3
Decarbonization of Energy Systems
Energy is a basic human need that is also essential for economic growth. The global energy demand today is about 155,000 TWh, 82 percent of which is currently supplied by fossil fuels (32 percent petroleum, 23 percent natural gas, and 27 percent coal) and the rest by nuclear (5 percent); wind and solar (3 percent); and other renewable energy sources, including hydroelectric, geothermal, and biomass (10 percent) (BP, 2019; IEA, 2021e). In 2018, the primary energy consumption by the various sectors was electricity generation (38 percent), transportation (28 percent), industrial processes (23 percent), and residential and commercial spaces (12 percent) (EIA, 2021h). The global energy supply mix will be altered substantially by the decarbonization efforts of various sectors, particularly in electricity generation, and by the greater adoption of electric vehicles (EVs) for light-duty transportation. Refineries are optimized for the production of gasoline or diesel, and substantial work and investment have gone into the use of biofuels for transportation. Widespread use of EVs will disrupt both the fossil and biofuels industries.
Chemical engineering has played an essential role in meeting society’s demands for economical and energy-efficient conversion of natural resources into liquid and gaseous energy carriers while addressing environmental challenges associated with their production and use. Between now and 2050, the world population is expected to grow from 7.5 billion to well over 9 billion (OECD, 2012; UN, 2017), and increasingly prosperous populations will demand more energy; by 2050, the global demand for energy is forecast to increase by almost 50 percent (EIA, 2020b). Chemical engineering will continue to enable the equitable delivery of increasing amounts of reliable and affordable energy while supporting efforts to address the existential threat of global climate change (e.g., AIChE, 2020). Doing so will require the development and scale-up of renewable energy
sources and carbon sequestration and utilization at an unprecedented rate and scale, as well as consideration of trade-offs in such areas as water consumption, cost, and environmental justice.
This chapter describes the impetus for prioritizing decarbonization of energy systems and the important role chemical engineers will have in advancing technologies that minimize the climate impact of the energy sector. The chapter is organized from sources to end uses. Opportunities for chemical engineers are explored in energy sources; energy carrier production; energy storage; energy conversion and efficiency; and carbon capture, use, and storage.
THE NEED FOR DECARBONIZATION
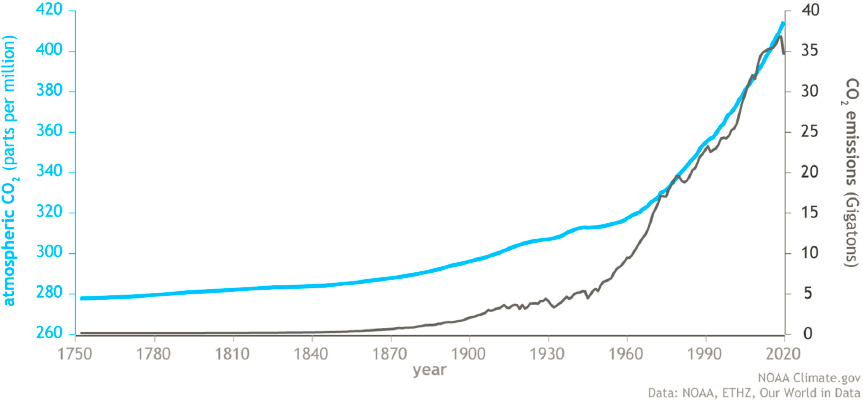
The international climate science community has established a link between global greenhouse gas (GHG) emissions and human actions and energy usage. The concentration of CO2 in the atmosphere has tracked closely its rate of anthropogenic production since the start of the Industrial Revolution in 1750 (Figure 3-1). Emissions rose to about 5 billion metric tons per year in the mid-20th century before reaching more than 35 billion metric tons per year by 2000 (NOAA, 2020). In parallel, global surface air temperatures have increased by 1 °C. Oceans absorb a large amount of CO2 released into the atmosphere. This absorbed CO2 reacts with seawater to form carbonic acid. Thus, increased CO2 levels in the atmosphere increase the acidity of the ocean, harming shellfish and other marine life.
International efforts to mitigate climate change began in 1992, culminating in 2015 with the Paris Agreement (UNFCC, 2015), whose primary goal is to keep the global
temperature rise during this century well below 2 °C above preindustrial levels. This is a monumental challenge that will require decarbonization of the energy sector, net-zero emissions, and fast-paced removal of GHG from the atmosphere. Transitioning to net-zero emissions in the energy sector is likely to cost trillions of dollars and will require efforts at all levels of government and across all sectors (e.g., the coordinated, systems-level approach proposed as part of the Sustainable Energy Corps; Alger et al., 2021). It will take decades, and may never be complete. The time required to decarbonize energy systems will depend on technological advances, government policies, changing economics of energy carrier options, and essential modifications in consumer behavior. Given the magnitude and complexity of the global energy system, no single energy carrier will be able to satisfy requirements across all sectors in the foreseeable future. Thus, achieving the goals of the Paris Agreement will require a wide range of energy sources and carriers.
The question of viable energy mixes was addressed in a comprehensive multimodel study coordinated by the Energy Modeling Forum 27 (EMF27), which examined 13 scenarios for keeping the global temperature increase below 2 °C in this century (Kriegler et al., 2014). Because of the significant number of uncertainties and assumptions involved in these 13 scenarios, it is best to use a notional average of the scenario outcomes to approximate the various energy trends. A notional average view suggests that in 2040,
- petroleum and natural gas will continue to play a significant role in the energy mix;
- coal usage will decrease significantly;
- energy carriers from nonbiogenic renewables (e.g., solar, wind, hydroelectric power) and biogenic sources (bioenergy) will grow significantly; and
- carbon capture, use, and storage (CCUS) will become a key technology for decreasing CO2 emissions.
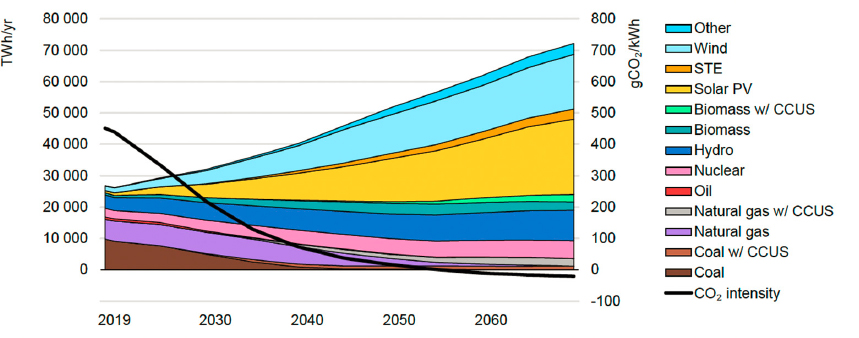
The generation, distribution, and use of electrons from renewable energy sources represent some of the most robust enablers of decarbonization. In its Sustainable Development Scenario for 2019–2070, the International Energy Agency (IEA, 2020d) concluded that the share of electricity in end-use energy demand will grow from about 20 percent to more than 50 percent. One-third of that electricity demand is expected to be met by solar power in the form of photovoltaic devices deployed at scale in decentralized form (Figure 3-2), with another 20 percent met by modular wind resources.
Opportunities exist worldwide across all sectors (electricity generation, industrial, transportation, and residential/commercial) to decrease energy-related emissions. Meeting the challenge of keeping the global temperature increase below 2 °C will require advances in four key areas:
- energy efficiency;
- increased use of lower-carbon energy sources;
- development and deployment of novel energy and energy storage technologies; and
- government policies to promote cost-effective solutions.
This chapter describes the critical role of chemical engineering in the transition from fossil fuels to renewable energy, with contributions ranging from energy carrier generation, storage, and distribution to energy use and conversion across various sectors. Some sectors, such as electricity generation, light- and medium-duty transportation, and heating and cooling for residential and commercial buildings, are less challenging to decarbonize than others (e.g., heavy-duty long-haul ground, aviation, and marine transportation; energy-intensive cement and steel production). Any low-carbon energy transition “bridging” strategy will rely on the greater use of a mix of energy carriers: electrons, hydrogen, and lower-emission liquid fuels (e.g., advanced biofuels, synthetic liquid fuels; Santiesteban and Degnan, 2021). The energy transition will require hybrid systems that combine different energy carriers to address the challenges of different usage sectors, challenges that chemical engineers are uniquely positioned to address. At the same time, it should be noted that, while chemical engineers can develop technological solutions and improve the economic competitiveness of those solutions, many of today’s barriers to addressing climate change are social and political, and chemical engineers work within that larger societal context.
ENERGY SOURCES
Chemical engineers play central roles in the recovery and development of energy sources and in energy distribution. This section focuses on the recovery and conversion of energy and opportunities for chemical engineering with respect to the two primary energy sources—solar and nuclear. The section on solar energy also includes opportunities for chemical engineers related to secondary sources (fossil fuels, biomass, and intermittent sources) derived from solar energy.
Solar Energy
The predominant source of energy for the planet is the flux of solar photons. Photons, in contrast to energy carriers, cannot be stored or “bottled.” Their energy is converted via natural processes into thermal, chemical, or electrical forms, with significant consequences for local and global climate and for human, animal, and plant life. Thermal capture acts as Earth’s thermostat, with surface temperatures balanced by albedo and greenhouse effects that also create the weather patterns from which energy is ultimately recovered in the form of wind and hydroelectric power. The energy of solar photons is also stored as chemical energy through photosynthetic cycles that convert CO2 and H2O (with photons as the energy source and coreactants) into biomass. Biogenic sources have been used as energy carriers throughout history—first soon after their formation as combustion fuels, but much more extensively in modern times as fossil fuels, well after geological chemical reactions have deoxygenated these photosynthetic residues and increased their energy density, forming natural gas, crude oil, and coal. The chemical reduction of primordial biomass that led to its deoxygenation and storage as fossil fuels is now being reversed, over much shorter periods of time, through their combustion and consequent conversion to CO2 and H2O, along with the release of heteroatoms sequestered within the biomass. These processes have had important local and global environmental consequences, but have also enabled the standard of living enjoyed today. This section describes solar energy as a primary energy source through the direct capture and conversion of photons to energy carriers (electrons; H2, NH3, or organic fuels; and heat), and as the source of fossil fuels (coal, natural gas, petroleum), biofuels (lignocellulosic and other sources), and intermittent sources (wind and marine).
Direct Capture and Conversion of Photons
Chemical engineering, the discipline most adept at transforming stored energy carriers into more convenient forms and into chemicals and materials, also brings an enabling skillset to the harvesting of photons and the storage of their energy until it is converted into its thermal, electrical, chemical, or mechanical forms. The discipline, closely collaborating with other disciplines, such as materials science, solid-state chemistry, and physics, will continue to contribute to the efficiency, durability, and reliability of photon capture materials and device components; the ability to manufacture and deploy them at scale; and the optimization, control, and systems-level strategies required for embedding modular devices within efficient electrical grids. Chemical engineers’ expertise in chemistry and catalysis and ubiquitous transport processes has been applied throughout the discipline’s history to improve the efficiency of chemical transformations. This expertise will be the enabling tool as photons are used, either directly or via electrons as intermediates, to affect the synthesis of chemicals or various energy carriers from CO2 and water. The domains of surface catalysis, photocatalysis, and electrocatalysis are firmly planted within the chemical engineering discipline.
In the case of photons, their capture and conversion to energy carriers (electrons; heat; chemical energy as H2, NH3, or small hydrocarbons/alcohols) that can be
stored and transported to consumers and markets are inseparable. The path to clean energy from photons, mediated by electrons and molecules that can be stored and transported, will require decentralized capture within “photon conversion factories,” akin in concept to the integrated refineries used to transform fossil resources into fuels and chemicals today, but in much smaller and modular forms and deployed at diverse points of photon capture. These factories will produce heat, electrons, energy carriers, and chemicals as part of modular integrated systems designed to capture the largest possible fraction of the solar flux with high quantum efficiencies and convert it into useful forms of chemical energy.
Direct photon capture and conversion to electrons as energy carriers.
There are several paths to photon capture and utilization. Direct solar conversion to electrons is carried out using semiconductors that first capture photons through electronic excitations across their band gap and then collect the excited electrons in the form of photovoltaic (PV) solar panels. Together, the decrease in the cost of PV panels and the expected increase in the electrification of energy systems are driving the deployment of global PV capacity at a rate that could not have been envisioned just a few years ago. This global capacity, only about 500 GW in 2018, will double by 2022 and is predicted to exceed 10 TW by 2030 and 30–70 TW by 2050 (the current global demand is 18 TW; Haegel et al., 2019).
Silicon (Si)-based PV cells represent about 80 percent of the currently installed solar capture capacity in the United States, the rest consisting of cadmium-telluride (CdTe) semiconducting thin-film PV cells (DOE, 2021a). High-purity amorphous and polycrystalline Si PV cells are manufactured using energy-intensive purification processes first developed for the processing of Si wafers for electronic devices. State-of-the-art Si PV cells operate at near-theoretical capture efficiencies (~30 percent), a limit set by the solar spectrum and the balance between the band gaps accessible by doping and the attainable current densities. The low-absorption cross-section of Si requires thick wafers, precluding the use of tandem devices designed to collect different components of the solar spectrum through systematic doping. Si PV cells will continue to evolve through incremental improvements in device architecture and design options at the cell/module scale, as well as through lower manufacturing costs; greater reliability/durability; and the development of infrastructure for their installation, maintenance, and seamless insertion into advanced electrical grids. Chemical engineers will continue to enable the evolution of Si PV cells, their deployment at a global scale, and the optimization and control strategies required to integrate them into the grid. Si PV cells represent the medium-term choice for deployment at scale in direct photon-to-electron conversion.
CdTe thin-film PV cells absorb photons at energies near the maximum flux in the solar spectrum. Recent improvements in efficiency and manufacturing costs have made them competitive with Si PV cells. These modules consist of micron-thick films of CdTe held within layers of conducting transparent oxides. Environmental concerns about the toxicity of the components in CdTe PV cells will need to be addressed through improvements in reliability and durability, thinner films, and higher efficiencies. These advances will enable greater market penetration as PV-based solar capture devices become more
prevalent in practice. Such advances in manufacturing, cell/module architecture, and systems integration will be driven by chemical engineering as an enabling discipline, as illustrated by recent efforts to coordinate research and manufacturing capabilities through a National Renewable Energy Laboratory–led consortium.1
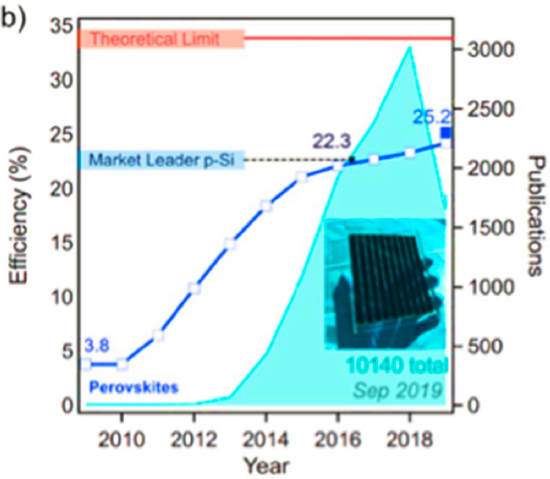
The parallel developments in dye-sensitized PV cells have recently been punctuated by the emergence of mesoscopic architectures, in which coatings of n-type semiconductor nanoparticles, such as titania, act as mesoporous anodes that provide 1,000-fold increases in dye-anode connectivity (Hardin et al., 2012; O’Regan and Grätzel, 1991). These molecular photovoltaics have emerged in parallel with perovskite solar cells (PSCs; Grätzel and Milić, 2019), leading to a significant disruption in the nature of research on PV cells and to very rapid advances in capture efficiencies. PSC devices also provide the benefits of roll-to-roll solution-based manufacturing processes, a tolerance for reagents lower in purity, and much smaller amounts of active materials relative to Si PV cells. PSC devices have evolved rapidly in photon capture efficiency, from 4 percent in 2010 to >25 percent in 2019 (Grätzel and Milić, 2019; Figure 3-3). These PSC devices are now approaching photocurrents near their theoretical maximum, but improvements in efficiency, open-circuit voltages, and long-term durability, as well as replacement of the toxic water-soluble components ubiquitous in the best-performing perovskite materials, need to be addressed before significant commercial deployment at scale can occur (Correa-Baena et al., 2017). These cells suffer from operational instabilities, short useful lives, and significant environmental and health concerns related to the long-term containment and ultimate disposal/recycling of their toxic constituents. These challenges are being addressed through significant funding from federal programs2 and an influx of entrepreneurial capital.
