4
Sustainable Engineering Solutions for Environmental Systems
By 2050, the Earth’s population is projected to grow to more than 9 billion, leading to a 60 percent increase in food demand, an 80 percent increase in energy demand, and a 55 percent increase in water demand (OECD, 2012; UN, 2017). Food, energy, and water are highly interconnected, with production or consumption of one usually directly linked to production or consumption of another. Agricultural crops produce biofuels and provide food for animals and humans. Energy is used to purify, transport, heat, or cool water; to produce fertilizers; and to power farm equipment, food processing, and cooking. Land and water diverted for energy production are no longer available for food production and vice versa. Water is used for the production of fuels and electricity, and for agriculture, food processing, livestock, and cooking. In addition to these complex relationships, air quality affects or is affected by all three sectors and has a direct impact on human health and well-being. Although water is a renewable resource that is conserved in the Earth, freshwater can be depleted locally, and the policies for its local allocation are set in a highly political context.
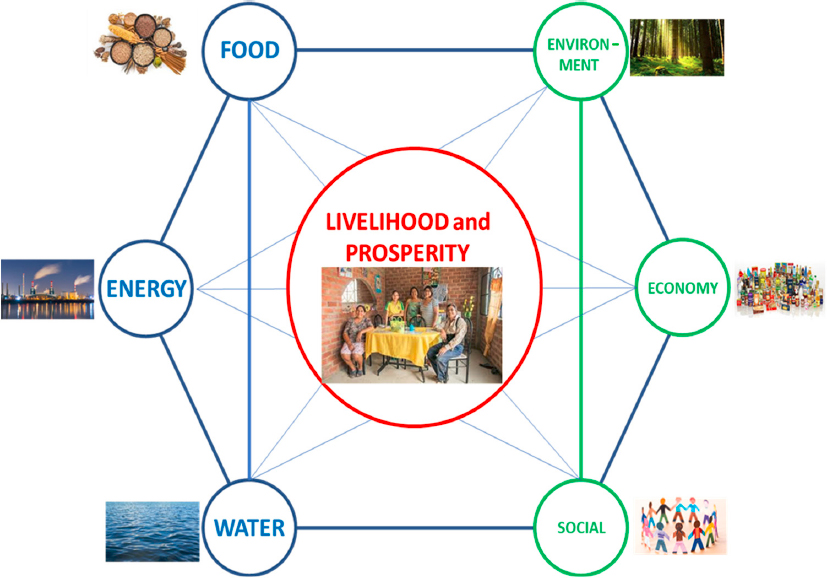
The concept of a water–energy–food (WEF) nexus was first introduced in 2011 by the World Economic Forum in Water Security: The Water-Energy-Food-Climate Nexus. To better contextualize this WEF nexus, it is important to intertwine an additional nexus, one that considers sustainability and environmental conditions (including climate), as well as economic and social (including human health) components, sometimes referred to as the “triple bottom line” (Das and Cabezas, 2018; Figure 4-1).
This chapter explores the role of chemical engineers in ensuring adequate food supplies and clean water and air. After a discussion of the WEF nexus, scientific gaps in understanding of the fundamental properties of water, as well as the need for engineering solutions for water quality and supply, are reviewed. Opportunities for chemical engineers to both pioneer and contribute to multidisciplinary efforts to advance agricultural and food processing technologies are then described, followed by a discussion of the research needs for understanding and improving air quality.
THE WATER–ENERGY–FOOD NEXUS
Solutions in the WEF nexus need to be sustainable; thus, environmental, economic, and social factors need to be carefully considered. Increased resource demand presents a monumental challenge for chemical engineers. They have historically played a central role in the energy sector, and their contributions in the interconnected space of water, food, and air quality have been important and are now growing. Fundamental insights from chemical engineering disciplines form the foundational knowledge necessary to understand and create solutions in the WEF nexus, which inherently spans multiple disciplines. Examples include the structure and dynamics of water, the nature and physics of aerosol particles, and the scaling of synthetic protein production. Additionally, chemical engineers’ knowledge of biochemical engineering and its applications to agriculture, and of separations with applications to water and air pollution, as well as their systems-level understanding, is critical to solving global problems.
Fossil fuels (petroleum, natural gas, and coal) make up most of the landscape of primary energy sources, contributing globally about 82 percent of the total energy produced in 2019 (IEA, 2021e). The continuing reliance on fossil fuels contributes to greenhouse gas (GHG) emissions and air pollution, as well as associated human health, environmental, and climate problems (D’Odorico et al., 2018). Today, one in five people lack
access to modern electricity in their homes, and 3 billion people use wood, coal, charcoal, or animal waste for heating and cooking, all of which have deleterious impacts on air quality (D’Odorico et al., 2018). Note that energy generation and consumption are covered extensively in Chapter 3 and are discussed in the remainder of this chapter only in the context of interdependencies with food, water, and air resources.
Over the past 50 years, global crop production has increased by more than 300 percent, and animal production by more than 250 percent. Dairy and meat production is expected to increase by more than 60 percent by 2050 (D’Odorico et al., 2018). All of these increases are attributable to the wider use of fertilizers and higher-yielding crop varieties, which enable greater food production. Of the global land surface, 38 percent is used for agriculture, with roughly one-third of that total used for crops and the other two-thirds used for animal grazing (FAO, 2020).
Food waste is a major problem around the world. In the United States, the percentage of food wasted ranges from 16 percent for meat to ~25–30 percent for grains, dairy, and eggs (Finley and Seiber, 2014). On a global scale, about one-quarter of the food produced for human consumption is lost or wasted in the supply chain; therefore, the percentage of arable land and freshwater resources used to produce that food is also wasted (D’Odorico et al., 2018).
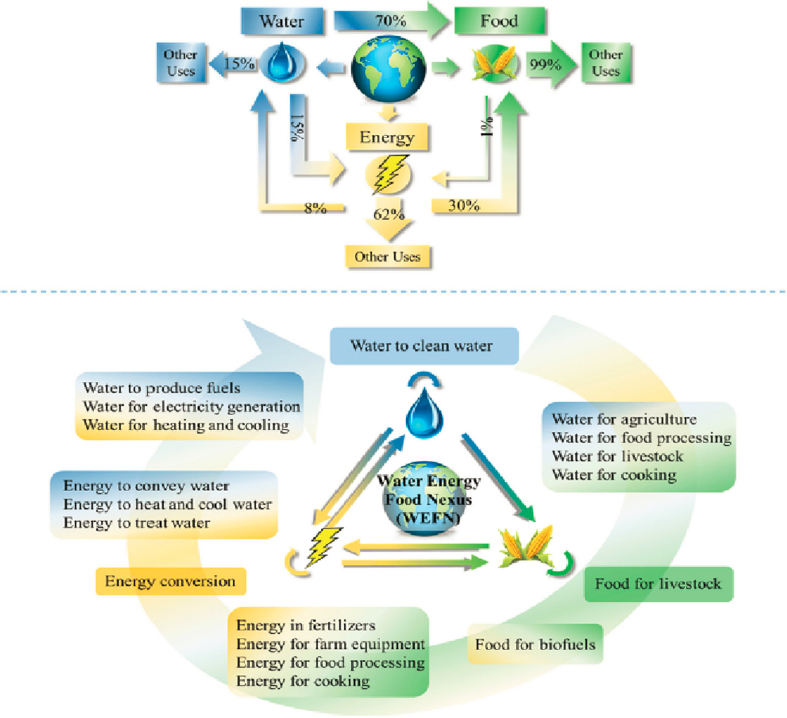
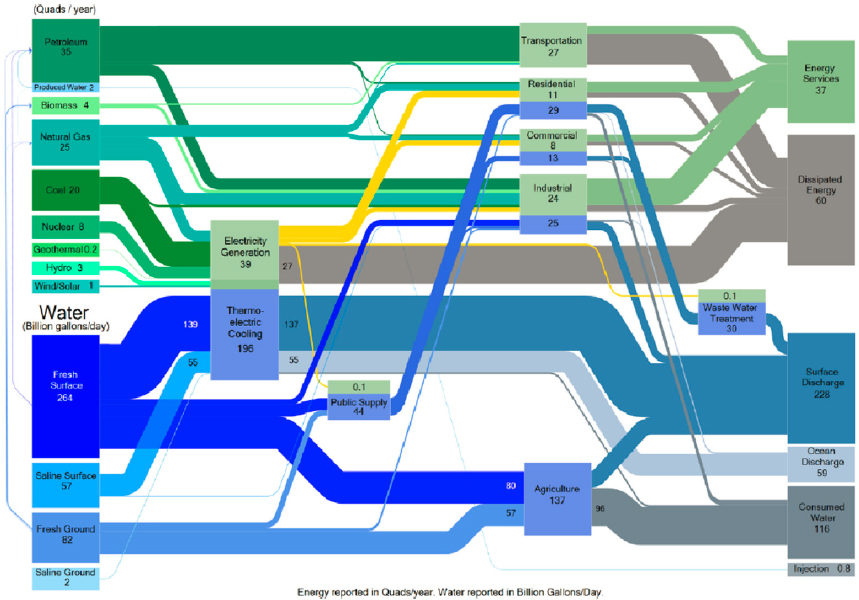
The interdependency within the WEF nexus is massive (Figure 4-2). Approximately 15 percent of global water withdrawals are used for energy, 70 percent for agriculture, and the remaining 15 percent for other applications. About 8 percent of global energy is used to transport, purify, and pump water, and about 30 percent to produce food. About 1 percent of all food is used to produce energy (Garcia and You, 2016). The interconnection between water and energy in various sectors of the U.S. economy in 2011 is depicted, from withdrawal and extraction through use, in the Sankey diagram in Figure 4-3 (DOE, 2014). The competition of water use for energy and food is at the core of the WEF nexus landscape: the growing population and the larger number of people now in the middle class drive demands for food, energy, and water, while at the same time freshwater resources are fixed and limited. This situation generates challenges for the environmental, economic, and social aspects of life on Earth (Albrecht et al., 2018; DOE, 2014; Simpson and Jewitt, 2019).
