5
Management of Chemicals in Indoor Environments
Effective management of chemicals in the indoor environment is critical to human health. This chapter considers management approaches to the control of pollutants in indoor air as well as the indoor chemistry associated with these management approaches. The chapter provides an overview of the hierarchy of controls as a framework for considering risk-reduction strategies. With this framework in mind, the committee first considers management approaches that result in minimal changes in indoor chemistry, followed by management approaches that rely on chemical transformations. This chapter also discusses environmental factors, human behavior, and other management considerations. In addition, the current state of design standards addressing management of chemicals is noted where applicable. Finally, the committee summarizes key knowledge gaps and recommendations for future research. The chapter is not intended to be an exhaustive review of methods for modifying indoor chemistry; instead, the committee’s goal is to highlight general approaches that may be considered, especially recent findings related to removal approaches. This chapter not only focuses on gas and particle removal but also provides insights into the impact of surface cleaning and pathogen inactivation on indoor air quality.
TYPES OF CONTROL
Approaches to the management of chemicals in the indoor environment are generally consistent with the industrial hygiene hierarchy of controls (Table 5-1) for chemical exposure (Schulte et al., 2013). This hierarchy of controls (Figure 5-1) includes elimination of the hazard, substitution of the hazard, engineering controls, administrative controls, and personal protective equipment (PPE)—each control approach has various advantages and disadvantages. Each control method can have different levels of success, with complete elimination being considered the most effective control. It should be noted that not every type of control may be feasible in a given situation, and the hierarchy of effectiveness is subject to variation. Additionally, some controls may be classified in more than one way. For example, elimination of indoor smoking in public buildings can be viewed both as source control and an administrative control depending on one’s perspective.
TABLE 5-1 Hierarchy of Controls in the Context of Indoor Chemistry
| Control Methodology | Examples | Comments |
|---|---|---|
| Elimination of the hazard | Elimination of lead paint use in indoor environments. | Often easiest to implement in the design or development stage of a process. Afterward, changes in equipment and procedures may be required to eliminate or substitute for a hazard. |
| Removal of flame retardants from furniture or clothing manufacturing. | ||
| Substitution | Reformulating cosmetic nail products to replace toxic or irritating ingredients. | |
| Engineering controls | Local exhaust including the use of cooking stove hoods. General dilution ventilation systems (e.g., HVAC and HEPA systems). Air cleaning using particle filters. Simultaneous heating and exhausting a building to increase chemical emission rates prior to building occupancy (“bake-out”). Installation of vapor barriers to reduce intrusion of VOCs or radon into a building. | Exhaust hoods are designed to remove the hazard at the source. |
| HVAC systems can dilute or filter air contaminants to reduce hazard. | ||
| Administrative controls | Educating consumers about chemical sources and their effects and operation of the ventilation system. | Administrative controls and PPE may be needed when hazards are poorly controlled. |
| Placing occupancy limits or other management rules in occupational settings. | ||
| PPE | Use of respirators and other PPE. |
NOTE: HEPA = high-efficiency particulate air; HVAC = heating, ventilation, and air conditioning; PPE = personal protective equipment; VOC = volatile organic compound.
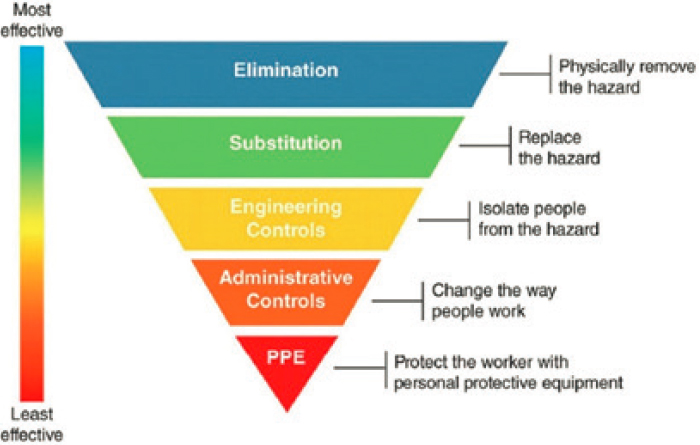
NOTE: PPE = personal protective equipment. SOURCE: NIOSH (2018).
Elimination of indoor contaminants could involve the removal of a chemical from a consumer product or another material, eliminating high emission rate sources (e.g., ozone generator). Elimination is also a viable approach to manage those chemicals (e.g., air fresheners or fragrances) found indoors that are introduced by human activity. Several specialized environments rely on eliminating chemicals of concern from construction materials. For example, the National Aeronautics and Space Administration has developed test procedures for the evaluation of off-gassing of chemicals from materials that could be used onboard the International Space Station or the Orion Multipurpose Crew Vehicle (NASA, 2011). Likewise, the U.S. Navy evaluates off-gassing from materials used in the construction of submarine decks (NAVSEA System Communications, 2016). Only deck materials that pass the testing requirements are permitted for subsequent use on a submarine. Both of these environments have limited access to replacement air and are associated with continuous exposure of occupants, making elimination of potentially hazardous materials from these specialized environments of paramount importance. The next most effective control method is substitution, which is the replacement of a chemical of concern found in a consumer product, furnishing, construction material, or other product with a less hazardous chemical. Because indoor chemistry is complex and often incompletely understood, caution is needed with this approach—replacement chemicals may contribute to unforeseen chemistry or later be found to be equally or more hazardous than the original chemical. Frameworks to guide the selection of alternative chemicals are available and need to be applied to decision making to reduce the likelihood of a regrettable substitution (NRC, 2014).
Engineering controls, such as mechanical ventilation or general exhaust systems that bring in outdoor air, filtration of gas-phase and particulate contaminants, and other types of air cleaners, further reduce the risk when elimination or substitution of a chemical hazard is not practical or incompletely mitigates the risk. Ventilation, exhaust, and filtration are commonly combined in heating, ventilation, and air-conditioning (HVAC) systems that also heat, cool, humidify, and dehumidify indoor spaces. Local exhausts, such as range hoods, control emissions from cooking at the source. For most offices and other non-manufacturing building work environments, these approaches have historically been the primary method to control indoor air quality (Woods, 1991). To reduce the entry of volatile organic compounds (VOCs) or radon through cracks or other defects in foundation slabs or basement walls into an overlying building (i.e., vapor intrusion), engineering controls include installation of fans, blowers, or physical and chemical barriers (EPA, 2015; Khan et al., 2019; Verginelli et al., 2017). Engineering controls seek to isolate and remove potentially hazardous material from the indoor environment.
Administrative controls include a range of regulations, policies, and procedures that limit exposure but require compliance on the part of the person. Reducing chemical exposure by altering human activity patterns can be an effective administrative control. For example, this could be accomplished by changing the application times for building maintenance chemicals and services (e.g., pesticide applications and air handler unit cleaning) to times that buildings are unoccupied. Other administrative controls include product safety labeling and training of end users to reduce chemical misuse. Another administrative control is the use of warning alarms (e.g., carbon monoxide or radon detectors) indoors.
PPE is considered the least effective of all control measures for chemical exposure in the workplace because it relies most heavily on individual compliance. Effective use of PPE requires individuals to be trained on its proper use and limitations. Well-fit respirators and other PPE offer direct protection for people who work with specific chemicals of concern. PPE is often used in occupational settings (Keer et al., 2018), with specialized masks for reducing exposure to paint fumes, volatile solvents, cleaning products, and other chemicals in a non-occupational setting. Masks and respirators can be effective for reducing exposure to some gases and particulate matter (PM), and the pollutants and pathogens associated with that PM.
However, no classification system is perfect and that this system is not always applicable. For example, while masks and respirators are typically considered PPE in terms of chemical exposure, they are more effectively considered source control in the case of exhaled airborne pathogens and thus are an engineering control.
MANAGEMENT THROUGH CAPTURE AND REMOVAL
Air contaminants can be removed, decomposed, or otherwise inactivated by ventilation, filtration, sorption, physical cleaning, and passive surface removal. These approaches have different levels of efficiency for removing gas- and particle-phase contaminants and are most effective for controlling risk when used in combination for comprehensive improvement of indoor air quality (i.e., the “swiss cheese model”). This section briefly describes each approach and summarizes its potential impacts on indoor chemistry.
Ventilation
For the purposes of this chapter, ventilation is defined as “intentional introduction of air from the outdoors into a building,” primarily for the purpose of removing indoor air contaminants (ASHRAE, 2021). In some cases, ventilation air may be brought into a building without conditioning, but often ventilation air is heated, cooled, dehumidified, or humidified to indoor conditions, which is a major energy demand on building HVAC systems. This energy load can cause building managers or homeowners to reduce usage and lower ventilation rates.
Ventilation is the primary control for indoor air quality in most buildings, and, in principle, it can remove any type of indoor air contaminant that can be entrained in air. Removal by ventilation of volatile pollutants from continuous sources results in dilution and a temporary reduction in their indoor concentrations (Hult et al., 2015) but cannot reduce concentrations to zero. Once ventilation ceases, many pollutants may return to their pre-ventilation values (Wang et al., 2020). This is generally the case for VOCs emitted from building materials. Thus, while ventilation can increase dilution-driven emission rates of semivolatile compounds, the efficacy of this process for surface cleaning is unlikely to be substantial. However, pre-occupancy ventilation, sometimes combined with use of HVAC systems to raise the indoor temperature, could help to remove contaminants emitted during the curing of various building materials, resulting in lower indoor concentrations at occupancy (Kang et al., 2010; Kim et al., 2008). Particle filtration (see the next section) is incorporated into ventilation systems to remove both particles from the outdoor air being brought in and particles from indoor sources. In addition to being a source of particle-phase contaminants, ventilation air also brings in ozone and other ambient air pollutants from outdoors, potentially degrading indoor air quality and promoting undesirable chemical transformations (see Chapter 4).
Ventilation rates can affect indoor chemistry in multiple ways. Higher air change rates increase the rate at which contaminants in outdoor air, such as ozone and PM, enter a building, but they also shorten the average residence time of air indoors, which reduces the time available for reactions to occur in air. Thus, the consequence of increased ventilation rates may be both lower concentrations of indoor contaminants and reaction products and higher concentrations of outdoor contaminants (Weschler and Shields, 2000). On the other hand, surface chemistry is less affected. Moreover, the type of ventilation system may impact chemical reactions between outdoor and indoor air. Some systems do not recirculate indoor air (i.e., they supply 100 percent outdoor air to conditioned spaces and remove an approximately equal amount of air). Other types of systems bring in outdoor air and mix it with a generally much larger volume flow rate of recirculated indoor air, which is then conditioned to heat or cool. This may have an effect on indoor chemistry because ozone-containing outdoor air mixes with and can react with recirculated indoor air in the HVAC system before the mixture is supplied to occupied spaces.
The effectiveness of ventilation is greatly influenced by how outdoor air is brought into a building and distributed within spaces. Some buildings have “natural ventilation,” in which air enters through openings in the building enclosure that may be engineered for that purpose or through operable windows (Izadyar et al., 2020). Natural ventilation via operable windows is more common in single-family residences and older buildings in the United States. Natural ventilation flow rate can be highly variable and depends on the placement and size of openings as well as the magnitude of driving forces due to wind and temperature differences (Li and Delsante, 2001). Larger, naturally ventilated buildings may have openings that are actively controlled (Saber et al., 2021). A primary reason for using natural ventilation is to reduce the energy used for cooling while maintaining occupant comfort and indoor air quality. Because natural ventilation could result in direct introduction of outdoor air into a building without any filtration or air treatment, its use in polluted areas raises serious concerns about impacts on indoor air quality (Chen et al., 2019).
