6
Effects of Oil in the Sea
6.1 INTRODUCTION
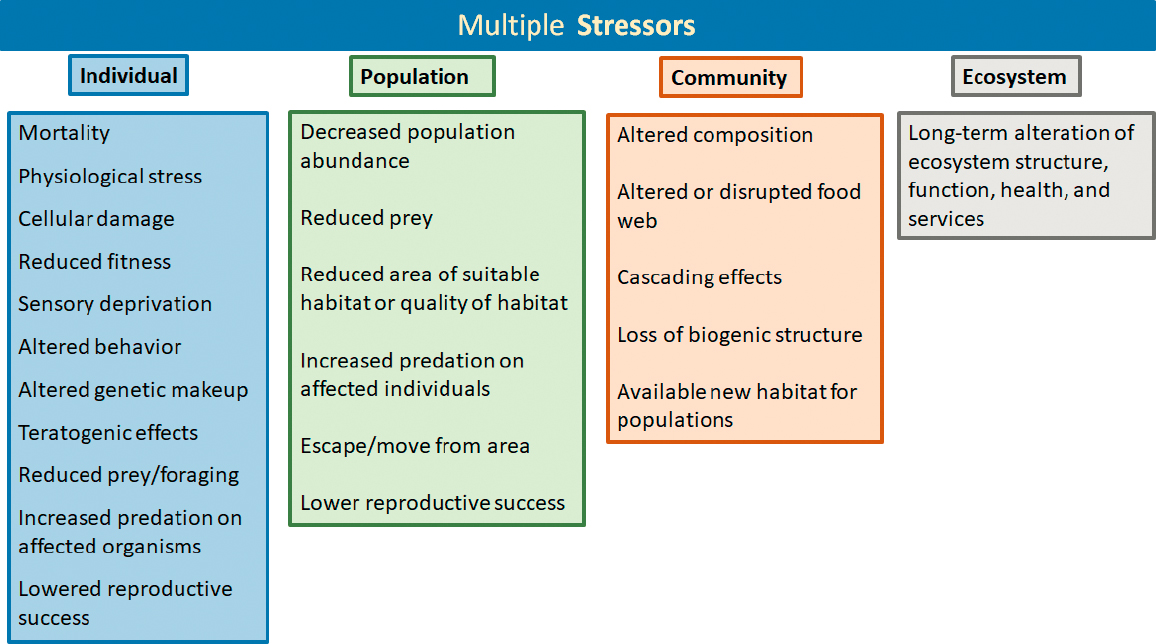
The variety of oil inputs to the marine environment challenges efforts to anticipate, respond to, understand, or even describe their potential biological and environmental effects. Although there are a number of ways oil may enter the sea (see Chapter 3), oil spills into the marine environment have received particular attention and public interest, in part due to images of oil-coated animals such as seabirds, marine turtles, and marine mammals. Oil may harm individual organisms, populations, or communities directly through adverse effects that impair survival or reproduction, and indirectly either through cascading consequences of direct effects or via impaired dependencies among different species, populations or trophic structure (see Figure 6.1). Effects from acute, short-term exposures may be limited, resulting in sublethal responses that may lead to mortality, or may involve longer-term or delayed responses on individuals, populations, communities, and ecosystems. Releases of oil into the sea, where smaller amounts are released over a protracted period (e.g., by natural seeps or leaking infrastructure) may also cause adverse effects for exposed organisms. Chronic exposure to oil may also occur after an oil spill in particular habitats and locations, such as armored beach sediments or entrainment in mangrove roots. The importance of long-term effects from acute and/or chronic oil inputs into the sea was recognized and a focus of Oil in the Sea III, and this chapter expands this further (see Highlights box and Figure 6.1), recognizing multigenerational, epigenetic, population, and community-level effects.
Since publication of Oil in the Sea III (NRC, 2003), molecular technologies and tools (‘omics) have advanced significantly and are increasingly being employed to study the presence, fate, and effects of environmental contaminants, including oil and its constituents. Omic approaches (i.e., genomics [DNA], transcriptomics [RNA], proteomics [proteins], lipidomics, and metabolomics), coupled with other disciplines have been used to provide a systems-wide approach
to monitor and assess ecosystem health and function (Beale et al., 2022; see Box 6.1). For example, microbes have been used as bioindicators of oil and in studying fate and impact for utility in pre-spill response planning, in oil spill response, restoration and in a predictive capacity (Harik et al., 2022; see Chapter 5). In higher organisms they have been used to confirm traditional and identify new mechanisms of actions (MOAs) of oil constituents (e.g., AhR receptor and cardiac toxicity mechanisms; see Box 6.1). Despite advances in ’omic tools, there are limitations and challenges, so further developments are required before these techniques can be recognized for their full potential and utility in oil spill science.
Each oil spill or input is unique, although we understand a series of general groupings of types of effects, for different types of organisms and populations, for oil in general and for certain oil types. Continuing discovery of the importance of exposure routes and indirect effects, new mechanisms of toxicity, the influence of environmental co-stressors in modifying toxicity, and effects at the community level or long-term effects after major oil spills confirms our incomplete knowledge of how oil can harm ecosystems, while spills of national significance (SONS) (e.g., Deepwater Horizon [DWH]) offer rare opportunities for substantial investigation.
Studies addressing the impact of oil have also used genomic tools at broader scales, such as using environmental DNA (eDNA) and metagenomics (i.e., the sequencing of genes to look for all organisms or targeted taxonomic groups in an environmental sample) to study biodiversity, and community composition shifts and/or to assess organisms’ metabolic potential to conduct important ecosystem functions and processes (e.g., carbon degradation). These analyses can be conducted in space and time in non-oiled areas (i.e., baseline studies) for comparison with oil-contaminated locations, including monitoring changes that expose recovery and/or long-term effects. A number of studies have suggested that eDNA surveys may be useful tools in future biomonitoring and impact assessments for oil, although further research is required to assess their utility (Cordier et al., 2019). Similarly, metatranscriptomics involving the sequencing of active or expressed genes could enable analysis of ecosystem functions and activities, although much more research is needed to develop these tools for use in oil spill assessments.
Genomic and transcriptomic approaches do not necessarily reflect functional components; hence their utility would be improved through combination with metabolomics, proteomics, and other monitoring approaches, used in an integrated eco-surveillance framework (Beale et al., 2022). Further research is required to assess the relevance of transcriptomic changes to adverse outcomes in individuals and their relation to population-level consequences, especially given concentration- and temporally-related responses. Metagenomics approaches (i.e., eDNA), while revealing many of the organisms present in the ecosystem under study, may not represent all species (given differences in DNA recovery or rare samples) and also do not provide quantitative data for each detected species. eDNA metabarcoding near oil and gas extraction locations has shown correlations with oil constituents in some but not all studies
(Cordier et al., 2019; Lanzen et al., 2021). However, as detailed above, many advances have been made using ‘omics approaches and will continue to advance oil spill science and contaminant environmental assessments in the future (Martyniuk, 2018). Additional research efforts are needed to translate and adapt laboratory findings for field application, particularly for predictions and oil spill preparedness and for damage assessment and recovery tools in higher organisms.
As described in Chapter 2, oils may be refined products of pure or limited oil types, or even more complicated mixtures, such as crude oils. Effects from exposure to these compounds arise from the interaction among the various chemical compounds in released oils, together with the complexity of the ecosystem where the oil input or chronic release has occurred. Toxicity, which is understood to mean a harmful quality, may result from physical effects such as coating or smothering, or chemical effects involving any of a variety of distinct toxicity mechanisms. The effects of oil on organisms vary widely, depending on initial oil composition and its subsequent weathering state and fate, the mode of exposure, environmental conditions at the time of exposure, the species and life stage of exposed organisms and their habitat, the mechanism(s) of toxicity, and the exposure concentration and duration due in part to the heterogeneity of oil distribution. The relevant mechanism of toxicity depends strongly on the life stage of the exposed species, the environmental conditions of their habitat, and the mode(s) of exposure. For example, oil slicks present a serious threat to adult seabirds (life stage and species) when they come into contact (mode of exposure) with a floating slick oil (habitat and oil composition), through impairment of their buoyancy, thermoregulation, and mobility (toxic mechanism) that increases risk of death by starvation or consumption by predators. If the oil is highly weathered floating tar balls, however, this threat is much attenuated. In contrast, the threat presented by physical contact with oil to the mobility of fish inhabiting surface waters immediately beneath a floating oil slick is negligible, although threats are presented to these same fish by other modes of exposure, and threats presented by different components of oil acting through other mechanisms of toxicity may be more substantial particularly for fish early-life stages in surface waters.
As a result, the effects of oil on organisms are both complicated and complex. They are complicated because of the intricate interactions among the numerous important factors that determine which effects occur and how serious these effects may be, and complex because often these interactions are non-linear, or depend on the scale in space and time of the exposure incident, or both. These intricacies seriously limit the applicability of most generalizations regarding the effects of oil to marine organisms and ecosystems. A simplified summary of the complex effects of oil on the marine environment is shown in Figure 6.3; each component is explained in detail within this chapter.