In 2016, the United Nations estimated that 800 million people suffer from food insecurity, approximately 1.2 billion people lack access to electricity, and 800 million people lack access to safe drinking water (Scanlon et al., 2017). The world’s population is projected to increase to well over 9 billion in 2050, and the global middle class will continue to expand. The resulting projected drain on WEF resources is so great that it will be necessary to address the nexus holistically rather than as separate sectors. Future resource security (defined as the uninterrupted availability of resources at an affordable price) will require not only the integrated management of water, energy, and food resources but also a transition to a circular economy, with special attention to challenges of sustainability and climate (Biggs et al., 2015; D’Odorico et al., 2018; see Chapter 6 for further discussion of the circular economy). This integrated management approach is at the core of the capabilities and focus of chemical engineers, from the chemical to the system scale, with respect to reducing demand (conservation), increasing supplies, and
managing storage and transport (i.e., managing the spatial and temporal imbalances of production and consumption) while working toward a circular economy in the WEF nexus. The U.S. Department of Energy (DOE, 2014) frames integrated solutions for the WEF nexus around six pillars:
- optimizing the freshwater efficiency of energy production, electricity generation, and end-use systems;
- optimizing the energy efficiency of water management, treatment, distribution, and end-use systems;
- enhancing the reliability and resilience of energy and water systems;
- increasing safe and productive use of nontraditional water sources;
- promoting responsible energy operations with respect to water quality, ecosystem, and seismic impacts; and
- exploiting productive synergies among water and energy systems.
Chemical engineers can lead and contribute to advances in the many technology vectors required within each of these pillars. Some key examples include the following:
- Reducing water demand (conservation)
- reduction of food waste and development of technologies that reduce spoilage, and use of food waste to produce chemicals (e.g., bio-oils) and to feed livestock (Balicka, 2020);
- implementation of a “more-crop-per-drop” (i.e., water productivity in agriculture) approach, use of engineered crops with higher water efficiency and/or drought tolerance, and development of better pesticides (Scanlon et al., 2017);
- use of brackish groundwater instead of freshwater for energy (D’Odorico et al., 2018; Scanlon et al., 2017);
- development of advanced sensors to avoid waste and improve process reliability, as well as use of relevant data collection, analysis, and reporting (DOE, 2014);
- development of alternative fluids to replace freshwater in various processes (D’Odorico et al., 2018; Scanlon et al., 2017);
-
- improvement of the water efficiency of industrial processes, and replacement of freshwater (e.g., with supercritical CO2, nitrogen, nanomaterials, or liquid hydrocarbons) in oil and gas extraction (D’Odorico et al., 2018);
- development of efficient, less expensive, and lower-water-use or waterless cooling options for thermoelectric power plants, and options for recovering waste heat and reducing the need for cooling in thermoelectric power plants (D’Odorico et al., 2018; Scanlon et al., 2017);
- improvement of water reuse within homes (e.g., new uses for greywater) and development of technologies for waterless products, processes, and activities (D’Odorico et al., 2018; Scanlon et al., 2017); and
- increased use of renewable energy (wind and solar) to reduce the water demand for electricity generation (DOE, 2014; IChemE, 2015).
- Increasing supplies
- Food
- closing the yield gap of crop productivity between low- and middle income countries and higher-income countries while minimizing environmental, social, and other impacts (“sustainable intensification”);
- producing genetically modified (GM) crops that are insect- and herbicide-resistant and tolerant to drought;
- producing GM livestock to change the fat content in milk;
- producing in vitro meat that does not involve raising livestock (cell-cultured meat) or plant protein–based meat; and
- developing hydroponics- and aquaponics-based technologies (D’Odorico et al., 2018; Scanlon et al., 2017).
- Water
- further developing desalination options for brackish groundwater or seawater so that desalination expands beyond specific uses that deal with small volumes of water (e.g., drinking water) and population groups that can accommodate the higher costs;
- capturing stormwater;
- developing advanced materials for removing chemical and biological contaminants from water (e.g., removing lead contamination from drinking water to address contamination scenarios such as those faced by residents of Flint, Michigan); and
- treating municipal wastewater (D’Odorico et al., 2018).
- Energy
- developing second- (i.e., from agricultural waste) and third- (i.e., from nonedible biomass) generation biofuels,1
- developing advanced energy crops,
- using waste for energy production,
- Food
___________________
1 Today’s ethanol from fermentation of sugars and biodiesel from plants are considered first-generation biofuels, and in both cases, the feedstocks can be used for food as well.
-
-
- improving the energy efficiency of production of chemicals and products, and
- developing technology for cost-effective recovery of dissipated energy from electricity generation that could also help with carbon capture and storage (DOE, 2014).
-
- Managing storage and transport
- developing technologies that enhance food preservation and storage (D’Odorico et al., 2018), and
- improving battery and other energy storage technologies (IChemE, 2015).
Interdisciplinary research that integrates the physical, agroecological, and social sciences, as well as economics, is needed, along with the involvement of academia, industry, and government. While chemical engineering’s role in the energy sector is discussed in Chapter 3, the ties among food, water, air, the environment, and energy are apparent and are key to the future of both the discipline and society writ large.
MOLECULAR SCIENCE AND ENGINEERING OF WATER SOLUTIONS
Chemical engineers have a leading role in molecular science and engineering that requires a fundamental understanding of water structure, dynamics, and interactions, as well as in the development of new complex separation processes. However, issues of water scarcity, preservation, and purification are on a global scale, and solutions to these complex issues therefore require an unprecedented ability to think across scales ranging from the atomic to the geologic. The ability of chemical engineers to solve problems from the molecular to the system-design level will be critical to meeting these challenges, but they will also have to learn from and interact with civil and environmental engineers and to understand system boundaries that go far beyond a unit operation, process, or plant scale.
Water Purification
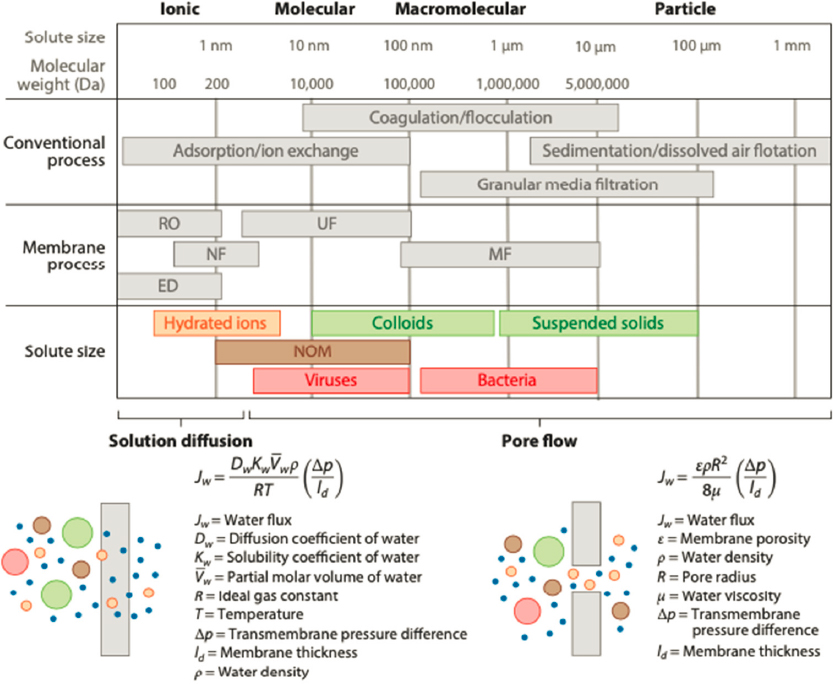
The purification of water involves the removal of a variety of chemicals or solid materials using a range of processes (Figure 4-4). Several water sources and associated opportunities for chemical engineering are highlighted below.
