3
Partitioning of Chemicals in Indoor Environments
Partitioning of chemicals plays an important role in indoor chemistry, indoor air quality, and occupant exposure to chemicals. Partitioning refers to the distribution of chemicals among phases or compartments. At the state of thermodynamic equilibrium, partitioning determines the concentration of a chemical in air, on surfaces, and elsewhere. Partitioning can also refer to the process of molecular exchange between phases. Chemicals in indoor environments are often not at equilibrium, and net transfer can occur between phases. Therefore, partitioning refers to both the thermodynamic state of chemicals distributed among phases in a system and the processes that transfer chemicals among phases, generally with a net tendency to approach equilibrium. Partitioning can also influence the rate of primary emissions (see Chapter 2) of some chemicals. Because of the very high surface-area-to-volume ratio of indoor environments, partitioning influences the manner, extent, and duration over which occupants are exposed to contaminants. The distribution of chemicals among phases and compartments also influences efforts to remove chemicals from indoor environments and mitigate exposure. While chemical transformations can subsequently occur, as discussed in Chapter 4, this chapter will focus on the role of partitioning of chemicals in indoor environments as it relates to indoor chemistry and indoor air quality.
Chemicals can have a greater affinity for one reservoir than another, and this is characterized by a “partition coefficient” defined later in this chapter. For example, nicotine has a high affinity for indoor material surfaces; therefore, there tends to be more nicotine on surfaces than in the air. The sizes of the reservoirs are also important. By combining partition coefficients with the sizes of reservoirs, one can determine where most of the mass of a chemical contaminant will be at equilibrium. However, partitioning does not occur instantaneously, and it can take time, sometimes years, for reservoirs to approach equilibrium with their neighbors owing to slow rates of molecular transport. As will be discussed in this chapter, challenges remain in understanding partitioning from the molecular to whole building scales and how partitioning influences exposure and chemistry. These challenges include limited information on the detailed composition of building materials across the building stock; complexity of spatial and temporal variations in environmental conditions; and poor understanding of the complexity of surfaces and indoor materials on molecular and nanometer (nm) length scales (Ault et al., 2020; Liu et al., 2020), as well as the molecular
interactions which drive the partitioning of a chemical from the gas to other phases (Abbatt and Wang, 2020; Ault et al., 2020).
This chapter first describes building reservoirs and surfaces as well as how contaminants interact with surfaces and bulk phases. Then the “partition coefficient” is defined along with a discussion of partitioning equilibria, chemical transport limitations on equilibria, and how environmental conditions influence partitioning. Specific reservoirs are discussed with a focus on the most recent studies and discoveries, and then the chapter concludes with a description of future research needs.
INDOOR ENVIRONMENTAL RESERVOIRS AND SURFACES
The indoor environment is often characterized by its different reservoirs. For the purposes of this report, reservoirs are defined as any surface or volume present in indoor environments to which chemicals can partition. Practically, when defining reservoirs for the purposes of talking about partitioning behavior, it is useful to combine materials of the same type into a reservoir. For example, it might be useful to define textiles as a reservoir or to distinguish textile reservoirs by type of fiber. Other useful reservoirs include paint films; the surfaces of glass, wood, dust, and indoor air; and even the volume of air surrounding a building. Important points to consider in the partitioning of chemicals are the fact that the physicochemical properties of these different materials are quite varied and the surfaces (see Box 3-1) of these materials, including their surface area, are often poorly defined. However, the properties of these materials are important to understand, as surface reservoirs of chemicals have recently been shown to be a dominant source of chemicals and potentially of chemical exposure (Wang et al., 2020b).
From a surface chemistry perspective, indoor environments provide a large variety of surfaces that a gas-phase molecule present in air can partition into (Figure 3-1). The available indoor surface area for this partitioning is many times greater than outdoors. The flat projected area is approximately 3 m2 for every cubic meter of room volume, for a room with contents such as furnishings (Manuja et al., 2019); much more area is available by accounting for the internal surface area of rough and porous materials.
Partitioning to the surface can be more or less important than the bulk, depending on the nature of the material and chemical. Surfaces can be impermeable, such as window glass, metal, and granite counters, or they can be highly permeable and porous, such as fabrics used in home furnishings, carpets, textiles used for clothing, and even painted walls.
For nonporous, solid surfaces, deposition of particles and partitioning of chemicals result in the buildup of thin films of material with thicknesses on the order of nanometers to micrometers (Eichler et al., 2019b; Liu et al., 2020; Weschler and Nazaroff, 2017). For porous materials or
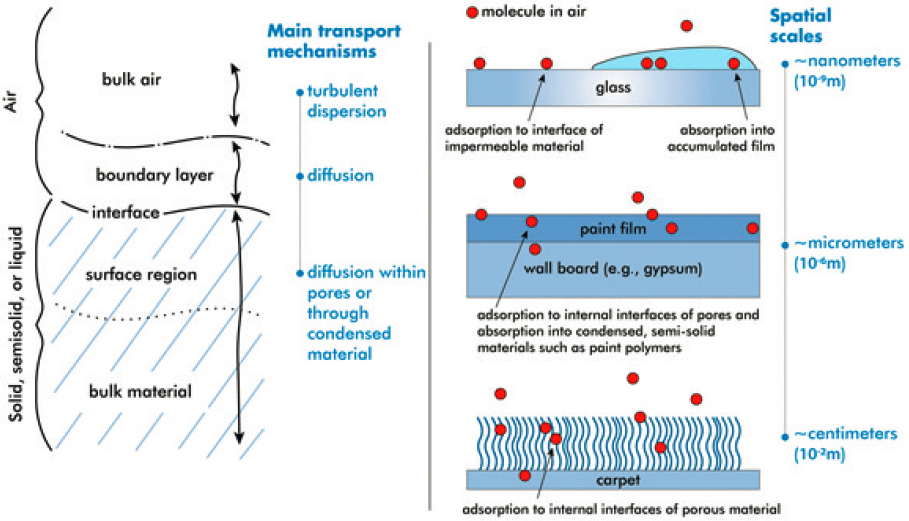
aqueous reservoirs, surfaces are the “gateway” into the bulk. Surfaces can control the ability of a chemical to partition from one phase into another. The surface is dynamic—in other words, changing over time. Surfaces can be cleaned, refurbished, or even repainted. Surfaces can soil through the buildup of organic thin films and dust particles, and the deposition of aerosols. In this region, thin films of organics can become surfaces themselves. Thus, there are complexities in understanding these surfaces, from clean to dirty, that are part of indoor environments.
PARTITIONING AMONG RESERVOIRS AND PHASES FOUND IN INDOOR ENVIRONMENTS
There are different phases and reservoirs in which gas-phase chemicals can partition (Figure 3-2). Gas-phase chemicals can partition to particles suspended in air (aerosol particles), impermeable solid surfaces like glass, thin films, and dust particles present on surfaces, permeable materials like wood, and water reservoirs (condensed aqueous phases; see Chapter 4). Partitioning is often regarded as an equilibrium process whereby the equilibrium in different phases (or different reservoirs) for a chemical is dictated by size of the reservoir and the nature and strength of the molecular interactions that occur. Thermodynamics govern these processes: the net transfer of molecules from one phase to another is due to a difference in chemical potential; thus, gradients drive this process until the chemical potential in each phase approaches equilibrium and is equal. This is similar to temperature differences or gradients that reach zero as they approach equilibrium. The equilibrium processes involving chemicals in the gas phase with other phases can be written generally as follows: M (phase 1) ⇄ M (phase 2), where, for example, phase 1 is the gas phase (air) and phase 2 is a solid phase (e.g., a wood surface). These equilibria can be written for a variety of processes involving different reservoirs and phases. If equilibrium is established, then all reservoirs and phases are in equilibrium with one another.
These equilibria can be characterized by partition coefficients. While there are numerous ways to define partition coefficients, here, two are considered: Karea and Kvol. The area-specific partition
SOURCE: Modified from Weschler and Nazaroff (2008).
coefficient, Karea, is the equilibrium ratio of the areal concentration on a surface, Cs (in μg/m2), to the gas-phase (air) concentration, Cg (in μg/m3), or Karea = Cs/Cg; its units are then m3/m2 or simply m. This parameter is useful when describing partitioning to surfaces like glass or metal. The volume-specific partition coefficient, Kvol, refers to partitioning to the bulk of a material and is defined as the equilibrium ratio of the volume concentration in that material, Cv (in μg/m3), to the concentration in air, or Kvol = Cv/Cg; its units are then m3/m3, but it is often shown without units. Partition coefficients are specific to the contaminant and the material it partitions to, but they can be influenced by environmental conditions, such as relative humidity (as discussed later in this chapter).
Gas- to aqueous-phase partitioning can be described by Henry’s law, KH = [A]/CA,g, where CA,g is the gas-phase concentration of species A, [A] is its concentration in the aqueous phase, and KH is Henry’s law constant. Note that the Henry’s law constant and the water-air partition coefficient, Kwa, are equivalent after accounting for the gas constant and temperature (Weschler and Nazaroff, 2008). For partitioning into organic materials, including organic films, which are prevalent on surfaces, the Koa value for a molecular species (i.e., the ratio of the amount of a chemical in octanol compared to air) is often chosen to approximate the equilibrium state. There have been several approaches formulated toward understanding chemical partitioning, especially of semivolatile organic compounds (SVOCs) (Wang et al., 2020b; Weschler and Nazaroff, 2008). Additional considerations of indoor dust/air partitioning models have also been described (Parnis et al., 2020).