Most new nonresidential buildings, and an increasing number of residential buildings, have “mechanical ventilation,” which utilizes fans to draw outdoor air into the building. In some cases, 100 percent outdoor air is delivered to spaces via ductwork; in others, it is mixed with recirculating air before being distributed. The most common approach for room air distribution from mechanical systems is to mix it thoroughly with indoor air to dilute contaminants to a relatively uniform concentration. Stratified ventilation pushes contaminated air upward and out of the occupied zone to be removed at the top of the space. In theory, this more effectively removes contaminants for the same ventilation rate as mixing ventilation (Arghand et al., 2015; ASHRAE, 2021; Fathollahzadeh et al., 2015). Personal ventilation supplies ventilation air directly to occupants, achieving further increases in efficiency. Our understanding of the influence of these ventilation approaches on exposure that results from indoor chemistry is still developing.
Ventilation rates are set by building codes that rely on consensus standards, such as ASHRAE Standard 62.1, to achieve “acceptable” indoor air quality. The current definition of “acceptability” could be described as striving for safety with respect to air contaminants with adverse health effects (but excluding allergens, pathogens, and other biocontaminants) and occupant satisfaction in terms of odor control (80 percent “not dissatisfied” in ASHRAE Standard 62.1). Based on extensive experimental studies of response to body odor, an outdoor air flow rate of roughly 7.5 L/s (15 ft3/min) per sedentary adult is required to achieve a rate of 20 percent of persons dissatisfied with air quality (Fanger and Berg-Munch, 1983). Although ASHRAE standards are the basis of codes primarily in the United States, ventilation standards used in other countries are similar in most respects, so ASHRAE standards are used as examples of the state of practice throughout this chapter (see Box 5-1). Historically, ventilation rates set in building regulations have at times been both much higher, when infection prevention was a consideration, and much lower, when influenced by the desire to reduce the energy consumption of buildings. As a result, minimum ventilation rates have varied by a factor of six since the 1830s (Janssen, 1999). Consideration of indoor chemistry has rarely entered into, or influenced, the standard-setting process. Very low ventilation rates adopted in the United States circa 1980 (as low as 2.5 L/s or 5 ft3/min per person) were in a range correlated with high incidence of sick building syndrome symptoms and contemporaneous with the emergence of sick building syndrome as a significant indoor air quality problem (Fisk et al., 2009). Although many factors, both social and environmental, may contribute to sick building syndrome, inadequate ventilation rate (a surrogate for high exposures to contaminants) has been suggested by multiple studies. Compliance with ventilation codes is mainly prescriptive, meaning that acceptable indoor air quality is assumed if requirements for ventilation rates are met; however, performance-based approaches that require documentation of control of specific contaminants also exist and could predominate in the future. Design of mechanical ventilation and HVAC systems to meet applicable codes is necessary but not sufficient for ensuring healthy indoor environments: by setting the thermostat, turning HVAC systems on or off, and opening or closing windows, building occupants and facility managers can exert strong influence over how both mechanical and natural ventilation are utilized in the spaces they occupy (see Box 5-2).
The role of ventilation in mitigating risk of respiratory infection transmission has been widely discussed during the COVID-19 pandemic. Ventilation lowers the airborne concentration of infectious aerosols. Risk is related to the quantity of pathogen-containing air exhaled by an infected person that is inhaled by a susceptible one. Rudnick and Milton (2003) derived a relationship between secondary cases of an airborne disease and the rebreathed fraction of air (air inhaled that was previously exhaled by another person) and showed that it could be related to indoor carbon dioxide concentration because occupants are indoor carbon dioxide sources. This approach has also been applied to assessment of COVID-19 infection risk (Peng and Jimenez, 2021). A typical approach is to determine the critical rebreathed fraction resulting in a basic reproductive number, R0, of 1. The basic reproductive number is the number of new infections resulting from a given
case and is a key parameter in the spread of an epidemic. Theoretically, if R0 is greater than 1, an epidemic will spread at a rate that increases with R0; if it is less than 1, it will die out. From the critical rebreathed air fraction, the necessary ventilation rate can be determined, as well as the resulting indoor carbon dioxide concentration that can be used in ventilation system control.
Outdoor air is a source of contaminants to the indoor environment, creating caveats for ventilation. Outdoor air has to meet certain minimum criteria to be considered suitable for use. For example, ASHRAE Standard 62.1 provides criteria for nonresidential buildings during regionally elevated ozone events. However, these standards do not apply to residential buildings and could be challenging to implement. Similarly, ASHRAE standards require enhanced particle filtration of outdoor air in nonresidential buildings when regional PM10 or PM2.5 exceed national maximum standards, but such practices are neither required nor logistically straightforward in residential buildings. Adding these filtration or sorption controls is straightforward for mechanical ventilation systems but may be difficult in naturally ventilated buildings, particularly those that rely on windows to admit outdoor air. Furthermore, the extent to which nonresidential buildings adhere to these standards requires consideration when evaluating exposure. Extreme pollution events introduce pollution episodically, but residential environments typically rely on occupants to implement mitigation approaches. For example, wildfire smoke occurs sporadically and can have substantial impacts on indoor air quality and human exposure to pollutants. Building resilience to such events is important. However, modifying buildings to reduce outdoor air pollution is an engineering control, while reduction of outdoor air pollution, including both background and episodic events, constitutes removal of the hazard; improving outdoor air quality is thus consistent with the hierarchy of controls.
Filtration
Filters used in indoor air quality applications remove a wide range of particles from the air from indoor and outdoor sources (described in detail in Chapter 2). The range of sizes of these particles can cover at least four orders of magnitude, from less than 0.01 to more than 100 micrometers (μm). Not only are larger particles easier to filter but they also settle out of the air (i.e., deposit) more rapidly than smaller particles. Particles of greatest concern are those with adverse health effects because of size or chemical composition. Exposure to PM is associated with premature death, cardiac arrhythmias and heart attacks, and respiratory effects including asthma and bronchitis (Anderson et al., 2012). Translocation of inhaled ultrafine particles to the brain and other tissues may also contribute to systemic disease (Schraufnagel, 2020). Mechanical filtration is most commonly used to remove smaller particles from air in indoor air quality applications; however, electrostatic capture is also used, either independently (electrostatic precipitation) or as an enhancement to mechanical filtration.
Mechanical filters remove particles by capturing them on filter media as air passes through. Most filter media are mats of randomly oriented fibers of glass, synthetic, metal, and other materials. Open cell foam is also used as a filter media. Depending upon particle size and mass, particle capture results from a number of mechanisms. Large particles may deviate from the flow path of air moving around a fiber and be captured by impaction. Particles may also be captured by impingement if the flow path they are following brings them within less than one particle radius from the filter surface. Both impingement and impaction efficiency (i.e., fraction of particles removed on a single pass) decrease as particle size decreases. For smaller particles, diffusion becomes the predominant mechanism of capture and increases in efficiency as particle size decreases. Ultimately, a typical mechanical filter has its highest efficiency for the largest and smallest particles with a lower efficiency in an intermediate size range, generally 0.1–1 μm.
The performance of mechanical filters varies depending on several factors, including material, density, thickness, and flow rate, and is measured most commonly using ASHRAE Standard 52.2,
which tests filters with particles of different sizes and rates them based on the minimum single-pass efficiency within each of three size ranges: 0.3–1, 1–3, and 3–10 μm (ASHRAE, 2017). Minimum Efficiency Reporting Value (MERV) varies from 1 to 16, with a higher MERV indicating better performance. The minimum requirement for nonresidential, non-health care buildings in ASHRAE Standard 62.1 is MERV 8, which has no minimum requirement for 0.3–1 μm particles, 20 percent for 1–3 μm, and 70 percent for 3–10 μm. Because of their low efficiency in the PM2.5 range, MERV 6 and 8 filters do little to remove the fine particles associated with health effects. Their primary purpose is to remove larger particles that can foul HVAC equipment. Studies of the potential impact of more efficient filtration on mortality have found that use of higher-efficiency filters could yield large annual economic benefits from morbidity and mortality reductions and reduce incidence of airborne respiratory diseases, such as seasonal influenza (Azimi and Stephens, 2013).
Different standards are used to rate higher-efficiency filters, generally with a single particle size that is near the most penetrating size. High-efficiency particulate air (HEPA) filters are used in small air cleaners, clean rooms, laboratories, and health care spaces designed to isolate or protect patients. While there is some variation across different standards that define multiple levels of HEPA and ultra-high efficiency performance, the definition of a HEPA filter is one that removes at least 99.97 percent of 0.3 μm particles (roughly the most penetrating particle size) (White, 2009). A widely used standard for high performance filters is published by the Institute of Environmental Sciences and Technology (IEST Contamination Control Division, 2016).
The performance of some mechanical filters is enhanced by placing an electrostatic charge on filter fibers during the manufacturing process (electret filters). This enhances the performance of the filter by attracting charged particles in the air stream. The benefit of charging mechanical filters is that a desired performance level can be achieved with a thinner filter that has lower resistance to flow. The use of electret filters is controversial, however, as they may rapidly lose their charge and perform at much lower levels (Lee and Kim, 2020), while other studies indicate that stable performance can be achieved through selection of appropriate filter materials (Cai et al., 2020).
Electrostatic precipitators (ESPs) rely solely on electrostatic forces to remove particles from the air. While mechanical filtration is most common, ESPs are used in both commercial and residential buildings. Typically, a high voltage is applied between wires or pins and collector plates. Corona discharge from the wires ionizes the air and charges particles in the air stream, which move transversely to the air stream and deposit on the collector plates. Because the corona discharge in ESPs may produce ozone (Poppendieck et al., 2014), they need to be tested if intended for indoor use. ESP performance may be adversely affected by fouling. Experiments in which siloxanes (found in a number of personal care products) are present in air created silicon oxide deposits on positive corona discharge wires that could reduce particle collection efficiency (Davidson and McKinney, 1998). Performance of ordinary mechanical filters can also be enhanced by ionizing air upstream of the filter to create charged particles.
Because they collect particles rather than destroy them, mechanical filters need to be replaced periodically when they reach their maximum loading. An additional motivation for regular maintenance is the potential for filters to become sources of indoor pollutants. Particles captured on filter media continue to be exposed to recirculating indoor air and, consequently, can themselves become chemical pollution sources, contributing to odors (Bekö et al., 2004, 2007; Hyttinen et al., 2001; Pasanen et al., 1994; Pejtersen, 1996; Schleibinger and Rüden, 1999) and other adverse effects (Bekö et al., 2004, 2007; Lin and Chen, 2014; Sidheswaran et al., 2013; Siegel, 2016). Additionally, because filter materials or the particles they collect may constitute a food source for bacteria and fungi, it is possible for growth to occur on filters when sufficient moisture is present, creating further potential for filters to be a secondary source of indoor air pollution (Forthomme et al., 2014; Perrier et al., 2008). This is a practical concern of sufficient importance, and use of antimicrobial materials to control growth has been considered (Foarde and Hanley, 2001; Verdenelli et al., 2003).
Antimicrobial coatings also have been shown to reduce airborne microbial levels (Watson et al., 2022) but may or may not contribute to the chemical burden of indoor air.