Desalination
The conversion of ocean or brackish water to drinking water is one of the great engineering advances of recent decades, and this technology is applied at scale in numerous locations (e.g., the Ashkelon Seawater Reverse Osmosis Plant in Israel2). The two main technologies applied to this problem are distillation, in several forms, and reverse
___________________
osmosis (RO) membrane systems, the latter of which account for 65 percent of global capacity (Abdelkareem et al., 2018; Bhojwani et al., 2019). Modern RO plants operate near the thermodynamic limit but are still very large consumers of energy. Opportunities to improve the overall energy efficiency of a seawater RO plant involve energy and waste-heat management as much, if not more, than the development of new membrane materials or processes. Many RO plants are located in regions where renewable power sources (wind or solar) can be contributors, or in remote regions where nuclear power is a viable alternative. In the latter case, it is possible to use an arrangement whereby the power plant runs at full capacity, with electricity fed into the grid, during periods of high demand, and is otherwise used to purify water, which is much easier to store than electricity.
Produced Water
While sea- or brackish-water desalination is well developed, current and future challenges relate to dealing with water contaminated in different ways. For example, oil production yields oil along with so-called produced water, which in many cases is larger in volume than the oil. This water contains suspended oil, additives, and solids, and in many cases, heavy metals or radioactive elements. If hydraulic fracturing has been used, at least some of the produced water will contain polymeric or surfactant-based “fracking” fluids (this water, sometimes called flowback, was, ironically, initially formulated with freshwater). The oil and water may be produced as an emulsion (either water-in-oil or oil-in-water, or as a multiple emulsion), which if stabilized by asphaltenes or resins can be quite stable. Economical treatment and reuse are very difficult, so this water is often reinjected into the formation. The development of technology for cost-effective treatment of this water, particularly on site, presents a substantial opportunity for chemical engineers.
Similar challenges continue to exist in, for example, managing the water produced during coal and other mining; the iron-ore treatment in the taconite process; and multicomponent radioactive liquid wastes such as those at the Hanford, Washington, nuclear reactor site (where 45 years of operation resulted in an estimated 440 billion gallons of wastewater [Washington State Department of Ecology, 2021]). Other challenges include the removal of boron and other neutral materials, as well as the removal of environmentally persistent perfluorocarbon molecules.
In the case of oil-field or mining water, the separations need to be carried out in steps to accomplish the sequential removal of colloidal and larger particles, then oils, and finally salts. Each of these steps could be considered a unit operation. The science of coagulation and flocculation is well known and practiced in municipal water treatment plants, but the implementation of new coagulants or flocculates is often too costly. Nonetheless, there are substantial opportunities to develop new polymer or surfactant chemistries that can address these challenges and to leverage the principles of self-assembly to treat these waste streams.
After solids and oils have been removed, membrane processes can be applied. The membranes used for water treatment are usually porous polymeric films with pore structures and sizes designed for the application at hand. The flux of water and solutes across the membrane always depends on a balance between permeability and selectivity, as outlined by Landsman and colleagues (2020). In this process, the design parameters are the pore size and distribution; solute size and shape; solute concentration; osmotic pressure; and, importantly, interactions between the solute and the membrane, which can be electrostatic, chemical, or biospecific (Landsman et al., 2020). Each of these parameters can be exploited to design separations for water contaminated in various ways.
A range of research-based approaches have been suggested for new membranes. Molecularly heterogeneous polymers (multiblock copolymers) can be designed to form bicontinuous solids, and then treated to make solids of controlled porosity or synthesized with carbon nanotube channels. Such membranes could be functionalized to be electroac-
tive or biomolecularly specific. They could incorporate elements of ion-specific exchange, or be catalytic and react with and separate target molecules simultaneously. This is a rich field for chemical engineers with an interest in transport, catalytic and reaction chemistry, thermodynamics, self-assembly, and materials science. To make an impact on real-world applications, research in this area needs to focus on the factors that typically limit existing technology solutions (e.g., biofouling, durability, cost). Moving forward, an important consideration will be management of the life cycle of such membranes and incorporation of recycle or upcycle characteristics into their design. For example, the production of polymeric membranes often relies on polar aprotic solvents, such as N,N-dimethylformamide (DMF), or 1,4-dioxane and tetrahydrofuran (THF). These solvents pose considerable environmental challenges, and it would be preferable to replace them with environmentally responsible alternatives.
Trace-Element Recovery
There is an increasing need to recover recyclable and reusable nutrients and minor or trace elements—particularly phosphorous, rare earth elements, and energy-related elements—from waste streams. In many cases, natural deposits of their associated minerals are limited within the United States (Jyothi et al., 2020). In addition, challenges remain for the extraction and/or destruction of both emerging and legacy trace contaminants that threaten human and ecological health. The low concentrations of trace elements make removal challenging, especially in waters containing high salinity or highly complex organic matrices. A wide variety of conventional separations have been employed (e.g., chemical precipitation, coagulation-flocculation, flotation, solvent extraction, ion exchange, adsorption, membrane processes, filtration, reverse osmosis, and electrochemical techniques), with varying levels of technoeconomic feasibility depending on the contaminant properties, background water composition, and treatment goals (Naidu et al., 2019; Pereao et al., 2018). Two of the more promising approaches, especially for recovery of trace contaminants, use sorption (including adsorption, absorption, ion exchange, and precipitation) and/or membrane processes. For many trace elements, however, significant advances in these technologies are needed to expand recovery and reuse and reduce treatment costs (Elbashier, et al., 2021; Li et al., 2021b). Several promising new techniques employing novel sorbents have emerged, including electrospun nanofibers with a highly specific surface for adsorbent applications (e.g., Sharma, 2013) and ionic imprinted polymers (e.g., Branger et al., 2013; Luo et al., 2015). In the case of membrane technologies, novel materials targeted for recovery of rare earth elements include metal organic frameworks and liquid membranes (Smith et al., 2019; Tursi et al., 2021).
The recovery of phosphorus from water presents formidable challenges. Reserves of phosphate rock are rapidly being depleted, and the current cost of recovering phosphorus from wastewater or agricultural runoff exceeds the cost of separating conventional phosphate from rock. Therefore, new and cost-effective technologies for recovering phosphorous from wastewater are needed. To this end, as Peng and colleagues (2018) indicate, more than 50 such technologies, including biological, chemical, and physical processes, have been developed. One of the most common end products for phosphorus recovery is
precipitated struvite or vivianite. Fluidized bed reactors that achieve upwards of 80 percent recovery from wastewater have been reported (Nelson et al., 2017), but the energy consumption, cost, and footprint of available processes are all too high to encourage their widespread adoption. Newer alternatives based on adsorption technologies, ranging from alginates to peptide-based materials, as well as biobased systems, are rapidly being explored, with encouraging results (e.g., Jama-Rodzenska et al., 2021; Su et al., 2020; Zhang et al., 2020a). Alternative approaches, such as Donnan dialysis with ion-exchange membranes, are also being investigated for concentrated water containing high concentrations of ions, organic matter, and suspended solids in concentrated waste streams (Shashvatt et al., 2021). However, much research is still needed to bring such technologies into practice.
The extraction of lithium or uranium from water streams poses similar challenges, including extraction from seawater and water produced during oil and gas operations. In the case of lithium, concentrations range from about 5 mg/L to about 500 mg/L, and removal can be accomplished using solar evaporation, adsorbents, membrane-based processes, and electrolysis-based systems (Kumar et al., 2019). Current challenges include accelerating concentration processes and dealing with lower lithium concentrations. In the case of oil and gas wastewater, metal oxide adsorbents and membrane technologies are promising. However, current membrane materials lack sufficient lithium-ion selectivity, and novel membrane approaches, such as 12-Crown-4-functionalized polymer membranes (Warnock et al., 2021) and Cu-m-phenylenediamine (MPD) membranes (Wang et al., 2021a), require further research.
Finally, concerns resulting from the widespread contamination of drinking water by legacy contaminants, such as perfluorinated compounds and lead released from water distribution systems, highlight the need for innovative treatment technologies that address recalcitrant organics and metals with known human health risks at trace concentrations. Per- and polyfluoroalkyl substances (PFAS) have no known half-life and have been found in more than 2,000 locations within the Unites States. The toxicity of the more than 4,000 PFAS compounds varies widely; state-level water quality regulations for some PFAS compounds, such as perfluorooctanoic acid (PFOA), are at the part-per-trillion level. Significant research into treatment strategies includes technologies focused on contaminant destruction (e.g., advanced oxidation and reduction, photolysis, electrochemical oxidation, and incineration) and those aimed at separating the compounds from water (e.g., activated carbon adsorption, polymeric adsorbents, ion exchange, ozofractionation, and membrane separation; Ross et al., 2018). To date, however, no single technology has been identified that is cost-effective, not energy intensive, and universally effective at treating the range of PFAS compounds (e.g., varying functionality and chain length) within the complex matrices of source waters (Crone et al., 2019; Gagliano et al., 2020). The development of treatment technologies for these persistent chemicals is a major opportunity for chemical engineers.