SIZE AND CAPACITY OF DIFFERENT INDOOR RESERVOIRS
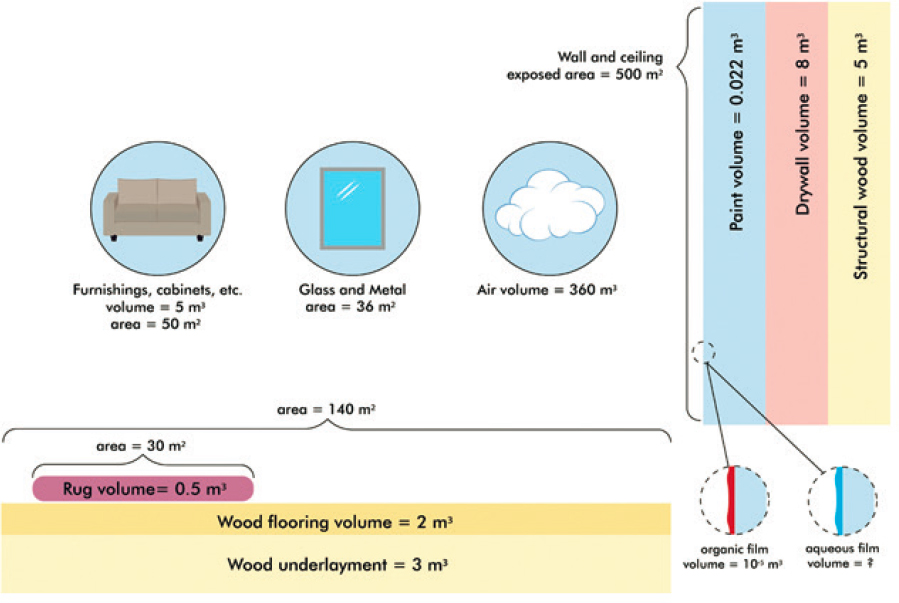
Buildings vary greatly in size, design, types of materials, and environment. To help put partitioning in perspective, this section uses the example of a typical U.S. residence with a floor area of 140 m2 and volume of 360 m3 (living space) (U.S. Census, 2021). The internal, exposed surface area is ~1,150 m2, with painted walls and ceiling, flooring, windows, and furnishings comprising the bulk of that area. Most of the volume is air, followed by the building shell and walls, then furnishings. An accounting of areas and volumes of all indoor materials is beyond the scope of this report, but Figure 3-3 provides a few for scale; here, values are estimated from multiple sources (Hodgson et al., 2005; Manuja et al., 2019; Nazaroff and Weschler, 2020).
An estimate of the amount of a chemical present in a specific reservoir relative to indoor air can be obtained by multiplying an area-specific partition coefficient (Karea) by the surface-area-to-volume ratio of that material indoors. For example, glass and metal are responsible for approximately 0.1 m2 of exposed surface area per cubic meter of residence volume. Therefore, using a value of Karea =1,000 m3/m2 for the partition coefficient of a typical SVOC with glass and steel (see the later section in this chapter on Impermeable Surfaces), ~100 times more contaminant mass will be present on these surfaces than in the air volume of the house. The mass of SVOCs can be orders of magnitude higher in the bulk of materials like paint, wood, and textile than at their outermost surface region. In general, the amount that can partition to interfacial reservoirs and organic films is much lower than the amount that can partition into bulk materials. Indoor reservoirs can accumulate very large amounts of contaminants relative to the amount in air.
PARTITIONING THERMODYNAMICS: EFFECTS OF TEMPERATURE AND RELATIVE HUMIDITY
The strength of the intermolecular interactions between a sorbing molecule and the chemicals in the condensed-phase reservoir play a very strong role in determining the degree to which gas-to-surface partitioning occurs. These interactions involve van der Waal’s, dipole-dipole, dipole-induced dipole, and H-bonding forces, whereas covalent bonds arise when chemical transformations occur (see Chapter 4). Together with entropic factors, the collective strength of these intermolecular forces—referred to as the sorption enthalpy—controls the magnitude of the equilibrium partition coefficient defined above.
An important factor to consider is the temperature dependence of the partition coefficient, which is controlled by the sorption enthalpy (i.e., larger sorption enthalpies are related to a stronger temperature dependence of the partitioning). Partitioning of SVOCs to indoor materials has been characterized as a function of temperature (Wei et al., 2018). The enthalpy varies by chemical and the reservoir to which it partitions, but it is typically ~100 kJ/mol for SVOCs (Liang and Xu, 2014). In this case, a 5 °C increase in the temperature (e.g., from 20 °C to 25 °C) results in a 2-fold change in the partition coefficient and thus a 2-fold increase in the concentration in air relative to the surface. For example, a recent field study showed a 1.25- to 2-fold increase in indoor SVOC concentrations when ambient temperature increased from 14 °C to 21 °C, due to the emissions from indoor surfaces (Kristensen et al., 2019).
Many factors, including relative humidity, impact a chemical’s partitioning behavior. Water vapor can partition to surfaces, and it has been shown that the amount of water associated with surfaces increases as relative humidity increases and as temperature decreases. The multiple roles of adsorbed water in the partitioning and chemistry of a variety of different surfaces have been previously discussed (Rubasinghege and Grassian, 2013). Water on surfaces can both increase and decrease the uptake of chemicals on surfaces. When increasing the amounts of water on the surface results in a decrease in the sorption of other chemicals, this suggests that there is competition between sorbed water (i.e., on surfaces or inside porous materials) and sorbed chemicals. As the amount of sorbed water on indoor surfaces increases with increasing relative humidity, the sorbed chemicals are displaced from the surface into the gas phase. The extent of this displacement depends on the relative energies of the specific molecular interactions that occur for water compared to the sorbed chemical (Frank et al., 2020; Huang et al., 2021). Several recent studies have shown that, for some organic compounds such as limonene, increasing relative humidity leads to a decrease in the amount of limonene adsorbed on silica (SiO2) particle surfaces and the presence of adsorbed water on the surface (Frank et al., 2020). However, for more oxygenated, polar chemicals, such as dihydromyrcenol, linalool, and α-terpineol, there is no displacement and little adsorbed water on the SiO2 particle surface at high relative humidity (Huang et al., 2021).
Studies on more complex indoor surface materials show that increasing relative humidity affects partitioning behavior in different ways depending on the chemical and the material. In some cases, increasing relative humidity has no effect, in other cases it decreases the amount of organics associated with the material, and in a few cases increasing relative humidity is shown to increase the amount of chemical sorbed in the material (Won et al., 2001). Most importantly, recent studies have made clear that a range of behaviors on the impacts of water on surfaces and materials can be found in indoor environments as it relates to the chemical partitioning of organic compounds (Wang et al., 2020b).
PARTITIONING DYNAMICS, TIMESCALES, AND LIMITATIONS ON THE EQUILIBRIUM CONCEPT
Although thermodynamics drive these equilibria, transport limitations and chemical kinetics play an important role in indoor partitioning of chemicals, as high physical and energy barriers for some processes result in nonequilibrium conditions. The amount of any chemical present within
reservoirs changes over time with changes in the conditions; these can occur over timescales of minutes to years. For example, SVOCs present in a new piece of furniture may require decades to approach equilibrium with the surrounding air and surfaces. Mechanisms that slow transfer of molecules from one reservoir to another include diffusion across a thin layer of slow-moving air adjacent to all surfaces (i.e., boundary layer) and diffusion into building materials and furnishings. These “mass transfer limitations” are central to understanding the dynamic development of contaminant concentrations in indoor reservoirs and will be highlighted several times in this report; however, because these mechanisms are well described in other sources, this report will not separately discuss mass transfer phenomena. The equilibrium partitioning concept is also somewhat limited, as it only describes scenarios where reactions do not occur. If an organic compound undergoes oxidation or other chemical reactions on the surface, it may or may not re-equilibrate back into the gas phase on relevant timescales.
The combination of reservoir capacity and transport limitations can strongly influence dynamic indoor concentrations. For example, surfaces and porous materials can become large reservoirs of many chemicals. Although air can be “cleaned” in minutes by indoor/outdoor air exchange (e.g., opening windows) or high ventilation rates (e.g., heating, ventilation, and air-conditioning [HVAC] systems and filtration), reservoirs release these chemicals back into indoor air (Uhde et al., 2019; Wang et al., 2020b), reducing the effectiveness of control efforts. This leads to continued exposure of these contaminants with only intermittent decreases under certain conditions. Similarly, low-volatility chemicals can remain on surfaces for durations much longer than timescales of ventilation; this can provide enough time for slow chemical reactions to occur. Eichler et al. (2019a) provide insights into these highly variable surfaces and a mechanistic framework in which to model SVOCs that are emitted from surfaces and materials indoors.
CURRENT SCIENCE ON PARTITIONING OF CHEMICALS IN INDOOR ENVIRONMENTS
Impermeable Surfaces
Impermeable indoor surfaces, to which chemicals can adsorb but not penetrate, include windows and mirrors, glazed tiles, ceramic-top stoves, polished stone counters, stainless steel, copper pipes, and enameled appliances. High-density plastics may sometimes be considered effectively impermeable to large organic chemicals. Indoor air is in direct contact with many such exposed surfaces, providing for rapid uptake and release of chemicals, including reactive species that may not otherwise penetrate below permeable material surfaces. Uptake to such indoor surfaces has been studied since it was recognized that adsorption to the surfaces of steel or glass chambers altered the dynamic air concentration of volatile organic compounds (VOCs) from building materials being studied for their emissions (Tichenor et al., 1991). In a review of SVOC partitioning indoors, Wei et al. (2018) reported that partition coefficients for impermeable surfaces ranged from 6 m to 7,500 m; the wide range is due to the range of temperature, relative humidity, and materials studied. Wu et al. (2017) observed that molecular-scale surface area was of central importance in the partitioning of phthalate esters to metal, glass, and acrylic.