Sorption
Sorbents are materials with high surface area that have a high capacity for adsorbing (or sometimes chemically reacting with) gas-phase contaminants. As an optional component of HVAC systems, sorbent filters may be included to help control odors and reduce VOC levels. Both physical and chemical sorption are used, with activated carbon being the primary physical adsorbent. Permanganate-impregnated alumina is a commercialized chemically sorbent medium (Han et al., 2017). Activated carbon may be impregnated with other materials to increase its effectiveness. The capacity of sorbents to hold various common air contaminants varies with the material; for example, activated carbon has a much higher capacity to adsorb toluene than permanganate-impregnated alumina but a significantly lower ability to adsorb formaldehyde (Spengler et al., 2001). All sorbents require maintenance as their capacity eventually becomes depleted and they need to be replaced. Quantified breakthrough times for formaldehyde at typical indoor concentrations with no competition from other chemicals through activated carbon filters is typically between 50 and 2,000 hours, depending on loading rates and activated carbon type (Ligotski et al., 2019; Zhu et al., 2019). Temperature and relative humidity can also impact sorbent capacity, with a 35 percent increase in relative humidity decreasing breakthrough times for perchloroethylene on activated carbon by a factor of two (ASTM D5160).
Some materials categorized as sorbents can be more accurately described as chemically transforming materials. For example, permanganate is an oxidant that can reduce concentrations of some VOCs; however, its potential to generate unwanted oxidized products requires more research. In addition to the impact of the sorbent medium, a number of other factors can affect sorbent performance, especially the concentration of chemicals in the air, composition of contaminants, and humidity. In general, low concentration, presence of competing species, and higher humidity all reduce sorbent effectiveness (Spengler et al., 2001; Underhill, 2001). In most HVAC systems, ventilation provides the only active means of removing gas-phase chemical contaminants from occupied spaces, with the possible exception of ambient ozone. Activated carbon can be used to remove ozone and is recommended by ASHRAE Standard 62.1 in circumstances where outdoor ozone levels exceed specified thresholds (Aldred et al., 2016a,b). By reducing indoor ozone concentrations, application of activated carbon filtration can therefore reduce ozone-initiated chemistry indoors (see Chapter 4).
Physical Cleaning of Surfaces
As described in Chapters 2, 3, and 4, surfaces play an important role in the chemical state of the indoor environment, leading to a variety of exposure mechanisms. Not only does touching surfaces or ingesting dust lead to chemical exposure but the gas-phase levels of many indoor air pollutants are controlled by partitioning interactions with a much larger quantity of those chemicals on the surfaces (see Chapter 3). Thus, a crucial point is that physical cleaning is important for lowering not only dermal and ingestion exposure but also inhalation exposure.
A number of processes occur with physical washing, with many relying on dissolving contaminants into the cleaning agent. For example, if the cleaning agent is water or water-based, water-soluble contaminants are easily removed. These include species such as small acids and bases, as well as highly oxygenated organics. If the cleaning agent is acidic (e.g., vinegar) or basic (e.g., ammonia-based), then it will be especially effective at removing basic and acidic molecules, respectively. An example is from cooking where basic amines can be formed. It is expected that
these species will be removed using an acidic cleaner. However, water-based cleaners are not effective at removing nonpolar, hydrocarbon-like materials, such as cooking oils. As a result, physical cleaning often employs surfactant-containing materials that are able to solubilize such materials.
While the examples above rely on solubility to remove surface pollutants, other classes of cleaning agents have chemically active agents that are typically designed to oxidize contaminants. Common examples are chlorine-based bleach and hydrogen peroxide solutions. Both of these cleaners contain strong oxidizing agents that are particularly effective against microorganisms but also drive complex chemical reactions on the surface, as described in Chapter 4. Importantly, while the primary contaminant may be effectively destroyed by cleaning with such agents, chemical transformation products are left on the surface and may partition into the gas phase (Collins and Farmer, 2021). In many cases, it is not known whether these products are more or less toxic than the original target. In addition, the unused cleaning agent remains on the surface and may partition to the gas phase. For example, Wong et al. (2017) demonstrated that repeated washing of a floor with chlorine bleach solution led to progressively more and more hypochlorous acid (HOCl) gas added to the air in the room. This occurred because the floor was becoming cleaner and cleaner, such that HOCl did not react on the floor as much, with more available for inhalation exposure in the room instead.
Surfaces Engineered to Improve Indoor Air Quality
As noted in previous chapters, chemicals can deposit, adsorb, absorb, and react with surfaces of building materials, furnishings, and occupants. These phenomena can, in theory, be leveraged, and the materials can be engineered to improve indoor air quality. Removal of pollutants at indoor surfaces is an attractive option because of passive transport of pollutants to the large available surface area. The effective clean air delivery rate for small molecules in an indoor environment is typically ~2.5 m3/h per square meter of engineered surface based on reported deposition velocities of ozone to passive removal materials (Darling et al., 2016). If all inner walls of a typical building removed contaminants at this rate, this would be equivalent to five or more air changes per hour of fresh air—but only for the specific contaminants removed at that surface.
In general, materials that only absorb or adsorb chemicals will eventually saturate and either require replacement or regeneration to continue to be effective. Products of this sort that have been promoted include odor removing paint and wallboard impregnated with activated carbon for VOC control. Upon saturation, dynamic changes in environmental conditions may effectively alter equilibrium conditions in a way that acts to periodically drive molecules off these materials, turning them into sources that increase exposure. However, it is also possible that day-night cycles of temperature or humidity could be used intentionally to regenerate the materials by desorbing them during non-occupied periods. Feasibility of this approach for carbon dioxide (CO2)-sorbing coatings has been demonstrated (Rajan et al., 2017) and moisture control (buffering) in buildings through use of hygroscopic materials is the subject of many studies (Zhang et al., 2017).
To overcome saturation, removal at surfaces by chemical transformation has been proposed. Ozone reacts readily on many surfaces, and many available materials already remove ozone, some with minimal formation of byproducts (Cros et al., 2012; Gall et al., 2011; Kunkel et al., 2010; Lamble et al., 2011). To remove VOCs and nitrogen oxides more effectively, photocatalytic paints have been designed and tested for indoor use. Because these paints generally rely on available indoor lighting, photolytic energy and flux is much lower than in photocatalytic oxidation (PCO) units that use intense UV light. Therefore, these paints have limited effectiveness, only partially oxidize molecules (if at all) (Salthammer and Fuhrmann, 2007), can result in the net formation and release of formaldehyde and other VOCs (Gandolfo et al., 2018), and have been shown to effectively convert nitrogen oxides to nitrous acid (Gandolfo et al., 2015). Periodic renewal of engineered surfaces will be necessary since any systems that rely on surface chemistry will degrade over time as the active sites become soiled or “poisoned” with permanent deposits of reaction products. Despite the good
intentions of designers, controls such as the use of plants or passive “green walls” are of limited effectiveness in improving air quality (Cummings and Waring, 2020; Irga et al., 2018).
Overall, there are possibilities for passive air cleaning through the use of indoor surfaces, but often the details are more complicated than just surfaces being effective adsorbents for indefinite periods of time. Saturation, re-emission, and reactive chemistry all play roles that require consideration before passive surfaces are implemented to remove indoor air pollutants.
MANAGEMENT THROUGH CHEMICAL TRANSFORMATIONS
Chemically modifying air pollutants to transform them into benign species or increase their removal rates is an increasingly used approach to improve indoor air quality. Devices that use chemical transformations can be additive (e.g., addition of an oxidant or other reactive chemical species to air), photolytic (e.g., application of UV light), or contained (e.g., photocatalysis systems that operate in a confined device, converting polluted air to “cleaner” air). The efficacy of these systems in removing air pollutants and their potential to create unintended byproducts require careful testing and investigation (Collins and Farmer, 2021; Siegel, 2016; Ye et al., 2021; Zhang et al., 2011). This section outlines each approach below.
Chemical Additions
The addition of chemical compounds to indoor air is a commonly proposed approach to air cleaning. These additions—whether through gas-phase or misting additions—raise concerns of secondary chemistry, which have been detailed by Collins and Farmer (2021).
Ozone is a biradical that reacts rapidly with alkenes and select inorganic species, notably including nitric oxide (NO) radicals. The concept behind ozone addition devices is to break down organic molecules, including those causing odors, through these oxidation reactions. However, there are few field studies of how the high levels of ozone from generators impact indoor chemistry. For example, Tang et al. (2021) showed that ozone could be effective at removing compounds associated with third-hand smoke but released other potential gases and induced ultrafine particle formation; as a result, that study proposed calculating minimum times before re-entry for occupants following ozone addition. Due to the chemistry described in Chapter 4, ozone addition to the built environment raises several concerns:
- The subsequent functionalization and fragmentation of organic molecules produces an array of oxygenated products, some of which are more toxic than the parent molecule. For example, ozonolysis of limonene, a common indoor VOC, produces formaldehyde (Weschler, 2006), while ozonolysis of skin oils and building materials produces an array of aldehydes and ketones (Wang and Morrison, 2010; Wisthaler and Weschler, 2010).
- Ozonolysis of VOCs is well established to produce condensable material that forms secondary organic aerosol (Hallquist et al., 2009), which has known health effects (Chowdhury et al., 2018).
- Ozone reacts with elastomers including natural rubbers, causing degradation and other unintended consequences, such as cracking of insulation on wiring.
- Ozone itself is a known air toxic, causing inflammation and cardiorespiratory effects.
Because of these concerns, the California Air Resources Board (CARB) recommends against the use of ozone generators. In 2010, CARB adopted a regulation requiring all indoor air cleaners sold in California to produce less than 50 ppb of ozone (California Code of Regulations, 2010).
Hydroxyl (OH) radicals are typically more broadly reactive with organic species than ozone, oxidizing not only alkenes by OH addition but also hydrocarbons by H abstraction to form water
and an alkoxy radical (R) that quickly forms a peroxy radical (RO2), which can undergo a series of reactions resulting in complex products, including peroxides, carbonyls, and carboxylic acids. Hydroxyl radical oxidation is not yet widely used in indoor environments to intentionally degrade VOCs but raises many of the same concerns as ozone, including the potential for production of secondary organic aerosol and other unintended byproducts due to its rapid oxidation chemistry (Friedman and Farmer, 2018; Lee et al., 2006). A recent study demonstrated that operating hydroxyl generator air-cleaning devices in an office environment increased PM and substantially enhanced oxidized organic compounds in the gas phase and secondary organic aerosol (Joo et al., 2021). However, chemically comprehensive studies of the emissions of these devices are lacking, and the extent to which ozone or other oxidants contributed to observed oxidation chemistry remains unexplored, particularly considering the short lifetime (order of seconds) of hydroxyl radicals in the indoor environment. Another investigation by Ye et al. (2021) studied multiple air cleaners that purported to remove VOCs by sorption and/or oxidative degradation and found that a range of byproducts was produced, including formaldehyde. The health effects of exposure to emissions and subsequent chemistry from these hydroxyl radical generators are not understood.
Odor-masking products and disinfectants are often added to indoor environments through fogging, spraying, or other vapor or droplet dispersal. The addition of scented products typically introduces reactive VOCs (e.g., monoterpenes) that can undergo chemical transformations and create unintended byproducts. The chemical mechanisms of oxidation reactions of individual VOCs are becoming better understood, but the interaction between these molecules and the chemical complexity present in indoor surfaces and air is not. A few commercial products act to trap odiferous compounds in cyclodextrin to reduce obnoxious smells (Hammer et al., 2013).
