3
Potential Uses of CO2 in Commercial Products
3.1 FRAMING, INTRODUCTION, AND SCOPE OF CHAPTER
Carbon is the central element of many manufactured products, notably plastics, fuels, and commodity chemicals; however, current production relies predominantly on fossil sources of carbon, carbon-emitting inputs such as fossil-fuel-powered heat, electricity, and transportation, and fossil-derived co-reagents such as hydrogen. These processes and, upon degradation, the embodied carbon in the products, result in greenhouse gas (GHG) emissions to the atmosphere. Emissions from chemicals and materials production and embodied carbon in products will need to be reduced to net zero for long-term sustainability. One route to net-zero emissions production of carbon-based chemicals and materials is to replace fossil sources of carbon with non-fossil-derived carbon dioxide (CO2), and reduce to zero the emissions associated with all other inputs to the utilization process.
In the context of this report, it is useful to categorize products made from CO2 into two tracks that are distinguished by the lifetime of the products: Track 1 with lifetime greater than 100 years and Track 2 with lifetime less than 100 years (Sick et al. 2022a). Although the exact lifetime to distinguish between the two tracks is debatable, 100 years is used here following a practice promoted by the United Nations Framework Convention on Climate Change (UNFCCC n.d.). The climate relevance of products in the tracks will be different. Track 1 products, especially concrete and aggregates, are considered durable storage options for CO2. Likewise, most polymers have lifetimes of hundreds of years and therefore can hold carbon long enough to provide a removal-related climate benefit, if appropriately treated to prevent GHG emissions through degradation or incineration. Track 2 products, on the other hand, decompose back into CO2 over a shorter timescale. Thus, their primary climate benefit is staked on avoiding the use of fossil carbon in their production (see Figure 3-1). CO2 utilization can replace fossil carbon embedded in chemicals, fuels, and materials with renewable carbon from CO2. The embedded carbon in chemicals and fuels will be released to the atmosphere upon degradation of chemicals and materials over short or long timescales, representing 450 million metric tons (MMT) carbon per year globally for chemicals and derived materials (Kahler et al. 2021), and 3,337 and 2,062 MMT carbon in oil and gas fuels, respectively, as of 2019 (Friedlingstein et al. 2022; Global Carbon Project 2021).1 In addition to embodied carbon, emissions associated with chemical and fuel production, along with other aspects of the product life cycle, are significant. Net-zero carbon use for chemicals and fuels can be feasible, provided that all inputs sum to a zero-carbon budget on a life cycle basis.
___________________
1 Embodied carbon in oil and gas fuels was estimated using the estimated emissions from combustion of such fuels in 2019.
The range of product categories accessible from CO2 feedstock is very large. A 2019 National Academies study of CO2 utilization status and research needs summarized the scope of technologies and products available for mineral, chemical, and biological processes (NASEM 2019). Mineralization processes were deemed the most highly developed potential growth areas for utilization, including carbonated precast and ready-mix concrete and aggregate materials. Chemical and biological processes are available to produce various chemicals and materials directly from CO2, and prospects under development include one-carbon (C1), two-carbon (C2), and multi-carbon (C2+) hydrocarbons and oxygenates, monomers and polymers, and other materials. A 2016 study examined a similar scope of possible products and projected the potential annual use of CO2 to make these products by 2030 to be between 2 and 8 gigatonnes (Gt), generating an annual revenue stream between $0.5 trillion and $2 trillion (Global CO2 Initiative 2018). Subsequent studies reported similar magnitudes of CO2 consumption and future market opportunity for CO2 utilization (Biniek et al. 2020; CSIRO 2022; Hepburn et al. 2019; NASEM 2019; Sick et al. 2022b; some of which are summarized in Table 3-1). At this time, the future scope of this emerging industry is highly uncertain and thus estimates of potential will vary widely. Local needs, resource availability, and regulatory and incentive frameworks also will lead to significantly different outcomes for different regions. In general, the role of CO2 utilization is to provide a sustainable source of carbon that can enable net-zero production of chemicals and materials, rather than contribute significantly to durable emissions mitigation (see, e.g., Mac Dowell et al. 2017).
CO2 utilization enables net-zero or net-negative chemicals and materials production and provides opportunities to design new products and processes with preferred attributes. In some cases, especially for Track 2 chemicals, CO2 substitutes for fossil carbon to produce the same product or products of similar function. Even in those cases, CO2 offers opportunities to re-design products and processes, especially taking advantage of the
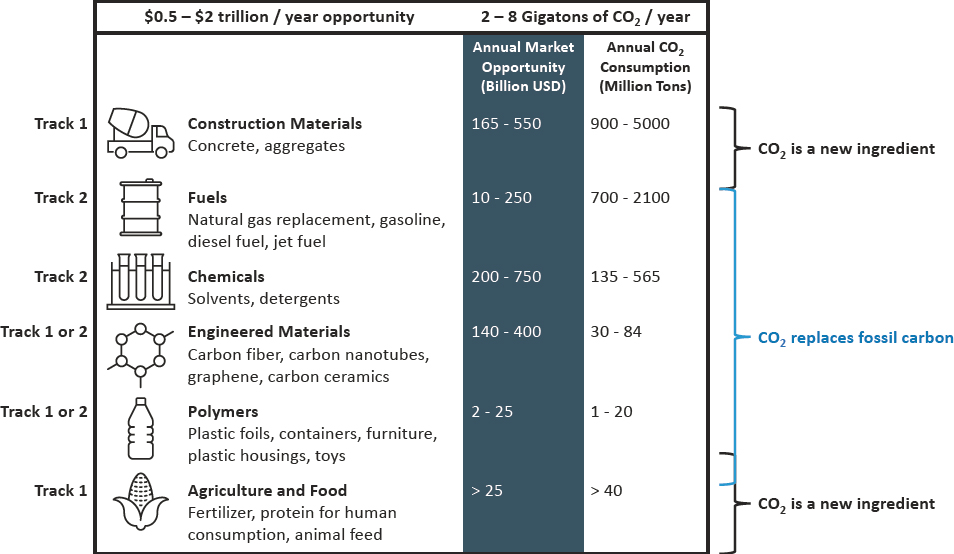
SOURCE: Committee generated based on data from V. Sick, G. Stokes, F. Mason, et al., 2022, Implementing CO2 Capture and Utilization at Scale and Speed: The Path to Achieving Its Potential, Technical Report, Global CO2 Initiative, University of Michigan, https://dx.doi.org/10.7302/5825. Reproduced with permission, Global CO2 Initiative. CC BY 4.0.
TABLE 3-1 Summary of Estimated and Projected Market Value and Volume of Products and CO2 Amounts Utilized for Various Products
| CO2 Utilization Market | Product Market Size | |
|---|---|---|
| Specific Product Example or Market Cluster | 2015 Total Market Size and 2030 Expected Total Market Sizea | 2030 Potential Market for Product Covered by Utilized Carbona |
| Building Materials | ||
| Concrete and concrete aggregates | 2015: 20–30 Gt 2030 projection: 40 Gt |
6.5–16.5 Gt |
| Carbonated aggregates | 2015: 25–35 Gt 2030 projection: 50 Gt |
1–10.5 Gt |
| Carbonated aggregates | 2015: 25–35 Gt 2030 projection: 50 Gt |
1–10.5 Gt |
| Fuels | ||
| Liquid fuels | 2015: 800 billion to 1 trillion gallons 2030 projection: 1 trillion+ gallons |
7–165 billion gallons |
| Jet fuel | ||
| Methane | 2015: 3–4 trillion cubic meters 2030 projection: over 4–5 trillion cubic meters |
4–165 billion cubic meters |
| Chemical Intermediates | ||
| All chemicals | ||
| Methanol | 2015: 60–70 Mt 2030 projection: 190 Mt |
4–34 Mt (for fuels), 1.3–9.3 Mt (for chemical intermediates) |
| Syngas | 2015: 130–150 GW 2030 projection: 500 GW |
15–265 GW |
| Formic acid | 2015: 0.5–0.7 Mt 2030 projection: 1 Mt |
10–475 kt |
| Polymers and Materials | ||
| Polyurethane | ||
| Polyhydroxyalkanoates | ||
| Polyols and polycarbonates | 2015: 8–10 Mt 2030 projection: 17 Mt |
0.4–6.8 Mt |
| Carbon fiber | ||
| Carbon black | ||
| Carbon nanotubes | ||
| Utilization of CO2 in Products (Mt CO2/year) | Market Value ($) | |
|---|---|---|
| 2030 Potential CO2 Utilization in Productb | 2050 Potential CO2 Utilization in Product | Market Value of Product in 2030, 2035, 2040,e and 2050d |
| 150 | 100–1,400c 24–1,300 (precast concrete)d |
2030: $50 billion 2035: $150 billion 2040: $450 billion |
| 1,000–9,500d | 2050: $182–$337 billion (aggregates), $623–$666 billion (precast concrete) | |
| 1,000–4,200c | ||
| 15 | 14–10,200d | 2030: $17 billion 2035: $25 billion 2040: $36 billion 2050: $5–$1,849 billion |
| 260–4,400d | 2050: $16–$214 billion | |
| 300–600c | ||
| 260–580d | 2030: $300 million 2035: $3 billion 2040: $25 billion 2050: $96–$183 billion |
|
| 0.5–1d | 2030: $64 million 2035: $200 million 2040: $300 million 2050: $700–$840 million |
|
| 10 (plastics, including polyurethane) | 1.8–13 | 2030: $13 billion 2035: $24 billion 2040: $40 billion 2050: $130–$191 billion |
| 2030: $10 million 2035: $30 million 2040: $60 million |
||
| 0.1 | ||
| 40–200 | 2050: $14–$66 billion | |
| 2030: $3 million 2035: $21 million 2040: $32 million |
||
| CO2 Utilization Market | Product Market Size | |
|---|---|---|
| Specific Product Example or Market Cluster | 2015 Total Market Size and 2030 Expected Total Market Sizea | 2030 Potential Market for Product Covered by Utilized Carbona |
| Algae and Other Microorganisms | ||
| Microalgae (processed to create biofuels or food additives) | ||
| Fish meal protein alternative | ||
NOTES: This table represents a collection of available data for several broad product volume and market value studies. Absences of values indicate that the studies did not examine or make estimates for the categories. The volumes and values across different studies are independent estimates with different assumptions and are not directly comparable.
SOURCES: a Global CO2 Initiative (2018); b Biniek et al. (2020); c Hepburn et al. (2019); d Sick et al. (2022b); e and Lux Research (2020).
oxygen content of CO2 in the production of oxygenated products like polycarbonates (Zimmerman et al. 2020). For others, the introduction of carbon into these products is new, namely construction materials in Track 1 such as CO2-cured concrete or carbonated aggregates. In addition to creating large-scale CO2 removal opportunities, mineral carbonation to form building materials offers the chance to develop entirely new materials with new properties and performance, though these may trigger the need for extensive documentation and updated codes and standards.
Maximum growth rates of CO2 utilization estimated in market studies have not been achieved to date, due to the high cost of CO2 and zero-emission energy versus fossil inputs, a lack of supportive legislation and policy to incentivize production and use of net-zero carbon products, a lack of infrastructure for CO2 and enabling inputs, and frequently a perception of immaturity of underlying technologies requiring a long duration to profitability.
Even with a supportive environment for producing net-zero carbon products, CO2 utilization will compete with other means of reducing the lifecycle emissions of products and materials, including switching from hydrocarbon fuels to electricity and hydrogen and replacing fossil-carbon feedstocks with bio-based or recycled carbon inputs. Replacing hydrocarbon fuels with hydrogen or electricity rather than CO2-based synthetic fuels, when feasible, requires less energy. The energy cost needed to convert CO2 into a fuel can be two to four times the amount required to use electricity or clean hydrogen directly as an energy carrier (Bazzanella and Ausfelder 2017; Herzog 2018). Therefore, the production of hydrocarbon fuels may be motivated primarily by use cases where electricity or/and hydrogen are not (currently) a feasible option, for example, long-haul aviation. On the other hand, many carbon-based chemicals and materials cannot be substituted readily with noncarbon alternatives. Where carbon-based materials are required, fossil-free production from CO2 (Kätelhön et al. 2019) will compete with alternative sources of carbon such as biomass and recycled products (Berg et al. 2020). Biomass feedstock, especially waste biomass as a lower-cost competitor to CO2, is likely to be an important source of carbon in the future, but there is limited available land to derive carbon feedstocks from plants, particularly if waste biomass is being used. Recycled products are also an important carbon feedstock competing with CO2, but even as challenges in chemical recycling are overcome, some processes will favor CO2 as a starting material, and in some instances, it will be impractical to collect all product material for recycling. In addition, the demand for carbon-based products and associated export opportunities will continue to increase globally, as economies grow to meet the needs of billions of currently underserved people.