Molecular interactions at the interface can be unique to the primarily inorganic materials that comprise impermeable indoor surfaces. For example, several authors have observed higher than anticipated partitioning of terpenes to SiO2, the primary component of glass (Chase et al., 2017; Fang et al., 2019b; Liu et al., 2019). Molecular dynamic simulations are consistent with spectroscopic measurements of limonene adsorption to SiO2, demonstrating the potential of computational methods to quantify molecular-level thermodynamics that have building-scale implications (Fang et al., 2019a). Partitioning behavior of terpenes is important because double bonds and other
substituents in terpenes can influence their orientation on the surface (Fang et al., 2019a; Ho et al., 2016) and the rate of surface chemistry (see Chapter 4; Ham and Wells, 2009; Shu and Morrison, 2011; Stokes et al., 2008, 2009).
Organic Thin Films
Film development is an extension of molecular adsorption onto pristine surfaces. As more molecules continue to adsorb to a surface, they can form “islands” or eventually films with thicknesses measured in nanometers. Deposition of particles can also contribute to surface films. Because the formation, composition, and aging of films is analogous to secondary organic aerosols, they have been described as secondary organic films (Ault et al., 2020). Clear evidence has been found for the heterogeneity of glass surfaces in indoor environments using new microspectroscopic methods to analyze surface films and deposited particles on glass surfaces. In particular, Or et al. (2018) developed atomic force microscopy coupled to photothermal infrared spectroscopy to analyze surfaces placed in different indoor environments (e.g., kitchen, garage, office, and copier room). These studies show the variability of these surfaces on nanometer to micrometer length scales. When glass is placed in a kitchen for 6 months, organic films are shown to contain several different types of organics, including fatty acids and oxidized organic compounds, such as nonanal.
Organic films were first identified and characterized in indoor spaces by Liu et al. (2003). The composition is dominated by plasticizers, mono- and dicarboxylic acids, and “cooking organic aerosol” components (Lim and Abbatt, 2020; Liu et al., 2003; O’Brien et al., 2021) but also includes many other SVOCs as discussed below. Weschler and Nazaroff (2017) proposed that indoor organic films develop from accumulation of existing indoor SVOCs. SVOCs with lower octanol-air coefficients (Koa) rapidly equilibrate with an existing thin film and maintain a constant concentration, even as the film grows as the result of absorption of chemicals with higher Koa. This process continues, and the film growth slows as chemicals of higher molecular weight reach equilibrium with the film. Eichler et al. (2019a) extended this model to include the formation of the first layer of the film, and Lakey et al. (2021) further extended that model to the influence of overlying turbulence, film viscosity, and chemical transformations. In experiments intended to follow film development on surfaces, Wallace et al. (2017) heated surfaces that had been deployed indoors and measured the number concentration of ultrafine particles that were generated as the result of evaporation and nucleation in air; they observed that the particle emission rate (proportional to film thickness) increased with exposure up to ~100 days of exposure. Lim and Abbatt (2020) deployed glass capillaries in various locations in a home and measured film growth and composition using direct analysis in real time mass spectrometry (DART-MS). They observed initial growth rates consistent with predictions and other measurements, at least 0.05 nm/day, with a highly homogeneous film composition from location to location throughout a residence. This is consistent with growth of the films by SVOC uptake. Taken as a whole, thin organic films eventually develop on all indoor surfaces, and this has been suggested as one reason why ozone decay rates among buildings are surprisingly similar despite the presence of very different kinds of materials (Weschler and Nazaroff, 2017). Based on a review of equilibrium measurements of compounds found on surfaces and air, Weschler and Nazaroff (2012) inferred that average organic surface film thicknesses range from 3 to 30 nm, which is consistent with direct measurements of organic matter and predictive models, although these films may not be of uniform thickness.
Importantly for this chapter, these films provide volume for absorption of chemicals. Surface films accumulate the many SVOCs that have been identified indoors including phthalates, flame retardants, polycyclic aromatic hydrocarbons, perfluorinated alkyl substances (PFAS), and others (Bennett et al., 2015; Butt et al., 2004a,b; Duigu et al., 2009; Gewurtz et al., 2009; Huo et al., 2016; Liu et al., 2019; Melymuk et al., 2016; Pan et al., 2012; Venier et al., 2016). For most of
these SVOCs, their presence in films dwarfs the amount present in the air (Weschler and Nazaroff, 2008), but the amount present in films is dwarfed by bulk reservoirs of permeable materials (see the next section).
Films that develop on exposed surfaces can be highly responsive to changes: there is little mass-transfer resistance for flux to and from bulk indoor air, and in the absence of a dramatic increase in viscosity, diffusion is fast (Lakey et al., 2021; O’Brien et al., 2021). This means that changes in indoor environmental conditions will result in a rapid response from these exposed reservoirs. For example, increasing ventilation by opening a window is usually expected to improve air quality by diluting chemicals that have indoor sources; however, in response to the disruption of equilibrium, near-instantaneous increased emissions of SVOCs stored in surface reservoirs will recharge the air phase, limiting the ability of a temporary increase in ventilation to decrease levels. Consistently high ventilation can eventually deplete these reservoirs, but the time required to lower indoor air concentrations can range from days to years.
Although films have the potential to respond rapidly, low-volatility chemicals take time to equilibrate with these films. Over timescales of weeks, chemicals with log10(Koa) <11 are likely to equilibrate with 10 nm-thick organic surface films, while those with log10(Koa) >14 will take years to approach equilibrium (Lim and Abbatt, 2020; Venier et al., 2016; Weschler and Nazaroff, 2008).
Permeable Materials
Permeable materials comprise the majority of surface reservoirs that are present indoors (Hodgson et al., 2005; Manuja et al., 2019). The term “permeable” here refers to materials that allow chemicals to penetrate below their interface by diffusing into the bulk of the material or via openings or pores that allow access to the internal surface area. Wood, textiles, concrete, plaster, drywall, plastics, and coatings have large surface areas for molecular adsorption and/or large volumes for absorption. Manuja et al. (2019) reported that painted walls and coated wood comprised the majority of exposed surface area in the 22 rooms they studied, in line with the findings of an earlier report (Hodgson et al., 2005). The capacity of some permeable materials relevant to the indoor environment to take up and release chemicals has been measured for VOCs (Algrim et al., 2020; Meininghaus and Uhde, 2002; Won et al., 2001) and SVOCs (Eftekhari and Morrison, 2018; Morrison et al., 2015a; Saini et al., 2016; Wei et al., 2018).
A notable recent advance has been in the application of real-time methods to observe the dynamics of VOC absorption into paint. Algrim et al. (2020) quantified partition and diffusion coefficients for carboxylic acids and related these parameters to volatility. Their framing of the results mirrors the way atmospheric chemists now parameterize partitioning into aerosols. These parameters can then be used in models to predict the time-dependent air concentrations of VOCs as influenced by their interactions with painted surfaces.
In-vitro evidence that clothing influences dermal uptake of indoor SVOCs (Bekö et al., 2018; Morrison et al., 2016, 2017a) has resulted in increased interest in partitioning to textiles (Licina et al., 2019). Because textiles can act as a sink, clothing can help reduce dermal uptake of chemicals at least until the materials become saturated; at that time, they become a net source and may even enhance dermal uptake of some chemicals (Cao et al., 2016; Morrison et al., 2016, 2017b). Measurements of partitioning of flame retardants (Saini et al., 2016; Venier et al., 2016), plasticizers (Cao et al., 2016; Morrison et al., 2015b, 2017b; Saini et al., 2016), and other chemicals (Morrison et al., 2015a) demonstrate that the textile reservoir is large. Textiles act not only as strong chemical sinks when first introduced to homes but also as long-term sources of exposure to those same chemicals. When equilibrated with a typical home, a single pair of cotton jeans has been demonstrated to absorb more phthalates, flame retardants, and other species than are present in the entire air volume of a home (Cao et al., 2016; Morrison et al., 2015a; Saini et al., 2016).
Airborne Particles
SVOCs of indoor origin have long been observed to partition to indoor particles (Weschler, 1980). Advanced measurements of aerosol composition have recently revealed a wealth of time-averaged and real-time compositional information. Several groups have deployed aerosol mass spectrometers, thermal desorption aerosol gas chromatography (TAG), and extractive electrospray ionization mass spectrometry systems to collect and generate detailed, real-time (or roughly hourly depending on the system), compositional information for aerosol particles (Avery et al., 2019; Brown et al., 2021; DeCarlo et al., 2018; Fortenberry et al., 2019; Lunderberg et al., 2020). Consistently across these studies, indoor sourced SVOCs are observed partitioning to particles that originate outdoors or are generated indoors during cooking or other activities. These include phthalates, siloxanes, paint additives, and components of third-hand smoke. Also evident is that partitioning to aerosol particles shifts the equilibrium between air and building surfaces, causing an increase in emission rates of some compounds.