Our understanding of the biological effects of petroleum released into the marine environment is informed by experimental laboratory and field studies, theoretical considerations, and observations of marine oil pollution incidents and natural oil seeps. Each of these have advantages and limitations. Studies of natural oil seeps provide insights into how communities of organisms adapt to chronic oil exposure. Laboratory and mesocosm experiments permit control of experimental conditions. However, accounting for differences between testing conditions in the laboratory or mesocosm and conditions in field settings is usually problematic, so these studies provide useful insights into what might happen but do not necessarily indicate that those events will occur during a particular oil pollution event. Laboratory studies are especially useful in identifying mechanisms of action and providing data for predictive models, but often fall short of providing environmentally relevant information useful for oil spill response or assessing the actual impacts under specific field conditions. Field studies provide wider integration of environmental factors, but extrapolating results to other combinations of environmental factors, oils or oil components, and species or life stages may be difficult. Research focused on theoretical considerations provides efficient testing and invaluable guidance for detecting adverse effects of oil pollution in the field, but predictions based on theory always require evidence for validation. Observations of effects during oil spills obviously provide reliable indications of harm to organisms and the environment, but establishing causal relationships with exposure to oil is often difficult. Moreover, each of these approaches to detecting effects of oil exposure is better suited for some organisms than others. Overall, the best combination of data for determining effects of an oil spill consists of oil composition and toxicity, and the response of organisms or habitats.
Environmental conditions of marine ecosystems around North America vary widely, as does our understanding of them. Extensive studies of the coastal waters of Mexico, the contiguous United States, and southern Canada provide a wealth of information about the physical and biological characteristics and functioning of these ecosystems, often including long-term data sets that are invaluable for detecting significant perturbations resulting from oil contamination. The Outer Continental Shelf Environmental Assessment Program during the 1970s provided the first extensive and detailed studies of the seas around Alaska, now augmented by studies prompted by the 1989 Exxon Valdez spill and continuing Arctic oil production in Alaska. Elsewhere, the marine ecosystems of the North American Arctic and sub-Arctic have received much less scientific study, even while trans-Arctic marine transportation and the attendant increased risk of oil contamination are expected to increase dramatically as global warming continues to shrink the Arctic ice cap. The Trans Mountain pipeline expansion from the Alberta oil sands to Vancouver, British Columbia, and the expected increased use of very low sulfur fuel oils in shipping opens up the potential for new types of oil spills (i.e., dilbit; NASEM, 2016a). Nonetheless, across all of these regions, it is the sea surface and shoreline habitats that are most vulnerable to the adverse effects of oil spills and chronic oil discharges. Seabirds, marine mammals, sea turtles, and
other surface-dwelling organisms are vulnerable to direct contact with oil from spills or other discharges, and once oil contaminates shorelines, it may linger for decades, presenting a long-term threat through contact and other mechanisms of toxicity.
Oil spills or pollution abatement response efforts provide yet another dimension of effects to organisms, populations, and communities (see Chapter 4). These efforts aim first to protect human life, health, and property, and second to minimize ecological harm. No oil spill response option is without ecological consequences, and these consequences must be considered as effects of oil spills or contamination as well. For example, whereas under some circumstances the use of chemical dispersants may be a valuable response tool, it may adversely affect aquatic organisms near dispersed oil plumes. Because this topic is thoroughly reviewed in a recent National Academies of Sciences, Engineering, and Medicine report (NASEM, 2020), our discussions on this topic only summarize pertinent literature since the National Academies report.
Once oil contamination begins to decline and degrade, affected organisms, populations, and habitats begin to recover. Some organisms, populations, or communities may recover relatively rapidly, but other effects may extend over a decade or much longer depending on the specific habitat and species. Although aspects of ecosystem recovery may be measured and monitored, debate continues regarding when, if, or how much ecosystems have recovered. Identifying which changes are consequences of particular perturbations, such as oil spills, and distinguishing them from natural changes that may have occurred anyway is extraordinarily challenging, but nonetheless necessary for determining when rehabilitation efforts are no longer worthwhile. Thus, determining when adverse effects of oil contamination have abated, or when the affected ecosystem has adapted, perhaps irreversibly, to such perturbations, remains an active aspect of research and management policy discussion regarding oil pollution effects.
Oil in the sea, as well as subsequent cleanup activities, affects humans as well. We consider these relationships briefly under the One Health framework, emphasizing that these mutual dependencies are inseparable. Similar to the greater awareness of interconnectedness in infectious disease ecology, where land use, socioeconomic status, and climate resiliency are directly related to risks of emerging disease, it is clear that the effects of oil in the sea are much more complex and multifactorial than previously appreciated. For instance, oil contamination affects the health and well-being of spill responders, local inhabitants, and coastal communities—which, in contrast to earlier Oil in the Sea reports, we explicitly address here. This chapter specifically addresses human medical health harm, including seafood safety, but recognizes other human stressors as well, including economic and social issues. Only through strong, collaborative science, bringing together experts from many different fields to work collectively, can we fully appreciate health-related impacts, and mitigate adverse consequences not only during a spill but also potentially, for effects in future events.
Finally, we present the current state of oil pollution effects modeling in Section 6.7. Although these models have advanced considerably over the past two decades, there remains considerable scope for improvements, in part in relation to the data required to build and validate these models. Traditionally, laboratory toxicity tests have been used to try to mimic or replicate field conditions during a spill, which is not feasible; however, they have been useful in establishing toxicity thresholds for a number of diverse taxa that have been exposed to numerous types of oils (at differing weathering states), hydrocarbon mixtures, or single hydrocarbon components. These data have been used to develop and validate various biological effects and toxicity models used to predict toxicity (especially to new and understudied species) and have been of use both in the National Resource Damage Assessment (NRDA) process and in oil spill decision making to determine the best response option. How these tests are conducted and reported defines their utility; over- or under-estimations of toxicity can occur depending on how test media are made, chemically verified, and the experiment conducted and reported. These issues led to the development of a standardized protocol that was published a couple years before the Oil in the Sea III was released (i.e., Chemical Response to Oil Spills: Ecological Research Forum [CROSERF]; Singer et al., 2000). As highlighted in Section 6.4, new knowledge and technical advances in analytical chemistry warrant assimilation into better understanding of effects.
This chapter further explores the significant potential effects of oil spills in marine and estuarine habitats, highlighting what has been learned since Oil in the Sea III (NRC, 2003), and recognizing critical reports on effects that were not included in Oil in the Sea III. The numerous studies conducted during and after the DWH incident significantly expanded and improved our understanding of oil spill effects, not just for that specific spill but for oil pollution in general. However, the studies also highlighted and uncovered many new data gaps and information/research needs. Furthermore, there are new oil types (see Chapter 2), including very-low sulfur fuel oils (VLSFOs) and diluted bitumen (dilbit; NASEM, 2016a), for which very little information exists regarding their effects. The chapter begins with Sections 6.2 and 6.3 summarizing the modes of oil exposure to organisms (including humans) and the mechanisms of oil toxicity by which oil may harm organisms, recognizing similarities and differences across taxonomic groups. Section 6.4 follows with a discussion on the limitations and challenges in interpreting the toxicity data. Next, we review the effects of oil contamination on marine habitats, communities, and ecosystems in Section 6.5. We then review research and critical research needs in the Arctic in Section 6.6, oil effects modeling in Section 6.7, and the One Health framework (along with human health effects) in Section 6.8. We end this chapter with conclusions and identification of data gaps and research needs in Section 6.9.
6.2 MODES OF EXPOSURE
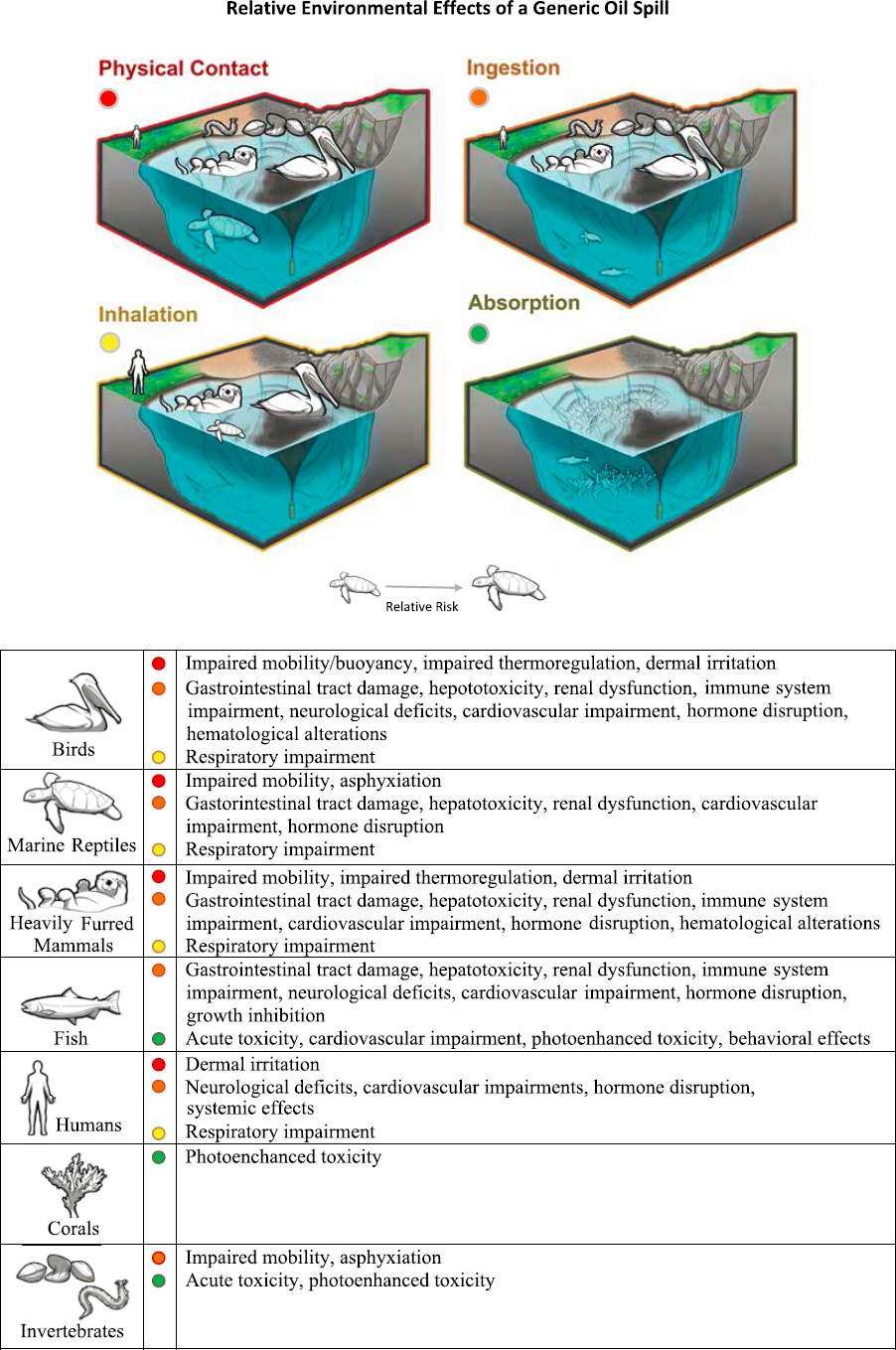
Oil can harm organisms through any combination of four major modes of exposure: physical contact, ingestion, inhalation of volatile components and oil droplets, or absorption of dissolved components (see Figure 6.3). The organisms are most susceptible to a particular combination of these modes of exposure depends on the species, life stage, habitat, initial oil discharge source, composition and subsequent weathering state, and environmental conditions. Here, we summarize the species, life stages and habitats most often affected by each mode of exposure.
