemical evolution spectrum spanning fully abiotic to fully biotic. Any unambiguous life detection discovery will require that all plausible abiotic and prebiotic formation mechanisms for the potential biosignature(s) be ruled out (Hoehler et al. 2020); the endmember case of greatest abiotic molecular complexity would serve as an important test for such a claim.
Q11.1c What Are the Relevant Chemical Pathways That Can Lead from Prebiotic Chemistry to Biochemistry, and How Does That Transition Depend on the Geochemical State of the Environment?
Chemical pathways (i.e., links of chemical reactions) in chemical networks are condition-specific; that is, certain pathways will proceed only under certain conditions, such as in the presence of a certain reactant or catalyst/substrate, within, for example, a specific pH, temperature, or pressure range. The physicochemical conditions of a habitable environment are intrinsically linked to the geochemical state of that environment, and as such the progression from prebiotic chemistry to biotic chemistry through evolution of specific chemical pathways is likely to be mediated by geochemical factors (Q9.2). Mapping which chemical pathways are most likely to lead to biochemistry requires a deep understanding of chemical reaction networks in a variety of geochemical regimes spanning past and present conditions of habitable environments in the solar system.
Strategic Research for Q11.1
- Determine the organic molecule inventory, including extent of molecular complexity and degree of organization, of currently or previously habitable environments throughout the solar system (e.g., Enceladus, Europa, Titan, Ceres, Mars—see Question 10 for full list) with spacecraft in situ and/or remote sensing observations, telescopic observations, or sample return.
- Identify relevant chemical pathways that can lead from prebiotic chemistry to biochemistry in currently or previously habitable environments throughout the solar system as a function of geochemical state by characterizing each environments’ geochemistry, physicochemical conditions, and associated prebiotic (or potentially biotic) reactants, intermediates, and products using spacecraft in situ and/or remote sensing observations, telescopic observations, and via laboratory/experimental and modeling/theoretical research.
- Characterize the extent of molecular complexity and degree of organization that can be achieved for an organic molecule inventory in the absence of life in currently or previously habitable environments throughout the solar system using laboratory/experimental and modeling/theoretical research as well as field work and astromaterial analysis to explore the parameter space (e.g., temperature, pressure, pH, reactants, and catalysts/substrates).
- Ascertain how levels of organic inventory, molecular complexity, and/or degree of organization that can be achieved in the absence of life might inform the search for life elsewhere using laboratory/experimental and modeling/theoretical research as well as field work and astromaterial analysis.
- Evaluate possible sources of false positives for organic chemical biosignatures with spacecraft in situ and/or remote sensing observations, telescopic observations, and sample return from worlds unlikely to host life (e.g., the Moon, comets, and asteroids) and uninhabited regions of previously or currently habitable environments (e.g., the surface of Mars) in an effort to assess the production and/or delivery rate of abiotic organic molecules that might mimic biological organic molecules and establish a baseline against which measurements in habitable environments can be compared. This also includes analysis of astromaterials, as well as theoretical and laboratory approaches.
Q11.2 BIOSIGNATURE POTENTIAL: WHAT IS THE BIOSIGNATURE POTENTIAL (I.E., THE RELIABILITY, DETECTABILITY, AND SURVIVABILITY OF BIOSIGNATURES) IN HABITABLE ENVIRONMENTS BEYOND EARTH? WHAT ARE THE POSSIBLE SOURCES OF FALSE POSITIVES AND FALSE NEGATIVES?
Here, the committee defines “biosignature potential” as relating to the properties of a given habitable environment that act to enhance the probability of biosignature preservation and detection, including whether the properties of the habitable environment lend themselves in some way to the discrimination of biosignatures from a background of nonbiological processes that might otherwise render them improbable or nonunique. Several important traits to be considered when assessing the functional merit of a potential biosignature include its
reliability, detectability, and survivability (Meadows 2017; Meadows et al. 2018; NASEM 2019). The reliability of a biosignature weighs the probability of life having produced it (i.e., its biological prevalence) against the improbability of nonbiological processes producing it (i.e., its abiotic prevalence). Currently, the basis set for evaluating the biological prevalence of a potential biosignature is limited to our experience with the terrestrial biosphere, which is potentially limiting when considering potential biosignatures of life on non-Earthlike worlds (see Q11.3c). On the other hand, the abiotic prevalence of a potential biosignature (or abiosignature) relies on our understanding of abiotic processes operating over a broad range of geochemical conditions (see Q11.1). The detectability of a biosignature depends on the quality and magnitude of its signal, which are functions of the biological potential of the environment (see Question 10), and the level to which that biosignature can be quantified with current technology. For example, the reported detection of phosphine (PH3), a potential biosignature, in the atmosphere of Venus (Greaves et al. 2020) has come under scrutiny as the absorption feature in the reprocessed data does not meet standard criteria for being statistically significant (Snellen et al. 2020). The survivability of a biosignature depends on whether it can be preserved long enough for analysis, or whether its decomposition products can be traced back to the identity of the original biosignature. Biosignature detectability and survivability are environment-specific; therefore, a thorough understanding of the environmental context is critical for biosignature evaluation.
Q11.2a What Characteristics Indicative of Life Can Serve as Definitive Biosignatures in Environments Beyond Earth?
As described in Question 9, biosignatures can be defined broadly as objects, substances, and/or patterns whose origin specifically requires a biological agent (Des Marais et al. 2003), and can manifest in a broad diversity of observables including, but not limited to, cell-like morphologies, sedimentary fabrics, organic molecular structures, abundance distributions of organic molecules, chirality in classes of molecules, mineral properties, stable isotope patterns in minerals and organic compounds, relative abundance of atmospheric gases, or temporal changes (e.g., daily and/or seasonal) in global planetary properties (Des Marais et al. 2008). Experience from the study of life on Earth suggests that these potential biosignatures can be grouped into three generic types: (1) chemical, (2) morphological, and (3) physiologic/metabolic. Chemical biosignatures result from life’s selectivity toward specific organic and inorganic compounds and from life’s capability to “invent” complex organic molecules that fill important biological roles (e.g., biopolymers). Morphological (i.e., structural) biosignatures may emerge from life’s fundamental cellular architecture (though may not be required; see Q11.4a), and/or from the complex web of competitive and cooperative interactions among organisms (e.g., sedimentary fabrics), and their interactions with the environment. Physiologic/metabolic biosignatures are direct manifestations of the activity of living organisms (e.g., mobility and growth) including when they have the capacity to interact with and alter the environment at rates that are significantly faster or different than equivalent abiotic processes (e.g., chemical catalysis). For a given element, the relative alteration of different isotopic forms for that element—most commonly 13C and 12C—have frequently been used as biosignatures, despite an increasing recognition of the range of abiotic processes that may produce similar depletions in the 13C signatures and hence “mimic” life (NASEM 2019). Studies of biosignatures produced by life on Earth provide a foundation for the search for similar biosignatures in other habitable environments of the solar system (Question 9).
The building blocks of life can have various abiotic origins and are likely to permeate the solar system and other planetary systems in the Milky Way galaxy. This is to be expected, as abiotic chemistry provided the feedstock for prebiotic chemical evolution that ultimately gave rise to life at some point in the early history of Earth. A complex abiotic organic chemical background represents one possible form of a biosignature—that is, chemicals, features, and/or processes that define the nonbiological background against which signs of life needs to be resolved. This is made even more challenging by the fact that, in some cases, such abiosignatures may mimic biosignatures. Thorough characterization of the environment, including the extent of molecular complexity and degree of organization achievable in abiotic conditions for that environment (Q11.1b), can support assessment of the biogenicity of a potential biosignature even in a complex abiotic chemical background.
Q11.2b What Are the Relationships Between the Physical and Chemical Properties and Processes Operating in a Habitable Environment and the Potential Amount of Biomass That Might Be Present? How Might This Drive the Detectability of Any Biosignatures Present?
The chemical inventory and fluxes of metabolically useful redox couples, the environmental conditions (e.g., pH, Eh, temperature, and the presence/absence of sunlight), and availability of substrates that can facilitate catalysis are among the important factors that will determine whether a habitable world supports the production of a large (in terms of total biomass) and productive biosphere, or a biosphere that is limited in terms of its productivity, total biomass, and areal/geographic extent. Predictions regarding the potential size and extent of the biosphere on a habitable world would provide useful guidance for mission planning, sampling, and instrument design, and inform assessments of the susceptibility of a biosignature search to false negative outcomes.