Development and deployment of CO2-derived product technologies needs to be accompanied and guided by rigorous life cycle assessments (LCAs), coupled with techno-economic assessments (TEAs), to evaluate
| Utilization of CO2 in Products (Mt CO2/year) | Market Value ($) | |
|---|---|---|
| 2030 Potential CO2 Utilization in Productb | 2050 Potential CO2 Utilization in Product | Market Value of Product in 2030, 2035, 2040,e and 2050d |
| 200–900 | ||
| 2030: $25 million 2035: $3 billion 2040: $13 billion 2050: $18–$921 billion |
||
benefits and risks to the environment as well as economic viability. LCAs and TEAs can be performed at different levels of detail appropriate to the project scope and stage, and the outcomes of such studies are inputs for decision making along the entire path from invention to full-scale commercialization. A recent international community effort developed CO2-specific guidelines for LCAs and TEAs (Zimmermann et al. 2018), and a web resource initiated and supported by the U.S. Department of Energy (DOE) was made available in 2021 (Global CO2 Initiative 2022a). The AssessCCUS resource includes databases of information as well as tools for conducting TEAs and LCAs. This effort begins to address 2022 guidance from the White House Council on Environmental Quality that called for consolidating and publishing a repository for LCA methodology, results, and information related to CO2 capture and utilization (CCU) and carbon dioxide removal (CDR) (CEQ, 2022).
Choosing research directions, determining priority criteria for environmental risks, or directing policy support involves multifactor decision processes of potentially high complexity and ambiguity (Cremonese et al. 2022). For CO2 utilization, comprehensive analyses and scenario studies are needed to identify technologies and processes that produce needed chemicals, offer environmental improvements, and have the best prospects of being cost competitive against other options for meeting the same goal (Fernández-Dacosta et al. 2017). Trade-offs need to be evaluated using a suitable unit of analysis that allows results to be compared. This applies both to comparing different CO2 utilization production pathways and to comparing CO2 utilization pathways with those that use other sustainable carbon feedstocks (Artz et al. 2018; Ravikumar et al. 2021b). Equally or more complex are comparisons between different products, such as concrete and chemicals, to identify the largest impact on CO2 emissions (Ravikumar et al. 2021a), or different ways of meeting a greater goal, such as net-zero emissions to the atmosphere.
The remainder of this chapter describes in more detail the products and processes relevant for CO2 utilization in a net-zero emissions future, in order to inform needs for infrastructure and policies described later in the report. The potential climate impact of net-zero CO2 utilization processes and products is assessed, with a focus on either durable products that can offer long-term storage of CO2 or nondurable products that will represent a large-scale displacement of fossil-based chemicals in a net-zero carbon economy. The chapter also enumerates needs for additional inputs such as energy, hydrogen, water, land, and facilities. As noted in Chapter 1, this report focuses on chemically transformative uses of CO2 for a net-zero carbon future and does not include an analysis of CO2 use for enhanced oil and gas recovery (EOR, EGR), as a process fluid (cooling, cutting, fire extinction), or for highly transient products (e.g., beverages, supercritical fluid extractions, low-temperature drying, insulation).
3.2 FUTURE SOURCES OF CO2 FOR UTILIZATION
Chapter 1 describes the need to reduce atmospheric concentrations of GHGs; the most likely path to reduce emissions is to decarbonize the energy system via a shift from fossil-fuel combustion to zero-emissions energy conversion and storage. Decarbonization of the energy system means that most sources of CO2 emissions today (see Section 2.1) are likely to be eliminated or significantly reduced by 2050. The sources that remain in the long term may be those that are most technically difficult or expensive to eliminate, including CO2 emissions from the combustion of fuels for heavy-duty transportation such as aviation and shipping, and industrial process emissions such as those from steel or cement manufacturing, among others. Some of these emissions may be from combustion of fossil fuels, representing linear flows of carbon from the ground to the atmosphere, and some may represent circular flows of carbon into and out of the atmosphere. A 2021 National Academies report on accelerating deep decarbonization in the United States estimated that there would be a constant level of remaining CO2 emissions at about 5 percent of 2005 levels, or about 300 MMT CO2, in a net-zero emissions scenario for 2050 (NASEM 2021a). The same report also noted that two comprehensive modeling studies of pathways for decarbonization each included >500 MMT of carbon capture and storage (CCS) in the United States per year (DDP n.d.; Larson et al. 2021). A 2022 study of active carbon management summarized several modeling studies’ requirements for carbon removal, finding a range of ~500 MMT to ~8 Gt carbon removal required annually worldwide to reach net-zero goals (ITIF 2022). Another method to estimate the possible scale of remaining emissions in a decarbonized future is to consider the “hard to abate” sectors such as aluminum, plastic, steel, and cement, as addressed by the Mission Possible study. That study estimated that the remaining global emissions for those products in a circular carbon scenario would be 5.6 Gt CO2 per year, 0.8 from aluminum, 0.9 from plastics, 1.9 from steel, and 2.0 from cement (Energy Transitions Commission 2018).
The remaining CO2 produced in a net-zero system is likely to have multiple potential fates, including CO2 utilization for durable storage (Track 1; see Figure 1-3-1) or circular processes (Track 2), but also CCS or non-utilization negative emissions processes such as land- or ocean-based enhanced weathering. Remaining fossil emissions can only be used sustainably for durable Track 1 products, while biogenic, direct air capture (DAC), and direct ocean capture (DOC) sources can be used sustainably for either Track 1 or Track 2 products. LCAs of complete CO2 utilization systems will determine a particular product’s carbon footprint, which includes considering the emissions impact of the CO2 source, as it relates to the product’s life cycle from sourcing of materials through manufacture, processing, use, and disposal. Such analyses require materials and processes to be accounted for and attributed, but that does not necessarily mean that different CO2 sources must be segregated according to their emissions impact. As with other commodities, sources of CO2 can be mixed, and the origin and fate of any particular molecule of CO2 need not be specifically known, as long as reliable bookkeeping allows for appropriate attributions to be made and the balance known and accounted for.
For the circular processes generating Track 2 products, where carbon from CO2 replaces fossil-origin carbon, CO2 utilization will compete with other potential sustainable carbon feedstocks including biomass and recycled materials. The interplay between these carbon feedstocks is beginning to be explored, particularly with regard to their role in “de-fossilizing” the chemical industry (Bazzanella and Ausfelder 2017; Gabrielli et al. 2020; Lange 2021). Recycling used carbon products can be a first step toward a circular economy, but since it inherently cannot achieve 100 percent efficiency, meeting the demand for chemical products will require additional carbon inputs from biomass or CO2 (Lange 2021). Among different routes to produce a carbon-neutral product, trade-offs exist between requirements for land, scarce materials and minerals, and water (Gabrielli et al. 2020). Efforts toward developing a circular economy are particularly strong in Europe, as evidenced by the European Commission’s Circular Economy Action Plan released in 2020 (EC 2020). A detailed analysis of the European chemical industry found that achieving carbon neutrality to meet the European Commission’s larger climate goals would require innovative research and development efforts, including through long-term public–private partnerships to facilitate deployment and de-risking of new technologies (Bazzanella and Ausfelder 2017). Other recommended policies to promote de-fossilizing the European chemical industry included establishing central, open-access databases for (1) LCA studies of low-carbon technologies and (2) carbon sources (biomass, CO2, and other gases) and existing infrastructure (Bazzanella and Ausfelder 2017).
3.3 POTENTIAL UTILIZATION PRODUCTS AND PROCESSES
Going forward, transformations of CO2 are likely to expand dramatically beyond the current limited set of chemically or biologically advantageous chemicals, described above, in order to synthesize many net-zero or net-negative carbon-containing products. Table 3-2 and the section that follows summarize classes of materials to consider for future CO2 utilization, drawing from a comprehensive research status assessment published by the National Academies in 2019. When planning infrastructure for future CO2 utilization, however, it is useful to consider not only what products can be made from CO2, but also what products will be a high priority in a future net-zero economy, and what fraction of the product market will be made with carbon from biomass and recycling. Section 3.5 highlights priority technologies for a net-zero future.
TABLE 3-2 Summary of Major Potential CO2 Utilization Opportunities Including Utilization Process and Infrastructure Aspects
| Chemical or Material | Utilization Process | Summary of Infrastructure Aspects by Product Class |
|---|---|---|
| Construction material | ||
| Concrete | Mineralization |
|
| Aggregates | Mineralization |
|
| Chemicals and fuels | ||
C1 Compounds
C2 Compounds
C2+ Compounds
|
Chemical and biological |
|
Niche Products
|
Chemical |
|
| Chemical or Material | Utilization Process | Summary of Infrastructure Aspects by Product Class |
|---|---|---|
| Polymers, polymer precursors, and other materials | ||
| Polymers and polymer precursors | Chemical and biological |
|
| 0-3D elemental carbon and engineered products | Chemical |
|
3.3.1 Construction Materials
3.3.1.1 Concrete
Concrete materials offer a particularly attractive opportunity for CO2 utilization. With an estimated annual global production of 20–40 Gt by 2030 (see Table 3-1) and resulting durable CO2 use of up to 1.41 Gt per year by 2050, the potential CO2 utilization could be at a climate-relevant scale. Furthermore, CO2 use in concrete production results in durable conversion to solid Track 1 materials and thus constitutes a CDR process. CO2 incorporation into concrete can occur via a variety of mechanisms, including curing via carbonation of the cement or supplemental cementitious material, as well as the use of fillers and aggregates that themselves have been manufactured from minerals or waste materials by carbonation with CO2 (Hills et al. 2020; Woodall et al. 2019). An example of a mineral carbonation reaction is CO2 interacting with portlandite, an alkaline mineral, to produce calcite, the mineral carbonate product, and water, Ca(OH)2 + CO2 à CaCO3 + H2O. This is a thermodynamically favored and exothermic reaction at ambient pressure and temperature.
The amount of CO2 incorporated into the material during curing varies substantially between so-called precast concrete products (>1 percent by mass) and ready-mix concrete (<1 percent by mass). Although the methods differ in applications and performance, given suitable production conditions, both can yield concrete materials that exhibit higher compressive strength. In building applications where compressive strength is important, then the same structure could be built with less concrete (Monkman and MacDonald 2017; Ravikumar et al. 2021b). Note that cement curing with CO2 reduces the pH of the resulting concrete compared to traditional concrete, which could be detrimental if steel reinforcements are used in the concrete, since corrosion of steel is suppressed only at higher pH levels. Solutions to this potential shortcoming could include use of alternative concrete materials that
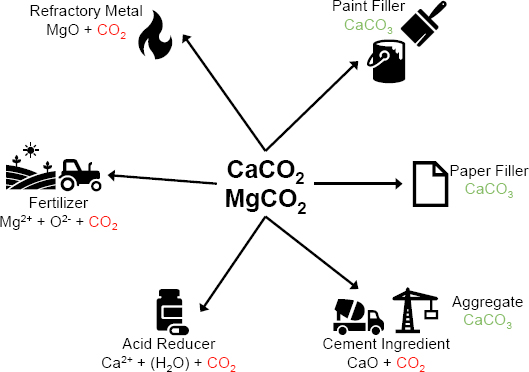
SOURCE: C.M. Woodall, N. McQueen, H. Pilorgé, and J. Wilcox, 2019, “Utilization of Mineral Carbonation Products: Current State and Potential,” Greenhouse Gases: Science and Technology 9(6):1096–1113, https://doi.org/10.1002/ghg.1940. CC BY 4.0.
seek to avoid the need for most, if not all, steel reinforcements via increased ductility and self-healing capability (Li 2019) or replacing steel rebar with sustainably sourced and produced carbon fiber bars. Concrete with modified properties often results in conflicts with existing codes, standards, and approved materials lists for procurement, making the introduction of these new concretes more difficult. Additionally, conventional and CO2-cured concrete absorb CO2 from the ambient air, so there has been some question of the impact of initial CO2 curing on the total lifetime absorption of CO2. Continued curing of concrete during its service life is slow and dependent on many factors, and quantitative understanding of the impact of CO2-curing concrete during construction on future CO2 uptake is not well understood (Zhang et al. 2020).
3.3.1.2 Aggregates
As with concrete materials, the carbonation of minerals and selected waste materials offers Track 1–type CO2 utilization opportunities, that is, providing durable storage of CO2. An inorganic material with sufficient alkalinity, typically from available Ca2+ or Mg2+ or their oxides, will react with CO2 to form durable carbonates that could be considered for use as aggregates, such as in concrete production, as a substitute for gravel, or as fillers in paper or porcelain production, as illustrated in Figure 3-2 (La Plante et al. 2021; Woodall et al. 2019). Materials such as steel slag, cement kiln dust, and fly ash contain a significant amount of alkaline material readily available for direct reaction with CO2, while some minerals require chemical processing to make the alkaline materials available. CO2 dissolved in natural bodies of water can also be utilized to form inorganic carbonates. Such processes also have been designed as chemical looping processes with the purpose of capturing CO2 (Adánez and Abad 2019).
3.3.2 Chemicals and Fuels
Using chemical or biological processes, CO2 can be converted into various carbon-based chemicals and/or fuels. Present global efforts focus on converting CO2 into C1 and C2 materials with single or multiple steps. The 2019 National Academies’ assessment of CO2 utilization status and research needs identified major opportunities for chemical and fuel formation via CO2 utilization. Figures 3-3 and 3-4 show the major products accessible via chemical and biological routes, respectively.