Concerns about toxicity and long-term durability continue to prevent PSC devices from displacing Si PV cells in the marketplace. Recent developments have led to more stable perovskite compositions, to the identification and mitigation of extrinsic degradation mechanisms, and to device configurations that ensure more reliable containment to prevent the release of toxic components in the most efficient perovskites (e.g., methylammonium lead trihalides and formamidinium analogs). Durability and containment, however, remain formidable challenges (Correa-Baena et al., 2017; Rong et al., 2018). Significant ongoing research focuses on perovskite compositions that minimize intrinsic degradation processes and on modular device architectures that ensure reliable long-term operations. As in the case of Si PV cells, chemical engineering is well positioned to address and resolve these challenges, and to deploy the improvements in practice at scale. The development of advanced solution-based processes for perovskite cell manufacture, integration of PSC systems into existing grids, and life-cycle assessment (LCA) of the full environmental impact of these devices are also encompassed by fundamentals and practice of chemical engineering.
___________________
1 See https://www.energy.gov/eere/solar/cadmium-telluride-research-and-development-consortium-coordination.
2 See https://www.energy.gov/eere/solar/solar-energy-technologies-office-fiscal-year-2020-perovskite-funding-program.
These PSC systems absorb light via direct electronic transitions, leading to high photon absorption cross-sections and to efficient capture using thin films, in contrast to the thick wafers required for Si PV cells, because of the low photon absorption cross-sections inherent in their indirect electronic transitions. Such thin films minimize the amounts of active components needed and provide significant opportunities for solution coating processes and for the scalable manufacturing of PSC devices (Li et al., 2018). Thin films also allow the synthesis of flexible devices suitable for curved surfaces; their transparent nature enables tandem cells with stacked layers of different perovskites designed to capture complementary wavelengths in the solar spectrum and a larger fraction of the impinging solar flux. A life-cycle analysis of Si-free tandem cells consisting of two perovskite layers recovered the energy required to manufacture them in 0.35 years, a much shorter period than the 1.44 years for perovskite-Si tandem cells (Tian et al., 2020a).
Conversion of photons to H2, NH3, or organic fuels as energy carriers.
The previous section addresses the conversion of solar energy via electronic excitations and ejection and capture of the emitted electrons. In this context, electrons are transported to markets or stored as chemical energy within batteries to mitigate the intermittency of solar flux. The capture of the energy of photons as energetic molecules provides alternative paths for transporting the photon energies in a different form. At the point of capture, such strategies also deal with the intermittency issues inherent in solar capture. In all cases, these strategies require modular architectures and significant integration and intensification of the photochemical and electrochemical processes involved.
Implementation requires one of the following strategies: (1) direct reduction of a common molecule (such as H2O, CO2, or N2) used as the vehicle for storing and transporting solar energy using photons directly within slurries of particulate photocatalysts (direct photocatalysis; Goto et al., 2018; Takata et al., 2020); (2) photoelectrochemical cells (PECs) that couple photovoltaic and electrochemical cells at the device scale to generate H2 from H2O, organic energy carriers or H2–CO mixtures from CO2–H2O reactants, or NH3 from N2–H2O mixtures (photoelectrochemical devices); or (3) spatially separate modules that use PV devices to generate electrons and electrocatalytic cells that use these electrons as reactants to reduce the carrier molecules (and their mixtures) to the end products listed in (2) (sequential processes). Such systems have the ultimate potential to deliver these energy carriers and chemicals at scale, but they have been demonstrated at practical scales only for H2 production via strategy (3)—the combination of commercial PV cells and H2O electrolysis modules, each at the state of the art. These strategies will require advances in the synthesis, characterization, and mechanistic assessment of catalytic solids, as well as the development of materials that can withstand severe chemical, photochemical, and electrochemical environments within complex hydrodynamics for systems that couple the required reactions through diffusional controls. Thus, the combination of, and advances in, various disciplines and such subdisciplines as catalysis, fluid mechanics, solid-state chemistry and physics, separations, advanced models and simulation at the microscopic and macroscale levels, process design, and process control will be important for future breakthroughs. These subdisciplines are all within the domains of chemical engineering research and practice.
H2 generation via photocatalytic water splitting represents the most direct route to the capture of solar energy as chemicals for either transport to markets or a means of addressing intermittency at the point of capture. The state of the art and competitiveness of the three strategies described above are discussed in several reviews (Ardo et al., 2018; The Royal Society, 2018a).
Direct catalytic water photolysis uses particulate photocatalysts consisting of an absorber (e.g., SrTiO3, Ta2N5) dispersed as aqueous suspensions. These photocatalysts generate electrons and holes that are collected separately at metal nanostructures present at their surfaces to form H2 and O2 at each location, in systems that are simple in design and applicable at larger scales than are possible with modular integrated photoelectrochemical devices. Collecting the H2 and O2 separately and preventing their recombination, extending the life of the metal-promoted semiconducting photocatalysts, and improving their capture efficiencies, however, pose significant safety, engineering, materials, and catalysis challenges (Ardo et al., 2018). This approach, and means of overcoming its challenges, are the current focus of the Japan Technological Research Association of Artificial Photosynthetic Chemical Process.
An extensive review of the challenges associated with reactor scale-up and synthesis, and of the efficiency of inorganic photoelectrodes provides the most detailed and up-to-date roadmap for the deployment of photoelectrochemical devices for water splitting. This review describes the trade-offs between efficiency and complexity as systems evolve from direct photocatalysis to integrated photoelectrochemical devices and ultimately to integrated systems with PV-electrolysis modules (Moss et al., 2021; Figure 3-4).
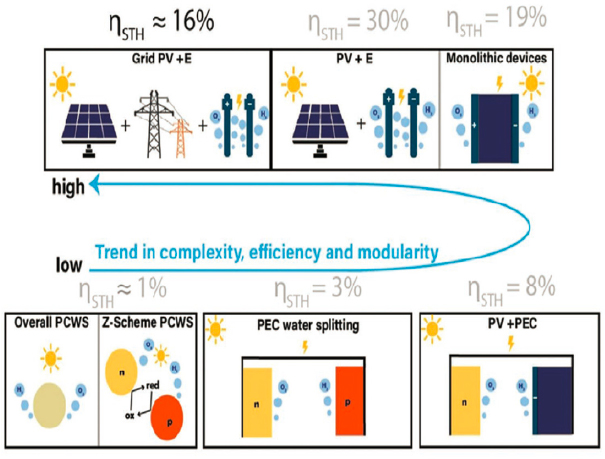
PEC devices, which combine photon capture and electron generation at the device scale, show higher capture efficiencies relative to direct photocatalytic water splitting. However, their complexity and modular architectures represent formidable hurdles for deployment at scale, as do the lifetime of the photoelectrodes and the delicate architectures required to integrate photon capture and electrolysis at the device scale. As in the case of direct photolysis, the efficiency of PEC devices decreases as more demands are placed on materials and interfaces to carry out the combined functions of photon capture, charge separation and collection, charge transport at a catalytic function, and the molecular-scale evolution of H2 and O2 via electron transfer at catalytic centers. Such PEC devices show very high photon capture efficiencies at the expense of greater cost and complexity; they represent solutions only for niche applications in the immediate future. Their ultimate use at scale remains uncertain, and any significant progress toward practical systems will require addressing engineering design, molecular and electronic transport, and materials discovery in concert.
These PEC devices for the synthesis of solar H2-based fuels, as well as their architectural analogs for artificial photosynthesis strategies for converting CO2–H2O mixtures to CO and organic energy carriers, remain at the proof-of-concept stage (Lewis, 2016). They will require the development of materials and interfaces that can efficiently induce charge separation upon photon-induced excitations and transfer these charges to catalysts that can form H2 and organic solar-derived fuels before recombination. These modules will need to be robust and relatively inexpensive for deployment at scale. Many
of these challenges are being addressed as part of the work of large multidisciplinary centers, such as the Joint Center for Artificial Photosynthesis3 and its recently announced successors, the Liquid Sunlight Alliance and the Center for Hybrid Approaches in Solar Energy to Liquid Fuels.4 These centers aim to design in concert the different components required and develop hybrid photoelectrodes that can combine photon capture and molecular catalysis to generate carriers from a broad range of wavelengths in the solar spectrum. These advances require a bridge between length and time scales inherent in photon-driven excitation and molecular transformations induced by emitted photoelectrons at a catalytic function. Systems-based integration, control, and design; reaction-transport formalisms; and knowledge of the catalytic properties of active surfaces and centers will play an enabling role in the design and selection of cost-effective devices for the direct generation of energy carriers from photons in a manner that avoids toxic and scarce elements, as well as containment and sustainability concerns (Montoya et al., 2017). Bringing such considerations into chemical, biochemical, and electron-driven processes is a domain of chemical engineers.
Formidable challenges remain for the development of electrochemical systems for direct or sequential conversion of photon energies into chemical energy in the form of H2 (from H2O), CO (from CO2), small alcohols and hydrocarbons or H2–CO mixtures for subsequent thermochemical conversion to such molecular carriers (from CO2–H2O), and ammonia (from N2–H2O). The most enduring and significant of these challenges are
- the modular nature and complex interconnections among functions and the integration of electrical and chemical processes at scale;
- the ubiquitous requirement for scarce precious metals as electrodes, and toxic or rare elements as semiconductors, dye sensitizers, dopants, and connectors;
- the need for process intensification and high photon capture efficiencies limited by transport processes within electrolytes, electrodes, or semiconductors;
- the durability of modules during extended field use and their recyclability after their useful life;
- the costly extraction of dissolved product molecules from dilute aqueous media and the separators required to avoid the recombination of photocatalytic or electrocatalytic products; and
- the energy requirements in fabrication and recovery of the component elements after use.
These matters involve catalysis and kinetics in complex and nonideal liquid systems (thermodynamic and hydrodynamic); transport of molecules, ions, and electrons in fluids and solids; materials assembly with precise nanoscale and mesoscale architectures; process integration, control, and optimization; and LCA. The challenges, fundamentals, and coping/solution strategies lie firmly within the domain of chemical engineering,
___________________
3 See https://solarfuelshub.org/.
4 See https://www.energy.gov/articles/department-energy-announces-100-million-artificial-photosynthesis-research.
which has in the past adeptly tackled challenges of similar character for complex chemical, biochemical, and electrochemical conversion processes mediated by heterogeneous, molecular, or biological catalysts.
Electrolysis remains the proven technology for electrochemical generation of H2 via modular systems based on acid polymer, liquid alkaline, or ionic-transport solid electrolytes (Ardo et al., 2018; Moss et al., 2021). Progress has recently been made in the scaling up of electrolysis, and chemical engineers have an opportunity to contribute to the development of applications at the scale required to disrupt the energy landscape. Electrolysis systems can be operated in acidic or alkaline regimes, although the rate-limiting nature of the O2 evolution half-reaction (H2O oxidation) has led to a preference for alkaline electrolyzers, which also avoid the platinum-based materials required to prevent electrode dissolution in acidic media, thus allowing the use of nonprecious metals (e.g., Ni, Fe, Cu) as electrodes. Acidic electrolyzers use polymer electrolyte membranes that minimize contact between H2 and O2 through fast proton transport and short anode–cathode distances. Alkaline electrolyzers have relied on microporous physical barriers that impose larger anode–cathode distances and ohmic losses. Recent developments in selective anion transport membranes prevent contact between H2 and O2 and have led to more compact membrane–electrode modules.
The challenges of deploying electrolyzers at scale include their efficient integration and control as multimodule stacks, the development of earth-abundant electrode materials, and thinner and more efficient separator membranes. The challenges are similar but even more formidable for electrochemical reduction of CO2 via concurrent electrolysis with H2O to form mixtures of H2, CO, alcohols, carboxylic acids, and hydrocarbons (Hori, 2008; Nitopi et al., 2019). These products can be used directly as energy carriers or as precursors to such carriers on heterogeneous catalysts (e.g., H2–CO conversion to liquid transportation fuels via Fischer-Tropsch or methanol synthesis). Electrochemical systems face similar challenges in meeting the scale required for impact:
- more efficient and robust electrodes based on earth-abundant elements;
- thinner and more selective membrane separators;
- higher-temperature electrolyzers;
- integration of electrocatalytic and thermocatalytic systems in sequence or within a single device to form more suitable energy carriers; and
- the development of supply chains to lower the costs of manufacturing and integrating the modular devices into molecular weight (MW)–scale distributed deployments for the synthesis of H2 and other energy carriers.
Conversion of photons to heat as an energy carrier.
The modular nature of PV and electrochemical cells, whether in separate or combined forms, poses significant challenges, including process integration and intensification and deployment at scale. These challenges can be addressed by using solar thermal strategies that capture the energy of photons as heat, which can be delivered to users as thermal energy, or converted to chemical energy for transport or for storage during diurnal or intermittent fluctuations in solar flux. The end use of the captured thermal energy depends on the temperatures accessible
through solar collectors and heat transfer media and on the location of markets relative to the point of capture. The specific end-use option of generating H2 at scale has been highlighted in recent reports because of its relevance to a hydrogen economy and its inherent advantages over electrolyzers in large-scale deployment (Gonzalez-Portillo et al., 2021; Moss et al., 2021; NREL, 2017).
This capture and storage of photon energies as heat can be used directly in ambient temperature control in commercial or residential spaces; as process heat in existing chemical processing plants or manufacturing operations; or for conversion into electrons or hydrogen, typically through the generation of high-pressure steam or through conversion cycles commonly termed “chemical looping.” The latter approach involves the design of reactors based on the chemical engineering principles of kinetics, transport, chemical absorbents, and construction materials that can withstand the extreme temperatures required for thermal and chemical efficiencies.
The achievement of very high temperatures (~1400 °C) has been enabled by advances in parabolic solar thermal concentrators (Sargent & Lundy LLC Consulting Group, 2003); these systems have in turn allowed the generation of very high–pressure steam for power generation in high-temperature turbines. At such high temperatures, chemical looping using redox-active oxides enables the cycling of such solids between a reduced state that reacts with water to form H2 and an oxidized state that evolves O2 in a spatially separate stage. Such processes use oxides of earth-abundant elements and form separate streams of H2 and O2 from H2O, thus avoiding the need for gas separators and the use of costly metals as electrodes, as well as the transport limitations inherent in the use of liquids as electrolytes.
These cyclic processes can be deployed via large-scale devices that allow process integration and intensification strategies unavailable for modular systems but ubiquitous in conventional refining, chemical manufacturing, and power generation processes, albeit at somewhat lower temperatures. The high temperatures required for efficient solar thermal capture pose significant challenges with respect to the design, use, and handling of the heat transfer media and the durability of the required redox-active oxides. Molten salts are typically used as heat transfer fluids, but solid particles in fluidized systems have recently emerged as attractive alternatives (Gonzalez-Portillo et al., 2021).
Fossil Fuels
Fossil fuels (coal, petroleum, and natural gas) have been essential for society’s development and progress, having powered the Industrial Revolution and shaped the modern world. Technological advances based largely on the ingenuity of chemical engineers have enabled the efficient extraction, processing, and conversion of fossil fuel raw materials into useful products. The expertise of chemical engineers remains essential in enabling a transition from the current energy landscape to one based on renewable and sustainable energy sources. However, most studies have concluded that fossil fuels will continue to play a key role in the energy mix at least until 2050, and in the meantime, the imperative is to reduce the carbon footprint of fossil fuels—a key opportunity for chemical engineers.
Coal.