Studies of Earth’s biosphere provide a framework for thinking about biological productivity, and ultimately the rate of biomass production that can be supported by an environment. A useful metric of biological productivity is “net primary productivity” (NPP): the quantity of CO2 fixed into organic matter per unit time, minus the quantity of organic carbon oxidized to CO2 by autotrophic respiration. Focusing specifically on Earth’s oceans, modern marine photoautotrophs can access virtually unlimited sources of water, sunlight, and inorganic carbon to support an NPP estimated at ~1013 kg of carbon/year (Field et al. 1998; Bender et al. 1994), such that marine NPP is limited by the availability of other nutrients including dissolved phosphorus, fixed nitrogen, and sometimes iron. Models of the early marine biosphere suggest that NPP would have been limited early on by the availability of inorganic geochemical electron donors, such as Fe2+ and H2, limiting NPP to ~1010–1012 kg of carbon/year (Kharecha et al. 2005; Canfield et al. 2006; Ward et al. 2019). The geological record of carbon isotopes in carbonate and organic carbon indicates that the fraction of Earth’s surface carbon budget buried as organic matter in sediments has increased through time (Krissansen-Totton et al. 2015), consistent with the notion of an increasingly productive biosphere. Similar assay and bioenergetics-based approaches to modern subsurface environments provide additional useful guidance for estimates of nonphotosynthetic subsurface biological productivity and biomass (e.g., Spear et al. 2005; Lollar et al. 2019), and these concepts have been extended to estimate the potential biomass of, for example, the modern martian subsurface (Weiss et al. 2000; Sholes et al. 2019), and other habitable environments beyond Earth (e.g., Hand et al. 2017; Cable et al. 2020; Higgins and Cockell 2020). While such modeling efforts require assumptions about the metabolic strategies employed by organisms living in the system, and the fluxes of nutrients available to them, they have the potential to yield estimates of biological productivity and perhaps even predictions regarding the composition of relevant stable isotopic reservoirs on other worlds. Such predictions can be useful in developing measurement requirements (e.g., biosignature type and expected concentration) for astrobiology-focused science objectives.
Q11.2c How Do the Physical and Chemical Properties and Processes in a Habitable World Affect the Survivability of Biosignatures?
When considering the likelihood that a biosignature can be detected in a habitable environment, it is also important to consider how the physical and chemical conditions that make an environment habitable also act to modify, enhance, or erase evidence of inhabitation. NASEM (2019) speaks to this issue in the context of the survivability of biosignatures, or “taphonomy,” which is the field of study concerned with those processes that result in the formation, preservation, alteration, and destruction of biosignatures. This issue is also discussed in detail in Question 9.5. Our understanding of the processes that affect the biosignature potential of a habitable environment necessarily have “baked in” assumptions about the nature of the biological system one suspects is or was present. The search for the remnants of an extinct, nonphotosynthetic, lithotrophic microbial biosphere on Mars, for example, would require an exploration of the unique chemical and physical conditions favoring biosignature production and preservation in that environment, distinct from those that might favor the successful search for an atmospheric biosignature on Venus or an exoplanet. Accordingly, the trade space for understanding how the chemical and physical processes in a habitable world affect its biosignature potential is vast, and needs to be narrowed on the basis of assumptions about the biosphere that
produced it, and the environment(s) in which the signature(s) of that biology have resided in order to avoid false negatives that result from an under-appreciation of the specific environmental factors that might act to modify or erase a potential biosignature.
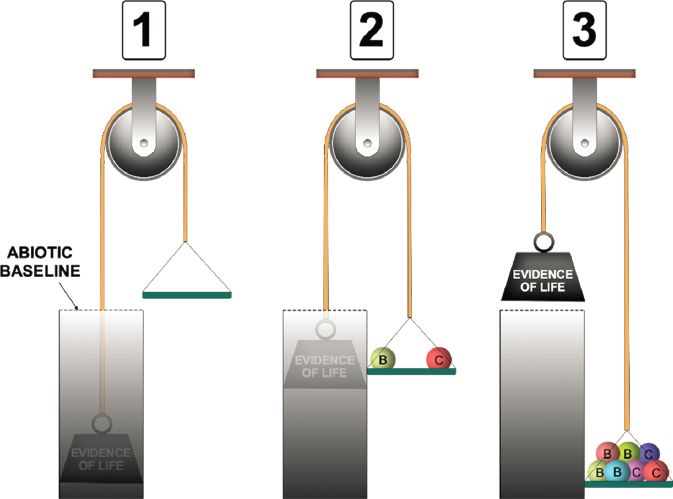
Q11.2d How Can We Best Devise a Formal Framework for the Interpretation of a Set of Biosignature Measurements in a Given Environment?
To account for uncertainties in biosignature potential and the possibly unique nature of life elsewhere (Q11.3c), a search for evidence of life is more likely to succeed if it is based on multiple, mutually supporting lines of evidence as well as on their environmental context (Figure 14-3). Building on well-established tenets such as multiple working hypotheses (where every rational explanation of the phenomenon is included and impartially evaluated; Chamberlin 1890, 1897), frameworks that allow correct interpretation of the significance of each potential biosignature, according to their own possible sources of false positive and false negative signals,
provide a more rigorous standard of proof. In addition, such frameworks can help identify sets of measurements that together discriminate between a biotic or abiotic origin for potential biosignatures with high statistical significance (NASEM 2019).
Some preliminary concepts for interpretive frameworks have been posited recently for possible life detection mission scenarios, considering uncertainties arising from environmental or instrumental factors and where valuable but limited opportunities exist to collect additional samples or conduct follow-up analyses. At the most basic level, a framework may be represented as a decision tree, wherein questions about acquired data may lead to increased or decreased probabilities or “scores” associated with life detection (e.g., Vago et al. 2017). Thorough, yet flexible, decision trees may prove especially useful during a mission, where selection of samples or measurement parameters can be guided by “real-time” analysis of collected data. Where data increase in volume and complexity and become increasingly cumbersome to manage, such as on remote missions with restricted communication, science autonomy will increasingly be required for life detection (Theiling et al. 2021). Machine learning and other tools may provide an expanded practical framework for interpreting biosignature measurement data in such conditions.
Strategic Research for Q11.2
- Determine which attributes of terrestrial life can serve as definitive biosignatures in the search for life beyond Earth by theoretical, field, and laboratory research activities that inform on the range of morphological and chemical (e.g., organic, isotopic, mineralogical, and atmospheric) signatures produced by living systems, with a complementary emphasis on what contextual evidence is required to confidently recognize a potential biosignature.
- Constrain the biomass and/or bioenergetic potential of habitable environments throughout the solar system through measurements of environmental factors (e.g., pH, temperature, salinity, and redox potential) and fluxes (e.g., energy and nutrients) with spacecraft in situ and/or remote sensing observations, or returned sample analysis; and theoretical, field, and laboratory research activities to understand how modern and ancient environmental properties and processes on Earth relate to biomass production to inform the development of life detection strategies and technology (see also Question 9).
- Characterize the range of processes that affect the production and preservation of detectable biosignatures in habitable environments by theoretical, field, and laboratory research activities that inform us about the pathways and rates of biosignature production, preservation, and destruction, be they morphological or chemical (e.g., organic, isotopic, mineralogical, and atmospheric) in nature. Tie those studies to in situ, remote, and/or telescopic measurements of specific environmental properties of habitable worlds in the solar system (e.g., Enceladus, Europa, Titan, Ceres, and Mars; see Question 10 for full list) that might act to enhance, preserve, or destroy biosignatures.
- Establish a comprehensive, standardized framework for evaluation of biosignatures, including the potential for abiosignatures, false positives, and false negatives, through community-level dialog and consensus, supported by laboratory/experimental and modeling/theoretical research as well as field work.
Q11.3 LIFE DETECTION: IS OR WAS THERE LIFE ELSEWHERE IN THE SOLAR SYSTEM?