The total volume and mass of airborne particles in buildings is generally quite small relative to the surrounding air. One cubic meter of air weighs approximately 1.2 kg, within which there is usually less than 0.1 mm3 of particles present (or less than 100 μg). Despite the relatively small volume of particles, partitioning ensures that some SVOCs can be present at a greater mass in particles than in air (Salthammer and Goss, 2019). Inhalation of airborne particles can therefore dominate total inhalation exposures despite making up a small fraction of inhaled mass or volume. However, this is influenced by particle concentration and chemical partition coefficients, which are themselves strongly influenced by environmental conditions (Zhou et al., 2021). Over a wide range of volatilities, most SVOCs are expected to equilibrate with particles in 1 hour or less (Liu et al., 2014).
Particle Deposition to Surfaces
Besides the partitioning of discrete chemical species, particle deposition also plays a role in the transfer of chemical mass from air to surfaces in the indoor environment. For example, the deposition of aerosols can contribute to organic film formation or mineral dust deposition containing reactive metals from indoor-outdoor air exchange. Deposited salts can contribute to material corrosion (Sinclair et al., 1990). Deposition also acts to reduce inhalation exposure; the concentration of particulate matter of outdoor origin is generally lower indoors than outdoors (Chen and Zhao, 2011). Particle deposition rates depend on particle size and density as well as indoor air mixing (Lai and Nazaroff, 2000; Nazaroff and Cass, 1987; Thatcher et al., 2002). The orientation of surfaces is an important factor in the total mass deposited (Nazaroff and Cass, 1987). Horizontal, upward-facing surfaces accumulate more particles than vertical surfaces, which contribute to organic films on surfaces (Deming and Ziemann, 2020). Walking indoors can resuspend some deposited mass, transferring it back into the air (Boor et al., 2013; Wang et al., 2020a).
Advanced methods are now being applied to probe the physical and chemical characteristics of single deposited particles to indoor surfaces. Microspectrochemical techniques that combine imaging and measurements of chemical speciation can improve our understanding of particle deposition. Or et al. (2018) revealed both organic particles and films on the surface of glass slides placed vertically in a kitchen for 6 months. Glass slides in other indoor environments, including a garage, a copier room, and an office, also showed particle deposition. The greatest number of particles were less than 500 nm in diameter, with the copier room having the greatest number of particles deposited on the glass surface after 6 months. Results from the House Observations of Microbial and Environmental Chemistry (HOMEChem) study showed that glass surfaces exposed to different kitchen activities again resulted in particle-covered surfaces, many of which were <100 nm in size, but that these activities led to different particle coverages (Or et al., 2020). Most interesting is that physical deposition models (Lai and Nazaroff, 2000) underestimated particle deposition
rates by an order of magnitude during the stir-fry experiments, suggesting that not all processes or conditions are being captured by the models. This model-measurement discrepancy is consistent with real-time particle measurements taken during HOMEChem that showed that traditional models underestimate deposition rates. Boedicker et al. (2021) used these measurements to propose using an alternate model for particle deposition to better account for interception processes to irregular collection surfaces. Physical measurements of individual particles (e.g., viscosity, phase, shape, and charge) contribute to our understanding of film formation, formation of hydrophobic or hydrophilic “islands,” and resuspension. This highlights the importance of probing the physicochemical properties and interactions between particles and common indoor surfaces that influence deposition in more detail.
Dust
Deposited particles eventually accumulate, collectively forming dust. Because dust can be a major source of exposure to chemicals, especially for small children, it has been studied extensively. As discussed in Chapter 2, thousands of chemicals, including flame retardants, pesticides, plasticizers, and chemical stabilizers, have been quantified in the dust of homes, offices, schools, and daycare centers (Lucattini et al., 2018). As such, this report includes dust as a distinct and significant reservoir for indoor chemicals.
Weschler and Nazaroff (2010) evaluated paired air and dust concentration measurements reported in the existing literature. They observed that the concentration in dust could be predicted reasonably well using the octanol-air partition coefficient, Koa. Deviations from such predictions may be due to temperature effects, kinetic limitations in achieving equilibrium with air, uncertainties in the values of thermodynamic parameters (like Koa), and the presence of particles of abraded materials (e.g., plastics) that contribute very large quantities of contaminants to dust. More recent studies confirm that many chemicals partition from air to dust in a generally predictable manner (Melymuk et al., 2016; Watkins et al., 2013), with the notable exception of high molecular weight species that take much longer to equilibrate (Melymuk et al., 2016; Parnis et al., 2020; Weschler and Nazaroff, 2010). Dust that deposits on a surface can more rapidly absorb a contaminant from that surface than from the air if that surface is a primary contaminant source (Rauert and Harrad, 2015; Schripp et al., 2010). This can overcome some mass-transfer limitations for equilibration but also can result in a higher concentration in the dust than would be expected based on the air concentration alone.
Condensed Water
Water is present in homes in many forms, including bulk water, water adsorbed to surfaces as thin films (see Chapter 4), perhaps leading to visible condensation, and water absorbed into materials. Because of the high surface-area-to-volume ratio of indoor spaces, the amount of condensed water available for partitioning is orders of magnitude higher than outdoors (Duncan et al., 2019). The amount of water available for partitioning per volume of air in an indoor space has been estimated to range from less than 10-6 l/m3 (for water adsorbed to indoor surfaces) up to 0.1 l/m3 (for bulk water present in a toilet) (Nazaroff and Weschler, 2020).
Water-soluble organic gases partition predictably into condensed water, but partitioning can be influenced by the pH of the water (Nazaroff and Weschler, 2020). The pH, in turn, is influenced by the presence of salts and absorbed gases such as ammonia, carbon dioxide, and nitric acid. People are an important indoor source of carbon dioxide, ammonia (Li et al., 2020), and organic acids; therefore, the presence of occupants likely influences partitioning of acids and bases to indoor materials and condensed water (Nazaroff and Weschler, 2020).
While the research community is far from understanding the full extent to which water influences indoor air concentrations of contaminants, some recent studies are suggestive. Duncan et al. (2019) observed that the air concentration of formic and acetic acids dropped when the air conditioner was operating in a test home. The concentrations rose again when the air conditioner was off. They interpreted this behavior as partitioning of these acids into the air-conditioning condensate that forms on cooling coils. Similar behavior has been observed for ammonia (Ampollini et al., 2019) and nitrous acid (Wang et al., 2020b) in the HOMEChem study. The HOMEChem study also revealed that the air concentration of acids rose rapidly upon closing windows during an airing-out perturbation experiment. This evidence of very large, highly accessible reservoirs of acids suggested that water or other polar materials on surfaces play an important role in storing acids (Collins et al., 2018a; Wang et al., 2020b).
Wang et al. (2020b) described partitioning among air, organic, and aqueous phases by relating the equilibrium proportion of a chemical distributed among phases as a function of the octanol-air partition coefficient and the water-air partition coefficient (or Henry’s law coefficient). They showed how pH of an aqueous phase controls the phase distribution of organic acids, with increasing pH driving acids from air or the organic phase into the aqueous phase. Experiments conducted at HOMEChem demonstrated that moderately strong acids (e.g., formic acid and HONO) present in indoor surfaces could be re-partitioned to the gas phase via acidification of the aqueous surface reservoirs, via application of acetic acid in a vinegar cleaner (Wang et al., 2020b).
Microorganisms and biofilms present on surfaces can be treated as a condensed phase composed primarily of water with a small amount of organic matter representing microorganisms. As an approximation, chemicals will partition based on the octanol-air partition and Henry’s law coefficients, proportional to the relative amount of organic matter and water. Microbial metabolism could shift conditions away from equilibrium, but the extent depends on relative rates of metabolism and chemical transport to and away from the microorganism.
Occupants
Occupants are an important source of carbon dioxide (CO2), ammonia (NH3), and numerous VOCs, as discussed in Chapter 2, and can act as sinks for chemicals that influence indoor concentrations and lead to chemical exposure. They also transfer skin and skin oils to surfaces by direct contact and desquamation. Unlike most other indoor reservoirs, occupants come and go, resulting in daily dynamics in source/sink behavior. The thin film of lipids (i.e., skin oils) found on the surface of skin can act much like cooking oils in their partitioning behavior. Weschler and Nazaroff (2012) compared measurements of SVOCs in skin lipids from the Children’s Total Exposure to Persistent Pesticides and Other Persistent Organic Pollutants study to values predicted based on air concentrations and octanol-air partition coefficients. They found good agreement, implying that SVOCs in skin lipids were roughly equilibrated with indoor air, allowing partitioning to be predicted from standard thermodynamic parameters. They combined this finding with existing models of transdermal uptake of chemicals to show that air-to-skin partitioning and net uptake could be an important component of chemical exposure in buildings. Experiments with human subjects have now verified this exposure pathway for phthalates (Morrison et al., 2016; Weschler et al., 2015) and nicotine (Bekö et al., 2018). Partitioning of ionizable compounds, like nicotine and methamphetamine, to skin lipids has not been well characterized and may be strongly influenced by the water content, acid concentration, and pH (Bekö et al., 2017; Morrison et al., 2015a; Parker and Morrison, 2016). Given the large capacity for absorbing SVOCs, a recent publication suggests that skin lipids on the hands can act as important agents of chemical transport among indoor surfaces (Diamond et al., 2021). Partitioning to clothing and subsequent exposure is also important, as discussed earlier in this chapter. Box 3-2 discusses third-hand smoke as one example of the complexity of partitioning.