Lead contamination of drinking water resulting from dissolution of lead solder, fixtures, and piping is another concern requiring innovative solutions, including consideration of point-of-use treatment technologies. Maintenance of lead scales within distribution systems is the typical control mechanism for ensuring water quality; however, changes in water quality can dramatically affect lead release and compromise drinking
water quality either across the distribution system or within premise plumbing (Wahman et al., 2021). Thus, the development of effective point-of-use technologies (e.g., reverse osmosis, adsorbents) that can remove both dissolved and particulate lead is needed (Brown et al., 2017; Verhougstraete et al., 2019). Nanoenabled technologies are also useful for lead and other metal contaminants, such as copper, Cu(II); chromium, Cr(VI); and arsenic, As(V). For example, Greenstein and colleagues (2019) have examined polymer-iron oxide nanofiber composites and iron oxide–coated polyacrylonitrile fibers for removal of lead, Pb(II), and these other metal ions, as well as suspended solids. In these cases and others, solutions to such separation problems depend on fundamental chemical engineering principles, such as thermodynamics, transport phenomena, chemical kinetics, engineering of nanoscale materials, and process design.
Removal of Microplastics
Chemical engineers have opportunities to address the challenges of remedying the damage done by pollution in large bodies of water. A significant challenge is the remediation of microplastics in oceans and large lakes. An estimated 8 million metric tons of plastic enters waterways annually (NOAA, 2021b), and a significant portion of that total is in the form of so-called microplastics, which are less than 5 mm in size. Microplastics enter the water directly as waste from consumer products, such as cosmetics, but are also produced by attrition of larger pieces of plastic or incomplete incineration of plastics. The Great Pacific Garbage Patch (GPGP) covers approximately 1.6 million square kilometers in the North Pacific (Lebreton et al., 2018). By comparison, the Deepwater Horizon oil spill covered about 150,000 square kilometers in the Gulf of Mexico, or 10 percent of the area of the GPGP (NOAA, 2021a). Microplastics pose a risk to larval fish and thus can potentially impact a significant source of food protein (Gove et al., 2019).
Remediation of such a large area of open sea is challenging. Some microbial treatments have been proposed (Auta et al., 2017), but appear to be applied more effectively in treatment plants. Open-sea cleanup of microplastics will likely have to rely on their physical removal. Such harvesting will require the concentration of microplastics into flocs, which may be promoted by highly effective flocculating agents (Roh et al., 2019). Such flocs could then also be subjected to bioremediation processes.
Removal of microplastics from water and other media presents opportunities for chemical engineers. Removal methods include physical sorption and filtration, biological removal and ingestion, and chemical treatments (Iyare et al., 2020; Padervand et al., 2020). Physical sorption methods include adsorption on green algae (depending on the microparticles’ surface charge), removal using membrane technology, and removal using filtration technology (e.g., ultrafiltration, disc filter, rapid sand filtration, and dissolved air flotation [Talvitie et al., 2017]). Chemical removal methods include coagulation, agglomeration, and settling processes (Lapointe et al., 2020) using Fe3+- and Al3+-based salts and other coagulants (e.g., polyacrylamide [PAM]); electrocoagulation; sol-gel reactions; and photocatalytic degradation. Biological removal and ingestion methods include biological degradation using microorganisms (Padervand et al., 2020).
Fundamental Properties of Water
The technological advances needed to secure a global freshwater supply, as described above, rely on fundamental breakthroughs in understanding the structure of water and its dynamics (Debenedetti and Klein, 2017). For example, the structuring of water near an interface plays a critical role in the fouling of that surface, whether it is on the outside of a naval vessel or the inside of a pipe in a water treatment plant. Similarly, whether ions, for example, are depleted or concentrated at the air–water interface is of considerable importance in the development of new water-purification technologies or of water transport models for agriculture or climate prediction. Chemical engineers, primarily in academia, are heavily involved in experimental and theoretical studies of water both with and without other additives. This section reviews and summarizes some of the opportunities and challenges therein.
Considerable advances have been made over the past two decades regarding the structure and dynamics of water under a wide range of conditions and environments. Much of that progress has been fueled by theoretical and computational advances, coupled with the development of experimental techniques capable of probing structure and dynamics over a wide range of length and time scales (Figure 4-5).
Structure of Pure Water
Some of the more intriguing developments in understanding the structure of pure water have been enabled by developments in synchrotron light sources and vibrational spectroscopy, coupled with progress in models and computation that has permitted a deeper interpretation of such measurements (Bjorneholm et al., 2016). Various types of vibrational spectroscopy, as well as elastic and inelastic neutron scattering, have gradually been refined to provide detailed insights into the structure and dynamic processes in bulk water and at interfaces, particularly when coupled with selective deuteration, at time scales ranging from fractions of a picosecond to tens of nanoseconds. These time scales encompass a wide range of characteristic molecular processes, from O–H bond vibrations to the motion of water and water-bound molecules, such as lipids in bilayer membranes or polymers in solution. Two-dimensional infrared spectroscopy, for example, continues to push its limits of sensitivity and applicability and has provided previously inaccessible information about how molecules, such as amyloid proteins, self-assemble in aqueous solution (Middleton et al., 2012; Shim et al., 2009). Sum frequency generation (SFG) is providing much-needed insights into chemical reactions at interfaces, including those occurring at electrodes for energy generation (Neri et al., 2017). In situ experiments, such as inelastic neutron scattering measurements, can now provide critical information about industrially relevant reactions as they occur in real-world processes, such as the formation of NaOH and Na2SiO3 activated in low-CO2 cements (Gong et al., 2019). Experiments at light sources (synchrotrons) have for decades been important for structural characterization, such as crystallographic studies of water, hydrates, and aqueous solutions of biomolecules, including proteins. Synchrotron light sources continue to be upgraded, and with
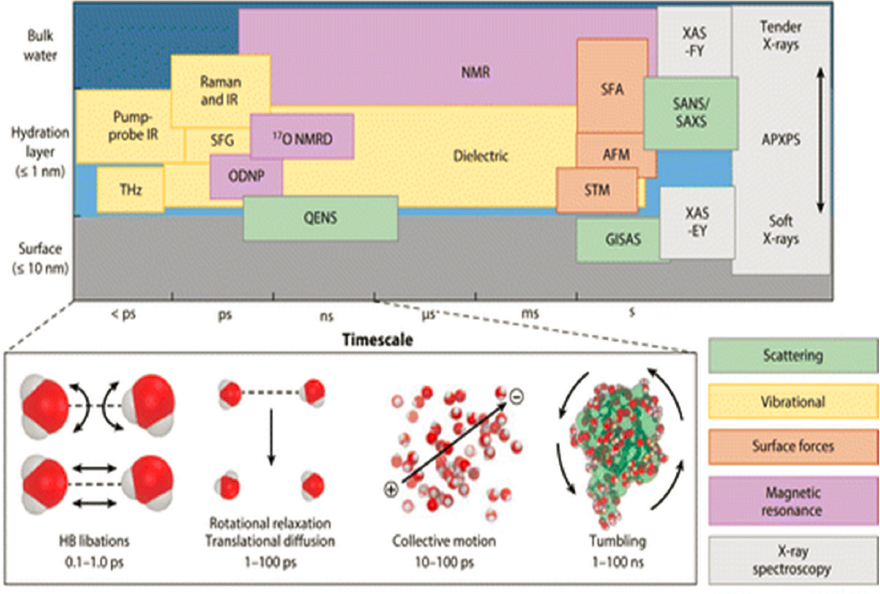
those upgrades, new methods are providing an unprecedented view into the structure of water in a wide range of scenarios, including as ultrathin films or in samples undergoing unusual phase transitions (Byrne et al., 2021).
Over the past 30 years, the issue of water polymorphism in pure water and aqueous solutions has gradually been fleshed out (Bachler et al., 2019; Debenedetti, 2003; Gallo et al., 2016; Handle et al., 2017). The phase diagram of water has been expanded to recognize the existence of several forms of amorphous ice, low-density amorphous (LDA) ice, and high-density amorphous (HDA) ice. LDA ice can be formed by rapid vapor deposition of water onto ultracold substrates (below 120 K); it has a density of 0.94 g/cm3, but its viscosity is higher than that of water. In contrast, HDA ice has a density of 1.17 g/cm3 and can be formed by pressurizing a particular phase of ice (ice Ih) at temperatures in the vicinity of 140 K. A third form of amorphous ice, referred to as very-high-density amorphous ice, has a density of 1.26 g/cm3 and can be formed by compressing HDA to pressures above 1 GPa. The existence and origin of these newly found forms of ice have
been understood largely based on sophisticated molecular simulations, many of which have been carried out by chemical engineers (Palmer et al., 2018). The discovery of these ices has helped explain several astrophysical observations, as they arise, for example, in comets or icy moons and in some of the coldest regions of the Earth’s atmosphere. Amorphous ice is also relevant for engineering applications and could be used for the chemical and structural stabilization of biological molecules at low temperatures (as needed, for example, in cryo-transmission electron microscopy) over extended periods. The discovery of LDA ice formed by vapor deposition has inspired the creation by chemists and chemical engineers of other classes of emerging engineering materials, such as ultrastable vapor-deposited glasses, which offer unusual mechanical characteristics and important advantages for applications in electronics, such as organic light-emitting diodes and polymorphic metallic films that respond to light.