6.2.1 Physical Contact
While physical contact with fresh or weathered oil most commonly occurs at the air-sea interface or on shorelines following a discharge of oil to marine waters, contact with organisms inhabiting the benthos, the water column, or the air may also occur. After an oil spill, the most visible hazard of oil is from oil slicks and sheens that may coat larger organisms that occupy or routinely traverse the oil-water interface—mainly seabirds, marine mammals, and marine turtles—and organisms that inhabit the intertidal surfaces of oiled shorelines, including humans (particularly oil spill response personnel and industry personnel working on oiled shorelines). These are the initial effects most commonly seen by the public and reported at the beginning of an oil spill. More energetic sea states promote entrainment of oil droplets into the water column and the air (see Section 5.2), promoting contact and possible coating of marine organisms inhabiting the mixed layer of the marine water column and perhaps seabirds such as petrels that may fly near breaking waves within oil slicks. As oil weathers, and possibly associates with inorganic sediments or organic matter in the upper water column, oil may sink to the benthos, contaminating coral reefs, epibenthic organisms, and eventually perhaps benthic infauna. However, restriction of released oil to mainly two dimensions at the air-sea interface and on intertidal shorelines leads to especially severe and widespread adverse effects from physical coating of organisms occupying these habitats. Consequently, the most significant acute effects of oil spills or other surface discharges on larger vertebrates are those related to physical coating based on contact with the oil in a surface slick.
The unique morphology of seabird feathers makes them particularly susceptible to contact with oil in surface slicks. The microscopic interlocking of barbules and barbicels creates a waterproof barrier that traps air next to the skin, providing critical insulation in species with high body temperatures (103–106°F), as well as buoyancy when in the water (Albers, 1995; Jessup and Leighton, 1996). Oil exposure causes the collapse of this microstructure (Hartung, 1967; Clark et al., 1968; Jenssen and Ekker, 1988). As a result, a comparatively lower surface tension can allow water to penetrate deeply into this insulative air layer (Stephenson and Andrews, 1997; Newman et al., 2000; O’Hara and Morandin, 2010).
In heavily furred aquatic mammals (e.g., sea otters, fur seals), the density and alignment of interlocking hair bundles can create an insulative air layer beneath the pelage in a manner very similar to a bird’s feathers (Tarasoff, 1972; Williams et al., 1992), in contrast to with the blubber or fat layer used by many other marine mammal species to remain warm. Upon exposure to oil products, this coat loses its ability to repel water, thereby decreasing insulation and buoyancy.
Sessile organisms inhabiting the intertidal reaches of shorelines are vulnerable to smothering when oil accumulates in relatively thick surface layers. Porous shoreline sediments may allow these surface accumulations to penetrate beneath the surface, coating infauna and rooted vegetation by direct contact with oil, especially when the tidal excursion range allows for partial dehydration of subsurface sediments that permit surface oil to flow downward through aerated sediment interstices. Resuspension of surface and subsurface oil through bioturbation—for example, when sea otters or starfish encounter oiled sediments while excavating shoreline sediments in search of infaunal prey organisms—may also lead to oil coating the external surfaces of these predators.
Although oil that settles to the seafloor could also coat organisms residing there, oil loadings on the seafloor surface are usually modest. After the Exxon Valdez oil spill, transport of oil mixed with inorganic sediment particles from heavily oiled shorelines to the shallow (<6 m depth) subtidal sediments by wave action resulted in measured oil loadings consistently less than 0.2 mg oil/g sediment in the uppermost 2 cm of benthic sediments, and loadings were consistently even lower in deeper subtidal sediments, based on a total of 39 polycyclic aromatic hydrocarbons (PAHs) congener classes and the ratio of this total PAH to the mass of 30.5% weathered Alaska North Slope oil (O’Clair et al., 1996; Wang et al., 2003). Oil loadings of benthic sediments following the DWH oil spill provide an extreme example, with maximum loadings of ~15 mg oil/g sediment in the uppermost 1–2 cm of benthic sediments across ~30 km2 in the vicinity of the well blowout on the seafloor, based on comparison of hopane measurements of seafloor surface sediments (Valentine et al., 2014) and the hopane concentration in 27.7% weathered South Louisiana crude oil (Wang et al., 2003). By comparison, a 0.5-mm thick oil slick on the sea surface at a density of 0.90 g/cm3 above the uppermost 2 cm of the sea has an equivalent loading of ~22 mg oil/cm3, and in the case of the DWH spill, slicks of this thickness or greater likely contaminated several hundred km2 of sea surface. Measurements of shoreline oil loadings 12 years after the Exxon Valdez spill imply initial loadings of the order of 100 mg/cm3 (Short et al., 2004). These comparisons imply that oil loadings at the sea surface or on heavily oiled shorelines usually present much more serious and widespread contact hazards to marine organisms than oil that sinks to the seafloor.
Oil droplets dispersed into the mixed layer of the marine water column may contact and coat a wide variety of pelagic marine organisms. Respiratory structures such as gills of fish and invertebrates are especially susceptible to such
contamination, as are particulate-collection structures of suspension-feeding organisms. Concentrations of oil droplets entrained into the water column largely depend on the surface mixing energy supplied by breaking waves (see Section 5.2), the presence of dispersants, and the viscosity of the oil. High winds during or immediately after spills promote and entrain dispersion of oil droplets into seawater, increasing effects on fish and wildlife. The 1993 MV Braer and the 1996 North Cape spills provide examples of serious effects attributed to oil naturally dispersed into the air and water. The MV Braer spill grounded on 5 January 1993 in 100+ km/hr winds, eventually releasing nearly 85,000 t of light crude oil just off the south coast of the Shetland Islands (Conroy et al., 1994). Amid a winter storm near the coast of Rhode Island the barge North Cape discharged ~3,000 t of No. 2 fuel oil. Storm winds above 100 km/h and breaking waves higher than 5 m spread the oil along the coast and into inshore salt ponds, and dispersed the oil throughout the water column (Reddy and Quinn, 2001). Concentrations of 26 PAC (polycyclic aromatic compounds)1 and of total petroleum hydrocarbons, existing as droplets of dispersed oil and dissolved compounds, in the water column reached 115 and 3,940 μg/L, respectively. These measurements are some of the highest concentrations of PAH in the water column ever recorded after an oil spill (Reddy and Quinn, 2001), causing substantial mortality to aquatic organisms (see Section 6.5.6.5).
6.2.2 Ingestion
Seabirds and sea otters may ingest oil while preening or grooming to remove oil from feathers or pelage. Sea turtles, other marine mammals, and particle-feeding fish and invertebrates may ingest oil directly while feeding. All these organisms, including sea turtles, may ingest oil through consumption of oil-contaminated prey organisms.
Preening or grooming by seabirds or heavily furred mammals is likely the second most important mode of oil exposure for these organisms. Hartung and Hunt (1966) estimated that ducks exposed to 7 g of oil would preen off 1.5 g in the first day, and Cunningham et al. (2017) calculated that a 20% covering in double-crested cormorants (the high limit to a “lightly oiled” category used in the DWH NRDA efforts) equated to 13 g of oil and, following previous work (Hartung, 1963), assumed that 50% of the oil would be preened off by day 8 of the trial.
Field studies have shown that sea turtles may consume oil-contaminated food (Hall et al., 1983; Camacho et al., 2013). Other suspension- or filter-feeding organisms also readily ingest dispersed oil droplets, including jellyfish; numerous shrimp, krill, and other crustaceans; sea butterflies (pteropods); barnacles; mussels; and oysters, among numerous other species. Deposit-feeding benthic infauna may also ingest oil (Gordon et al., 1978). Once contaminated, consumption of these organisms by higher-order consumer species provides a secondary route of exposure for these predators. For example, sea otters have an extremely high metabolic rate to maintain basal body temperatures, estimated at 2.4 times that of a comparable terrestrial mammal (Costa and Kooyman, 1982), and eat up to 25% of their body weight per day (Kenyon, 1969). This dietary intake can result in additional internal exposure to PAHs and petroleum compounds contained in prey species in affected environments (Neff et al., 1987; Jaouen-Madoulet et al., 2000; Bodkin et al., 2012).
