4
Chemical Transformations
Chemical transformations can be defined as chemical processes that lead to the loss or removal of certain substances (e.g., reactants) and the generation or formation of new substances (e.g., products). The products that arise from these reactions frequently have very different properties from the reactants in terms of partitioning, toxicity, etc. There are different types of chemical reactions that are relevant indoors, including photolysis, hydrolysis, acid-base reactions, and redox reactions. Some of these processes are irreversible, leading to permanent loss of species, while others are reversible, resulting in temporary loss and eventual regeneration of reactants. These chemical processes are complex and extensive, with numerous species involved as precursors, intermediates, or products.
As outlined in Chapter 3, indoor chemical compounds partition into a variety of compartments that may contain a variety of phases; hence, chemical transformations occur at different locations indoors, including the gas phase, airborne particles, and indoor surfaces, as well as hidden places such as ducts and the heating, ventilation, and air-conditioning (HVAC) system. The partitioning of semivolatile and low-volatility molecules to indoor surfaces can increase their indoor residence times. Surface-adsorbed molecules may diffuse into the bulk of indoor surfaces and materials, where they may undergo chemical transformations. The relative rates of ventilation, gas-phase loss, and loss to surfaces are important to compare when evaluating the fate of an indoor air molecule. Reactions on surfaces can be very important, even if relatively slow, if the species is partitioned strongly to the surface.
This chapter covers the chemical transformations that occur in indoor environments, starting with those in the air and followed by those that occur on surfaces, noting the different classes of important multiphase processes. It discusses the modeling of indoor environments that needs to incorporate our knowledge of chemical reactions and partitioning processes described in Chapter 3. It concludes by listing several priority research areas in indoor chemical transformations.
AIRBORNE CHEMISTRY
In the outdoor atmosphere, chemical transformations are mostly driven by photochemistry. In contrast, indoor settings are generally dark, with much less ultraviolet and overall light (levels
a couple of orders of magnitude lower than outdoors), even during daylight hours (Abbatt and Wang, 2020). An important exception is direct sunlight, which can drive chemistry on window glass and other directly illuminated surfaces, as this is where the solar flux, although still diminished in intensity relative to outdoors, is greatest. Note that solar radiation at wavelengths shorter than ~330 nanometers (nm) is completely attenuated by windows, which precludes many photochemical reactions that are important outdoors, including the formation of hydroxyl (OH) radicals by ozone (O3) photolysis (Young et al., 2019). Furthermore, some indoor photochemistry can also be promoted by specific light sources, such as some bare fluorescent lights (Kowal et al., 2017). The spatial, temporal, and spectral variability need to be taken into account when considering the role of photochemistry in indoor environments (Kowal et al., 2017; Weschler and Carslaw, 2018; Zhou et al., 2020).
The most important indoor oxidant is considered to be O3, which is largely transported from outdoors: an indoor-to-outdoor ratio of O3 is commonly between 0.2 and 0.7 as a function of the air exchange rate (Nazaroff and Weschler, 2022). Depending on outdoor O3 levels, the O3 mixing ratio indoors is typically around 1 to 30 ppb, which is sufficiently high to trigger gas-phase oxidation of unsaturated volatile organic compounds (VOCs). This represents a major source of OH radicals in dark conditions, leading to typical OH concentrations of 1 to 5 × 105 cm−3. OH concentrations can be elevated as high as 106 to 107 cm−3 for special events, such as cooking and bleach cleaning with substantial release of nitrous acid (HONO) and hypochlorous acid (HOCl), which may go on to be photolyzed in air that is directly illuminated by sunlight (and not by reflected light) to form OH radicals (Young et al., 2019). Due to their very high reactivity, OH radicals can drive indoor chemistry at relatively low concentrations by oxidizing both saturated and unsaturated compounds. Chlorine radicals can be generated via photolysis of a number of inorganic chlorinated species (e.g., HOCl, chlorine [Cl2], nitryl chloride [ClNO2]) (Wong et al., 2017). Given its high reactivity, Cl-initiated chemistry may lead to the formation of oxygenated semivolatile products as well as secondary organic aerosol (SOA; Mattila et al., 2020a). The concentration of nitrate (NO3) radicals, which are important outdoors for nighttime chemistry, is likely negligible (<0.01 ppt) in residential indoor settings for most conditions with low O3 and relatively high NO levels (Arata et al., 2018; Young et al., 2019).
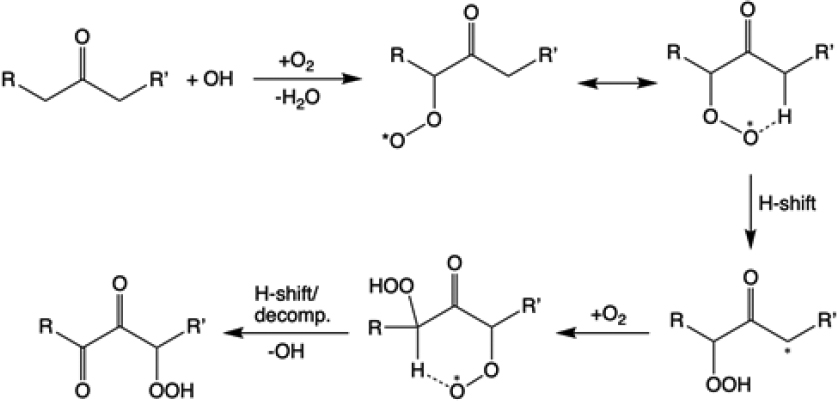
Gas-phase oxidation of VOCs leads to the formation of a myriad of semivolatile compounds, driven by complex reactions of peroxy and alkoxy radicals involving hydrogen (HOx) and nitrogen oxides (NOx). Recently, it has been shown that peroxy radicals can undergo isomerization by internal hydrogen shifts, resulting in the generation of highly oxygenated organic molecules (HOMs) (Crounse et al., 2013) (see Figure 4-1). These compounds are extremely low volatility, contributing to new particle formation and growth of SOA particles (Ehn et al., 2014). A recent theoretical study calculated the rate coefficients of the possible unimolecular reactions of the first-generation peroxy radicals formed by limonene ozonolysis, finding that they react unimolecularly with rates that are competitive indoors, especially with low concentrations of hydroperoxy (HO2) and nitric oxide (NO) (Chen et al., 2021). HOMs generated by O3-initiated autoxidation of limonene were indeed detected in an art museum; the HOM molar yield of 11 percent and the SOA mass yield of 47 percent were determined, indicating that limonene autoxidation efficiently forms SOA indoors (Pagonis et al., 2019). Inclusion of HOM formation improved the performance of an indoor chemistry model for simulating SOA mass concentrations against measurements (Kruza et al., 2020). Organic hydroperoxides may be labile in the condensed phase, potentially undergoing decomposition to yield reactive oxygen species (ROS) including OH and superoxide (Wei et al., 2021); quantification of ROS in indoor aerosols would be important for evaluation of human exposure (Morrison et al., 2021).
Indoor organic aerosol (OA) may generally be dominated by transport of outdoor OA and primary emissions by cooking, while some specific events can lead to substantial formation of indoor SOA. For example, formation of indoor SOA can be triggered by indoor illumination of bleach
SOURCE: Reprinted (adapted) with permission from Crounse, J. D., L. B. Nielsen, S. Jørgensen, H. G. Kjærgaard, and P. O. Wennberg. 2013. Autoxidation of Organic Compounds in the Atmosphere. The Journal of Physical Chemistry Letters 4(20):3513–3520. DOI: 10.1021/jz4019207. Copyright 2013 American Chemical Society.
emissions via chlorine and OH oxidation of terpenes (Wang et al., 2019), and one study showed that ozonolysis of human skin lipids can lead to new particle formation (Yang et al., 2021). A modeling study indicated that oxidative aging can affect indoor OA concentrations when air temperature and OH concentrations are high, and air exchange rates and OA concentrations are low (Cummings and Waring, 2019). Indoor OA may often exist as amorphous semisolids, reflecting low water content under low or moderate relative humidity indoors (Cummings et al., 2020). This may call the assumption of equilibrium SOA partitioning into question, and kinetic limitations of bulk diffusion may need to be properly accounted for. Indoor OA properties, including morphology, mixing state, and phase state, are still largely unexplored and further studies are desired.
While incompletely mixed conditions of indoor air constituents have been recognized as a topic of interest and concern for indoor air quality (e.g., Lambert et al., 1993), indoor air constituents often have been treated as well mixed and homogeneously distributed in ventilated indoor environments. Hence, indoor measurements are mostly conducted at a single location in a room and at a fixed height, and indoor chemistry models often employ a box model assuming homogeneous mixing. A recent study demonstrated, however, that heterogeneous distributions of indoor air pollutants arise indoors, dependent on their temporal and spatial scales as controlled by chemical reactions and deposition rates, as well as indoor air flow and ventilation (Lakey et al., 2021). Short-lived radical species (e.g., OH, Cl, NO3) exhibit sharp spatial gradients, and their temporal scales are determined mainly by reaction rates, affected only marginally by deposition and ventilation. Moderately long-lived species such as ammonia (NH3), Cl2, and O3 will exhibit spatial gradients within a room as controlled by both chemical processes and indoor air flow conditions. Long-lived species, including carbon dioxide (CO2) and VOCs, will be mostly well mixed within the indoor space, possibly affecting other rooms and the environment surrounding the building by circulation and transport outdoors. The widely applied concept of deposition velocity, which expresses the species’ flux density to the surface divided by its concentration in the uniformly mixed core region (Nazaroff et al., 1993), may need to be revisited for compounds exhibiting spatial gradients. Note that spatial gradients can also arise from localized primary emissions sources even for chemically inert species
(Mahyuddin et al., 2014; Song et al., 2021). A better understanding of spatial distributions of indoor species is critical for accurate assessments of human exposure to indoor oxidants and pollutants. Surface interactions (see Chapter 3) can impact spatial distributions but are still poorly characterized, despite their importance becoming increasingly clear (Ault et al., 2020).
SURFACE CHEMISTRY
This section considers important classes of reactions that occur via surface chemistry. Especially with rapid gas-to-surface loss, the overall rate of the process may be controlled by mass transfer in the gas phase (i.e., the movement of the molecule through the gas-phase concentration boundary layer to the surface interface). However, if the multiphase reaction rate is slow, then the overall rate is determined by the surface chemistry and not by the gas-phase mass transfer.
Oxidation Reactions
Oxidation reactions are driven by a variety of atmospheric oxidants, including ozone, hydroxyl (OH), and nitrate (NO3) (Gligorovski and Weschler, 2013; Young et al., 2019). This section explores common oxidation reactions occurring on indoor surfaces.