The addition of vaporized disinfectants, such as hydrogen peroxide, hypochlorous acid, and chlorine dioxide, has been used to decontaminate buildings and materials. Vaporized hydrogen peroxide can be effective in deactivating bacterial contamination (Johnston et al., 2005; Kahnert et al., 2005; Rudnick et al., 2009) but may be photolyzed to produce hydroxyl and peroxy radicals (Zhou et al., 2020), which can undergo further reactions indoors. The deposition of vaporized hydrogen peroxide on building materials induces the emission of various VOCs, although the extent to which this release is the result of reactive chemistry or simple displacement reactions remains unknown (Poppendieck et al., 2021). Hypochlorous acid (HOCl) is the conjugate acid of hypochlorite (OCl–), the key ingredient in bleach and a well-established disinfectant. Fogging indoor environments with hypochlorous acid has emerged as a disinfectant strategy during the COVID-19 pandemic, but this approach has not yet been established in independent literature to effectively deactivate microbes. Box 5-3 briefly discusses other challenges of evaluating chemically transformative air-cleaning devices that have emerged during the pandemic. The addition of hypochlorous acid into indoor environments will initiate a series of reactions that can produce secondary aerosol (Mattila et al., 2020; Wang et al., 2019) and unintended byproducts, including cyanogen chloride, molecular chlorine, and organic isocyanates (Mattila et al., 2020; Wong et al., 2017). Research is beginning to elucidate the chemical reactions related to hypochlorous acid in the indoor environment, but the underlying chemical mechanisms and subsequent health consequences of these byproducts are largely unknown. HOCl reacts with skin oils (Schwartz-Narbonne et al., 2019) and may react similarly with lung or other tissue, also causing direct health impacts—and producing additional secondary products. Vaporized triethylene glycol (TEG) has long been known to destroy airborne pathogens (Lester Jr et al., 1952; Rosebury et al., 1947) and is far less reactive than the other surface disinfectants described here. The potential for TEG to react with other molecules in the indoor environment has not been substantively explored in the scientific literature, although the high boiling point and viscous nature of TEG suggests that it may accumulate on surfaces and impact surface-air partitioning.
Fumigation with gas-phase chlorine dioxide (ClO2) has also been used as a building-wide disinfectant for bacteria and fungi, including in response to anthrax attacks or mold remediation
(Hsu et al., 2015; Hubbard et al., 2009). However, ClO2 is both photolabile and reactive, thus raising concerns over byproduct formation. ClO2 can also be sorbed to, and react with, material surfaces, raising concerns over material degradation (Derkits et al., 2010).
In summary, the literature suggests that chemical additions and modifications to indoor environments are sometimes effective tools in the arsenal for decontamination, but they can enable secondary chemistry: the potential formation of unintended byproducts with unknown implications for exposure and health. The physical and chemical mechanisms responsible for byproduct formation—and the health effects of those byproducts in real-world indoor environments—warrants further study.
Ultraviolet Light
Photolysis, the decomposition of molecules due to interaction with light, can also be used to control chemical air contaminants and inactivate or kill microorganisms. Indeed, indoor air quality applications of UV light date to at least the 1930s, when it was first used for air disinfection in operating rooms (Hart, 1936) and schools (Wells et al., 1942), and it was in use even earlier for drinking water disinfection (von Recklinghausen, 1914). Because it has been a subject of intensive study for more than a century, much is known about the effectiveness of UV. In practice, photolysis is employed for indoor air quality control via ultraviolet germicidal irradiation (UVGI) systems. UVGI is an accepted adjunct to ventilation and filtration for control of tuberculosis as noted in the Centers for Disease Control and Prevention’s guidelines (Jensen et al., 2005; NIOSH, 2009).
Most UVGI systems utilize 254 nanometer (nm) UV-C, the predominant wavelength produced by low pressure mercury vapor lamps. While less hazardous than light in the UV-B range, which can
penetrate more deeply into the skin and increase skin cancer risk, direct exposure to UV-C can cause painful, although transient, eye and skin irritation and has a lower (but nonzero) level of carcinogenicity (Sliney and Stuck, 2021). Emerging light-emitting diode (LED) and excimer lamp technology offers the prospect of a wider range of available wavelengths in the future that will lead to safer and more effective UVGI systems (Buonanno et al., 2020; Ma et al., 2021). UV-C air disinfection using 254 nm sources is applied in a number of ways in indoor environments (Kowalski, 2010):
- Upper room systems, in which fixtures are placed in an occupied space to create a disinfection zone above the occupied zone to protect occupants from exposure. Such systems continuously expose air and surfaces in the space. The effectiveness of an upper room system depends on air circulation between the occupied and disinfection zones in a space. This can be driven by thermal plumes from people and equipment and by ventilation systems.
- Airstream disinfection, in which lamps are placed in air distribution ducts or in air-handling units. They may be positioned to simultaneously prevent microbial growth on the wetted surfaces of cooling coils, which not only control air temperature but also dehumidify by condensing moisture on their surfaces.
- Standalone air cleaners (i.e., enclosed air cleaners located in occupied spaces that typically include fans). Some air cleaners that rely primarily on high-efficiency mechanical filters or other technologies to disinfect air include UV lamps for the purpose of disinfecting filter surfaces.
Research into the indoor chemistry associated with the use of UVGI systems is sparse and primarily limited to ozone production. However, very little is known about production of other byproducts by UVGI systems as they are typically applied. UV light is known to photolyze some compounds, but little real-world testing has been done to identify the extent to which this affects indoor air. Degradation of materials that are exposed to UV-C in HVAC systems has been studied but not the impact of these processes on indoor air quality (Kauffman and Wolf, 2012). One study has investigated secondary organic aerosol formation resulting from exposure of toluene to 254 nm UV-C (Choi et al., 2019). Particle formation was observed in a test chamber at high toluene concentration (55 to 85 mg/m3) and UV doses (19.5 mW/cm2). However, tests in a bathroom with an upper room fixture yielded no measurable particle generation, possibly owing to both VOC concentrations and UV doses being orders of magnitude smaller than in the test chamber.
Photocatalysis
PCO is an approach that relies on the UV activation of catalysts, such as titanium dioxide (TiO2), to convert molecular oxygen to hydroxyl and superoxide radicals, which then oxidize organic and inorganic gases (Chen et al., 2012; Hay et al., 2015; Huang et al., 2016). In general, the molecule has to adsorb to the catalyst, where it becomes sequentially oxidized. If the molecule and/or resulting oxidized intermediates are retained on the catalyst for a sufficiently long time, the final products are CO2 and water (Tompkins et al., 2005). However, application of PCO is challenged in indoor applications by the formation and release of partially oxidized byproducts, including formaldehyde (Destaillats et al., 2012; Farhanian and Haghighat, 2014; Haghighatmamaghani et al., 2019; Hay et al., 2015; Sleiman et al., 2009). Transformation of nitrogen oxides to nitrous acid has also been demonstrated (Gligorovski, 2016). Removal efficiency decreases and byproduct formation increases as humidity and VOC concentrations increase (Sleiman et al., 2009; Yu et al., 2006). Some light-activated systems rely on visible rather than UV light; these may be even more susceptible to incomplete oxidation and byproduct formation. Fouling of the catalyst from an array
of compounds present in indoor air, including benzaldehyde, benzoic acid, and volatile siloxanes, can decrease the lifespan of photocatalytic devices, decrease removal efficiency, and increase byproduct formation, further reducing their effectiveness (Cao et al., 2000; Hay et al., 2015). While PCO efficiency and byproduct formation have been investigated extensively in controlled laboratory environments, more studies of effectiveness, longevity, and byproducts in real-world indoor environments are needed to understand the potential for subsequent chemical transformations. In particular, more accurate evaluations of these devices would take place in occupied buildings, because occupants are a major source of siloxanes that can decrease efficiency and increase byproduct formation (Tang et al., 2015).
Ionizers
Ions are positively or negatively charged species. At ground level, total ion concentrations in the atmosphere are thought to be on the order of hundreds of ions per cubic centimeter (Beig and Brasseur, 2000). In the gas phase, these species are reactive in the atmosphere, with tropospheric lifetimes measured in seconds. This reactivity means that ions can react with trace gases and particles, potentially transforming pollutants into either new or more easily removed forms. For example, charging particles may enhance particle agglomeration processes, leading to larger particles that deposit more rapidly or are filtered more easily than the original size distribution. However, few peer-reviewed studies have investigated whether these ionizers work in real-world environments (Pushpawela et al., 2017), with questions raised over their ability to enhance particle removal (Tang et al., 2021). In addition to questions of efficacy, ion generators face several challenges: negative health effects from exposure to high ion levels (Liu et al., 2021), the potential for ozone formation following ionization of molecular oxygen, the potential for byproduct formation from ion-molecule reactions, and the potential for nucleation of new particles through reactions of ions and molecules (Zeng et al., 2021).
OTHER CONSIDERATIONS FOR MANAGEMENT OF CHEMICALS
Environmental Factors
Ambient and indoor environmental factors can impact the effectiveness and choice of control technologies. While mechanical or natural ventilation are utilized in virtually all buildings, the extent to which they are relied upon is strongly dependent on local climate, which impacts the amount of energy consumption necessary to bring outdoor air to indoor conditions. Particularly in extreme climates, there is a strong economic disincentive to use increased outdoor air supply as a means of enhanced control. Air-to-air energy recovery between outdoor air intake and exhaust streams is required in some systems in most climate zones (ASHRAE, 2019) to mitigate the energy cost of ventilation, but it remains a major contributor to the total energy use of a building. Additionally, as noted earlier in this chapter, outdoor air can be a significant source of contaminants. High ambient humidity is also a concern in some climates because some types of HVAC equipment have limited ability to dehumidify, which can lead to indoor moisture problems affecting chemistry (see Chapter 4) and mold growth. Likewise, temperature and humidity can affect the performance of sorbents. They also affect natural sorption/desorption processes leading to variable emission rates in indoor spaces (Haghighat and De Bellis, 1998; Markowicz and Larsson, 2015).
Occupational Risk and Specialized Environments
Specific built environments have distinct air-cleaning needs that may provide insight into more general settings. While industrial settings are not within the scope of this report, and typically
follow the hierarchy of controls summarized earlier, air quality in aircraft has been considered in some detail (NRC, 2002; Spengler and Wilson, 2003; Zhang et al., 2011). Aircraft cabins without ozone catalysts are often subject to high ozone levels, with peak ranges of 30–275 ppb at cruising altitude (Bhangar and Nazaroff, 2013; Bhangar et al., 2008; Bekö et al., 2015; Spengler et al., 2004; Spicer et al., 2004; Weisel et al., 2013). Newer airplanes with properly serviced ozone catalyst systems can have peak ozone concentrations below 50 ppb (Bhangar et al., 2008; Weisel et al., 2013). Ozone concentrations in airplane cabins can be high enough to raise concern over exposure as both a primary air pollutant and initiator of secondary chemistry (Coleman et al., 2008; Gao et al., 2015; Weisel et al., 2013). Aircraft typically moderate air quality with enhanced ventilation and filtration, although chemically additive approaches have been considered. For example, Wisthaler et al. (2007) investigated various PCO and sorption air purifiers in aircraft cabins and found that while many were effective at reducing total VOC loading, photocatalytic devices incompletely oxidized ethanol into unacceptable levels of acetaldehyde and formaldehyde. However, the high ozone levels present in some aircraft can react with soiled filters and sorptive surfaces (Spengler et al., 2004; Spicer et al., 2004; also see Chapter 4 for more on ozone chemistry). Other unique environments that require specialized approaches to air cleaning include submarines and the International Space Station.