MODELS FOR PARTITIONING BEHAVIOR
Although partitioning occurs via both adsorption and absorption mechanisms to many indoor reservoirs with a vast array of chemical and morphological complexity, models of human exposure to indoor chemicals typically rely on simplified representations of these interactions that use partition coefficients. In particular, such exposure models require predictions of the distribution of the chemicals across indoor spaces in order to assess different exposure mechanisms. For example, a recent model describes how Koa and Kwa are used to assess the degree of inhalation, dermal, and dietary and non-dietary ingestion exposures that arise for many organic chemicals in the indoor environment as a function of the fundamental properties of the molecules (Li et al., 2019). See Chapter 6 for a more detailed discussion of exposure assessment and modeling.
Advanced techniques to estimate partition coefficients are an active area of research. This is especially important given the tens of thousands of commercial chemicals released indoors, because it is not realistic to experimentally measure partitioning parameters for each of these chemicals to each reservoir. These methods include advanced polyparameter linear free energy relationships for different materials (Endo and Goss, 2014) and quantum chemical methods that have been applied to thousands of chemicals (Wang et al., 2017). Furthermore, at the individual molecule level, a highly detailed understanding is emerging of how important indoor chemicals (e.g., terpenes and some oxygenated VOCs) interact with select surface components (e.g., silica) through coupled
experimental-theoretical approaches (Fang et al., 2019a,b; Huang et al., 2021). It will be valuable to compare the performance of estimated parameters currently used in exposure models with results from these detailed studies. Because of the implications for indoor exposure and health, partitioning models for surfactant species, such as PFAS, need further development.
CONCLUSIONS
Chemical contaminants move from one reservoir to another with a net tendency toward approaching, but not necessarily always attaining, an equilibrium condition. The nature of the equilibrium condition may change with time as environmental parameters such as temperature and relative humidity evolve. This partitioning process distributes chemicals from their initial sources throughout indoor spaces, to air, building materials, furnishings, dust, and so forth. These reservoirs buffer the air concentrations of chemicals, reducing the short-term effectiveness of controls by ventilation or filtration, compared to long-term effectiveness. Because the indoor partitioning capacity is so large, many molecules that are considered to be entirely volatile in the outdoor environment exhibit SVOC behavior indoors (Wang et al., 2020b). Partitioning of indoor chemicals to aerosols increases inhalation exposure, while partitioning to dust and surfaces increases ingestion exposure, especially for toddlers. Partitioning can also result in chemicals moving to locations and conditions favorable for chemical transformations.
Despite a rapidly growing base of knowledge about indoor partitioning, important gaps remain. The materials that are present in buildings, or comprise buildings, are not physically or chemically well characterized. Partition coefficients have been measured only for very few pairs of chemical contaminants and materials. Models to predict thermodynamic parameters exist, but their application to real indoor materials has not been widely demonstrated. Furthermore, models have not yet successfully been applied to many chemical classes important in indoor environments, such as surfactants. The extent to which environmental and other building factors, occupant activities, and control systems influence partitioning and exposure remain to be explored. Acting on the following recommendations could help bridge these gaps.
RESEARCH NEEDS
Given its findings about the current state of the science, the committee has identified priority research areas to help drive future advances in chemical partitioning relevant to indoor environments:
- Expand equilibrium and nonequilibrium (dynamic and steady-state) partitioning studies to include a larger variety of materials present in buildings. Detailed studies of partitioning of SVOCs to both impermeable and permeable materials, such as glass, clothing, and paint, have illustrated the sophistication of currently available laboratory techniques that allow for measurement of both uptake kinetics and equilibrium partitioning to representative indoor materials. These studies could be extended to additional indoor materials—wood, concrete, home furnishings, carpet, clothing, etc.—as well as those found in interstitial spaces to develop a more comprehensive understanding of the capacity and chemical affinities of different surface reservoirs for a wide range of sorbing chemicals. This is important to determine lifetime exposure levels and the overall mass flow of chemicals through the built environment. Better understanding of diffusion timescales through materials would inform whether their full volume is available for partitioning and the concentration gradients that may exist with them.
- Examine the influence of environmental conditions and occupant activities on equilibrium and nonequilibrium partitioning and the influence of partitioning on contamination management. The effects of seasonal and daily temperature changes and relative humidity are important, including studies of extreme conditions, for example, associated with
- air-conditioning systems. Regional differences in building types and building climate control are important to assess, given their connections to total contaminant exposure. Building occupants can add, remove, or modify reservoirs and change conditions that influence partitioning phenomena. Management of indoor environments does not typically account for partitioning phenomena but may improve with such consideration. For example, the effectiveness of ventilation strategies could improve if their timing accounted for partitioning dynamics.
- Develop a molecular-level understanding of partitioning among indoor reservoirs. This may arise from use of advanced physical chemistry techniques and models. In particular, soiled surfaces, airborne particles, dust, and surface coatings are important. Laboratory-scale partitioning experiments could be complemented by experiments in genuine indoor experiments, such as when chemicals are added in a controlled manner to an indoor space or as mixtures of chemicals to investigate synergistic effects. Spectrochemical analysis, imaging, and depth profiling could provide important insights into molecular interactions and spatial gradients in indoor materials.
- Improve predictive models of equilibrium and nonequilibrium partitioning and compare them with observations from laboratory experiments and real-world, occupied buildings. Multimedia partitioning models that assess human exposure typically use a limited number of partitioning volumes, such as air, polar surface reservoirs (e.g., water), and mildly polar reservoirs (e.g., n-octanol). It is important to establish how well this simplified approach represents the full chemical complexity of the system, when assessed against the additional uncertainties associated with source fluxes, human behavior and diversity, and reactive loss rates. Advanced computational methods, including quantum chemical methods and molecular dynamic simulations, can be used to determine partitioning coefficients. Relevant model systems may play a role in providing important insights and a more detailed understanding of the more complex home environment. These studies can also probe temperature, relative humidity, and other important factors that impact chemical partitioning.
- Identify key species, materials, and partitioning phenomena that strongly influence exposure. Given that most chemicals are predominantly partitioned to indoor surfaces, it is important for exposure modeling to consider carefully the distribution of chemicals among these surface reservoirs, the gas phase, and aerosol particles. Compositional changes occurring due to partitioning to airborne particles remains an important research topic. For SVOCs, the gas phase acts as a conduit between large surface reservoirs and aerosol particles, which can be inhaled and increase exposure. While this phenomenon has been illustrated by third-hand tobacco smoke, the degree and timescales for other contaminants residing in surface reservoirs to partition to aerosol particles need to be determined. Particles and other contaminants are complex and have many sources, and indoor environments themselves are diverse; the very few predictive models that are now including such complexity have not been well vetted for real environments and will continue to need more comprehensive primary input data (e.g., source terms), a better fundamental understanding of partitioning, and evaluation in real indoor environments.
REFERENCES
Abbatt, J. P. D., and C. Wang. 2020. The atmospheric chemistry of indoor environments. Environmental Science: Processes & Impacts 22(1): 25–48. https://doi.org/10.1039/c9em00386j.
Algrim, L. B., D. Pagonis, J. A. de Gouw, J. L. Jimenez, and P. J. Ziemann. 2020. Measurements and modeling of absorptive partitioning of volatile organic compounds to painted surfaces. Indoor Air 30(4): 745–756. https://doi.org/10.1111/ina.12654.
Ampollini, L., E. F. Katz, S. Bourne, Y. Tian, A. Novoselac, A. H. Goldstein, G. Lucic, M. S. Waring, and P. F. DeCarlo. 2019. Observations and contributions of real-time indoor ammonia concentrations during HOMEChem. Environmental Science & Technology 53(15): 8591–8598. https://doi.org/10.1021/acs.est.9b02157.
Ault, A. P., V. H. Grassian, N. Carslaw, D. B. Collins, H. Destaillats, D. J. Donaldson, D. K. Farmer, J. L. Jimenez, V. F. McNeill, G. C. Morrison, R. E. O’Brien, M. Shiraiwa, M. E. Vance, J. R. Wells, and W. Xiong. 2020. Indoor surface chemistry: Developing a molecular picture of reactions on indoor interfaces. Chem 6(12): 3203–3218. https://doi.org/10.1016/j.chempr.2020.08.023.
Avery, A. M., M. S. Waring, and P. F. DeCarlo. 2019. Human occupant contribution to secondary aerosol mass in the indoor environment. Environmental Science: Processes & Impacts 21(8): 1301–1312. https://doi.org/10.1039/c9em00097f.
Bekö, G., G. Morrison, C. J. Weschler, H. M. Koch, C. Palmke, T. Salthammer, T. Schripp, A. Eftekhari, J. Toftum, and G. Clausen. 2018. Dermal uptake of nicotine from air and clothing: Experimental verification. Indoor Air 28(2): 247–257. https://doi.org/10.1111/ina.12437.
Bekö, G., G. Morrison, C. J. Weschler, H. M. Koch, C. Palmke, T. Salthammer, T. Schripp, J. Toftum, and G. Clausen. 2017. Measurements of dermal uptake of nicotine directly from air and clothing. Indoor Air 27(2): 427–433. https://doi.org/10.1111/ina.12327.
Bennett, D. H., R. E. Moran, X. M. Wu, N. S. Tulve, M. S. Clifton, M. Colon, W. Weathers, A. Sjodin, R. Jones, and I. Hertz-Picciotto. 2015. Polybrominated diphenyl ether (PBDE) concentrations and resulting exposure in homes in California: Relationships among passive air, surface wipe and dust concentrations, and temporal variability. Indoor Air 25(2): 220–229. https://doi.org/10.1111/ina.12130.