Structure of Water at Interfaces
Considerable progress has also been made in understanding the structure of water in inhomogeneous environments—for example, water at interfaces and under extreme confinement. Consensus has gradually emerged, primarily from various types of infrared measurements and molecular models, that an individual —OH group from the molecule is freed from the hydrogen-bonding network and points toward the vapor phase. Water molecules near the interfacial “layers” are believed to be slightly more disorganized than bulk, fully hydrogen-bonded water, and considerable debate continues around the acid/base characteristics of interfacial water. This last issue is of particular significance to electrochemistry and, more generally, to chemical reactions at interfaces, and presents exciting opportunities for chemical engineering. It is also important for the interpretation and design of adsorption processes at aqueous interfaces. Clathrate formation, for example, with both methane and CO2, is believed to be nucleated at the water interface (Li et al., 2020a; Liang et al., 2019). A better understanding of such interfaces would therefore enable the discovery and development of clathrate formation inhibitors or stabilizers.
Beyond pure water, the structure of aqueous solutions at interfaces presents important challenges (Bjorneholm et al., 2016). The issue of whether ions, for example, are depleted or concentrated at the air–water interface is still being debated. Current thinking is that large, more polarizable halide anions are depleted from the air–water interface to a lesser extent relative to smaller and less polarizable ions (Ghosal, 2005; Jungwirth and Tobias, 2006; Tong et al., 2018). Iodide, however, is believed to be enriched at that interface. These trends are strongly influenced by the presence of molecular solutes, and depending on the polarity and size of such solutes, it is difficult to predict the structure of an aqueous solution at an interface, posing considerable challenges for the design of adsorption operations, for example.
Theory and simulation have the potential to provide the tools needed to describe such systems, but molecular models of water and ions are currently unable to describe the solubility of ions in water or to predict the segregation or enrichment effects observed in experiments (Mester and Panagiotopoulos, 2015). Such models have been developed for bulk pure water, and the effects of ions have been included as an afterthought, thereby
restricting the models’ ability to describe solutions or mixtures and interfaces. Attempts to circumvent this problem by relying on quantum mechanical methods have been hindered by the computational demands of such calculations, which continue to exceed available resources. Recent approaches involving various combinations of advanced sampling concepts from statistical mechanics, quantum mechanical calculations of intermolecular interactions, and emerging concepts from machine learning offer considerable promise for reducing the description of water and aqueous solutions and their interfaces to a tractable problem. As chemical engineers strive to conceive and design chemical processes involving aqueous interfaces, it will be important for such predictive models to be developed and brought to bear on the design and optimization of modern water-based technologies.
The challenges and gaps in understanding that arise at the air–water interface are exacerbated at solid interfaces with metals; oxides; or organic matter, including biomolecules. Water–metal interfaces are central to catalysis and electrochemistry, and probes capable of directly reporting the structure of interfacial water are severely limited. Importantly, existing measurements require interpretation based on molecular models, and such models continue to suffer from the same shortcomings highlighted in the context of air–water interfaces. Much work remains to be done not only in understanding the structure of water at such interfaces but also in manipulating that structure to control reactivity and transport (Ruiz-Lopez et al., 2020).
Considerable effort has been focused on understanding both hydrophobicity and hydrophobic surfaces. Experimental evidence from SFG experiments has now shown that, as with the air–water interface, water molecules have an individual “dangling” —OH group at a hydrophobic surface (Bjorneholm et al., 2016). Results of experiments on individual surfaces also indicate that, beyond the first monolayer of water molecules at such an interface, water rapidly adopts a bulk, isotropic structure. Those findings are in conflict with measurements of the forces between two hydrophobic surfaces, which indicate that an attractive force can already be felt at separations on the order of 20 nm (Kekicheff et al., 2018), suggesting that some level of structural influence is felt at relatively large distances. Simulations of water structure and forces between hydrophobic surfaces have added some clarity to this ongoing debate, with some calculations finding evidence of long-range attractions and others finding only short-range interactions, depending on the type of surface and water model adopted in the calculations (Altabet and Debenedetti, 2017; Eun and Berkowitz, 2011; Hua et al., 2009).
Even less is known about how these interactions might change in the presence of ionic species, including ions or charged molecules. Recently, however, chemists and chemical engineers have made important advances by relying on synthesis of carefully conceived organic molecules and surfaces that present hydrophobic groups and charges in precise arrangements, coupled with atomic- or surface-force apparatus measurements (Ma et al., 2015). Those experiments have revealed that chemical heterogeneity at the nanometer level plays a significant role in the structure of water and the resulting hydrophobic interactions. A key advance is the realization that hydrophobicity is not an inherent characteristic of nonpolar domains but is instead controlled by functional groups separated by nanometer scales. Cleverly designed simulations have helped explain and exploit
some of these observations (Kim et al., 2015). From an engineering perspective, these findings suggest that judicious positioning of charges next to hydrophobic domains represents a strategy for tuning hydrophobic forces, with a wide range of implications, from the design of water-repellent coatings, antifreeze proteins, and self-assembling systems to the stabilization of colloidal suspensions.
Dynamics of Water
For chemical engineering, the relevance and excitement of many of these new discoveries surrounding the structure of water are matched only by new observations pertaining to the dynamics of water under confinement or near interfaces. Early experiments with membranes consisting of carbon nanotube pores (less than 2 nm in diameter) revealed that flow rates through such pores are roughly three orders of magnitude faster than anticipated by continuum flow calculations (Holt et al., 2006). Fast transport of gases in nanopores had been anticipated on the basis of simulations by chemical engineers (Skoulidas et al., 2002), and the experimental observations were explained by invoking the formation of a frictionless interface at the nanotube wall. It has also been reported that such a phenomenon occurs with both organic solvents (e.g., alkanes) and hydrogen-bonding solvents, and that in the case of water, the flow rate gradually drops over time, presumably as a result of some level of ordering in the pore. Subsequent experiments and simulations have shown that such ordering can in fact lead to even faster transport of water—as fast as 1 meter per second, and at small dimensions, in the range of a nanometer (Marbach et al., 2018; Striolo, 2006; Tunuguntla et al., 2017).
Chemical engineers have been particularly adept at drawing inspiration from biology to develop solutions for problems ranging from reengineering proteins to co-opting bacteria for water reuse. Research on technologies for water transport through protein pores, such as ion pumps or aquaporins, has been used in the design of the high-flux systems mentioned above or selective pores for ion transport. Fundamental theoretical and computational research has paved the way for the design of intriguing separation or energy-generation processes, including recent discoveries about the transport of aqueous ionic solutions through Janus nanopores—designer pores consisting of adjacent sections with different diameters and surface charges—that could potentially become important sources of energy from simple salinity gradients (Yang et al., 2018; Zhang et al., 2017; Zhu et al., 2018). More work is needed to scale up and optimize observations that have thus far been limited to laboratory-scale observations, but these and other “tailored-pore” approaches offer considerable promise.
Similarly, additional research on aqueous electrocatalysis, photocatalysis, and photoelectrocatalysis (Li et al., 2020b; McMichael et al., 2021; Ochedi et al., 2020)—for which the basic idea is to use electrons and/or photons to transform readily available substances, such as water and CO2, into sustainable chemical fuels such as hydrogen and methanol—is likely in the near future to see application in commercial projects that could shape the industrial landscape for generations. In the face of rapidly changing climate patterns, fundamental research on topics related to ice nucleation, clathrate formation or destabilization, and the fate of aerosols can inform technologies aimed at mitigating the
impacts of climate change. As water sources change and conservation is accelerated, monitoring will be of critical importance at a global scale. For example, “dating” efforts to gauge the age of aquifers using atom trap trace analysis of krypton and noble gases are only a few years old and are starting to yield intriguing data, but are as yet not widely known or utilized (Gerber et al., 2017).
FEEDING A GROWING POPULATION
The advent of farming-based agriculture was an essential enabler of human civilization. Innovative strides in agriculture in the 20th century, many of which were enabled by chemical engineers or researchers working in what would ultimately become the chemical engineering discipline, allowed substantial improvements in food yields per land area, the ability to efficiently prolong the life of foods, and the ability to deliver food to consumers over long distances. These innovations are exemplified by the development and scale-up of the Haber–Bosch process for ammonia production, which rivals global biological nitrogen fixation in magnitude (Galloway et al., 2008). The development of refrigeration, today a standard lesson in chemical engineering’s thermodynamics courses, is another critical example of the technological march toward improved efficiency in food production.
