6
Flexible Manufacturing and the Circular Economy
The chemical engineering discipline is broadly concerned with enabling realistic, cost-effective, efficient, and safe physical and chemical transformations of matter into more useful molecules or materials. In the last century, the discipline of chemical engineering enabled transformations of the entire landscape of both modern society and the planet. This chapter focuses on some key examples of the challenges and opportunities for chemical engineering in moving toward more flexible manufacturing and a circular economy.
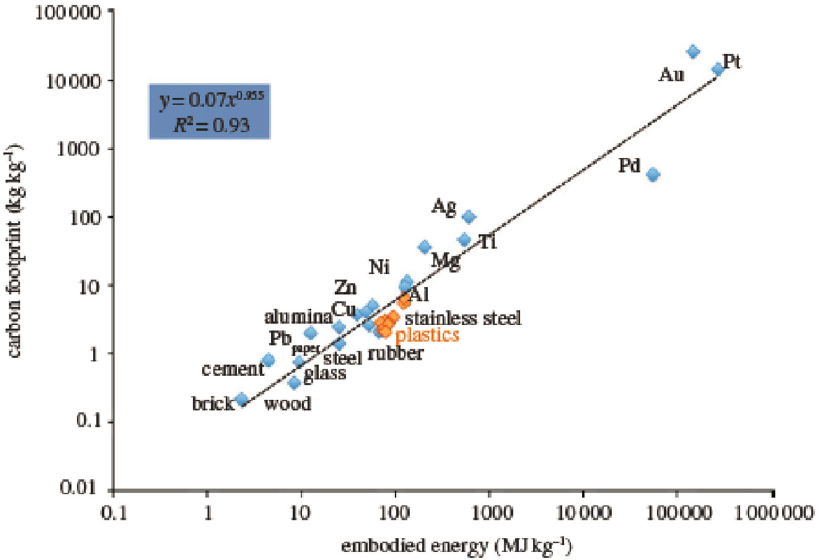
Manufacturing is generally defined as the synthesis and formulation of useful products. In the last century, chemical engineers revolutionized manufacturing across all sectors of the economy, including agrochemicals and fertilizers, cement, consumer goods, flavors and fragrances, food and feed, fuels, paints and coatings, paper and pulp, pharmaceuticals and biologics, polymers, semiconductors, and many others. To give some idea of scale, for plastics alone—a prominent example discussed later in this chapter—the mass of these synthetic materials manufactured in just the last 70 years now exceeds the mass of all living animals on the planet (Elhacham et al., 2020). Additionally, the chemicals and materials manufactured at scale today contain substantial embodied energy and produce significant greenhouse gas (GHG) emissions (Figure 6-1). These two metrics are important benchmarks, along with issues of environmental and social justice and supply chain resilience, for calibrating new technology development that can mitigate anthropogenic climate change.
Given their critical role in manufacturing, chemical engineers have many opportunities to increase its environmental sustainability. This chapter provides an overview of the intersection of manufacturing and chemical engineering, followed by a discussion of feedstock flexibility, distributed manufacturing and process intensification, and the importance of transitioning from a linear to a circular economy.
INTERSECTION OF MANUFACTURING AND CHEMICAL ENGINEERING
The intersection of manufacturing and chemical engineering is founded on the systems-level, quantitative approach intrinsic to the profession (Peters et al., 2002; Turton et al., 2012). This approach includes the ability to conduct rigorous mass and energy balances, coupled with appropriate technoeconomic assessment (TEA) and life-cycle assessment (LCA). The tools of TEA and LCA enable detailed analysis of developed processes to identify potential efficiency gains from reducing cost, energy use, or material inputs. These efficiency gains are accomplished by an improved ability to recycle materials or by the transition from batch to continuous processes, among other changes. TEA and LCA tools are also critical in manufacturing to identify “leapfrog” processes that can displace current methodologies (TEA and LCA are described in more detail in Chapter 8). More recently, consideration of environmental and social justice has become increasingly important for chemical engineers in these analyses.
In addition to TEA and LCA, the overall principles of green chemistry (Anastas and Warner, 1998) and green engineering (Anastas and Zimmerman, 2003), highlighted in Box 6-1, provide qualitative guidelines that chemical engineers can place in a quantitative, objective context with systems-level approaches. This capability ultimately enables more efficient and responsible manufacturing practices and better decision making regarding trade-offs.
Safety and safe operations are the most critical responsibility of the chemical engineering field. Safety is more important than reaching the goals of improving efficiency, increasing cost-effectiveness, and lowering environmental impacts of manufacturing processes. Simply put, many of the centralized industrial manufacturing facilities that chemical engineers have enabled over the last century handle gases, liquids, and solids at such scales and under such operating conditions that an accident can harm people and damage local and regional environments. Strict adherence to safety, including its inclusion in chemical engineering education, is essential to the discipline and needs to be rigorously maintained from the laboratory to the refinery.
Most conventional manufacturing processes in a chemical engineering context are operated at extremely large scale and in capital- and operating-intensive centralized facilities to harness economies of scale. However, advances in the valorization of flexible feedstocks, electrification of the manufacturing sector (Schiffer and Manthiram, 2017), and the concepts of scale-out and distributed manufacturing will play a key role for chemical engineering going forward. Some of these concepts are likely to play major roles in the deployment of manufacturing to low- and middle-income countries and to economically depressed regions of higher-income countries, as well as in various efforts at reshoring of manufacturing through new technologies. Indeed, industrial manufacturing has the potential in this century to at least partially transform physically from the scale of the petrochemical complexes studied by today’s undergraduate chemical engineers to more heterogeneous intensified and distributed manufacturing sites, including those with electrically driven power sources. Notably, flexible and distributed manufacturing have al-
ready been a major focus of research and development among the international community, but substantial opportunities remain for the U.S. chemical engineering community to contribute meaningfully in these areas.
Lastly, the concept of the circular economy is commonplace in today’s vernacular, including across many STEM (science, technology, engineering, and mathematics) professions. The Ellen MacArthur Foundation defines the circular economy as a systemic approach to economic development designed to benefit businesses, society, and the environment; in contrast to the “take-make-waste” linear model, a circular economy is regenerative by design and aims to gradually decouple growth from the consumption of finite resources (EMF, 2021). This broad concept has a figurative home squarely in the chemical engineering approach to manufacturing and green engineering principles because the ideas of materials recycling and, more generally, the efficient use of matter and energy are central to the field’s systems-level thinking. Transitioning manufacturing from a linear to a circular economy is a key opportunity for chemical engineers.