Boedicker, E. K., E. W. Emerson, G. R. McMeeking, S. Patel, M. E. Vance, and D. K. Farmer. 2021. Fates and spatial variations of accumulation mode particles in a multi-zone indoor environment during the HOMEChem campaign. Environmental Science: Processes & Impacts 23(7): 1029–1039. https://doi.org/10.1039/d1em00087j.
Boor, B. E., J. A. Siegel, and A. Novoselac. 2013. Monolayer and multilayer particle deposits on hard surfaces: Literature review and implications for particle resuspension in the indoor environment. Aerosol Science and Technology 47(8): 831–847. https://doi.org/10.1080/02786826.2013.794928.
Brown, W. L., D. A. Day, H. Stark, D. Pagonis, J. E. Krechmer, X. Liu, D. J. Price, E. F. Katz, P. F. DeCarlo, C. G. Masoud, D. S. Wang, L. Hildebrandt Ruiz, C. Arata, D. M. Lunderberg, A. H. Goldstein, D. K. Farmer, M. E. Vance, and J. L. Jimenez. 2021. Real-time organic aerosol chemical speciation in the indoor environment using extractive electrospray ionization mass spectrometry. Indoor Air 31(1): 141–155. https://doi.org/10.1111/ina.12721.
Butt, C. M., M. L. Diamond, J. Truong, M. G. Ikonomou, P. A. Helm, and G. A. Stern. 2004a. Semivolatile organic compounds in window films from lower Manhattan after the September 11th World Trade Center attacks. Environmental Science & Technology 38(13): 3514–3524. https://doi.org/10.1021/es0498282.
Butt, C. M., M. L. Diamond, J. Truong, M. G. Ikonomou, and A. F. ter Schure. 2004b. Spatial distribution of polybrominated diphenyl ethers in southern Ontario as measured in indoor and outdoor window organic films. Environmental Science & Technology 38(3): 724–731. https://doi.org/10.1021/es034670r.
Cao, J., C. J. Weschler, J. Luo, and Y. Zhang. 2016. Cm-history method, a novel approach to simultaneously measure source and sink parameters important for estimating indoor exposures to phthalates. Environmental Science & Technology 50(2): 825–834. https://doi.org/10.1021/acs.est.5b04404.
Chase, H. M., J. Ho, M. A. Upshur, R. J. Thomson, V. S. Batista, and F. M. Geiger. 2017. Unanticipated stickiness of alphaPinene. Journal of Physical Chemistry A 121(17): 3239–3246. https://doi.org/10.1021/acs.jpca.6b12653.
Chen, C., and B. Zhao. 2011. Review of relationship between indoor and outdoor particles: I/O ratio, infiltration factor and penetration factor. Atmospheric Environment 45(2): 275–288. https://doi.org/10.1016/j.atmosenv.2010.09.048.
Collins, D. B., R. F. Hems, S. Zhou, C. Wang, E. Grignon, M. Alavy, J. A. Siegel, and J. P. D. Abbatt. 2018a. Evidence for gas-surface equilibrium control of indoor nitrous acid. Environmental Science & Technology 52(21): 12419–12427. https://doi.org/10.1021/acs.est.8b04512.
Collins, D. B., C. Wang, and J. P. D. Abbatt. 2018b. Selective uptake of third-hand tobacco smoke components to inorganic and organic aerosol particles. Environmental Science & Technology 52(22): 13195–13201. https://doi.org/10.1021/acs.est.8b03880.
DeCarlo, P. F., A. M. Avery, and M. S. Waring. 2018. Thirdhand smoke uptake to aerosol particles in the indoor environment. Science Advances 4(5): eaap8368. https://doi.org/10.1126/sciadv.aap8368.
Deming, B. L., and P. J. Ziemann. 2020. Quantification of alkenes on indoor surfaces and implications for chemical sources and sinks. Indoor Air 30(5): 914–924. https://doi.org/10.1111/ina.12662.
Diamond, M. L., J. O. Okeme, and L. Melymuk. 2021. Hands as agents of chemical transport in the indoor environment. Environmental Science & Technology Letters 8(4): 326–332. https://doi.org/10.1021/acs.estlett.0c01006.
Duigu, J. R., G. A. Ayoko, and S. Kokot. 2009. The relationship between building characteristics and the chemical composition of surface films found on glass windows in Brisbane, Australia. Building and Environment 44(11): 2228–2235. https://doi.org/10.1016/j.buildenv.2009.02.019.
Duncan, S. M., S. Tomaz, G. Morrison, M. Webb, J. Atkin, J. D. Surratt, and B. J. Turpin. 2019. Dynamics of residential water-soluble organic gases: Insights into sources and sinks. Environmental Science & Technology 53(4): 1812–1821. https://doi.org/10.1021/acs.est.8b05852.
Eftekhari, A., and G. C. Morrison. 2018. A high throughput method for measuring cloth-air equilibrium distribution ratios for SVOCs present in indoor environments. Talanta 183:250–257. https://doi.org/10.1016/j.talanta.2018.02.061.
Eichler, C. M. A., J. Cao, G. Isaacman-VanWertz, and J. C. Little. 2019a. Modeling the formation and growth of organic films on indoor surfaces. Indoor Air 29(1): 17–29. https://doi.org/10.1111/ina.12518.
Eichler, C. M. A., E. A. Cohen Hubal, and J. C. Little. 2019b. Assessing human exposure to chemicals in materials, products and articles: The international risk management landscape for phthalates. Environmental Science & Technology 53(23): 13583–13597. https://doi.org/10.1021/acs.est.9b03794.
Endo, S., and K. U. Goss. 2014. Applications of polyparameter linear free energy relationships in environmental chemistry. Environmental Science & Technology 48(21): 12477–12491. https://doi.org/10.1021/es503369t.
Fang, Y., P. S. J. Lakey, S. Riahi, A. T. McDonald, M. Shrestha, D. J. Tobias, M. Shiraiwa, and V. H. Grassian. 2019a. A molecular picture of surface interactions of organic compounds on prevalent indoor surfaces: Limonene adsorption on SiO2. Chemical Science 10(10): 2906–2914. https://doi.org/10.1039/c8sc05560b.
Fang, Y., S. Riahi, A. T. McDonald, M. Shrestha, D. J. Tobias, and V. H. Grassian. 2019b. What is the driving force behind the adsorption of hydrophilic surfaces? Journal of Physical Chemistry Letters 10(3): 468–473. https://doi.org/10.1021/acs.jpclett.8b03484.
Fortenberry, C., M. Walker, A. Dang, A. Loka, G. Date, K. Cysneiros de Carvalho, G. Morrison, and B. Williams. 2019. Analysis of indoor particles and gases and their evolution with natural ventilation. Indoor Air 29(5): 761–779. https://doi.org/10.1111/ina.12584.
Frank, E. S., H. Fan, M. Shrestha, S. Riahi, D. J. Tobias, and V. H. Grassian. 2020. Impact of adsorbed water on the interaction of limonene with hydroxylated SiO2: Implications of π-hydrogen bonding for surfaces in humid environments. Journal of Physical Chemistry A 124(50): 10592–10599. https://doi.org/10.1021/acs.jpca.0c08600.
Gewurtz, S. B., S. P. Bhavsar, P. W. Crozier, M. L. Diamond, P. A. Helm, C. H. Marvin, and E. J. Reiner. 2009. Perfluoroalkyl contaminants in window film: Indoor/outdoor, urban/rural, and winter/summer contamination and assessment of carpet as a possible source. Environmental Science & Technology 43(19): 7317–7323. https://doi.org/10.1021/es9002718.
Ham, J. E., and J. R. Wells. 2009. Surface chemistry of dihydromyrcenol (2,6-dimethyl-7-octen-2-ol) with ozone on silanized glass, glass, and vinyl flooring tiles. Atmospheric Environment 43(26): 4023–4032. https://doi.org/10.1016/j.atmosenv.2009.05.007.
Ho, J., B. T. Psciuk, H. M. Chase, B. Rudshteyn, M. A. Upshur, L. Fu, R. J. Thomson, H.-F. Wang, F. M. Geiger, and V. S. Batista. 2016. Sum frequency generation spectroscopy and molecular dynamics simulations reveal a rotationally fluid adsorption state of a-pinene on silica. The Journal of Physical Chemistry C 120(23): 12578–12589. https://doi.org/10.1021/acs.jpcc.6b03158.
Hodgson, A. T., K. Y. Ming, and B. C. Singer. 2005. Quantifying Object and Material Surface Areas in Residences. Lawrence Berkeley National Laboratory Technical Report LBNL-56786. https://doi.org/10.2172/861239.
Huang, L., E. S. Frank, M. Shrestha, S. Riahi, D. J. Tobias, and V. H. Grassian. 2021. Heterogeneous interactions of prevalent indoor oxygenated organic compounds on hydroxylated SiO2 surfaces. Environmental Science & Technology 55(10): 6623–6630. https://doi.org/10.1021/acs.est.1c00067.
Huo, C. Y., L. Y. Liu, Z. F. Zhang, W. L. Ma, W. W. Song, H. L. Li, W. L. Li, K. Kannan, Y. K. Wu, Y. M. Han, Z. X. Peng, and Y. F. Li. 2016. Phthalate esters in indoor window films in a Northeastern Chinese urban center: Film growth and implications for human exposure. Environmental Science & Technology 50(14): 7743–7751. https://doi.org/10.1021/acs.est.5b06371.