Common Atmospheric Oxidants, Including Ozone
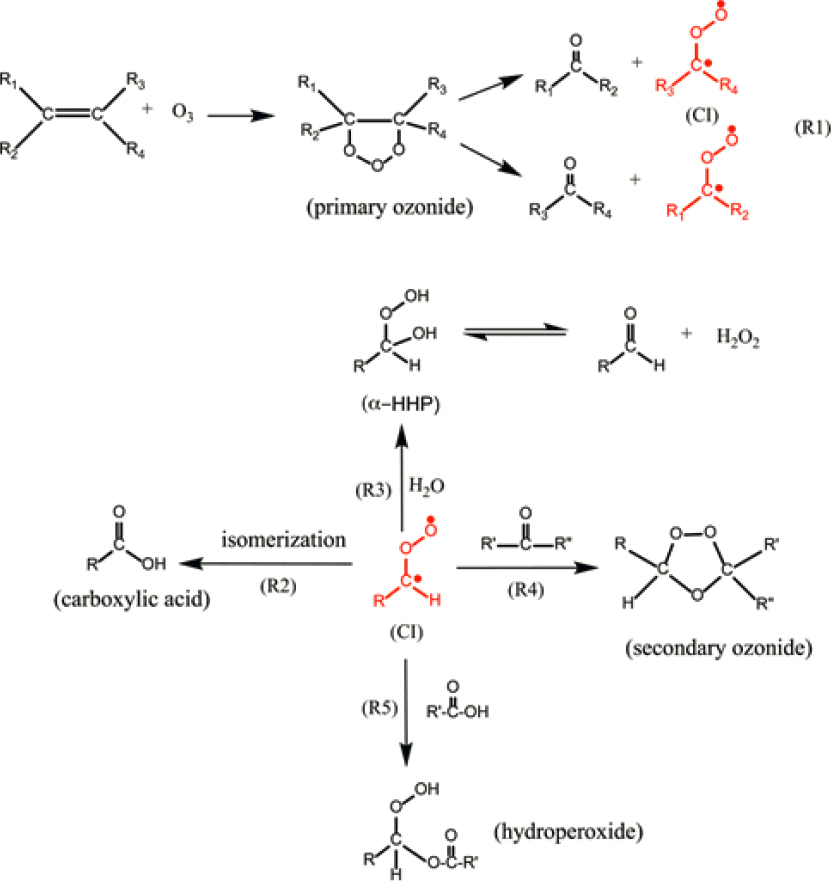
Given a sizable supply arising from outdoor-to-indoor air exchange (Stephens et al., 2012), ozone multiphase chemistry has been studied extensively with numerous recent findings. The new work builds upon extensive previous literature that established that gas-phase ozone is irreversibly lost with variable deposition velocities upon exposure to a wide range of building materials (Grøntoft and Raychaudhuri, 2004). Most indoor ozone is removed via reactions with indoor surfaces as opposed to gas-phase reactions (Nazaroff and Weschler, 2022; Weschler, 2000). Ozone can be lost on some inorganic surfaces, such as components of mineral dust (Hanisch and Crowley, 2003; Mogili et al., 2006) and manganese oxide-based catalysts (Li et al., 2021; Lian et al., 2015), forming molecular oxygen. Ozone reactions with molecules containing carbon-carbon double bonds are notable because they lead to significant chemical transformation. Reactions of this type with electron-rich functional groups are referred to as ozonolysis reactions, for which a general mechanism is provided in Figure 4-2.
As seen in Figure 4-2, a wide range of oxygenated products arises in such reactions:
| O3 + alkene → functionalized products (e.g., organic acids, carbonyls, secondary ozonides, peroxides) |
(1) |
As described below, major advances have arisen in our understanding of the multiphase ozonolysis of unsaturated oils, which are frequently present on the surface of human skin. These molecules also contaminate our clothing, which acts as both a shield to prevent ozone from reaching the underlying skin and a potential source of semivolatile oxidation products close to the body (Lakey et al., 2019; Licina et al., 2019; Morrison et al., 2016). Similar chemistry has also been studied as it pertains to natural products present in cooking oils, essential oils, cannabis, and some cleaning products (Huang et al., 2012; Liu et al., 2017; Shu and Morrison, 2012; Springs et al., 2011; Wylie and Abbatt, 2020; Zhou et al., 2019a). Many studies have recognized that these interactions drive a large reactive flux of ozone to indoor surfaces, with the simultaneous formation of numerous carbonyl-containing products (Abbass et al., 2017; Coleman et al., 2008; Gall et al., 2013; Rai et al., 2014; Wang et al., 2012). While this work focused largely on building materials and furnishings, other research has focused on detailed studies associated with the role of human occupants as the
SOURCE: Reprinted (adapted) with permission from Zhou, S., M. W. Forbes, and J. P. D. Abbatt. 2016. Kinetics and Products from Heterogeneous Oxidation of Squalene with Ozone. Environmental Science & Technology 50(21):11688-11697. DOI: 10.1021/acs.est.6b03270. Copyright 2016 American Chemical Society.
source reaction sites (Coleman et al., 2008; Pandrangi and Morrison, 2008; Tamás et al., 2006; Weschler et al., 2007; Wisthaler and Weschler, 2010; Wisthaler et al., 2005; Zannoni et al., 2021).
Pivotal experiments have involved the exposure of human subjects (or their contaminated clothing) to ozone in controlled settings, clearly demonstrating significant loss of ozone and simultaneous formation of oxygenated VOCs via laboratory, field, and modeling studies (Bekö et al., 2020; Lakey et al., 2017; Morrison et al., 2021; Wisthaler and Weschler, 2010). One study demonstrated across a range of human subjects that ozonolysis occurs not only with human sebum materials but also with exogenous compounds (e.g., lipids from cooking) (Morrison et al., 2021). At a mechanistic level, it is now known that ozone undergoes multiphase chemistry with squalene, a highly unsaturated alkene that is a major component of skin oil (Fu et al., 2013; Petrick and
Dubowski, 2009; Wells et al., 2008; Zhou et al., 2016a), and with unsaturated triglycerides, which are also present in cooking oil as well as skin oil (Zhou et al., 2019c). The chemistry is fast, with significant chemical change in the composition of skin oil occurring on timescales of hours under ambient ozone mixing ratios (Zhou et al., 2016b). Oxygenated VOCs and hydrogen peroxide are formed (Arata et al., 2019; Zhou and Abbatt, 2021), some of which are able to react in the gas phase and form SOA (Avery et al., 2019; Wang and Waring, 2014), leaving behind less volatile, highly oxygenated species on the surface (Zhou et al., 2016a). Importantly, this chemistry has been shown to occur in genuine indoor environments (see Box 4-1).
The detailed mechanism and product distribution for multiphase ozonolysis reactions of molecules containing carbon-carbon double bonds hinges on the behavior of the highly reactive Criegee intermediate. Although long recognized to be important for organic synthesis and in gas-phase atmospheric chemistry, the chemistry of condensed-phase Criegee intermediates under indoor conditions is now being explored. Studies performed as a function of relative humidity have shown that formation yields of VOCs and hydrogen peroxide (H2O2) are higher when there is more water present in the gas phase and presumably on surfaces as well (Arata et al., 2019; Zhou and Abbatt, 2021). The form that this surface water may take indoors is described in this chapter’s section on Reactions Involving Water. By contrast, when water abundance is low, multiphase loss of Criegee intermediates leads to the formation of secondary ozonides and other complex organic products arising from the Criegee intermediate combining with protic molecules, such as alcohols and carboxylic acids (Heine et al., 2017; Zhao et al., 2018; Zhou et al., 2019b). The lifetimes and toxicity of these highly oxygenated, functionalized, and higher molecular weight products on surfaces are poorly understood, with additional oxidation reactions possible, along with slow self-reactions, hydrolytic, and/or photochemical reactions. The formation of organic surface films described in Chapter 3 may occur not only through gas-to-surface partitioning of semivolatile molecules but also through contributions of such high molecular weight oxidation products and deposition of particles.
An additional class of ozonolysis reactions long explored to understand outdoor chemistry is the reaction of polycyclic aromatic hydrocarbons (PAHs) with ozone. These molecules arise from incomplete combustion activities, such as those that occur during cooking and smoking. They are especially prevalent in poorly ventilated residential settings where open burning and cookstoves are used. PAHs from outdoor wildfires can contribute to indoor concentrations as well (Messier et al., 2019). The larger PAHs are expected to partition strongly to indoor surfaces, making them susceptible to ozonolysis reactions and formation of a suite of products, including redox-active species, such as quinones. A recent study has shown that the surface reactivity of PAHs can be controlled by the viscosity and phase of the surface film, with non-reactive layers of oxidation products impeding
the reactivity of buried PAHs (Zhou et al., 2019a). This is an illustration of the importance of mass transfer processes in controlling the rates of multiphase reactive chemistry. The products of these reactions and their toxicity deserve additional attention. For example, ozonolysis of benzo[a]pyrene by indoor air produced a class of highly reactive products with both epoxide and di-alcohol functional groups (Zhou et al., 2017). This is the first demonstration that abiotic, multiphase oxidation processes can also form these carcinogenic compounds, widely known to form biotically in humans (Xue and Warshawsky, 2005).
In addition to reactions with unsaturated organic molecules, ozone reacts with reduced forms of heteroatoms, such as nitrogen, as present in nicotine, forming species such as nicotine oxide and SOA (Destaillats et al., 2006; Petrick et al., 2010; Sleiman et al., 2010; Wang et al., 2018). Indeed, commercial ozonolysis is a process employed in the removal of unwanted third-hand smoking odors from contaminated spaces (see Chapter 5).
By contrast to O3, gas-phase OH and NO3 are too short-lived to be transported from outside; rather, they are generated indoors at lower mixing ratios than outdoors (Young et al., 2019). The potential for multiphase indoor oxidation by OH has been explored recently, where potential impacts were examined for the slow (i.e., weeks or longer) loss of low-volatility species, such as phthalates and long-chain carboxylic acids that exist in organic surface films (Alwarda et al., 2018). An area that is in need of more study is the potential for autoxidation of unsaturated oils, initiated by OH oxidation and propagated by Criegee radicals (Zeng et al., 2020). As described above, it is important to assess the rate of air-to-surface mass transfer for reactive species like OH that can be generated within a concentration boundary layer close to a surface (Morrison et al., 2019). The multiphase oxidation chemistry of NO3 has not been addressed indoors, despite studies showing fast, multiphase reactions with certain classes of molecules, such as those containing carbon-carbon double bonds.
Cleaning Agents, Including Chlorine Bleach
Oxidizing cleaning agents are frequently applied indoors in an aqueous form, either as mists, through surface wipes, or via mopping. They are also sometimes used in dishwashers and washing machines. The molecular oxidants in these cleaning agents include hypochlorite (OCl−), in commercial chlorine bleach, and H2O2, in peroxide-based cleaners. Chlorine dioxide (OClO) has also been used in select environments, usually for microbial remediation.