FEEDSTOCK FLEXIBILITY FOR MANUFACTURING OF EXISTING AND ADVANTAGED PRODUCTS
The chemical engineering profession emerged in large part to confront the urgent challenges faced more than a century ago in the then-burgeoning petroleum refining industry. Petroleum is a highly heterogeneous organic resource. The global-scale petrochemical refining industry, in concert with the chemical engineering profession, was thus born out of the ability to characterize, fractionate, and ultimately valorize feed streams that are highly diverse in chemistry and that change as a function of time and source. Indeed, many of the original fractionated streams that could be derived from petroleum processing as a consequence of producing the original target fuels were considered waste. The ingenuity of chemists and chemical engineers, however, gave rise to uses for these waste compounds, including such diverse applications as asphalt, building blocks for synthetic polymers, and such formulated products as lubricants and processing fluids. These and myriad other high-value chemical applications today form the highly profitable chemicals backbone of the petrochemical business. The scale of the petrochemical industry worldwide is staggering: in 2019 its annual production volumes were 5–5.5 billion metric tons (100.69 million barrels per day) of crude oil, 4.1 trillion cubic meters (3.6 billion tons of oil equivalent) of natural gas, and 7.96 billion metric tons of coal (IEA, 2021c,d; EIA, 2021d).
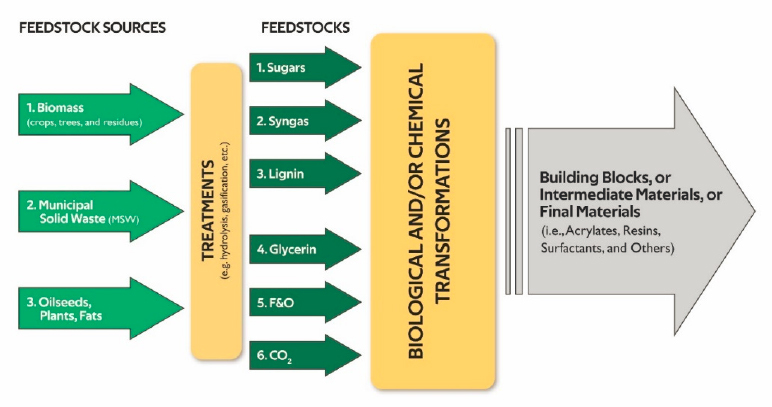
The continued drive toward more efficient, environmentally friendly, and cost-effective manufacturing processes will likely benefit greatly from a much wider range of available feedstocks for use as building blocks to produce the chemicals and materials demanded by modern society (Figure 6-2). This concept of feedstock flexibility can be broadly defined as the input of diverse feedstocks into a transformation process that is able to process various starting compounds or mixtures of compounds to produce the target product. Petrochemical refineries already do this today. Importantly, today’s petroleum feedstock requires oxidative chemistry to produce oxygenated molecules from hydrocarbons. For a different feedstock that is already oxygenated, such as lignocellulosic
biomass, reductive chemistry would be needed to manufacture such products as hydrocarbons.
The use of a flexible feedstock also influences ideas around distributed manufacturing, as discussed below. For example, the availability of stranded natural gas resources, along with the potential harm of leakage of GHGs from those resources, makes conversion of these streams via chemical, biological, electrochemical, or other means a key opportunity for chemical engineers. The use of nonthermal approaches requiring minimal utility infrastructure may be critical for the ultimate feasibility of small-scale and distributed harnessing of such feedstocks as stranded natural gas; industrial waste gases; and industrial, commercial, or municipal wastewater (Khalilpour and Karimi, 2012; Tuck et al., 2012). This work also includes, as shown in Figure 6-2, the use of selective transformations afforded by biological and chemocatalytic transformations. Indeed, biological transformations of conventional feedstocks, combined with knowledge from the environmental bioremediation community, can show how bioprocessing can be incorporated into the petrochemical industry.
Flexible feedstock sources beyond those derived from fossil fuels include large amounts of available biogenic and waste carbon inputs, such as municipal solid waste (MSW). The goal of integrating biobased pyrolysis oil made from lignocellulosic biomass or MSW into a petroleum refinery has been pursued for several decades (Chen et al., 2014; Talmadge et al., 2014), despite challenges with inorganic foulants and catalyst poisons common in biogenic and waste carbon. The chemical engineering community has a key opportunity to understand how biogenic and waste-based substrates affect current manufacturing infrastructure. And much more room is available to explore the concepts of “refinery integration” of stabilized, biogenic and/or waste-based, carbon-rich streams into the existing, mostly amortized petrochemical complex.
More broadly, existing waste streams of biogenic and waste-based carbon could serve as useful feed streams for leapfrog technologies. Chemical engineers are already playing critical roles in the harvesting, densification, conversion, and scale-up of innovative processes for leveraging these biogenic and waste-based feedstocks. As discussed in Chapter 3, by 2030 there will be an estimated 1 billion dry tons of lignocellulosic biomass annually in the United States alone that can potentially be sustainably harvested (DOE, 2016). This potentially large feedstock, along with large amounts of other available wet waste (e.g., food waste, manure, sludge, fats, oils, and greases total are ~700 million tons per year; Milbrandt et al., 2018), is distributed across the United States. Not only does its use offer the potential to produce meaningful amounts of transportation biofuels to offset substantial GHG emissions (Chapter 3), but it also could serve as a key feedstock for chemical manufacturing. Products could include both direct-replacement biochemicals (Nikolau et al., 2008) and biochemicals that do not resemble their fossil-based counterparts but offer a performance advantage (Cywar et al., 2021; DOE, 2018a). MSW also offers a substantial and important feedstock, which again is highly heterogeneous.
The manufacturing of direct-replacement chemicals offers substantial opportunities for chemical engineers to develop scaled-out, distributed manufacturing systems and innovative, large-scale processes that can compete with the conversion of fossil resources. Conversely, performance-advantaged bioproducts could also serve as economic incentives to invest in new capital infrastructure at scale to displace the fossil carbon-based feed streams that dominate chemicals and materials production at scale today. These target bioproducts are an opportunity for chemical engineers to develop fully integrated, novel processes for transforming typically highly oxygenated feedstocks (e.g., sugars, aromatics derived from lignin, algae biomass) into novel molecules and new materials for which the properties often are not known a priori. Indeed, performance-advantaged bioproducts can offer potential benefits along the entire value chain (from feedstock to manufacturing, use, and end of life) relative to incumbent chemicals and materials, thus providing product design, economic, and environmental benefits (Cywar et al., 2021; DOE, 2018a). Systems-level approaches will be necessary to understand the ultimate potential of a given process concept or early demonstrations to reach scalability for manufacturing processes (Cywar et al., 2021).