Kristensen, K., D. M. Lunderberg, Y. Liu, P. K. Misztal, Y. Tian, C. Arata, W. W. Nazaroff, and A. H. Goldstein. 2019. Sources and dynamics of semivolatile organic compounds in a single-family residence in northern California. Indoor Air 29(4): 645–655. https://doi.org/10.1111/ina.12561.
Lai, A. C., and W. W. Nazaroff. 2000. Modeling indoor particle deposition from turbulent flow onto smooth surfaces. Journal of Aerosol Science 31(4): 463–476. https://doi.org/10.1016/S0021-8502(99)00536-4.
Lakey, P. S. J., C. M. A. Eichler, C. Wang, J. C. Little, and M. Shiraiwa. 2021. Kinetic multi-layer model of film formation, growth, and chemistry (KM-FILM): Boundary layer processes, multi-layer adsorption, bulk diffusion, and heterogeneous reactions. Indoor Air 31(6): 2070–2083. https://doi.org/10.1111/ina.12854.
Li, L., J. A. Arnot, and F. Wania. 2019. How are humans exposed to organic chemicals released to indoor air? Environmental Science & Technology 53(19):11276–11284. https://doi.org/10.1021/acs.est.9b02036.
Li, M., C. J. Weschler, G. Beko, P. Wargocki, G. Lucic, and J. Williams. 2020. Human ammonia emission rates under various indoor environmental conditions. Environmental Science & Technology 54(9): 5419–5428. https://doi.org/10.1021/acs.est.0c00094.
Liang, Y., and Y. Xu. 2014. Emission of phthalates and phthalate alternatives from vinyl flooring and crib mattress covers: The influence of temperature. Environmental Science & Technology 48(24): 14228–14237. https://doi.org/10.1021/es504801x.
Licina, D., G. C. Morrison, G. Beko, C. J. Weschler, and W. W. Nazaroff. 2019. Clothing-mediated exposures to chemicals and particles. Environmental Science & Technology 53(10): 5559–5575. https://doi.org/10.1021/acs.est.9b00272.
Lim, C. Y., and J. P. Abbatt. 2020. Chemical composition, spatial homogeneity, and growth of indoor surface films. Environmental Science & Technology 54(22): 14372–14379. https://doi.org/10.1021/acs.est.0c04163.
Liu, C., Y. Zhang, and C. J. Weschler. 2014. The impact of mass transfer limitations on size distributions of particle associated SVOCs in outdoor and indoor environments. Science of the Total Environment 497–498:401–411. https://doi.org/10.1016/j.scitotenv.2014.07.095.
Liu, Q. T., R. Chen, B. E. McCarry, M. L. Diamond, and B. Bahavar. 2003. Characterization of polar organic compounds in the organic film on indoor and outdoor glass windows. Environmental Science & Technology 37(11): 2340–2349. https://doi.org/10.1021/es020848i.
Liu, Y., A. G. Bé, V. W. Or, M. R. Alves, V. H. Grassian, and F. M. Geiger. 2020. Challenges and opportunities in molecular-level indoor surface chemistry and physics. Cell Reports Physical Science 1:100256. https://doi.org/10.1016/j.xcrp.2020.100256.
Liu, Y., H. M. Chase, and F. M. Geiger. 2019. Partially (resp. fully) reversible adsorption of monoterpenes (resp. alkanes and cycloalkanes) to fused silica. Journal of Chemical Physics 150(7): 074701. https://doi.org/10.1063/1.5083585.
Lucattini, L., G. Poma, A. Covaci, J. de Boer, M. H. Lamoree, and P. E. G. Leonards. 2018. A review of semi-volatile organic compounds (SVOCs) in the indoor environment: Occurrence in consumer products, indoor air and dust. Chemosphere 201:466–482. https://doi.org/10.1016/j.chemosphere.2018.02.161.
Lunderberg, D. M., K. Kristensen, Y. Tian, C. Arata, P. K. Misztal, Y. Liu, N. Kreisberg, E. F. Katz, P. F. DeCarlo, S. Patel, M. E. Vance, W. W. Nazaroff, and A. H. Goldstein. 2020. Surface emissions modulate indoor SVOC concentrations through volatility-dependent partitioning. Environmental Science & Technology 54(11): 6751–6760. https://doi.org/10.1021/acs.est.0c00966.
Manuja, A., J. Ritchie, K. Buch, Y. Wu, C. M. A. Eichler, J. C. Little, and L. C. Marr. 2019. Total surface area in indoor environments. Environmental Science: Processes & Impacts 21(8): 1384–1392. https://doi.org/10.1039/c9em00157c.
Meininghaus, R., and E. Uhde. 2002. Diffusion studies of VOC mixtures in a building material. Indoor Air 12(4): 215–222. https://doi.org/10.1034/j.1600-0668.2002.01131.x.
Melymuk, L., P. Bohlin-Nizzetto, S. Vojta, M. Kratka, P. Kukucka, O. Audy, P. Pribylova, and J. Klanova. 2016. Distribution of legacy and emerging semivolatile organic compounds in five indoor matrices in a residential environment. Chemosphere 153:179–186. https://doi.org/10.1016/j.chemosphere.2016.03.012.
Morrison, G., H. Li, S. Mishra, and M. Buechlein. 2015a. Airborne phthalate partitioning to cotton clothing. Atmospheric Environment 115:149–152. https://doi.org/10.1016/j.atmosenv.2015.05.051.
Morrison, G., N. V. Shakila, and K. Parker. 2015b. Accumulation of gas-phase methamphetamine on clothing, toy fabrics, and skin oil. Indoor Air 25(4): 405–414. https://doi.org/10.1111/ina.12159.
Morrison, G. C., G. Bekö, C. J. Weschler, T. Schripp, T. Salthammer, J. Hill, A.-M. Andersson, J. Toftum, G. Clausen, and H. Frederiksen. 2017a. Dermal uptake of benzophenone-3 from clothing. Environmental Science & Technology 51(19): 11371–11379. https://doi.org/10.1021/acs.est.7b02623.
Morrison, G. C., C. J. Weschler, and G. Beko. 2017b. Dermal uptake of phthalates from clothing: Comparison of model to human participant results. Indoor Air 27(3): 642–649. https://doi.org/10.1111/ina.12354.
Morrison, G. C., C. J. Weschler, G. Beko, H. M. Koch, T. Salthammer, T. Schripp, J. Toftum, and G. Clausen. 2016. Role of clothing in both accelerating and impeding dermal absorption of airborne SVOCs. Journal of Exposure Science & Environmental Epidemiology 26(1): 113–118. https://doi.org/10.1038/jes.2015.42.
Nazaroff, W. W., and G. R. Cass. 1987. Particle deposition from a natural convection flow onto a vertical isothermal flat plate. Journal of Aerosol Science 18(4): 445–455. https://doi.org/10.1016/0021-8502(87)90042-5.
Nazaroff, W. W., and C. J. Weschler. 2020. Indoor acids and bases. Indoor Air 30(4): 559–644. https://doi.org/10.1111/ina.12670.
O’Brien, R. E., Y. Li, K. J. Kiland, E. F. Katz, V. W. Or, E. Legaard, E. Q. Walhout, C. Thrasher, V. H. Grassian, P. F. DeCarlo, A. K. Bertram, and M. Shiraiwa. 2021. Emerging investigator series: Chemical and physical properties of organic mixtures on indoor surfaces during HOMEChem. Environmental Science: Processes & Impacts 23(4): 559–568. https://doi.org/10.1039/d1em00060h.
Ongwandee, M., and P. Sawanyapanich. 2012. Influence of relative humidity and gaseous ammonia on the nicotine sorption to indoor materials. Indoor Air 22(1): 54–63. https://doi.org/10.1111/j.1600-0668.2011.00737.x.
Or, V. W., M. R. Alves, M. Wade, S. Schwab, R. L. Corsi, and V. H. Grassian. 2018. Crystal clear? Microspectroscopic imaging and physicochemical characterization of indoor depositions on window glass. Environmental Science & Technology Letters 5(8): 514–519. https://doi.org/10.1021/acs.estlett.8b00355.
Or, V. W., M. Wade, S. Patel, M. R. Alves, D. Kim, S. Schwab, H. Przelomski, R. O’Brien, D. Rim, R. L. Corsi, M. E. Vance, D. K. Farmer, and V. H. Grassian. 2020. Glass surface evolution following gas adsorption and particle deposition from indoor cooking events as probed by microspectroscopic analysis. Environmental Science: Processes & Impacts 22:1698–1709. https://doi.org/10.1039/d0em00156b.
Pan, S. H., J. Li, T. Lin, G. Zhang, X. D. Li, and H. Yin. 2012. Polycyclic aromatic hydrocarbons on indoor/outdoor glass window surfaces in Guangzhou and Hong Kong, south China. Environmental Pollution 169:190–195. https://doi.org/10.1016/j.envpol.2012.03.015.
Parker, K., and G. Morrison. 2016. Methamphetamine absorption by skin lipids: accumulated mass, partition coefficients, and the influence of fatty acids. Indoor Air 26(4):634–641. https://doi.org/10.1111/ina.12229.
Parnis, J. M., T. Taskovic, A. K. D. Celsie, and D. Mackay. 2020. Indoor dust/air partitioning: Evidence for kinetic delay in equilibration for low-volatility SVOCs. Environmental Science & Technology 54(11): 6723–6729. https://doi.org/10.1021/acs.est.0c00632.