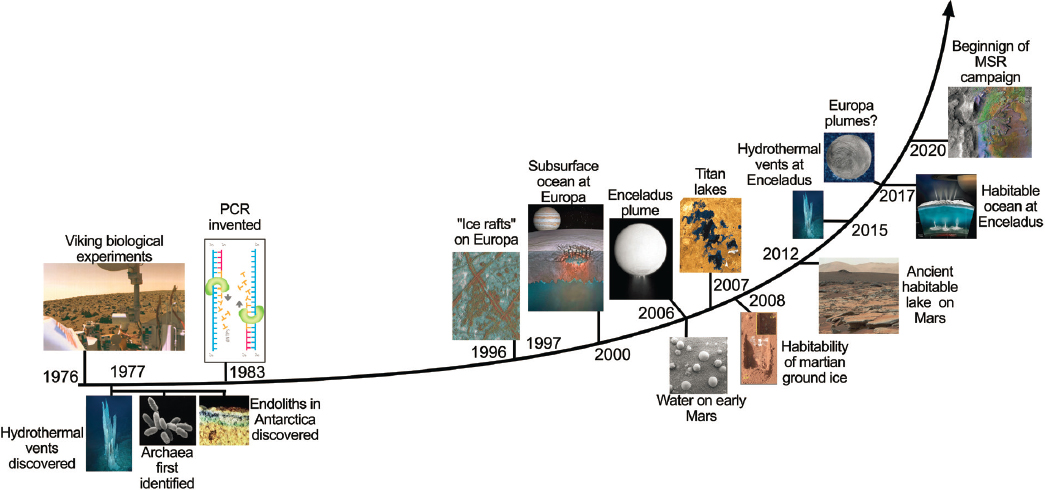
Recent decades of planetary exploration have witnessed a small revolution in our understanding of habitability conditions elsewhere in the solar system (Figure 14-4). It is now widely accepted that long-lived habitable environments existed on the surface of Mars early in the planet’s history, and that habitable conditions may have persisted until the present in the subsurface. There is compelling evidence that a habitable ocean exists beneath the surface of Enceladus, and possibly also beneath the surfaces of Europa and Titan. Motivated by these discoveries, we are poised to address the question of whether there is, or ever was, life elsewhere in the solar system, with scientific rigor.
The search for evidence of life beyond Earth builds on and extends the traditional objectives of planetary sciences, complementing comparative planetology and extending to a new potential field: comparative biology (Q11.4a). The magnitude of the question requires the coming together of scientists from a wide range of disciplines, and the combination of a diversity of perspectives and approaches. The search also needs to satisfy the scientific criteria of experimentation, address testable hypotheses, and allow modification of those hypotheses based on observation and measurement (NASEM 2019). This applies equally to cases where life may have gone extinct, detectable through its imprint preserved over time, or cases where life is currently viable, and may even comprise a population of metabolizing organisms, albeit not necessarily in a way immediately familiar to us (Q11.3c).
To minimize the likelihood of a false negative or a false positive result, optimal life-detection strategies would target multiple, orthogonal, mutually supporting lines of evidence, and carefully select sets of candidate biosignatures based on several key criteria (Q11.2). Assessments of the presence or absence of potential biosignatures in a habitable environment (present or past) need to be congruent with the physicochemical setting and ought to be informed by the biological potential of that environment (Figure 14-5 and Question 10). In addition, life-detection mission designs need to include comprehensive contamination control approaches informed by acceptable levels and types of contamination derived from top-level science and mission requirements. This effort would start as part of mission concept development, and contamination awareness and control would be addressed in detail throughout all mission phases. Last, the novelty of the measurements needed for life detection and the rigorous standards of evidence mandate tailored approaches in the conception, maturation, and deployment of instruments and instrument suites (Hoehler et al. 2020).
Q11.3a Are There Chemical, Morphological, and/or Physiologic/Metabolic or Other Biosignatures in Currently Habitable Environments in the Solar System?
The continued exploration of planetary bodies of the solar system is revealing a broader range of potentially habitable solar system environments than previously anticipated (Question 10). Data gathered by the Cassini spacecraft suggests that the subsurface ocean of Enceladus currently meets the requirements to sustain life (Cable et al. 2020). The Europa Clipper and Dragonfly missions will help constrain the biological potential of Europa’s and Titan’s subsurface oceans, respectively. The exploration of Venus (e.g., by VERITAS and DAVINCI) and Mars (e.g., by Curiosity and Perseverance) will help establish whether localized habitable regions currently exist within these seemingly uninhabitable worlds. Once habitable environments are identified, the search for evidence of life represents the logical next step and the greatest challenge.
The search needs to be conducted thoughtfully and with an open mind concerning potential outcomes, balancing the stringency and inclusivity of the observational strategy applied to a given environment. Stringency sets criteria for the quality and robustness of a biosignature detection, amidst potentially confounding conditions or background signals from the planetary environment, and thus seeks to minimize potential false positive results such as a “life-like” abiotic pattern or response. Inclusivity emphasizes consideration of a wide range of possible alien biosignatures (e.g., chemical, morphological, and/or physiologic/metabolic), not relying solely on Earth life as a guide, as well as their prevalence and detectability in the given environment. As such, inclusivity seeks to minimize potential false negative results, where life could be “missed” for lack of the ability to detect or recognize it. These concepts apply equally to cases where life may have gone extinct but remain detectable through its imprint preserved over time (Q11.3b).
The search for evidence of life requires tailored technology solutions (see Chapter 21). The novelty of the measurements needed for life detection and the rigorous standards of evidence we will demand of them mandate new approaches in the conception, maturation, and deployment of instruments and instrument suites. Specifically, because the preferred route to arriving at defensible conclusions is to employ multiple orthogonal lines of life detection, future missions will need to be designed holistically, potentially having instrument providers engage with each other very early in the process (i.e., pre-Phase A) to propose integrated instrument suites. The most sensitive life-detection experiments require sample acquisition, sample handling, and sample analysis (Figure 14-6);
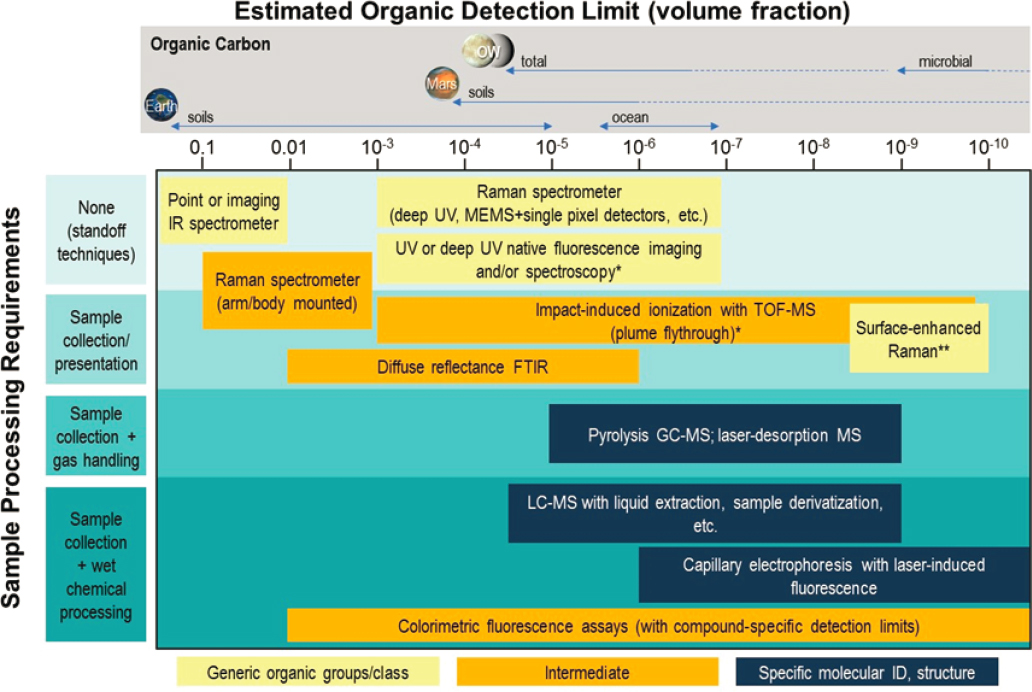
therefore, in situ detection of life is best advanced by integrated suites of instruments or single instruments that permit multiple analytical techniques, including nondestructive approaches, to be applied to the same collected materials (NASEM 2019) (see Chapter 17). Each step forward in this effort will also require a continuing vigilance against forward contamination from Earth (McKay et al. 2020).
Q11.3b Are There Chemical, Morphological, or Other Biosignatures in Previously Habitable Environments in the Solar System?