From a process perspective, the conversion of heterogeneous feedstocks of essentially any type into valorized end products can be broadly categorized into the “fractionate-first” approach that the petrochemical complex has successfully adopted or a “one-pot” approach that attempts to convert all feedstocks simultaneously. The latter includes such conversion approaches as hydrothermal liquefaction, pyrolysis, and gasification. While TEA and LCA, along with the demonstrable technical feasibility of a given process, will ultimately and quantitatively inform how various processes are adopted, scaled, and enabled, many opportunities exist to define new flowsheets using emerging tools in electrochemistry, photochemistry, synthetic biology, integrated separations and catalysis, and many other tools that are familiar to chemical engineers. These approaches, and combinations thereof, present opportunities to significantly change the manufacturing landscape.
From a chemistry perspective, new feedstocks—especially those related to lignocellulosic and algal biomass, wet organic waste, and CO2 and other waste gases—are often highly oxidized relative to conventional fossil feedstocks. Many common existing transformations add heteroatoms (e.g., nitrogen and oxygen) to hydrocarbon feedstocks to manufacture products. The use of new feedstocks that are more oxygen rich (and potentially contain other heteroatoms) offers the opportunity to develop new and novel transformation processes. These transformations could also take place in a condensed rather than a gas phase, the latter of which is typical of most conventional hydrocarbon processing. New separation technologies will be critical to realizing these transformations, as will catalysts that can enable the necessary reductive chemistry while remaining stable in aqueous environments.
Beyond innovative process and chemistry developments, there are fundamental research problems for chemical engineers to solve in the feedstock flexibility arena. A prominent example that hinders systems-level work is the lack of robust thermodynamic data in common process-modeling packages for the molecules found in biobased or waste-based feedstocks. Such data are plentiful for the hydrocarbon-rich feedstocks of relevance to petrochemical refining. However, the ability to model important thermodynamic properties of other molecules, which are often richer in oxygen and other heteroatoms, poses a substantial challenge, as does their incorporation into process simulators.
PROCESS INTENSIFICATION AND DISTRIBUTED MANUFACTURING
Many of chemical engineering’s historical successes involve the efficient production of chemicals on a large and therefore economical scale. A world-scale ammonia plant, for example, generates 1,000 metric tons of ammonia each day. The design tools needed to scale up individual unit operations in these processes are well established and are a key focus of undergraduate chemical engineering education. Many opportunities exist to develop novel processes, which can potentially improve performance but have not always been used in commodity-scale plants.
Process intensification (PI) is designed to create improved chemical processes by moving beyond the idea that a single piece of equipment performs a single unit operation. In a traditional chemical plant, for example, a reactor and a distillation column might be used separately for a reaction and a separation step in an overall process. Reactive distillation (e.g., Taylor and Krishna, 2000) and membrane reactors (e.g., Iulianelli et al., 2016) are two alternative PI strategies that combine these two steps into a single process. PI also encompasses efforts to use nontraditional driving forces to accomplish unit operations—for example, microwave heating in reactions (e.g., Goyal et al., 2019) or the use of structured contactors in adsorption-based separations (e.g., Koros and Lively, 2012). Chemical engineers have numerous opportunities to use these strategies to develop innovative new processes, as well as to remove bottlenecks from existing large-scale processes. (Examples relevant to electronic-materials manufacturing are highlighted in Box 6-2). While PI presents many opportunities for innovation, it is also necessary to acknowledge that there are often trade-offs between PI and process flexibility, which may be more important for the flexible feedstocks discussed elsewhere in this chapter.
Four principles for PI design have guided thinking about how chemical processes are developed (Harmsen, 2007; Tian et al., 2018):
- Maximize the effectiveness of intra- and intermolecular events. Improving process kinetics is a major principle for obtaining higher process performance, as it is usually the underlying limiting factor for low conversion and selectivity.
- Give each molecule the same processing experience, which results in products with uniform properties. Uniform product distributions facilitate waste reduction, which in turn reduces the efforts required for product separation.
- Optimize the driving forces at every scale, and maximize the specific surface area to which these forces apply. Doing so results in more efficient processes that use lower amounts of enabling materials, which then leads to reductions in equipment sizes.
- Maximize the synergistic effects of partial processes that enable multitasking. By combining several processing tasks, higher process efficiencies can be achieved compared with their stand-alone counterparts.
The general categories of applicable technologies include structured devices (e.g., structured catalyst-based reactors, microreactors, nonselective membrane reactors), hybrid processes (e.g., extractive crystallization, heat-integrated distillation, reactive distillation, selective/catalytic membrane reactors), energy transfer processes (e.g., rotating packed beds, sonochemical reactors, microwave-enhanced operations), dynamic processes (e.g., oscillatory baffled reactors, reverse flow reactor operation), and others (e.g., supercritical reactions, cryogenic separations; Harmsen, 2007). Many catalytic processes are also good candidates for PI technologies (e.g., Boger et al., 2003; Broekhuis et al., 2001; Cybulski and Moulijn, 1994; Kapteijn and Moulijn, 2020; Machado and Broekhuis, 2003; Machado et al., 2005; Nordquist et al., 2002; Welp et al., 2006, 2009). For example, slurry catalytic processes in the specialty chemical and pharmaceutical industry with gas and one or two liquid phases can be intensified by replacing the slurry catalyst with a fixed monolith catalytic reactor. These reactors can be installed in a pump-around loop to existing classic stirred-tank reactors to allow modularization and the ability to operate a single stirred tank with multiple beds using a monolith-loop reactor arrangement. Finally, microreactors are a more recent trend in chemical reactor synthesis. Their small channels allow for extremely accurate temperature control and high mixing intensity for single- and multiphase reaction processes. Scale is achieved by increasing the numbers of micro-reactor systems. The continuous flow reaction and separation networks allow for adequate production to meet most demand.
Traditional large processes increase product volume by scaling up. An alternative strategy achieves product volume by scaling out the deployment of many compact processing units in parallel. This is the key aim of modular manufacturing. Scaling out has benefits over scaling up in such processes as
- water treatment, both in municipal settings and for produced water from oil or gas wells;
- upgrading of natural gas from remote wells where pipeline infrastructure for gas transport is problematic;
- processing of bioproducts where transportation costs play a significant role in net GHG emissions;
- pyrolysis of waste polyolefins where the size of the pyrolysis reactors is limited by heat transfer considerations; and
- industrial sectors, such as pharmaceuticals or electronic materials, where highly valuable products are often produced in small quantities.