Indoor surfaces are sufficiently acidic to form HOCl when bleach washing occurs (Mattila et al., 2020b; Wong et al., 2017). As a result, recent work has addressed the multiphase reactions of HOCl. Well-known within the water treatment community, this oxidant reacts with unsaturated organic molecules, as well as with other functional groups such as thiols. Unlike the case with ozone, the carbon-carbon double bond in unsaturated organics is not broken by this chemistry. Rather, the organic molecules become chlorinated with, for example, chlorohydrins forming when HOCl reacts with alkenes such as squalene (Schwartz-Narbonne et al., 2019b), as shown in Reaction (2):
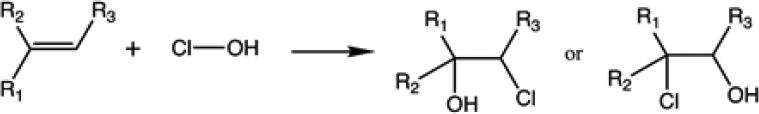 |
(2) |
This multiphase chemistry has been found to be rapid with squalene and oleic acids, demonstrating that bleach washing is likely leading to chlorination of skin and cooking oils (Schwartz-Narbonne et al., 2019b). The chlorohydrins are reactive, forming ester linkages with carboxylic acids, and lead to higher molecular weight substances that are likely to contribute to organic film growth. HOCl also reacts with terpenes, such as limonene, forming higher molecular weight
substances and potentially SOA in the presence of light (Wang et al., 2019). It is known to be reactive with reduced nitrogen as well, forming chloraldimines, for example (Finewax et al., 2021).
Chlorine bleach can promote additional reactions in the indoor environment. Although chloramines are found in headspace analysis of bleach (Wong et al., 2017), they also form when ammonia dissolves in the cleaning solution, leading to the production of monochloramine (NH2Cl), chlorimide (NHCl2), and nitrogen trichloride (NCl3) from sequential reactions with aqueous HOCl (Mattila et al., 2020b). This chemistry is well documented in the water treatment field but has only recently been demonstrated to occur in indoor environments including an indoor aquatic center (Wu et al., 2021). In addition, HOCl can react with surface nitrite, forming ClNO2, which evaporates to the gas phase and lowers HONO levels (Mattila et al., 2020b; Wang et al., 2020). Similarly, Cl2 is present in the headspace of bleach solutions (Wong et al., 2017), but it can also be formed by HOCl reacting with chloride, either present in indoor surface reservoirs or aerosol particles (Mattila et al., 2020b). Cl2, ClNO2, and HOCl are generally inert in the gas phase but can be potential sources of radicals in air that is directly exposed to sunlight or some forms of fluorescent lights, as described in the earlier section on Airborne Chemistry.
H2O2 is a known antimicrobial agent, indicating its ability to react with organic matter. Gas-phase H2O2 is readily lost to surfaces (Zhou et al., 2020), but the specific reactions in which it participates with indoor organic surface molecules are unknown. Specifically, the degree to which Fenton chemistry, which involves the reactions of iron with condensed-phase peroxides, can generate sufficient OH radicals within surface reservoirs to promote additional transformation processes is unknown. Another set of reactions, involving H2O2 reacting with carbonyls (especially aldehydes) to form more oxygenated organic products (Zhao et al., 2013), also may potentially occur on surfaces.
Reactions Involving Water
In addition to gas-phase reactions of water vapor with Criegee intermediates arising from ozonolysis reactions, aqueous-phase reactions play an important role in indoor environments. Water can adsorb onto surfaces from the gas phase in amounts determined by temperature and relative humidity. This water adsorption can lead to microscopic thin films of water or water adsorbed in nanometer- to micrometer-sized pores. Water vapor can also absorb into permeable surfaces (Schwartz-Narbonne and Donaldson, 2019). Observable water in the form of macroscopic thick films and bulk water reservoirs can also be present on window glass, in bathrooms, and in other areas within indoor spaces. Several different fields of chemistry are beginning to recognize that water in confined space environments, such as thin films, microdroplets, and inside the pores of porous materials, exhibits unique physicochemical properties compared to bulk phase water (Knight et al., 2020; Wei et al., 2020; Wilson et al., 2020). These differences between the properties of bulk water and water in confined systems (thin films, microdroplets, and pores) also need to be considered in indoor reactions involving water.
Aqueous-phase bulk reactions often involve acid-base chemistry. Acidic conditions are defined by pH values less than 7, whereas basic conditions occur at pH values above 7. These are environments where typically hydroxide ion (OH−), a base, or hydronium ion (H3O+), an acid, play a role in the reaction chemistry. However, there are other important bases in indoor environments, including ammonia, amines, and nicotine, and a number of soluble acidic substances including CO2 and formic acid (Nazaroff and Weschler, 2020). A major challenge in understanding aqueous-phase reactions in indoor environments is that, besides macroscopic thick films of water and bulk water reservoirs, thin water films and water adsorbed in small pores may be present; the concept of “pH” breaks down in these confined-space environments, as pH, defined as −log[H+], is applied to ideal dilute bulk water solutions. In these confined scenarios, activities, aH+, not concentrations, [H+], need to be considered. Furthermore, it has been proposed that stable pH gradients can exist on micrometer length scales, making these concepts of equilibrium pH measurements more difficult to apply to indoor environments where porous materials and thin water films are prevalent (Wei et al., 2018).
The above paragraphs outline the challenges in fully understanding chemical reactions of water in indoor environments. Although many of the concepts above have been implied in different review articles, these issues have not been investigated experimentally to any great extent. However, there have been studies of some reactions involving water that are relevant to indoor environments. A few cases are discussed in more detail below.
Reactions with Water and Common Indoor Inorganic Gases
It is well known that trace atmospheric gases can partition into water droplets or films and hydrolyze to yield acids, which will acidify water films (Nazaroff and Weschler, 2020). This includes reactions of carbon dioxide plus water to yield carbonic acid, H2CO3, which dissociates to carbonate and bicarbonate in water depending on solution pH. It also includes sulfur dioxide plus water to yield sulfurous acid (H2SO3), although sulfur dioxide levels in homes are relatively low compared to outdoors (Spengler et al., 1979). H2SO3 then oxidizes to sulfuric acid, leading to sulfate and bisulfate in solution, the exact speciation again depending on solution pH. In the House Observations of Microbial and Environmental Chemistry (HOMEChem) study, addition of acetic acid (in the form of vinegar) sufficiently acidified surfaces so that moderately strong acids such as formic acid, fulminic acid, and nitrous acid were strongly re-partitioned to the gas phase, implying they existed in dissociated forms in polar surface reservoirs (Wang et al., 2020).
Besides partitioning into water phases present indoors, one of the most consequential reactions with water in indoor environments that has been studied for many decades involves the disproportionation reaction of nitrogen dioxide with water (Pitts et al., 1985). Interestingly, the reaction can occur much more readily on surfaces than in the gas phase. Spectroscopic (Finlayson-Pitts et al., 2003; Goodman et al., 1999) and theoretical studies (Finlayson-Pitts, 2009) have shown that the reaction on hydrated silica surfaces leads to two products, nitrous acid and nitric acid, as in Equation (3).
| 2NO2 + H2O → HONO + HNO3 | (3) |
HONO in indoor environments has long been recognized as an important indoor air pollutant (Gligorovski, 2016), for which there is still intense interest. Indoor measurements find that HONO sometimes correlates with indoor NOx levels and anticorrelates with ozone (e.g., Lee et al., 2002), perhaps because of the reaction of nitrite on surfaces. The correlation with NOx does not necessarily occur on short timescales (Collins et al., 2018). HONO readily partitions between the surface and gas compartments, and it can undergo gas-phase photodissociation, as described in the earlier section on Airborne Chemistry. Important aspects of HONO multiphase chemistry are emerging. For example, a recent study showed that, similar to many organic compounds present indoors (Wang et al., 2020), large indoor surface reservoirs exist that lead to emissions of gas-phase HONO (Collins et al., 2018). The molecular form in which HONO exists on surfaces is not well known (i.e., whether it is as nitrite or some other species). In addition, gas-phase HONO is known to be reactive with a variety of materials on surfaces, including metal oxides and mineral dust, forming NOx (El Zein et al., 2013). A prominent example of its multiphase reactivity with surface organics is with nicotine, leading to the production of carcinogenic nitrosamines (Sleiman et al., 2010).
Reactions with Water: Ester Hydrolysis
Because of their properties as plasticizers and flame retardants, esters are used in many consumer products and are present in large quantities indoors (Wensing et al., 2005). For example, Wang et al. (2012) found different esters and their hydrolysis products in indoor dust samples collected in North America and Asia. Furthermore, the formation of alcohols from the hydrolysis of different phthalates has been observed (Castagnoli et al., 2019; Sjoberg and Ramnas, 2007). The
hydrolysis reaction results in smaller, lower molar mass compounds, an alcohol and carboxylic acid, as the forward direction of Reaction (4) illustrates.
| RCOOR’ + H2O ↔ RCOOH + R’OH | (4) |
The rate of this reaction depends on the availability of water (Bope et al., 2019). Although this process has been identified for a long time, its importance in indoor environments still has not been fully determined.
Overall, the products are more volatile than the parent ester and have the potential to partition into the gas phase. Although esters and their hydrolysis products have been detected in indoor dust samples, few studies have measured the rates of these reactions under conditions found in indoor environments. Notably, this hydrolysis reaction in bulk water is catalyzed by acids and bases, but little is known about the rates of these reactions in thin water films and small pores present in indoor materials. Initial findings of acidity effects have been indicated by fast reaction on wet alkaline concrete surfaces (Uhde and Salthammer, 2007), and with faster emission of formaldehyde from urea formaldehyde glues and resins that are used in building materials (Wolkoff and Kjaergaard, 2007).
Photochemical Reactions
The daytime chemistry of the outdoor environment is driven by photochemical reactions. In polluted environments, the term “photochemical smog” describes the interplay between sunlight (solar radiation) and the presence of hydrocarbons, reactive nitrogen oxides, and oxidants in the atmosphere that lead to particle formation and unhealthy air. Similarly, photochemical reactions can occur indoors to drive chemistry that is not thermally activated (Kowal et al., 2017; Young et al., 2019). Photosensitizers present in various forms, including semiconductor oxide particles such as titanium dioxide (TiO2), play an important role in photochemical reactions in outdoor chemistry (Chen et al., 2012) as well as indoor environments (Gligorovski, 2016) as discussed in more detail below.
Case Studies of Nitrogen Oxide Photochemistry
One of the most investigated reactions involving light-initiated chemistry is the formation of HONO from other nitrogen-containing species. In particular, photochemical conversion of both nitrogen dioxide and surface-adsorbed nitrates can be important in the formation of HONO indoors. For example, photolysis of surface nitrate leads to nitrogen dioxide (NO2) and nitrite (NO2−), the latter of which is then protonated to form gas-phase HONO. The photochemical loss of nitrate is enhanced on semiconductor oxides such as TiO2 relative to insulator oxides (Gankanda and Grassian, 2013). The formation of nitrogen oxides from irradiation of nitrates mixed with TiO2 using all widely available indoor light sources has been suggested as a potential NOx source indoors (Schwartz-Narbonne et al., 2019a). Additionally, solar light in the range from 330 nm to 400 nm passes through glass windows and can initiate light-mediated conversion of NO2 to HONO on indoor surfaces, such as gypsum (Gligorovski, 2016; Pandit et al., 2021), and potentially on the skin via TiO2 in personal care products. Incandescent and fluorescent light sources can drive this chemistry as well (Pandit et al., 2021). Langridge et al. (2009) showed that self-cleaning window glass with a TiO2 nanoparticle coating can be a strong daytime source of indoor HONO. Several studies have demonstrated photo-induced HONO formation on white wall paints (with 7 percent TiO2) by both direct solar light and indoor light bulbs near the sources (Gandolfo et al., 2015, 2020; Gemayel et al., 2017). Additionally, photocatalytic paints can degrade NO2 and formaldehyde, with selective efficiency for other VOCs (Salthammer and Fuhrmann, 2007). Bartolomei et al. (2014) have seen UV-induced HONO formation from NO2 and common household products, including detergents and lacquers, although
the exact photoactive chemicals in these household products were not identified. In one study by Depoorter et al. (2021), specific organic photosensitizers such as furfural were shown to contribute to HONO formation in indoor environments. Therefore, wavelengths less than 400 nm, surface acidity, and the light-absorbing properties of photosensitizers (which can be organic compounds and inorganic materials) are important parameters that can alter indoor HONO concentrations.