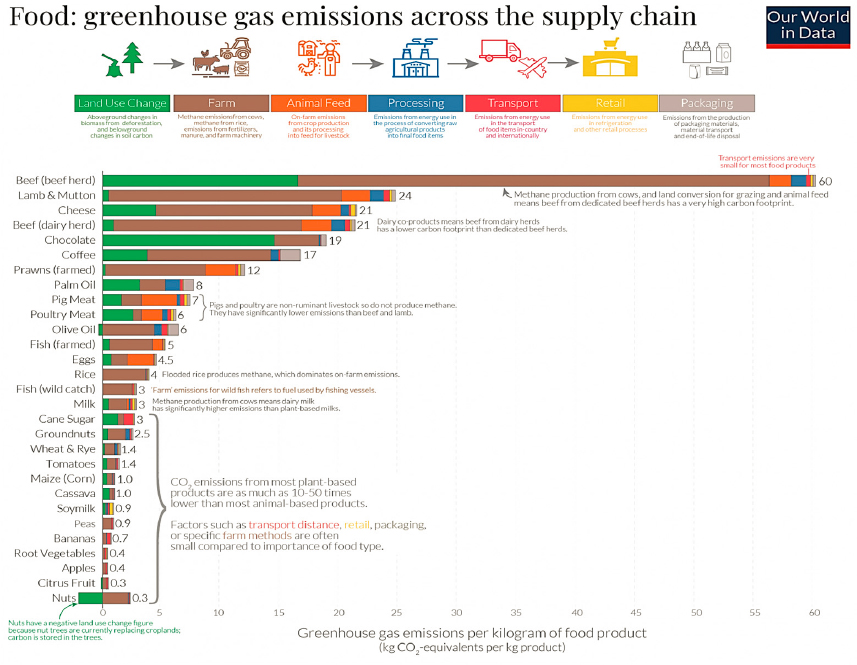
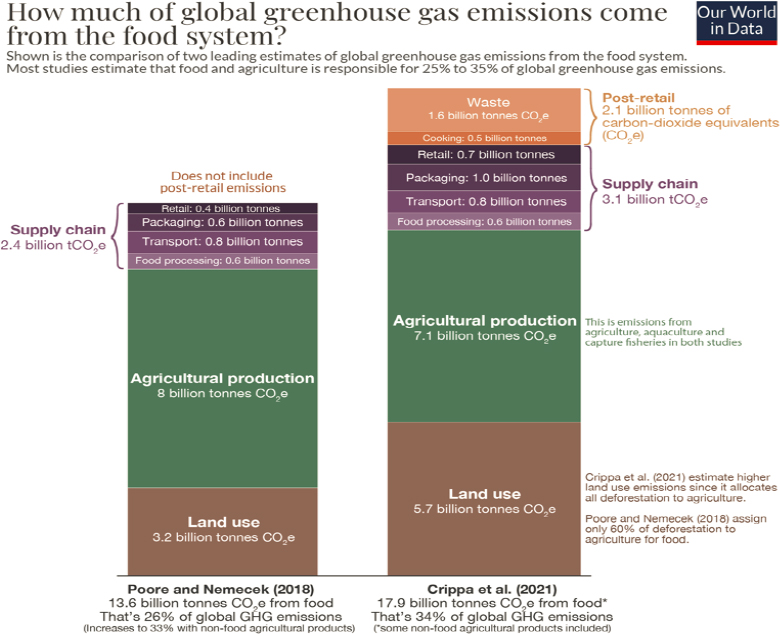
Today, agricultural pursuits use an astounding 38 percent of all land on the planet outside of Antarctica (FAO, 2020), and the raising of livestock is responsible for 14.5 percent of global GHG emissions (FAO, 2021). Researchers estimate that besides decarbonizing the energy sector (as discussed in Chapter 3), better management of food production and agriculture represents the other primary way to reduce global GHG emissions (Mbow et al., 2019; see Figures 4-6 and 4-7). As a result of continued population growth and anthropogenic climate change, humankind will need to rethink and reinvent agricultural and food production practices toward more sustainable land and resource use (Tilman et al., 2011).
These global-scale challenges present significant opportunities for chemical engineers, especially in the application of systems-level approaches to evaluate and optimize products and processes. Examples of specific challenges are mentioned below, but are by no means exhaustive. Generally, opportunities for innovation can be viewed in terms of increasing efficiency in agricultural practices and developing “leapfrog” technologies that are more sustainable than current agricultural methods.
Improving Agricultural Efficiency
The last three decades have seen significant technological innovations in agriculture, resulting in more precise application of resources, such as fertilizer; the advent of modern biomolecular techniques for engineering improvements in plant- and animal-based products; and the use of automated machinery and, more recently, robotics to replace manual labor. These and other agricultural advances will inevitably continue.
Since the emergence of metabolic engineering and synthetic biology, tools of modern molecular biology have become commonplace in chemical engineering. Tremendous opportunities exist to combine these tools with systems-level approaches to improve per-land-area crop yields; enhance the efficiency of water and nutrient use; and increase resistance to fungal infections, insect infestations, drought, and other adverse climate/weather and biological effects. While the public has generally viewed genetic modifications of plants with mistrust, techniques based on CRISPR (clustered regularly interspaced short palindromic repeats) technology may ultimately enable precise genomic editing without being considered genetic modification, representing a major boon for application in food crops. The use of genome-wide association studies, especially in the broad gene pools that exist in undomesticated crops, and translation to domesticated food crops present an opportunity to apply computational approaches common to chemical engineering curricula in concert with analysis-driven research.
Protein-rich products—including dairy, eggs, fish, meat, and poultry—account for an increasing amount of the human diet, with demand growing rapidly in low- and middle-income countries. In higher-income countries, protein-rich foods are often available in excess and make up a substantial fraction of food-related consumer waste (Spiker et al., 2017). Reducing net consumption of animal-based protein is likely to be an important contributor to improving sustainability and curbing methane emissions. Achieving this reduction will require, among many other efforts, both the development of substitutes for animal-based proteins and, in cases in which animals are still harvested for meat products, understanding of methods for mitigating related emissions. Exciting avenues of research in the latter area include understanding of the microbiome interaction of agricultural animals with feed. Examples include recent research wherein dairy cows were fed small amounts of seaweed, which reduced overall per-animal methane emissions (Roque et al., 2021).
Beyond the development of improved agricultural practices for plants and animals, improved water usage is a core element of the role of chemical engineering in the future of agriculture. Anthropogenic climate change, among other forces, is already shifting the global and regional balances of water resources. Systems-level approaches, including life-cycle assessment, will play a critical role in meeting this local, regional, and global challenge. Technological approaches to the use of water in agriculture are a prime example for which modular processes will be vital, as deployment is likely to require many units operating in a localized and autonomous manner rather than large, centralized facilities. Many of the same technical challenges apply to reducing the energy use associated with agriculture.
The judicious application and effective recycling of reactive nutrients, traditionally exemplified by ammonia and urea as fertilizer but also including phosphorous and potassium, is another area presenting multiple chemical engineering challenges. The dead zone in the Gulf of Mexico (and in bodies of water around the globe) due to fertilizer runoff is a legacy issue presenting an opportunity for chemical engineers to contribute to environmental protection through targeted applications of nutrients to enhance nitrogen management. Chemical engineers can also pioneer more efficient production of ammonia (beyond the Haber–Bosch process), which has reemerged as a grand challenge and has been the focus of intense research in the past decade (e.g., Boerner, 2019; Garnier, 2014; Smith et al., 2020). Any potential technology for this process needs to ultimately produce ammonia in very large quantities to be globally relevant. Addressing challenges in catalysis, reaction engineering, and process development overall will be critical to this end. Some nutrients beyond ammonia/reactive nitrogen are finite relative to the expected growth in agriculture, and their availability today still relies on mining, as was the case with ammonia in the late 19th century. These finite nutrients include phosphorous and potassium, which, along with ammonia, are commonly applied to feed and food crops. Meeting challenges in their economical and efficient recovery along multiple supply chains will be critical as agricultural demands increase.
Modern agriculture and food processing produce massive amounts of industrial and postconsumer waste. For example, nut processing results in the production of remarkable amounts of waste lignocellulose. These waste streams offer a direct and immediate pathway for the needed development of biofuels, biochemicals, and biomaterials—areas in which chemical engineers already play a leadership role. Going beyond anaerobic digestion or simple combustion of organic waste to produce products of higher value than methane (often used for combustion) or enable direct production of power is an area ripe for immediate contributions from chemical engineers.
The above discussion includes but a sampling of ways to improve modern agricultural practices in which chemical engineers can play pivotal roles. There are undoubtedly many others. The problem of food production is inherently global in nature, and systems-level thinking at multiple scales—a hallmark of the chemical engineering profession—will be critical to enable positive change toward a more sustainable agricultural system.
Reinventing Agriculture
Today’s agricultural practices are inherently land, water, and nutrient intensive. Almost universally, agriculture today is practiced in two dimensions and with batch processes. The use of land means that agricultural productivity scales with surface area, whereas most chemical engineering manufacturing processes, as described further in Chapter 6, scale volumetrically. Moreover, crops and animal products are typically not harvested continuously but in a manner that depends on the time of year, with many external factors (e.g., weather, climate, disease, fire, drought) affecting the process conversion efficiency, yield, and rate. For many generations, these observations appeared to be intrinsic to the production of food and therefore barely worth stating. However, now is the time to consider thoughtfully how immutable these notions are. Examples of this shift in thinking can already be seen with the surge of companies focused on the production of non–animal-based meat, dairy, egg, and oil substitutes. In various ways, these companies are exploring the concept of transitioning agriculture from an areal farming practice to a volumetric, continuous industrial manufacturing process. The outcomes of this approach will likely have effects across the entire food and agriculture supply chain.