Although examples in pharmaceutical processes typically use highly controlled feedstocks, the other examples are cases in which modular processes need to function despite significant variations in the availability and location of the process feedstock. Additionally, scaling out is sometimes necessary because of technical realities, while in other cases it offers an economic advantage. Pyrolysis of waste polyolefins is operated in scaled-out plants because of the heat transfer considerations; however, the price of the pyrolysis oil increases significantly in this context. On the other hand, and in the same example, collecting plastic waste over a large area and transporting it to a pyrolysis plant add cost that the benefit of a scaled-up plant might not be able to counterbalance. In this latter case, a distributed network of pyrolysis plants makes economic sense.
PI and modular manufacturing are important areas in which the chemical engineering research community can provide intellectual leadership. A challenge for the academic community is that the successes (or failures) of work in either area are inherently determined at a process scale. Both demonstrating that a process is possible at “lab scale” and combining such work with process modeling and/or LCA are needed to support large investments. Also critical is the development of new materials and processes that can be deployed readily at the requisite scale and cost for use in the target processes. To give just one example, individual membrane modules used in current water treatment applications have surface areas measured in hundreds or thousands of square meters. Any putative new membrane that cannot be produced readily and economically at this scale will simply have no meaningful impact in water treatment, regardless of how superlative its performance may be (Koros and Zhang, 2017). This observation highlights the importance of pursuing research focused on the manufacturability of modular components, not just on the development of new high-performance materials. This is also an area in which close working relationships between academic researchers and industrial practitioners can be fruitful.
Rapid advances in additive manufacturing (e.g., 3-D printing; see Box 6-3) have also opened up a wide range of possibilities for generating new devices used in chemical processes. These possibilities are perhaps especially rich in the realm of PI and modular manufacturing. Because much work in additive manufacturing is taking place in the mechanical engineering and materials science communities, chemical engineers have numerous opportunities to combine expertise from these adjacent fields with application-specific needs in chemical processing.
Lastly, many modular processes (e.g., the treatment of stranded natural gas) are likely to occur in remote locations where monitoring or access by highly trained operators
is limited. This fact, together with the issue of feedstock flexibility discussed above, highlights the need to develop modular processes that are inherently robust to process upsets and that take full advantage of advanced instrumentation and control strategies. Modular processes could leverage electrochemical power generated at small scale and on-site and/or biological manufacturing, which can often require lower heat and power inputs.
THE CIRCULAR ECONOMY AND DESIGN FOR END OF LIFE
The Industrial Revolution, beginning with the invention of the steam engine in the 17th century, enabled the use of raw materials and energy—which seemed to be infinitely available at the time—to make and eventually to mass produce products. The resulting economy was thus primarily linear (i.e., take → make → dispose), with resources being extracted or harvested, energy being used to make products, and products being disposed of at their end of life (Collias et al., 2021). The world’s linear economy annually generated about 110 million metric tons of MSW in 1900, more than 1 billion metric tons in 2000 (including about 80 percent of consumer products, excluding packaging, disposed of after a single use), and about 2 billion metric tons in 2016, and this number is forecast to grow to 3.4 billion metric tons by 2050 (Hoornweg et al., 2013; Kaza et al., 2018). The consequences of the growth of the current economy are unsustainable.
The Circular Economy
A sustainable future requires a transition to a circular economy. In that model, resources are managed differently than in the linear economy, the way products are made and used changes, and new consideration is given to the fate of products and materials at their end of life. The circular economy uses waste streams as sources of secondary resources, and it incorporates the principles of green chemistry and engineering (Box 6-1; Anastas and Warner, 1998; Anastas and Zimmerman, 2003; Collias et al., 2021).
In the circular economy model, materials and products are made efficiently and reused, thus preventing waste. If new raw materials are needed, they are produced sustainably. This model represents a paradigm shift that is consistent with emerging consumer preferences for recycling and reducing nonrecyclable waste (Nielsen, 2015), as well as new government restrictions on pollution and waste. A circular economy can be facilitated by emerging digital technologies and product designs that track materials and products and extend their lives. Decoupled from the consumption of finite resources, it can drive economic growth with business, societal, and environmental benefits. In the long term, the circular economy will achieve cost savings in materials that result in cost savings in products and packaging. For consumers, the benefits of the circular economy will come from new developments and trends in such areas as urbanization, technologies (e.g., anaerobic digestion), information technology capabilities, online retail sales, business models, and packaging technologies (EMF, 2014).
The circular economy model encompasses two cycles: biological and technical (Figure 6-3). In the biological cycle, food and biological materials (e.g., wood) feed back into the system through recycling processes, such as composting and anaerobic digestion, that regenerate living systems, such as the soil. In the technical cycle, such strategies as recycling, reuse, repair, and remanufacturing allow the recovery and restoration of products, components, and materials (EMF, 2013a,b, 2014).
The circular economy is based on three strategies (EMF, 2017a,b) that are consistent with the principles of green chemistry and engineering:
- Strategy 1: Design to avoid pollution and waste—Reduce or eliminate GHG emissions and hazardous substances and the resulting pollution of air, land, and water. Also limit, if not eliminate, waste of materials during the manufacturing of products and packaging, as well as during the discarding of products and packages at their end of life.
- Strategy 2: Extend useful life—Design for durability, reuse, remanufacturing, and recycling to keep materials, products, and packaging circulating in the economy as long as possible, thus preserving energy and materials.
- Strategy 3: Regenerate natural systems—There is synergy between a circular economy and a biobased economy. In the biobased economy, biobased materials are made from biobased resources and/or with the use of biobased energy, and these biobased materials cycle between the economy and natural systems (to become biobased resources). Ideally, the circular economy uses renewable and avoids the use of nonrenewable resources.
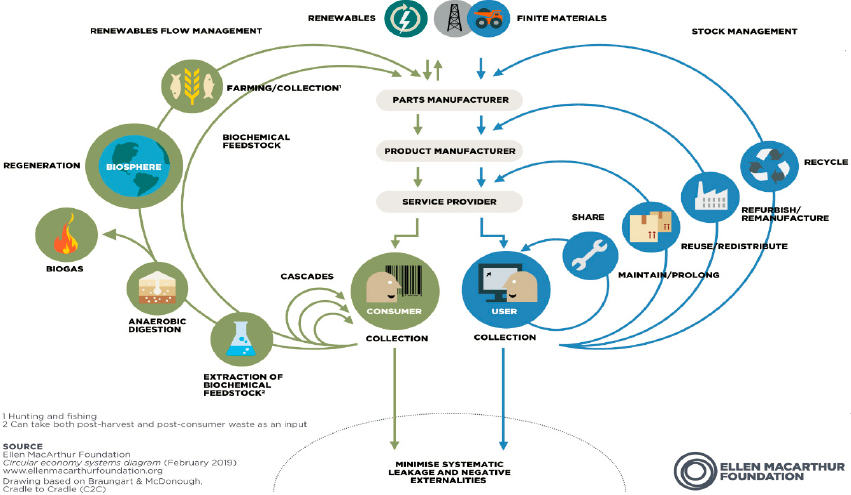
Each of these strategies is described further below, with a focus on plastics as an example of an area in which chemical engineers will play a key role. The essential principles of the circular economy, however, apply beyond the manufacture of synthetic polymers.