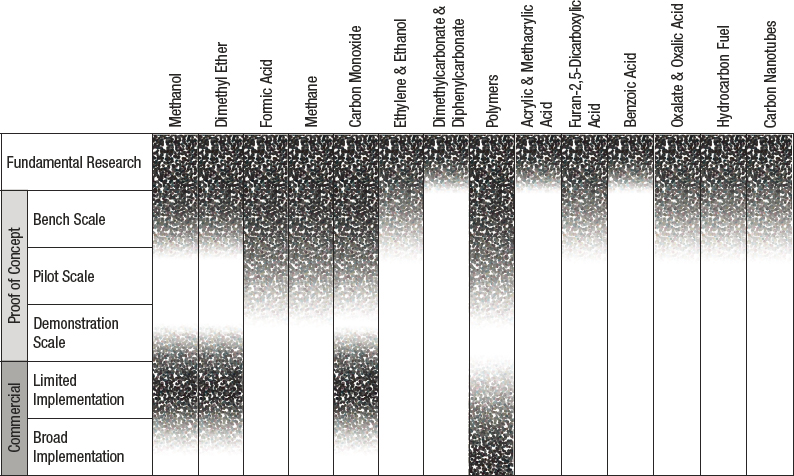
SOURCE: National Academies of Sciences, Engineering, and Medicine, 2019, Gaseous Carbon Waste Streams Utilization: Status and Research Needs, Washington, DC: The National Academies Press, https://doi.org/10.17226/25232.
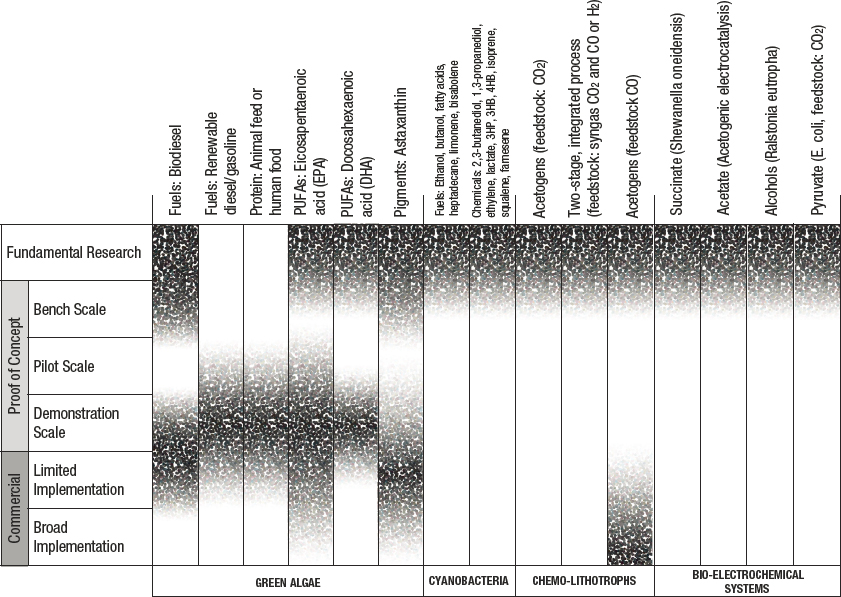
SOURCE: National Academies of Sciences, Engineering, and Medicine, 2019, Gaseous Carbon Waste Streams Utilization: Status and Research Needs, Washington, DC: The National Academies Press, https://doi.org/10.17226/25232.
3.3.2.1 C1 Compounds
C1 compounds are important targets for CO2 utilization because they are major commodity chemicals or intermediates and are more easily chemically synthesized from CO2 than chemicals that require the formation of carbon-carbon bonds. CO2 could be converted directly into partially or fully hydrogenated C1 compounds such as formic acid (HCOOH), methanol (CH3OH), and methane (CH4), as well as other important C1 products such as carbon monoxide (CO) and urea ((H2N)2CO). Many of these products are used on their own as is or for industrial processes, and some serve as feedstocks for other chemical syntheses.
Urea, an input to fertilizer production and other industrial processes, is currently the largest global chemical consumer of CO2, and urea synthesis is expected to remain a major consumer. Carbon monoxide is also an important intermediate product of CO2 utilization, because it may become an important intermediate to sustainable fuel production via Fischer-Tropsch pathways, or for other chemical processes, in a net-zero emissions future. Of the partially and fully hydrogenated products of CO2, methane and methanol could serve as energy carriers; however, the toxicity of methanol may limit its distributed uses (chemeurope n.d.; IRENA and Methanol Institute 2021). Methanol and formic acid are commodity chemicals and could serve as intermediates for other sustainable chemical and fuel production, including methanol-to-gasoline via, for example, the Mobil zeolite catalysis process.
3.3.2.2 C2+ Compounds
C2+ compounds are important targets for CO2 utilization because they constitute fuels, commodity chemicals, and chemical feedstocks. Key C2 compound targets are ethylene, ethanol, oxalate, and oxalic acid. Other C2+ compounds that could be synthesized from CO2 include alcohols, carboxylic acids, fuels, pigments, and proteins. Both chemical and biological systems can produce C2+ chemicals, with biological systems being especially adept at forming multicarbon products. For example, a process using microbes to convert CO2 to ethanol has been commercialized (Köpke et al. 2011; McCoy 2022). In addition to ethanol, C2+ products accessible via biological processes include various alcohols and hydrocarbons and their mixtures, diols, and carboxylic acids, particularly carboxylic acids that are also metabolic biomolecules. Both chemical and biological processes can produce individual chemicals and mixtures that may be used as fuels, such as C2–C8 hydrocarbons and alcohols. Processes to create synthetic fuels and biofuels from CO2 can serve the potential markets for sustainable fuels for heavy-duty transportation, particularly aviation, and for storage of renewable energy in chemical bonds (so-called Power-to-X).
3.3.3 Polymers, Polymer Precursors, and Composites
Polymers and polymer precursors are high-demand commodities. The global market for polymers is expected to grow 5.1 percent annually and will reach more than $1 trillion by 2030 (Research and Markets 2020). The major driver of the rapid market growth has been the fast transfer from reusable packaging to one-time utilization of polymer-based plastics. While there are efforts to reduce single-use plastics, some single-use, disposable plastic requirements will remain, especially for medical applications. Other polymer uses include in fibers, rubbers, and fabrics, and for some durable plastic applications, such as materials in vehicles and appliances.
Chemical syntheses of polymers from CO2 can occur via direct or indirect routes. CO2 is directly incorporated into aliphatic polycarbonates as carbonate groups via copolymerization with epoxides, or indirectly incorporated via conversion to other monomers, such as methanol, carbon monoxide, ethylene, organic carbonates, or urea (NASEM 2019). CO2 integration into polyols to produce polyurethanes has been demonstrated and scaled to pilot-size plants (Langanke et al. 2014). Benzene may be another polymer precursor of interest, as it is currently used to synthesize derivatives that are polymerized to form polystyrene and synthetic rubber, among others. Only a part of the carbon in polycarbonates is CO2-derived when CO2 is copolymerized with epoxides. Research is exploring renewable feedstocks such as cyclohexadiene oxide, limonene oxide, and α-pinene oxide. CO2-derived polymers can be modified for more applications by blending with natural and synthetic compounds, integrating with fillers, and crosslinking (e.g., vulcanization).
In addition to chemical means of producing polymers from CO2 utilization, biological systems can produce polymer precursors and biopolymers. Biologically derived polymer precursors can be used to synthesize polyesters, typically via an esterification reaction using a four-carbon dicarboxylic acid monomer and a diol monomer. Many dicarboxylic acids have been thoroughly investigated as valuable fermentation products that can be made efficiently in various microorganisms. Most of these dicarboxylic acids are found in the citric acid cycle and include succinate, fumarate, and malate. Other carboxylic acids of note include 2,5-furan dicarboxylic acids such as 2,5-dioxopentanoate. Research into the microbial production of the diol monomers is still in its infancy, including of 2,3-butanediol and 1,3-propanediol, but the promise of making industrially viable bioplastics has led to increased research investigating the production of various diols. Other biological polymer precursors include ethylene, for making polyethylene (NASEM 2019). Cyanobacteria can also produce biodegradable polymers, especially polyhydroxy alkanoates, and work is ongoing to produce this material from CO2, rather than sugars (Troschl et al. 2017).
3.3.4 Elemental Carbon Materials
Conventional and advanced carbon materials with 0-3D structures, for example, carbon quantum dots, carbon nanotubes, graphene, graphite, amorphous carbon, carbon fiber, carbon black, and carbon-carbon composites, offer opportunities for advanced properties and new uses. The carbon materials are stable, although their stabilities are affected by the conditions in which they are utilized. The markets of carbon quantum dots, carbon nanotubes, graphene, graphite, amorphous carbon, carbon fiber, carbon black, and carbon-carbon composites in 2020 were $0.652B (Mordor Intelligence 2021), $0.877B (Emergen Research 2022), $0.286B (Fortune Business Insights 2022), $13.6B (Fortune Business Insights 2021), $0.226B (Research Reports World 2021a), $16.0B (Global Market Insights 2021), $2.523B (Research Reports World 2021b), respectively. Markets for carbon materials are expected to rapidly increase. A recent study of CO2 utilization potential that examined carbon black estimated a market value of $14B–$66B in 2050 and a potential for CO2 use of between 40 and 200 MMT (Sick et al. 2022b). Outside of carbon black production, technologies for the conversion of CO2 to other carbon materials are less mature, and market projections are difficult. Carbon materials are rich in structure, texture, and properties. Zero-dimensional, or 0-D, carbon materials include graphene quantum dots, carbon quantum dots, nano-diamond, fullerenes (e.g., C60), carbon black, and carbon-coated metal nanoparticles. Representative 1D, 2D, and 3D carbon materials include carbon nanotubes and carbon nanofibers; graphene; and carbon foam and expanded graphite, respectively. Different dimensional materials have correspondingly different applications based on their structure and properties. For example, due to their tiny size, quantum confinement effects, desirable properties, and biocompatibility, 0D carbon materials have great potential in sensing applications (Uygun and Uygun 2020; Wang et al. 2020). 1D carbon nanotubes and 2D graphene are being explored for a plethora of advanced technologies. Three-dimensional hierarchical carbons are inexpensive, lightweight, have a high specific surface area and well-ordered channels, and have outstanding electronic and ionic conductivity, and thus are promising materials for energy conversion and storage for high-strength material applications. Carbon black applications range from filler materials, for example, for tires and toners, to additives for concrete. Using carbon black as a filler in durable materials offers opportunities for growing Track 1 type utilization of CO2 with durable carbon storage. Finally, carbon-carbon composites, made from carbon fiber and graphite, are lightweight and very strong, enabling their use as structural materials in vehicles and buildings.
3.3.5 Niche Products: Diamonds, Perfumes, and Liquor
Consumer-facing products made with CO2 might not have a large CO2 utilization potential by volume but can serve as signaling products to increase consumer familiarity and comfort with CO2-derived products (Arning et al. 2020; Lutzke and Árvai 2021). Examples of such products include diamonds, high-purity ethanol for the production of perfumes and vodka, personal care products, and food (Aether n.d.; Air Protein 2022; Gallon 2022; Kiverdi 2019; Köpke et al. 2011; Pace and Sheehan 2021). In some cases, these products also may provide a greater profit margin than the respective commodity chemicals or intermediates and may allow producers to successfully commercialize other higher-volume processes in tandem to bring down overall costs to compete in a larger market.
3.4 EMERGING, PILOT, AND COMMERCIAL FACILITIES UTILIZING CO2
In 2016, less than 200 active entities were identified as working on CO2 utilization (Global CO2 Initiative 2018) and respective technology readiness levels were mostly below 5. Since then, more developers have entered the field, but the numbers are only now beginning to grow more rapidly. AirMiners and the Circular Carbon Network keep listings of carbon capture, utilization, and storage (CCUS) developers, which include 403 companies (as of May 13, 2022) that work on capture technologies or services, or utilization (AirMiners 2022; Circular Carbon Network 2022). A visual database that includes additional information, such as publications and policies, is also available (Global CO2 Initiative 2022b). Figure 3-5 shows locations of startup CO2 utilization companies in the United States.