Coal is the most abundant and least expensive of all fossil fuel resources. It can be converted into gas or liquids to produce chemicals and fuels, but is used primarily for direct combustion. As a solid fuel, coal generates more CO2 per unit energy than other fossil fuels—from approximately 30 percent more than diesel to nearly twice as much as natural gas (EIA, 2021i).5 Coal resources are used mainly to generate electricity, and while reliance on coal has increased in low- and middle-income countries (e.g., China and India), the opposite has been true in higher-income countries (e.g., United States, European Union, Japan). In the United States, electricity generation from coal has been declining since 2008, with the biggest drop (~16 percent) taking place in 2019; in contrast, the use of natural gas has increased dramatically since 1995, and the use of renewables has increased since 2005, albeit at a slower pace (Figure 3-5).6
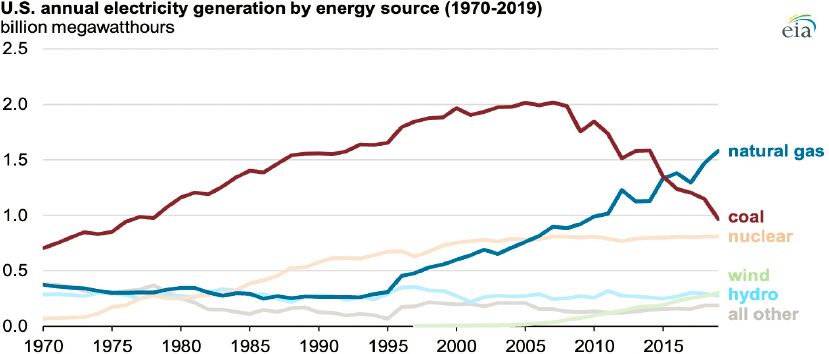
Most investments in coal-fired plants in 2019 (almost 90 percent) were for higher-efficiency (supercritical and ultrasupercritical) plants; the remaining small portion of investments were in inefficient subcritical plants, mainly in Indonesia (IEA, 2020c). High-efficiency coal-fired power plants use water at high, above-critical temperatures and pressures (373 °C and 220 bar, respectively). The efficiency gains thus achieved reduce by about 20 percent both the amount of coal needed and CO2 emissions. These plants also emit substantially lower amounts of nitrogen oxides and sulfur oxides (IEA, 2012).
___________________
5 Pounds of CO2 emitted per million Btu (gJ): coal, 215 (227); diesel, 161 (170); gasoline, 157 (166); propane, 139 (147); natural gas, 117 (123) (EIA, 2021).
6 Preliminary IEA analysis indicates a sharp drop in power-sector demand in 2020 as a result of the COVID-19 pandemic, with demand for coal having the greatest uncertainty of all fuels used for power.
To capitalize on coal’s advantages and help mitigate its disadvantages, research and development (R&D) is needed to increase thermal efficiency, demonstrate cost-effective and secure carbon capture and storage, further improve emission controls, and reduce water consumption. Meeting these challenges will require research to improve existing and develop new breakthrough technologies. The Electric Power Research Institute has recommended the following key goals for such efforts, all areas in which chemical engineers can play a role (Maxson and Phillips, 2011):
- improved plant efficiency via high-temperature materials and higher turbine inlet temperatures;
- cost-effective and scalable CO2 capture in new or retrofitted applications;
- environmentally safe and permanent storage of CO2;
- improved emission control systems that can achieve near-zero emissions of all pollutants; and
- advanced cooling and water management methods to reduce water demand and pollutant discharges.
Progress has been made in several of these areas (e.g., improved plant efficiency, improved emission control systems, and water management), but less so in the implementation of viable CCUS processes.
Natural gas.
Natural gas contains mostly methane, but also small amounts of ethane and varying amounts of heavier hydrocarbons, including propane, butane, and pentane. The ethane and heavier hydrocarbons in natural gas are typically referred to as natural gas liquids (NGLs). Natural gas can also contain CO2, sulfur, helium, nitrogen, hydrogen sulfide, and water, which are removed before it is used as an energy source.
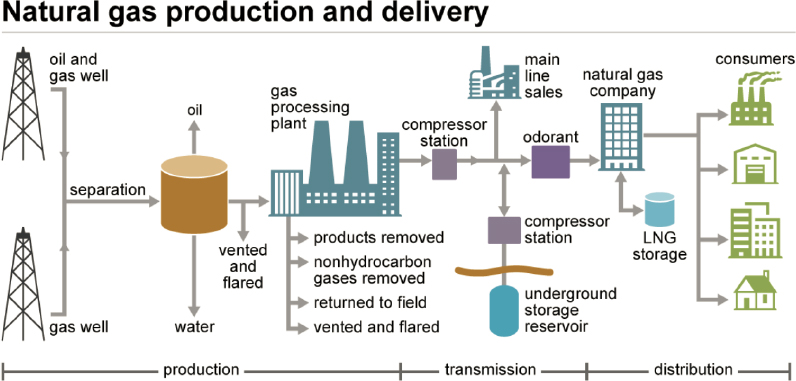
Processing plants remove water vapor and nonhydrocarbon compounds, and the NGLs are separated from the wet gas and sold separately. The separated NGLs are called natural gas plant liquids, while the processed natural gas is called dry, consumer-grade, or pipeline-quality natural gas. Some natural gas is dry enough to satisfy pipeline transportation standards without processing. Odorants (light mercaptan compounds) are added to aid in the detection of pipeline leaks. Pipelines transport dry natural gas to underground storage fields or to distribution companies and eventually to consumers (EIA, 2021g; Figure 3-6).
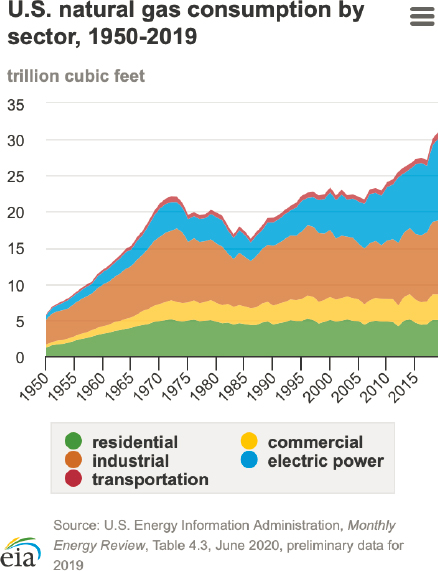
In the mid-2000s, a step change in natural gas and shale oil production occurred in the United States. Often referred to as the shale revolution, this innovation was made possible by a combination of hydraulic fracturing and horizontal drilling techniques that enabled economical oil and gas production from shale formations. The result has been a near doubling of U.S. natural gas production, from 18 trillion cubic feet in 2005 to ~34 trillion cubic feet in 2019. The United States is now a world leader in natural gas and oil production, and a global supplier. In the United States, natural gas is used primarily for electricity generation (power sector) and for heating (industry, residential, and commercial), with a small fraction used for transportation. Figure 3-7 shows natural gas consumption by the various sectors in 2019 and its evolution over the period 1950–2019.
It is generally accepted that, relative to other fossil fuels, natural gas provides a cleaner bridge to a renewable energy future, and it is the only fossil energy source projected to grow in the coming decades (DOE, 2018b). However, the longer-term future for natural gas is less certain. Innovation throughout the entire value chain will be required if natural gas is to continue being a key contributor to the future of the low-carbon energy mix. Areas in which chemical engineers will play a key role include the following:
- Production
- Advances in water-quality management; water recycling for shale or unconventional gas production
- Further reduction of methane venting to the atmosphere
- Accelerated development of CO2 to replace water as a fracturing agent
- Processing
- Development of low-energy processes for natural gas separation and purification
- Storage and transportation
- Methane leakage control
- Higher-efficiency compressors and heat exchangers
- Smart sensors for pipeline operational efficiency
- Materials for intercontinental transport via pipeline versus the current practice, which involves liquefaction and regasification
- Low-cost pipeline materials to enable cotransport of natural gas and high concentrations of hydrogen (>20 percent)
- Use
- Development of commercially viable CCUS technologies
- Improved efficiency of the overall natural gas system, including increased combustion efficiency and waste-heat recovery, and development of innovative controls and low-cost sensors that enable data-driven operations
- Development of technologies for trigeneration (combined cooling, heating, and power systems
- Design of novel processes for integration of natural gas with renewables, particularly solar and wind
- Design of novel processes for production of low- or zero-CO2 hydrogen (e.g., “blue” hydrogen [with CCUS] and “purple” hydrogen [with black carbon and/or carbon nanotubes coproduction]; see the discussion of hydrogen below)
Petroleum.
Chemical engineers have played a central role in the oil industry from its beginning, initially converting crude oil into useful products in small and simple refineries, and subsequently optimizing large and integrated refineries to address energy efficiency and environmental concerns in the manufacture and use of transportation fuels and chemicals. Chemical engineers have also worked closely with geologists to maximize the recovery of conventional and unconventional fossil resources.
The oil industry and chemical engineering evolved together. Many advances in chemical engineering science and technology were driven by the needs of the oil industry, and these advances in turn have spurred growth in the oil industry. With increasing global focus on the need to accelerate the transition to low-carbon energy to mitigate climate change, the expertise of chemical engineers is required now more than ever to help the oil industry minimize its carbon footprint. As discussed previously, the energy system is enormous and complex, and the transition to a low-carbon energy mix will take decades; in the near term, the need for petroleum and its derivative products will continue.
Petroleum is the largest energy source in the United States, used both as a fuel for transportation (road, aviation, marine, and rail) and as a feedstock for the manufacturing of such products as plastics, fibers, lubricants, paints, and solvents. In 2020, U.S. crude oil consumption averaged about 18 million barrels per day, with the transportation sector accounting for 66 percent and the industrial sector for 28 percent of this total (EIA, 2021k).
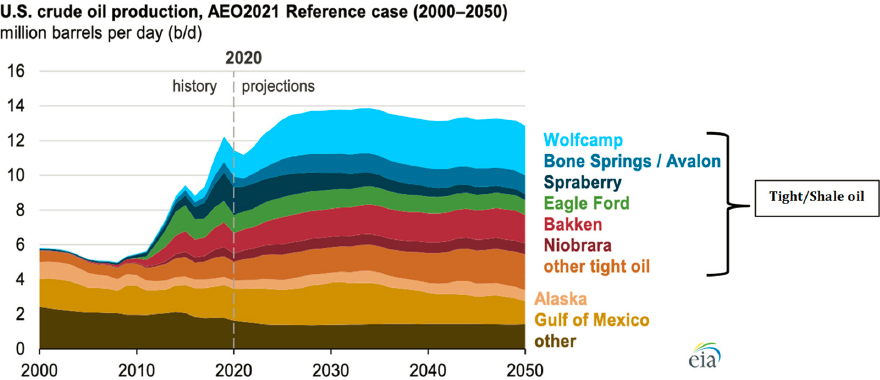
U.S. oil production totaled 9.6 million barrels per day in 1970 and declined over the subsequent 35 years. Production in 2005 was 5.2 million barrels per day, and imports reached more than 10 million barrels per day—just under 50 percent of total U.S. crude oil consumption. Since 2010, however, the combination of hydraulic fracturing and horizontal drilling has enabled access to oil trapped in shale, making the United States a top world producer of crude oil (Figure 3-8).
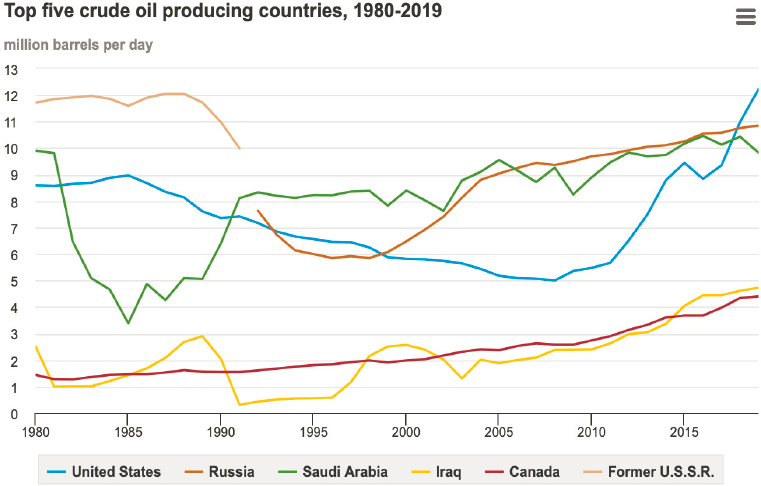
In 2020, the United States produced more than 11 million barrels of crude oil per day, with tight/shale oil accounting for about 65 percent of this total (EIA, 2021c). The U.S. Energy Information Administration, projects that tight/shale oil will remain the main source of crude oil produced in the United States (EIA, 2021b; Figure 3-9). Relative to conventional crude oil, tight/shale oils contain lighter hydrocarbons, have higher H/C ratios, and are generally very light crude (API [American Petroleum Institute] gravity 45–50) and sweet (<0.1 percent sulfur). They also require less energy to process into desired products. Thus, they are positioned to play an important role in the oil industry’s efforts to minimize its carbon footprint.
The challenge of unconventional tight/shale oil lies in improving its extraction, as it is stranded within geological features that are difficult to image and access because of their low permeability. Thus, the reservoirs need to be hydraulically fractured to create paths for the flow of oil and gas. This process requires either hydraulic fracturing of the geological systems to create paths for flow or horizontal drilling over long distances before fracturing, using explosives or high-pressure water containing various proppants (small particles such as sand or ceramic beads) and chemicals (Geoscience News and Information, 2021). Proppants, as their name implies, prop the fractures open, and the chemicals create a viscoelastic fluid to carry the proppants. The U.S. Department of Energy (DOE) is sponsoring research aimed at enhancing the ultimate recovery of oil and gas from both existing and new wells in mature and emerging basins (DOE, 2021b). Areas in which chemical engineers can contribute to innovation in tight/shale oil production are described below.
Improved water management.
As discussed in the above section on natural gas, extraction of tight/shale oil and gas requires a large amount of water and produces a large amount of water that requires treatment or disposal. Low-cost technologies for produced-water treatment are required to maximize water usage recycling, thus minimizing freshwater usage. Produced water may contain injected chemicals plus naturally occurring materials such as brines, metals, radionuclides, and hydrocarbons. The flowback and produced water are usually stored in tanks or pits before treatment or disposal, often through underground injection (DOE, 2021b; EPA, 2021b). Potential options for either replacing water as a working fluid or minimizing its use include the use of liquefied propane gas (LPG; e.g., API, 2021), supercritical CO2 (Song et al., 2019), or microwave fracking (e.g., Aresco, 2021).
Increased recovery to extend well life.
The amount of oil produced in primary recovery from an unconventional reservoir is much smaller than that produced from a conventional reservoir. In addition, production rates from unconventional wells often decline by more than 50 percent in the first year. Improved fracturing technology to create more efficient and durable oil and gas flow pathways is therefore needed. Chemical engineers can contribute to meeting this challenge by applying their understanding of mass transport in porous materials.
Data-driven approaches.
The DOE national laboratories, in collaboration with universities and industry, are leading an effort to integrate physics-informed statistical models; inverse models, such as neural networks; natural language processing; big data analytics; and other emerging artificial intelligence/machine learning (AI/ML) technologies to draw meaningful insights from reservoir data for real-time rapid visualization and prediction to enable effective decision making (DOE, 2021b).
Methane management.
The atmospheric concentration of methane, a more potent GHG than CO2, has risen steadily for more than a decade (Nisbet et al., 2019). This trend reflects the increased production of shale oil and gas, as well as the natural (e.g., from wetlands and other flood zones) and biogenic (e.g., from agriculture or waste) emissions that also play a role. As a result of regulations, the oil industry has made good progress in reducing methane emissions; however, more progress is needed. The main source of fugitive methane emissions is well venting, followed by pneumatic devices that use natural gas as the operating fluid, as well as storage and transport venting and leaks.
Chemical engineers can enable significant reductions in methane emissions by
- developing low-cost modular technologies for conversion of methane to liquid products to replace venting;
- developing methods for using air instead of natural gas as the operating fluid for pneumatic controllers;
- improving techniques and developing smart sensors for methane leakage control and detection; and
- improving methods for deploying higher-efficiency compressors.