Chemical engineers are uniquely positioned to solve problems associated with the three strategies of the circular economy. Their contributions could include redesigning processes and products to reduce or eliminate pollution, developing new ways to reduce and utilize waste, designing products to be used longer, and designing processes and products using sustainable feedstocks. Besides providing technical expertise and leadership in various areas of the circular economy, chemical engineers have opportunities to address challenges in the following four more specific technology areas:
- purification of materials with a large volume and a high rate of collection, such as paper, cardboard, polyethylene terephthalate (PET), glass, and steel;
- recycling of polymers with a large volume and a low rate of collection;
- utilization of by-products of manufacturing processes, such as used concrete, CO2, and food waste; and
- development of materials with high value that currently have a small volume and a low rate of collection, such as 3-D printing materials and biobased materials.
Design to Avoid Pollution and Waste
Pollution and waste are associated with the production of all materials, products, and packaging. To show how concepts associated with the circular economy can be applied to reducing energy consumption and GHG emissions, it is useful to consider the opportunities for plastics.1 More than 90 percent of the feedstock for the plastics industry is petroleum or natural gas. About 6 percent of the world’s production of petroleum and natural gas liquids (NGLs) is used to produce plastics, with about half used as feedstock and the other half as fuel in the production processes (EMF, 2016). Another significant percentage of natural gas production is used as feedstock and fuel for plastics manufacturing.
Since the early 1950s, plastics have dramatically changed people’s way of life, but at the same time, they pose an environmental challenge because of the means used for their disposal in various parts of the world. Since 1950, 8,300 million metric tons of synthetic polymers has been produced, and 4,900 million metric tons has been discarded (Figure 6-4). Resistance to degradation—one of the most important properties of many
___________________
1 Here, for the purposes of simplicity, the terms “plastics” and “polymers” are used interchangeably. IUPAC (the International Union of Pure and Applied Chemistry) defines plastics as “polymeric materials that may contain other substances to improve performance and/or reduce costs.” Plastics are manmade and include both thermoplastics and thermosets, whereas polymers can be manmade or occur naturally.
plastics—is the main cause of their persistence in the environment. Thus, a circular economy for plastics provides the best opportunity to continue enjoying the benefits of plastics in everyday life while reducing the environmental impact of their mismanaged disposal. This circular economy of plastics addresses both aspects of the first strategy listed above (i.e., both pollution and waste reduction).
The primary goal of the circular economy of plastics is that plastics never become waste, and instead reenter the economy. There are two main strategies for achieving this goal: create an effective after-use plastics economy, and drastically reduce the leakage of plastics into natural systems and other negative externalities (EMF, 2016). Steps to reduce the pollution and waste of plastics include selecting from and executing various options that are depicted in typical waste hierarchies (Billiet and Trenor, 2020). The most preferred option is rethinking and redesigning the product and package. If that option is not possible, other options, in order of preference, are as follows:
- Reduce the amount of plastic used in the product and package.
- Reuse the product and package.
- Recycle the product and package.
- Use the plastic in the product and/or package as fuel.
- Dispose of the plastic in the product and/or package in a managed landfill.
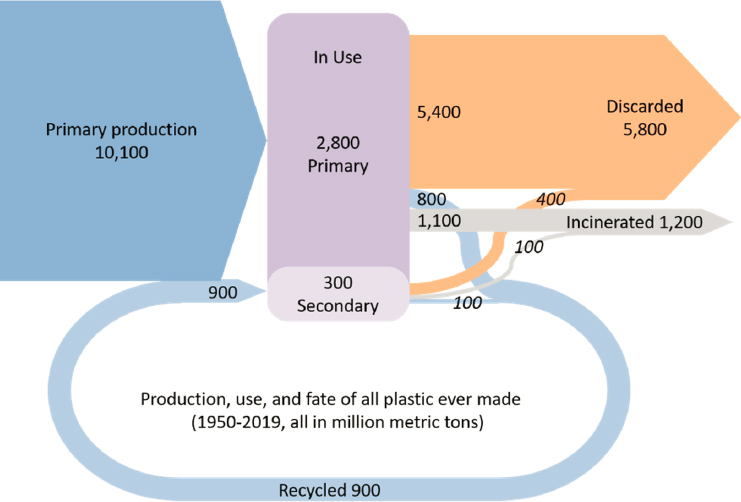
The least desirable outcome is disposal of the plastic in a mismanaged way. Replacing fossil-derived plastics with biobased plastics (totally or partly) and using renewable energy in the production of fossil-derived plastics are some alternative options for reducing the carbon footprint of plastics. Chemical engineers have an opportunity to apply quantitative, systems-level thinking to this problem through the application of TEA and LCA to determine which options optimize emissions reductions while considering other trade-offs, such as water consumption, cost, and environmental justice. Chemical engineers also have an important role to play in increasing and improving the recycling of plastics. Opportunities exist in all recycling technologies, such as mechanical recycling; dissolution recycling; and advanced recycling methods, such as depolymerization, pyrolysis, and gasification (Figure 6-5).