Importantly, photosensitizers produce ROS, including OH. It has been suggested that on TiO2, adsorbed nitrogen dioxide can photodissociate to NO and O (Gligorovski, 2016). This is followed by the reaction of adsorbed forms of NO and OH, present on the surface from reactions of water on irradiated TiO2, to yield HONO. ROS can also readily react with organic compounds to yield more oxygenated, less volatile organic compounds that will have a higher affinity for indoor surfaces.
MODELING INDOOR CHEMISTRY
Indoor chemistry models are essential for quantifying chemical transformations and partitioning by treating a variety of highly complex chemical and physical processes. Models can be used to
- assess gaps in our fundamental understanding of indoor chemistry processes and evaluate major uncertainties,
- guide measurements and design laboratory experiments through identification of key parameters and estimates of expected concentrations of species,
- predict under what conditions indoor air chemistry processes might cause deleterious impacts to human health and well-being,
- design effective operation of buildings to mitigate such risks, and
- provide a foundation for chemical exposure assessment (see Chapter 6).
More than 30 years ago, Nazaroff and Cass (1986, 1989) conducted a pioneering study to develop a general mathematical model of reactive gas-phase species and particulate matter, accounting for gas-phase chemical reactions and photolysis as well as the effects of ventilation, filtration, deposition onto surfaces, direct emission, and coagulation. They further developed a conceptual model for the rate of deposition of reactive gas-phase pollutants by combining mass transport and surface kinetics under different airflow conditions, including laminar convection flow and homogeneous turbulence (Cano-Ruiz et al., 1993).
Later investigators developed increasingly detailed models of gas chemistry and particle composition (Carslaw, 2007; Sarwar et al., 2003; Wang and Waring, 2014). Some models adopt representations for outdoor atmospheric chemistry including detailed gas-phase chemistry mechanisms and OA model frameworks, further developing them for indoor scenarios by adding indoor-relevant processes (Carslaw et al., 2012; Cummings and Waring, 2019). Models improve aerosol representation by providing a better understanding of different aerosol fractions by season or region, as well as by exploring whether the assumption of equilibrium is relevant for typical buildings with short residence times and large temperature variations (Weschler et al., 2008). The impacts of the wide variety of indoor sources such as cooking and cleaning on indoor aerosol composition warrant further investigation.
Efforts to develop and apply molecular- and process-based models to a variety of indoor chemical and physical processes are increasing. Molecular dynamics simulations are used to investigate interactions of oxidants and organic compounds with indoor surfaces. Numerical and analytical approaches are used to describe mass transport of species through the indoor boundary layer as controlled by diffusion and turbulence (Morrison et al., 2019). The formation and growth of indoor organic surface films have also been modeled with respect to partitioning (Weschler and Nazaroff, 2017), multilayer adsorption (Eichler et al., 2019), and heterogeneous reactions (Lakey et al., 2021).
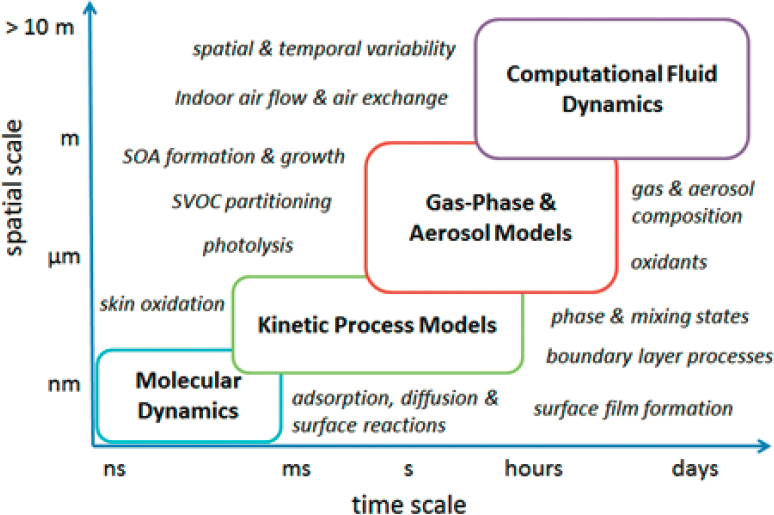
There are emerging and concerted efforts to coordinate the different types of models into integrated indoor chemistry modeling frameworks (see Figure 4-3). This effort requires expertise in chemistry, engineering, and building science; proficiency in modeling approaches that operate across a wide range of timescales and space scales; and active integration of building- and people-related factors, such as air exchange rate, ventilation strategy, and occupants’ behavior and activities. Recent modeling efforts on ozone reactions with human skin lipids represent a good example for integration of different modeling approaches with molecular-to-room scales (von Domaros et al., 2020) as well as model applications to experimental observations showing human impacts on ozone and semivolatile organic compound concentrations (Wisthaler and Weschler, 2010). Molecular dynamics simulations were applied to simulate ozone interactions with squalene, determining key kinetic parameters that can be used directly in a kinetic process model to resolve mass transport and chemical reactions in the gas phase, in clothing, and on skin (Lakey et al., 2019). In clothing, diffusion can be slowed down due to partitioning of species to skin oils and other substances covering the fibers, as simulated by human envelope models (Morrison et al., 2017). For examination of the spatial distributions of ozone and reaction products, computational fluid dynamics (CFD) modeling can account for convection, diffusion, chemical reactions, and source emissions (Won et al., 2020). As it is computationally too expensive to resolve detailed surface interactions within the CFD model, outputs from the kinetic flux model including the ozone uptake coefficient and the product yields are input to constrain the model and alleviate the computational burden. The model results show that primary ozonolysis products are concentrated in the human envelope and the breathing zone, while secondary products are relatively well distributed throughout the room (Won et al., 2020). Such a combined approach can simulate complex indoor chemical processes and evaluate human exposure to secondary pollutants.
To validate and evaluate indoor chemistry models, comparisons with observations and experiments are crucial. Recently, a growing number of targeted laboratory experiments and indoor field
SOURCE: Modified from Shiraiwa et al. (2019).
NOTE: SOA = secondary organic aerosol; SVOC = semivolatile organic compounds.
observations have been conducted, such as the HOMEChem study and the Indoor Chemical Human Emissions and Reactivity experiments, providing unique datasets measured by state-of-the-art experimental techniques. Combining models with such experimental data is beneficial to test hypotheses and gain mechanistic and quantitative interpretation of observations, as demonstrated by several recent advances. For example, kinetic modeling revealed that multiphase chemistry in aqueous bleach and aerosol/surface uptake are essential in controlling reactive chlorine and nitrogen species after bleach applications in HOMEChem (Mattila et al., 2020b). Integration of surface spectroscopic measurements with molecular dynamics simulations and kinetic modeling has provided a molecular picture of the interaction of hydrophobic molecules with hydrophilic surfaces (Fang et al., 2019). A combined spectroscopic and atomistic modeling approach has also elucidated the conformational and orientational preferences of squalene at the air/oil interface and their implications for reactions with ozone (von Domaros et al., 2020). Applications of kinetic and thermodynamic modeling revealed that the multiphase reactivity of PAHs is driven by phase separation and diffusion limitations, affecting their fates in indoor environments (Zhou et al., 2019a).
Models also allow for extrapolation of experimental results to indoor conditions, spaces, and scenarios that are inaccessible by measurement. Laboratory experiments are often conducted at high concentrations with short reaction times, which contrasts the relatively low concentrations with longer reaction times in real indoor environments. Once the model is constrained, it can simulate concentrations and species properties under indoor-relevant conditions. While measurements are often conducted at one location, applications of CFD modeling can visualize spatial distributions of indoor species. If experiments and observations are unavailable to validate models, probabilistic modeling can be applied, whereby model input parameters are varied over their most likely values and the model sensitivity to these changes is explored (Cummings et al., 2020). As well as providing an understanding of the model sensitivity to specific parameters, this technique allows ranking of model parameters in terms of importance for prioritizing future measurements and experiments.
CONCLUSIONS
Major findings from the past several years have illustrated the complexity of chemical reactions that occur in indoor environments. In particular, gas-phase oxidation reactions, some occurring via auto-oxidation mechanisms, lead to the formation of a suite of highly oxygenated gas-phase species that may form SOA. In addition, much of reactive indoor chemistry occurs on surfaces via multiphase chemistry. Although long acknowledged to be important, ozonolysis reactions of unsaturated organics have now been demonstrated to form highly oxygenated species, such as secondary ozonides and volatile oxygenates, on surfaces. This chemistry is now known conclusively to occur on humans and their clothing and on other surfaces contaminated by cooking or smoking.
Another important conclusion is that the complexity of such reactions presently precludes a quantitative understanding of these processes under genuine indoor conditions, where substrate composition and environmental parameters (e.g., relative humidity) have been shown to affect the mechanisms and kinetics. Without a better understanding of the identities and amounts of many indoor chemicals, especially in surface reservoirs, an accurate toxicological and epidemiological evaluation of chemical dose and health outcomes is not yet possible in indoor environments. Furthermore, such uncertainties in reactive chemistry when coupled to uncertainties in partitioning make it challenging to determine the relative importance of the major exposure pathways for many indoor chemicals.
Recently, new chemistry has been identified when chemical cleaning agents, such as chlorine bleach, are used on indoor surfaces. The suite of chemical products that arise from such activities is only just starting to be studied. The reactive chemistry that occurs with some other common cleaning agents, such as hydrogen peroxide, has yet to be investigated under indoor conditions.
Photochemistry has yet to be definitely demonstrated to be of importance in genuine indoor settings, except when the air or surfaces are directly illuminated with sunlight. While infrequent in many indoor settings, high levels of oxidants can be generated, and other reactive photochemistry can occur in such situations. It is possible that important, yet slow, photochemistry occurs elsewhere on indoor surfaces that are not exposed to direct sunlight, but this has yet to be confirmed.
Water is an important molecule indoors for facilitating chemical transformations. These can include acid-base reactions, slow hydrolysis of organic compounds such as esters, reactions with Criegee intermediates that form during ozonolysis of unsaturated organics, and the NO2 disproportionation reaction.