Biofuels
The production of biofuels from biogenic carbon, such as waste plant matter, algae, and organic waste, has long been heralded as a means of offsetting GHG emissions from the combustion of fossil fuels (e.g., Lynd et al., 1991; Pacala and Socolow, 2004). The combustion of waste plant matter—lignocellulose—and other biogenic carbon for cooking and home heating has been practiced since before recorded history. The well-known conversion of carbohydrates into fuel ethanol has also been pursued for more than a century. The use of ethanol from starch-based sugars as a fuel was advocated by Henry Ford in the early years of the automobile industry, an approach superseded by the development of crude oil production and refining. Fuel ethanol has been produced successfully at scale since the 1970s in Brazil and later in other countries, predominantly from sugars derived from sugarcane and cornstarch. Annual production in 2019 reached nearly 18 billion gallons (430 million bbl) in the United States (EIA, 2020a) and 29 billion gallons (690 million bbl) worldwide (EIA, 2021a).
By 2019, the annual production and use of biodiesel, mainly from waste oils and fats, had risen to about 2.5 billion gallons (60 million bbl) per year in the United States, with a global production of 10.9 billion gallons (260 million bbl; EIA, 2021a). The COVID-19 pandemic notwithstanding, the contribution of biofuels to the global transportation sector has increased each year over the last two decades. Though reasonably mature today, starch- and sugar-based ethanol and biodiesel still pose challenges for chemical engineers in the areas of extracting value from by-products (e.g., glycerol in biodiesel production), capturing and sequestering CO2 from ethanol production processes, and expanding the range of fuel products beyond ethanol at a scale of impact. These challenges remain significant barriers to improving the economic feasibility of at-scale biofuel production.
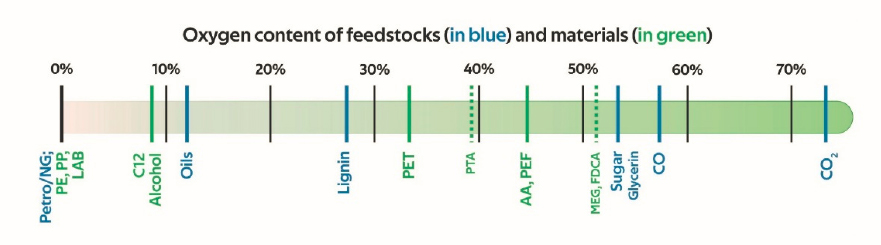
Interest in biofuel production as a potential strategy for offseting GHG emissions and decreasing dependence on fossil resources has seen a resurgence, prompting substantial debate about the long-term viability and utility of biofuels. From a technical perspective, most biogenic feedstocks have lower energy content than their fossil-based counterparts because of the high oxygen content of lignin and plant-derived polysaccharides (e.g., sugars contain about 54 percent oxygen by mass; Figure 3-10). Thus, for nearly all processes for converting biogenic, oxygenated compounds to energy-dense liquid fuels, the production of high-density fuels at a reasonable cost relative to the amortized, technologically mature petroleum industry represents a significant challenge.
The above technological challenges combine with the potential environmental consequences of harvesting crops for energy use to generate considerable controversy (Searchinger et al., 2008). The environmental cost of clearing agricultural land and its loss for growing food crops, complications related to water use, and the potential for an uncertain landscape and diverse mix of political and tax boundaries around the world make the future of biofuels uncertain. Furthermore, the potential rapid growth in the adoption of EVs may reduce demand for transportation fuels more broadly. The impact of biofuels in the energy sector will depend critically on bringing judicious, rigorous, and transparent
economic, environmental, and technical analyses—hallmarks of the chemical engineering profession—to bear on the selection of viable options for biofuel production.
Lignocellulosic feedstocks.
An estimated 1 billion tons of lignocellulose could be sustainably harvested for biofuel production annually in North America (DOE, 2016). The conversion of lignocellulosic biomass into biofuels and other organic coproducts—which represents the foundation of the biorefinery concept—poses considerable challenges for chemical engineers. Lignocellulose is a complex and heterogeneous composite material whose carbon content is predominantly in the form of two polysaccharides—cellulose and hemicellulose—as well as the aromatic polymer lignin. Numerous processing options have been considered over many decades; they entail either fractionating biomass into its constituents (thus enabling selective processing options, akin conceptually to the methods used in petroleum processing) or processing biomass directly into liquid or gaseous intermediates for subsequent conversion to transportation fuels and chemicals. Unlike petroleum, biomass is a solid, polymeric material that can be substantially heterogeneous, a feature that presents feedstock-associated challenges beyond the processing of liquids and gases that form the bedrock of the chemical engineering discipline.
The combined challenges of converting lignocellulosic biomass and offsetting fossil resources as a feedstock for transportation fuels create considerable opportunities for chemical engineering to continue making enabling contributions to the at-scale conversion of lignocellulose into biofuels and other products (flexible feedstocks are discussed in Chapter 6). These opportunities begin with the crops themselves. Now that biology is a core component of the chemical engineering discipline, modern molecular biology techniques are now ubiquitous in the toolkit of many chemical engineers. These techniques can be used to modify plants so they can be more efficient photon collectors (Kromdijk et al., 2016), to produce target chemicals in planta (Yang et al., 2020), and to
reduce the recalcitrance of plant components in chemical conversion in a biorefinery (Chen and Dixon, 2007).
Once plants have been harvested and transported to a biorefinery or centralized depot, there are many opportunities for overcoming recalcitrance to enable conversion of plant-based biomass to biofuels. The diversity of conversion pathways precludes a comprehensive review; therefore, this section focuses on the challenges and opportunities for chemical engineers to achieve cost-effective and sustainable biofuel production.
In the conventional biochemical conversion pathways, thermochemical treatment processes increase the reactivity of biomass, mainly by improving physical access to polysaccharides. These processes apply acid, base, steam, organic solvents, ionic liquids, or deep eutectic solvents, usually at temperatures in excess of 100 °C. Pretreatment approaches can also take the form of fractionation methods that separate polysaccharides from lignin for more direct and selective processing in parallel process trains. Polysaccharides are subsequently converted into monomeric sugars via carbohydrate-active enzymes or sugars and dehydration products, such as furanics or levulinic acid, through further use of acid catalysts. Soluble carbohydrates and derivatives are then converted into fuel molecules or precursors through biological and/or chemical catalysis. In the pioneer cellulosic ethanol plants built in the 2000s, lignin is commonly used to provide heat and power via on-site combustion.
Many attempts have been made to bring biochemical conversion–focused biorefineries to scale (1,000 to 2,000 metric tons/day), especially with the aim of converting nonfood crops or agricultural residues (e.g., wheat straw, corn stover), supported by substantial government investments. Yet the formidable challenges of economical feedstock collection, feedstock handling, biomass pretreatment, aseptic solid–liquid separation, and sterile bioconversion continue to prevent these facilities from achieving the required capacity factors for economic viability at scale of impact.
The lessons learned from these early facilities inform the many opportunities for chemical engineers to advance this field in moving from the process paradigm described above. For example, because on-site lignin combustion is estimated to be the most expensive single unit operation in a biorefinery, as well as a major source of non-GHG emissions, the development of alternative uses for lignin, 40 percent of which can be made of biomass carbon, is a major opportunity for research (Davis et al., 2013; Eberle et al., 2017). Successful conversion of lignin into value-added biofuels or biorefinery coproducts represents a major frontier for the chemical engineering community. Process intensification (PI) through the consolidation of biomass deconstruction into fewer unit operations and a focus on the elimination of costly steps is critical. An important component of PI involves separations, which are often key cost drivers in biorefineries. The challenges in lignocellulosic separations differ from those in petroleum processes because they involve the handling of solids and because biomass-derived compounds consist of high–boiling point oxygenates. Thus, separation technologies that operate wholly in the condensed phase will likely be necessary for the biorefinery.
Beyond biochemical conversion strategies, other lignocellulosic conversion routes employ fast, thermal deconstruction of the whole biomass or fractions thereof. Hydrothermal liquefaction uses liquid water at temperatures above 250 °C to produce a liquid
biocrude stream that can be catalytically converted into biofuel molecules. Alternative biomass pyrolysis routes use oxygen-free environments at or above 500 °C to produce bio-oil, light gases, and char from lignocellulosic biomass; some of these products are deoxygenated catalytically, either in the pyrolysis reactor or in subsequent process steps. At an even higher temperature (>700 °C), synthesis gas (CO and H2) can be produced from biomass via gasification in mildly oxidizing hydrothermal environments. Research opportunities common to these high-temperature biomass conversion processes include the need to understand the complex reaction networks, the design of catalysts and catalytic processes for substantial deoxygenation and operation in the presence of common catalyst poisons entrained in and originating from biomass, and the challenges of operating continuous high-pressure processes.
In most biofuel production processes, chemical coproducts are often invoked as a requirement for economic viability, with the associated challenges of the very large–scale disparity between these two value streams. Even ethylene, the chemical produced in largest amounts from fossil sources, is produced in amounts approximately an order of magnitude smaller than diesel and gasoline, while other commodity-scale chemicals are dwarfed by the scale of ethylene. Ultimately, expensive, small-volume coproducts cannot serve as adequate justification for expensive biofuel production processes, and chemical engineers will play an important role in finding realistic, scalable solutions. It is important to note the annual scale of global petroleum production: 5.0–5.5 billion metric tons (100.69 million bbl per day) of crude oil and 4.1 trillion cubic meters (3.6 billion tons of oil equivalent) of natural gas (IEA, 2021c,d; EIA, 2021d). Petroleum refineries have scales ranging from 10 million to 130 million metric tons per year globally. For biofuels to compete economically with fossil fuels, they need to be produced at similar scales, and even that may not be sufficient because of their unfavorable (oxygen) stoichiometry. Nonetheless, government regulations and/or the implementation of a carbon tax may bring the production of biofuels to a scale of impact, as in the recent case of ethanol in the United States and Brazil.
Feedstocks beyond lignocellulose.
Waste plant biomass is not the only source of renewable or waste carbon for producing biofuels, as is evident from global efforts to use algae, which can grow on marginal lands and in ocean or brackish water. The economical conversion of algae to lipids and other biofuel precursors has not been achieved, however, despite extensive research over decades. Many engineering challenges remain, including cost-effective cultivation in open ponds or controlled photobioreactors, greater CO2 and photon capture efficiency, separation of target products from cells, and catalysts and processes for the downstream conversion of algae-based intermediates (e.g., fatty acids and carbohydrates) to biofuels.
Some organic waste feedstocks are also of potential use in biofuel production (see the discussion of feedstock flexibility in Chapter 6). Given that municipal solid waste (MSW) contains substantial organic matter (e.g., food waste, paper, and cardboard), chemical engineering has many opportunities to increase the use of such feedstocks through research in fractionation and separations, as well as combined conversion approaches. Similarly, industrial and consumer-based food production yields substantial, often highly reduced (deoxygenated) waste feedstocks, such as oils and fats, that can be
catalytically converted to biofuels, although that process poses substantial challenges. In moving toward a zero-waste society, these organic waste feedstocks, among others, offer substantial opportunities for chemical process development.
Lastly, it is noteworthy that the conversion of CO2 or gaseous mixtures, such as flue gas from coal-fired power plants or gases from steel processing, is of interest to the chemical engineering community. Considerable effort is currently devoted to realizing the potential of CO2 and other gas conversions via biological, electro-, and thermal catalysis routes (e.g., Ye et al., 2019). It will be critical to consider substrate concentrations (e.g., direct CO2 air capture and conversion is a major challenge), the source of reducing equivalents, and the cost and sustainability of any type of process in this vein, as discussed earlier in this chapter in the context of photon capture.
Overall, biofuels will play a role in reducing GHG emissions associated with transportation fuels. However, judicious analyses of process feasibility, economics, and environmental impact will be critical for deciding among the many options; LCA will provide the rigor needed for such analyses. The challenge for chemical engineers is to identify those options that will ultimately be economically successful and sustainable at the scale required to meet society’s fuel needs.
Intermittent Energy Sources
Wind.
Wind has provided a source of power for centuries. Three main types of wind turbines are used today:
- distributed or “small” wind turbines (<100 kW), which are used to power a home, farm, or small business directly and are not integrated into the electrical grid;
- utility-scale wind turbines (100 kW to several MW), which deliver electricity to the grid for distribution to end users; and
- larger offshore wind turbines (up to 15 MW).
Wind energy, a niche option a few decades ago, is now the largest source of renewable electricity in the United States. In 2019, wind energy output represented about 7 percent of the U.S. electricity supply, with 100 GW of capacity—equivalent to powering about 32 million homes (AWEA, 2020). On a global scale, wind energy accounts for about 5 percent of electricity demand (IEA, 2020d).
Chemical engineers are involved in several areas of wind energy production (Veers et al., 2019). Specific challenges in materials research, development, and implementation include
- carbon composites and/or recycled materials for turbine blades;
- cement and steel manufacturing with lower CO2 emissions for wind turbine structural units, such as motors and gear boxes; and
- metallurgy and lubricants for state-of-the-art wind turbines.
The large diameter of modern turbines poses significant manufacturing and transportation challenges and the need for modular manufacturing and on-site assembly for both onshore and offshore installations. The decentralized deployment of installations for capturing wind energy also requires local energy storage and robust sensor and control systems, and often creates environmental concerns regarding the impact on coastal ecosystems and land and ocean animal life (NREL, 2020). Finally, as with all renewable energy sources, challenges exist with respect to the integration of wind energy into chemical production (Centi et al., 2019) and end-of-life considerations for turbine components.
Marine.
Marine energy includes energy derived from ocean waves, tidal movements, ocean and river currents, salinity gradients (i.e., where a river empties into the sea), and thermal conversion (i.e., based on the temperature difference between surface seawater and deep [~1 km] seawater). Economical production of tidal energy requires tidal waves larger than 3 m. The United States has several demonstration projects in tidal-energy power production, but none are producing power at commercial scale. Overall, marine energy’s development level is similar to that of wind energy roughly 30 years ago, which is to say that wind and solar energy are at commercial scale, while wave energy is at precommercial scale (see IRENA, 2020, for marine energy status and prospects). Chemical engineers can potentially make contributions to marine energy through the development of
- materials capable of withstanding seawater corrosion;
- flexible materials capable of handling the fatigue loads imparted by waves with their fast cycles of 8–10 s;
- antifouling coatings for submerged equipment; and
- electroactive polymers—polymers that generate electricity from mechanical stimuli (e.g., dielectric elastomers, piezoelectric materials, ionic polymer metal composites, and triboelectric materials)
Nuclear Energy
Nuclear power plants use heat produced during nuclear fission to produce steam, which is used to spin large turbines that generate electricity. The current technology is based on nuclear fission in pressurized water-moderated reactors (light water reactors). Fast breeder reactors have been in development for several decades, and some are now in commercial operation in Russia. While there is renewed interest in nuclear fusion, its potential commercial deployment is still decades away, and the role of chemical engineering in this area is likely to be marginal and is therefore not discussed here.
The United States is the world’s largest producer of nuclear power, accounting for more than 30 percent of worldwide nuclear electricity generation. Nuclear power has contributed almost 20 percent of electricity generation in the United States reliably and economically over the past two decades. It has been the single-largest contributor (more than 70 percent) of U.S. non-GHG-emitting electric power generation (DOE, 2021c). However, the actual and/or perceived hazards of nuclear power plants and the public’s negative perception of nuclear power have contributed to its slow growth. As of January
2021, the United States had 94 operable reactors (96,550 MW); 39 inactive reactors (18,140 MW); and two new reactors under construction in Georgia, with a planned electricity generation capacity of about 1,100 MW each (WNA, 2021).
Advanced nuclear industrial cogeneration offers a potential pathway with sufficient heat and energy intensity to address the problems of industrial emissions at scale. Traditional nuclear reactors rely on large light water reactors operating at maximum temperatures below 300 °C—a temperature high enough to make steam for power generation but too low to drive industrial processes. Consequently, the nuclear power industry is currently focused solely on power generation. Advanced reactors have higher output temperatures relative to light water reactors—up to 600 °C for molten salt reactors and 900 °C for high-temperature gas reactors. These higher temperatures are sufficient to drive most petrochemical processes. During the past decade, DOE has explored using this heat for industrial processes with the Next Generation Nuclear Plant.