3.5 PRIORITY NEEDS FOR CO2-DERIVED PRODUCTS THAT COULD CONTRIBUTE TO A NET-ZERO CARBON FUTURE
3.5.1 CO2-Derived Product Priorities
Products from CO2 utilization that will be of highest importance and significance in a net-zero carbon future (IPCC 2022) include Track 1 materials that offer durable CO2 storage and Track 2 materials for which the climate benefit comes from replacing fossil carbon with CO2-based carbon in a circular fashion for carbon-containing products that continue to be necessary and desirable. These products are summarized in Table 3-2, above. Even-
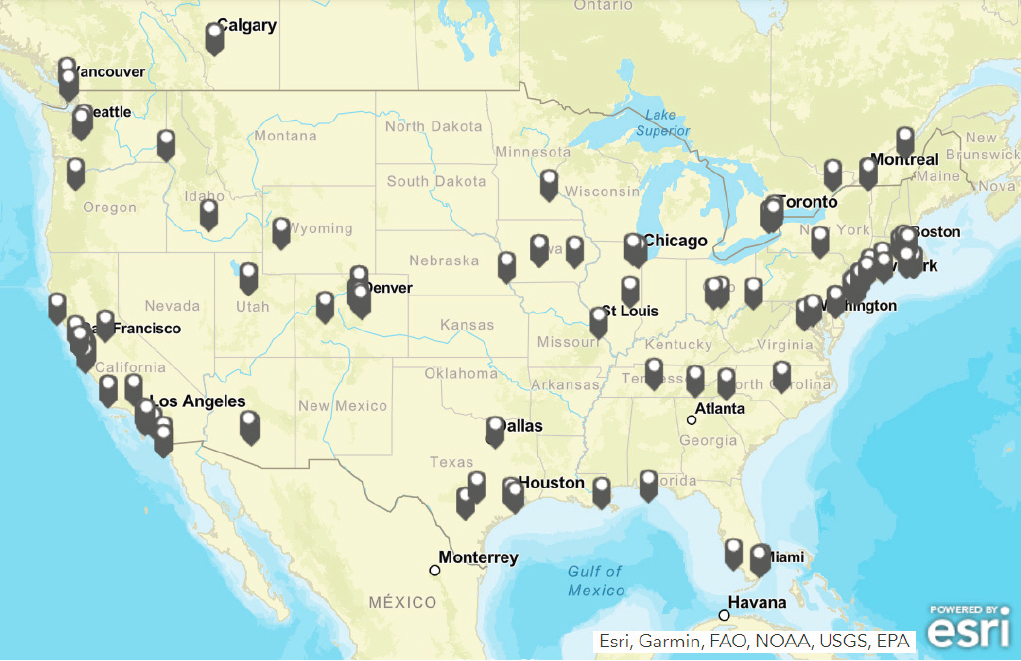
SOURCE: Global CO2 Initiative, 2022, “CCU Activity Hub,” University of Michigan, https://www.globalco2initiative.org/evaluation/carbon-capture-activity-hub.
tually, products in both tracks could conceivably displace the incumbent products completely. However, this outcome will depend on many factors related not only to manufacturability, cost, and other externalities, but also to the development of alternative processes and products that may replace incumbent fossil-carbon products. Examples of such alternative products include electricity and clean hydrogen as energy carriers for mobility applications.
3.5.2 Requirements for Other Inputs and Feedstocks
Success for CO2 utilization depends not only on access to CO2, but also on an array of other inputs and feedstocks, all of which must contribute to a net-zero or net-negative system. In particular, CO2 utilization systems require scale-up of carbon-emission-free energy for both utilization processes and other parts of the CO2 utilization system, including capturing and processing (separating and purifying) CO2, generating other inputs, and transporting reactants and products. Additionally, some processes may require clean hydrogen, water, and land. Large-scale deployment of CO2 conversion technology could be problematic if the technology relies on catalysts that require difficult-to-source and -secure access to raw materials (including some metals).
3.5.2.1 Electricity and Fuel Requirements for Carbon Capture
Figure 3-6 shows the electric and fuel energies, which, summed together, are required to capture CO2 from various sources. For point sources, the amount of electric energy and/or fuel required for carbon dioxide capture and storage (CCS) is taken from the 2019 National Petroleum Council Carbon Capture study. For DAC, the energy requirements for liquid and solid capture come from IEA (2022).
Capital costs for carbon capture depend on CO2 concentration and pressure of the waste gas stream and vary by more than 10-fold for the industrial processes shown. For all technologies other than DAC, the energy required
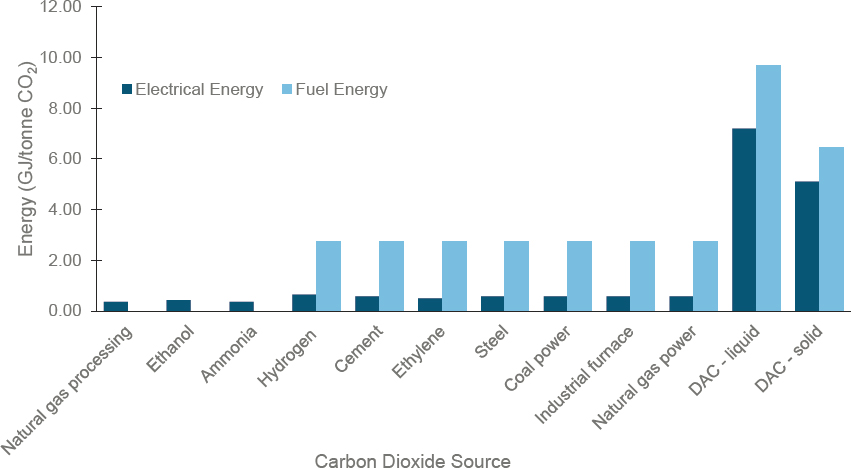
SOURCES: Committee generated based on data from National Petroleum Council, 2019, “Meeting the Dual Challenge: A Roadmap to At-Scale Deployment of Carbon Capture, Use, and Storage,” https://dualchallenge.npc.org; and International Energy Agency, 2022, Direct Air Capture: A Key Technology for Net Zero, Paris: IEA, https://www.iea.org/reports/direct-aircapture-2022. All rights reserved; as modified by the National Academies of Sciences, Engineering, and Medicine.
for CO2 capture is less than 20 percent of the energy released via fossil-fuel combustion that generates the CO2 (e.g., 18 GJ/ton of CO2 released from methane combustion). Because of very low (~420 ppm) CO2 concentrations in air, DAC requires a greater amount of energy than post-combustion carbon capture (IEA 2022). Current DAC research therefore is examining use of waste heat, or pressure or humidity swings, to improve the return on energy investment. Figure 3-6 shows that selecting a CO2 source is important in assessing the electric power and fuel requirements for capture. CO2 also must be captured from the fuel used in process heating and electricity generation. In the future, as electrical grids decarbonize, low- or zero-carbon electricity may replace the fuel portion of energy for capture (Müller et al. 2020).
3.5.2.2 Hydrogen Requirements for CO2 Utilization Products
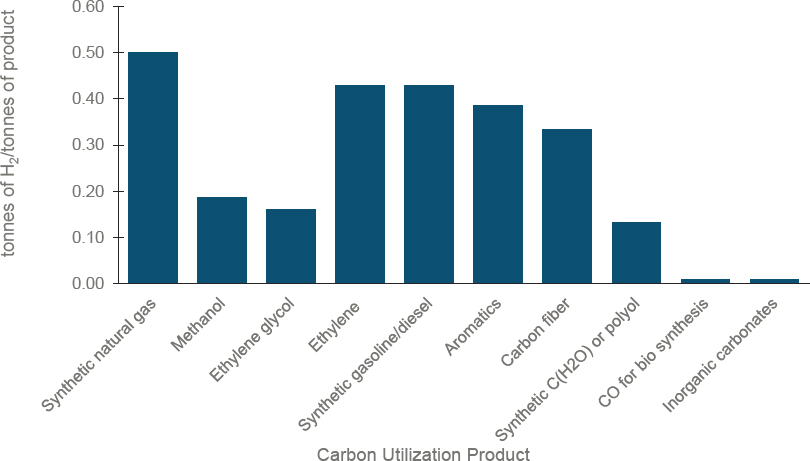
Once the CO2 is captured, many different products can be made. Figure 3-7 depicts the inputs of hydrogen required for various classes of products formed based on reaction stoichiometries. Less hydrogen is required for materials that incorporate oxygen into the final product.
3.5.2.2.1 Electricity and Feedstock Requirements for Hydrogen Production
Figure 3-8 shows a tabulation of the amount of water, electricity, and natural gas to make clean hydrogen via water electrolysis, steam methane reforming (SMR), autothermal reforming (ATR), partial oxidation (POx) of methane, and methane pyrolysis (MP). Multiplying these inputs per unit of hydrogen by the hydrogen consumption per ton of product (see Figure 3-7) yields the inputs required per ton of final product. Note that hydrogen can be manufactured on-site or remotely and supplied by pipeline, truck, or rail. Chapter 4 discusses the infrastructure needs to deliver hydrogen and electricity to the utilization site.
The endothermic SMR process involves the following chemical reaction:
SMR: CH4 + H2O → CO + 3H2.
The exothermic partial oxidation (POx) reaction is
POx: CH4 + ½ O2 → 2H2 + CO.
Autothermal reforming seeks to balance exothermic and endothermic reactions to be energy neutral (Luque and Speight 2015):
ATR: CH4 + ¼ O2 + ½ H2O → CO + 5/2 H2.
CO produced in the above processes can be reacted with water to form more H2 via the mildly exothermic water-gas shift (WGS) reaction:
WGS: CO + H2O ⇌ CO2 + H2.
Though this section is about production of hydrogen as an input to CO2 utilization, it should also be noted that the WGS reaction is a reversible equilibrium and can be used to convert captured CO2 to CO via the reverse, or “backward,” reaction. CO in mixtures with H2 constitutes “synthesis gas,” from which the entire fuels and petrochemical economy can be reproduced via Fischer-Tropsch synthesis (FTS) or methanol synthesis (plus subsequent conversion reactions). FTS and methanol synthesis are fully developed commercial processes that have been practiced for more than 100 years, at scales equivalent to full commercial petroleum refineries (e.g., gas-to-liquids production in Qatar). While catalysts are known and demonstration facilities have been operated, the reverse WGS reaction is the only step that is not fully optimized and practiced at commercial scale to enable CO2 utilization, but technology readiness is already at a high level. Existing technology thus is proven and demonstrated for use of CO2 as a full replacement for the fossil-based fuels-and-chemicals economy, if desired.
To avoid formation of gaseous CO2 product, endothermic MP can be conducted to form solid carbon via
MP: CH4 → C + 2H2.
The process has been practiced for nearly a century to form carbon black or sometimes hydrogen. Current research and development (R&D) seeks to efficiently and continuously co-produce both, including formation of structured carbons for building materials and generation of hydrogen as an energy carrier for the clean energy input needed to drive the endothermic reaction. A portion (50 percent) of the hydrogen generated can be used for this purpose. Technology varies widely from thermal to thermo-catalytic, to plasmas.
The above stoichiometric reactions do not describe the full inputs required to generate hydrogen. Required water inputs can increase up to 10-fold above the stoichiometric need due to water purification (e.g., for electrolysis), treatment, and process cooling steps (Blanco 2021; Lampert et al. 2015, 2016), as well as the need to inject additional water (steam) to prevent coking of catalysts and equipment for natural gas–based synthesis. A similar problem holds for energy and hydrogen inputs above the thermodynamic or stoichiometric minimum. SMR requires energy inputs to run process equipment and heating steps for carbon capture, in addition to driving the endothermic reaction itself. ATR seeks to combust a portion of the natural gas feed to provide the endothermic reaction heat, but also requires electricity and heat (for capture solvent/sorbent regeneration) to run carbon capture, as well as water feed and final product purification steps. POx combusts additional natural gas feed to produce excess heat, which is used to generate electricity to power all process steps, such that all CO2 is generated in one stream for capture, reducing capital costs (Liu 2021). If individual input materials and processes are net-emitting, then they can be paired with a separate, verifiable negative emissions process (e.g., DAC plus storage), which will allow the entire system to be net-zero or net-negative emissions.
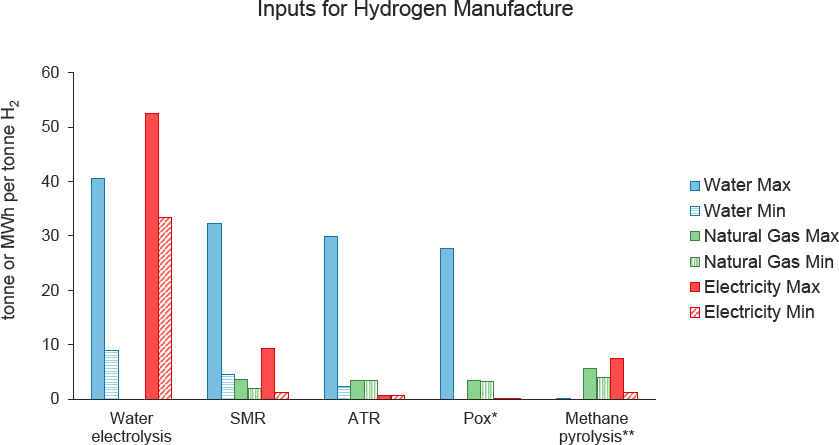
SOURCES: Committee generated using data from Bazzanella and Ausfelder (2017); Beswick et al. (2021); Blanco (2021); Collodi et al. (2017); Hopcroft and Papadamou (2021); IEAGHG (2017); Lamb et al. (2020); Lampert et al. (2015, 2016); Liu (2021); Marchese et al. (2020); Oni et al. (2022); Tenhumberg and Büker (2020); and Timmerberg et al. (2020).