6.2.3 Inhalation
Inhalation mainly involves fractionation of oil components into the air, so the composition of inhaled oil components is determined by components that have substantial partial pressures. These more volatile oil components include the BTEX (benzene, toluene, ethylbenzene, and xylene) and other alkyl-substituted monocyclic aromatic compounds, and alkane hydrocarbons containing 10 or fewer carbon atoms (see Section 2.1.3). Less frequently, microdroplets of whole oil may also be inhaled once oil is atomized by breaking waves (see Section 5.2.3) or by remediation methods such as high-pressure washing of oiled shorelines (see Section 4.2.4, Table 4.4). The addition of chemical dispersants can potentially increase the formation of aerosolized oil, with smaller droplet size distribution leading to greater droplet numbers (Afshar-Mohajer et al., 2018). The toxicity of the oil aerosols decreases with the use of chemical dispersant, with the dispersant creating a higher surface-to-volume ratio of the droplets, suggesting increased dissolution. Drozd et al. (2015) developed a composition-based model including evaporation, characterizing oil components out to a maximum of 30 carbons, though this model requires further examination before it is utilized this operationally.
Seabirds, marine mammals, and sea turtles are all susceptible to inhalation of volatile oil components in the air above oil slicks on the sea surface. Of these, cetaceans and deep-diving pinnipeds (e.g., elephant seals) are likely the most vulnerable, because they often breathe explosively immediately after returning to the sea surface following a dive, and show little inclination to avoid oil slicks (e.g., Smultea and Würsig, 1995). Inhalation is an important potential exposure pathway for humans too, especially oil spill response personnel and industry personnel working near accidental oil discharges.
6.2.4 Absorption
Oil components that dissolve into seawater may be absorbed by aquatic organisms, and this mode of exposure has been the most extensively studied. Like inhalation, absorption involves fractionation of oil components from the oil phase into the receiving medium, in this case seawater. At equilibrium, the composition of dissolved oil components is
___________________
1 Although PACS and PAHs are sometimes used interchangeably in the literature, PAHs containing only carbon and hydrogen are a subset of PACs. See Section 2.1.3 for more details.
determined mainly by a particular compound’s mole fraction in the discharged oil, and its partition coefficient KD, which is the ratio of the chemical’s equilibrium concentration in the oil and in the seawater phase. Following actual oil discharges, however, equilibrium conditions are almost never approached, so the composition of compounds in oil that dissolve into seawater is determined by their relative dissolution rates (see Section 5.2.5), and are always lower than the equilibrium concentrations. These dissolution rates are directly related by the relative surface area of the discharged oil in receiving waters, so that dissolution from naturally or chemically dispersed oil droplets is usually faster than dissolution from surface oil slicks, because the surface area of dispersed oil droplets is usually considerably greater than the surface area of surface oil slicks for an equivalent mass of oil in the two cases (see Section 5.2.5).
The compounds in oil that dissolve most readily include the BTEX compounds, other mono-, di-, and polycyclic aromatic compounds and their alkyl-substituted congeners (see Chapter 2). Concentrations of BTEX compounds that dissolve into surface waters tend to be ephemeral, owing to their high vapor pressures that favor evaporative losses to the atmosphere. Also, BTEX concentrations in the water column beneath oil discharges to the sea surface are often rapidly diluted within the mixed layer of the water column. For example, benzene is the most water-soluble hydrocarbon at 1,340 mg/L seawater (Mackay and Shiu, 1975), and has a partition coefficient of KD (as approximated by the octanol-water partition coefficient Kow) of about 135. Its concentration in South Louisiana crude oil is about 1.87 g/L. The equilibrium concentration of benzene after partitioning into a fixed volume of water from a fixed volume of oil may be approximately estimated as Cw = Co/(Kow + Vw/Vo), where V refers to volume and the subscripts w and o refer to the water and oil phases (Shiu et al., 1988). A 1-mm surface slick of this oil floating on a 10-cm thick seawater column will result in an equilibrium benzene concentration of 7.9 mg/L in the seawater, whereas this same slick will result in an equilibrium benzene concentration of only 0.18 mg/L in a 10-cm thick mixed seawater layer. Even lower concentrations result from less soluble hydrocarbons in non-equilibrium conditions, especially the PACs. These results are consistent with measurements of dissolved PACs in surface waters contaminated by oil slicks following major oil spills. After the 1989 Exxon Valdez oil spill, the highest combined concentration of dissolved PACs measured in surface waters was less than 0.015 mg/L (Neff and Stubblefield, 1995; Short et al., 1996), and after the 2010 DWH spill this concentration was rarely exceeded even in surface waters above the well break.2
Dissolved oil compounds may be absorbed by almost all aquatic organisms that inhabit or come into contact with oil-contaminated waters. Absorption by most organisms is through respiratory or other gas-exchange organs, and secondarily through epidermal tissues. The ambient concentration of a chemical primarily determines the rate of absorption, although this may be modified somewhat by an organism’s movement through the water or of its appendages or other structures.
6.3 MECHANISMS OF TOXICITY
Oil may harm biota through a variety of toxic mechanisms, involving both adverse effects from physical contact and poisoning from toxic compounds derived from oil. The vulnerability of organisms varies widely, depending on species, life-stages, their habitats, the mode(s) of exposure and the toxic mechanisms involved. Furthermore, environmental parameters (e.g., temperature, pressure, and UV light), the presence of other co-stressors (e.g., chemical contaminants), and complex toxicity relationships, such as, oil driven alterations to an organisms microbiome and ultimate impacts to health (i.e., immune system) increase the complexity of determining the effects of oil exposure on marine organisms. Groups of toxic mechanisms are strongly or even exclusively associated with particular modes of exposure. Therefore, the known toxic mechanisms of oil are reviewed together within each exposure mode category of Section 6.2, along with the most vulnerable species associated with each toxic mechanism.
6.3.1 Toxicity from Physical Contact
Organisms that come into contact with oil may suffer impaired mobility, impaired thermoregulation, dermal irritation and increased susceptibility to infection, and asphyxiation.
6.3.1.1 Impaired Mobility
Physical contact with oil may drastically reduce the mobility of affected organisms, impairing the ability of affected organisms to locate, capture and consume food, to avoid or escape from predators; and in some cases to avoid sinking and subsequent death by drowning. At sea, the most vulnerable organisms are those that inhabit or traverse the air-sea interface, such as seabirds, marine mammals, and sea turtles.
In birds, flight capability and capacity rely on the orderly structure of the remiges (e.g., flight feathers) to provide both lift and thrust. The physical presence of oil on these feathers interferes with the feathers’ ability to interlock, thereby decreasing their ability to promote optimal flight dynamics as well as increasing body weight (Holmes et al., 1978; Leighton, 1993). These effects have been experimentally shown in Western sandpipers (Calidris mauri) to decrease takeoff speed by 29%, reduce takeoff angle by 10 degrees, require increased energy needs for flight (20% increase for lightly oiled, 41% increase for moderately oiled), and result in greater wingbeat frequencies and amplitudes (Maggini et al., 2017). Similarly, in experimentally oiled homing pigeons (Columba livia), it was found that oiling birds at 20% levels (on the wing and tail surface) resulted in a 1.6 times greater return time when compared to baseline flights (Perez et al., 2017). Furthermore, oiled
___________________
2 See https://www.diver.orr.noaa.gov/web/guest/diver-explorer.
birds flew significantly longer distances, at slower speeds, had more drastic elevation changes, and had greater maximal elevations reached, reflecting behavioral changes necessary to return to their original location. These impacts on flight capacity can lead to wild birds needing to expend increased energy stores and/or alter flight behaviors (e.g., increased wingbeat frequency/amplitude, flying at greater elevations and/or closer to ridgelines) to enable flight to occur. This loss of energy stores, in combination with that due to increased metabolic demands from hypothermia, can lead to significant loss of pectoral muscle mass that further affects normal flight characteristics. Alterations in flight capabilities can directly cause a number of different injurious outcomes, including an inability to evade predators (Burns and Ydenberg, 2002) and delayed arrival at breeding grounds. Also, the need to expend additional nutritive resources (in combination with increased heat loss from external oiling and decreased uptake of energy from food items due to internal exposure) can rapidly cause decreased fat and lean mass, leading to mortality. In addition, the removal of the air layer next to the skin can cause birds to lose the capability to swim or float in the water (McEwan and Koelink, 1973; Vermeer and Vermeer, 1975), leading to drowning at sea or, if they are able to make it to shore, increased vulnerability to dehydration, starvation, and/or predation.
6.3.1.2 Impaired Thermoregulation
Physical contact with oil may reduce the ability of seabirds and marine mammals to limit heat loss, leading to increased energy expenditure to maintain body temperature. These increased energy demands require increased food consumption needs, which may be more difficult to meet if contacted oil also impairs mobility as well as limiting their ability to remain in an aquatic habitat. Seabirds and heavily furred marine mammals that inhabit or traverse the air–sea interface are especially vulnerable to adverse effects from increased heat loss.
Oil penetration into the insulating air layer next to seabird skin results in increased heat loss from the skin and a much greater challenge to remain euthermic (e.g., tendency to become hypo- or hyper-thermic). Experimentally, in studies on double-crested cormorants (Phalacrocorax auritus) following the DWH oil spill, externally dosed birds were found to lose heat but able to maintain core body temperature (as opposed to orally dosed birds, which had difficulty maintaining internal temperature; Cunningham et al., 2017). Most often, birds exhibit a significantly greater basal metabolic rate to maintain core body temperature (Jenssen and Ekker, 1991; Jenssen, 1994), which was estimated recently to be as much as a 13–18% increase even in sublethal exposures to oil (Mathewson et al., 2018). However, should sufficient food stores be present, this increase in energy demand can be offset for some time (Oka and Okuyama, 2000) and can even lead to preservation of core body temperatures through increased foraging.