Mars witnessed a protracted period of habitable conditions early in its history, with relatively mild surface temperatures and widespread bodies of liquid water with physical and chemical conditions compatible with biology. A detailed geologic record of this early habitable period has been preserved on the surface, as shown by data gathered by the Curiosity rover at Gale crater. A search for evidence of past life on Mars is now under way at Jezero crater with the Perseverance rover and is a top priority objective for the analysis of samples cached by the rover, which will eventually be brought to Earth as part of the Mars Sample Return campaign. While physiologic/-
metabolic biosignatures (which would indicate organisms that are alive today) may no longer be present, evidence of past life in the returned samples can be ascertained from chemical and morphological fingerprints left behind. To support that objective, data gathered by Perseverance may help constrain biosignature survivability (Q11.2) and offer a deeper understanding of the geologic evolution of that environment, particularly in terms of physical (e.g., temperature, pressure, and transport) and chemical (e.g., oxidation, photodissociation, and radiolysis) processing that biosignatures may have undergone over geologic timescales. Studies of biosignatures produced by ancient life on Earth (Question 9), such as in Archaean sediments, can inform the search for similar biosignatures in martian sedimentary deposits.
Q11.3c How Might We Develop the Scientific Understanding to Recognize Life “Not as We Know It”?
The search for evidence of life beyond Earth is necessarily informed by terran life (i.e., life “as we know it,” the particular case of terrestrial life, Question 9), which increases the potential likelihood that we would not detect nonterran life in an otherwise inhabited world. Acknowledging that terrestrial life might be but one example of all possible forms of life in the universe, we need to recognize the possibility of life with biology that differs in a significant way from the terrestrial model in form and/or function. There is a recognized need to improve our understanding of which biosignatures are likely to be prevalent across all forms of life, in order to reduce the risk that our missions and technologies will be too narrowly focused on the features of terran life. The goal of such research is to expand the ability to search for any life through exploration of a broader definition of life based on activity, with less dependence on assumptions about structure and specific biogeochemistry—specifically, more universal or agnostic biosignatures (NASEM 2019). From least to most radical (and therefore most to least likely), potential biochemical differences include (NRC 2007):
- Different lipid molecules for membranes. Major categories of living organisms on Earth are distinguished in part by their use of different lipids, suggesting that alternative patterns compatible with other metabolic pathways are likely.
- Different sets of amino acids or nucleobases other than the ~20 amino acids and 5 nucleobases used by terrestrial life, to encode information (Young and Schultz 2010; Hoshika et al. 2019).
- The opposite enantiomer of amino acid and sugar chirality (i.e., mirror life), and/or a genetic polymer other than a polyanionic backbone.
- Different genetic molecules. Life on Earth has switched genetic molecules at least once (from RNA to DNA); alternatives may be numerous (Cleaves et al. 2019; Zhou et al. 2021).
- Alternative essential elements with similar chemical properties (e.g., elements within the same column of the periodic table or transition metals with similar oxidation states, reactivities, and/or binding affinities).
- A solvent that is liquid at temperatures and pressures other than those of liquid water. In the solar system, two environments with liquids not dominated by water are the hydrocarbon (methane-ethane) lakes found in Titan’s polar regions and sulfuric acid clouds in the atmosphere of Venus. Both have been hypothesized to be able to harbor life as we do not know it (Limaye et al. 2018; Lunine et al. 2020), although limitations of organic compounds in these environments (such as low solubility in Titan’s lakes and instability in the Venus’s atmosphere) may pose insurmountable challenges even to novel/alien biochemistries. Other potential solvents include formamide, ammonia, and carbon dioxide, but no planetary environments are yet known to contain such liquids as the dominant component.
Accordingly, nonterran life can fall along a sliding scale from less to more radical departures from the basic terrestrial model. Strategies to search for evidence of life need to strike the right balance between inclusiveness of alternative biologies (e.g., by considering novel and/or agnostic biosignatures) while adhering to known biological principles (e.g., a chemical system that can undergo Darwinian evolution). Understanding the spectrum of possible departures from the terrestrial model, and how those might impact our selection of potentially habitable environments and the interpretation of potential biosignatures therein, is an important aspect of astrobiology.
Q11.3d If We Do Not Find Evidence of Life in a Habitable Environment, What Would It Take to Convince Ourselves That There Truly Is or Was No Life Present There, Rather Than Possibly Not Having Detected It (a False Negative)?
When searching for evidence of life, the probability of a false negative result is highest in environments where potential biosignatures occur at very low abundance (e.g., owing to low productivity or to degradation/destruction processes), operating at a very low (or even dormant) metabolic state, or where life is not distributed homogeneously (i.e., biological oases amidst an abiotic landscape). False negatives owing to low biological signals can be constrained based on contextual information. But false negatives owing to heterogeneous distributions of biological signals are more difficult to recognize, because spatial heterogeneity can occur at any scale, up to planet wide. Research in various environments on Earth suggests that heterogeneous distributions of life are nevertheless not arbitrary. Biological oases typically occur in areas where resources (e.g., water, nutrients, and energy) are locally more abundant, or where lethal environmental conditions (e.g., radiation and excessive temperatures) are somehow mitigated. Life signatures can be relatively diverse and abundant in those oases, but quickly vanish with distance or time. Often, biological oases are associated with specific substrates or physical environments (e.g., rocks, sediments, subsurface layers, and fracture surfaces) whose chemical or physical properties provide a survival advantage to organisms. As such, correct interpretations of a negative result require adequate understanding of the spatial variability in resources and environmental conditions. Research in terrestrial environments can inform how spatial variations in resources and environmental conditions can shape the distribution of life in the landscape (Question 9). From these studies, models can be developed that predict potential “hotspots” or blooms of life as a function of resources and environmental conditions. Such models can then inform the most likely locations to find evidence of life on a planetary body, and how the likelihood of finding evidence of life changes spatially. Similarly, models considering putative metabolisms in a certain environment could inform protocols for instigating a “bloom” in a collected sample, if nutrient-starved organisms were lying dormant.
Strategic Research for Q11.3
- Develop and validate effective life detection payloads that support the search for evidence of life beyond Earth by maturing end-to-end technologies for sample acquisition, sample handling/preparation, and sample analysis, and by prioritizing the early integration and validation of these technologies and instrument suites.
- Search for evidence of present life in environments beyond Earth that currently have a high biological potential by looking for multiple, independent biosignatures with spacecraft in situ observations or in samples returned to Earth, informed by laboratory/experimental and modeling/theoretical research as well as field studies of currently habitable environments on Earth.
- Search for evidence of past life in environments beyond Earth that had a high biological potential in the past by looking for multiple, independent biosignatures with spacecraft in situ observations or in samples returned to Earth, informed by laboratory/experimental and modeling/theoretical research as well as field studies of ancient life on Earth.
- Search for evidence of nonterran life in environments beyond Earth by looking for multiple, independent biosignatures with spacecraft in situ observations or in samples returned to Earth, informed by laboratory/experimental and modeling/theoretical research of putative organisms that might utilize unique biochemistry or alternative essential elements or solvents.
- Determine the optimal sampling strategy to minimize the likelihood of a false negative life detection measurement (or set of measurements) owing to heterogeneous distributions of biological signals or other factors by employing laboratory studies and modeling/theoretical research in conjunction with field studies of biological oases and environments with severe nutrient/energy limitations on Earth.
- Develop and implement analysis techniques to evaluate the likelihood of forward contamination in life-detection missions through models of contamination transfer from spacecraft surfaces and by improving hardware protection/cleaning methods, in order to meet the stringent contamination requirements imposed by life-detection science.
Q11.4 LIFE CHARACTERIZATION: WHAT IS THE NATURE OF LIFE ELSEWHERE, IF IT EXISTS?
With terrestrial life as the sole example, biology is unlikely to evolve into a universal science in the same way as physics, chemistry, and geology have evolved with the respective advent of telescopic astronomy (17th century), spectroscopy (19th century), and planetary exploration by spacecraft (1960s). For these fields, we now have a predictive understanding. But the discovery of life on another world would allow us, for the first time, to conduct comparative biology beyond the boundaries of Earth, and to address some of the most fundamental questions in biology: Is there a general theory of living systems? Is life a common cosmic phenomenon? What is life, how does it emerge, and what are its universal means of operation?