Advanced nuclear reactors have the potential to provide the heat required by various industrial processes. Significant cost reduction is required for this advanced technology to be affordable for industrial heat generation, but with some new concepts based on low-cost natural gas, competitive, cost-effective solutions are not out of reach. Efforts to drive down cost are focused in three areas:
- New qualified fuels—TRISO (TRi-structural ISOtropic) particle fuel and molten salts—offer fundamentally better process safety profiles. Each TRISO particle is made up of a uranium, carbon, and oxygen fuel kernel, which is encapsulated by three layers of carbon- and ceramic-based materials that prevent the release of radioactive fission products. These fuels are designed so that processing shuts down automatically if they overheat, thus allowing for inherently safer reactors that are much simpler to operate relative to traditional reactors.
- Safer fuel allows for extensive or complete automation, which significantly lowers operating costs.
- Well-supervised factory production of standard reactors attempts to drive down unit costs by applying the fixed manufacturing facility costs over many units and driving annual improvements in efficiency. This factory-built approach has been used to achieve dramatic cost improvements in the wind and solar industries.
Increased demand for nuclear reactors and efficiency gains associated with the corresponding manufacturing learning curve could lower the cost of nuclear reactors. The petrochemical industry sector is capable of driving demand for these units for decades. High-temperature reactors provide high-quality heat directly to industrial facilities, and the integration of this heat will require chemical engineers working within an interdisciplinary team that understands process safety, integration, and intensification.
While existing as a separate discipline, nuclear engineering borrows heavily from physics, as well as from mechanical and chemical engineering. Setting aside the particle physics associated with fission and fusion reactions, a nuclear reactor is effectively a
chemical reactor that takes in fuel as a feed, produces fission or fusion products as waste, and produces heat as a product. Process integration and process design are necessary to extract energy most efficiently from the steam that is generated by a nuclear reactor. Viewed through the lens of the fundamental pillars of chemical engineering (transport phenomena, reaction engineering, thermodynamics, and applied mathematics), the design and safe operation of nuclear power plants are a good match for the skillset of a well-trained chemical engineer. Optimal thermodynamics and heat transfer are key to an efficient process design, as is process control to operate a power plant effectively and safely. Further development of advanced reactor designs, as well as storage solutions for nuclear waste, will also benefit from the same chemical engineering fundamentals. Advances in nuclear energy present a clear opportunity for chemical engineers to collaborate with nuclear and other engineering disciplines.
ENERGY CARRIER PRODUCTION
Energy carriers are intermediates in the energy-supply chain, located between primary and/or secondary sources (Thollander et al., 2020) and end-use applications. For convenience and economy, energy carriers have shifted continually from solids to liquids, recently from liquids to gases, and more recently to electricity, a trend that is expected to continue and even accelerate to address climate change concerns. Currently, about one-third of final energy carriers reach consumers in solid form (as coal and biomass), one-third in liquid form (consisting primarily of oil products used in transportation), and one-third through distribution grids in the form of electricity and gas. It is projected that the share of all grid-oriented energy carriers could increase to about 50 percent of all consumer energy by 2100 (Sims et al., 2007).
The following sections describe the opportunities for chemical engineers to contribute to electricity generation and the production of low-carbon fossil fuels, hydrogen, and synthetic fuels; production of advanced liquid biofuels was discussed previously in this chapter.
Electricity
Reduction of GHG emissions during electricity generation, as well as electrification of light- and medium-duty vehicles and residential/commercial heating, is crucial for decarbonization of the energy sector in the near and medium terms.
Electricity generation from coal-fired plants, the largest CO2 emitters, has decreased in the United States since 2009, while electricity generation from both natural gas and renewable energy sources has increased. In 2020, about 4,000 TWh of electricity was generated at utility-scale electricity generation facilities in the United States, with about 60 percent of that total being generated from fossil fuels—coal, natural gas, petroleum, and other gases; about 20 percent from nuclear energy; and about 20 percent from renewable energy sources (EIA, 2021b).
The shift from coal to natural gas and renewables was made possible by a steep decline in the cost of key technologies associated with shale gas, wind power, solar power,
and grid-connected electricity storage (DOE, 2015). New wind and solar technologies offer the lowest levelized cost7 of electricity over most of the Earth’s surface (IRENA, 2020). Since 2009, the levelized cost of wind has declined by 70 percent and that of solar photovoltaics by almost 90 percent, providing an important means of supplying electricity with no direct CO2 emissions (Lazard, 2019).
Recent decarbonization studies (e.g., IEA, 2021b; NASEM, 2021a) indicate that deep decarbonization of electricity generation can be accelerated, but further innovation is required. Chemical engineers are contributing to the scale-up, cost reduction, and reliability of improved and novel technologies, particularly in the solar and wind energy sectors. (Specific opportunities in these sectors were discussed earlier in this chapter.)
Low-Carbon Liquid Fossil Fuels
Liquid hydrocarbons from crude oil have been the preferred energy carrier for the transportation sector because of their high energy density, easy distribution and storage, low cost, and well-established and extensive infrastructure along the value chain. If they are to play a role in the low-carbon energy mix of the future, however their carbon footprint will need to be significantly reduced (Figure 3-11).
In 2019, the global demand for liquid hydrocarbons was about 100 million barrels per day, approximately 58 percent of which was for the transportation sector, 14 percent for feedstock for chemicals, 12 percent for power generation/residential/buildings, and 16 percent for other industrial use (ExxonMobil, 2019). Demand is expected to increase until at least 2040, although not uniformly across all sectors. Demand for hydrocarbon liquid fuels for industrial use and for power generation, residential uses, and buildings is projected to decrease, being replaced by energy carriers from renewable sources. In the chemical sector, demand for liquid hydrocarbons used as feedstock to manufacture consumer products is expected to increase. In the transportation sector, overall demand is projected to grow, but not uniformly across transportation types. Gasoline demand for light-duty vehicles is projected to decrease as a result of greater market penetration of EVs, while demand for liquid fuels in commercial transportation (heavy-duty, aviation, and marine) is expected to increase, particularly in the heavy-duty long-haul sector. Changes in global demand for oil, based on new policy scenarios, are projected to follow similar trends (IEA, 2021a).
Petroleum Refining
Most of the CO2 emissions associated with liquid fuels come from the use/combustion of the fuel itself, but there are also opportunities to reduce emissions during oil production, extraction, and refining. The majority of current refineries were designed and optimized to manufacture primarily gasoline; diesel, aviation, and other heavy fuels and
___________________
7 Levelized cost is the sum of total lifetime costs divided by the amount of energy produced, and thus represents the present value of the total cost of building and operating a power plant over an assumed lifetime.
chemicals are normally secondary products. To maintain a low cost of production, the amount of lower-cost heavy (high C/H ratio) and “dirty” crude oils in the feedstock mix is maximized. Refineries will require significant reconfiguration not only to satisfy product demand and meet challenges associated with GHG emissions, but also to maintain low production costs for liquid fuels. Chemical engineering is already playing a central role in addressing these challenges.
Opportunities to reduce CO2 emissions during refinery operations include the following:
- Increase energy efficiency through further improvement of energy management systems to ensure that refineries are run according to the most energy-efficient standards.
- Replace combustion of liquid fuel for heat generation with renewable sources (e.g., green electricity).
- Produce renewable (green) hydrogen with electrolyzers using imported or self-generated renewable electricity.
- Use low-grade heat generated during operations to produce electricity for internal and external use.
- Eliminate flaring in refineries.
- Replace steam-driven rotating machines and fired heaters with electric counterparts.
- Deploy CCUS from refinery flue gases.
Opportunities for chemical engineers to contribute to reductions in the carbon footprint of refinery feedstocks include the following:
- Use lighter and sweeter crude oil (higher H/C ratio and fewer heteroatom contaminants, such as sulfur and nitrogen compounds) instead of carbon-intensive and harder-to-process heavy oils (lower H/C ratio and more heteroatom contaminants).
- Coprocess crude oil with biomass.
- Integrate bio- and oil and refineries.
- Produce and use renewable (green) hydrogen for hydroprocessing needs and/or heat generation.
- Integrate electrofuels (e-fuels) within existing refineries to decrease low-carbon liquid fuel production costs.
- Further integrate production of petrochemicals to optimize the product slate (i.e., minimize production of gasoline/distillate and maximize that of petrochemicals and higher-quality lubricants).
Hydrogen
Hydrogen is a versatile energy carrier with significant potential to contribute to a clean, low-carbon energy system. To realize this potential, chemical engineering and related fields can contribute in the following ways:
- Increase production from nonfossil sources.
- Significantly increase clean hydrogen production via water hydrolysis using clean electricity.
- Develop a hydrogen infrastructure for distribution and storage at scale to satisfy demand.
Globally, 96 percent of hydrogen is produced from fossil sources (48 percent natural gas, 30 percent liquid hydrocarbons, and 18 percent coal), with only about 4 percent produced from electrolysis of water (IRENA, 2018). In 2018, the global demand for pure hydrogen was above 70 million metric tons, and the demand for hydrogen as part of a mixture of gases, such as synthesis gas, was about 45 million metric tons. The vast majority of this hydrogen (~95 percent) was used for production of chemicals (mainly ammonia for fertilizers and methanol) and for oil refining (IEA, 2019). Hydrogen is currently produced in large quantities, but a vast expansion of the world’s production capacity would be needed for hydrogen to replace a significant fraction of oil in transportation. Current hydrogen demand corresponds to ~8.4 EJ (2,333 TWh), or about 6 percent of the annual global transportation energy demand (IRENA, 2021).
A growing number of countries have policies that directly support investment in low-carbon hydrogen technologies, and global demand for hydrogen as an energy carrier is projected to increase significantly after 2030 (IEA, 2020e). This increase is projected mainly in sectors that are relatively more challenging to decarbonize and that have needs
that cannot be met by electrification, including fuel for heavy-duty long-haul transportation; synthetic fuels for aviation and shipping, for which available low-carbon fuel options are limited; ammonia as fuel for shipping; and a source for heat generation in the industrial and buildings sector. Hydrogen is a promising option for storing renewable energy.
Today, hydrogen is produced primarily by steam reforming of natural gas; partial oxidation (catalytic and not) and autothermal reforming of natural gas technologies are also used to a lesser extent. In some countries, particularly in Asia, gasification is used commercially for hydrogen production from coal. Production by water electrolysis is also a commercial technology, but at a much smaller scale and higher cost. Methane pyrolysis, or methane splitting of natural gas using renewable electricity to produce hydrogen and black carbon, is currently in the commercial demonstration scale.8 Many other routes to low-carbon hydrogen, such as thermochemical water splitting, direct photocatalysis, and biological production from microorganisms, are in various stages of R&D.
It is generally accepted that hydrogen from fossil fuels without CCUS will remain the main source of hydrogen production. After 2030, it is estimated that almost all of the growth in hydrogen production will come from low-carbon hydrogen (IEA, 2020d) made from renewables-based electricity or from fossil fuels, particularly natural gas, in combination with CCUS. By 2070, electrolytic hydrogen is projected to account for nearly 60 percent of global hydrogen production (IEA, 2020d).
Hydrogen produced from different feedstocks is identified by colors. “Black,” “gray,” and “brown” refer to hydrogen produced from coal, natural gas, and biomass, respectively. “Blue” is commonly used for hydrogen produced from fossil fuels, with CO2 emissions reduced by the use of CCUS. “Green” hydrogen is produced from water electrolysis using renewable electricity.
Blue and green hydrogen have a path to competitiveness with gray hydrogen (Hydrogen Council, 2019). The competitiveness of blue hydrogen depends primarily on scale-up of CCUS facilities and the value attributed to sequestered CO2. Carbon prices or taxes of USD 50/ton—a figure consistent with near-term milestones of major economies with net-zero commitments (e.g., in the European Union by 2030; Argus, 2020)—would make blue hydrogen competitive. The competitiveness of green hydrogen will require steep cost reductions for electrolyzers, as well as reductions in renewable energy costs. The production of green hydrogen is projected to break even with that of gray hydrogen before 2030 in regions with low renewable-energy costs, and before 2035 in regions with average renewable-energy costs (Hydrogen Council, 2019). A combination of green and blue hydrogen production pathways will be required to satisfy the potential demand for low-carbon hydrogen. Their supply mix will depend on a range of technical and societal factors, production costs, existing infrastructure (such as power supply and transmission networks), and emerging hydrogen trade routes.
Many challenges and opportunities related to the production of low-carbon hydrogen can be addressed by chemical engineers. For blue hydrogen, the challenges and opportunities center on improved CCUS (i.e., at lower cost) and assessment of geological
___________________
sites for long-term CO2 storage; for green hydrogen, they center on the need for a source of sustainable, low-cost, renewable electricity and access to freshwater.
Larger-scale electrolysis plants, whose development will depend on achieving lower costs and improved electrical efficiency for the electrolyzers, are also needed. Three main electrolyzer technologies exist today: alkaline electrolysis, proton exchange membrane (PEM) electrolysis, and solid oxide electrolysis cells (SOECs). Alkaline electrolysis is a mature, commercial-scale technology with relatively low capital costs. PEM electrolyzers use pure water as an electrolyte solution and avoid the recovery and recycling of electrolyte solution necessary with alkaline electrolysis; however, they use expensive electrode catalysts and membrane materials. Lifetimes of PEM electrolyzers are currently shorter than those of alkaline electrolyzers, and overall costs are higher. SOECs are the least-developed electrolysis technology and not yet commercialized. Ceramics serve as the electrolyte, resulting in lower material costs. Because steam is used for electrolysis, SOECs require a heat source. If the hydrogen produced were to be used for the production of synthetic hydrocarbons (in power-to-liquid or power-to-gas schemes), the waste heat from these synthesis processes (e.g., Fischer-Tropsch synthesis and methanation) could be recovered to produce steam for further SOEC electrolysis (IEA, 2019).
Synthetic Fuels
Synthetic fuels are produced by converting hydrogen and a carbon source into compounds that can be used as energy carriers, such as methane; methanol; ethanol; and such higher-carbon-number products as gasoline, diesel, and aviation fuel. Ammonia is also increasingly seen as a non–CO2-generating synthetic fuel, although ammonia production itself is currently a major source of CO2 emissions. Electrification and advances in electrochemical routes to ammonia synthesis may increase the potential for ammonia as a promising fuel. Low-carbon or carbon-neutral synthetic fuels require green hydrogen and carbon from a bioenergy source or from CO2 captured from flue gases or the atmosphere using direct air capture (DAC) technologies. In the case of DAC, however, significant energy and technology advances will be necessary to make this route viable. The low-carbon or carbon-neutral synthetic fuels are referred as power-to-fuels (PtF) or electrofuels (e-fuels).
The production of e-fuels requires significant amounts of electricity, and from a thermodynamic point of view, the electricity should be used directly. For example, 25 kWh of energy is required to produce 1 L of synthetic kerosene from electrolytic hydrogen together with CO2 captured through DAC. More than 80 percent of the energy is used to produce hydrogen; around 15 percent is used for the capture of CO2 through DAC; and the rest is used in the Fischer-Tropsch synthesis. Currently, only about 40 percent of the energy input is stored in the final liquid product, although with process optimization, the overall conversion efficiency could potentially increase beyond 45 percent (IEA, 2020d). However, e-fuels have the potential to help some sectors—particularly the aviation transportation sector, which is difficult to decarbonize and for which electrification is not an
option because of high energy-density requirements for the energy carrier. These considerations would need to be important enough to justify the thermodynamic inefficiencies, however.
A few aviation e-fuel demonstration plants have been announced. For example, the Norsk e-fuel project is planning a first plant in Herøya, Norway. That plant is expected to become operational in 2023, with a production capacity of 10 million L annually, scaling up to 100 million L annually by 2026.9 Cost reduction for e-fuel production is expected to benefit from the economy of scale and experienced manufacturing learning curve. It should be emphasized that DAC technology is in its infancy, with substantial room for technology and process improvement and cost reduction. For e-fuels to be competitive with conventional fossil fuels and even bio–jet fuel, a combination of low electricity costs and a high CO2 tax or cost will be needed.