Important progress has been made in the past few years to develop models that integrate our growing knowledge of chemical transformations, partitioning between different indoor reservoirs, mass transfer, and indoor-outdoor air exchange. However, these models remain limited in their predictive capabilities owing to uncertainties in the underlying fundamental chemistry, especially on surfaces.
RESEARCH NEEDS
Given its findings about the current state of the science, the committee has identified priority research areas to help drive future advances in chemical transformations relevant to indoor environments:
- Expand research into the chemistry associated with human occupancy, behavior, and activities, especially to identify processes that alter exposure to chemicals. Common human activities, such as cooking, cleaning, smoking, and personal care product use, lead to chemical change that needs to be fully investigated. The complete suite of transformation products that arise when these primary emissions react in the indoor environment is unknown.
- Investigate transformations of long-lived contaminants. Many chemical contaminants, such as phthalates, are frequently viewed as being chemically inert, with ventilation a more important removal process for semivolatile species. This may not be the case for some species. There is a need to assess the degree to which potentially toxic contaminants, especially those with low volatility that strongly partition to indoor surfaces, are removed and transformed via chemical reactions. As described in Chapter 2, the indoor environment is the receptor of thousands of new chemicals used in consumer products. It needs to be determined which of these species are chemically unstable or reactive, and which can transform into potentially more toxic chemicals.
- Apply advanced instrumentation and analytical techniques to study chemistry taking place in a broader range of building types, including their air, contents, and surfaces. The recent use of highly advanced techniques in analytical science has illustrated how reactive chemistry can drive the chemical complexity of indoor environments. These studies include measurements of the gas phase and suspended particles, with instruments providing detailed chemical information in situ and with high time resolution. It is recommended that such detailed studies are used to characterize a wider range of indoor environments. While prior work has frequently characterized indoor constituents in a time-averaged sense, chemical reactions occur in a dynamic manner with their rates dependent on changing environmental conditions and oxidant levels. It is important to examine the dynamical behavior of indoor environments that may give rise to spatial and temporal composition gradients and, consequently, variable exposure. Detailed studies of the surface composition of passively collected samples from indoor environments could
- also be expanded. Given the dynamic nature of indoor environments, it will be fruitful to apply advanced surface analysis techniques in situ to outstanding questions such as the identification of nitrogen-containing compounds on surfaces that lead to the formation of nitrous acid and the assessment of the dynamic mass balance of skin oil materials that are always being deposited and chemically transformed.
- Broaden our understanding of chemistry taking place on and within the complex surface materials and interfaces present within buildings. Surface materials are highly complex, with variable chemical composition, morphology, and porosity. It is not known how the structure of such surfaces affects the rates and products of surface reactions. For example, studies in microdroplets and micron-thick water films have shown chemical reactions to be greatly accelerated relative to the bulk, which may be due to the unique environment of the microdroplet, especially the interface. This could be due to partial solvation of reactants, fast diffusion of reactants and products, different chemical speciation at the interface compared to the bulk, orientation of interfacial reactants, and surface potential. It is important to determine how such driving forces apply to porous materials indoors. It is likely that comparable effects arise with organic surface films and materials.
- Expand, improve, and integrate models across different timescales and spatial scales. Timescales with direct relevance to human exposure can range from short, with variations in indoor air composition on the scale of seconds to minutes, to very long, with slow release from indoor surface reservoirs occurring over decades. Characterization of reactive behavior at the molecular level, especially on surfaces, needs to be increasingly coupled to models that describe the overall, room-, and building-scale behavior. Multiphase modeling could capture the coupled, complex condensed-phase mass transfer and chemistry that occurs within permeable surface reservoirs. To resolve the spatial and temporal gradients that exist for chemicals indoors, there are new opportunities to couple CFD to detailed chemistry models. Models are also limited due to uncertainties regarding the parameterization of surface interactions, the propagation of light through indoor environments, and the concentrations and identity of a suite of secondary pollutants formed through indoor chemical reactions.
REFERENCES
Abbass, O. A., D. J. Sailor, and E. T. Gall. 2017. Effect of fiber material on ozone removal and carbonyl production from carpets. Atmospheric Environment 148:42–48. https://doi.org/10.1016/j.atmosenv.2016.10.034.
Abbatt, J. P. D., and C. Wang. 2020. The atmospheric chemistry of indoor environments. Environmental Science: Processes & Impacts 22(1):25–48. https://doi.org/10.1039/c9em00386j.
Alwarda, R., S. Zhou, and J. P. D. Abbatt. 2018. Heterogeneous oxidation of indoor surfaces by gas-phase hydroxyl radicals. Indoor Air 28(5):655–664. https://doi.org/10.1111/ina.12476.
Arata, C., N. Heine, N. Wang, P. K. Misztal, P. Wargocki, G. Beko, J. Williams, W. W. Nazaroff, K. R. Wilson, and A. H. Goldstein. 2019. Heterogeneous ozonolysis of squalene: Gas-phase products depend on water vapor concentration. Environmental Science & Technology 53(24):14441–14448. https://doi.org/10.1021/acs.est.9b05957.
Arata, C., K. J. Zarzana, P. K. Misztal, Y. Liu, S. S. Brown, W. W. Nazaroff, and A. H. Goldstein. 2018. Measurement of NO3 and N2O5 in a residential kitchen. Environmental Science & Technology Letters 5(10):595–599. https://doi.org/10.1021/acs.estlett.8b00415.
Ault, A. P., V. H. Grassian, N. Carslaw, D. B. Collins, H. Destaillats, D. J. Donaldson, D. K. Farmer, J. L. Jimenez, V. F. McNeill, G. C. Morrison, R. E. O’Brien, M. Shiraiwa, M. E. Vance, J. R. Wells, and W. Xiong. 2020. Indoor surface chemistry: Developing a molecular picture of reactions on indoor interfaces. Chem 6(12):3203–3218. https://doi.org/10.1016/j.chempr.2020.08.023.
Avery, A. M., M. S. Waring, and P. F. DeCarlo. 2019. Human occupant contribution to secondary aerosol mass in the indoor environment. Environmental Science: Processes & Impacts 21(8):1301–1312. https://doi.org/10.1039/c9em00097f.
Bartolomei, V., M. Sorgel, S. Gligorovski, E. G. Alvarez, A. Gandolfo, R. Strekowski, E. Quivet, A. Held, C. Zetzsch, and H. Wortham. 2014. Formation of indoor nitrous acid (HONO) by light-induced NO2 heterogeneous reactions with white wall paint. Environmental Science and Pollution Research International 21(15):9259–9269. https://doi.org/10.1007/s11356-014-2836-5.
Bekö, G., P. Wargocki, N. Wang, M. Li, C. J. Weschler, G. Morrison, S. Langer, L. Ernle, D. Licina, S. Yang, N. Zannoni, and J. Williams. 2020. The Indoor Chemical Human Emissions and Reactivity (ICHEAR) project: Overview of experimental methodology and preliminary results. Indoor Air 30(6):1213–1228. https://doi.org/10.1111/ina.12687.
Bope, A., S. R. Haines, B. Hegarty, C. J. Weschler, J. Peccia, and K. C. Dannemiller. 2019. Degradation of phthalate esters in floor dust at elevated relative humidity. Environmental Science: Processes & Impacts 21(8):1268–1279. https://doi.org/10.1039/c9em00050j.
Cano-Ruiz, J. A., D. Kong, R. Balas, and W. W. Nazaroff. 1993. Removal of reactive gases at indoor surfaces: Combining mass transport and surface kinetics. Atmospheric Environment. Part A. General Topics 27:2039–2050. https://doi.org/10.1016/0960-1686(93)90276-5.
Carslaw, N. 2007. A new detailed chemical model for indoor air pollution. Atmospheric Environment 41(6):1164–1179. https://doi.org/10.1016/j.atmosenv.2006.09.038.
Carslaw, N., T. Mota, M. E. Jenkin, M. H. Barley, and G. McFiggans. 2012. A significant role for nitrate and peroxide groups on indoor secondary organic aerosol. Environmental Science & Technology 46(17):9290–9298. https://doi.org/10.1021/es301350x.
Castagnoli, E., P. Backlund, O. Talvitie, T. Tuomi, A. Valtanen, R. Mikkola, H. Hovi, K. Leino, J. Kurnitski, and H. Salonen. 2019. Emissions of DEHP-free PVC flooring. Indoor Air 29(6):903–912. https://doi.org/10.1111/ina.12591.
Chen, H., C. E. Nanayakkara, and V. H. Grassian. 2012. Titanium dioxide photocatalysis in atmospheric chemistry. Chem Rev 112(11):5919–5948. https://doi.org/10.1021/cr3002092.
Chen, J., K. H. Møller, P. O. Wennberg, and H. G. Kjaergaard. 2021. Unimolecular reactions following indoor and outdoor limonene ozonolysis. The Journal of Physical Chemistry A 125(2):669–680. https://doi.org/10.1021/acs.jpca.0c09882.
Coleman, B. K., H. Destaillats, A. T. Hodgson, and W. W. Nazaroff. 2008. Ozone consumption and volatile byproduct formation from surface reactions with aircraft cabin materials and clothing fabrics. Atmospheric Environment 42(4):642–654. https://doi.org/10.1016/j.atmosenv.2007.10.001.
Collins, D. B., R. F. Hems, S. Zhou, C. Wang, E. Grignon, M. Alavy, J. A. Siegel, and J. P. D. Abbatt. 2018. Evidence for gas-surface equilibrium control of indoor nitrous acid. Environmental Science & Technology 52(21):12419–12427. https://doi.org/10.1021/acs.est.8b04512.
Crounse, J. D., L. B. Nielsen, S. Jørgensen, H. G. Kjærgaard, and P. O. Wennberg. 2013. Autoxidation of organic compounds in the atmosphere. The Journal of Physical Chemistry Letters 4(20):3513–3520. https://doi.org/10.1021/jz4019207.
Cummings, B. E., Y. Li, P. F. DeCarlo, M. Shiraiwa, and M. S. Waring. 2020. Indoor aerosol water content and phase state in U.S. residences: Impacts of relative humidity, aerosol mass and composition, and mechanical system operation. Environmental Science: Processes & Impacts 22(10):2031–2057. https://doi.org/10.1039/D0EM00122H.
Cummings, B. E., and M. S. Waring. 2019. Predicting the importance of oxidative aging on indoor organic aerosol concentrations using the two-dimensional volatility basis set (2D-VBS). Indoor Air 29(4):616–629. https://doi.org/10.1111/ina.12552.
Depoorter, A., C. Kalalian, C. Emmelin, C. Lorentz, and C. George. 2021. Indoor heterogeneous photochemistry of furfural drives emissions of nitrous acid. Indoor Air 31(3):682–692. https://doi.org/10.1111/ina.12758.
Destaillats, H., B. C. Singer, S. K. Lee, and L. A. Gundel. 2006. Effect of ozone on nicotine desorption from model surfaces: Evidence for heterogeneous chemistry. Environmental Science & Technology 40(6):1799–1805. https://doi.org/10.1021/es050914r.