Economics and Sustainability
While the primary criteria for selection of any indoor environmental control technology are efficacy and safety, other considerations also weigh heavily in such decisions. The most important of these factors is economics, which includes the cost of equipment, operating costs, and energy use. Costs have always been a major concern for owners of indoor air quality control systems, but, increasingly, emission of greenhouse gases in the production of energy used to operate building systems is also a factor in decisions. What is considered an acceptable cost depends on the purpose of the facility. For example, high expenditures may be justified for systems protecting vulnerable occupants, such as health care facilities. Because buildings are required by building codes to meet minimum outdoor air supply requirements, the cost of a ventilation system is unavoidable. However, this can range from a mechanical ventilation system that delivers outdoor air as required whenever a building is occupied to operable windows that provide a much lower level of reliability because they depend on occupant behavior. Chemical filtration and air cleaning, on the other hand, is not mandatory for most buildings, and some types of equipment add significant initial costs and ongoing maintenance costs to replace consumable components (e.g., sorbent filter media). On the other hand, because outdoor air brought in to control contaminant levels needs to be conditioned to indoor temperature and humidity levels, increasing ventilation rates above minimum requirements for the purpose of control could, depending on climate, result in greater incremental energy use and incur higher operating costs than filtration and cleaning of recirculated air. For the same reason, air cleaners may be viewed as a more sustainable alternative to ventilation for control of indoor contaminants. Despite the attractiveness of filtration and air cleaning, ventilation remains the predominant means of control because of the difficulty of properly specifying air-cleaning systems to achieve overall indoor air quality goals. In order to apply air-cleaning technologies effectively, a definition of indoor air quality in terms of measurable contaminants is needed. An addendum to ASHRAE Standard 62.1 that proposes threshold values for 14 compounds and PM2.5 was approved in 2021. Given the possible presence of a much larger number of compounds in indoor environments, however, there is a clear need for advances in both analytical instrumentation and our understanding of indoor chemistry to move such efforts forward. Applied research on energy-optimal use of combinations of control technologies is also needed.
CONCLUSIONS
The management of chemical contaminants in indoor environments includes removal (through ventilation, filtration, sorption, physical cleaning, and passive surface removal) and chemical transformations (including photolysis, ionizers, chemical additions, and photocatalysis). No single management approach can remove all contaminants that are present indoors; therefore, source elimination is always the preferred method of control. However, combinations of management approaches can also be effective at reducing exposure, as can situation-specific choices, such as increasing ventilation to reduce air contaminant exposure. There are different chemical consequences of every management approach. Approaches that include oxidation are particularly prone to generating products of concern. While this chapter highlights the scientific community’s knowledge of underlying physical and chemical principles of air cleaning, several knowledge gaps remain, including the fundamental chemistry of many air-cleaning technologies. With the exception of ventilation, particle filtration, and sorption, few air-cleaning approaches are tested in real-world environments, which contain a far more complicated mixture of compounds than most laboratory studies. As outlined in previous chapters, chemical reactions in indoor environments can follow complex mechanisms and result in numerous different products. This makes predicting chemical reactions and the efficacy of air-cleaning devices challenging—and highlights the need for better testing standards for air-cleaning efficacy and chemistry that account for this complexity. There is insufficient chemical research to truly understand and prioritize chemical byproducts in terms of toxicology and health effects, and to identify safe and effective levels of chemical additives for air-cleaning technologies.
RESEARCH NEEDS
In particular, the committee highlights the need for research incorporating real-world testing of management approaches that are anticipated to induce chemical transformations, specifically the following:
- Testing approaches need to be developed that consider both efficacy and byproduct formation in a representative range of real-world environments (e.g., ultrafine particles, PM2.5, oxygenated VOCs including formaldehyde). Different chemicals induce different types of chemistry, so any testing approach has to be flexible enough to account for likely products, and the complexity of indoor chemistry means that non-targeted analysis approaches could be useful. These tests and measurements can help inform a quantitative assessment of thresholds for health effects for relevant compounds.
- Developers of air-cleaning technologies need to recognize that many gas-phase molecules in indoor air partition with indoor surface reservoirs. Cleaning the air may not substantially remove the contamination if a large amount of that molecule remains on the surfaces and re-partitions to the gas phase after air cleaning stops. Surface cleaning must thus be coincident with air cleaning, although comprehensive surface removal is rarely feasible for compounds adsorbed to, for example, paint surfaces.
- Controlled field experiments are necessary to better understand the fundamental chemistry of emerging air-cleaning technologies, as well as mold and smoke remediation schemes.
Finally, given the recent public interest in indoor air quality, device manufacturers, researchers, and public health professionals need to communicate clearly to consumers about the efficacy and chemical consequences of different air-cleaning approaches. The lack of testing and regulation has
led to rampant unsubstantiated claims about efficacy and health benefits of devices. The potential health risks and benefits resulting from their use warrant further investigation and potential certification or regulatory oversight. Based on the current state of knowledge, the committee cautions against approaches that induce secondary chemistry in occupied settings, unless the benefits demonstrably outweigh the risks of exposure to chemical reactants and byproducts.
REFERENCES
Aldred, J. R., E. Darling, G. Morrison, J. Siegel, and R. Corsi. 2016a. Benefit-cost analysis of commercially available activated carbon filters for indoor ozone removal in single-family homes. Indoor Air 26:501–512.
Aldred, J. R., E. Darling, G. Morrison, J. Siegel, and R. L. Corsi. 2016b. Analysis of the cost effectiveness of combined particle and activated carbon filters for indoor ozone removal in buildings. Science and Technology for the Built Environment 22:227–236.
Andersen, R., V. Fabi, J. Toftum, S. P. Corgnati, and B. W. Olesen. 2013. Window opening behaviour modelled from measurements in Danish dwellings. Building and Environment 69:101–113. https://doi.org/10.1016/j.buildenv.2013.07.005.
Anderson, J. O., J. G. Thundiyil, and A. Stolbach. 2012. Clearing the air: A review of the effects of particulate matter air pollution on human health. Journal of Medical Toxicology 8:166–175. https://doi.org/10.1007/s13181-011-0203-1.
Arghand, T., T. Karimipanah, H. B. Awbi, M. Cehlin, U. Larsson, and E. Linden. 2015. An experimental investigation of the flow and comfort parameters for under-floor, confluent jets and mixing ventilation systems in an open-plan office. Building and Environment 92:48–60. https://doi.org/10.1016/j.buildenv.2015.04.019.
ASHRAE. 2017. Standard 52.2—Method of Testing General Ventilation Air-Cleaning Devices for Removal Efficiency by Particle Size.
ASHRAE. 2019. Standard 62.1—Ventilation for Acceptable Indoor Air Quality.
ASHRAE. 2021. ASHRAE Handbook—Fundamentals. Atlanta, GA: ASHRAE.
ASTM International. 2019. ASTM D5160-95 Standard Guide for Gas-Phase Adsorption Testing of Activated Carbon.
Azimi, P., and B. Stephens. 2013. HVAC filtration for controlling infectious airborne disease transmission in indoor environments: Predicting risk reductions and operational costs. Building and Environment 70:150–160. https://doi.org/10.1016/j.buildenv.2013.08.025.
Becker, R., G. Haquin, and K. Kovler. 2014. Air change rates and radon accumulation in rooms with various levels of window and door closure. Journal of Building Physics 38(3):234–261. https://doi.org/10.1177/1744259113506071.
Beig, G., and G. P. Brasseur. 2000. Model of tropospheric ion composition: A first attempt. Journal of Geophysical Research: Atmospheres 105(D18):22671–22684. https://doi.org/10.1029/2000JD900119.
Bekö, G., J. G. Allen, C. J. Weschler, J. Vallarino, and J. D. Spengler. 2015. Impact of cabin ozone concentrations on passenger reported symptoms in commercial aircraft. PLOS ONE 10(5):e0128454. https://doi.org/10.1371/journal.pone.0128454.
Bekö, G., G. Clausen, and C. J. Weschler. 2007. Further studies of oxidation processes on filter surfaces: Evidence for oxidation products and the influence of time in service. Atmospheric Environment 41(25):5202–5212. https://doi.org/10.1016/j.atmosenv.2006.07.063.
Bekö, G., O. Halás, G. Clausen, and C. J. Weschler. 2004. Ventilation filters as sources of air pollution–Processes occurring on surfaces of used filters. Presented at Indoor Climate of Buildings ‘04, Strbske Pleso, November 21–24, 2004.
Bhangar, S., S. C. Cowlin, B. C. Singer, R. G. Sextro, and W. W. Nazaroff. 2008. Ozone levels in passenger cabins of commercial aircraft on North American and transoceanic routes. Environmental Science & Technology 42(11):3938–3943. https://doi.org/10.1021/es702967k.
Bhangar, S., and W. W. Nazaroff. 2013. Atmospheric ozone levels encountered by commercial aircraft on transatlantic routes. Environmental Research Letters 8(1):014006. https://doi.org/10.1088/1748-9326/8/1/014006.
Buonanno, M., D. Welch, I. Shuryak, and D. J. Brenner. 2020. Far-UVC light (222 nm) efficiently and safely inactivates airborne human coronaviruses. Scientific Reports 10:10285. https://doi.org/10.1038/s41598-020-67211-2.
Cai, R.-R., S.-Z. Li, L.-Z. Zhang, and Y. Lei. 2020. Fabrication and performance of a stable micro/nano composite electret filter for effective PM2.5 capture. Science of the Total Environment 725:138297. https://doi.org/10.1016/j.scitotenv.2020.138297.
Calì, D., R. K. Andersen, D. Müller, and B. W. Olesen. 2016. Analysis of occupants’ behavior related to the use of windows in German households. Building and Environment 103:54–69. https://doi.org/10.1016/j.buildenv.2016.03.024.
California Code of Regulations. 2010. Regulation for Limiting Ozone Emissions from Indoor Air Cleaning Devices. California Code of Regulations Title 17. Public Health Division 3. Air Resources Chapter 1. Air Resources Board Subchapter 8.7. Indoor Air Cleaning Devices Article 1. Indoor Air Cleaning Devices. https://ww2.arb.ca.gov/sites/default/files/2020-03/air-cleaner-regulation.pdf.
Cao, L., Z. Gao, S. L. Suib, T. N. Obee, S. O. Hay, and J. D. Freihaut. 2000. Photocatalytic oxidation of toluene on nanoscale TiO2 catalysts: Studies of deactivation and regeneration. Journal of Catalysis 196(2): 253–261. https://doi.org/10.1006/jcat.2000.3050.
Chen, H., C. E. Nanayakkara, and V. H. Grassian. 2012. Titanium dioxide photocatalysis in atmospheric chemistry. Chemical Reviews 112(11): 5919–5948. https://doi.org/10.1021/cr3002092.
Chen, J., G. S. Brager, G. Augenbroe, and X. Song. 2019. Impact of outdoor air quality on the natural ventilation usage of commercial buildings in the US. Applied Energy 235:673–684. https://doi.org/10.1016/j.apenergy.2018.11.020.
Choi, E., Z. Tan, and W. A. Anderson. 2019. Formation of secondary organic aerosols by germicidal ultraviolet light. Environments 6(2):17. https://doi.org/10.3390/environments6020017.
Chowdhury, P. H., Q. He, T. Lasitza Male, W. H. Brune, Y. Rudich, and M. Pardo. 2018. Exposure of lung epithelial cells to photochemically aged secondary organic aerosol shows increased toxic effects. Environmental Science & Technology Letters 5(7):424–430. https://doi.org/10.1021/acs.estlett.8b00256.
Coleman, B. K., H. Destaillats, A. T. Hodgson, and W. W. Nazaroff. 2008. Ozone consumption and volatile byproduct formation from surface reactions with aircraft cabin materials and clothing fabrics. Atmospheric Environment 42(4):642–654. https://doi.org/10.1016/j.atmosenv.2007.10.001.
Collins, D. B., and D. K. Farmer. 2021. Unintended consequences of air cleaning chemistry Environmental Science & Technology 55(18):12172–12179. https://doi.org/10.1021/acs.est.1c02582.
Cros, C., G. Morrison, J. Siegel, and R. Corsi. 2012. Long-term performance of passive materials for removal of ozone from indoor air. Indoor Air 22(1):43–53. https://doi.org/10.1111/j.1600-0668.2011.00734.x.