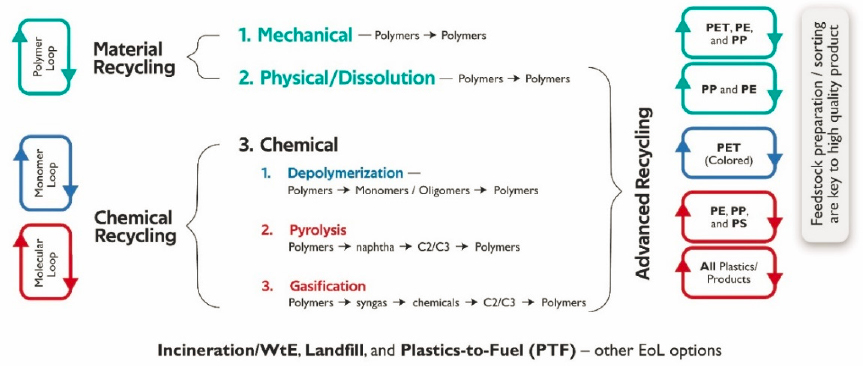
There are numerous opportunities to improve the quality of plastics produced and increase the range of plastics that can be mechanically recycled. Technologies that clean waste plastics and remove surface and bulk contamination are also the focus of chemical engineering work. Mechanical recycling of mixed polymers leads to the formation of polymer blends, and the associated technologies for compatibilization are important. Plastics present an additional challenge in that, unlike metals or glass in recycling, polymers change their structure. Advanced recycling will help recycle the polymers that mechanical recycling cannot, and could provide infinite recycling loops. An example of an LCA of mechanical recycling processes is described by Franklin Associates (2018).
Scaling up of dissolution recycling processes that produce food-grade plastics from waste plastics presents another opportunity for chemical engineers. Examples of such processes include Newcycling® by APK AG, PureCycle Technologies (Layman,
2019a,b), and CreaSolv® by CreaCycle GmbH (Maeurer et al., 2012). The main unit operations that chemical engineers can further develop, optimize, scale up, and commercialize are polymer dissolution, extraction, processing, layer separation, filtration, contaminant migration, and solvent diffusion and recycling (Pappa et al., 2001; Walker et al., 2020; Zhao et al., 2018).
Depolymerization of condensation polymers is applicable to polyesters (e.g., PET and polylactic acid [PLA]) and polyamides (e.g., nylon), among others. Hydrolysis, methanolysis, glycolysis, ammonolysis, aminolysis, and hydrogenation are typical chemical processes that, depending on the chemical used for the PET chain scission, degrade the starting polymers to either their respective monomers or oligomers. The product of depolymerization is purified to remove contamination and colorants, and then used to form new polymers. Catalyst or enzyme development, process optimization and scale-up, purification, and polymer processing are key areas with an intense chemical engineering focus. Examples of LCA for PET recycling are found in Shen et al. (2010) and Singh et al. (2021).
Pyrolysis is used for polyolefins, multilayer packaging, fiber-reinforced composites, polyurethanes, and other polymers that are difficult to depolymerize. Pyrolysis takes place at moderate to high temperatures and produces various hydrocarbons. Chemical engineers play a central role in the design of the reactor and its modeling. Catalytic pyrolysis (Cocchi et al., 2020; Miandad et al., 2016; Ratnasari et al., 2017; Zero Waste Scotland, 2013) is another technology that presents chemical engineering challenges and opportunities. The objective of this technology is to achieve C–C bond breaking at lower temperatures and reaction times relative to thermal pyrolysis, and to produce a higher-volume and higher-quality liquid fraction. Catalysts that have been explored include FCC (fluid catalytic cracking) catalyst, spent FCC catalyst, ZSM-5 (Zeolite Socony Mobil-5), HZSM-5, Cu-Al2O3, zeolites, Fe2O3, MCM-41 (Mobil Composition of Matter No. 41), coal fly ash, and mixtures of the above (Miandad et al., 2016; Ratnasari et al., 2017). In addition to thermal and catalytic pyrolysis technology vectors, plasma pyrolysis, microwave-assisted pyrolysis, and hydrocracking are other pathways for converting feedstock to pyrolysis oil for further conversion. A chemical engineering challenge in pyrolysis is to achieve process scale-up rather than process scale-out (i.e., scale via parallel units/modular design), which is common today, including with the advent of electrochemically driven reactors. This scale-up challenge results from the difficulty of achieving adequate heat transfer to the plastic (which acts as an insulator) as the volume of the reactor increases and its surface area does not increase proportionally. As a result, the typical annual capacity of pyrolysis reactors/plants is 5–10 thousand tons, and larger-capacity plants are constructed with many reactors in parallel rather than the more economical option of using larger reactors.
Gasification converts plastics to a gaseous mixture of CO and H2 (syngas), CO2, CH4, and other light hydrocarbons via partial oxidation in the presence of steam and oxygen or air at less than the stoichiometric ratio (Higman and van der Burgt, 2008; Rezaiyan and Cheremisinoff, 2005).
Incineration can be useful for end-of-life plastics and other waste. In waste-to-energy (WtE) processes, for example, MSW is combusted, and the heat produced is used
to make steam for generating electricity or to heat buildings. Besides producing electricity, WtE contributes to reducing the amount of material that would otherwise go to landfills and the resulting landfill emissions. In 2018 in the United States, about 12 percent of the 292 million tons of MSW was burned in WtE plants, generating 13 billion kWh of electricity in 2019 (EIA, 2020c). The percentage of MSW that feeds WtE installations ranges from 12 percent in the United States to 74 percent in Japan. Many large landfills generate electricity from the methane gas that is produced from the decomposition of biomass. Incineration and WtE processes can also potentially release hazardous chemicals and particles. These chemicals can affect air quality, affecting the neurological, respiratory, and reproductive systems of the human body and damaging the environment. Removing these chemicals from the incineration process is an important chemical engineering challenge.
Other potential solutions to the plastics disposal problem beyond the recycling technologies discussed above include the following:
- Use of biodegradable plastics and various enzymes and biodegrading organisms for plastics—LCAs are necessary to determine whether in some environments, the negative effects of the uncontrolled release of the biodegradation products into the atmosphere outweigh the benefits of using biodegradable plastics.
- Closed-loop recycling of polymers synthesized with in-chain functional groups that act as break points—For example, Häuβler and colleagues (2021) prepared a redesigned version of polyethylene with renewable polycarbonate and polyester in-chain functional groups that can be recycled chemically by solvolysis with a recovery rate exceeding 96 percent.
- Composting and anaerobic digestion—Compositing is a managed process of controlled decomposition of organic material by aerobic microorganisms producing compost (also called humus), CO2, water, and heat. Anaerobic digestion is used primarily to process wet waste material with microorganisms that break down organic material in the absence of oxygen and produce biogas.
Extension of Useful Life
In addition to reducing environmental impact, there is also an economic incentive for keeping materials, products, and packaging in use as long as possible. The economic value of plastic packaging that is lost after a single use is estimated to be between $80–120 billion, and the environmental cost of plastic packaging is estimated to be $40 billion—more than the industry’s total profits (McKinsey Sustainability, 2016). In designing products for longevity, the entire life cycle of the product and the value chain need to be managed. For example, if a physical unit is needed, design thinking might suggest making it more durable or making it easy to maintain by using designs that allow critical components to be replaced when worn out.