3.5.2.3 Enabling Inputs Required for Mineral Products
Steel slag, cement kiln dust, fly ash, or some mined minerals (serpentines) can react exothermically with CO2 to create valuable products (cements, aggregates). Crushing, grinding, and water exposure pretreatment of native minerals makes suitable cations available for reaction with CO2 and requires relatively little energy. The reaction between CO2, water, and the pretreated minerals is exothermic and also requires little energy input, so the total energy consumption needed for CO2 mineralization is low (Dipple et al. 2021; Woodall et al. 2019). As discussed further in Section 4.4, concrete and aggregate production and use are highly distributed due to the difficulty in transporting both inputs and produced materials, which are large volume and heavy, and the inherent distribution of the final products, which are used in a wide variety of buildings and infrastructure, including roads and other major public works projects.
The primary enabling infrastructure requirement for carbon mineralization processes is the heavy-duty power for mining and comminution of minerals to a particle size that can react with CO2 at acceptable rates. Hydrogen could be a zero-carbon fuel option for mining trucks and equipment, as battery-electric vehicles may lack the power density needed for the very heavy-duty cycles of equipment. Hydrogen can be supplied directly by pipeline to a refueling terminal, or can be generated on-site by supplying water and clean electricity from the grid. Running handling equipment and operations will require some additional electricity. For solids handling, these power requirements are generally higher per ton of product than from a facility producing a gas or liquid product.
3.5.3 Consumer Sentiment
Public awareness of the origin, production methods, and even the component ingredients in many everyday products is low. Acceptance or rejection of products and services therefore might be guided by perceptions and misconceptions. It is thus critical to include research on societal aspects in any planning for technology development and deployment (Buck 2016). Risk-benefit assessments point to CCU acceptance as being influenced by perceptions of CCS (Arning et al. 2020, 2021; Engelmann et al. 2020). Nature-based solutions for carbon removal might find preferential approval over technology (Wolske et al. 2019). A representative survey of adult U.S. citizens showed that a two-thirds majority would be inclined or comfortable with using CO2-made products. Subtle differences with respect to product type, gender, and political preference of those surveyed had a mild impact on the responses (Lutzke and Arvai 2021).
3.5.4 Future Market Volumes and Current Price Points
Estimates for market penetration (rates) and price points for CO2-derived products depend on supporting policy, competition from bio-based carbon sources, and alternative solutions, for example, electricity or hydrogen as an energy carrier instead of hydrocarbons. CO2 utilization will compete with other sources of carbon (biomass, recycling) as well as other technologies to replace the current use of carbon-based products, for example, electricity or hydrogen for mobility applications. Table 3-1 describes various market projections for products of CO2 utilization.
Projections for market volumes and CO2 utilization potential vary widely between published studies, sensitively reacting to the assumptions made regarding technologies, cost, and other competing factors. A recent study projects CO2 utilization for production of aggregates and precast concrete to grow substantially by 2050, reaching between 1 and nearly 11 gigatonnes per year (see Figures 3-9 and 3-10). While market shares for aggregates are projected to only reach 14–18 percent by 2050, CO2-cured precast concrete could capture as much as 75–80 percent of the market in 2050. Figure 3-11 shows projections of market size through 2040 for various CO2 utilization products, including building materials, fuels, polymers, chemicals, protein, and carbon additives.
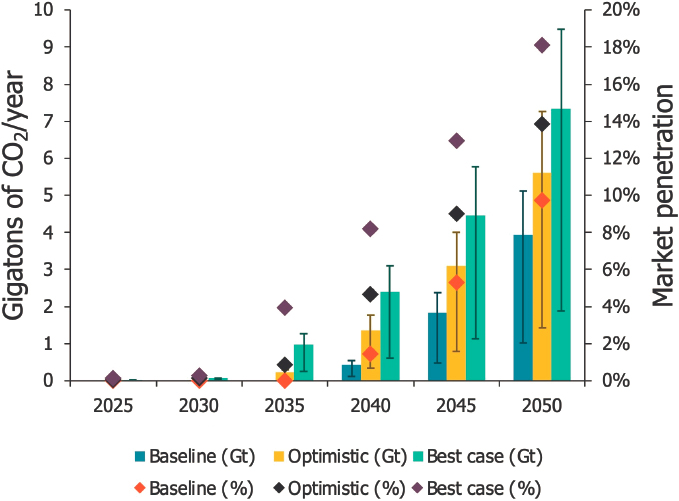
SOURCE: V. Sick, G. Stokes, and F.C. Mason, 2022, “CO2 Utilization and Market Size Projection for CO2-Treated Construction Materials,” Frontiers in Climate 4(May):878756, https://doi.org/10.3389/fclim.2022.878756. CC BY 4.0.
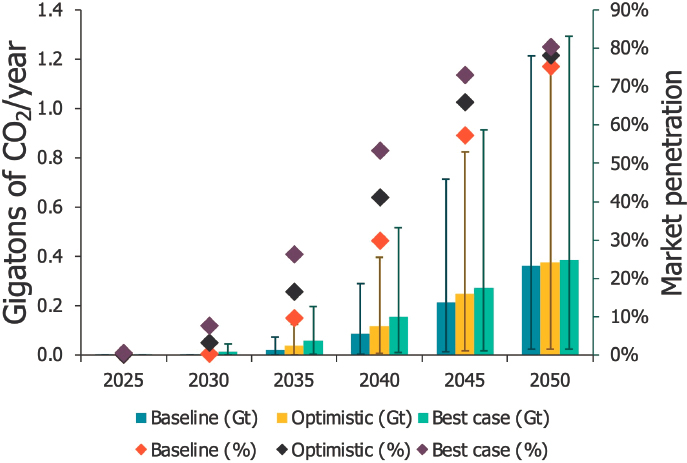
SOURCE: V. Sick, G. Stokes, and F.C. Mason, 2022, “CO2 Utilization and Market Size Projection for CO2-Treated Construction Materials,” Frontiers in Climate 4(May):878756, https://doi.org/10.3389/fclim.2022.878756. CC BY 4.0.
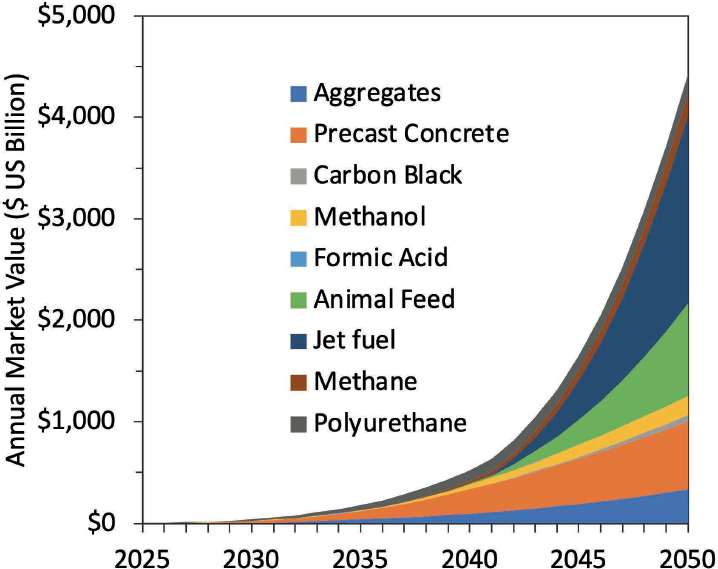
SOURCE: Committee generated based on data from V. Sick, G. Stokes, F. Mason, et al., 2022, Implementing CO2 Capture and Utilization at Scale and Speed: The Path to Achieving Its Potential, Technical Report, Global CO2 Initiative, University of Michigan, https://dx.doi.org/10.7302/5825. Reproduced with permission, Global CO2 Initiative. CC BY 4.0.
3.6 NEAR-TERM OPPORTUNITIES, SYNERGIES, AND NEEDS
Significant opportunities exist to use CO2 successfully at large scale to make products. While all of these products can have commercial value, the climate benefit from their production depends on their lifetime, as noted in Section 3.1. Categorizing products as Track 1 or Track 2 is a convenient way to identify what the climate benefits of a given product will be. Responsible launch of the CO2 utilization industry requires that life cycle assessments in combination with societal impact and techno-economic assessments accompany and guide decision making from early stages of research to commercial deployments.
A CO2 utilization industry as a whole does not yet exist, but components of it are emerging. Technology readiness for several product categories is at a level where commercial deployments are imminent. Supply and value chains are not established, and work is needed to ensure that infrastructure investments for CO2 capture and transport strategically and synergistically enable CO2 capture and utilization. Specifically, the government support enacted in the Infrastructure Investment and Jobs Act of 2021, calling for hubs for DAC and hydrogen, could be leveraged for synergies with CO2 utilization. Developing such synergies may include, for instance, co-locating at least one DAC and hydrogen hub pair, or locating hubs in regions with an industrial focus to facilitate the adoption of CO2 utilization and spur private investment. DOE’s Notice of Intent for the hydrogen hubs calls for such integration with industrial uses of hydrogen, which could include CO2 utilization: “End-use diversity—at least one hub shall demonstrate the end-use of clean hydrogen in the electric power generation sector, one in the industrial sector, one in the residential and commercial heating sector, and one in the transportation sector” (DOE-OCED 2022). Such co-located hubs and other concentrations of CO2 utilization could create nuclei where private investment may find a need for pipelines and other shared infrastructure. Centralized production and use of various fossil fuels and derived chemicals is the norm in the existing petrochemical industry and could be best for CO2 and hydrogen production and use as well.
The largest amounts of CO2 could be used to produce construction materials, polymers, fuels, and commodity chemicals. Track 1 CO2 utilization may serve a need for consuming and storing large volumes of CO2 that would otherwise be emitted to the atmosphere, such as in construction materials and some durable products. Track 2 CO2 utilization will be a part of ensuring access to necessary chemicals in a circular carbon economy, including for large volumes of fuels, polymers, and commodity chemicals. The following sections detail opportunities, synergies, and needs for the important, large-volume products in Track 1 and Track 2.
3.6.1 Construction Materials
Opportunities for large-scale CO2 utilization potential exist for the production of Track 1 construction materials that constitute a means of durable CO2 storage, equivalent to CCS, yet with the potential for higher societal value and reduced cost.
CO2 utilization that produces Track 1 construction materials can create synergies to address multiple pressing societal needs given the urgent need to rebuild a substantial portion of U.S. built environment infrastructure, addressing the acute housing shortage and the significant carbon footprint of the built environment. It is instructive to note how slowly durable products penetrate the market due to 10+-year use phases for legacy technology. Rapid launch of CO2-based construction materials is therefore essential, since infrastructure investments are made for even longer-term use, and CO2 removal opportunities would be lost with delayed deployments. Mineralization reactions such as by-product use of steel slag or fly ash to reduce energy requirements for concrete (IEA 2019; Mangan 2010) or mining of serpentine minerals for production of aggregates represent some of the most likely options for viable CO2 utilization with very large potential for CO2 removal.
To establish CO2 utilization for concrete and aggregate production, distributed CO2 sources will be needed, given the fragmented and very localized production requirements for this sector. Co-location with CO2 point sources needs to be explored as much as possible to reduce transportation requirements. Properties of the new construction materials may differ from incumbents, triggering the need for testing and validation of the new materials, creation of new environmental product declarations, and adaptation of building codes and standards. The latter is nontrivial, given the conservative nature of building codes.
3.6.2 Synthetic Fuels
CO2 utilization to produce synthetic fuels would provide energy-dense hydrocarbons derived from renewable, rather than fossil, carbon to power existing and future combustion processes. Synthetic fuels are a Track 2 chemical, and therefore their production must use DAC, DOC, or biogenic CO2 sources because circular carbon flows are required for sustainability. Synthetic fuels may be particularly important for applications where hydrogen- or electric-powered vehicles will be challenging to produce or operate, such as in long-distance, heavy-freight applications in aviation and shipping. Synthetic fuels may also offer a daily, monthly, or seasonal energy storage opportunity. Additionally, local synthetic hydrocarbon fuel production may provide safe and secure access to fuels during overseas deployments, a potentially important application as continued use of existing defense-related vehicle fleets is a significant concern for national security. Such production methods could be cost-competitive relative to logistics costs of supplying today’s fuels (Poland 2021). Finally, developing CO2 utilization for synthetic fuel production affords an opportunity not only to use existing infrastructure but also existing workers, many of whose skills will be translatable from the current fossil-fuel system to a synthetic fuel system.
Despite the opportunities and synergies in using aspects of the current energy system, synthetic fuels will be challenged to compete with alternative methods of de-fossilizing or de-carbonizing combustion applications in transportation, industry, and buildings. Synthetic fuels are expensive to produce, because of both the high capital costs of equipment needed for electrolysis and fuel synthesis and the high electricity consumption of the processes (Searle et al. 2021). Other potential sources of sustainable drop-in, energy-dense fuels include biomass- or waste-derived hydrocarbons. All drop-in fuels will compete with hydrogen and/or electricity to power equipment and processes, especially because combustion of hydrocarbon fuels is less efficient for powering transportation or other processes than use of hydrogen for combustion or in fuel cells, or direct electrification. A 2021 study of light-duty vehicle fuel economy examined the emissions and energy-use implications of various alternative fuels compared to conventional internal combustion engine vehicles powered with standard gasoline with 10 percent ethanol. Using the GREET model to estimate energy use of alternative-fueled vehicles, the study found that electric cars use 45 percent of the energy of conventional vehicles, fuel-cell vehicles powered with electrolytic hydrogen generated from renewable energy use 78 percent of the energy of conventional vehicles, and conventional vehicles powered by synthetic fuels synthesized from CO2 and electrolytic hydrogen using renewable energy use 297 percent of the energy of conventional vehicles (NASEM 2021b).