One example—among many—of plant-based products used today is lipids in oil-producing crops. The production of palm oil, for example, is quite energy intensive, leading to substantial land-use change effects, especially in tropical regions. The application of synthetic biology, metabolic engineering, bioprocess development, and analysis-driven research to produce oil products that can displace plant-based oils in a cost-effective and sustainable manner will likely have positive effects on reforestation and preservation of the natural environment (Parsons et al., 2020). Producing food in a continuous, industrial manufacturing setting could also vastly increase the number of feedstocks available for food production beyond CO2, NH3, and sunlight. As discussed in the section on feedstock flexibility in Chapter 6, the use of waste-based feedstocks for valuable products, especially for food production enabled by biological and catalytic transformations pioneered by the chemical engineering community, offers a clear path toward a more circular carbon and nitrogen economy that is more sustainable than today’s agricultural practices, and chemical engineers will play a critical role in this much-needed transition.
Food Engineering and Processing and Storage
Supplying the world’s population with food that is nutritious, affordable, and sustainable is a global challenge. Societal-scale pressures associated with climate change and population growth and distribution will demand substantial changes in the world’s food sources in the coming decades. Despite the large number of chemical engineers working in the food industry, food science has historically not been an area of strong emphasis in U.S. chemical engineering research. Multiple opportunities exist for chemical engineers to play a critical role in the future of food engineering and processing. These topics share several features with more traditional chemical manufacturing, including the need to operate at very large scales at low cost and with stringent safety, sustainability, and quality
standards. Any proposed solution that fails to meet any of these criteria will be ineffective in leading to real change in the world’s food system. Of critical importance is to evaluate the cost and sustainability of new concepts using systems-level life-cycle assessment while taking account of the role of local environmental conditions and traditions.
Food Engineering
Chemical engineers have led in adapting the tools of molecular and systems biology for applications in diverse areas; however, the application of these methods to food engineering is in its early stages. Although genetic modifications can be controversial, molecular biology has the potential to enable enormous advances in crop science. An example is golden rice, which could eliminate vitamin A deficiencies in large populations. The pursuit of research and development (R&D) in chemical engineering with plants rather than single-celled organisms, such as yeast, poses both technical and nontechnical challenges. The cell walls in plants make extracting molecules from plant cells more challenging relative to animal or microbial cells, and the longer life cycles of plants compared with single-celled organisms or model organisms such as C. elegans create logistical challenges for research. Nevertheless, the potential for chemical engineers to combine their skills in systems thinking with a rich set of biomolecular tools to improve food-related plants is great, particularly if they make concerted efforts to work in concert with related fields of biological and agricultural science.
In addition to improvements in food at the molecular level, the coming decades are likely to see significant changes in the macroscopic sources of food, particularly protein and lipid sources. Meat-free protein alternatives are already becoming mainstream in the United States, but considerable advances will be required if these kinds of foods are to be used on a global scale. Scaling up the volume of products that involve processing solids and liquids at low cost and with high reliability has been a core focus of chemical engineering since the field’s inception, and chemical engineers will have a major impact if they can adapt their existing expertise in chemical engineering and biotechnology to the challenge of producing new foods at scale. Valuable opportunities exist for collaboration between chemical engineers and researchers who are pioneering initial demonstrations of “lab-grown” foods on small scales and for diversification in such areas as self-assembly into food-relevant applications.
Although the impact of new food sources will accelerate, the scale of primary crops and agriculture will remain vast. Chemical engineers can play a role in continuing developments in precision agriculture, defined as the precise delivery of fertilizer, pesticides, and herbicides to minimize environmental impact. In the past, only a limited connection has existed in this area between the ambitions and needs of industry and farmers and the scope of academic research in the U.S. chemical engineering community.
Food Processing and Storage
While producing food in better ways is critical to meeting global food needs, reducing food waste could also have an enormous impact. The challenges associated with
food waste differ around the world: broadly, in higher-income countries, food waste is dominated by high-quality food that is not completely used by consumers, whereas in low- and middle-income countries, food waste more often results from a shortfall in the delivery of food from its original source to consumers. Consequentially, although significant advances in packaging and food treatment to prolong shelf life have the potential to make a major impact in both low- and middle-income countries and higher-income countries, there can be no single solution to the overall challenge of reducing food waste. Even so, chemical engineers have the potential to enable important advances in this globally important area. To ensure impact in low- and middle-income countries, researchers need to focus on ultra–low-cost solutions and appropriate technologies that account for local context and cultural traditions. In higher-income countries, the sustainability of food production and packaging is a major concern that can be addressed through the use of nonfood containers to hold food during shipping or storage and edible coatings that can be applied to food to prolong shelf life. Chemical engineers are well suited to the challenging task of applying systems-level life-cycle assessment to the development of solutions in these areas.
UNDERSTANDING AND IMPROVING AIR QUALITY
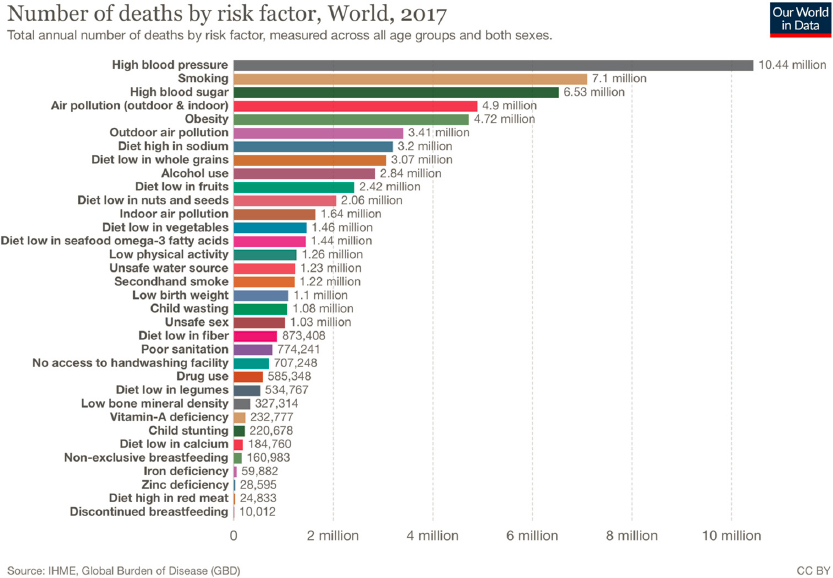
Even when air pollutants are at very low levels—and despite the progress that has been made in this area since 1970—air pollution continues to harm the environment, affect the climate, and harm human health (Figure 4-8; EPA, 2021a). On a global scale, the World Health Organization (WHO) estimates that about 4.2 million people died prematurely worldwide in 2016 because of outdoor air pollution in cities and rural areas, and 91 percent of those deaths occurred in low- and middle-income countries (WHO, 2018). The causes of death were heart disease, stroke, chronic obstructive pulmonary disease, and lung cancer. Additionally, the WHO estimates that of the 3 billion people who still use solid fuels (such as wood, crop wastes, charcoal, coal, and dung) and kerosene for cooking, about 3.8 million died prematurely in 2016 from diseases attributable to indoor air pollution caused by these types of cooking practices (WHO, 2016). Globally, air pollution is the fourth-highest contributing risk factor for death, behind high blood pressure, smoking, and high blood sugar (Figure 4-8). Indoor air quality3 is far less well studied and not as uniformly regulated as outdoor, yet people in the United States and other high-income countries spend more than 85 percent of their time indoors (EPA, 2018). Efforts to address indoor air pollution are complicated by the highly heterogeneous building and ventilation types that exist across even relatively small regions. Building codes related to ventilation have focused thus far on energy efficiency and less on air quality, and in many cases, improvements related to energy efficiency have led to less air exchange and thus poorer indoor air quality (EPA, 2020).
___________________
3 The National Academies’ Committee on Emerging Science on Indoor Chemistry is currently examining the links among chemical exposure, air quality, and human health in indoor environments.