All life on Earth is genetically related and likely represents only one data point among the possible instances of biology. Characterizing life elsewhere, if it exists, would allow us to begin distinguishing attributes specific to life on Earth from those that are shared across instances of biology (Figure 14-7). An important challenge in this process will be to recognize life beyond Earth as a truly independent biology, with no ancestral links to life on Earth due, for example, to biological exchange by impacts. Our ability to characterize life beyond Earth if/once it is discovered will be key to addressing this challenge. Three scenarios could be possible. First, life could be discovered that is so similar to Earth life that it would be challenging to rule out terrestrial contamination without extensive phylogenetic analysis. Second, identification of extraterrestrial life whose biochemistry overlaps significantly (but not completely) with terrestrial life might point to a shared origin and later dispersion through planetary exchange. Third, we may find extraterrestrial life that has sufficiently different biochemical attributes from any known Earth organism. This case may point to a separate origin of life and imply that living systems can readily emerge under appropriate physical and chemical conditions; alternatively, the two systems could share a common origin but have diverged so greatly over time that any trace of common evolutionary heritage has been obscured. Each scenario would have significantly different implications about the prevalence of life in the universe or the likelihood of it being transported across planetary havens. Coupled with a detailed understanding of its environment, inspection of biological properties and processes beyond Earth would provide insight into how a biosphere arises and co-evolves with its host world. Such insights into universal properties, emergence, prevalence, and workings at scales from microscopic to global could bring within reach a definition of life and a universal theory of biology.
Since the onset of robotic exploration of the solar system, the knowledge gained via such exploration and concomitant advances in the understanding of what life requires, what constitutes evidence of life, and how to build instruments to search for it has poised humanity on the verge of finding and characterizing either life beyond Earth, or habitable worlds with no evidence of life. Either way, these discoveries, for which the groundwork can be undertaken in this coming decade, are bound to bring answers to some of humanity’s most profound questions.
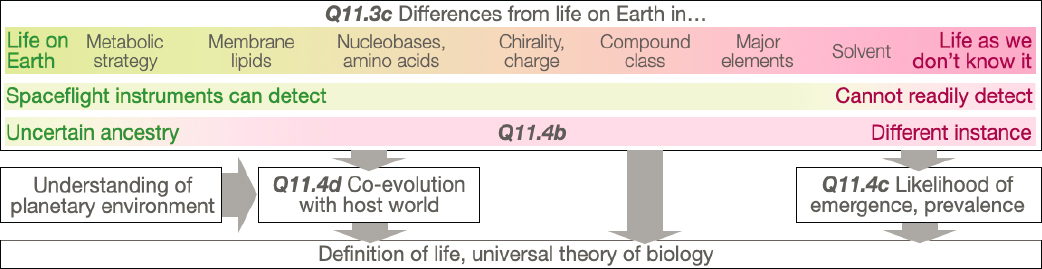
Q11.4a How Might Comparative Biology Be Implemented on a Small Sample Size (N = 2) If Evidence of Life Is Discovered Elsewhere in the Solar System?
Characterizations of life beyond Earth would take place at multiple levels (e.g., biochemically, structurally, and metabolically), each one likely to produce profound insights on the nature of biological systems. For example, the cell is the basic structural, functional, and biological unit of all modern terrestrial organisms. However, life elsewhere could exist in an acellular evolutionary form (e.g., with only a protein coat as in viruses, see Q9.2), or it could be based on a different cellular architecture. It is unknown whether all living systems require a cellular organization altogether. Similarly, for all their metabolic diversity, terrestrial organisms extract energy from a limited subset of physical and chemical sources (see Question 9). However, life elsewhere might be able to exploit other sources of energy (e.g., different wavelengths and intensities of radiation) reflecting adaptation to their local environment. Last, all life on Earth shares the same biochemical framework based on a universal set of molecular building blocks (e.g., amino acids, nucleobases, sugars, and lipids) decorated with essential elements such as N, P, and S that confer a broad range of molecular structures and functionalities. This particular biochemical framework operates optimally within a range of physical and chemical conditions (e.g., temperature, salinity, and pH) (Q9.2). The biochemistry of life elsewhere could be identical to Earth’s and be bound by a similar range of environmental conditions, or could be based on a different subset of building blocks and essential elements, and operate optimally under conditions that are prohibitive to terrestrial life. To understand this potential variability, the discovery of evidence of life beyond Earth ought to be followed by dedicated investigations into the nature of those life forms.
Q11.4b How Do We Discriminate Between an Independent Origin of Life Elsewhere in the Solar System and a Shared Ancestry with Earth Life?
The above alternative means by which biological structure and function may be achieved could be used to distinguish life that shares ancestry with Earth life (up to the point of a last universal common ancestor—LUCA; Q9.1f) from life with a more distant relationship or independent emergence. Finding a new metabolic strategy but the same biomolecules as on Earth would suggest adaptation of terrestrial life transported to a new world. The identification of a cell membrane with new lipids would suggest a new category of organism, but one not necessarily more distinct than the three categories of organisms on Earth are from one another. In contrast, alternative biomolecules or elements would point to a more distantly related or unique instance of life.
More generally, among the different types of biosignatures (e.g., chemical, structural, and metabolic/physiological; Q11.2a), only certain chemical (organic) biosignatures, or informational evidence derived from them, might truly reveal an independent emergence. The latter include biopolymers whose sequence changes randomly through time (e.g., nucleic acids, peptides, and membrane lipids) and their specific building blocks (including specific molecular properties such as chirality). Even if alternatives as described in Q11.3c are found, there will be a degree of uncertainty in our assessment of an independent origin of life owing to our limited understanding of the pre-LUCA biochemical nature of life on Earth.
Another clue can be taken from the likelihood of material transfer (and of biological viability in this material during the transfer) between Earth and another inhabited world. The presence of rocks from Mars and other bodies in the meteorite collection show that this likelihood is nonzero; in the case of Mars specifically, the time when Mars was most habitable (late Noachian) would have coincided with a period of large impacts occurring on Earth, meaning that life could have spread from one potentially habitable world to another. Transfer between Earth and any inhabited environments in outer solar system ocean worlds and dwarf planets is less likely, however, owing to low impact probabilities and high impact velocities of ejected material, as well as the barrier presented by ice shells that encase subsurface oceans (Question 5).
Q11.4c What Does the Presence or Absence of Life on Other Worlds Tell Us About the Emergence of Life in General?
Proposed environments for the emergence of life on Earth, likely during the early Archaean, include surface aquatic environments (e.g., lakes and shallow marine settings), ephemeral playas with wet-dry cycling, hydrothermal volcanic fields at the surface, hydrothermal vents on the ocean floor, and subsurface fracture networks (Q9.1). All have attributes
that would be favorable or challenging to prebiotic chemical evolution (e.g., stability, longevity, energy supply, and dilution), and the scarcity of early Archaean geologic and fossil records limits our capacity to constrain the actual environmental setting where life could have begun on our planet. The search for evidence of life on other worlds is a means to test some of these origin of life hypotheses. For example, the absence of life in an otherwise habitable ocean world would rekindle ideas for an origin of life in environments with low water–rock ratios (Deamer and Damer 2017). But the discovery of life in an ocean world would lend credibility to theories that life on Earth could have originated in deep hydrothermal vents on the ocean floor. Therefore, characterizing life beyond Earth could shed light on the unknown circumstances of life’s emergence on our own planet, at least in terms of likelihood and environmental setting. The likelihood of life emerging and persisting on a habitable world is unknown, but our presence on Earth shows that it is not zero. An instance of biology on another world would help us understand whether life is an unavoidable outcome in the evolution of worlds that harbor habitable environments for sufficient time (1 billion years or less; van Kranendonk et al. 2018), whether it requires an improbable combination of circumstances (Ward and Brownlee 2000), and/or how frequently life transported from another world takes hold.
Q11.4d How Have Life and Its Host World Co-Evolved?
Determining the biochemical and metabolic characteristics of any extraterrestrial life would help us understand how it may have adapted to its environment, and perhaps the impact it has had on that environment from microscopic to global scales. Life on Earth has been shaped by the environment, including multiple mass extinction events that profoundly changed the phylogeny and ecology of the surviving groups. But terrestrial life has also had a profound impact on its environment, causing significant changes in the composition of the atmosphere, ocean sediments, and the upper layers of the crust (Q9.4). Is this co-evolution of life and the environment an intrinsic characteristic of inhabited worlds? If life ever existed on Mars, whose geologic history is in some sense comparable to Earth’s, was it able to significantly modify the environment? Did it retreat to cryptic niches as the planet became increasingly colder and dryer? Or did it succumb to one or more mass extinction events? If life exists in an ocean world, has it modified the chemistry of the ocean? Has it been shaped by events outside its sheltered confines? Or does it represent an endmember instance of static biology in a largely unchangeable environment?