In addition to the challenges associated with higher energy use, combustion of hydrocarbon fuels is a source of particulate matter, nitrogen oxides, and ozone (leading to smog in the presence of hydrocarbons), which adversely impact human health with disproportionate impact on disadvantaged communities, unlike zero-emission electrical power or hydrogen-powered fuel cells.2 Because synthetic fuels may be designed to have lower criteria emissions than current fuels such as gasoline and diesel, they typically have fewer contaminants than fossil fuels and can be made as custom blends of chemicals. Aviation fuel or perhaps marine propulsion are possibly the best uses for synthetic hydrocarbon fuels, since local air quality impacts would be less severe and electrification is difficult due to the low energy and power densities afforded by batteries. Particularly for aviation, the ability to use existing aircraft and distribution infrastructure together with a decrease in manufacturing costs for synthetic aviation fuel may enable CO2-derived synthetic fuels to be nearly cost-competitive with existing hydrocarbon fuels and other alternative hydrogen options by 2050, if there is a price on CO2 emissions (Third Derivative n.d.).
3.6.3 Commodity Chemicals
CO2 utilization can be one way to de-fossilize production and use of carbon-based commodity chemicals. Unlike the fuels described above, there are few options for replacing carbon-based commodity chemicals with carbon-free alternatives.
Commodity chemicals from CO2, such as synthetic fuels, have synergy with the existing infrastructure for distribution and use of the final chemical or material product. The chemical industry is designed to make and use
___________________
2 Hydrogen, when combusted rather than used in a fuel cell, can lead to criteria pollutant NOx, if not done judiciously.
several “building block” chemicals to synthesize a large number and volume of chemicals, and so if CO2 utilization could be used to produce these central intermediates, then much of the same infrastructure and capital could be used for sustainable carbon-based chemical production. Similar to the fuels discussion above, the existing workforce will remain effective for the new circular carbon economy.
CO2 utilization will compete with other sources of sustainable carbon, including biomass and waste-based resources. For hydrocarbon chemicals production, the same molecules can sometimes be made from bio-based feedstocks with lower specific energy needs, but higher land use (Haider 2022). Whether or not CO2-derived hydrocarbon chemicals will be competitive with bio-based chemicals will depend on reduction in the cost of DAC and sufficient availability of low-cost, zero-carbon-emission energy. Hydrocarbon chemical products have sequestration lifetimes of less than 100 years (IEA 2019); thus, their chemical synthesis must not involve current fossil emission point sources.
3.6.4 Polymers
Polymers and polymer precursors are a major class of chemicals and materials, used for plastics, rubbers, fibers, and foams. CO2 utilization has significant potential to displace existing polymer syntheses from fossil fuels and for CO2 to be a feedstock for other, different polymers optimized for CO2 utilization. Currently, there is some commercial-scale synthesis of polycarbonates from CO2, though copolymerization methods and use of fossil-fuel–derived feedstocks result in only a portion of the carbon in the product being derived from CO2. Biological and chemical systems can produce polymer precursors and polymers.
CO2 utilization in the production of polymers can fill a need for materials in a circular economy, addressing huge existing markets and offering opportunities to improve polymer syntheses in some cases. However, challenges exist for converting CO2 to polymers. The reaction conditions for producing polymer precursors, including ethylene and benzene, differ from those of conventional ethylene and benzene production technologies. Thus, the existing ethylene and benzene manufacturing infrastructures need to be modified. Also, the contaminants (trace gases, especially NOx/SOx, and particulates) in the captured CO2 could lower the activities of the chemical catalysts used in CO2-to-polymer synthesis. Contaminants in CO2 feedstock streams are less of a problem for biological CO2-to-polymer synthesis (Kondaveeti et al. 2020).
Production of polymers from CO2 in a cost-effective and environmentally friendly manner will require synergistic efforts. First, CO2 capture and purification will need to be available to provide high-purity CO2, which not only will improve CO2 utilization efficiency but also will increase the purity and productivity of polymers and thus the marketability of CO2-derived polymers. Co-location of CO2 and hydrogen sources could lower chemical polymer production costs. The choices for specific chemical and biological pathways need to be considered together with the capability of the existing polymer synthesis infrastructures. Polymers are a high-volume material class, accessible from CO2, recycled material, and biomass feedstocks. CO2 utilization will be favorable for certain polymer syntheses, including those of polycarbonates and polyurethanes, and will provide an opportunity to produce polymers that replace those lost in recycling processes, which are not 100 percent efficient. Biodegradability of polymers can be an asset for products that are frequently discarded and unlikely to be recycled, and CO2 utilization can produce those polymers in a circular carbon economy. Some polymers are durable and can represent Track 1–type utilization if products are sufficiently long-lived (>100 years), such as some building materials.
3.6.5 Conclusion
Overall, it is clear that CO2 utilization is not a silver bullet either to counter the effects of climate change or to meet the needs for carbon-based products. However, it is one of many tools that will be used to accomplish these goals, and in some cases, it is likely to be favorable over alternatives. For example, CO2 utilization may offer opportunities for CDR at lower cost for Track 1 chemical products compared to CCS, access to Track 2 carbon products with lower land-use requirements than biomass-derived ones, and, for both product tracks, a new industry that may address environmental justice and other negative impacts related to the production of incumbent chemicals and materials. Forecasts for the magnitude of the emerging CCU industry necessarily cover a wide range, but even at
lower estimates, in 2050, nearly 2 Gt CO2 could be utilized in products, generating $1.1 trillion in revenues. If conditions favor CO2 utilization over other alternatives, a high estimate of 27 Gt CO2 utilized and $4.4 trillion revenues has been projected. Policy guidance will be essential to ensure an expedited and just launch of the CCU industry.
3.7 FINDINGS AND RECOMMENDATIONS ON POTENTIAL USES OF CO2 IN COMMERCIAL PRODUCTS
FINDING 3.1 Product Lifetime. Some CO2-derived products, such as CO2-cured concrete, carbonated aggregates, carbon fibers used in structural materials, and long-lived polymers, have lifetimes that will lead to durable CO2 sequestration if handled appropriately. In contrast, short-lived products, for example, fuels, fertilizers, and many other chemicals, will decompose quickly and return the CO2 to the atmosphere, presenting the opportunity to participate in a circular carbon economy.
FINDING 3.2 Sustainability of Products Based on Carbon Source. Long- and short-lived CO2-derived products have fundamentally different relationships to carbon sources, resulting in different climate impacts. Long-lived products may be net-negative compatible if non-fossil CO2 is used, or net-zero compatible if sourced from fossil CO2. Short-lived products may be net-zero compatible if the CO2 is sourced from non-fossil sources. If emissions cannot be reduced to be compatible with net-zero or net-negative pathways, such as use of fossil CO2 for short-lived products, then the resulting product may be considered unsustainable relative to alternative options such as production from biomass or recycled material feedstocks, carbon sequestration, or use of non-carbon-based technologies, such as replacing hydrocarbon fuels with hydrogen or electricity. The long-term sustainability of different CO2 utilization processes is an important consideration for infrastructure planning and policy.
FINDING 3.3 Carbon Accounting. Carbon accounting across the value chain will be important to assessing sustainability and climate impact of carbon management technologies, including CO2 utilization.
RECOMMENDATION 3.1. The U.S. Department of Energy should fund research to quantify the dynamic impact of CO2-derived products, for example, their specific lifetime, on the CO2 balance in the atmosphere. The United States should incorporate knowledge acquired from European projects and regulatory activities in addressing circular carbon economies and net-negative emissions.
FINDING 3.4 Life Cycle, Techno-economic, and Societal Factors Assessment. For all assessments of net-zero or net-negative emissions status of CO2 utilization products, it is important to estimate the full life cycle impact of the process, including upstream and downstream greenhouse gas emissions associated with the process, feedstock origin, energy use, product fate, co-product fate, and associated waste. To ensure simultaneous economic viability and environmental justice, techno-economic assessments and societal factors have to be fully integrated with life cycle assessments.
RECOMMENDATION 3.2. The U.S. Department of Energy (DOE) should build on ongoing efforts to harmonize and standardize life cycle assessment (LCA) for carbon capture, utilization, and storage projects. In addition to LCA, techno-economic assessment and analysis of societal impacts including environmental justice should be used by DOE to prioritize investments in project demonstrations and loan commitments.
FINDING 3.5 CO2 Utilization Target Products. Targets for net-zero-emissions carbon products include concrete, aggregates, commodity carbon-containing chemicals, polymers, elemental carbon products, and high-value niche products. Many needs for carbon-based products will remain or grow in a net-zero-emissions economy, but a major class of materials, carbon-based fuels, is likely to decrease in use due to vehicle electrification. The use of zero-emission carbon fuels for heavy-duty transport, especially aviation, likely will remain substantial.
FINDING 3.6 Product Equivalence. Many CO2-derived products (e.g., fuels, materials, chemicals) are chemically identical to their fossil-derived counterparts. These products can utilize existing infrastructure and systems and may displace incumbent fossil-derived products. Some CO2-derived products, such as CO2-cured concrete and carbonated aggregates, may have different composition, properties, and performance than the incumbent material, which could yield benefits or disadvantages.
FINDING 3.7 Displaced Emissions. Displacing fossil-derived products with CO2 utilization-derived products may lead to avoidance of CO2 emissions, if the CO2 utilization processes are net-zero emissions. However, not all emissions may be displaced, particularly criteria emissions from combustion of fuels, such as nitrogen oxides and soot, which have local air quality and human health impacts.
FINDING 3.8 Net-Zero Emissions Enabling Inputs. For net-zero-emissions CO2 utilization, all inputs—including but not limited to electricity, hydrogen, heat, and transportation—will need to have net-zero emissions on a life cycle basis. Electricity and hydrogen inputs can be substantially decarbonized by adding carbon capture and storage to existing infrastructure for generation from fossil fuels, or by building new infrastructure using zero-carbon-emissions options such as solar, wind, nuclear, or geothermal power.
FINDING 3.9 Energy and Hydrogen Requirements. Utilizing CO2 as a starting material in chemical processes to produce fuels, commodity chemicals, and other hydrocarbon-based chemicals requires more external energy and often hydrogen inputs than generating the same products from fossil carbon sources. CO2 utilization to produce hydrocarbon substitutes for fossil fuels will also require substantially more power and hydrogen than needed for direct use of electricity or hydrogen to power transportation and other energy services.
FINDING 3.10 Market Potential. The global utilization potential for CO2 to make products is several gigatonnes per year. The volume of CO2 utilized in a net-zero economy will be driven by the market value of carbon-based products and the competitiveness of CO2 as a feedstock. These factors depend on (1) demand for services provided by carbon-based products; (2) their relative cost compared to fossil-based products, non-carbon-based alternatives such as electricity and hydrogen, and products derived from carbon sources other than CO2, such as biomaterial carbon and recycled waste carbon products; (3) availability of inputs that enable net-zero product status, including clean hydrogen and energy; and (4) policy incentives and regulatory frameworks, including CO2 abatement incentives. The potential for and cost of CO2 removal will affect market demand directly in some cases—such as when the marketable product also provides carbon removal (e.g., concrete and aggregates or carbon fiber)—and indirectly in other cases, by changing the attractiveness of using CO2 removal as an alternative to de-fossilization of chemical products.
3.8 REFERENCES
Adánez, J., and A. Abad. 2019. “Chemical-Looping Combustion: Status and Research Needs.” Proceedings of the Combustion Institute 37(4):4303–4317. https://doi.org/10.1016/j.proci.2018.09.002.
Aether. n.d. “Diamonds from Thin Air.” https://aetherdiamonds.com/?gclid=Cj0KCQjwg_iTBhDrARIsAD3Ib5iCN7jkzfw-7cO6eoWJNb8VOS9WJVb_p6U-wgnv3joI6XG-cIVTQHr0aApmYEALw_wcB.
Agora Verkehrswende, Agora Energiewende, and Frontier Economics. 2018. The Future Cost of Electricity-Based Synthetic Fuels. https://static.agora-energiewende.de/fileadmin/Projekte/2017/SynKost_2050/Agora_SynKost_Study_EN_WEB.pdf.
Air Protein. 2022. “How We’ll Feed the Future.” https://www.airprotein.com/making-air-meat.
AirMiners. 2022. “AirMiners Index.” https://airminers.org/explore.
Arning, K., J. Offermann-van Heek, A. Sternberg, A. Bardow, and M. Ziefle. 2020. “Risk-Benefit Perceptions and Public Acceptance of Carbon Capture and Utilization.” Environmental Innovation and Societal Transitions 35(June):292–308. https://doi.org/10.1016/j.eist.2019.05.003.