Ehn, M., J. A. Thornton, E. Kleist, M. Sipilä, H. Junninen, I. Pullinen, M. Springer, F. Rubach, R. Tillmann, B. Lee, F. LopezHilfiker, S. Andres, I.-H. Acir, M. Rissanen, T. Jokinen, S. Schobesberger, J. Kangasluoma, J. Kontkanen, T. Nieminen, T. Kurtén, L. B. Nielsen, S. Jørgensen, H. G. Kjærgaard, M. Canagaratna, M. D. Maso, T. Berndt, T. Petäjä, A. Wahner, V.-M. Kerminen, M. Kulmala, D. R. Worsnop, J. Wildt, and T. F. Mentel. 2014. A large source of low-volatility secondary organic aerosol. Nature 506(7489):476–479. https://doi.org/10.1038/nature13032.
Eichler, C. M. A., J. Cao, G. Isaacman-VanWertz, and J. C. Little. 2019. Modeling the formation and growth of organic films on indoor surfaces. Indoor Air 29(1):17–29. https://doi.org/10.1111/ina.12518.
El Zein, A., M. N. Romanias, and Y. Bedjanian. 2013. Kinetics and products of heterogeneous reaction of HONO with Fe2O3 and Arizona Test Dust. Environmental Science & Technology 47(12):6325–6331. https://doi.org/10.1021/es400794c.
Fang, Y., P. S. J. Lakey, S. Riahi, A. T. McDonald, M. Shrestha, D. J. Tobias, M. Shiraiwa, and V. H. Grassian. 2019. A molecular picture of surface interactions of organic compounds on prevalent indoor surfaces: Limonene adsorption on SiO2. Chemical Science 10(10):2906–2914. https://doi.org/10.1039/C8SC05560B.
Finewax, Z., D. Pagonis, M. S. Claflin, A. V. Handschy, W. L. Brown, O. Jenks, B. A. Nault, D. A. Day, B. M. Lerner, J. L. Jimenez, P. J. Ziemann, and J. A. de Gouw. 2021. Quantification and source characterization of volatile organic compounds from exercising and application of chlorine-based cleaning products in a university athletic center. Indoor Air 31(5):1323–1339. https://doi.org/10.1111/ina.12781.
Finlayson-Pitts, B. J. 2009. Reactions at surfaces in the atmosphere: Integration of experiments and theory as necessary (but not necessarily sufficient) for predicting the physical chemistry of aerosols. Physical Chemistry Chemical Physics 11(36):7760–7779. https://doi.org/10.1039/b906540g.
Finlayson-Pitts, B. J., L. M. Wingen, A. L. Sumner, D. Syomin, and K. A. Ramazan. 2003. The heterogeneous hydrolysis of NO2 in laboratory systems and in outdoor and indoor atmospheres: An integrated mechanism. Physical Chemistry Chemical Physics 5(2):223–242. https://doi.org/10.1039/B208564J.
Fu, D., C. Leng, J. Kelley, G. Zeng, Y. Zhang, and Y. Liu. 2013. ATR-IR study of ozone initiated heterogeneous oxidation of squalene in an indoor environment. Environmental Science & Technology 47(18):10611–10618. https://doi.org/10.1021/es4019018.
Gall, E., E. Darling, J. Siegel, G. Morrison, and R. Corsi. 2013. Evaluation of three common green building materials for ozone removal, and primary and secondary emissions of aldehydes. Atmospheric Environment 77. https://doi.org/10.1016/j.atmosenv.2013.06.014.
Gandolfo, A., V. Bartolomei, E. Alvarez, T. Sabrine, S. Gligorovski, J. Kleffmann, and H. Wortham. 2015. The effectiveness of indoor photocatalytic paints on NOx and HONO levels. Applied Catalysis B: Environmental 166-167:84–90. https://doi.org/10.1016/j.apcatb.2014.11.011.
Gandolfo, A., V. Bartolomei, D. Truffier-Boutry, B. Temime-Roussel, G. Brochard, V. Berge, H. Wortham, and S. Gligorovski. 2020. The impact of photocatalytic paint porosity on indoor NOx and HONO levels. Physical Chemistry Chemical Physics 22(2):589–598. https://doi.org/10.1039/c9cp05477d.
Gankanda, A., and V. H. Grassian. 2013. Nitrate photochemistry in NaY zeolite: Product formation and product stability under different environmental conditions. Journal of Physical Chemistry A 117(10):2205–2212. https://doi.org/10.1021/jp312247m.
Gemayel, R., B. Temime-Roussel, N. Hayeck, A. Gandolfo, S. Hellebust, S. Gligorovski, and H. Wortham. 2017. Development of an analytical methodology for obtaining quantitative mass concentrations from LAAP-ToF-MS measurements. Talanta 174:715–724. https://doi.org/10.1016/j.talanta.2017.06.050.
Gligorovski, S. 2016. Nitrous acid (HONO): An emerging indoor pollutant. Journal of Photochemistry and Photobiology A: Chemistry 314:1–5. https://doi.org/10.1016/j.jphotochem.2015.06.008.
Gligorovski, S., and C. J. Weschler. 2013. The oxidative capacity of indoor atmospheres. Environmental Science & Technology 47(24):13905–13906. https://doi.org/10.1021/es404928t.
Goodman, A. L., G. M. Underwood, and V. H. Grassian. 1999. Heterogeneous reaction of NO2: Characterization of gas-phase and adsorbed products from the reaction, 2NO2(g) + H2O(a) → HONO(g) + HNO3(a) on hydrated silica particles. The Journal of Physical Chemistry A 103(36):7217–7223. https://doi.org/10.1021/jp9910688.
Grøntoft, T., and M. R. Raychaudhuri. 2004. Compilation of tables of surface deposition velocities for O3, NO2 and SO2 to a range of indoor surfaces. Atmospheric Environment 38(4):533–544. https://doi.org/10.1016/j.atmosenv.2003.10.010.
Hanisch, F., and J. N. Crowley. 2003. Ozone decomposition on Saharan dust: An experimental investigation. Atmospheric Chemistry and Physics 3(1):119–130. https://doi.org/10.5194/acp-3-119-2003.
Heine, N., F. A. Houle, and K. R. Wilson. 2017. Connecting the elementary reaction pathways of Criegee intermediates to the chemical erosion of squalene interfaces during ozonolysis. Environmental Science & Technology 51(23):13740–13748. https://doi.org/10.1021/acs.est.7b04197.
Huang, H.-L., T.-J. Tsai, N.-Y. Hsu, C.-C. Lee, P.-C. Wu, and H.-J. Su. 2012. Effects of essential oils on the formation of formaldehyde and secondary organic aerosols in an aromatherapy environment. Building and Environment 57:120–125. https://doi.org/10.1016/j.buildenv.2012.04.020.
Knight, A. W., P. Ilani-Kashkouli, J. A. Harvey, J. A. Greathouse, T. A. Ho, N. Kabengi, and A. G. Ilgen. 2020. Interfacial reactions of Cu(ii) adsorption and hydrolysis driven by nano-scale confinement. Environmental Science: Nano 7(1):68–80. https://doi.org/10.1039/C9EN00855A.
Kowal, S. F., S. R. Allen, and T. F. Kahan. 2017. Wavelength-resolved photon fluxes of indoor light sources: Implications for HOx production. Environmental Science & Technology 51(18):10423–10430. https://doi.org/10.1021/acs.est.7b02015.
Kruza, M., G. McFiggans, M. S. Waring, J. R. Wells, and N. Carslaw. 2020. Indoor secondary organic aerosols: Towards an improved representation of their formation and composition in models. Atmospheric Environment 240:117784. https://doi.org/10.1016/j.atmosenv.2020.117784.
Lakey, P. S. J., G. C. Morrison, Y. Won, K. M. Parry, M. von Domaros, D. J. Tobias, D. Rim, and M. Shiraiwa. 2019. The impact of clothing on ozone and squalene ozonolysis products in indoor environments. Communications Chemistry 2(1):56. https://doi.org/10.1038/s42004-019-0159-7.
Lakey, P. S. J., A. Wisthaler, T. Berkemeier, T. Mikoviny, U. Poschl, and M. Shiraiwa. 2017. Chemical kinetics of multiphase reactions between ozone and human skin lipids: Implications for indoor air quality and health effects. Indoor Air 27(4):816–828. https://doi.org/10.1111/ina.12360.
Lakey, P. S. J., Y. Won, D. Shaw, F. F. Østerstrøm, J. Mattila, E. Reidy, B. Bottorff, C. Rosales, C. Wang, L. Ampollini, S. Zhou, A. Novoselac, T. F. Kahan, P. F. DeCarlo, J. P. D. Abbatt, P. S. Stevens, D. K. Farmer, N. Carslaw, D. Rim, and M. Shiraiwa. 2021. Spatial and temporal scales of variability for indoor air constituents. Communications Chemistry 4(1):110. https://doi.org/10.1038/s42004-021-00548-5.
Lambert, W. E., J. M. Samet, and J. D. Spengler. 1993. Environmental tobacco smoke concentrations in no-smoking and smoking sections of restaurants. American Journal of Public Health 83:1339–1341. https://doi.org/10.2105/AJPH.83.9.1339.
Langridge, J., R. Gustafsson, P. Griffiths, R. Cox, R. Lambert, and R. Jones. 2009. Solar driven nitrous acid formation on building material surfaces containing titanium dioxide: A concern for air quality in urban areas? Atmospheric Environment 43:5128–5131. https://doi.org/10.1016/j.atmosenv.2009.06.046.
Li, L., R. Gao, and P. Zhang. 2021. Catalytic decomposition of gaseous ozone at room temperature. Progress in Chemistry 33(7):1174–1186. https://doi.org/10.7536/PC200716.
Lian, Z., J. Ma, and H. He. 2015. Decomposition of high-level ozone under high humidity over Mn–Fe catalyst: The influence of iron precursors. Catalysis Communications 59:156–160. https://doi.org/10.1016/j.catcom.2014.10.005.
Licina, D., G. C. Morrison, G. Beko, C. J. Weschler, and W. W. Nazaroff. 2019. Clothing-mediated exposures to chemicals and particles. Environmental Science & Technology 53(10):5559–5575. https://doi.org/10.1021/acs.est.9b00272.
Liu, S., R. Li, R. J. Wild, C. Warneke, J. A. de Gouw, S. S. Brown, S. L. Miller, J. C. Luongo, J. L. Jimenez, and P. J. Ziemann. 2016. Contribution of human-related sources to indoor volatile organic compounds in a university classroom. Indoor Air 26(6):925–938. https://doi.org/10.1111/ina.12272.
Liu, T., Z. Li, M. Chan, and C. K. Chan. 2017. Formation of secondary organic aerosols from gas-phase emissions of heated cooking oils. Atmospheric Chemistry and Physics 17(12):7333–7344. https://doi.org/10.5194/acp-17-7333-2017.
Liu, Y., P. K. Misztal, C. Arata, C. J. Weschler, W. W. Nazaroff, and A. H. Goldstein. 2021. Observing ozone chemistry in an occupied residence. Proceedings of the National Academy of Sciences USA 118(6). https://doi.org/10.1073/pnas.2018140118.