Cummings, B. E., and M. S. Waring. 2020. Potted plants do not improve indoor air quality: A review and analysis of reported VOC removal efficiencies. Journal of Exposure Science & Environmental Epidemiology 30(2):253–261. https://doi.org/10.1038/s41370-019-0175-9.
Darling, E., G. C. Morrison, and R. L. Corsi. 2016. Passive removal materials for indoor ozone control. Building and Environment 106:33–44. https://doi.org/10.1016/j.buildenv.2016.06.018.
Davidson, J. H., and P. J. McKinney. 1998. Chemical vapor deposition in the corona discharge of electrostatic air cleaners. Aerosol Science and Technology 29(2):102–110.
Derkits, G. E., M. L. Mandich, W. D. Reents, J. P. Franey, C. Xu, D. Fleming, R. Kopf, and S. Ryan. 2010. Reliability of electronic equipment exposed to chlorine dioxide used for biological decontamination. 2010 IEEE International Reliability Physics Symposium, Anaheim, CA, May 2-6-2010. https://doi.org/10.1109/IRPS.2010.5488715.
Destaillats, H., M. Sleiman, D. P. Sullivan, C. Jacquiod, J. Sablayrolles, and L. Molins. 2012. Key parameters influencing the performance of photocatalytic oxidation (PCO) air purification under realistic indoor conditions. Applied Catalysis B: Environmental 128:159–170. https://doi.org/10.1016/j.apcatb.2012.03.014.
EPA (U.S. Environmental Protection Agency). 2015. OSWER technical guide for assessing and mitigating the vapor intrusion pathway from subsurface vapor sources to indoor air. EPA OSWER Publication 9200:2–154.
Fanger, P. O., and B. Berg-Munch. 1983. Ventilation and body odor. Proceedings of an Engineering Foundation Conference on Management of Atmospheres in Tightly Enclosed Spaces, Santa Barbara, CA, October 17–21, 1983. https://www.aivc.org/resource/ventilation-and-body-odor.
Farhanian, D., and F. Haghighat. 2014. Photocatalytic oxidation air cleaner: Identification and quantification of by-products. Building and Environment 72:34–43. https://doi.org/10.1016/j.buildenv.2013.10.014.
Fathollahzadeh, M. H., G. Heidarinejad, and H. Pasdarshahri. 2015. Prediction of thermal comfort, IAQ, and energy consumption in a dense occupancy environment with the under floor air distribution system. Building and Environment 90:96–104. https://doi.org/10.1016/j.buildenv.2015.03.019.
Fisk, W. J., A. G. Mirer, and M. J. Mendell. 2009. Quantitative relationship of sick building syndrome symptoms with ventilation rates. Indoor Air 19(2):159–165. https://doi.org/10.1111/j.1600-0668.2008.00575.x.
Foarde, K. K., and J. T. Hanley. 2001. Determine the efficacy of antimicrobial treatments of fibrous air filters. ASHRAE Transactions 107:156.
Forthomme, A., A. Joubert, Y. Andrès, X. Simon, P. Duquenne, D. Bemer, and L. Le Coq. 2014. Microbial aerosol filtration: Growth and release of a bacteria–fungi consortium collected by fibrous filters in different operating conditions. Journal of Aerosol Science 72:32–46. https://doi.org/10.1016/j.jaerosci.2014.02.004.
Friedman, B., and D. K. Farmer. 2018. SOA and gas phase organic acid yields from the sequential photooxidation of seven monoterpenes. Atmospheric Environment 187:335–345. https://doi.org/10.1016/j.atmosenv.2018.06.003.
Gall, E. T., R. L. Corsi, and J. A. Siegel. 2011. Barriers and opportunities for passive removal of indoor ozone. Atmospheric Environment 45(19):3338–3341. https://doi.org/10.1016/j.atmosenv.2011.03.032.
Gandolfo, A., V. Bartolomei, E. G. Alvarez, S. Tlili, S. Gligorovski, J. Kleffmann, and H. Wortham. 2015. The effectiveness of indoor photocatalytic paints on NOx and HONO levels. Applied Catalysis B: Environmental 166:84–90. https://doi.org/10.1016/j.apcatb.2014.11.011.
Gandolfo, A., S. Marque, B. Temime-Roussel, R. Gemayel, H. Wortham, D. Truffier-Boutry, V. Bartolomei, and S. Gligorovski. 2018. Unexpectedly high levels of organic compounds released by indoor photocatalytic paints. Environmental Science & Technology 52(19):11328–11337. https://doi.org/10.1021/acs.est.8b03865.
Gao, K., J. Xie, and X. Yang. 2015. Estimation of the contribution of human skin and ozone reaction to volatile organic compounds (VOC) concentration in aircraft cabins. Building and Environment 94:12–20. https://doi.org/10.1016/j.buildenv.2015.07.022.
Gligorovski, S. 2016. Nitrous acid (HONO): An emerging indoor pollutant. Journal of Photochemistry and Photobiology A: Chemistry 314:1–5. https://doi.org/10.1016/j.jphotochem.2015.06.008.
Haghighat, F., and L. De Bellis. 1998. Material emission rates: Literature review, and the impact of indoor air temperature and relative humidity. Building and Environment 33(5):261–277. https://doi.org/10.1016/S0360-1323(97)00060-7.
Haghighatmamaghani, A., F. Haghighat, and C.-S. Lee. 2019. Performance of various commercial TiO2 in photocatalytic degradation of a mixture of indoor air pollutants: Effect of photocatalyst and operating parameters. Science and Technology for the Built Environment 25(5):600–614. https://doi.org/10.1080/23744731.2018.1556051.
Hallquist, M., J. C. Wenger, U. Baltensperger, Y. Rudich, D. Simpson, M. Claeys, J. Dommen, N. M. Donahue, C. George, A. H. Goldstein, J. F. Hamilton, H. Herrmann, T. Hoffmann, Y. Iinuma, M. Jang, M. E. Jenkin, J. L. Jimenez, A. Kiendler-Scharr, W. Maenhaut, G. McFiggans, T. F. Mentel, A. Monod, A. S. H. Prévôt, J. H. Seinfeld, J. D. Surratt, R. Szmigielski, and J. Wildt. 2009. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmospheric Chemistry and Physics 9(14):5155–5236. https://doi.org/10.5194/acp-9-5155-2009.
Hammer, T. R., N. Berner-Dannenmann, and D. Hoefer. 2013. Quantitative and sensory evaluation of malodour retention of fibre types by use of artificial skin, sweat and radiolabelled isovaleric acid. Flavour and Fragrance Journal 28:238–244.
Han, K. H., J. S. Zhang, and B. Guo. 2017. Toward effective design and adoption of catalyst-based filter for indoor hazards: Formaldehyde abatement under realistic conditions. Journal of Hazardous Materials 331:161–170. https://doi.org/10.1016/j.jhazmat.2017.02.021.
Hart, D. 1936. Sterilization of the air in the operating room by special bactericidal radiant energy: Results of its use in extrapleural thoracoplasties. Journal of Thoracic Surgery 6(1):45–81.
Hay, S. O., T. Obee, Z. Luo, T. Jiang, Y. Meng, J. He, S. C. Murphy, and S. Suib. 2015. The viability of photocatalysis for air purification. Molecules 20(1):1319–1356. https://doi.org/10.3390/molecules20011319.
Howard, J., A. Huang, Z. Li, Z. Tufekci, V. Zdimal, H.-M. van der Westhuizen, A. von Delft, A. Price, L. Fridman, and L.-H. Tang. 2021. An evidence review of face masks against COVID-19. Proceedings of the National Academy of Sciences 118(4). https://doi.org/10.1073/pnas.2014564118.
Howard-Reed, C., L. A. Wallace, and W. R. Ott. 2002. The effect of opening windows on air change rates in two homes. Journal of the Air & Waste Management Association 52(2):147–159. https://doi.org/10.1080/10473289.2002.10470775.
Hsu, C.-S., M.-C. Lu, and D.-J. Huang. 2015. Disinfection of indoor air microorganisms in stack room of university library using gaseous chlorine dioxide. Environmental Monitoring and Assessment 187(2):1–11. https://doi.org/10.1007/s10661-014-4235-2.
Huang, Y., S. S. H. Ho, Y. Lu, R. Niu, L. Xu, J. Cao, and S. Lee. 2016. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 21(1):56. https://doi.org/10.3390/molecules21010056.
Hubbard, H., D. Poppendieck, and R. L. Corsi. 2009. Chlorine dioxide reactions with indoor materials during building disinfection: Surface uptake. Environmental Science & Technology 43(5):1329–1335. https://doi.org/10.1021/es801930c.
Hult, E. L., H. Willem, P. N. Price, T. Hotchi, M. L. Russell, and B. C. Singer. 2015. Formaldehyde and acetaldehyde exposure mitigation in US residences: In-home measurements of ventilation control and source control. Indoor Air 25(5):523–535. https://doi.org/10.1111/ina.12160.
Hyttinen, M., P. Pasanen, and P. Kalliokoski. 2001. Adsorption and desorption of selected VOCs in dust collected on air filters. Atmospheric Environment 35(33):5709–5716. https://doi.org/10.1016/S1352-2310(01)00376-4.
IEST (Institute of Environmental Sciences and Technology) Contamination Control Division. 2016. IEST-RP-CC001: HEPA and ULPA Filters.
Irga, P. J., T. J. Pettit, and F. R. Torpy. 2018. The phytoremediation of indoor air pollution: A review on the technology development from the potted plant through to functional green wall biofilters. Reviews in Environmental Science and Bio/Technology 17(2):395–415. https://doi.org/10.1007/s11157-018-9465-2.
Iwashita, G., and H. Akasaka. 1997. The effects of human behavior on natural ventilation rate and indoor air environment in summer—A field study in southern Japan. Energy and Buildings 25(3):195–205. https://doi.org/10.1016/S0378-7788(96)00994-2.
Izadyar, N., W. Miller, B. Rismanchi, and V. Garcia-Hansen. 2020. Impacts of façade openings’ geometry on natural ventilation and occupants’ perception: A review. Building and Environment 170:106613. https://doi.org/10.1016/j.buildenv.2019.106613.
Janssen, J. E. 1999. The history of ventilation and temperature control. ASHRAE Journal 41:48–72.
Jensen, P. A., L. A. Lambert, M. F. Iademarco, and R. Ridzon. 2005. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings. Morbidity and Mortality Weekly Report: Recommendations and Reports 54(RR-17):1–141.
Johnson, T., and T. Long. 2005. Determining the frequency of open windows in residences: A pilot study in Durham, North Carolina during varying temperature conditions. Journal of Exposure Science & Environmental Epidemiology 15(4):329–349. https://doi.org/10.1038/sj.jea.7500409.
Johnston, M. D., S. Lawson, and J. A. Otter. 2005. Evaluation of hydrogen peroxide vapour as a method for the decontamination of surfaces contaminated with Clostridium botulinum spores. Journal of Microbiological Methods 60(3):403–411. https://doi.org/10.1016/j.mimet.2004.10.021.
Joo, T., J. C. Rivera-Rios, D. Alvarado-Velez, S. Westgate, and N. L. Ng. 2021. Formation of oxidized gases and secondary organic aerosol from a commercial oxidant-generating electronic air cleaner. Environmental Science & Technology Letters 8(8):691–698. https://doi.org/10.1021/acs.estlett.1c00416.
Kahnert, A., P. Seiler, M. Stein, B. Aze, G. McDonnell, and S. H. E. Kaufmann. 2005. Decontamination with vaporized hydrogen peroxide is effective against Mycobacterium tuberculosis. Letters in Applied Microbiology 40(6):448–452. https://doi.org/10.1111/j.1472-765X.2005.01683.x.