Arning, K., J. Offermann-van Heek, and M. Ziefle. 2021. “What Drives Public Acceptance of Sustainable CO2-Derived Building Materials? A Conjoint-Analysis of Eco-Benefits vs. Health Concerns.” Renewable and Sustainable Energy Reviews 144(July):110873. https://doi.org/10.1016/j.rser.2021.110873.
Artz, J., T.E. Müller, K. Thenert, J. Kleinekorte, R. Meys, A. Sternberg, A. Bardow, and W. Leitner. 2018. “Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment.” Chemical Reviews 118(2):434–504. https://doi.org/10.1021/acs.chemrev.7b00435.
Bazzanella, A.M., and F. Ausfelder. 2017. Low Carbon Energy and Feedstock for the European Chemical Industry. Technology Study. Germany: DECHEMA. https://dechema.de/dechema_media/Downloads/Positionspapiere/Technology_study_Low_carbon_energy_and_feedstock_for_the_European_chemical_industry-p-20002750.pdf.
Berg, M.C., D. Vural, S. Bhagia, L. Liang, Z. Yang, X. Meng, N. Gallego, N. Bryant, S.V. Pingali, and H.M. O’Neill. 2020. Polymer and Structural Science Behind Valorizing Lignin Using Solvents. Research Report. Oak Ridge National Laboratory. https://genomicscience.energy.gov/research-summaries-genomic-science-annual-pi-meeting-abstract-book-feb-2020.
Beswick, R.R., A.M. Oliveira, and Y. Yan. 2021. “Does the Green Hydrogen Economy Have a Water Problem?” ACS Energy Letters 6(9):3167–3169. https://doi.org/10.1021/acsenergylett.1c01375.
Biniek, K., K. Henderson, M. Rogers, and G. Santoni. 2020. “Driving CO2 Emissions to Zero (and Beyond) with Carbon Capture, Use, and Storage.” McKinsey, June 30. https://www.mckinsey.com/business-functions/sustainability/our-insights/driving-co2-emissions-to-zero-and-beyond-with-carbon-capture-use-and-storage.
Blanco, H. 2021.” Hydrogen Production in 2050: How Much Water Will 74EJ Need?” energypost.eu July 22. https://energypost.eu/hydrogen-production-in-2050-how-much-water-will-74ej-need.
Buck, H.J. 2016. “Rapid Scale-up of Negative Emissions Technologies: Social Barriers and Social Implications.” Climatic Change 139(2):155–167. https://doi.org/10.1007/s10584-016-1770-6.
CEQ (Council on Environmental Quality). 2022. “Carbon Capture, Utilization, and Sequestration Guidance.” Federal Register 87(32):8808–8811.
chemeurope. n.d. “Methanol Economy.” In Encyclopedia of Chemistry. https://www.chemeurope.com/en/encyclopedia/Methanol_economy.html.
Circular Carbon Network. 2022. “Innovator Index.” https://circularcarbon.org/innovator-index.
Collodi, G., G. Azzaro, N. Ferrari, and S. Santos. 2017. “Techno-Economic Evaluation of Deploying CCS in SMR Based Merchant H2 Production with NG as Feedstock and Fuel.” Energy Procedia 114(July):2690–2712. https://doi.org/10.1016/j.egypro.2017.03.1533.
Cremonese, L., T. Strunge, B. Olfe-Kräutlein, S. Jahilo, T. Langhorst, S. McCord, L. Müller, et al. 2022. Making Sense of Techno-Economic and Life Cycle Assessment Studies for CO2 Utilization. Ann Arbor, MI: Global CO2 Initiative. https://doi.org/10.7302/4202.
CSIRO (Commonwealth Scientific and Industrial Research Organization). 2022. “CO2 Utilisation Roadmap.” https://www.csiro.au/en/work-with-us/services/consultancy-strategic-advice-services/CSIRO-futures/Energy-and-Resources/CO2-Utilisation-Roadmap.
DDP (Deep Decarbonization Pathways). n.d. “The DDP Initiative.” https://ddpinitiative.org.
Dipple, G., P. Keleman, and C.M. Woodall. 2021. “The Building Blocks of CDR Systems.” Chapter 2 in Carbon Dioxide Removal Primer, J. Wilcox, B. Kolosz, and J. Freeman, eds. CDR Primer. https://cdrprimer.org/read/chapter-2.
DOE-HFTO (U.S. Department of Energy Hydrogen and Fuel Cell Technologies Office). n.d. “DOE Technical Targets for Hydrogen Production from Electrolysis.” https://www.energy.gov/eere/fuelcells/doe-technical-targets-hydrogen-production-electrolysis.
DOE-OCED (Office of Clean Energy Demonstrations). 2022. “DE-FOA-0002768: Notice of Intent to Issue Funding Opportunity Announcement No DE-FOA-0002779—Bipartisan Infrastructure Law: Additional Clean Hydrogen Programs (Section 40314): Regional Clean Hydrogen Hubs.” https://oced-exchange.energy.gov/Default.aspx#FoaId4e674498-618c-4f1a-9013-1a1ce56e5bd3.
EC (European Commission). 2020. “A New Circular Economy Action Plan for a Cleaner and More Competitive Europe. Brussels, Belgium: EC.” https://eur-lex.europa.eu/resource.html?uri=cellar:9903b325-6388-11ea-b735-01aa75ed71a1.0017.02/DOC_1&format=PDF.
Emergen Research. 2022. “Carbon Nanotube Market Size to Reach USD 3,027.4 Million in 2030: Increasing Application of Carbon Nanotubes in Electric Vehicles Is a Major Factor Driving Industry Demand, Says Emergen Research.” PR Newswire, April 25. https://www.prnewswire.com/news-releases/carbon-nanotube-market-size-to-reach-usd-3-027-4-million-in-2030--increasing-application-of-carbon-nanotubes-in-electric-vehicles-is-a-major-factor-driving-industry-demand-says-emergen-research-301531992.html.
Energy Transitions Commission. 2018. Mission Possible: Reaching Net-Zero Carbon Emissions from Harder-to-Abate Sectors by Mid-Century. London.
Engelmann, L., K. Arning, A. Linzenich, and M. Ziefle. 2020. “Risk Assessment Regarding Perceived Toxicity and Acceptance of Carbon Dioxide-Based Fuel by Laypeople for Its Use in Road Traffic and Aviation.” Frontiers in Energy Research 8(November):579814. https://doi.org/10.3389/fenrg.2020.579814.
Fernández-Dacosta, C., M. van der Spek, C.R. Hung, G.D. Oregionni, R. Skagestad, P. Parihar, D.T. Gokak, A.H. Strømman, and A. Ramirez. 2017. “Prospective Techno-Economic and Environmental Assessment of Carbon Capture at a Refinery and CO2 Utilisation in Polyol Synthesis.” Journal of CO2 Utilization 21(October):405–422. https://doi.org/10.1016/j.jcou.2017.08.005.
Fortune Business Insights. 2021. Graphite Market Size, Share & COVID-19 Impact Analysis, by Product (Synthetic, and Natural), by Application (Refractories, Foundries). Research Report. https://www.fortunebusinessinsights.com/graphite-market-105322.
Fortune Business Insights. 2022. Graphene Market Size, Share & COVID-19 Impact Analysis, by Product (Graphene Oxide, Graphene Nanoplatelets (GNP), and Others), by End-Use Industry (Electronics, Aerospace & Defense, Energy, Automotive, and Others), and Regional Forecast, 2022-2029. Research Report. https://www.fortunebusinessinsights.com/graphene-market-102930.
Friedlingstein, P., M.W. Jones, M. O’Sullivan, R.M. Andrew, D.C.E. Bakker, J. Hauck, C. Le Quéré, et al. 2022. “Global Carbon Budget 2021.” Earth System Science Data 14(4):1917–2005. https://doi.org/10.5194/essd-14-1917-2022.
Gabrielli, P., M. Gazzani, and M. Mazzotti. 2020. “The Role of Carbon Capture and Utilization, Carbon Capture and Storage, and Biomass to Enable a Net-Zero-CO2 Emissions Chemical Industry.” Industrial & Engineering Chemistry Research 59(15):7033–7045. https://doi.org/10.1021/acs.iecr.9b06579.
Gallon, V. 2022. “Beiersdorf to Launch the World’s First Skincare Product with Recycled CO2.” Premium Beauty News April 11. https://www.premiumbeautynews.com/en/beiersdorf-to-launch-the-world-s,20148.
Global Carbon Project. 2021. “Supplemental Data of Global Carbon Budget 2021 (Version 1.0) [Data set].” https://doi.org/10.18160/gcp-2021.
Global CO2 Initiative. 2018. Global Roadmap Study of CO2U Technologies. Technical Report. Ann Arbor: University of Michigan. https://hdl.handle.net/2027.42/146529.
Global CO2 Initiative. 2022a. “AssessCCUS—Techno-Economic and Life Cycle Assessment for Carbon Capture, Utilization, and Storage.” University of Michigan. https://assessccus.globalco2initiative.org.
Global CO2 Initiative. 2022b. “CCU Activity Hub.” University of Michigan. https://www.globalco2initiative.org/evaluation/carbon-capture-activity-hub.
Global Market Insights. 2021. Carbon Black Market Size and Share: Statistics—2027. Research Report. Selbyville, DE. https://www.gminsights.com/industry-analysis/carbon-black-market.
Haider, K. 2022. “CO2-Derived Products.” Panel discussion presentation at the Carbon Utilization Infrastructure, Markets, Research and Development Meeting #2. March 1. https://www.nationalacademies.org/event/03-01-2022/carbon-utilization-infrastructure-markets-research-and-development-meeting-2#sectionEventMaterials.
Hepburn, C., E. Adlen, J. Beddington, E.A. Carter, S. Fuss, N. Mac Dowell, J.C. Minx, P. Smith, and C. K. Williams. 2019. “The Technological and Economic Prospects for CO2 Utilization and Removal.” Nature 575(7781):87–97. https://doi.org/10.1038/s41586-019-1681-6.
Herzog, H.J. 2018. Carbon Capture. Cambridge, MA: MIT Press.
Hills, C.D., N. Tripathi, R.S. Singh, P.J. Carey, and F. Lowry. 2020. “Valorisation of Agricultural Biomass-Ash with CO2.” Scientific Reports 10(1):13801. https://doi.org/10.1038/s41598-020-70504-1.
Hopcroft, T., and A. Papadamou. 2021. “The Water Industry in a Hydrogen Economy.” The Water Report, July 12. https://www.paconsulting.com/newsroom/expert-opinion/the-water-report-the-water-industry-in-a-hydrogen-economy-12-july-2021.
IEA (International Energy Agency). 2019. Putting CO2 to Use: Creating Value from Emissions. Paris: IEA. https://iea.blob.core.windows.net/assets/50652405-26db-4c41-82dc-c23657893059/Putting_CO2_to_Use.pdf.
IEA. 2022. Direct Air Capture 2022. Technical Report. Paris. CC BY 4.0. https://www.iea.org/reports/direct-air-capture-2022.
IEAGHG (IEA Greenhouse Gas R&D Programme). 2017. Techno-Economic Evaluation of SMF Based Standalone (Merchant) Hydrogen Plant with CCS. Technical Report. Cheltenham, UK. https://ieaghg.org/publications/technical-reports.
IPCC (Intergovernmental Panel on Climate Change). 2022. Climate Change 2022: Mitigation of Climate Change. Working Group III Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press https://report.ipcc.ch/ar6wg3/pdf/IPCC_AR6_WGIII_FinalDraft_FullReport.pdf.
IRENA (International Renewable Energy Agency) and Methanol Institute. 2021. Innovation Outlook: Renewable Methanol. Abu Dhabi: International Renewable Energy Agency. https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2021/Jan/IRENA_Innovation_Renewable_Methanol_2021.pdf.
ITIF (Information Technology & Innovation Foundation). 2022. “Active Carbon Management: Critical Tools in the Climate Toolbox.” https://itif.org/publications/2022/04/18/active-carbon-management-critical-tools-climate-toolbox.
Kahler, F., M. Carus, O. Porc, and C. vom Berg. 2021. Turning Off the Tap for Fossil Carbon—Future Prospects for a Global Chemical and Derived Material Sector Based on Renewable Carbon. Hurth, Germany: Nova Institute for Ecology and Innovation. https://renewable-carbon.eu/publications/product/turning-off-the-tap-for-fossil-carbon-future-prospects-for-a-global-chemical-and-derived-material-sector-based-on-renewable-carbon.
Kätelhön, A., R. Meys, S. Deutz, S. Suh, and A. Bardow. 2019. “Climate Change Mitigation Potential of Carbon Capture and Utilization in the Chemical Industry.” Proceedings of the National Academy of Sciences 116(23):11187–11194. https://doi.org/10.1073/pnas.1821029116.
Kiverdi. 2019. “Carbon Transformation in Action.” https://www.kiverdi.com/about.