ENERGY STORAGE
Energy storage occurs when an energy carrier is held in a fixed location until it can be deployed. When the energy carrier uses chemical bonds or potential energy, as is the case for liquid fuels and pumped hydropower, respectively, energy storage is conceptually simple. When the energy carrier is electrons, storage requires batteries or capacitors or conversion of the electrical energy into another energy carrier. Because many of these are mature technologies, chemical engineers have limited potential to impact some modes of energy storage, especially those involving mechanical energy, such as pumped hydropower and compressed gas storage. For energy carriers that involve electrons or chemical bonds, however, chemical engineers can play a critical role in the development and deployment of scalable and economical energy storage.
The changing nature of the world’s energy system has continued to create significant demand for energy storage, and this trend is likely to accelerate in the coming years. One obvious example involves intermittent renewable electricity sources such as solar and wind, whose full contribution to decarbonization of electricity generation can be realized only if they are coupled with grid-scale storage. A second example is the trend toward electrification of light-duty vehicles, which will require massive deployment of on-board batteries.
The properties of an energy storage system can vary with both scale and application. In vehicle applications, for example, the weight and size of batteries are critical; in grid-scale storage, by contrast, the battery weight is unimportant. The typical storage time and desired charging and discharging rates are also very different for these two applications. This example illustrates why future energy storage needs will be met by a wide array of technologies—there is no one-size-fits-all approach to energy storage. The rapid deployment of electrical storage systems is underpinned by the commercial sector’s large investments in technology development. If academic research is to have an impact in this environment, researchers will have to seek solutions in frontier areas in which well-
___________________
funded development efforts in industry are less likely to overwhelm the scale of any academic effort.
An important distinction between energy storage in electrons and chemical bonds lies in the number of times a particular piece of storage medium can be cycled. For fuels based on chemical bonds, storage simply involves a tank, and little to no change in the storage medium is expected even after thousands of cycles. Here, chemical engineers have a role to play in understanding evaporation and erosion, as well as improving leak detection methods. Batteries, however, tend to degrade during cycling. Many of these degradation processes stem from chemical reactions or nucleation and growth of phases that are driven by fundamental chemical engineering principles. This observation indicates that chemical engineers can play a key role in developing concepts that increase battery lifetimes. At the same time, for batteries to be a core part of a truly sustainable energy system, their end-of-life disposal or recycling needs to be considered. Similarly, the global availability of battery components (e.g., lithium) is an important consideration in LCA comparisons of competing technologies. The intentional design of batteries for end-of-life disposal and the use of earth-abundant elements are areas in which chemical engineering researchers are poised to play an important role.
As discussed in the previous section, hydrogen is a versatile energy carrier with significant potential to contribute to a clean, low-carbon energy system. Given its low energy density, however, its long-distance distribution and storage poses challenges, particularly if it needs to be shipped overseas. It has been estimated (IEA, 2019) that for distances above ~1,000 miles, shipping hydrogen as ammonia or liquid organic hydrogen carriers (LOHCs) is likely to be more cost-effective than shipping liquified hydrogen. Chemical engineers have opportunities to significantly lower the cost of conversion before export and reconversion back to hydrogen in the case of LOHCs, and before consumption or the direct use of ammonia as fuel.
Vast sectors of the global energy system rely on liquid or solid energy carriers, such as gasoline and coal. The energy density and ease of transporting these carriers relative to gases or electrons give them strong intrinsic advantages. Chemical engineering played a central role in what might be termed the hydrocarbon economy of the 20th century, and the discipline will also be central to the deployment of more sustainable and environmentally benign liquid energy carriers in the 21st century. Unlike high-value-added products such as pharmaceuticals, new or improved liquid energy carriers need to be competitive with low-cost alternatives and deployable at massive scale in order to be viable. In many cases, achieving these goals will require integrated process development rather than a singular focus on improved catalysts or similar chemical steps. The ability of chemical engineers to use LCAs and technoeconomic assessments (TEAs) to focus research on approaches with a plausible path to economic viability ensures that their contributions will have impact in the domain of energy storage.
ENERGY CONVERSION AND EFFICIENCY
Ultimately, whatever the source and carrier, energy is converted to heat or work, commonly referred to as energy use or consumption. This section is organized by application within the transportation, industry, residential, and commercial sectors, and focuses on opportunities for chemical engineers to contribute to energy efficiency and decarbonization in those sectors.
Transportation Sector
Transportation is a large and diverse sector that encompasses road (passenger and freight vehicles), aviation, marine, and rail transportation. In 2018, the transportation sector accounted for nearly a quarter of global CO2 emissions (IEA, 2021a), and efforts to decarbonize the transportation sector are therefore critical to achieving the goals of the Paris Agreement. As noted previously, the transition to a net-zero emissions transportation sector will take decades and cost hundreds of billions of dollars, and may never be complete (Ogden et al., 2016). Net-zero emissions aside, just reducing transportation CO2 emissions significantly in the coming decades is a formidable challenge. Tackling this challenge will require structural shifts in the transportation of people and freight, much larger gains in energy efficiency, major advances in technology, effective government policies, significant levels of investment in infrastructure for low-carbon energy carriers, and the manufacture of low-carbon and zero-emission vehicles.
No single energy carrier can, in the foreseeable future, satisfy the requirements across all segments of the transportation sector (EPA, 2021a). Some segments of the sector are easier to decarbonize (light- and medium-duty vehicles) than others that require high-energy-density fuels (heavy-duty long-haul, aviation, marine). A “bridging” low-carbon energy transition strategy will rely on the combined increased use of such energy carriers as electrons, hydrogen, and low-carbon liquid fuels, particularly advanced biofuels and synthetic liquid fuels (Santiesteban and Degnan, 2021). This section focuses on opportunities for chemical engineers to contribute to technology improvements in EVs, hydrogen-powered fuel cell engines, and internal combustion engines.
Electric Vehicles
The global EV market has grown over the past decade as a result of both technological advances and supportive government policies. About 7.2 million EVs were in use in 2019, and this number is predicted to increase to nearly 140 million within the next decade (IEA, 2020a). Many automobile manufacturers have announced plans to stop production of internal combustion engines by 2030. The key technological enabler of the growth in EVs is the progress made in lithium-ion batteries, in terms of both improved performance and cost reduction (BloombergNEF, 2020; Figure 3-12). To accelerate market penetration of EVs, several remaining challenges for lithium-ion batteries need to be overcome, many of which chemical engineers are poised to help address, as described below.
The chemical industry has played a critical role in the successful development and manufacturing of lithium-ion–based batteries and continues to drive innovation to meet booming demand. Improvements are being pursued in mining, metals processing and purification, battery design and manufacturing, battery chemistries, and performance.
The advances needed for lithium-ion batteries in automotive applications are described by Masias and colleagues (2021):
- further increases in energy density per unit weight and volume;
- continued cost reduction on a $/kWh basis, which is both challenging and necessary to increase the opportunity for wide-scale adoption of electric transportation;
- the ability to validate and predict long battery life quickly and accurately;
- fast recharging while preserving long battery life and overall safety; and
- direct recycling of cathodes and anodes to reduce both the costly geological extraction and initial material processing steps of rare elements.
Lithium-ion–based batteries are approaching the theoretical energy density limit imposed by their inherent chemistry; therefore, they may be unable to satisfy future EV demand, and several next-generation lithium batteries are being investigated (Wu et al., 2020), the three main technologies being lithium-air, lithium-sulfur, and lithium-metal. Despite some research progress, however, development and scale-up have been slow. The lithium-air battery has faced battery life and energy efficiency challenges. The lithium-sulfur battery has shown more promise than the lithium-air, but it, too, has been limited by life and volume concerns because of its low energy density. Lithium-metal batteries have advanced the furthest in the past decade but are still in the development stage (Masias et al., 2021).
All of the lithium battery types described above use a liquid electrolyte. One strategy for improving overall performance is the use of a solid electrolyte material. Such batteries are referred to as solid-state or all-solid-state batteries (ASSBs). The discovery of highly conductive solid-state electrolytes has led to tremendous progress in the development of ASSBs (Tan et al., 2020a), making it possible to overcome such technical challenges as poor interfacial stability, scalability, preservation of high energy density, production safety, and cost reduction.
Solid-state batteries with lithium-metal anodes have the potential to achieve high energy densities. Several start-ups, many in close collaboration with large automakers, are focusing on the development of technologies required to optimize solid-state electrolytes. Substantial increases in the energy density of solid-state batteries, together with improvements in other performance metrics, such as cost and durability, could make electrification a viable and more attractive commercial option for medium- and heavy-duty regional and long-haul vehicles. These advances could also have a significant impact on short-distance and small-freight marine and aviation transportation.
Hydrogen-Powered Fuel Cell Vehicles
The two primary options for zero-emissions transportation are electric drivetrains powered by batteries (battery electric vehicles [BEVs]) and by hydrogen fuel cells (fuel cell electric vehicles [FCEVs]). Both are used for light-, medium-, and heavy-duty vehicles. These two technologies have complementary strengths and meet different application and customer needs. BEVs are emerging as the technology of choice for light- and medium-duty vehicles, while FCEVs are better suited for heavy-duty commercial vehicles that are driven long distances, carry heavy loads, and require relatively quick refueling.
PEM fuel cells are used in FCEVs because they offer higher power density, lower overall weight, and lower total volume compared with other fuel cell types (NRC, 2015). They are also easily scaled for different vehicle classes. Because these cells operate at fairly low temperatures (<100 °C), they have a short warm-up time and better durability relative to other fuel cell types. These features also contribute to much greater overall efficiency compared with high-temperature solid oxide or molten carbonate fuel cells in a frequent start-up/shutdown vehicle application.
In a PEM fuel cell, the anode–separator–cathode structure is known as the membrane electrode assembly (MEA). It consists of a solid polymer electrolyte membrane (e.g., Nafion®) with a catalyst layer and a gas diffusion layer on each side. The catalyst layers are platinum-group metal nanoparticles (usually platinum or platinum alloy) on a porous carbon/ionomer support. The gas diffusion layers are multilayer carbon fiber/polytetrafluorethylene paper sheets that facilitate mass transfer of reactants and products. The MEA is sandwiched between bipolar plates that have channels for gas flow and conduct the electric current. Like batteries in an EV, the fuel cell unit in the vehicle is actually a stack of many individual fuel cells arranged in series to provide sufficient voltage. The stack is rated by maximum power output.
The key challenges and opportunities to which chemical engineers are contributing in this domain are as follows:
- Reducing costs—The cost of platinum-based catalysts is the primary driver of fuel cell costs, a fact that has motivated research on technologies for reducing the platinum content of MEA catalysts and even developing platinum-free MEA architectures.
- Increasing durability—The on-road durability of PEM fuel cells is currently less than desirable for large-scale commercial deployment. The primary cause of degradation is loss of catalyst activity (e.g., due to sintering) and deterioration of the PEM. One important lever for increasing durability is to increase catalyst loading (i.e., add more platinum), but doing so increases cost. A better alternative is to design sintering-resistant catalysts or replace platinum with lower-cost metals.
- Increasing efficiency—PEM fuel cells for on-road FCEVs have a peak power efficiency of up to 60 percent in terms of converting the available fuel energy of hydrogen into electrical energy, well below the theoretical maximum efficiency of approximately 80 percent.
Hydrogen is stored on the vehicle as a compressed gas at a pressure of 700 bar in one or two carbon-fiber composite tanks. Since even highly compressed hydrogen has a much lower energy density than gasoline and diesel fuel, the volume of the tank(s) needs to be large enough to enable a driving range comparable to that of conventional internal combustion engine (ICE) vehicles. Research, development, and demonstration (RD&D) in this area targets primarily the cost of the composite tanks (e.g., cheaper carbon fibers or other materials and cheaper construction methods) and auxiliary components. Early-stage research also is being conducted on other technologies for storing hydrogen on FCEVs, such as
- adsorbents (e.g., metal organic framework materials [MOFs] and metal hydrides) that would allow lower hydrogen pressures;
- cold/cryocompressed conditions in insulated tanks (e.g., −75 °C/500 bar or −235 °C/70 bar); and
- chemical storage, in which a hydrogen-dense chemical (e.g., NH3BH3, methylcyclohexane) is loaded into the tank, H2 is disassociated through heat or chemical reaction, and then spent chemical is collected and regenerated at a central facility.
Without a breakthrough, vehicle manufacturers would be unlikely to opt for these alternatives in the foreseeable future because of the need to develop additional technologies and/or fueling infrastructures to accommodate them.
Internal Combustion Engine Powertrain Vehicles
For both heavy- and light-duty ICE powertrain vehicles, chemical engineers can play important roles in advancing technology for increasing fuel efficiency, thereby re-
ducing CO2 emissions. Advances in ICE technology have traditionally been led by mechanical engineers, making this a key opportunity for chemical engineers to collaborate with another engineering discipline. Areas for such collaboration include
- advanced combustion schemes (e.g., low-temperature combustion);
- electrified accessories and waste-heat recovery;
- hybridization;
- lightweighting, with new, aerodynamic cabs and trailer components;
- advanced communication and logistics;
- energy-efficient tires and monitoring systems; and
- advanced GPS-based predictive control technology.
GHG emissions could be reduced in ICE-powered heavy-duty vehicles by the use of low-carbon liquid fuels. Codevelopment of more efficient fuels and vehicle engines is a major collaborative opportunity for chemical and mechanical engineers, with chemical engineers bringing expertise in reaction mechanisms and transport and mechanical engineers bringing expertise in transport, computational fluid dynamics, and engine design. Opportunities in this area include
- blends of high-quality, low-carbon fossil-diesel fuel with advanced biofuels and/or synthetic fuels (e.g., e-fuels) to achieve specific tailpipe GHG emission levels; and
- increased control of the variability of diesel-fuel properties.
Industrial Sector
The industrial sector produces the goods and raw materials people use every day. Industry is energy intensive—nearly half of the world’s energy is dedicated to industrial activity; accordingly, it is also responsible for a large portion of global CO2 emissions. Production of cement, steel, and chemicals accounts for the largest portion of industrial CO2 emissions—about 70 percent (IEA, 2020d). Reducing manufacturing-related emissions in the United States and China will be critical to reducing CO2 global emissions from manufacturing (Figure 3-13).
The lack of commercially available and scalable low-carbon alternatives to fossil fuels makes deep reductions in CO2 emissions from industry highly challenging in the short and medium terms. This fact is reflected in the Sustainable Development Scenario projections (IEA, 2020d), in which the industrial sector emerges as the second-largest CO2 emitter in 2070, after the transportation sector, accounting for around 40 percent of residual emissions, even though its emissions are projected to be 90 percent lower overall than in 2019 (Figure 3-13). Currently, energy inputs to the industrial sector are approximately 70 percent from fossil fuels (IEA, 2020d). In the Sustainable Development Scenario projections, the use of fossil fuels in industry would be reduced by more than 60 percent by
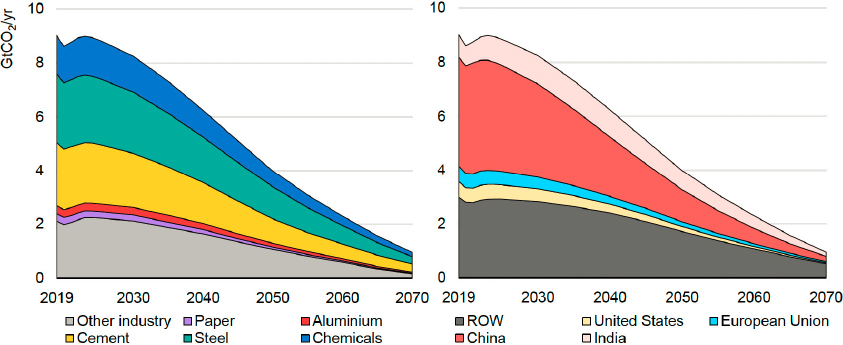
2070, being replaced primarily by electricity and bioenergy, while more than 75 percent of the remaining CO2 emissions would be captured and stored permanently. The following sections focus on opportunities for chemical engineers in the cement, steel, and chemical production subsectors, as well as cross-cutting approaches for decarbonization of the industrial sector.