The decreased insulation, however, typically also increases risk of starvation because oiling increases the rate at which stored body fat is used (Hartung, 1967; Fry and Lowenstine, 1985) and, subsequently, muscle wasting (Leighton, 1993; Bursian et al., 2017; Perez et al., 2017). The degree and speed of morbidity/mortality associated with loss of insulation is dependent on, among other factors, species, degree of oiling, and environmental conditions. The timing of mortalities of birds due to external oiling is more likely extended in lesser oiled categories (e.g., trace to light) and for those species whose habits do not require being in water for foraging or other normal functions. However, the cumulative effects of these issues (in combination with many of the internal effects discussed in the following sections) eventually can exhaust body energy stores to a point that, even in trace to lightly oiled birds cannot maintain physiological function.
In heavily furred aquatic mammals (e.g., sea otters, fur seals), the density and alignment of interlocking hair bundles can create an insulative air layer beneath the pelage in a manner very similar to birds’ feathers (Tarasoff, 1972; Williams et al., 1992), and in contrast to the blubber or fat layer used by many other marine mammal species to remain warm. Exposure to oil products causes this coat to lose its ability to repel water, which decreases the animal’s insulation and buoyancy, and can lead to hypothermia and associated physiological problems similar to those seen in birds (Davis et al., 1988).
6.3.1.3 Dermal Irritation
Physical contact with oil may irritate the skin of most wildlife. In addition to reducing their ability to locate and capture prey, this may also increase their susceptibility to infection if their behavioral response to irritation leads to dermal abrasion or lesions. Oil contacting the skin or gills of fish may similarly induce lesions that also increase their susceptibility to infections. The physical presence of oil on vertebrates’ skin, mucous membranes, and other sensitive tissues has been shown to cause irritation, burning, and permanent damage or loss of function to the skin and eyes in some species. These lesions have included the presence of inflamed, ulcerated, thickened, or sloughing skin and/or an inability to hear or see normally (Mazet et al., 2002; Tseng, 2006; Camacho et al., 2013). The skin of birds is particularly sensitive, being extremely thin and fragile over most of the body surface (Bauck et al., 1997). Petroleum products, depending on their constituent fractions, weathering, and other physical properties, can have a number of physical effects on tissues, causing both acute and chronic physical damage to the epidermis and the underlying layers. In particular, more highly refined products (e.g., gasoline, kerosene) can cause significant damage if not cleaned off—particularly in areas where bare skin may be present (such as the junction of the lower to upper leg in birds), regions where the product may accumulate (such as the patagium in birds and inner thigh or axillary areas), and sensitive tissues such as the corneum (Engelhardt, 1983; Massey, 2006; Helm et al., 2014; Cunningham et al., 2017). Effects to vertebrates relate not only to the specific elements of the product, but also to the adherence of the oil to the animal. Birds and furred mammals have been both experimentally and anecdotally proven
to have oil adhere readily to their outer pelage (summarized in Engelhardt, 1982), where animals with no pelage or feathers (e.g., cetaceans, sea turtles) are more resistant. Similarly, experimental studies have shown cetacean skin may also bar petroleum compounds from causing adverse effects. In one study, dolphin skin was directly exposed to gasoline for 75 minutes with no observed acute effects. In addition, the healing ability of superficial cuts, when massaged with either crude oil or gasoline for 30 minutes, was not significantly different (Geraci and St. Aubin, 1982; Geraci, 1990). Exposure of the skin to petroleum in susceptible areas can also allow dermal absorption of BTEX compounds and some smaller PACs, leading to potential chronic health effects (Peakall et al., 1982, 1983; Pérez et al., 2008). In fish, prolonged exposure to oiled sediment followed by exposure to high titers of the pathogen Vibrio anguillarm caused dermal lesions through apparent immunosuppression (Bayha et al. 2017; see Section 6.3.2.4).
Exposure to oil from the DWH oil spill has been proposed as the cause of dermal lesions in fish (Murawski et al., 2014, 2021; Romero et al., 2018, 2020; Pultser et al., 2020), but corroborating chemical evidence of exposure to Macondo oil was not conclusive. The presence of a time series from 2010 to 2016 of apparently increasing and decreasing PAH concentrations with a petrogenic or mixed petrogenic and pyrogenic signature reported for mesopelagic fish and cephalopods in the northern Gulf of Mexico illustrates the need for a better understanding of the dynamics of sources, fates, and effects of PAHs/PACs, and other petroleum chemicals in deep water column biota and ecosystems.
6.3.1.4 Asphyxiation
Intertidal organisms that become covered by oil may be unable to respire, resulting in death by asphyxiation. Asphyxiation may also kill seabirds, shorebirds, marine mammals and sea turtles if their behavioral response to contact with oil leads to occlusion of their nostrils or airways by oil (Camacho et al., 2013). Necropsy findings during the DWH incident for heavily oiled animals collected during directed field capture efforts found asphyxiation by oil as the proximate cause of death (n=2/7), with oil found obstructing the glottis or in the trachea and bronchi in five of 10 dead turtles in the stranding, and significant amounts of oil in the mouth and esophagus (Stacy et al., 2012).
Oil smothering may also cause asphyxiation in plants growing in salt-water marshes or other intertidal habitats. Widespread mortality of mangrove forests following two large oil spills in Panama was attributed to asphyxiation and possibly to other toxic effects (Duke et al., 1997), although chemically toxic effects on intertidal plants have not been well studied. Early assessments for the DWH spill (in July 2010) in southeast Louisiana salt marshes clearly documented the dieback of all marsh vegetation in heavily oiled areas. The oiled marshes no longer contained living vegetation instead there were only dead stems layering the exposed, oiled sediments (Lin and Mendelssohn, 2012; Silliman et al., 2012; Zengel et al., 2015). As with the mangroves, it is not clear whether the ultimate mortality was due to asphyxiation or toxic compounds.
6.3.2 Toxicity from Ingestion
Attempts of contaminated animals to rid themselves of oil (feathers of seabirds or the pelage of marine mammals) often leads to ingestion of substantial amounts of oil. Sea turtles can also ingest harmful amounts of oil while breathing at the sea surface or through ingestion of oil-contaminated prey, as can fish through consumption of dispersed oil droplets, oil-contaminated prey, or for some species when gulping air at the sea surface. Ingestion of oil can cause numerous toxic effects, including damage to the gastrointestinal tract, liver, and kidney, and to the immune, neurological, cardiovascular, and hormonal (i.e., adrenal, hypothalamic, thyroid) systems, as well as causing anemia and inhibiting growth.
6.3.2.1 Gastrointestinal Tract Damage
Ingestion of oil via preening or grooming can initially cause significant alterations in gastrointestinal function, elimination of gastric microbiota, and direct damage to tissues of the gastrointestinal tract. These effects, due either to physical presence of oil or to direct damage to the gastrointestinal system, can first manifest in animals via watery stools, diarrhea, and wasting in the presence of increased food/water uptake (Rebar et al., 1995; Briggs et al., 1996; Massey, 2006; Cunningham et al., 2017). If the subsequent damage is severe, it has been shown to lead to gastric erosion/hemorrhagic enteritis and degeneration of intestinal villi (Hartung and Hunt, 1966; Fry and Lowenstine, 1985; Lipscomb et al., 1993; Camacho et al., 2013). Malabsorption and maldigestion of fluids and nutrients can lead acutely to cachexia, wasting, and severe dehydration (Briggs et al., 1996; Newman et al., 2000; Holmes and Cronshaw, 2013) and, should food again become available, can lead to “refeeding syndrome” in which altered electrolyte balances and increased extracellular fluid volumes can lead to tetany/seizures, hemolytic anemia, dysrhythmias/cardiac failure, and even death (Orosz, 2013; Fravel et al., 2016). Marine iguanas appeared to be especially sensitive to gastrointestinal tract damage following a small oil spill near the Galapagos Islands (Wikelski et al., 2002). Increasing evidence has highlighted the importance of organisms’ relationships with bacteria, not just for symbiotic organisms like corals but also in an organism’s microbiome (e.g., epithelial and GI tracts), alterations in which can have ramifications for the immune, metabolic, and other systems and ultimate toxicity of oil constituents through microbial metabolism (Adamovsky et al., 2018; Duperron et al., 2020). Recent studies have shown changes in gut microbiota from oil exposure in zebrafish (González-Penagos et al., 2020) and in southern flounder following exposure to DWH-oil contaminated sediments (Brown-Peterson et al., 2017); such changes have also been proposed for use as potential biomarkers for oil contamination (Walter et al., 2019).