Mahyuddin, N., H. B. Awbi, and M. Alshitawi. 2014. The spatial distribution of carbon dioxide in rooms with particular application to classrooms. Indoor and Built Environment 23:433–448.
Mattila, J. M., C. Arata, C. Wang, E. F. Katz, A. Abeleira, Y. Zhou, S. Zhou, A. H. Goldstein, J. P. D. Abbatt, P. F. DeCarlo, and D. K. Farmer. 2020a. Dark chemistry during bleach cleaning enhances oxidation of organics and secondary organic aerosol production indoors. Environmental Science & Technology Letters 7(11):795–801. https://doi.org/10.1021/acs.estlett.0c00573.
Mattila, J. M., P. S. J. Lakey, M. Shiraiwa, C. Wang, J. P. D. Abbatt, C. Arata, A. H. Goldstein, L. Ampollini, E. F. Katz, P. F. DeCarlo, S. Zhou, T. F. Kahan, F. J. Cardoso-Saldana, L. H. Ruiz, A. Abeleira, E. K. Boedicker, M. E. Vance, and D. K. Farmer. 2020b. Multiphase chemistry controls inorganic chlorinated and nitrogenated compounds in indoor air during bleach cleaning. Environmental Science & Technology 54(3):1730–1739. https://doi.org/10.1021/acs.est.9b05767.
Messier, K. P., L. G. Tidwell, C. C. Ghetu, D. Rohlman, R. P. Scott, L. M. Bramer, H. M. Dixon, K. M. Waters, and K. A. Anderson. 2019. Indoor versus outdoor air quality during wildfires. Environmental Science & Technology Letters 6(12):696–701. https://doi.org/10.1021/acs.estlett.9b00599.
Mogili, P. K., P. D. Kleiber, M. A. Young, and V. H. Grassian. 2006. Heterogeneous uptake of ozone on reactive components of mineral dust aerosol: An environmental aerosol reaction chamber study. The Journal of Physical Chemistry A 110(51):13799–13807. https://doi.org/10.1021/jp063620g.
Morrison, G., P. S. J. Lakey, J. Abbatt, and M. Shiraiwa. 2019. Indoor boundary layer chemistry modeling. Indoor Air 29(6):956–967. https://doi.org/10.1111/ina.12601.
Morrison, G. C., A. Eftekhari, F. Majluf, and J. E. Krechmer. 2021. Yields and variability of ozone reaction products from human skin. Environmental Science & Technology 55(1):179–187. https://doi.org/10.1021/acs.est.0c05262.
Morrison, G. C., C. J. Weschler, and G. Bekö. 2017. Dermal uptake of phthalates from clothing: Comparison of model to human participant results. Indoor Air 27(3):642–649. https://doi.org/10.1111/ina.12354.
Morrison, G. C., C. J. Weschler, G. Bekö, H. M. Koch, T. Salthammer, T. Schripp, J. Toftum, and G. Clausen. 2016. Role of clothing in both accelerating and impeding dermal absorption of airborne SVOCs. Journal of Exposure Science & Environmental Epidemiology 26(1):113–118. https://doi.org/10.1038/jes.2015.42.
Nazaroff, W. W., and G. R. Cass. 1986. Mathematical modeling of chemically reactive pollutants in indoor air. Environmental Science & Technology 20(9):924–934. https://doi.org/10.1021/es00151a012.
Nazaroff, W. W., and G. R. Cass. 1989. Mathematical modeling of indoor aerosol dynamics. Environmental Science & Technology 23(2):157–166. https://doi.org/10.1021/es00179a003.
Nazaroff, W. W., A. J. Gadgil, and C. J. Weschler. 1993. Critique of the use of deposition velocity in modeling indoor air quality. Modeling of Indoor Air Quality and Exposure. ASTM International. https://www.astm.org/stp13101s.html.
Nazaroff, W. W., and C. J. Weschler. 2020. Indoor acids and bases. Indoor Air 30(4):559–644. https://doi.org/10.1111/ina.12670.
Nazaroff, W. W., and C. J. Weschler. 2022. Indoor ozone: Concentrations and influencing factors. Indoor Air 32:e12942.
Pagonis, D., L. B. Algrim, D. J. Price, D. A. Day, A. V. Handschy, H. Stark, S. L. Miller, J. A. de Gouw, J. L. Jimenez, and P. J. Ziemann. 2019. Autoxidation of limonene emitted in a university art museum. Environmental Science & Technology Letters 6(9):520–524. https://doi.org/10.1021/acs.estlett.9b00425.
Pandit, S., S. L. Mora Garcia, and V. H. Grassian. 2021. HONO Production from gypsum surfaces following exposure to NO2 and HNO3: Roles of relative humidity and light source. Environmental Science & Technology 55(14):9761–9772. https://doi.org/10.1021/acs.est.1c01359.
Pandrangi, L. S., and G. C. Morrison. 2008. Ozone interactions with human hair: Ozone uptake rates and product formation. Atmospheric Environment 42:5079–5089. https://doi.org/10.1016/j.atmosenv.2008.02.009.
Petrick, L., H. Destaillats, I. Zouev, S. Sabach, and Y. Dubowski. 2010. Sorption, desorption, and surface oxidative fate of nicotine. Physical Chemistry Chemical Physics 12(35):10356–10364. https://doi.org/10.1039/c002643c.
Petrick, L., and Y. Dubowski. 2009. Heterogeneous oxidation of squalene film by ozone under various indoor conditions. Indoor Air 19(5):381–391. https://doi.org/10.1111/j.1600-0668.2009.00599.x.
Pitts, J. N., T. J. Wallington, H. W. Biermann, and A. M. Winer. 1985. Identification and measurement of nitrous acid in an indoor environment. Atmospheric Environment 19(5):763–767. https://doi.org/10.1016/0004-6981(85)90064-2.
Rai, A. C., B. Guo, C. H. Lin, J. Zhang, J. Pei, and Q. Chen. 2014. Ozone reaction with clothing and its initiated VOC emissions in an environmental chamber. Indoor Air 24(1):49–58. https://doi.org/10.1111/ina.12058.
Salthammer, T., and F. Fuhrmann. 2007. Photocatalytic surface reactions on indoor wall paint. Environmental Science & Technology 41(18):6573–6578. https://doi.org/10.1021/es070057m.
Sarwar, G., R. Corsi, D. Allen, and C. Weschler. 2003. The significance of secondary organic aerosol formation and growth in buildings: Experimental and computational evidence. Atmospheric Environment 37(9):1365–1381. https://doi.org/10.1016/S1352-2310(02)01013-0.
Schwartz-Narbonne, H., and D. J. Donaldson. 2019. Water uptake by indoor surface films. Scientific Reports 9(1):11089. https://doi.org/10.1038/s41598-019-47590-x.
Schwartz-Narbonne, H., S. H. Jones, and D. J. Donaldson. 2019a. Indoor lighting releases gas phase nitrogen oxides from indoor painted surfaces. Environmental Science & Technology Letters 6(2):92–97. https://doi.org/10.1021/acs.estlett.8b00685.
Schwartz-Narbonne, H., C. Wang, S. Zhou, J. P. D. Abbatt, and J. Faust. 2019b. Heterogeneous chlorination of squalene and oleic acid. Environmental Science & Technology 53(3):1217–1224. https://doi.org/10.1021/acs.est.8b04248.
Shiraiwa, M., N. Carslaw, D. J. Tobias, M. S. Waring, D. Rim, G. Morrison, P. S. J. Lakey, M. Kruza, M. von Domaros, B. E. Cummings, and Y. Won. 2019. Modelling consortium for chemistry of indoor environments (MOCCIE): Integrating chemical processes from molecular to room scales. Environmental Science: Processes & Impacts 21(8):1240–1254. https://doi.org/10.1039/c9em00123a.
Shu, S., and G. Morrison. 2012. Rate and reaction probability of the surface reaction between ozone and dihydromyrcenol measured in a bench scale reactor and a room-sized chamber. Atmospheric Environment 47:421–427. https://doi.org/10.1016/j.atmosenv.2011.10.068.
Sjoberg, A., and O. Ramnas. 2007. An experimental parametric study of VOC from flooring systems exposed to alkaline solutions. Indoor Air 17(6):450–457. https://doi.org/10.1111/j.1600-0668.2007.00492.x.
Sleiman, M., L. A. Gundel, J. F. Pankow, P. Jacob, 3rd, B. C. Singer, and H. Destaillats. 2010. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proceedings of the National Academy of Sciences USA 107(15):6576–6581. https://doi.org/10.1073/pnas.0912820107.
Song, Y., Q. Yang, H. Li, and S. Shen. 2021. Simulation of indoor cigarette smoke particles in a ventilated room. Air Quality Atmosphere & Health 14:1837–1847.
Spengler, J. D., B. G. Ferris, and D. W. Dockery. 1979. Sulfur dioxide and nitrogen dioxide levels inside and outside homes and the implications on health effects research. Environmental Science & Technology 13(10):1276–1280. https://doi.org/10.1021/es60158a013.
Springs, M., J. R. Wells, and G. C. Morrison. 2011. Reaction rates of ozone and terpenes adsorbed to model indoor surfaces. Indoor Air 21(4):319–327. https://doi.org/10.1111/j.1600-0668.2010.00707.x.
Stephens, B., E. T. Gall, and J. A. Siegel. 2012. Measuring the penetration of ambient ozone into residential buildings. Environmental Science & Technology 46(2):929–936. https://doi.org/10.1021/es2028795.
Tamás, G., C. J. Weschler, Z. Bakó-Biró, D. P. Wyon, and P. Strøm-Tejsen. 2006. Factors affecting ozone removal rates in a simulated aircraft cabin environment. Atmospheric Environment 40(32):6122–6133. https://doi.org/10.1016/j.atmosenv.2006.05.034.
Uhde, E., and T. Salthammer. 2007. Impact of reaction products from building materials and furnishings on indoor air quality—A review of recent advances in indoor chemistry. Atmospheric Environment 41:3111–3128. https://doi.org/10.1016/j.atmosenv.2006.05.082.
von Domaros, M., P. S. J. Lakey, M. Shiraiwa, and D. J. Tobias. 2020. Multiscale modeling of human skin oil-induced indoor air chemistry: Combining kinetic models and molecular dynamics. The Journal of Physical Chemistry B 124(18):3836–3843. https://doi.org/10.1021/acs.jpcb.0c02818.
Wang, C., B. Bottorff, E. Reidy, C. M. F. Rosales, D. B. Collins, A. Novoselac, D. K. Farmer, M. E. Vance, P. S. Stevens, and J. P. D. Abbatt. 2020. Cooking, bleach cleaning, and air conditioning strongly impact levels of HONO in a house. Environmental Science & Technology 54(21):13488–13497. https://doi.org/10.1021/acs.est.0c05356.
Wang, C., D. B. Collins, and J. P. D. Abbatt. 2019. Indoor illumination of terpenes and bleach emissions leads to particle formation and growth. Environmental Science & Technology 53(20):11792–11800. https://doi.org/10.1021/acs.est.9b04261.