The extent of a biosphere and of its effects likely depends on its overall energy and nutrient supply (see Question 10). Evidence to date suggests that any life elsewhere in the solar system has not, and cannot have, had as much of an effect on its host world as—even microbial—life on Earth, which has shaped our oxygen-rich atmosphere (Q6.2). However, the global influence of biology is the prime line of evidence being pursued to search for life on extrasolar planets. The next (ultimate?) level of study would examine the biochemistry and physiology of any discovered organisms. Given the experimental rather than observational or analytical nature of such investigations, current and foreseeable technology likely limits them to approaches involving humans, either with returned samples or, for Mars, in situ human exploration, rather than solely via in situ robotic exploration.
Strategic Research for Q11.4
- Investigate the chemical environments of Europa and Enceladus that are relevant to potential biochemistry through measurements of possible metabolic reactants and products (e.g., organic and inorganic compounds), reaction conditions (e.g., temperature, pressure, Eh, and pH), rates and catalysis (e.g., mineral surfaces and trace elements), as well as the structure of biomolecules at the surface and in waters sourced from the subsurface.
- Determine whether any life present in martian materials might share ancestry with Earth through measurement of any biomolecules as part of the organic chemical inventory to assess their function (including, for ancient life, their predegradation form) and what they reveal about the co-evolution of Mars’s life and climate.
- Prepare for characterizing life in the subsurface of ocean worlds by determining the heterogeneity of thicknesses of ice shells via planetary mission data as well as validating and deploying emerging technologies for life characterization, and maturing technology for accessing the subsurface for exploration, by work in the field and in the laboratory.
- Develop viable solutions to technical challenges for curation of samples to be returned from Mars and ocean worlds that will preserve their scientific integrity, including biosafe curation coupled (for ocean worlds) with cold conditions.
- Characterize any form of life discovered beyond Earth, and investigate its relatedness, or lack thereof to life on Earth through measurements of its biochemical, morphological, and/or physiological/metabolic traits with spacecraft in situ observations or in samples returned to Earth.
REFERENCES
Altwegg, K., H. Balsiger, A. Bar-Nun, J.-J. Berthelier, A. Bieler, P. Bochsler, C. Briois, et al. 2016. “Prebiotic Chemicals—Amino Acid and Phosphorus—in the Coma of Comet 67P/Churyumov-Gerasimenko.” Science Advances 2(5):e1600285. https://doi.org/10.1126/sciadv.1600285.
Barge, L.M., L. Rodriguez, J.M. Weber, and B. Theiling. 2020. “Beyond ‘Biosignatures’: Importance of Applying Abiotic/Prebiotic Chemistry to the Search for Extraterrestrial Life.” White paper #173 submitted to the Planetary Science and Astrobiology Decadal Survey 2023–2032. Bulletin of the AAS 53(4). https://doi.org/10.3847/25c2cfeb.b66ffca4.
Bender, M., T. Sowers, and L. Labeyrie. 1994. “The Dole Effect and Its Variations During the Last 130,000 Years as Measured in the Vostok Ice Core.” Global Biogeochemical Cycles 8(3):363–376. https://doi.org/10.1029/94GB00724.
Brown, R.H., J.-P. Lebreton, and J.H. Waite, eds. 2010. Titan from Cassini-Huygens. Dordrecht, Netherlands: Springer. https://doi.org/10.1007/978-1-4020-9215-2.
Cable, M.L., M. Neveu, H. Hsu, and T. Hoehler. 2020. “Enceladus.” Pp. 217–246 in Planetary Astrobiology, V.S. Meadows, G.N. Arney, B.E. Schmidt, and D.J. Des Marais, eds. Tucson: University of Arizona Press. https://doi.org/10.2458/azu_uapress_9780816540068-ch009.
Canfield, D.E., M.T. Rosing, and C. Bjerrum. 2006. “Early Anaerobic Metabolisms.” Philosophical Transactions of the Royal Society of London B: Biological Sciences 361:1819–1836. https://doi.org/10.1098/rstb.2006.1906.
Cartwright, R.J., J.P. Emery, N. Pinilla-Alonso, M.P. Lucas, A.S. Rivkin, and D.E. Trilling. 2018. “Red Material on the Large Moons of Uranus: Dust from the Irregular Satellites?” Icarus 314:210–231. https://doi.org/10.1016/j.icarus.2018.06.004.
Chamberlin, T.C. 1890. “The Method of Multiple Working Hypotheses.” Science (old series) 15:92–96. Reprint Science 148(3671):754–759, May 1965. https://doi.org/10.1126/science.148.3671.754.
Chamberlin, T.C. 1897. “The Method of Multiple Working Hypotheses.” Journal of Geology 5:837–848.
Chan, Q.H.S., A. Stephant, A. Franchi, X. Zhao, R. Brunetto, Y. Kebukawa, T. Noguchi, et al. 2021. “Organic Matter and Water from Asteroid Itokawa.” Scientific Reports 11:5125. https://doi.org/10.1038/s41598-021-84517-x.
Chyba, C.F., and C.B. Phillips. 2002. “Europa as an Abode of Life.” Origins of Life and Evolution of the Biosphere 32:47–67. https://doi.org/10.1023/A:1013958519734.
Cleaves, H.J., C. Butch, P.B. Burger, J. Goodwin, and M. Meringer. 2019. “One Among Millions: The Chemical Space of Nucleic Acid-Like Molecules.” Journal of Chemical Information and Modeling 59:4266–4277. https://doi.org/10.1021/acs.jcim.9b00632.
Clemett, S.J., S.A. Sandford, K. Nakamura-Messenger, F. Horz, D. and McKay. 2010. “Complex Aromatic Hydrocarbons in Stardust Samples Collected from Comet 81P/Wild 2.” Meteoritics and Planetary Science 45:701–722. https://doi.org/10.1111/j.1945-5100.2010.01062.x.
Colaprete, A., P. Schultz, J. Heldmann, D. Wooden, M. Shirley, K. Ennico, B. Hermalyn, et al. 2010. “Detection of Water in the LCROSS Ejecta Plume.” Science 330:463–468. https://doi.org/10.1126/science.1186986.
Cruikshank, D.P., and R.H. Brown. 1987. “Organic Matter on Asteroid 130 Elektra.” Science 238(4824):183–184. https://doi.org/10.1126/science.238.4824.183.
Cruikshank, D.P., C.K. Materese, Y.J. Pendleton, P.J. Boston, W.M. Grundy, B. Schmitt, C.M. Lisse, et al. 2019. “Prebiotic Chemistry of Pluto.” Astrobiology 19(7):831–848. https://doi.org/10.1089/ast.2018.1927.
De Sanctis, M.C., V. Vinogradoff, A. Raponi, E. Ammannito, M. Ciarniello, F.G. Carrozzo, S. De Angelis, et al. 2018. “Characteristics of Organic Matter on Ceres from VIR/Dawn High Spatial Resolution Spectra.” Monthly Notices of the Royal Astronomical Society 482(2):2407–2421. https://doi.org/10.1093/mnras/sty2772.
Deamer, D., and B. Damer. 2017. “Can Life Begin on Enceladus? A Perspective from Hydrothermal Chemistry.” Astrobiology 17(9):834–839. https://doi.org/10.1089/ast.2016.1610.
Delitsky, M.L., D.A. Paige, M.A. Siegler, E.R. Harju, D. Schriver, R.E. Johnson, and P. Travnicek. 2017. “Ices on Mercury: Chemistry of Volatiles in Permanently Cold Areas of Mercury’s North Polar Region.” Icarus 281:19–31. https://doi.org/10.1016/j.icarus.2016.08.006.
Des Marais, D.J., L.J. Allamandola, S.A. Benner, A.P. Boss, D. Deamer, P.G. Falkowski, J.D. Farmer, et al. 2003. “The NASA Astrobiology Roadmap.” Astrobiology 3(2):219–235. https://doi.org/10.1089/153110703769016299.