6.3.2.2 Hepatotoxicity
Once petroleum-related compounds are absorbed via the gastrointestinal system, “first-pass metabolism,” where compounds are transported via the portal vein to the liver, becomes important for their removal. The liver is the key organ responsible for xenobiotic metabolism, and oral exposure to oil has been shown in numerous studies to cause significant damage and alterations to this system; such studies included sea turtles in the Canary Islands (Camacho et al., 2013) and more recently a host of bird exposure studies stemming from the DWH spill (Bursian et al., 2017; Harr et al., 2017; Horak et al., 2017). Aryl hydrocarbon (Ah) receptor/cytochrome P450 enzymes, necessary for eliminating deleterious compounds from animals, have been proven to be activated in the presence of PAH congeners (Lee et al., 1985; Peakall et al., 1989; Trust et al., 2000; Schwartz et al., 2004a; Esler et al., 2010), but this metabolism can also lead to producing toxic and carcinogenic reactive intermediate compounds (Harvey, 1991) including oxygen radicals (Gutteridge and Halliwell, 2010), and it is unclear if it is the metabolic activity or the compounds themselves that cause pathological findings. Decreases in liver function, no matter if related to direct or indirect damage to the liver, can cause alterations in plasma protein levels and function (e.g., decreases in albumin and immunoglobulins), leading to immune dysfunction (Briggs et al., 1997; Newman et al., 2000); decreased protein synthesis and carbohydrate/lipid metabolism, leading to altered nutritive homeostasis (Hazelwood, 1986); decreased production of clotting factors, leading to increased health risk from injuries (Hochleithner et al., 2006); and impaired detoxification capacity, leading to an inability to eliminate PAH congeners (Leighton, 1993; Troisi et al., 2006). Additionally, the hemolytic anemia produced by oil exposure (see Section 6.3.2.9) can cause an accumulation of iron in the Kupffer cells of the liver, leading to hemosiderosis or hemochromatosis that, if severe enough, can decrease the liver’s functional capacity (Fry and Lowenstine, 1985; Khan and Nag, 1993; Balseiro et al., 2005).
6.3.2.3 Renal Dysfunction
Once petroleum compounds enter the circulatory system, significant damage and alterations have been seen in the renal system, manifested by alterations in kidney metabolic function from both direct effects of PAHs leading to glomerulonephritis (Fry and Lowenstine, 1985; Couillard and Leighton, 1990) or other renal structural changes (Dean et al., 2017; Harr et al., 2017), or the presence/effects of oil and PAHs to the gastrointestinal system leading to intestinal inflammation and damage to villi, severe dehydration, and, thus, renal damage (Leighton et al., 1986). Decreases in kidney function can cause significant and deleterious alterations to blood electrolyte balances (e.g., hyperkalemia, hypochloremia), thereby affecting intra- and extracellular fluid volumes, blood pressure, and acidosis (and subsequent changes to cardiac function) (Tseng and Ziccardi, 2019), and can also lead to a decreased capacity to eliminate metabolic waste, reduced hemostasis, and generalized debilitation (Echols, 2006).
6.3.2.4 Immune System Impairment
Similar to the impacts of oil absorption via the gastrointestinal system on red blood cells, leukocyte (white blood cell) presence and composition can be seriously affected, leading to significant effects on immune function. Alterations in white blood counts and distributions of types of cells have been noted in numerous experimental exposures (Rocke et al., 1984; Briggs et al., 1997; Schwartz et al., 2004b; Troisi, 2013; Dean et al., 2017). These changes appear to be due to a number of different primary and secondary immunosuppressive factors, including a shift of emphasis in cellular production from leukocytes to erythrocytes (due to anemia), malabsorption of nutrients from the gut, abnormal concentrations of corticosteroids due to stress, and potentially immunosuppressive action due to PAH induction of the Ah receptor/Cytochrome P450 metabolic system producing reactive intermediate compounds. A depression in the number and distribution of leukocytes (specifically, decreases in lymphocytes and often a concomitant increase in monocytes/heterophils) in association with depletion in lymphoid tissues appear to be the most common findings (Leighton, 1986; Briggs et al., 1997; Schwartz et al., 2004b; Dean et al., 2017). However, it has also been postulated that these declines are more linked to non-specific reactions (driven by the multifactorial nature of the immune system and its interactions with nutrition, stress, and other biological factors) than direct reactions to oil exposure, leading to an inconsistency in results in the published literature. As discussed in the preceding gastrointestinal section, recent new knowledge has evolved regarding the importance of an organism’s microbiome, with studies highlighting secondary consequences to the immune (and other) systems as a result of oil-induced changes to the organism’s external and/or internal microbiome, including dysbiosis (Bayha et al., 2017; Tarnecki et al., 2022). In any event, immune dysfunction, if significant, can lead to significant morbidity and/or mortality due to the animal’s inability to combat bacterial, fungal, viral, or parasitic infections, leading to increased energy demands.
6.3.2.5 Neurological Deficits
Neurological deficits have also been observed in live oiled animals (Massey, 2006; Helm et al., 2014), though it is unclear if the deficits noted were directly related to petroleum exposure or from other biomedical causes (e.g., hypoglycemia or from other nutritional causes, trauma-related, associated with liver dysfunction). If neuropathies were due to oil exposure, changes were likely due to either direct narcotic-type effects of single-ring aromatic hydrocarbons on the central nervous system, alterations in neurotransmitter levels in the brain (ATSDR, 1995), or direct morphological changes
in neuronal tissues (Peterson et al., 2003). Alterations in behavioral function can lead to lack or avoidance of predators, inability to forage, decreased reproductive efforts, decreased migratory habits, and other secondary but significant effects.
6.3.2.6 Cardiovascular Impairments
The ingestion of oil may also be linked to alterations in cardiovascular function, manifested by visually observable cardiac abnormalities (e.g., flaccid heart musculature), changes to cardiac morphology (e.g., increased ejection velocities and volumes), and decreased perfusion/blood pressure (Harr et al., 2017). While this issue is just now becoming evident in birds and marine mammals, there is broad evidence of cardiac-associated pathology in developing fish species (Incardona et al., 2013; Whitehead, 2013; Incardona et al., 2014; see also Section 6.3.4.2). If these effects apply to bird and/or mammal species, it is currently unclear whether these changes might be due to direct effects of PAHs on heart muscle (Ou and Ramos, 1992), alterations in cardiac conduction (Brette et al., 2014), activation of the Ah receptor/cytochrome P450 causing ventricular remodeling (Incardona et al., 2004), secondary changes due to other oil-related pathology (e.g., hematological, renal, and gastrointestinal effects causing increased blood pressure needs) (Leighton et al., 1985), or a combination of the above. Cardiovascular impairment from PACs absorbed by fish have been particularly well studied (see Section 6.3.4.2).
6.3.2.7 Hormonal System Disruption
Changes in hormonal systems (similar to dysfunction noted previously above for dolphins) can also occur, appearing to be driven primarily through direct or indirect effects on the adrenal gland (Peakall et al., 1983), followed by increases in plasma corticosterone levels (Holmes et al., 1979; Rattner and Eastin, 1981; Lattin et al., 2014). Less direct evidence has been found on direct and/or indirect effects on thyroid function (Rattner et al., 1984; Jenssen et al., 1990), possibly due to direct effects of PAHs (and the metabolism of such compounds by cytochrome P450 systems) on the hypothalamus-pituitary-adrenal (HPA) axis (Fairbrother et al., 2004; Mohr et al., 2010; Schwacke et al., 2013) and/or effects of reactive metabolites (from hepatic activity) on these tissues (Rolland, 2000). Oil ingestion can also lead to direct alterations in reproductive function, manifested by changes in reproductive behavior, embryo/fetal mortality, teratogenesis, failed hatching/births, and increased chick/pup abandonment (well summarized regarding birds by Leighton, 1993).
6.3.2.8 Anemia
Should the metabolites of petroleum compounds pass into the blood, significant direct alterations to erythrocyte (e.g., red blood cell) presence and function, manifested by regenerative and non-regenerative anemias, can occur. Consistently during oiled wildlife response, birds and sea otters that have been collected and cared for have exhibited significant anemias (reflected by low packed cell volumes/hematocrits) (Rebar et al., 1995; Tseng, 1999), though the source of such anemias in oil spill settings is unclear (e.g., potentially lack of production due to nutritive issues, destruction due to damage to cells, or a combination of factors). Numerous (though not all) experimental studies have shown significant destructive anemias associated with oral oil exposure in birds (Leighton, 1985; Balseiro et al., 2005; Troisi et al., 2007; Harr et al., 2017) and in mink as a model for sea otters (Mazet et al., 2000; Beckett et al., 2002; Schwartz et al., 2004a). When destructive anemias occur, they appear to be driven primarily through oxidative damage to the cell membranes via exposure to oxygen radicals formed in the metabolism of PAHs (Leighton, 1986; Troisi et al., 2006; Harr et al., 2017), leading to the denaturation of hemoglobin and, in the case of birds, the formation of so-called Heinz bodies. Hemolytic anemias, however, are not universal in non-laboratory exposures, and are likely one component of a more complex host of factors (including lack of erythrocyte production due to stress and poor nutrition from reduced foraging and lack of absorption) that lead to significant challenges in oiled animals for oxygenation of tissues.
6.3.2.9 Growth Inhibition
Ingestion of oil or oil-contaminated food inhibits growth in fish (e.g., Carls et al., 1996) and birds (e.g., Butler and Lukasiewicz, 1979), and likely has comparable effects on marine mammal growth. Ingestion of alkane hydrocarbons retards growth in fish (Luquet et al., 1983, 1984), which may make them more vulnerable to consumption by predators (e.g., Craig et al., 2006). The ability of juvenile fish to avoid their predators is a strong function of their body size, so faster-growing juveniles spend less time reaching maturity, growing out of the most vulnerable smaller body sizes of younger life stages.