Wang, C., D. B. Collins, R. F. Hems, N. Borduas, M. Antinolo, and J. P. D. Abbatt. 2018. Exploring conditions for ultrafine particle formation from oxidation of cigarette smoke in indoor environments. Environmental Science & Technology 52(8):4623–4631. https://doi.org/10.1021/acs.est.7b06608.
Wang, C., and M. S. Waring. 2014. Secondary organic aerosol formation initiated from reactions between ozone and surface-sorbed squalene. Atmospheric Environment 84:222–229. https://doi.org/10.1016/j.atmosenv.2013.11.009.
Wang, L., C. Liao, F. Liu, Q. Wu, Y. Guo, H. B. Moon, H. Nakata, and K. Kannan. 2012. Occurrence and human exposure of p-hydroxybenzoic acid esters (parabens), bisphenol A diglycidyl ether (BADGE), and their hydrolysis products in indoor dust from the United States and three East Asian countries. Environmental Science & Technology 46(21):11584–11593. https://doi.org/10.1021/es303516u.
Wei, H., E. P. Vejerano, W. Leng, Q. Huang, M. R. Willner, L. C. Marr, and P. J. Vikesland. 2018. Aerosol microdroplets exhibit a stable pH gradient. Proceedings of the National Academy of Sciences USA 115(28):7272–7277. https://doi.org/10.1073/pnas.1720488115.
Wei, J., T. Fang, C. Wong, P. S. J. Lakey, S. A. Nizkorodov, and M. Shiraiwa. 2021. Superoxide formation from aqueous reactions of biogenic secondary organic aerosols. Environmental Science & Technology 55(1):260–270. https://doi.org/10.1021/acs.est.0c07789.
Wei, Z., Y. Li, R. G. Cooks, and X. Yan. 2020. Accelerated reaction kinetics in microdroplets: Overview and recent developments. Annual Review of Physical Chemistry 71:31–51. https://doi.org/10.1146/annurev-physchem-121319-110654.
Wells, J., G. C. Morrison, and B. K. Coleman. 2008. Kinetics and reaction products of ozone and surface-bound squalene. Journal of ASTM International 5(7):1–12. https://doi.org/10.1520/JAI101629.
Wensing, M., E. Uhde, and T. Salthammer. 2005. Plastics additives in the indoor environment—flame retardants and plasticizers. Science of the Total Environment 339(1–3):19–40. https://doi.org/10.1016/j.scitotenv.2004.10.028.
Weschler, C. J. 2000. Ozone in indoor environments: Concentration and chemistry. Indoor Air 10(4):269–288. https://doi.org/10.1034/j.1600-0668.2000.010004269.x.
Weschler, C. J., and N. Carslaw. 2018. Indoor chemistry. Environmental Science & Technology 52(5):2419–2428. https://doi.org/10.1021/acs.est.7b06387.
Weschler, C. J., and W. W. Nazaroff. 2017. Growth of organic films on indoor surfaces. Indoor Air 27:1101–1112.
Weschler, C. J., T. Salthammer, and H. Fromme. 2008. Partitioning of phthalates among the gas phase, airborne particles and settled dust in indoor environments. Atmospheric Environment 42(7):1449–1460. https://doi.org/10.1016/j.atmosenv.2007.11.014.
Weschler, C. J., A. Wisthaler, S. Cowlin, G. Tamas, P. Strom-Tejsen, A. T. Hodgson, H. Destaillats, J. Herrington, J. Zhang, and W. W. Nazaroff. 2007. Ozone-initiated chemistry in an occupied simulated aircraft cabin. Environmental Science & Technology 41(17):6177–6184. https://doi.org/10.1021/es0708520.
Wilson, K. R., A. M. Prophet, G. Rovelli, M. D. Willis, R. J. Rapf, and M. I. Jacobs. 2020. A kinetic description of how interfaces accelerate reactions in micro-compartments. Chemical Science 11(32):8533–8545. https://doi.org/10.1039/d0sc03189e.
Wisthaler, A., G. Tamas, D. P. Wyon, P. Strom-Tejsen, D. Space, J. Beauchamp, A. Hansel, T. D. Mark, and C. J. Weschler. 2005. Products of ozone-initiated chemistry in a simulated aircraft environment. Environmental Science & Technology 39(13):4823–4832. https://doi.org/10.1021/es047992j.
Wisthaler, A., and C. J. Weschler. 2010. Reactions of ozone with human skin lipids: Sources of carbonyls, dicarbonyls, and hydroxycarbonyls in indoor air. Proceedings of the National Academy of Sciences USA 107(15):6568–6575. https://doi.org/10.1073/pnas.0904498106.
Wolkoff, P., and S. K. Kjaergaard. 2007. The dichotomy of relative humidity on indoor air quality. Environment International 33(6):850–857. https://doi.org/10.1016/j.envint.2007.04.004.
Won, Y., P. S. J. Lakey, G. Morrison, M. Shiraiwa, and D. Rim. 2020. Spatial distributions of ozonolysis products from human surfaces in ventilated rooms. Indoor Air 30(6):1229–1240. https://doi.org/10.1111/ina.12700.
Wong, J. P. S., N. Carslaw, R. Zhao, S. Zhou, and J. P. D. Abbatt. 2017. Observations and impacts of bleach washing on indoor chlorine chemistry. Indoor Air 27(6):1082–1090. https://doi.org/10.1111/ina.12402.
Wu, T., T. Földes, L. T. Lee, D. N. Wagner, J. Jiang, A. Tasoglou, B. E. Boor, and E. R. Blatchley. 2021. Real-time measurements of gas-phase trichloramine (NCl3) in an indoor aquatic center. Environmental Science & Technology 55:8097–8107. https://doi.org/10.1021/acs.est.0c07413.
Wylie, A. D. L., and J. P. D. Abbatt. 2020. Heterogeneous ozonolysis of tetrahydrocannabinol: Implications for thirdhand cannabis smoke. Environmental Science & Technology 54(22):14215–14223. https://doi.org/10.1021/acs.est.0c03728.
Xue, W., and D. Warshawsky. 2005. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: A review. Toxicology and Applied Pharmacology 206(1):73–93. https://doi.org/10.1016/j.taap.2004.11.006.
Yao, M., C. J. Weschler, B. Zhao, L. Zhang, and R. Ma. 2020. Breathing-rate adjusted population exposure to ozone and its oxidation products in 333 cities in China. Environment International 138:105617. https://doi.org/10.1016/j.envint.2020.105617.
Yang, S., D. Licina, C. J. Weschler, N. Wang, N. Zannoni, M. Li, J. Vanhanen, S. Langer, P. Wargocki, J. Williams, and G. Bekö. 2021. Ozone initiates human-derived emission of nanocluster aerosols. Environmental Science & Technology 55(21):14536–14545. https://doi.org/10.1021/acs.est.1c03379.
Young, C. J., S. Zhou, J. A. Siegel, and T. F. Kahan. 2019. Illuminating the dark side of indoor oxidants. Environmental Science: Processes & Impacts 21(8):1229–1239. https://doi.org/10.1039/c9em00111e.
Zannoni, N., M. Li, N. Wang, L. Ernle, G. Bekö, P. Wargocki, S. Langer, C. J. Weschler, G. Morrison, and J. Williams. 2021. Effect of ozone, clothing, temperature, and humidity on the total OH reactivity emitted from humans. Environmental Science & Technology 55:13614–13624. https://doi.org/10.1021/acs.est.1c01831.
Zeng, M., N. Heine, and K. R. Wilson. 2020. Evidence that Criegee intermediates drive autoxidation in unsaturated lipids. Proceedings of the National Academy of Sciences 117(9):4486–4490. https://doi.org/10.1073/pnas.1920765117.
Zhao, R., C. M. Kenseth, Y. Huang, N. F. Dalleska, X. M. Kuang, J. Chen, S. E. Paulson, and J. H. Seinfeld. 2018. Rapid aqueous-phase hydrolysis of ester hydroperoxides arising from Criegee intermediates and organic acids. Journal of Physical Chemistry A 122(23):5190–5201. https://doi.org/10.1021/acs.jpca.8b02195.
Zhao, R., A. K. Y. Lee, R. Soong, A. J. Simpson, and J. P. D. Abbatt. 2013. Formation of aqueous-phase α-hydroxyhydroperoxides (α-HHP): Potential atmospheric impacts. Atmospheric Chemistry and Physics 13(12):5857–5872. https://doi.org/10.5194/acp-13-5857-2013.
Zhou, Z., and J. P. D. Abbatt. 2021. Formation of gas-phase hydrogen peroxide via multiphase ozonolysis of unsaturated lipids. Environmental Science & Technology Letters 8(2):114–120. https://doi.org/10.1021/acs.estlett.0c00757.
Zhou, S., M. W. Forbes, and J. P. Abbatt. 2016a. Kinetics and products from heterogeneous oxidation of squalene with ozone. Environmental Science & Technology 50(21):11688–11697. https://doi.org/10.1021/acs.est.6b03270.
Zhou, S., M. W. Forbes, Y. Katrib, and J. P. D. Abbatt. 2016b. Rapid oxidation of skin oil by ozone. Environmental Science & Technology Letters 3(4):170–174. https://doi.org/10.1021/acs.estlett.6b00086.
Zhou, S., B. C. H. Hwang, P. S. J. Lakey, A. Zuend, J. P. D. Abbatt, and M. Shiraiwa. 2019a. Multiphase reactivity of polycyclic aromatic hydrocarbons is driven by phase separation and diffusion limitations. Proceedings of the National Academy of Sciences USA 116(24):11658–11663. https://doi.org/10.1073/pnas.1902517116.
Zhou, S., S. Joudan, M. W. Forbes, Z. Zhou, and J. P. D. Abbatt. 2019b. Reaction of condensed-phase Criegee intermediates with carboxylic acids and perfluoroalkyl carboxylic acids. Environmental Science & Technology Letters 6(4):243–250. https://doi.org/10.1021/acs.estlett.9b00165.
Zhou, S., Z. Liu, Z. Wang, C. J. Young, T. C. VandenBoer, B. B. Guo, J. Zhang, N. Carslaw, and T. F. Kahan. 2020. Hydrogen peroxide emission and fate indoors during non-bleach cleaning: A chamber and modeling study. Environmental Science & Technology 54(24):15643–15651. https://doi.org/10.1021/acs.est.0c04702.
Zhou, S., L. W. Y. Yeung, M. W. Forbes, S. Mabury, and J. P. D. Abbatt. 2017. Epoxide formation from heterogeneous oxidation of benzo[a]pyrene with gas-phase ozone and indoor air. Environmental Science: Processes & Impacts 19(10):1292–1299. https://doi.org/10.1039/c7em00181a.
Zhou, Z., S. Zhou, and J. P. D. Abbatt. 2019c. Kinetics and condensed-phase products in multiphase ozonolysis of an unsaturated triglyceride. Environmental Science & Technology 53(21):12467–12475. https://doi.org/10.1021/acs.est.9b04460.






