Kang, D. H., D. H. Choi, S. M. Lee, M. S. Yeo, and K. W. Kim. 2010. Effect of bake-out on reducing VOC emissions and concentrations in a residential housing unit with a radiant floor heating system. Building and Environment 45(8):1816–1825. https://doi.org/10.1016/j.buildenv.2010.02.010.
Kauffman, R. E., and J. D. Wolf. 2012. Study of the degradation of typical HVAC materials, filters and components irradiated by UVC energy—Part II: polymers. ASHRAE Transactions 118(2).
Keer, S., D. McLean, B. Glass, and J. Douwes. 2018. Effects of personal protective equipment use and good workplace hygiene on symptoms of neurotoxicity in solvent-exposed vehicle spray painters. Annals of Work Exposures and Health 62(3): 307–320. https://doi.org/10.1093/annweh/wxx100.
Khan, S. M., J. Gomes, and D. R. Krewski. 2019. Radon interventions around the globe: A systematic review. Heliyon 5(5):e01737. https://doi.org/10.1016/j.heliyon.2019.e01737.
Kim, S.-S., D.-H. Kang, D.-H. Choi, M.-S. Yeo, and K.-W. Kim. 2008. Comparison of strategies to improve indoor air quality at the pre-occupancy stage in new apartment buildings. Building and Environment 43(3):320–328. https://doi.org/10.1016/j.buildenv.2006.03.026.
Kowalski, W. 2010. Ultraviolet Germicidal Irradiation Handbook: UVGI for Air and Surface Disinfection. University Park, USA. Springer Science & Business Media.
Kunkel, D. A., E. T. Gall, J. A. Siegel, A. Novoselac, G. C. Morrison, and R. L. Corsi. 2010. Passive reduction of human exposure to indoor ozone. Building and Environment 45(2):445–452. https://doi.org/10.1016/j.buildenv.2009.06.024.
Lai, D., S. Jia, Y. Qi, and J. Liu. 2018. Window-opening behavior in Chinese residential buildings across different climate zones. Building and Environment 142:234–243. https://doi.org/10.1016/j.buildenv.2018.06.030.
Lamble, S., R. Corsi, and G. Morrison. 2011. Ozone deposition velocities, reaction probabilities and product yields for green building materials. Atmospheric Environment 45(38):6965–6972. https://doi.org/10.1016/j.atmosenv.2011.09.025.
Lee, A., A. H. Goldstein, J. H. Kroll, N. L. Ng, V. Varutbangkul, R. C. Flagan, and J. H. Seinfeld. 2006. Gas-phase products and secondary aerosol yields from the photooxidation of 16 different terpenes. Journal of Geophysical Research: Atmospheres 111(D17). https://doi.org/10.1029/2006JD007050.
Lee, J., and J. Kim. 2020. Material properties influencing the charge decay of electret filters and their impact on filtration performance. Polymers 12(3). https://doi.org/10.3390/polym12030721.
Lester Jr, W., E. Dunklin, and O. Robertson. 1952. Bactericidal effects of propylene and triethylene glycol vapors on airborne Escherichia coli. Science 115(2988):379–382. https://doi.org/10.1126/science.115.2988.379.
Li, Y., and A. Delsante. 2001. Natural ventilation induced by combined wind and thermal forces. Building and Environment 36(1):59–71. https://doi.org/10.1016/S0360-1323(99)00070-0.
Ligotski, R., U. Sager, U. Schneiderwind, C. Asbach, and F. Schmidt. 2019. Prediction of VOC adsorption performance for estimation of service life of activated carbon based filter media for indoor air purification. Building and Environment 149:146–156. https://doi.org/10.1016/j.buildenv.2018.12.001.
Lin, C.-C., and H.-Y. Chen. 2014. Impact of HVAC filter on indoor air quality in terms of ozone removal and carbonyls generation. Atmospheric Environment 89:29–34. https://doi.org/10.1016/j.atmosenv.2014.02.020.
Liu, W., J. Huang, Y. Lin, C. Cai, Y. Zhao, Y. Teng, J. Mo, L. Xue, L. Liu, W. Xu, X. Guo, Y. Zhang, and J. Zhang. 2021. Negative ions offset cardiorespiratory benefits of PM2.5 reduction from residential use of negative ion air purifiers. Indoor Air 31(1):220–228. https://doi.org/10.1111/ina.12728.
Ma, B., P. M. Gundy, C. P. Gerba, M. D. Sobsey, and K. G. Linden. 2021. UV inactivation of SARS-CoV-2 across the UVC spectrum: KrCl* excimer, mercury-vapor, and light-emitting-diode (LED) sources. Applied and Environmental Microbiology 87(22):e01532–21. https://doi.org/10.1128/AEM.01532-21.
Markowicz, P., and L. Larsson. 2015. Influence of relative humidity on VOC concentrations in indoor air. Environmental Science and Pollution Research 22(8):5772–5779. https://doi.org/10.1007/s11356-014-3678-x.
Mattila, J. M., C. Arata, C. Wang, E. F. Katz, A. Abeleira, Y. Zhou, S. Zhou, A. H. Goldstein, J. P. D. Abbatt, P. F. DeCarlo, and D. K. Farmer. 2020. Dark chemistry during bleach cleaning enhances oxidation of organics and secondary organic aerosol production indoors. Environmental Science & Technology Letters 7(11):795–801. https://doi.org/10.1021/acs.estlett.0c00573.
Morrison, G., J. Cagle, and G. Date. 2022. A national survey of window-opening behavior in United States homes. Indoor Air 32(1):e12932. https://doi.org/10.1111/ina.12932.
NASA (National Aeronautics and Space Administration). 2011. Flammability, Offgassing, and Compatibility Requirements and Test Procedures. https://standards.nasa.gov/standard/nasa/nasa-std-6001.
NAVSEA (Naval Sea Systems Command) System Communications. 2016. Approved Deck Coverings. Washington, DC: SUBJ/NAVSEA.
NIOSH (National Institute for Occupational Safety and Health). 2009. Environmental Control for Tuberculosis: Basic Upper-room Ultraviolet Germicidal Irradiation Guidelines for Healthcare Settings. https://www.cdc.gov/niosh/docs/2009-105/.
NIOSH. 2018. Deck covering materials, interior, cosmetic polymeric. In Performance Specification. http://everyspec.com/MIL-PRF/MIL-PRF-010000-29999/MIL-PRF-24613A_7535/.
NRC (National Research Council). 2002. The Airliner Cabin Environment and the Health of Passengers and Crew. Washington, DC: The National Academies Press. https://doi.org/10.17226/10238.
NRC. 2014. A Framework to Guide Selection of Chemical Alternatives. Washington, DC: The National Academies Press.
Offermann, F. J. 2009. Ventilation and indoor air quality in new homes. PIER Collaborative Report. https://ww2.arb.ca.gov/sites/default/files/classic/research/apr/past/04-310.pdf.
Pasanen, P. O., J. Teijonsalo, O. Seppänen, J. Ruuskanen, and P. Kalliokoski. 1994. Increase in perceived odor emissions with loading of ventilation filters. Indoor Air 4(2):106–113. https://doi.org/10.1111/j.1600-0668.1994.t01-2-00005.x.
Pejtersen, J. 1996. Sensory pollution and microbial contamination of ventilation filters. Indoor Air 6(4):239–248. https://doi.org/10.1111/j.1600-0668.1996.00003.x.
Peng, Z., and J. L. Jimenez. 2021. Exhaled CO2 as a COVID-19 infection risk proxy for different indoor environments and activities. Environmental Science & Technology Letters 8(5):392–397. https://doi.org/10.1021/acs.estlett.1c00183.
Perrier, J. C. B., L. Le Coq, Y. Andres, and P. Le Cloirec. 2008. SFGP 2007-Microbial growth onto filter media used in air treatment devices. International Journal of Chemical Reactor Engineering 6(1). https://doi.org/10.2202/1542-6580.1675.
Poppendieck, D., H. Hubbard, and R. L. Corsi. 2021. Hydrogen peroxide vapor as an indoor disinfectant: Removal to indoor materials and associated emissions of organic compounds. Environmental Science & Technology Letters 8(4):320–325. https://doi.org/10.1021/acs.estlett.0c00948.
Poppendieck, D. G., D. Rim, and A. K. Persily. 2014. Ultrafine particle removal and ozone generation by in-duct electrostatic precipitators. Environmental Science & Technology 48(3):2067–2074.
Price, P. N., and M. H. Sherman. 2006. Ventilation Behavior and Household Characteristics in New California Houses. Berkeley, CA: Ernest Orlando Lawrence Berkeley National Laboratory.
Pushpawela, B., R. Jayaratne, A. Nguy, and L. Morawska. 2017. Efficiency of ionizers in removing airborne particles in indoor environments. Journal of Electrostatics 90:79–84. https://doi.org/10.1016/j.elstat.2017.10.002.
Rajan, P. E., A. Krishnamurthy, G. Morrison, and F. Rezaei. 2017. Advanced buffer materials for indoor air CO2 control in commercial buildings. Indoor Air 27(6):1213–1223. https://doi.org/10.1111/ina.12386.
Rijal, H. B., M. A. Humphreys, and J. F. Nicol. 2018. Development of a window opening algorithm based on adaptive thermal comfort to predict occupant behavior in Japanese dwellings. Japan Architectural Review 1(3):310–321. https://doi.org/10.1002/2475-8876.12043.
Rijal, H. B., P. Tuohy, M. A. Humphreys, J. F. Nicol, A. Samuel, I. A. Raja, and J. Clarke. 2008. Development of adaptive algorithms for the operation of windows, fans, and doors to predict thermal comfort and energy use in Pakistani buildings. American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) Transactions 114(2):555–573.
Rosebury, T., G. Meiklejohn, L. C. Kingsland, and M. H. Boldt. 1947. Disinfection of clouds of meningopneumonitis and psittacosis viruses with triethylene glycol vapor. The Journal of Experimental Medicine 85(1):65–76. https://doi.org/10.1084/jem.85.1.65.
Rudnick, S., and D. Milton. 2003. Risk of indoor airborne infection transmission estimated from carbon dioxide concentration. Indoor Air 13(3): 237–245. https://doi.org/10.1034/j.1600-0668.2003.00189.x.
Rudnick, S. N., J. J. McDevitt, M. W. First, and J. D. Spengler. 2009. Inactivating influenza viruses on surfaces using hydrogen peroxide or triethylene glycol at low vapor concentrations. American Journal of Infection Control 37(10):813–819. https://doi.org/10.1016/j.ajic.2009.06.007.
Saber, E. M., I. Chaer, A. Gillich, and B. G. Ekpeti. 2021. Review of intelligent control systems for natural ventilation as passive cooling strategy for UK buildings and similar climatic conditions. Energies 14(15):4388. https://doi.org/10.3390/en14154388.
Salthammer, T., and F. Fuhrmann. 2007. Photocatalytic surface reactions on indoor wall paint. Environmental Science & Technology 41(18):6573–6578. https://doi.org/10.1021/es070057m.
Schleibinger, H., and H. Rüden. 1999. Air filters from HVAC systems as possible source of volatile organic compounds (VOC)–laboratory and field assays. Atmospheric Environment 33(28):4571–4577. https://doi.org/10.1016/S1352-2310(99)00274-5.
Schraufnagel, D. E. 2020. The health effects of ultrafine particles. Experimental & Molecular Medicine 52(3):311–317. https://doi.org/10.1038/s12276-020-0403-3.