Rauert, C., and S. Harrad. 2015. Mass transfer of PBDEs from plastic TV casing to indoor dust via three migration pathways—A test chamber investigation. Science of the Total Environment 536:568–574. https://doi.org/10.1016/j.scitotenv.2015.07.050.
Rubasinghege, G., and V. H. Grassian. 2013. Role(s) of adsorbed water in the surface chemistry of environmental interfaces. Chemical Communications 49(30): 3071–3094. https://doi.org/10.1039/c3cc38872g.
Saini, A., C. Rauert, M. J. Simpson, S. Harrad, and M. L. Diamond. 2016. Characterizing the sorption of polybrominated diphenyl ethers (PBDEs) to cotton and polyester fabrics under controlled conditions. Science of the Total Environment 563–564:99–107. https://doi.org/10.1016/j.scitotenv.2016.04.099.
Salthammer, T., and K. U. Goss. 2019. Predicting the gas/particle distribution of SVOCs in the indoor environment using poly parameter linear free energy relationships. Environmental Science & Technology 53(5): 2491–2499. https://doi.org/10.1021/acs.est.8b06585.
Schripp, T., C. Fauck, and T. Salthammer. 2010. Chamber studies on mass-transfer of di(2-ethylhexyl)phthalate (DEHP) and di-n-butylphthalate (DnBP) from emission sources into house dust. Atmospheric Environment 44(24): 2840–2845. https://doi.org/10.1016/j.atmosenv.2010.04.054.
Sheu, R., C. Stonner, J. C. Ditto, T. Klupfel, J. Williams, and D. R. Gentner. 2020. Human transport of thirdhand tobacco smoke: A prominent source of hazardous air pollutants into indoor nonsmoking environments. Science Advances 6(10): eaay4109. https://doi.org/10.1126/sciadv.aay4109.
Shu, S., and G. C. Morrison. 2011. Surface reaction rate and probability of ozone and alpha-terpineol on glass, polyvinyl chloride, and latex paint surfaces. Environmental Science & Technology 45(10): 4285–4292. https://doi.org/10.1021/es200194e.
Sinclair, J. D., L. A. Psota-Kelty, C. J. Weschler, and H. C. Shields. 1990. Deposition of airborne sulfate, nitrate, and chloride salts as it relates to corrosion of electronics. Journal of the Electrochemical Society 137:1200–1206. https://doi.org/10.1149/1.2086631.
Sleiman, M., L. A. Gundel, J. F. Pankow, P. Jacob, 3rd, B. C. Singer, and H. Destaillats. 2010. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proceedings of the National Academy of Sciences USA 107(15): 6576–6581. https://doi.org/10.1073/pnas.0912820107.
Stokes, G. Y., A. M. Buchbinder, J. M. Gibbs-Davis, K. A. Scheidt, and F. M. Geiger. 2008. Heterogeneous ozone oxidation reactions of 1-pentene, cyclopentene, cyclohexene, and a menthenol derivative studied by sum frequency generation. Journal of Physical Chemistry A 112(46): 11688–11698. https://doi.org/10.1021/jp803277s.
Stokes, G. Y., E. H. Chen, S. R. Walter, and F. M. Geiger. 2009. Two reactivity modes in the heterogeneous cyclohexene ozonolysis under tropospherically relevant ozone-rich and ozone-limited conditions. Journal of Physical Chemistry A 113(31): 8985–8993. https://doi.org/10.1021/jp904104s.
Thatcher, T. L., A. C. Lai, R. Moreno-Jackson, R. G. Sextro, and W. W. Nazaroff. 2002. Effects of room furnishings and air speed on particle deposition rates indoors. Atmospheric Environment 36(11): 1811–1819. https://doi.org/10.1016/S1352-2310(02)00157-7.
Tichenor, B., Z Guo, J. Dunn, L. Sparks, and M A. Mason. 1991. The interaction of vapour phase organic compounds with indoor sinks. Indoor Air 1(1): 23–35. https://doi.org/10.1111/j.1600-0668.1991.03-11.x.
Uhde, E., D. Varol, B. Mull, and T. Salthammer. 2019. Distribution of five SVOCs in a model room: Effect of vacuuming and air cleaning measures. Environmental Science: Processes & Impacts 21(8): 1353–1363. https://doi.org/10.1039/c9em00121b.
U.S. Census. 2021. American Housing Survey. https://www.census.gov/programs-surveys/ahs.html.
Van Loy, M. D., W. W. Nazaroff, and J. M. Daisey. 1998. Nicotine as a marker for environmental tobacco smoke: Implications of sorption on indoor surface materials. Journal of the Air & Waste Management Association 48(10): 959–968. https://doi.org/10.1080/10473289.1998.10463742.
Venier, M., O. Audy, S. Vojta, J. Becanova, K. Romanak, L. Melymuk, M. Kratka, P. Kukucka, J. Okeme, A. Saini, M. L. Diamond, and J. Klanova. 2016. Brominated flame retardants in the indoor environment—Comparative study of indoor contamination from three countries. Environment International 94:150–160. https://doi.org/10.1016/j.envint.2016.04.029.
Wallace, L. A., W. R. Ott, C. J. Weschler, and A. C. K. Lai. 2017. Desorption of SVOCs from heated surfaces in the form of ultrafine particles. Environmental Science & Technology 51(3): 1140–1146. https://doi.org/10.1021/acs.est.6b03248.
Wang, C., B. Bottorff, E. Reidy, C. M. F. Rosales, D. B. Collins, A. Novoselac, D. K. Farmer, M. E. Vance, P. S. Stevens, and J. P. D. Abbatt. 2020a. Cooking, bleach cleaning, and air conditioning strongly impact levels of HONO in a house. Environmental Science & Technology 54(21): 13488–13497. https://doi.org/10.1021/acs.est.0c05356.
Wang, C., D. B. Collins, C. Arata, A. H. Goldstein, J. M. Mattila, D. K. Farmer, L. Ampollini, P. F. DeCarlo, A. Novoselac, M. E. Vance, W. W. Nazaroff, and J. P. D. Abbatt. 2020b. Surface reservoirs dominate dynamic gas-surface partitioning of many indoor air constituents. Science Advances 6(8): eaay8973. https://doi.org/10.1126/sciadv.aay8973.
Wang, C., T. Yuan, S. A. Wood, K. U. Goss, J. Li, Q. Ying, and F. Wania. 2017. Uncertain Henry’s law constants compromise equilibrium partitioning calculations of atmospheric oxidation products. Atmospheric Chemistry and Physics 17(12): 7529–7540. https://doi.org/10.5194/acp-17-7529-2017.
Watkins, D. J., M. D. McClean, A. J. Fraser, J. Weinberg, H. M. Stapleton, and T. F. Webster. 2013. Associations between PBDEs in office air, dust, and surface wipes. Environ Int 59:124–132. https://doi.org/10.1016/j.envint.2013.06.001.
Wei, W., C. Mandin, and O. Ramalho. 2018. Influence of indoor environmental factors on mass transfer parameters and concentrations of semi-volatile organic compounds. Chemosphere 195:223–235. https://doi.org/10.1016/j.chemosphere.2017.12.072.
Weschler, C. J. 1980. Characterization of selected organics in size-fractionated indoor aerosols. Environmental Science & Technology 14(4): 428–431. https://doi.org/10.1021/es60164a008.
Weschler, C. J., G. Beko, H. M. Koch, T. Salthammer, T. Schripp, J. Toftum, and G. Clausen. 2015. Transdermal uptake of diethyl phthalate and di(n-butyl) phthalate directly from air: Experimental verification. Environmental Health Perspectives 123(10): 928–934. https://doi.org/10.1289/ehp.1409151.
Weschler, C. J., and W. W. Nazaroff. 2008. Semivolatile organic compounds in indoor environments. Atmospheric Environment 42(40): 9018–9040. https://doi.org/10.1016/j.atmosenv.2008.09.052.
Weschler, C. J., and W. W. Nazaroff. 2010. SVOC partitioning between the gas phase and settled dust indoors. Atmospheric Environment 44(30): 3609–3620. https://doi.org/10.1016/j.atmosenv.2010.06.029.
Weschler, C. J., and W. W. Nazaroff. 2012. SVOC exposure indoors: Fresh look at dermal pathways. Indoor Air 22(5): 356–377. https://doi.org/10.1111/j.1600-0668.2012.00772.x.
Weschler, C. J., and W. W. Nazaroff. 2017. Growth of organic films on indoor surfaces. Indoor Air 27(6): 1101–1112. https://doi.org/10.1111/ina.12396.
Won, D., R. L. Corsi, and M. Rynes. 2001. Sorptive interactions between VOCs and indoor materials. Indoor Air 11(4): 246–256. https://doi.org/10.1034/j.1600-0668.2001.110406.x.
Wu, Y., C. M. Eichler, W. Leng, S. S. Cox, L. C. Marr, and J. C. Little. 2017. Adsorption of phthalates on impervious indoor surfaces. Environmental Science & Technology 51(5): 2907–2913. https://doi.org/10.1021/acs.est.6b05853.
Zhou, X., J. Lian, Y. Cheng, and X. Wang. 2021. The gas/particle partitioning behavior of phthalate esters in indoor environment: Effects of temperature and humidity. Environmental Research 194:110681. https://doi.org/10.1016/j.envres.2020.110681.




