6.3.3 Toxicity from Inhalation
Nearly all of the toxicological effects of inhaled hydrocarbons on seabirds, marine mammals, and sea turtles are inferred from field studies, although a few laboratory studies have been conducted on seabirds (e.g., Bruner et al. 1984; Olsgard et al., 2008; Cruz-Martinez, 2015). Toxicity from inhaled hydrocarbons in field studies is usually inferred from a combination of pathological and chemical evidence, such as evidence of direct damage to pulmonary epithelial cells following known exposure to hydrocarbon vapors, perhaps coupled with analysis for hydrocarbons that shows the presence of volatile hydrocarbons in pulmonary tissues at relative concentrations consistent with a vapor phase fraction of a petroleum source (see Chapter 2). However, distinguishing the toxic effects caused by inhalation from those caused by ingestion is usually problematic, as both modes of exposure are often significant, and inhaled hydrocarbons rapidly enter the bloodstream where they cause the same
suite of effects as hydrocarbons that enter the bloodstream, following ingestion of oil. We summarize toxic effects associated with inhalation of petroleum or fractions of petroleum, with reference to the toxicity mechanisms noted earlier for ingestion (see Section 6.3.2).
Marine mammals, and probably sea turtles, are especially vulnerable to toxic effects following inhalation of hydrocarbon vapors. Many marine mammal and sea turtle species inhale deeply when surfacing immediately after a protracted dive. If this happens when an animal surfaces through a relatively fresh oil slick that has hydrocarbon vapor pressures sufficient to substantially displace atmospheric oxygen, inhalation of the gas mixture above the slick may cause the animal to lose consciousness and drown. Although direct evidence of this is not available for marine mammals, accidental inhalation of high concentrations of hydrocarbon vapors among oil and gas extraction workers has led to sudden deaths attributed to oxygen deprivation and toxic effects (Harrison et al., 2016). Marine mammals and sea turtles that surface within a large and relatively fresh oil slick may be susceptible to similar risks; however, there is a paucity of data, especially for sea turtles, so future research should be directed at addressing this knowledge gap.
In sea otters, direct respiratory damage due to oil exposure was observed as one of the most significant findings in animals during the Exxon Valdez oil spill (Lipscomb et al., 1993). Overall, interstitial pulmonary emphysema was seen in 73% of heavily contaminated, 45% of moderately contaminated, and 15% of lightly contaminated animals necropsied during the event, with dyspnea and subcutaneous emphysema also diagnosed in live otters in the rehabilitation facility. The underlying etiology of this emphysema remains unclear, as it has not been reproduced in subsequent laboratory exposure trials on surrogate species, but otters may be anatomically predisposed due to well-developed interlobular septa in their lungs.
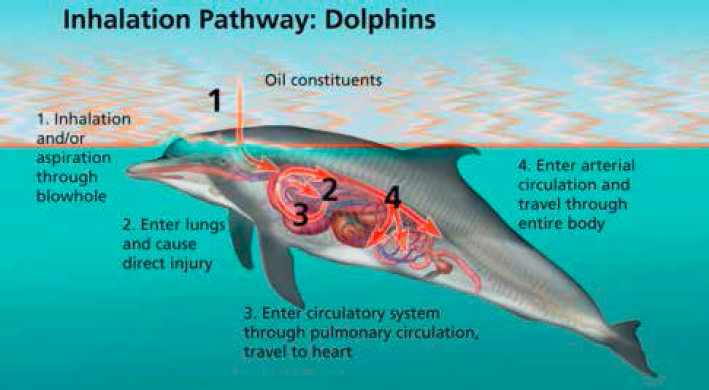
Similarly, following the DWH oil spill, a number of studies focused on the short- and long-term health impacts on coastal bottlenose dolphins (Tursiops truncatus) in heavily oiled Barataria Bay, Louisiana, due largely to exposure to oil and volatile compounds produced from the dispersing product. In 2011, 43% of the evaluated dolphins were considered “unhealthy” and 17% were given a poor or grave diagnosis, meaning they were not likely to survive (Schwacke et al., 2014). Furthermore, Barataria Bay dolphins were five times as likely to have moderate to severe lung disease, mostly described by substantial alveolar interstitial syndrome, lung masses, and pulmonary consolidation, compared to those found in a control group (Sarasota Bay, Florida). Additionally, strong evidence of adrenal compromise and an impaired stress response, leading to an overall increased susceptibility to infectious disease, was found in this dolphin population (reflected by low blood cortisol levels and associated low aldosterone values), as well as evidence of inflammation, hypoglycemia, and altered iron metabolism (Schwacke et al., 2014; Venn-Watson et al., 2015; Smith et al., 2017). While it is unclear what role ingestion and subsequent metabolism of petroleum had in dolphins with impaired health, inhalation/aspiration and subsequent transfer of toxins from the respiratory system to the blood is strongly suspected as a driving factor (see Figure 6.4). Necropsy results on dolphins found within the spill region revealed potentially lethal changes to their adrenal gland (33%; including unusually thin adrenal cortices) and primary bacterial pneumonia (22%) in agreement with earlier findings in live-sampled dolphins (Venn-Watson et al., 2015). Follow-up health assessments of this population in 2013 and 2014 indicated that, although overall improvements were seen in population health, pulmonary abnormalities (e.g., moderate to severe lung disease evidenced by pleural effusion, alveolar-interstitial syndrome, and pulmonary masses, nodules, and consolidation) and impaired stress responses continued for at least 4 years after the spill (Smith et al., 2017). This heightened risk of pulmonary effects (and adrenal compromise via subsequent transfer to the blood) is likely due to the uniqueness of cetacean physiology, with
SOURCE: NOAA.
the short trachea in Tursiops causing them to be explosive breathers that can exchange 70–90% of total lung capacity in 0.3 seconds, leading to rapid gas exchange and high air flow (Aksenov et al., 2014). Previous experimental studies hypothesized that cetaceans, on the whole, would avoid surface slicks (Geraci et al., 1983); however, in practice, this appears not to be the case in calm waters (Smultea and Würsig, 1995), leading to significant risk in these species.
Health impacts on marine mammals and sea turtles exposed at the air-sea interface, primarily those with rapid gas exchange and high air flow (cetaceans) or prolonged exposure (sea otters) manifest in short- and long-term effects (at least 4 years post spill for bottle-nosed dolphins in the DWH oil spill). Lack of avoidance of oil (Smultea and Würsig, 1995; Stacy et al., 2017), especially in calm waters, leads to significant risk in these species. Continued monitoring and determination of health effects require long-term studies for the assessment of population impacts.
Changes in respiratory function of birds through inhalation are manifested by increased respiratory effort, physiological damage, and decreased ability to fly/ambulate/dive, leading to decreased oxygenation of the blood and necessary changes in normal foraging behaviors. Avian respiratory physiology is unique, with the lack of a diaphragm and air sacs requiring large volumes of air to move across respiratory surfaces to allow the needed oxygenation of blood. Additionally, due to very efficient gas exchange across thin respiratory tissues to the bloodstream (in tandem to the increased tidal volume), the transfer of lower molecular size PACs to the bloodstream as a preface to systemic effects is comparatively higher in birds (Duncker, 1974; Brown et al., 1997). Direct respiratory damage to birds is not frequently reported in the literature; however, pathological consequences are likely hidden by the more overt causes of morbidity and mortality due to external coating and ingestion from preening. Rehabilitated birds during oil spills have regularly been reported to have respiratory distress in captivity (Mazet et al., 2002), which can increase the propensity for significant fungal infections (e.g., aspergillosis). Experimental studies in laughing gulls (Leucophaeus atricilla) exposed orally to DWH oil found respiratory inflammation (pneumonia and/or air sacculitis) in nearly one-third of the subjects (Horak et al., 2017).
6.3.4 Toxicity from Absorption of Soluble Oil Components
Oil components that dissolve into the water column may harm organisms by causing acute (i.e., short-term) toxicity that can lead to death, cardiovascular impairment that decreases fitness, and for organisms with translucent tissues that inhabit the near (~1-m depth) surface layer of oil-contaminated waters illuminated by strong sunlight, photo-enhanced toxicity. As vertebrates, fish may also suffer the systemic effects of inhaled hydrocarbons experienced by seabirds, marine mammals, and sea turtles (Takeshita et al., 2021) (see Section 6.3.3).
6.3.4.1 Acute Toxicity
Bioassays typically measure acute toxicity as the concentration of a toxicant that will kill 50% of exposed aquatic organisms within some specified exposure period, often 96 hours, and typically summarized as 96-h LC50 (shorthand for “96-hour lethal concentration for 50% of the test organisms”). In these tests, test organisms are exposed to each of several different concentrations of the toxicant solution, and a concentration that would kill 50% of the test organisms is estimated from the number found dead at a specified time for each exposure concentration. The same approach is used to evaluate sublethal toxicity endpoints such as abnormalities associated with fish embryotoxicity (e.g., Turcotte et al., 2011; Lin et al., 2015), in which case the effective concentration causing 50% of the test organisms to display the response being evaluated is estimated, and denoted as EC50.
Extensive laboratory tests conducted with the water-soluble fractions (WSFs) of many kinds of crude and refined oils have found that 96-h LC50 values usually exceed ~0.1 mg of total dissolved hydrocarbons measured per liter of seawater (Anderson et al., 1974; Rice et al., 1977; Fuller and Bonner, 2001; Mitchelmore et al., 2020a,b). Most of these dissolved hydrocarbons are aromatic compounds because of their higher water solubility relative to the saturated alkane, resin and asphaltene fractions of oil (see Chapter 2). Refined oils such as diesel fuels are somewhat more toxic than crude or heavier refined oils (Anderson et al., 1974; Rice et al., 1977; NASEM, 2016a; Adams et al., 2017; Hodson et al., 2019). In most organisms tested, the toxicity of aromatic compounds increases with the number of aromatic rings and the extent of alkyl substitution (Rice et al., 1977; Turcotte et al., 2011; Lin et al., 2015). Apparent sensitivity varies by more than two orders of magnitude among species, and within species, sensitivity may vary unpredictably among life stages (Rice et al., 1977; Mitchelmore et al., 2020b), although embryonic life stages of fish are especially sensitive (see Section 6.3.4.2).