Des Marais, D.J., J.A. Nuth, L.J. Allamandola, A.P. Boss, J.D. Farmer, T.M. Hoehler, B.N. Jakosky, et al. 2008. “The NASA Astrobiology Roadmap.” Astrobiology 8(4):715–730. https://doi.org/10.1089/ast.2008.0819.
Ditzler, M.A., A.C. Rios, M. Nuevo, M. Popovic, R. Mancinelli, D. Summers, J.T. Broddrick, et al. 2020. “Beyond Targeted Searches: The Need for System-Level Approaches to Understanding the Connection Between Astrochemistry and the Emergence of Life.” White paper #269 submitted to the Planetary Science and Astrobiology Decadal Survey 2023–2032. Bulletin of the AAS 53(4). https://doi.org/10.3847/25c2cfeb.7a06f84d.
Eigenbrode, J.E., R.E. Summons, A. Steele, C. Freissinet, M. Millan, R. Navarro-Gonzalez, B. Sutter, et al. 2018. “Organic Matter Preserved in 3-Billion-Year-Old Mudstones at Gale Crater, Mars.” Science 360:1096–1101. https://doi.org/10.1126/science.aas9185.
Field, C.B., M.J. Behrenfeld, J.T. Randerson, and P. Falkowski. 1998. “Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components.” Science 281(5374):237–240. https://doi.org/10.1126/science.281.5374.237.
Fink, U., M. Hoffmann, W. Grundry, M. Hicks, and W. Sears. 1992. “The Steep Red Spectrum of 1992 AD: An Asteroid Covered with Organic Material?” Icarus 97(1):145–149. https://doi.org/10.1016/0019-1035(92)90064-E.
Freissinet, C., D.P. Glavin, P.R. Mahaffy, K.E. Miller, J.L. Eigenbrode, R.E. Summons, A.E. Brunner, et al. 2015. “Organic Molecules in the Sheepbed Mudstone, Gale Crater, Mars.” Journal of Geophysical Research: Planets 120(3):495–514. https://doi.org/10.1002/2014JE004737.
Greaves, J.S., A.M.S. Richards, W. Bains, P.B. Rimmer, H. Sagawa, D.L. Clements, S. Seager, et al. 2020. “Phosphine Gas in the Cloud Decks of Venus.” Nature Astronomy 5:655–664. https://doi.org/10.1038/s41550-020-1174-4.
Hamill, C.D., N.L. Chabot, E. Mazarico, M.A. Siegler, M.K. Barker, and J.M. Martinez Camacho. 2020. “New Illumination and Temperature Constraints of Mercury’s Volatile Polar Deposits.” Planetary Science Journal 1(3):57. https://doi.org/10.3847/PSJ/abb1c2.
Hand, K.P., A.E. Murray, J.B. Garvin, W.B. Brinckerhoff, B.C. Christner, K.S. Edgett, B.L. Ehlmann, et al. 2017. Report of the Europa Lander Science Definition Team. Washington, DC: National Aeronautics and Space Administration. https://solarsystem.nasa.gov/docs/Europa_Lander_SDT_Report_2016.pdf.
Higgins, P.M., and C.S. Cockell. 2020. “A Bioenergetic Model to Predict Habitability, Biomass and Biosignatures in Astrobiology and Extreme Conditions.” Journal of the Royal Society Interface 17(171):1–13. https://doi.org/10.1098/rsif.2020.0588.
Hoehler, T., W. Brinckerhoff, A. Davila, D. Des Marais, S. Getty, D. Glavin, A. Pohorille, et al. 2020. “Groundwork for Life Detection.” White paper #202 submitted to the Planetary Science and Astrobiology Decadal Survey 2023–2032. Bulletin of the AAS 53(4). https://doi.org/10.3847/25c2cfeb.bd9172f9.
Hoshika, S., N.A. Leal, M.J. Kim, M.S. Kim, N.B. Karalkar, H.J. Kim, A.M. Bates, et al. 2019. “Hachimoji DNA and RNA: A Genetic System with Eight Building Blocks.” Science 363(6429):884–887. https://doi.org/10.1126/science.aat0971.
Johnson, N.M., and M.R.R. de Oliveira. 2019. “Venus Atmospheric Composition in Situ Data: A Compilation.” Earth and Space Science 6(7):1299–1318. https://doi.org/10.1029/2018EA000536.
Kharecha, P., J. Kasting, and J. Siefert. 2005. “A Coupled Atmosphere-Ecosystem Model of the Early Archean Earth.” Geobiology 3(2):53–76. https://doi.org/10.1111/j.1472-4669.2005.00049.x.
Krissansen-Totton, J., R. Buick, and D.C. Catling. 2015. “A Statistical Analysis of the Carbon Isotope Record from the Archean to Phanerozoic and Implications for the Rise of Oxygen.” American Journal of Science 315(4):275–316. https://doi.org/10.2475/04.2015.01.
Lang, S.Q., G.L. Früh-Green, S.M. Bernasconi, W.J. Brazelton, M.O. Schrenk, and J.M. McGonigle. 2018. “Deeply-Sourced Formate Fuels Sulfate Reducers But Not Methanogens at Lost City Hydrothermal Field.” Scientific Reports 8:755. https://doi.org/10.1038/s41598-017-19002-5.
Limaye, S.S., R. Mogul, D.J. Smith, A.F. Ansari, G.P. Slowik, and P. Vaishampayan. 2018. “Venus’ Spectral Signatures and the Potential for Life in the Clouds.” Astrobiology 18(9):1181–1198. https://doi.org/10.1089/ast.2017.1783.
Lollar, G.S., O. Warr, J. Telling, M.R. Osburn, and B. Sherwood Lollar. 2019. “‘Follow the Water’: Hydrogeochemical Constraints on Microbial Investigations 2.4 km Below Surface at the Kidd Creek Deep Fluid and Deep Life Observatory.” Geomicrobiology Journal 36:859–872. https://doi.org/10.1080/01490451.2019.1641770.
Lunine, J.I., M.L. Cable, S.M. Horst, and M. Rahm. 2020. “The Astrobiology of Titan.” Pp. 247–266 in Planetary Astrobiology, V.S. Meadows, G.N. Arney, B.E. Schmidt, and D.J. Des Marais, eds. Tucson: University of Arizona Press. https://doi.org/10.2458/azu_uapress_9780816540068-ch010.
Lyons, T.W., K. Rogers, R. Krishnamurthy, L. Williams, S. Marchi, E. Schwieterman, N. Planavsky, and C. Reinhard. 2020. “Constraining Prebiotic Chemistry Through a Better Understanding of Earth’s Earliest Environments.” White paper #143 submitted to the Planetary Science and Astrobiology Decadal Survey 2023–2032. https://doi.org/10.3847/25c2cfeb.7a898b.
Marshall, S.M., A.R.G. Murray, and L. Cronin. 2017. “A Probabilistic Framework for Identifying Biosignatures Using Pathway Complexity.” Royal Society Philosophical Transactions A 375(2109). https://doi.org/10.1098/rsta.2016.0342.
McCord, T.B., R.W. Carlson, W.D. Smythe, G.B. Hanson, R.N. Clark, C.A. Hibbitts, F.P. Fanale, et al. 1997. “Organics and Other Molecules in the Surfaces of Callisto and Ganymede.” Science 278(5336):271–275. https://doi.org/10.1126/science.278.5336.271.
McCord, T.B., G.B. Hansen, R.N. Clark, P.D. Martin, C.A. Hibbitts, F.P. Fanale, J.C. Granahan, et al. 1998. “Non-Water-Ice Constituents in the Surface Material of the Icy Galilean Satellites from the Galileo Near-Infrared Mapping Spectrometer Investigation.” Journal of Geophysical Research: Planets 103(E4):8603–8626. https://doi.org/10.1029/98JE00788.
McDermott, J.M., J.S. Seewald, C.G. German, and S.P. Sylva. 2015. “Pathways for Abiotic Organic Synthesis at Submarine Hydrothermal Fields.” Proceedings of the National Academy of Sciences 112(25):7668–7672. https://doi.org/10.1073/pnas.1506295112.
McKay, C., A. Davila, J. Eigenbrode, C. Lorentson, R. Gold, J. Canham, A. Dazzo, et al. 2020. “Contamination Control Technology Study for Achieving the Science Objectives of Life-Detection Missions.” NASA/TM-20205008709. Washington, DC: National Aeronautics and Space Administration.