Schulte, P. A., L. T. McKernan, D. S. Heidel, A. H. Okun, G. S. Dotson, T. J. Lentz, C. L. Geraci, P. E. Heckel, and C. M. Branche. 2013. Occupational safety and health, green chemistry, and sustainability: A review of areas of convergence. Environmental Health 12(1):1–9. https://doi.org/10.1186/1476-069X-12-31.
Schwartz-Narbonne, H., C. Wang, S. Zhou, J. P. D. Abbatt, and J. Faust. 2019. Heterogeneous chlorination of squalene and oleic acid. Environmental Science & Technology 53(3):1217–1224. https://doi.org/10.1021/acs.est.8b04248.
Sidheswaran, M., W. Chen, A. Chang, R. Miller, S. Cohn, D. Sullivan, W. J. Fisk, K. Kumagai, and H. Destaillats. 2013. Formaldehyde emissions from ventilation filters under different relative humidity conditions. Environmental Science & Technology 47(10):5336–5343. https://doi.org/10.1021/es400290p.
Siegel, J. 2016. Primary and secondary consequences of indoor air cleaners. Indoor Air 26(1):88–96. https://doi.org/10.1111/ina.12194.
Sleiman, M., P. Conchon, C. Ferronato, and J.-M. Chovelon. 2009. Photocatalytic oxidation of toluene at indoor air levels (ppbv): Towards a better assessment of conversion, reaction intermediates and mineralization. Applied Catalysis B: Environmental 86(3–4):159–165. https://doi.org/10.1016/j.apcatb.2008.08.003.
Sliney, D. H., and B. E. Stuck. 2021. A need to revise human exposure limits for ultraviolet UV-C radiation. Photochemistry and Photobiology 97(3):485–492. https://doi.org/10.1111/php.13402.
Spengler, J., S. Ludwig, and R. Weker. 2004. Ozone exposures during trans-continental and trans-Pacific flights. Indoor Air 14:67–73. https://doi.org/10.1111/j.1600-0668.2004.00275.x.
Spengler, J. D., J. M. Samet, and J. F. McCarthy. 2001. Indoor Air Quality Handbook. New York. McGraw-Hill Education. https://www.accessengineeringlibrary.com/content/book/9780074455494.
Spengler, J. D., and D. G. Wilson. 2003. Air quality in aircraft. Proceedings of the Institution of Mechanical Engineers, Part E: Journal of Process Mechanical Engineering 217(4):323–335. https://doi.org/10.1243/095440803322611688.
Spicer, C. W., M. J. Murphy, M. W. Holdren, J. D. Myers, I. C. MacGregor, C. Holloman, R. R. James, K. Tucker, and R. Zaborski. 2004. Relate Air Quality and Other Factors to Comfort and Health Symptoms Reported by Passengers and Crew on Commercial Transport Aircraft (Part I) (ASHRAE Project 1262-TRP). Atlanta: American Society for Heating, Refrigerating and Air-Conditioning Engineers. https://www.techstreet.com/ashrae/standards/rp-1262-relate-air-quality-and-other-factors-to-comfort-and-health-symptoms-reported-by-passengers-and-crew-on-commercial-transport-aircraft-part-i?product_id=1717909.
Stephens, B., J. A. Siegel, and A. Novoselac. 2011. Operational characteristics of residential and light-commercial air-conditioning systems in a hot and humid climate zone. Building and Environment 46(10):1972–1983. https://doi.org/10.1016/j.buildenv.2011.04.005.
Tang, X., N. R. González, M. L. Russell, R. L. Maddalena, L. A. Gundel, and H. Destaillats. 2021. Chemical changes in thirdhand smoke associated with remediation using an ozone generator. Environmental Research 198:110462. https://doi.org/10.1016/j.envres.2020.110462.
Tang, X., P. K. Misztal, W. W. Nazaroff, and A. H. Goldstein. 2015. Siloxanes are the most abundant volatile organic compound emitted from engineering students in a classroom. Environmental Science & Technology Letters 2(11):303–307. https://doi.org/10.1021/acs.estlett.5b00256.
Tompkins, D. T., W. A. Zeltner, B. J. Lawnicki, and M. A. Anderson. 2005. Evaluation of photocatalysis for gas-phase air cleaning-Part 1: Process, technical, and sizing considerations. ASHRAE Transactions 111:60.
Tsang, A. M., and N. E. Klepeis. 1996. Descriptive Statistics Tables from a Detailed Analysis of the National Human Activity Pattern Survey (NHAPS) Data. Las Vegas, NV: Lockheed Martin Environmental Systems and Technologies.
Underhill, D. 2001. Removal of gases and vapors. In Indoor Air Quality Handbook 1st ed., edited by J. D. Spengler, J. M. Samet, and J. F. McCarthy. New York: McGRAW-HILL.
Verdenelli, M., C. Cecchini, C. Orpianesi, G. Dadea, and A. Cresci. 2003. Efficacy of antimicrobial filter treatments on microbial colonization of air panel filters. Journal of Applied Microbiology 94(1):9–15. https://doi.org/10.1046/j.1365-2672.2003.01820.x.
Verginelli, I., O. Capobianco, N. Hartog, and R. Baciocchi. 2017. Analytical model for the design of in situ horizontal permeable reactive barriers (HPRBs) for the mitigation of chlorinated solvent vapors in the unsaturated zone. Journal of Contaminant Hydrology 197:50–61. https://doi.org/10.1016/j.jconhyd.2016.12.010.
Von Recklinghausen, M. 1914. The ultra-violet rays and their application for the sterilization of water. Journal of the Franklin Institute 178(6):681–704.
Wang, C., D. B. Collins, and J. P. Abbatt. 2019. Indoor illumination of terpenes and bleach emissions leads to particle formation and growth. Environmental Science & Technology 53(20):11792–11800. https://doi.org/10.1021/acs.est.9b04261.
Wang, H., and G. Morrison. 2010. Ozone-surface reactions in five homes: Surface reaction probabilities, aldehyde yields, and trends. Indoor Air 20(3):224–234. https://doi.org/10.1111/j.1600-0668.2010.00648.x.
Wang, Z., S. F. Kowal, N. Carslaw, and T. F. Kahan. 2020. Photolysis-driven indoor air chemistry following cleaning of hospital wards. Indoor Air 30(6):1241–1255. https://doi.org/10.1111/ina.12702.
Watson, R., M. Oldfield, J. A. Bryant, L. Riordan, H. J. Hill, J. A. Watts, M. R. Alexander, M. J. Cox, Z. Stamataki, D. J. Scurr, and F. de Cogan. 2022. Efficacy of antimicrobial and anti-viral coated air filters to prevent the spread of airborne pathogens. Scientific Reports 12:2803. https://doi.org/10.1038/s41598-022-06579-9.
Weisel, C., C. J. Weschler, K. Mohan, J. Vallarino, and J. D. Spengler. 2013. Ozone and ozone byproducts in the cabins of commercial aircraft. Environmental Science & Technology 47(9):4711–4717. https://doi.org/10.1021/es3046795.
Wells, W.F., M. W. Wells, and T. S. Wilder. 1942. The environmental control of epidemic contagion. I. An epidemiologic study of radiant disinfection of air in day schools American Journal of Epidemiology 35(1):97–121.
Weschler, C. J. 2006. Ozone’s impact on public health: Contributions from indoor exposures to ozone and products of ozone-initiated chemistry. Environmental Health Perspectives 114(10):1489–1496. https://doi.org/10.1289/ehp.9256.
Weschler, C. J., and H. C. Shields. 2000. The influence of ventilation on reactions among indoor pollutants: Modeling and experimental observations. Indoor Air 10(2): 92–100.
White, E. 2009. HEPA and ULPA filters. Journal of Validation Technology 15(3):48–55. https://www.proquest.com/openview/955ada5d217bd22cf9a31441253b38ba/1?pq-origsite=gscholar&cbl=29232.
Wisthaler, A., P. Strom-Tejsen, L. Fang, T. J. Arnaud, A. Hansel, T. D. Mark, and D. P. Wyon. 2007. PTR-MS assessment of photocatalytic and sorption-based purification of recirculated cabin air during simulated 7-h flights with high passenger density. Environmental Science & Technology 41(1):229–234. https://doi.org/10.1021/es060424e.
Wisthaler, A., and C. J. Weschler. 2010. Reactions of ozone with human skin lipids: Sources of carbonyls, dicarbonyls, and hydroxycarbonyls in indoor air. Proceedings of the National Academy of Sciences 107(15):6568–6575. https://doi.org/10.1073/pnas.0904498106.
Wong, J. P. S., N. Carslaw, R. Zhao, S. Zhou, and J. P. D. Abbatt. 2017. Observations and impacts of bleach washing on indoor chlorine chemistry. Indoor Air 27(6):1082–1090. https://doi.org/10.1111/ina.12402.
Woods, J. E. 1991. An engineering approach to controlling indoor air quality. Environmental Health Perspectives 95:15–21. https://doi.org/10.1289/ehp.919515.
Yao, M., and B. Zhao. 2017. Window opening behavior of occupants in residential buildings in Beijing. Building and Environment 124:441–449. https://doi.org/10.1016/j.buildenv.2017.08.035.
Ye, Q., J. E. Krechmer, J. D. Shutter, V. P. Barber, Y. Li, E. Helstrom, L. J. Franco, J. L. Cox, A. I. H. Hrdina, M. B. Goss, N. Tahsini, M. Canagaratna, F. N. Keutsch, and J. H. Kroll. 2021. Real-time laboratory measurements of VOC emissions, removal rates, and byproduct formation from consumer-grade oxidation-based air cleaners. Environmental Science & Technology Letters 8(12):1020–1025. https://doi.org/10.1021/acs.estlett.1c00773.
Yu, K.-P., G. W. Lee, W.-M. Huang, C. Wu, and S. Yang. 2006. The correlation between photocatalytic oxidation performance and chemical/physical properties of indoor volatile organic compounds. Atmospheric Environment 40(2): 375–385. https://doi.org/10.1016/j.atmosenv.2005.09.045.
Zeng, Y., P. Manwatkar, A. Laguerre, M. Beke, I. Kang, A. S. Ali, D. K. Farmer, E. T. Gall, M. Heidarinejad, and B. Stephens. 2021. Evaluating a commercially available in-duct bipolar ionization device for pollutant removal and potential byproduct formation. Building and Environment 195:107750. https://doi.org/10.1016/j.buildenv.2021.107750.
Zhang, M., M. Qin, C. Rode, and Z. Chen. 2017. Moisture buffering phenomenon and its impact on building energy consumption. Applied Thermal Engineering 124:337–345. https://doi.org/10.1016/j.applthermaleng.2017.05.173.
Zhang, Y., J. Mo, Y. Li, J. Sundell, P. Wargocki, J. Zhang, J. C. Little, R. Corsi, Q. Deng, and M. H. Leung. 2011. Can commonly-used fan-driven air cleaning technologies improve indoor air quality? A literature review. Atmospheric Environment 45(26):4329–4343. https://doi.org/10.1016/j.atmosenv.2011.05.041.
Zhou, S., Z. Liu, Z. Wang, C. J. Young, T. C. VandenBoer, B. B. Guo, J. Zhang, N. Carslaw, and T. F. Kahan. 2020. Hydrogen peroxide emission and fate indoors during non-bleach cleaning: a chamber and modeling study. Environmental Science & Technology 54(24):15643–15651. https://doi.org/10.1021/acs.est.0c04702.
Zhu, X., M. Lv, and X. Yang. 2019. A test-based method for estimating the service life of adsorptive portable air cleaners in removing indoor formaldehyde. Building and Environment 154:89–96. https://doi.org/10.1016/j.buildenv.2019.03.018.
