Cement Production
More than 70 percent of the energy used in the U.S. cement industry comes from coal and petroleum coke. More than 50 percent of the total CO2 emissions attributable to cement production are process related (from calcination of limestone in the kiln), not energy related. To advance decarbonization of the cement industry, in addition to energy-efficiency improvements, these process-related CO2 emissions will need to be reduced. Key strategies for deep decarbonization of the cement industry are clinker substitution (supplementary cementitious materials [SCMs]), a switch to lower-carbon fuels, CCUS, low-carbon cement and concrete chemistries, and kiln electrification. While several of these strategies can be combined to approach net-zero emissions, decarbonization efforts related to demand reduction, use of SCMs, and waste-carbon utilization will also be needed. For example, Hasanbeigi and Springer (2019) recently showed that, compared with 2015, total CO2 emissions from California’s cement industry could decrease by 68 percent by 2040 even though the state’s cement production is projected to increase by 42 percent (Figure 3-14).
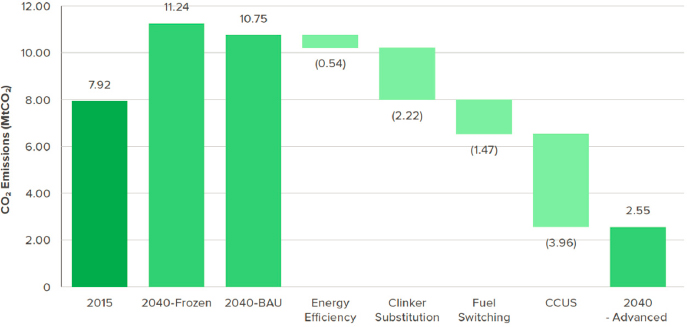
Further RD&D is necessary to decarbonize cement processes, including technologies to
- improve concrete performance and durability using alternative raw materials while meeting construction codes and standards, especially for natural SCMs (e.g., pozzolans, calcined clay);
- develop innovative ways of using large-scale nonpurified CO2 for different applications and product streams with low energy penalty and cost;
- develop electrified kilns capable of operating at very high temperatures suitable for producing cement;
- advance the use of waste biomass and green hydrogen in cement kilns and improve understanding of resulting effects on the kiln and final product;
- achieve high carbon-capture efficiency (more than 90 percent) on retrofitted and new cement plants;
- develop better catalysts and process designs to deliver higher efficiency levels, reduce costs, and lower material consumption or waste production for CCUS in the cement plant; and
- improve CO2 transportation and storage infrastructure for CCUS.
Steel Production
Around 70 percent of steel in the United States is produced by electric arc furnaces; the remainder is produced by blast furnaces (BFs) or basic oxygen furnaces. Key technologies needed to decarbonize the steel industry include improvements in energy efficiency; a switch to low-carbon fuels; use of green hydrogen instead of natural gas in direct reduction of iron (DRI); production of iron by electrolysis of iron ore using only renewable electrical energy; postcombustion CCUS (such as top-gas recycling in BFs); DRI with CCUS; HIsarna (smelting reduction) with CCUS; carbon utilization (carbon to
ethanol or chemicals); and green hydrogen plasma smelting reduction. Although some of these decarbonization technologies have been commercialized, some require further RD&D, such as
- industrial-scale plant design for producing iron by electrolysis, and development of detailed cost models for assessing the commercial viability of the process; and
- development of methods to analyze and evaluate the BF operation continuously when extending the use of hydrogen to all tuyeres (injection nozzles for air/H2) in BFs.
Chemical Manufacturing
The chemical industry is highly diverse, producing more than 70,000 products globally. Yet 18 large-volume chemicals—including light olefins, ammonia, BTX (benzene, toluene, xylene) aromatics, and methanol—account for 80 percent of the energy demand and 75 percent of the total GHG emissions attributable to global chemical manufacturing (IEA, ICCA, and DECHEMA, 2013). For perspective, the global production volumes in 2012 were 220 million metric tons for ethylene and propylene, 198 million metric tons for ammonia, 58 million metric tons for methanol, and 43 million metric tons for benzene. Together, production of these four products used about 7.1 EJ of energy per year (or a specific energy consumption of about 13.7 MJ/kg of product), and the top 18 large-volume products used a total of about 9.4 EJ of energy per year (Figure 3-15).
Because about 90 percent of chemical manufacturing processes use catalysts, catalyst and catalyst-related process improvements could reduce the energy intensity of these 18 products by 20–40 percent by 2050, amounting to energy savings of about 13 EJ and a CO2 emissions reduction of 1 metric gigaton (IEA, ICCA, and DECHEMA, 2013). Incremental improvements will suffice in the short to medium term; in the longer term, however, the deployment of new technologies, such as biomass feedstocks and green hydrogen, will be necessary. The challenges for chemical engineers are clear: to identify top catalyst and catalyst-related process opportunities for these 18 and other chemicals.
Key technologies for decarbonization of the chemical industry include energy systems engineering; fuel switching; novel catalytic processes (e.g., olefin production via catalytic cracking of naphtha or renewable methanol); advanced separation processes; electrification; transitions to low-carbon feedstocks and processes (e.g., biomass, hydrogen-based production of ammonia and methanol, artificial photosynthesis, renewable-energy electrochemistry, biobased plastics production, gas-to-liquids gas); and CCUS. Chemical engineers can contribute to the RD&D necessary to achieve these technological advances, including the following examples:
- Achieve viability for natural gas crackers and improve catalyst production; improve the efficiency of olefin production via catalytic cracking of naphtha or via methanol; and address environmental issues for used catalysts, including regeneration and catalyst selectivity and lifetime.
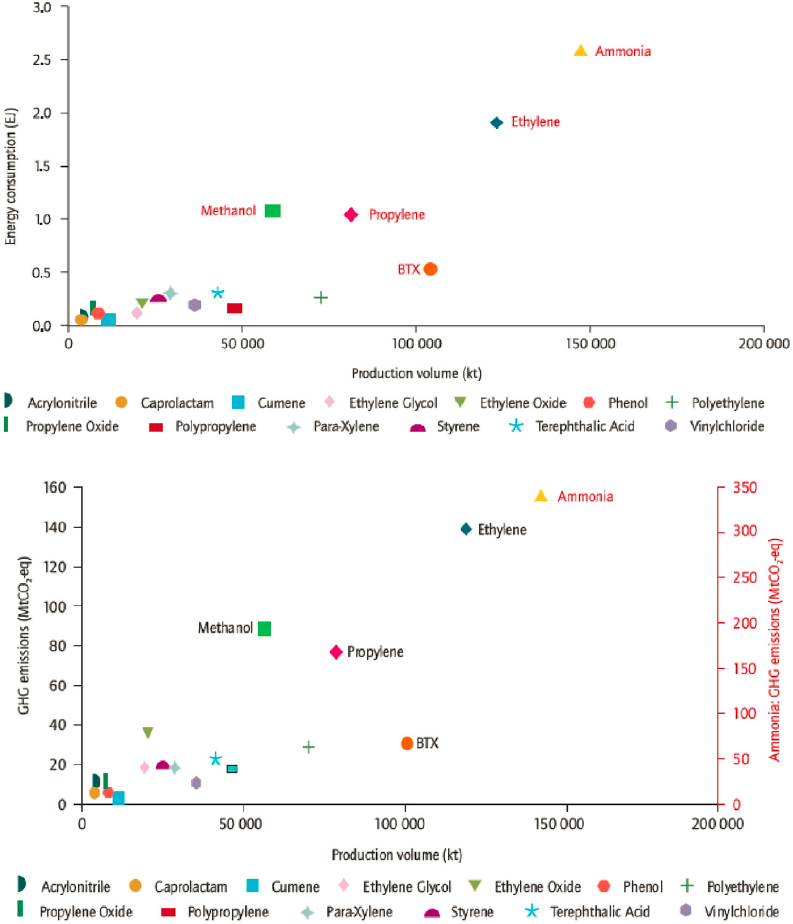
- Improve the hydrogen peroxide propylene oxide process—oxidation of propylene with hydrogen peroxide yields propylene oxide and water as a byproduct; its energy consumption could be about 35 percent lower than that of the traditional process.
- Resolve waste management issues for selective membranes, including membrane washing/cleaning, and drive step-change advances in separations, including the use of ceramic membranes.
- Reduce technical and economic hurdles for such low-carbon processes as anaerobic digestion and gasification, biobased production of plastics, and hydrogen-based production of ammonia and methanol.
- Advance processes related to water electrolysis, such as optimized processes for variable operation, improved stability for operations under pressure (30–40 bar), electrodes with low-content noble metals and other rare elements, and photocatalytic water splitting (e.g., non–noble metal electrodes, corrosion-resistant photoelectrode materials improved over potential).
- Reduce technical hurdles for fuel switching to hydrogen and other lower-carbon fuels, electrification, and CCUS.
Petroleum Refining
The United States is one of the largest producers of liquid transportation fuels and refined petroleum products in the world, and energy engineering could reduce the fuel used in producing these products by 50 percent (Morrow et al., 2015). Key technologies for decarbonization of petroleum refining include fuel switching, electrification, biomass hydrothermal liquefaction and biocrude oil, synthetic fuel synthesis, reduction or elimination of flaring, selective membranes, advanced control and improved monitoring, CCUS, catalytic cracking, progressive distillation, self-heat recuperation, and biodesulfurization. Further RD&D is critical for these technologies, and chemical engineers can make contributions to
- advance synthetic fuel synthesis;
- integrate biofeedstocks;
- scale up technology for conversion of CO2 and H2 to hydrocarbons with lower electricity consumption;
- improve chemical separations with lower energy demand for selective membranes;
- apply advanced modular nuclear reactors for low-temperature steam generation; and
- improve electric heating to achieve high temperatures and large scales efficiently and economically.
Food and Beverage Industry
The food and beverage industry is the third-largest consumer of energy in the United States; its energy consumption is dominated by mechanical systems, compressed air, refrigeration, and process heat in the moderate-to-low temperature range. Key technologies for decarbonization of the food and beverage industry include fuel switching to lower-GHG sources, such as electricity and renewables, and plant efficiency measures, particularly because many of these plants tend to be operated by small- and medium-sized manufacturers with limited energy-management capacity. Electrification of dewatering,
drying, and process-heating applications using heat pumps, hybrid boilers, induction heating, dielectric heating, and advanced cooling/refrigeration represents an important opportunity for this subsector. In addition, advanced processing and preservation to reduce degradation in processing, along with improvements at the supply chain and consumer levels, are important because on a global scale, about a quarter of the food supply is wasted (see Chapter 4; Buzby et al., 2014; D’Odorico et al., 2018; Finley and Seiber, 2014). A key challenge for this subsector is that food and beverage processors must comply with multiple regulations that complicate the implementation and slow the adoption of new technologies. Although some decarbonization technologies are commercially available, further RD&D is needed in such areas as
- shifting from steam and fossil fuels to electric and solar heating technologies;
- demonstrating and certifying alternative processing technologies to reduce GHG emissions while maintaining product safety and quality, and reducing degradation in the supply chain;
- using waste to produce bioenergy; and
- adopting fuel switching and expanded implementation of energy efficiency.
Cross-Cutting Approaches for Decarbonization of the Industrial Sector
To achieve the net-zero emissions goal for industry, five broadly applicable decarbonization pillars require vigorous pursuit in parallel over the next decades: demand reduction, energy efficiency, fuel switching and electrification, transformative technologies in sectors, and abatement. The applicability and selection of these pillars will vary across sectors, with weighing of trade-offs in costs and accessible resources (de Pee et al., 2018). Except for sector-specific transformative technologies, these approaches can be considered cross-cutting and can facilitate reduction of GHG emissions across multiple sectors.
Barriers abound across the landscape of deployment, development, scale-up, and whole-system integration of current, emerging, and transformative technologies that can advance these cross-cutting approaches. Nonetheless, chemical engineers have opportunities to help overcome these barriers (e.g., in the areas of competitiveness, carbon capture and use, and advanced materials). Many low-carbon technologies are in the early stages of development and will require extensive RD&D for effective deployment. RD&D needs range from advancing cross-cutting technologies (e.g., improved electrolysis of water to lower-cost H2) to making radical changes (e.g., applying high-temperature heat for ethane crackers). Figure 3-16 summarizes a more comprehensive set of recommendations from a recent study by the National Academies of Sciences, Engineering, and Medicine on low-carbon technologies, approaches, and infrastructure needing RD&D investment in the 2020–2050 period (NASEM, 2021a). A portfolio of collaborative RD&D initiatives, multigeneration plans, agile management, and durable support will be needed to face these challenges successfully and drive progress going forward (NASEM, 2019f, 2021b).
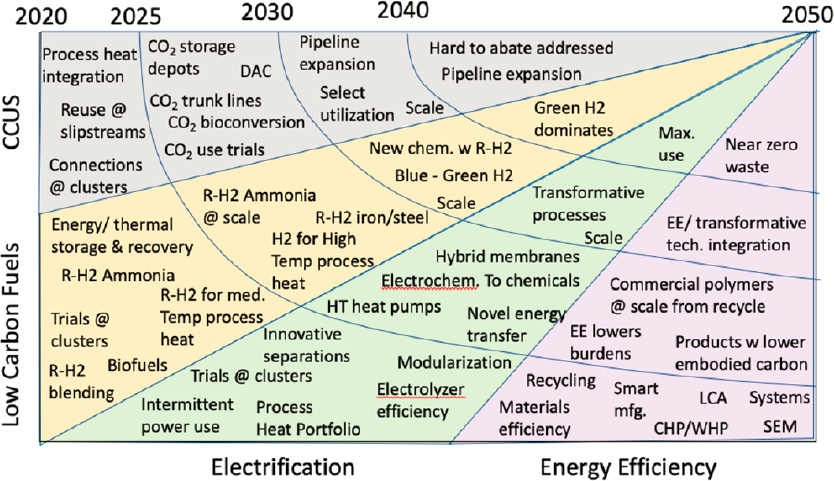
Commercial and Residential Sectors
The impact of the commercial and residential sectors on net energy use and GHG emissions in the United States is similar in scale to that of the transportation sector. Although significant opportunities exist for improving energy efficiency in commercial and residential settings, the highly dispersed nature of this sector creates challenges not shared by the industrial subsectors discussed in the previous section, which have large, fixed facilities. A recent National Academies report on decarbonization identifies key strategies that employ existing technologies, including electrification of energy use and dramatically increased use of electric heat pumps for heating and hot water (NASEM, 2021a). The future energy needs of residential buildings in the United States and elsewhere in the world are quite different. In the United States, the critical need is for more efficient use of energy for heating and air conditioning as existing systems for these purposes are replaced or updated. Low- and middle-income nations, on the other hand, are likely to see enormous growth in the use of air conditioning, driven by rising prosperity and, to a lesser extent, by the impact of climate change.
To contribute to significantly reducing energy use in the commercial and residential sectors, chemical engineers will need to work closely with other engineers, including mechanical and civil engineers, who are more closely affiliated with these sectors, in the
development of technologies that can drive progress. As is the case with other topics discussed in this chapter, putative technologies will need to be deployable at low cost with high reliability to have any chance of success.
Although systems for refrigeration and air conditioning are ubiquitous, improvements in their efficiency and sustainability will require overcoming key technological challenges. Chemical engineers can play a pivotal role in the development of new heat-transfer fluids that are nontoxic and nonflammable and lack the very high GHG intensity of many chlorofluorocarbons (CFCs) and other halocarbons. Nontraditional heat-transfer cycles, such as adsorption cooling, and materials with caloric properties (e.g., Moya and Mathur, 2020) also have considerable potential that intersects strongly with chemical engineering applications in other domains.
Improving the properties of building materials is a key path toward improving commercial and residential energy efficiency. Opportunities include the development of passive materials, such as paints for roofs that reflect the solar spectrum more effectively and, as a result, reduce the cooling load required for buildings (Li et al., 2021a); and active materials, such as “smart glass,” which adapts to external sunlight to reduce the net energy demands in buildings (Alias et al., 2019). Chemical engineers have many opportunities to contribute creatively in these areas, provided that researchers remain focused on meeting the cultural and economic needs of end users rather than on fashioning “pure technology” solutions.