Kondaveeti, S., I.M. Abu-Reesh, G. Mohanakrishna, M. Bulut, and D. Pant. 2020. “Advanced Routes of Biological and BioElectrocatalytic Carbon Dioxide (CO2) Mitigation Toward Carbon Neutrality.” Frontiers in Energy Research 8(June):94. https://doi.org/10.3389/fenrg.2020.00094.
Köpke, M., C. Mihalcea, J.C. Bromley, and S.D. Simpson. 2011. “Fermentative Production of Ethanol from Carbon Monoxide.” Current Opinion in Biotechnology 22(3):320–325. https://doi.org/10.1016/j.copbio.2011.01.005.
La Plante, E.C., I. Mehdipour, I. Shortt, K. Yang, D. Simonetti, M. Bauchy, and G.N. Sant. 2021. “Controls on CO2 Mineralization Using Natural and Industrial Alkaline Solids Under Ambient Conditions.” ACS Sustainable Chemistry & Engineering 9(32):10727–10739. https://doi.org/10.1021/acssuschemeng.1c00838.
Lamb, J.J., M. Hillestad, E. Rytter, R. Bock, A.S.R. Nordgård, K.M. Lien, O.S. Burheim, and B.G. Pollet. 2020. “Chapter 3 – Traditional Routes for Hydrogen Production and Carbon Conversion.” Pp. 21–53 in Hydrogen, Biomass and Bioenergy, J.J. Lamb and B.G. Pollet, eds. Cambridge, MA: Academic Press. https://doi.org/10.1016/B978-0-08-102629-8.00003-7.
Lampert, D.J., H. Cai, Z. Wang, J. Keisman, M. Wu, J. Han, J. Dunn, et al. 2015. Development of a Life Cycle Inventory of Water Consumption Associated with the Production of Transportation Fuels. ANL/ESD-15/27. Argonne, IL: Argonne National Laboratory. https://doi.org/10.2172/1224980.
Lampert, D.J., H. Cai, and A. Elgowainy. 2016. “Wells to Wheels: Water Consumption for Transportation Fuels in the United States.” Energy & Environmental Science 9(3):787–802. https://doi.org/10.1039/C5EE03254G.
Langanke, J., A. Wolf, J. Hofmann, K. Böhm, M.A. Subhani, T.E. Müller, W. Leitner, and C. Gürtler. 2014. “Carbon Dioxide (CO2) as Sustainable Feedstock for Polyurethane Production.” Green Chemistry 16(4):865–870. https://doi.org/10.1039/C3GC41788C.
Lange, J.-P. 2021. “Towards Circular Carbo-Chemicals—the Metamorphosis of Petrochemicals.” Energy & Environmental Science 14(8):4358–4376. https://doi.org/10.1039/D1EE00532D.
Larson, E., C. Greig, J. Jenkins, E. Mayfield, A. Pascale, C. Zhang, J. Drossman, et al. 2021. Net-Zero America: Potential Pathways, Infrastructure, and Impacts. Carbon Mitigation Initiative at Princeton University. Princeton, NJ. https://netzeroamerica.princeton.edu/the-report.
Li, V.C. 2019. Engineered Cementitious Composites (ECC): Bendable Concrete for Sustainable and Resilient Infrastructure. Berlin, Heidelberg: Springer. https://doi.org/10.1007/978-3-662-58438-5.
Liu, N. 2021. “Increasing Blue Hydrogen Production Affordability.” Hydrocarbon Processing June. https://www.hydrocarbonprocessing.com/magazine/2021/june-2021/special-focus-process-optimization/increasing-blue-hydrogen-production-affordability.
Luque, R.L., and J.G. Speight. 2015. Gasification for Synthetic Fuel Production: Fundamentals, Processes, and Applications. Cambridge, UK: Woodhead Publishing. https://doi.org/10.1016/C2013-0-16368-4.
Lutzke, L., and J. Árvai. 2021. “Consumer Acceptance of Products from Carbon Capture and Utilization.” Climatic Change 166(1–2):15. https://doi.org/10.1007/s10584-021-03110-3.
Lux Research. 2020. CO2 Capture & Utilization: The Emergence of a Carbon Economy. State of the Market Report. Boston, MA: Lux Research. https://members.luxresearchinc.com/research/report/35957.
Mac Dowell, N., P.S. Fennell, N. Shah, and G.C. Maitland. 2017. “The Role of CO2 Capture and Utilization in Mitigating Climate Change.” Nature Climate Change 7:243–247.
Mangan, A. 2010. “By-Product Synergy Networks: Driving Innovation Through Waste Reduction and Carbon Mitigation.” Chapter 6 in Sustainable Development in the Process Industries: Cases and Impact, J. Harmsen and J.B. Powell, eds. New York: John Wiley & Sons.
Marchese, M., E. Giglio, M. Santarelli, and A. Lanzini. 2020. “Energy Performance of Power-to-Liquid Applications Integrating Biogas Upgrading, Reverse Water Gas Shift, Solid Oxide Electrolysis and Fischer-Tropsch Technologies.” Energy Conversion and Management: X 6(April):100041. https://doi.org/10.1016/j.ecmx.2020.100041.
McCoy, M. 2022. “Green Chemical Maker LanzaTech to Go Public via Merger.” Chemical & Engineering News March 9. https://cen.acs.org/business/biobased-chemicals/Green-chemical-maker-LanzaTech-to-go-public-via-merger/100/web/2022/03.
Monkman, S., and M. MacDonald. 2017. “On Carbon Dioxide Utilization as a Means to Improve the Sustainability of Ready-Mixed Concrete.” Journal of Cleaner Production 167(November):365–375. https://doi.org/10.1016/j.jclepro.2017.08.194.
Mordor Intelligence. 2021. “Quantum Dots Market – Growth, Trends, COVID-19 Impact, and Forecasts (2022-2027).” Industry Forecast. https://www.mordorintelligence.com/industry-reports/quantum-dots-market-industry.
Müller, L.J., A. Kätelhön, S. Bringezu, S. McCoy, S. Suh, R. Edwards, V. Sick, et al. 2020. “The Carbon Footprint of the Carbon Feedstock CO2.” Energy & Environmental Science 13(9):2979–2992. https://doi.org/10.1039/D0EE01530J.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2019. Gaseous Carbon Waste Streams Utilization: Status and Research Needs. Washington, DC: The National Academies Press. https://doi.org/10.17226/25232.
NASEM. 2021a. Accelerating Decarbonization of the U.S. Energy System. Washington, DC: The National Academies Press. https://doi.org/10.17226/25932.
NASEM. 2021b. Assessment of Technologies for Improving Light-Duty Vehicle Fuel Economy—2025–2035. Washington, DC: The National Academies Press. https://doi.org/10.17226/26092.
NPC (National Petroleum Council). 2019. Meeting the Dual Challenge: A Roadmap to At-Scale Deployment of Carbon Capture, Use, and Storage. Technical Report. Washington, DC. https://dualchallenge.npc.org.
Oni, A.O., K. Anaya, T. Giwa, G. Di Lullo, and A. Kumar. 2022. “Comparative Assessment of Blue Hydrogen from Steam Methane Reforming, Autothermal Reforming, and Natural Gas Decomposition Technologies for Natural Gas-Producing Regions.” Energy Conversion and Management 254(February):115245. https://doi.org/10.1016/j.enconman.2022.115245.
Pace, G., and S.W. Sheehan. 2021. “Scaling CO2 Capture with Downstream Flow CO2 Conversion to Ethanol.” Frontiers in Climate 3(May):656108. https://doi.org/10.3389/fclim.2021.656108.
Poland, C. 2021. “The Air Force Partners with Twelve, Proves It’s Possible to Make Jet Fuel Out of Thin Air.” Air Force, October 22. https://www.af.mil/News/Article-Display/Article/2819999/the-air-force-partners-with-twelveproves-its-possible-to-make-jet-fuel-out-of.
Ravikumar, D., G.A. Keoleian, S.A. Miller, and V. Sick. 2021a. “Assessing the Relative Climate Impact of Carbon Utilization for Concrete, Chemical, and Mineral Production.” Environmental Science & Technology 55(17):12019–12031. https://doi.org/10.1021/acs.est.1c01109.
Ravikumar, D., D. Zhang, G. Keoleian, S. Miller, V. Sick, and V. Li. 2021b. “Carbon Dioxide Utilization in Concrete Curing or Mixing Might Not Produce a Net Climate Benefit.” Nature Communications 12(1):855. https://doi.org/10.1038/s41467-021-21148-w.
Research and Markets. 2020. “Polymers Market—Forecast (2020–2025).” https://www.researchandmarkets.com/reports/5021563/polymers-market-forecast-2020-2025.
Research Reports World. 2021a. “Global Amorphous Graphite Market Research Report: Growth Trends and Competitive Analysis 2021–2027.” https://www.researchreportsworld.com/global-amorphous-graphite-industry-18347737.
Research Reports World. 2021b. “Global Carbon Carbon Composites Industry Research Report: Growth Trends and Competitive Analysis 2021–2027.” https://www.researchreportsworld.com/global-carbon-carbon-composites-industry-18344501.
Searle, S., G. Bieker, and C. Baldino. 2021. Decarbonizing Road Transport by 2050: Zero-Emission Pathways for Passenger Vehicles. Briefing Paper. Washington, DC: International Council on Clean Transportation. https://theicct.org/wp-content/uploads/2021/12/zevtc-decarbonizing-by-2050-Jul2021%E2%80%AF.pdf.
Sick, V., G. Stokes, and F.C. Mason. 2022a. “CO2 Utilization and Market Size Projection for CO2-Treated Construction Materials.” Frontiers in Climate 4(May):878756. https://doi.org/10.3389/fclim.2022.878756.
Sick, V., G. Stokes, F. Mason, Y.-S. Yu, A. Van Berkel, R. Daliah, O. Gamez, C. Gee, and M. Kaushik. 2022b. Implementing CO2 Capture and Utilization at Scale and Speed: The Path to Achieving Its Potential. Technical Report. Global CO2 Initiative, University of Michigan. https://dx.doi.org/10.7302/5825.
Tenhumberg, N., and K. Büker. 2020. “Ecological and Economic Evaluation of Hydrogen Production by Different Water Electrolysis Technologies.” Chemie Ingenieur Technik 92(10):1586–1595. https://doi.org/10.1002/cite.202000090.
Third Derivative. n.d. “First Gigaton Captured.” https://www.third-derivative.org/first-gigaton-captured.
Timmerberg, S., M. Kaltschmitt, and M. Finkbeiner. 2020. “Hydrogen and Hydrogen-Derived Fuels Through Methane Decomposition of Natural Gas—GHG Emissions and Costs.” Energy Conversion and Management: X 7(September):100043. https://doi.org/10.1016/j.ecmx.2020.100043.
Troschl, C., K. Meixner, and B. Drosg. 2017. “Cyanobacterial PHA Production—Review of Recent Advances and a Summary of Three Years’ Working Experience Running a Pilot Plant.” Bioengineering 4(2):26. https://doi.org/10.3390/bioengineering4020026.
UNFCCC (United Nations Framework Convention on Climate Change). n.d. “Common Metrics.” https://unfccc.int/process-and-meetings/transparency-and-reporting/methods-for-climate-change-transparency/common-metrics.
Uygun, H.D.E., and Z.O. Uygun. 2020. “Fullerene Based Sensor and Biosensor Technologies.” Chapter 4 in Smart Nanosystems for Biomedicine, Optoelectronics and Catalysis. Rijeka: IntechOpen. https://doi.org/10.5772/intechopen.93316.
Wang, Z., T. Hu, R. Liang, and M. Wei. 2020. “Application of Zero-Dimensional Nanomaterials in Biosensing.” Frontiers in Chemistry 8. https://doi.org/10.3389/fchem.2020.00320.
Wolske, K.S., K.T. Raimi, V. Campbell-Arvai, and P.S. Hart. 2019. “Public Support for Carbon Dioxide Removal Strategies: The Role of Tampering with Nature Perceptions.” Climatic Change 152:345–361. https://doi.org/10.1007/s10584-019-02375-z.
Woodall, C.M., N. McQueen, H. Pilorgé, and J. Wilcox. 2019. “Utilization of Mineral Carbonation Products: Current State and Potential.” Greenhouse Gases: Science and Technology 9(6):1096–1113. https://doi.org/10.1002/ghg.1940.
Zhang, D., T. Liu, and Y. Shao. 2020. “Weathering Carbonation Behavior of Concrete Subject to Early-Age Carbonation Curing.” Journal of Materials in Civil Engineering 32(4):04020038. https://doi.org/10.1061/(ASCE)MT.1943-5533.0003087.
Zimmermann, A., L. Müller, A. Marxen, K. Armstrong, G. Buchner, J. Wunderlich, S. Michailos, et al. 2018. Techno-Economic Assessment & Life Cycle Assessment Guidelines for CO2 Utilization. Global CO2 Initiative, University of Michigan. https://doi.org/10.3998/2027.42/145436.
Zimmerman, J.B., P.T. Anastas, H.C. Erythropel, and W. Leitner. 2020. “Designing for a Green Chemistry Future.” Science 367(6476):397–400. https://doi.org/10.1126/science.aay3060.






