Apart from designing for easy disassembly, use of single-material packaging is at the top of the list of desirable options for a product at the end of life. Multimaterial packaging does not allow for mechanical and other types of recycling because the various materials cannot easily be separated. Such multimaterial packaging might include blends of different plastics or multilayer films, with each layer made of a different polymer. Separation technologies for multimaterial films (e.g., biomimicry-based adhesives between the materials or layers) and the development of monomaterial solutions that achieve the same performance as multimaterial solutions are two key areas in which chemical engineers will continue to contribute.
Regeneration of Natural Systems
A biobased economy includes both biobased materials and biobased fuels (fuel uses are discussed in Chapter 3). Sugars (e.g., glucose) are the typical feedstocks for a biobased economy, with biomass being the preferred primary source. Biomass is typically classified as generation 1 (e.g., sugarcane, corn, sugar beet, potato) or generation 2 (e.g., crop residues, grass, straw, wood, MSW). Because generation 1 biomass as a sugar source competes with food uses, generation 2 biomass is a better long-term source of sugars.
Generation 2 biomass contains three natural polymers: cellulose (35–50 percent of the biomass), hemicellulose (25–30 percent), and lignin (15–30 percent). Cellulose is a linear polymer composed of (1,4)-d-glucopyranose units linked by β-1,4-glycosidic bonds. Hemicellulose is a branched heteropolymer composed of hexoses, pentoses, and uronic acids linked by different bonds. Lignin is a high-molecular-weight, amorphous polymer composed of aromatic rings of phenyl propane. Various processes are required to separate biomass into its three main components, and then convert cellulose and hemicellulose to sugars, and lignin to other smaller chemical units. The challenges and opportunities for chemical engineering in the use of biomass are significant. Technically and economically successful hydrolysis of biomass and the production of low-cost sugars are key to enabling the biobased economy. Chemical and enzymatic hydrolytic processes need to be advanced in this space. Also, valorization of lignin is key to making overall biomass utilization successful.
Use of biomass for fuels, products, and power has been recognized by the U.S. Department of Energy (DOE) as a critical component of reducing dependence on volatile supplies and prices of imported oil (Biddy, 2016). The importance of chemicals in the economy is disproportionate to the amount of oil used to produce them. For example, only about 3–4 percent of petroleum is used to make chemicals, whereas the pretax value of the petrochemical products (e.g., plastics, detergents, paints, adhesives, cosmetics, pesticides) was about $375 billion in 2005—about the same as the pretax value of transportation fuels, which use about 71 percent of petroleum (Marshall, 2007). The production cost of biobased chemicals and its comparison with that of the corresponding petroleum-derived chemicals depend on the prices of biomass, petroleum, and natural gas, as well as production capacity, technology maturity, and other factors. As the current price of natural
gas is relatively low (compared with the price of petroleum on an energy basis), the current production cost of the incumbent chemicals is relatively low relative to that of the biobased chemicals.
Over the past two decades, biorefineries have been proposed as a way to achieve favorable economics compared with typical petroleum refineries and downstream processing plants. The products of biorefineries can be fuels, chemicals, or both. The International Energy Agency (IEA, 2020b; IEA, ICCA, and DECHEMA, 2013) presented a vision of biorefineries based on eight feedstock platforms:
- Syngas—Biomass gasification yields syngas, which is converted into products using fermentation or other chemical processes.
- Biogas—Anaerobic digestion of high-moisture-content biomass (such as manure and waste streams from food processing plants) yields primarily methane, which can be used as a chemical feedstock.
- C6 and C5 sugars—C6 sugar streams and, to a lesser extent, C5/C6 mixed sugar streams can be used in fermentation processes to produce various chemicals.
- Plant-based oil—The basis of the oleochemical industry, with a by-product of glycerin, plant-based oil has been explored as feedstock for the production of chemicals.
- Algae oil—Algae has a higher productivity than plants because the entire biomass can be used to produce high-value products, such as high-protein food or feed, pigments, antioxidants, vitamins, and sterols.
- Organic solutions—When fresh wet biomass (such as grass or clover) is dewatered, an organic solution (nutrient-rich press juice) and a press cake (fiber-rich) are produced, and both can be used to make other chemicals.
- Lignin—Lignin’s native structure suggests that it can be used to produce aromatic molecules; however, only a limited number of products from its derivatives, lignosulfonates and kraft lignin, have been produced so far.
- Pyrolysis oil—Biomass pyrolysis produces pyrolysis oil, which can then be upgraded to different chemicals.
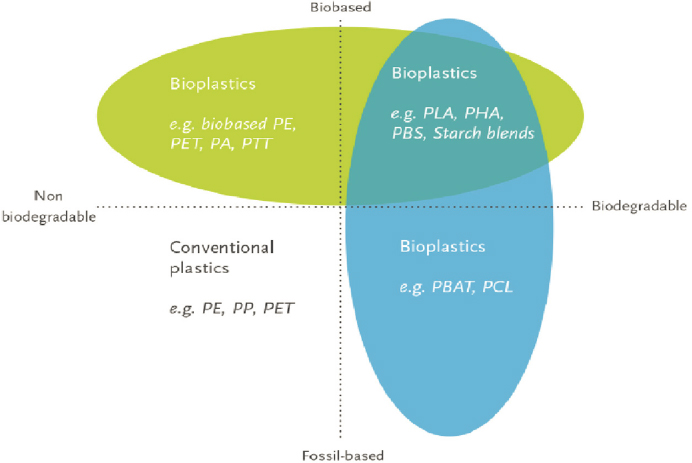
Biobased plastics are also part of the circular economy of plastics. Currently, there is a biobased plastic-alternative material (either biobased, biodegradable, or both) for many fossil-derived conventional plastics, at least at laboratory or pilot scale (Siracusa and Blanco, 2020). There are three main groups of biobased plastics (Figure 6-6):
- biobased or partially biobased and nonbiodegradable plastics, such as biobased polyethylene (PE; e.g., Braskem’s I’m green™), polypropylene, and PET and biobased polymers (e.g., polytrimethylene terephthalate [PTT]) (top left quadrant of Figure 6-6);
- biobased and biodegradable plastics, such as PLA, polyhydroxy alkanoate (PHA), polybutylene succinate (PBS), and starch-based blends (top right quadrant of Figure 6-6); and
- biodegradable plastics from fossil resources, such as polybutyrate adipate terephthalate (PBAT), polyvinyl alcohol (PVOH), and polycaprolactone (PCL) (bottom right quadrant of Figure 6-6).