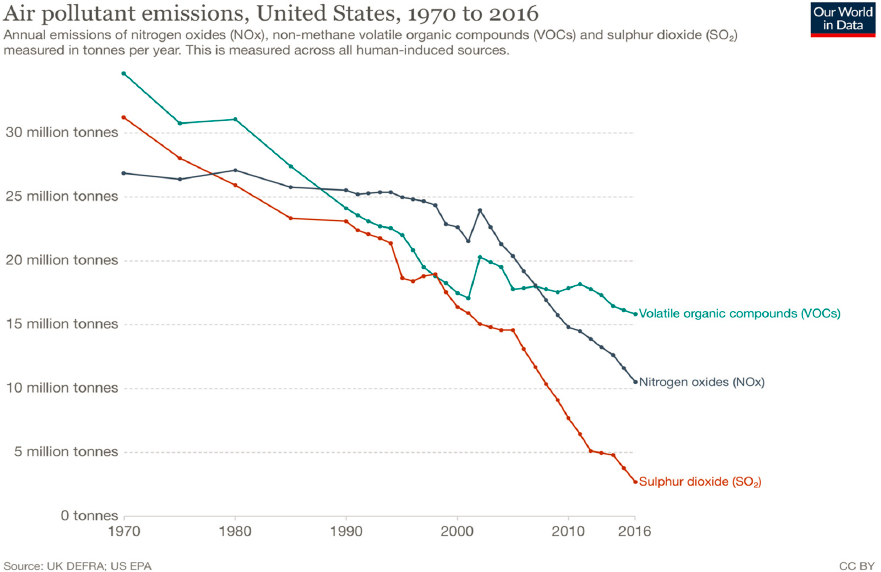
The six most common outdoor air pollutants are particulate matter (PM), ground-level ozone (O3), sulfur dioxide (SO2), nitrogen dioxide (NO2), airborne lead, and carbon monoxide (CO). While many of these pollutants are still present in the air, their concentrations in the United States have declined significantly since 1970 (Figure 4-9). In addition, 187 toxic air pollutants that can cause cancer and birth defects—including such chemicals as acetaldehyde, asbestos, cadmium and chromium compounds, benzene, dioxin, epichlorohydrin, and methanol—are listed in the U.S. Clean Air Act (42 U.S.C. § 85 [1955]); some of these chemical species form atmospheric aerosols, while the rest remain in the gaseous phase. Another air pollution challenge is to protect stratospheric ozone, which was achieved to a great extent by banning chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), and other harmful compounds (UNEP, 2020).
Still another challenge is posed by acid rain, caused by the reaction of atmospheric SO2 and NOx with water, oxygen, and other chemicals to form nitric and sulfuric acids. Acid rain can turn lakes and streams acidic, harming fish and wildlife, and flowing through soil can leach aluminum and remove minerals and nutrients from the soil. The impact of acid rain in the United States and Europe has largely been reduced by the regulation of sulfur and nitrogen emissions. Other air pollutants include CO2 and other GHGs, which are discussed further in the context of energy (Chapter 3) and the circular economy (Chapter 6).
Atmospheric aerosols are suspensions of microscopic and inhalable solid or liquid (typically, aqueous) particles in the air. About 90 percent of aerosols, by mass, occur naturally (e.g., from volcanic eruptions, ocean phytoplankton emissions, sea and salt sprays, forest and grassland fires, or dust aerosols from wind erosion of arid land; Voiland and Simmon, 2010). Anthropogenic aerosols include those from fossil fuels (e.g., those composed of sulfates) and agricultural waste burning, road dust, cooling towers, industrial processes, and deforestation fires (which are composed primarily of carbon). Aerosol concentrations can be as high as 106–107 particles per cm3. The typical size of aerosol particles is 1 nm to 10 µm; they are classified as PM10 when their diameter is less than 10 µm and PM2.5 when it is less than 2.5 µm. Aerosols greater than 10 µm in diameter settle to the ground in a matter of hours because of gravity, but those less than 1 µm in diameter remain in the atmosphere for weeks and are ultimately removed by impacting the surface of the Earth (dry deposition) or via precipitation (wet deposition). Aerosols are characterized as either primary (i.e., emitted as particles at the source) or secondary (i.e., formed from gaseous precursors; Haywood, 2016). This class of pollutants has garnered considerable attention with respect to indoor air quality during the COVID-19 pandemic because of the ability of exhaled virus-laden aerosols to cause infection.
Aerosols have both direct and indirect effects on climate. Models estimate that direct cooling effects have counteracted about half of the warming caused by GHGs since the 1880s (Voiland and Simmon, 2010). Indirect effects include nucleating the formation
of clouds and controlling their numbers and lifetimes. For humans, inhalation of PM and its subsequent deposition in the lungs and even entrainment into the bloodstream are the main causes of aerosol-related health problems. Specifically, PM2.5 can cause cardiovascular, mental, dermatological, and reproductive health problems (McNeill, 2020).
A major challenge for improving air quality is bridging the molecular scale of a chemical reaction and the massive scale of an atmospheric model. Some of the foundations of chemical engineering education (e.g., transport phenomena, thermodynamics, and chemical reaction engineering), along with a systems approach focused on complexity and scale, can be applied directly to solving environmental engineering problems. In many respects, the atmosphere (more generally, the environment) functions as a large chemical reactor in which pollutants are transported, mixed, and transformed while being distributed across the atmosphere, land, and water. More specifically, the extremely wide ranges of the temporal and spatial scales of the atmosphere are well suited to a chemical engineering modeling approach. Spatial scales span about 15 orders of magnitude, from the nanometer range of molecular dimensions to thousands of kilometers, and temporal scales span about 12 orders of magnitude, from the millisecond range of fast chemical reactions to the thousand-year scale of waste disintegration.
The chemical engineering profession has made key contributions to improving air quality, including the development of catalytic converters for vehicles (Box 4-1), cleaner-burning fuels, flue gas desulfurization and selective catalytic reduction systems for NOx conversion to nitrogen gas and water, wet flue gas scrubbing methods, and better coal gasification technologies (Haywood, 2016). Furthermore, most chemical industries are required to control their aerosol emissions, and the principal equipment for that control includes cyclonic separators, fabric filter collectors (baghouses), electrostatic filters and precipitators, and wet scrubbers, all of which were developed with the help of chemical engineers.
Moving forward, chemical engineers have an opportunity to minimize and eliminate the formation of air pollutants at the source. When “benign by design” is not possible, chemical engineers can play a role in minimizing or eliminating emissions of these pollutants into the environment or even apply treatments to make the emissions safe for the environment. Ultimately, it may even be possible to treat the environment to remove air pollutants (AIChE, 2017; Sánchez, 2019). Specific opportunities for chemical engineering include the following:
- reduction of emissions from smokestack and exhaust tailpipes through improvements in engines and industrial plants (e.g., engines with better computer control and fuel economy, and multivalve engines), advanced monitoring and diagnostics, enhanced maintenance, development of catalytic pathways to destroy pollutants, use of cleaner fuels (e.g., low-sulfur and renewable fuels) and filters (e.g., diesel filters), and development of electric and fuel cell vehicles;
- better management of waste, such as the use of environmentally friendly solvents, and equipment for capture of methane gas emitted from waste sites and its use as biogas (e.g., Cao et al., 2018b);
- use of clean energy solutions for power generation, cooking, heating, and lighting;
- use of strict emissions control in waste incineration sites; and
- capture of CO2 emissions.
In addition to these mitigating actions, chemical engineers can contribute to the fundamental understanding of aerosol formation, aerosol dynamics in the atmosphere, and their chemical characterization (e.g., Seinfeld, 1991; Seinfeld and Pandis, 2016). Sensor technologies that would allow better monitoring, chemical characterization, and knowledge of the spatial distributions of aerosols would also help address air pollution. Finally, the use of data science and multiscale models to illustrate atmospheric chemistry and the incorporation of process modeling to bridge the gap between observations and theory are opportune areas for the engagement of chemical engineers.
CHALLENGES AND OPPORTUNITIES
Food, energy, and water are highly interconnected, and solutions in this complex system need to be both environmentally sustainable and economically viable. A continued increase in the global population will lead to increased resource demands, posing a broad set of challenges for chemical engineers to address in the coming decades. Across all these
challenges, interdisciplinary and cross-sector collaborations will be critical, as will coordination across the federal agencies with responsibilities in these areas.
Chemical engineers can support water conservation by both designing higher-efficiency processes and developing methods for using alternative fluids to freshwater. Specific research opportunities range from better understanding of the fundamentals of water structure and dynamics to the development of membranes and other separation methods. In the domain of water use and water purification, U.S. chemical engineers would benefit from collaborations with civil engineers and other scientists and engineers in arid regions that have more experience with desalination.
Global pressures associated with climate change and population growth will require substantial changes in the world’s food sources, a need that chemical engineers can help address through enabling technologies. Specific opportunities for chemical engineers include precision agriculture, non–animal-based food and low-carbon-intensity food production, and reduction or elimination of food waste. Advanced agricultural practices designed to improve yield while reducing demand for both energy and water will require collaboration with other disciplines, as well as systems-level approaches such as life-cycle assessment. A particularly valuable opportunity for collaboration is with researchers who are pioneering initial demonstrations of lab-grown foods on small scales.
Chemical engineers have an opportunity to help in improving air quality by advancing understanding of the nature and physics of aerosol particles and applying separation technologies, as well as the molecular- and systems-level understanding that will be necessary to address this global challenge. Atmospheric science is already an interdisciplinary field that includes chemistry, physics, meteorology, and climatology, making it a promising area in which chemical engineering can contribute through increased collaboration.
Recommendation 4-1: Federal research funding should be directed to both basic and applied research to advance fundamental understanding of the structure and dynamics of water and develop the advanced separation technologies necessary to remove and recover increasingly challenging contaminants.
Recommendation 4-2: To minimize the land, water, and nutrient demands of agriculture and food production, researchers in academic and government laboratories and industry practitioners should form interdisciplinary, cross-sector collaborations focused on the scale-up of innovations in metabolic engineering, bioprocess development, precision agriculture, and lab-grown foods, as well as the development of sustainable technologies for improved food preservation, storage, and packaging.






