Meadows, V.S. 2017. “Reflections on O2 as a Biosignature in Exoplanetary Atmospheres.” Astrobiology 17(10):1022–1052. https://doi.org/10.1089/ast.2016.1578.
Meadows, V.S., C.T. Reinhard, G.N. Arney, M.N. Parenteau, E.W. Schwieterman, S.D. Domagal-Goldman, et al. 2018. “Exoplanet Biosignatures: Understanding Oxygen as a Biosignature in the Context of Its Environment.” Astrobiology 18(6):630–662. https://doi.org/10.1089/ast.2017.1727.
Mogul, R., S.S. Limaye, M.J. Way, and J.A. Cordova. 2021. “Venus’ Mass Spectra Show Signs of Disequilibria in the Middle Clouds.” Geophysical Research Letters 48(7). https://doi.org/10.1029/2020GL091327.
Mustard, J.F., M. Adler, A. Allwood, D.S. Bass, D.W. Beaty, J.F. Bell III, W.B. Brinckerhoff, et al. 2013. Report of the Mars 2020 Science Definition Team. Pasadena, CA: Mars Exploration Program Analysis Group. https://mars.nasa.gov/mars2020/files/mars2020/SDT-Report%20Finalv6.pdf.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2019. An Astrobiology Strategy for the Search for Life in the Universe. Washington, DC: The National Academies Press. https://doi.org/10.17226/25252.
NRC (National Research Council). 2007. The Limits of Organic Life in Planetary Systems. Washington, DC: The National Academies Press. https://doi.org/10.17226/11919.
Postberg, F., N. Khawaja, B. Abel, G. Cholet, C.R. Glein, M.S. Gudipati, B.L. Henderson, et al. 2018. “Macromolecular Organic Compounds from the Depths of Enceladus.” Nature 558:564–568. https://doi.org/10.1038/s41586-018-0246-4.
Quirico, E., S. Doute, B. Schmitt, C. de Bergh, D.P. Cruikshank, T.C. Owen, T.R. Geballe, and T.L. Roush. 1999. “Composition, Physical State, and Distribution of Ices at the Surface of Triton.” Icarus 139(2):159–178. https://doi.org/10.1006/icar.1999.6111.
Raponi, A., M.S. De Sanctis, F.G. Carrozzo, M. Ciarniello, B. Rousseau, M. Ferrari, E. Ammannito, et al. 2021. “Organic Material on Ceres: Insights from Visible and Infrared Space Observations.” Life 11(1):9. https://doi.org/10.3390/life11010009.
Sherwood Lollar, B., T.C. Onstott, G. Lacrampe-Couloume, and C.J. Ballentine. 2014. “The Contribution of the Precambrian Continental Lithosphere to Global H2 Production.” Nature 516:379–382. https://doi.org/10.1038/nature14017.
Sholes, S.F., J. Krissansen-Totton, and D.C. Catling. 2019. “A Maximum Subsurface Biomass on Mars from Untapped Free Energy: CO and H2 as Potential Antibiosignatures.” Astrobiology 19(5):655–668. https://doi.org/10.1089/ast.2018.1835.
Snellen, I.A.G., L. Guzman-Ramirez, M.R. Hogerheijde, A.P.S. Hygate, and F.F.S. van der Tak. 2020. “Re-Analysis of the 267 GHz ALMA Observations of Venus.” Astronomy & Astrophysics 644:1–3. https://doi.org/10.1051/0004-6361/202039717.
Spear, J.R., J.J. Walker, T.M. McCollom, and N.R. Pace. 2005. “Hydrogen and Bioenergetics in the Yellowstone Geothermal Ecosystem.” Proceedings of the National Academy of Sciences 102(7):2555–2560. https://doi.org/10.1073/pnas.0409574102.
Steel, E.L., A. Davila, and C.P. McKay. 2017. “Abiotic and Biotic Formation of Amino Acids in the Enceladus Ocean.” Astrobiology 17(9):862–875. https://doi.org/10.1089/ast.2017.1673.
Sutherland, J.D. 2017. “Opinion: Studies on the Origin of Life—the End of the Beginning.” Nature Reviews Chemistry 1:0012. https://doi.org/10.1038/s41570-016-0012.
Teolis, B.D., D.Y. Wyrick, A. Bouquet, B.A. Magee, and J.H. Waite. 2017. “Plume and Surface Feature Structure and Compositional Effects on Europa’s Global Exosphere: Preliminary Europa Mission Predictions,” Icarus 284:18–29. https://doi.org/10.1016/j.icarus.2016.10.027.
Theiling, B., W. Brinckerhoff, J. Castillo-Rogez, L. Chou, V. Da Poian, H. Graham, S.S. Hosseini, et al. 2021. “Non-Robotic Science Autonomy Development.” White paper #048 submitted to the Planetary Science and Astrobiology Decadal Survey 2023–2032. Bulletin of the AAS 53(4). https://doi.org/10.3847/25c2cfeb.ee4e6b64.
Thompson, W.R., S.K. Singh, B.N. Khare, and C. Sagan. 1989. “Triton: Stratospheric Molecules and Organic Sediments.” Geophysical Research Letters 16(8):981–984. https://doi.org/10.1029/GL016i008p00981.
Vago, J.L., F. Westall, Pasteur Instrument Teams, Landing Site Selection Working Group, et al. 2017. “Habitability on Early Mars and the Search for Biosignatures with the ExoMars Rover.” Astrobiology 17(6–7):471–510. https://doi.org/10.1089/ast.2016.1533.
van Kranendonk, M.J., V. Bennett, and E. Hoffmann, eds. 2018. Earth’s Oldest Rocks, 2nd ed. Cambridge, MA: Elsevier Science. https://doi.org/10.1016/B978-0-444-63901-1.12001-5.
Villalobos, Y., P. Rayner, S. Thomas, and J. Silver. 2020. “The Potential of Orbiting Carbon Observatory-2 Data to Reduce the Uncertainties in CO2 Surface Fluxes Over Australia Using a Variational Assimilation Scheme,” Atmospheric Chemistry and Physics 20(14):8473–8500. https://doi.org/10.5194/acp-20-8473-2020.
Waite, J.H., M.R. Combi, W.-H. Ip, T.E. Cravens, R.L. McNutt, W. Kasprzak, R. Yelle, et al. 2006. “Cassini Ion and Neutral Mass Spectrometer: Enceladus Plume Composition and Structure.” Science 311(5766):1419–1422. https://doi.org/10.1126/science.1121290.
Ward, L.M., B. Rasmussen, and W.W. Fischer. 2019. “Primary Productivity Was Limited by Electron Donors Prior to the Advent of Oxygenic Photosynthesis.” Journal of Geophysical Research: Biogeosciences 124(2):211–226. https://doi.org/10.1029/2018JG004679.
Ward, P.D., and D. Brownlee. 2000. Rare Earth: Why Complex Life Is Uncommon in the Universe. New York: Copernicus Books. https://doi.org/10.1007/b97646.
Weiss, B.P., Y.L. Yung, and K.H. Nealson. 2000. “Atmospheric Energy for Subsurface Life on Mars?” Proceedings of the National Academy of Sciences 97(4):1395–1399. https://doi.org/10.1073/pnas.030538097.
Wickramasinghe, D.T., and D.A. Allen. 1986. “Discovery of Organic Grains in Comet Halley.” Nature 323:44–46. https://doi.org/10.1038/323044a0.
Young, T.S., and P.G. Schultz. 2010. “Beyond the Canonical 20 Amino Acids: Expanding the Genetic Lexicon.” Journal of Biological Chemistry 285(15):11039–11044. https://doi.org/10.1074/jbc.R109.091306.
Zhang, J.A., and D.A. Paige. 2009. “Cold-Trapped Organic Compounds at the Poles of the Moon and Mercury: Implications for Origins.” Geophysical Research Letters 36(16):6. https://doi.org/10.1029/2009GL038614.
Zhou, Y., X. Xu, Y. Wei, Y. Cheng, Y. Guo, I. Khudyakov, F. Liu, et al. 2021. “A Widespread Pathway for Substitution of Adenine by Diaminopurine in Phage Genomes.” Science 372(6541):512–516. https://doi.org/10.1126/science.abe4882.
This page intentionally left blank.

