CARBON CAPTURE, USE, AND STORAGE
CCUS will be centrally important to controlling the concentration of carbon in the atmosphere. Achieving net-zero emissions to halt growth in the concentration of atmospheric CO2 will not require achieving zero anthropogenic CO2 emissions, but rather balancing anthropogenic CO2 emissions with natural and anthropogenic CO2 sinks. An extensive portfolio of mitigation strategies for GHG emissions could contribute to the achievement of net-zero emissions (Figure 3-17). Six negative emissions technologies (NETs) remove carbon from the atmosphere and sequester it (Fuss et al., 2018; NASEM, 2019b): coastal blue carbon, terrestrial carbon removal and sequestration, bioenergy with carbon capture and sequestration, DAC, carbon mineralization, and geological sequestration. Other approaches have been proposed, as well, such as cloud alkalinity, biomass burial, enhanced ocean upwelling and downwelling, DAC by freezing, marine bioenergy with carbon capture and sequestration, and electrochemical lining (The Royal Society, 2018b). The removal of other gases, such as methane, N2O, and CFCs with significant global warming potential will also be important in the overall effort; however, the concentration of these gases in the atmosphere is much smaller than that of CO2, making them very difficult to remove once they have been released.
The broad area of CCUS is a very rich one for chemical engineering; it presents numerous opportunities for major contributions, as well as challenges to address over the next years and decades, in the technical, economic, LCA, and integrated assessment management (IAM) areas. IAM is a quantitative tool for combining information from diverse
fields (i.e., science, economics, and policy) to assess the impact of emissions or their reduction. To address these challenges effectively, however, chemical engineers will need to partner with engineers from other disciplines, such as environmental engineering (e.g., NASEM, 2019c).
DOE has identified priority research directions for CCUS (DOE, 2018c). Of these priorities, chemical engineers could contribute in the following areas:
- Capture—designing high-performance solvents and developing environmentally friendly solvent processes, designing sorbent materials and integrated processes, developing membranes and related processes, and producing hydrogen from fossil fuels with CO2 capture.
- Utilization—designing interfaces for enhanced hydrocarbon recovery with carbon storage; valorizing CO2 from catalytic, electrochemical, and photochemical transformations to fuels, chemicals, and new materials; and tailoring microbial and bioinspired approaches to CO2 conversion.
- Storage—advancing multiphysics and multiscale fluid flow to achieve Gt-per-year capacity; locating, evaluating, and remediating existing and abandoned wells; and optimizing injection of CO2.
- Cross-cutting—integrating experiments, simulations, and machine learning across multiple length scales to guide the development of materials and processes; intensifying CCUS processes; incorporating social aspects into decision making; and integrating LCA and technoeconomic assessment (TEA), along with environmental and social considerations, to guide technology portfolio optimization.
Near-zero- and positive-emissions technologies emit almost zero GHGs or emit GHGs to a lesser extent compared with alternative technologies, respectively. They include enhanced energy efficiency, clean or renewable electrification, bioenergy, hydrogen and hydrogen-based fuels, and CCUS technologies. LCA and TEA (including scalability of the specific technology) are two primary tools used to evaluate and rank these technology options. Chemical engineers can play a role in the development of many, technologies that have been reviewed extensively elsewhere (e.g., Bui et al., 2018; Fuss et al., 2018; Hepburn et al., 2019; IEA, 2020d; NASEM, 2019b). Opportunities for chemical engineers in the areas of DAC and CO2 and CH4 utilization are briefly summarized in this section.
Direct Air Capture
DAC appears to be a relatively easy fix for climate change and has the additional advantage that it can be located close to the sequestration reservoir, thus avoiding the need to use a pipeline for CO2 transportation. However, dilute systems, such as those with CO2 in the air at a concentration of about 400 ppm, require more energy than concentrated systems, such as those with CO2 in flue gas from ammonia manufacture at a concentration exceeding 98 percent; from coal-fired power plants at a concentration of 12–15 percent; from cement, iron/steel, and glass production at a concentration of 20–35 percent; and
from natural gas–fired power plants at a concentration of 3–4 percent on a volume basis (NASEM, 2019a). Thus, the concentration of CO2 in flue gases is between almost 100 and 300 times that of CO2 in the air, and as a result, the energy required to capture CO2 from the air is 2 to 3 times greater than that required to capture CO2 from the flue gases (Bui et al., 2018).
Areas on which chemical engineers will focus in the future include the development of low-cost solid sorbents, highly CO2-selective materials that require reduced regeneration energy, materials that are highly active in ambient conditions, and processes with increased mass-transfer coefficient and high throughput and low pressure drop (NASEM, 2019b). Other areas of focus will include packing designs, process intensification, catalytic additives, and long-term stability of sorbent matrices.
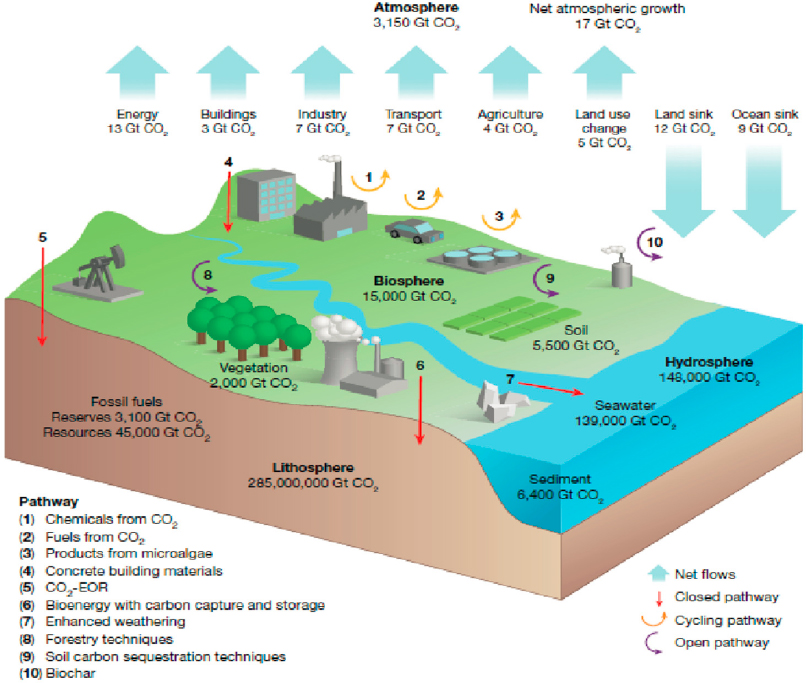
Carbon Utilization
Use of CO2 and CH4 as feedstocks is an important mechanism for CCUS. However, only a small amount of CO2 and CH4 emitted each year is currently being captured and used. The main pathways for CO2 utilization include mineral carbonation, chemical utilization, and biological utilization, while the main pathways for CH4 utilization are chemical utilization, biological utilization, and direct uses as fuel. Most carbon utilization technologies are early in their development phase. A number of research areas are described in the above-referenced National Academies report (NASEM, 2019a); specific opportunities for chemical engineers are highlighted in the following sections.
Mineral Carbonation
CO2 is used to make carbonates, such as cement and concrete, for use in the construction sector, as well as in paper and food production. The conversion of CO2, which is a low-energy molecule, into solid mineral carbonates in near-ambient temperatures is one of the few thermodynamically favorable reactions of CO2 (NASEM, 2019a). For this reason, as well as the sheer size of the construction materials market, the use of CO2 for mineral carbonation is considered the largest and most favorable CO2 utilization pathway. Challenges and opportunities for chemical engineers in this area include control of carbonation reactions, process design, accelerated carbonation and crystal growth, green synthesis routes for alkaline reactants, structure property relationships, analytical and characterization tools, and construction methodologies.
Chemical Conversion of CO2
Urea, polycarbonate, ethylene and propylene carbonates, salicylic acid, and polyether carbonate are currently produced from CO2 at commercial scales; however, the CO2 used is not derived from carbon capture processes. Commodity and fine chemicals and fuels currently produced from CO2 at pilot plants are methanol, methane, CO, fuel via a CO2-based Fischer-Tropsch process or direct pathway from CO2 to fuels, diphenyl carbonate, and oxalic acid (NASEM., 2019a). Specific challenges and opportunities for chemical engineers in the area of CO2 conversion to chemicals and fuels include the development of long-lasting and stable catalysts that can also work when the CO2 feed stream contains the impurities typically present in flue gases, low-temperature electrochemical conversion processes, enhanced conversion per pass and avoidance of carbonate formation, and lower energy requirements for the anode in the electrochemical reduction of CO2.
Chemical Conversion of CH4
Sources of methane waste gas include emissions from oil and gas plants, landfills, sewage, manure, and other waste operations. The methane waste gas from oil and gas
plants is primarily methane, whereas that from waste management operations, called biogas, is a mixture of CO2 and methane. In contrast with CO2, methane is a high-value and high-energy chemical and has no equivalent pathways to mineral carbonation. Because of its high energy, it is used primarily as fuel, and thus any conversion to chemicals needs to compete with the fuel value of methane. The cost trade-offs are likely to change with increased use of CCUS technologies. Challenges and opportunities for chemical engineers in this area include the development of catalysts, integration of catalyst and reactor technology, and identification of new chemical targets. Tools such as LCA and TEA will also be critical in identifying situations in which methane conversion is cost-competitive with the use of methane as fuel.
Biological Conversion of CO2
Biological conversion of CO2 holds great promise because some microorganisms have a natural ability to capture and covert CO2. Photosynthetic pathways to CO2 utilization include approaches using algae (products include biofuels, dietary protein and food additives, and commodity and specialized chemicals), green algae (products include biodiesel, dietary protein, polyunsaturated fatty acids, pigments, lipids, and terpenoids), and cyanobacteria (products include ethanol, butanol, fatty acids, heptadecane, limonene, bisabolene, ethylene, isoprene, squalene, and farnesene). By using CO2 as feedstock, these photosynthetic pathways mitigate the problem of the high-cost sugar feedstocks needed for microbial pathways; however, the slow growth rates of algae and cyanobacteria prevent them from achieving industrially relevant productivity and scale-up. Challenges and opportunities for chemical engineers in this area include bioreactor and cultivation optimization, analytical and monitoring tools, genome-scale modeling and improvement of metabolic efficiency, bioprospecting, valorization of coproducts, genetic tools, and pathways to new products.
Biological Conversion of CH4
Methanotrophs can use methane as their carbon and energy source. However, significant challenges arise, such as the risk of contamination during fermentation, buildup of toxic intermediates, and the high cost of additives. Some commercial activity has taken place in this space. Calysta™ has commercialized FeedKind® protein as an alternative feed for fish, livestock, and pets, using no agricultural land and less water than is required for similar agricultural products. Intrexon™ has used methanotrophs to produce high-value chemicals, such as isobutanol and farnesene. And Mango Materials™ plans to convert biogas into polyhydroxyalkanoate (PHA), which is a biodegradable plastic. Challenges and opportunities for chemical engineers in this area are the same as those for biological conversion of CO2.
CHALLENGES AND OPPORTUNITIES
In the energy sector, the overarching challenge for chemical engineers is to address the environmental and climate impacts of current energy systems, particularly the use of fossil-based energy sources. The transition to a low-carbon energy system will require a bridging strategy that relies on a hybrid system with a mix of energy carriers. Chemical engineering is rooted in the transformation of stored energy carriers into more convenient forms and into chemicals and materials. Chemical engineers have an important opportunity to continue to apply their skillsets to non–fossil-fuel-based energy sources and carriers and thus contribute to the decarbonization of the energy sector.
In the long term, achieving net-zero carbon emissions will require significant advances in photochemistry, electrochemistry, and engineering to enable efficient use of the predominant source of energy for Earth—the solar flux. To this end, novel systems will be required to improve the efficiency of photon capture and conversion to electrons; improve the storage of electrons; and advance the direct and/or sequential conversion of photons to energy carriers via reactions with H2O, N2, and CO2 to produce H2, NH3, and liquid fuels, respectively.
Specifically, greater market penetration of PV solar panels will require a continued decrease in the cost of these panels, as well as increased electrification of energy systems. Chemical engineers have an opportunity to play an enabling role in addressing this challenge by advancing incremental improvements in device architecture and design, lowering manufacturing costs, and improving reliability and durability. Beyond PV technologies, critical challenges hindering the greater use of PSC systems include operational instabilities, short useful lives, and the containment and ultimate disposal of some toxic components. Research conducted by chemical engineers and others will be critical to developing perovskite compositions that minimize degradation and ensure reliable long-term operation. Conversion of photons to H2, NH3, or organic fuels will require advances in the synthesis, characterization, and mechanistic assessment of catalytic solids, as well as the development of materials that can withstand severe chemical, photochemical, and electrochemical environments within complex hydrodynamics for systems that couple the required reactions through diffusional controls, all of which are research opportunities for chemical engineers.
Successfully mitigating climate change will require a long-term transition to renewable and sustainable sources of energy. In the short term, however, chemical engineers have many opportunities to reduce the carbon footprint of fossil fuels. For coal, these opportunities include research and technological advances to increase thermal efficiency, further improve emission controls, and reduce water consumption. While natural gas is a cleaner bridge fuel compared with other fossil fuels, innovations are still needed throughout the value chain. Chemical engineers can enable advances that will minimize or replace water use as a fracturing agent, improve storage and transportation, and better integrate natural gas with renewable energy sources. For petroleum, challenges and related opportunities for chemical engineers include improved water management,
increased recovery to extend well life, data-driven approaches to reservoir management, and improved methane management. Decreasing the GHG emissions associated with all types of fossil fuels will require the demonstration of cost-effective and secure carbon capture and storage methods, a critical opportunity for chemical engineers.
Because most biogenic feedstocks have lower energy content than their fossil-based counterparts, the greatest challenge for increased use of biofuels is the production of high-density fuels at a reasonable cost that is competitive with that of existing, fossil-based fuels. This challenge, combined with the need to account for the environmental consequences of harvesting crops for energy use, creates opportunities for chemical engineers to use systems-level economic, environmental, and technical analyses to select the most viable biofuel options. Furthermore, increasing the market penetration of EVs for personal transportation will require reimagining petroleum refineries that were designed to produce gasoline or diesel fuel as their main products. Refineries will require reconfiguration to shift their product slate toward petrochemicals and low-carbon liquid fuels needed for the difficult-to-decarbonize commercial transportation sector (e.g., heavy-duty long-haul ground, aviation, and marine transportation). Full integration of existing petroleum refinery assets with biorefineries and the greater use of renewable energy will enable significant reductions in carbon footprints and lower-cost low-carbon liquid fuels, another area that presents considerable opportunities for chemical engineers.
For intermittent energy sources, chemical engineers can contribute to the development of advanced materials that can increase the viability of wind and marine energy. Chemical engineering research will also be critical to advancing low-carbon fuels; improving petroleum refining; advancing clean hydrogen production; and developing improved synthetic fuels for sectors, such as aviation, that are difficult to decarbonize. A successful transition to low-carbon energy systems presents the challenge of energy storage. Chemical engineers can enable the development of new battery materials, as well as contribute to LCAs of competing battery technologies and the design of batteries for safe end-of-life disposal.
For end uses, the production of cement, steel, and chemicals presents the clearest opportunities for chemical engineers to contribute to decarbonization of the industrial sector. To achieve the net-zero emissions goal for industry, five broadly applicable decarbonization pillars require vigorous pursuit in parallel over the next decades: demand reduction, energy efficiency, fuel switching and electrification, transformative technologies in sectors, and abatement. All will benefit from the contributions of chemical engineers.
Finally, CCUS will be centrally important to controlling the concentration of carbon in the atmosphere. This broad area is rich with opportunities for chemical engineers, including LCA, integrated assessment management, and the science and technology advances necessary to advance direct air capture and carbon utilization.
Recommendation 3-1: Across the energy value chain, federal research funding should be directed to advancing technologies that shift the energy mix to lower-carbon-intensity sources; developing novel low- or zero-carbon energy technologies; advancing the field of photochemistry; minimizing water use associated with energy
systems; and developing cost-effective and secure carbon capture, use, and storage methods.
Recommendation 3-2: Researchers in academic and government laboratories and industry practitioners should form interdisciplinary, cross-sector collaborations focused on pilot- and demonstration-scale projects and modeling and analysis for low-carbon energy technologies.























