Compared with the conventional fossil-derived plastics (bottom left quadrant of Figure 6-6), biobased plastics can potentially have a lower carbon footprint and additional end-of-life options, such as composting. The global production of biobased plastics was 2.1 million metric tons in 2020, representing less than 1 percent of total plastics production. Biodegradable plastics made up about 58 percent of the biobased plastics volume, with the remainder comprising biobased and nonbiodegradable plastics (European Bioplastics, 2021). LCA can be a valuable tool for evaluating the trade-offs between conventional fossil-derived and biobased plastics.
The economics of the processes used to make biobased plastics depend heavily on the stoichiometry of the feedstock conversion to the product, and particularly on the oxygen content of the feedstock. For example, the theoretical mass yield of PE from sugar is about 30 percent, and the rest (70 percent) of the sugar mass is lost as water and CO2 (see the oxygen chart in Figure 3-10 in Chapter 3). This process starts with sugar fermentation to ethanol, dehydration of ethanol to ethylene, and polymerization of ethylene to PE. This bio-PE cannot compete in price with fossil-derived PE because of the stoichiometry of the conversion reaction from carbohydrate to hydrocarbon and the economy of
scale present in the production of fossil-derived PE. A similar argument holds for the production of biofuels from sugar or biomass. Note that the capacity of a typical petroleum refinery is 10–130 million metric tons per year (measured as the amount of petroleum that can be distilled in the atmospheric distillation units), whereas the capacity of an average ethanol fermentation plant is about 0.25 million metric tons, and that for ethanol dehydration to ethylene is smaller.
The biobased chemicals that are economically advantageous to produce from sugars are those that have an oxygen content similar to that of sugar or biomass, such as monoethylene glycol (MEG) and furan-2,5-dicarboxylic acid (FDCA), with mass theoretical yields of about 100 percent; acrylic acid (AA), with mass theoretical yield of 80 percent; and polyethylene furanoate (PEF), purified terephthalic acid (PTA), and PET, with theoretical mass yields exceeding 50 percent. Use of such feedstocks as lignin, CO, CO2, and vegetable oils will be advantageous to produce chemicals with similar oxygen contents. Various LCA studies on biobased plastics have been reviewed (Walker and Rothman, 2020).
It is important for chemical engineers to be involved in the development of the biobased economy. More specifically, challenges and opportunities for chemical engineers in this domain include
- scalable and economical processes for producing biobased plastics (e.g., PEF and PBS) and biobased monomers (e.g., biobased terephthalic acid; Collias et al., 2014);
- new biodegradable plastics for the marine environment;
- scalable and economical technologies for deconstructing lignin to molecules that can be used as feedstocks for various chemicals, such as
- bacterial, enzymatic, fungal, photocatalytic, hydrogenolysis, pyrolysis, oxidation via ionic liquids, and hydrolysis deconstruction technologies (Cao et al., 2018a; Glasser, 2019; Kellett and Collias, 2016; Ragauskas et al., 2014; Xu et al., 2019; Zakzeski et al., 2010); and
- engineering of lignin to make it more amenable to low-energy chemical deconstruction, such as by using monolignol ferulate transferase to introduce chemically labile ester linkages into the lignin in poplar trees (Wilkerson et al., 2014), and the proposed work on use of CRISPR (clustered regularly interspaced short palindromic repeats)–based genome engineering to edit multiple genes simultaneously to optimize biomass processing (Chanoca et al., 2019);
- technologies that use CO2 as a feedstock for various chemicals;
- reduction technologies for carbohydrate feedstocks to produce less oxygenated chemicals and hydrocarbons (e.g., dehydration of lactic acid to acrylic acid [Collias et al., 2018; Godlewski et al., 2014]; see Bozell and Petersen [2010] for the top 10 chemicals of the bioeconomy and the National Renewable Energy Laboratory [Biddy, 2016] for the 12 chemicals with prospects for near-term deployment);
- technologies that avoid energy-intensive processes, such as steam cracking; and
- technologies for converting waste plastics to higher-value chemicals or structures (upcycling), such as PE waste upcycling to long-chain alkylaromatics using tandem catalytic conversion by Pt supported on γ-alumina (Zhang et al., 2020b), lubricant and waxes (Celik et al., 2019; Rorrer et al., 2021), diacids (e.g. succinic acid, adipic acid) via oxidation, hydrogen (Uekert et al., 2020), and lithium-ion battery anodes (Villagomez-Salas et al., 2018).
CHALLENGES AND OPPORTUNITIES
A sustainable future will require a shift to a circular economy in which the end of life of products is accounted for, incorporating green chemistry and engineering. The chemical engineering profession emerged in large part to confront the urgent challenges faced more than a century ago in the then-burgeoning petroleum-refining industry. Petroleum is a highly heterogeneous organic resource. The continued drive toward more efficient, environmentally friendly, and cost-effective manufacturing processes will benefit from a much wider range of available feedstocks for use as building blocks to produce chemicals and materials. The challenge of feedstock flexibility offers chemical engineers an opportunity to develop advances in reductive chemistry and processes that will allow the use of oxygenated feedstocks, such as lignocellulosic biomass. Chemical engineers also have substantial opportunities develop scaled-out, distributed manufacturing systems and innovative, large-scale processes that can compete with the conversion of fossil resources. Fundamental research opportunities include the collection of robust thermodynamic data to improve the modeling of feedstock molecules that include oxygen and other heteroatoms.
Current challenges in process design include the need for improvements in distributed manufacturing and process intensification—areas in which the chemical engineering research community can provide intellectual leadership. Collaborations between academic researchers and industrial practitioners will be important for demonstration at process scale. In the transition from a linear to a circular economy, specific opportunities for chemical engineers include redesigning processes and products to reduce or eliminate pollution (e.g., Shi et al., 2021), developing new ways to reduce and utilize waste, designing products to be used longer, and designing processes and products using sustainable feedstocks.
Recommendation 6-1: Federal research funding should be directed to both basic and applied research to advance distributed manufacturing and process intensification, as well as the innovative technologies, including improved product designs and recycling processes, necessary to transition to a circular economy.
Recommendation 6-2: Researchers in academic and government laboratories and industry practitioners should form interdisciplinary, cross-sector collaborations fo-
cused on pilot- and demonstration-scale projects in advanced manufacturing, including scaled-down and scaled-out processes; process intensification; and the transition from fossil-based organic feedstocks and virgin-extracted inorganic feedstocks to new, more sustainable feedstocks for chemical and materials manufacturing.

