Much of the wide variability in apparent sensitivity among species may be the result of differences in acute bioassay test conditions. Mixing conditions can greatly affect the proportions of compounds that dissolve from the test oil into seawater. Analysis methods used to characterize the composition and concentrations of these compounds vary widely, and quality assurance measures range from absent to extensive. Characterizing the effective doses of test solutions is particularly challenging, because the concentrations of the dissolved compounds usually decline with time because of volatility losses, microbial degradation (see Section 5.1.7), and possibly catabolism of accumulated compounds by the test organisms. Under static test conditions, where the exposure solutions containing the test organisms are left undisturbed for the duration of the exposure, concentrations of dissolved aromatic hydrocarbons may decline to less than half of the initial concentrations. These declines may be mitigated by “static renewal” tests, in which the test solution is replaced at intervals (usually daily), or flow-through or partition-controlled dosing systems that maintain nearly constant concentrations are used (e.g., Turcotte, 2011). Also, most
acute toxicity bioassays reported in the literature have been conducted under incandescent or fluorescent illumination, so effects from photoenhanced toxicity (see Section 6.3.4.3) and from photo-oxidized compounds that result from exposure to the UV component of sunlight, now known to be important (Ward et al., 2018b; see Section 5.2.5), are largely precluded unless the tests are conducted outdoors. Taken together, these differences in experimental details may account for substantial proportions of the variability in toxicity reported for a given life stage of the species tested (see Section 6.4). Moreover, evaluation of the toxicity of photo-oxidized products of compounds that dissolve from oil into seawater would assist in determining how much they contribute to acutely toxic effects on test organisms.
6.3.4.2 Cardiovascular Impairment
Differences in the survival of pink salmon embryos in oiled compared with unoiled spawning habitat following the 1989 Exxon Valdez oil spill prompted laboratory studies confirming toxicity from exposure to water that had contacted oil (reviewed by Rice et al., 2001). Oil in the Sea III (NRC, 2003) noted that these findings remained controversial, in part because the concentrations of dissolved PAHs thought to be responsible for these effects were so low (~10 μg/L total of 39 PACs) compared with 96-h LC50s, and because no known mechanism of toxicity could account for the high toxicity and the associated sublethal effects (Brannon et al., 2001). These sublethal effects include deformed jaws, missing or deformed fins, spinal curvature, and pericardial and yolk sac edema that appeared in larvae after exposure to oil had ceased. Results from experiments published 1 year after Oil in the Sea III (NRC, 2003) found that these sublethal effects resulted from impaired development of the embryonic heart following exposure to three ring PACs (Incardona et al., 2004). This insight was a major advance in oil toxicology, and led to a considerable body of ongoing research establishing the details and environmental ramifications of embryonic cardiac impairment following exposure to PACs, as well as independent confirmation of these effects in several species of fish by researchers in Canada (Hodson, 2017), China (Zhang et al., 2012), Korea (Jung et al., 2013), and Norway (Sørhus et al., 2015).
Two distinct general toxicological mechanisms accounting for embryonic cardiac impairment in developing fish embryos are now clearly established. The aryl hydrocarbon receptor-dependent (AhR-dependent) mechanism involves initial intracellular binding of alkyl-substituted PACs with three or more rings, or unsubstituted PACs having four or more rings (Barron et al., 2004). This initiates induction of cytochrome P450-1A (CYP1A), a PAC-detoxifying enzyme that oxidizes PACs to more water-soluble and excretable products, along with genetic transcriptional effects wherein up- or down-regulate genes associated with cardiac development or function, leading to impaired cardiac development and function in embryos that persist in later surviving life stages (see Figure 6.5A). A second AhR-independent mechanism involves direct interference in calcium and potassium ion cycling of excitation-contraction coupling in developing cardiomyocytes by un- and alkyl-substituted three-ring PACs, along with somewhat different genetic transcriptional effects of up- or down-regulating genes associated with cardiac development or function, again leading to persistent impaired cardiac development and function in embryos (see Figure 6.5B). Phenotypic expression of the AhR-dependent pathway (e.g., pyrene in Figure 6.5 and Box 6.2) is distinctly different from that of the AhR-independent pathway (e.g., fluorene, phenanthrene and dibenzothiophene in Figure 6.5 and Box 6.2).
Although only a subset of PACs that dissolve from oil into water act through the AhR-dependent mechanism leading to CYP1A induction, once induced, CYP1A may act on other PACs as well, as most PACs are substrates for CYP1A (Incardona, 2017). This accounts for why PACs typically do not persist in vertebrate tissues (Meador et al., 1995). Though protective, induction of CYP1A may not entirely offset the combined effects of PAC mixtures that act through both the AhR-dependent and -independent mechanisms simultaneously.
Aqueous extracts of PACs from oils may be toxic at total PAC concentrations of less than 1μg/L, considerably lower than the potency of any of the individual PACs tested (e.g., Turcotte et al., 2011). While this may suggest synergistic interaction between the AhR-dependent and -independent mechanisms, PAC mixtures may be highly cardiotoxic without inducing CYP1A in endo- or myocardial cells (Jung et al., 2013; Sørhus et al., 2016). Harmful effects of these PAC extracts have been found at concentrations as low as 0.23 μg/L (Incardona et al., 2015), far lower than toxic thresholds associated with acute toxicity (see Section 6.2.4.1). See Figure 6.6.
Subtle interactions among PACs that act through either or both of the AhR-dependent and -independent mechanisms may also account for differences in effects stemming from genetic up- and down-regulation associated with fish that occupy different habitats. Jung et al. (2017) found considerable differences in the up- and down-regulation of genes of olive flounder (Paralichthys olivaceus) compared with those of spotted sea bass (Lateolabrax maculatus) exposed to PACs from Iranian heavy crude oil, and these differences also depended on the weathering state of the oil. From these and other related studies (summarized in Hodson, 2017, and Incardona, 2017), it is clear that the embryological effects of dissolved PACs on fish are intricate, complex, and dependent on numerous interacting factors, substantially complicating efforts to anticipate the results for particular exposures and species.
The <1 μg/L concentrations of three-ring PACs that can inflict substantial damage to developing fish embryos implies that these effects may be widespread after oil spills, or in association with chronic discharges such as stormwater runoff to marine receiving waters (e.g., McIntyre et al., 2016). Fish spawning and rearing habitats are especially vulnerable, including surface waters immediately beneath or near oil slicks for species that broadcast buoyant eggs (e.g., Jung et al., 2015), intertidal reaches of shorelines oiled by spills or oil-derived PACs from chronic discharges (e.g., pink salmon spawning streams after the Exxon Valdez; Bue et al., 1996), and perhaps the surface of the seafloor susceptible to contamination from produced water discharges for species that deposit their eggs there (see Section 6.5.6.6).
Fish, seabirds, and probably other vertebrates are susceptible to cardiovascular impairment caused by three and four ring PACs accumulated by any exposure route, including absorption through the skin and gastrointestinal tract, through respiratory surfaces of aquatic organisms, or by inhalation of hydrocarbon vapors (reviewed by Takeshita et al., 2021). Because at least some of the intracellular binding sites associated with the AhR-dependent and -independent toxicity mechanisms are highly conserved across vertebrates (Incardona, 2017), cardiotoxicity of PACs may be commensurately widespread. For example, Brette et al. (2017) suggest that environmental exposure to phenanthrene may account for some of the acute cardiac impacts of air pollution.
While the AhR-dependent and -independent cardiotoxicity effects of PACs may provide the basis for a unified toxicological model that accounts for cardiotoxic effects across wide ranges of oils, exposure conditions, habitats, species and life stages, it is likely that additional toxic effects of oil-derived PACs remain to be discovered. For example, naphthalene, a two-ring PAH, is toxic to early life stages of fish (Hodson, 2017), but not through either of the AhR-dependent or -independent mechanisms. In light of the likelihood that results may have additional implications for human health, this is an important area of future research.
6.3.4.3 Photoenhanced Toxicity
Photoenhanced toxicity refers to catalysis of the production of singlet oxygen by certain PACs absorbed within cells and exposed to UV light. Whereas most of the molecules within the Earth’s biosphere have paired electrons that have zero net spin (called a “singlet” state, denoted as 1X, where X is a molecule), molecular oxygen is highly unusual in having two unpaired electrons that have one of three spin states (called a “triplet” state, denoted as 3O2), making molecular oxygen a diradical molecule. Conservation of angular momentum strongly inhibits reactions between singlet and triplet molecules. Absorption of UV light by PACs may lead to promotion of a PAC electron into a higher-energy triplet state, which can readily transfer this energy to triplet oxygen when the energy differences are similar, converting the triplet oxygen into higher-energy singlet oxygen. This exchange returns the PAC to its lower-energy singlet state, and the higher-energy singlet oxygen is extremely reactive with singlet organic molecules, allowing this process to repeat indefinitely as long as (singlet) oxygen is available and the PAC is not oxidized by it (see Figure 6.7). This pathway is usually denoted as Type II photosensitization (e.g., Wang et al., 2009).
In a separate pathway, denoted as Type I, the excited triplet PAC molecule may directly oxidize biological substrates such as lipids, amino acids, or DNA/RNA bases, producing a highly reactive PAC anion radical (PAC•−), which may then react with other biological substrates, or donate its radical electron to 3O2, producing a superoxide anion (O2•−), which can then induce generation or reactive oxygen species (ROS) such as peroxides or hydroxyl radicals (Wang et al., 2009) (see Figure 6.7). This pathway also returns the excited-state PAC back to its ground (singlet) state. Consequently, one PAC molecule may catalyze production of many
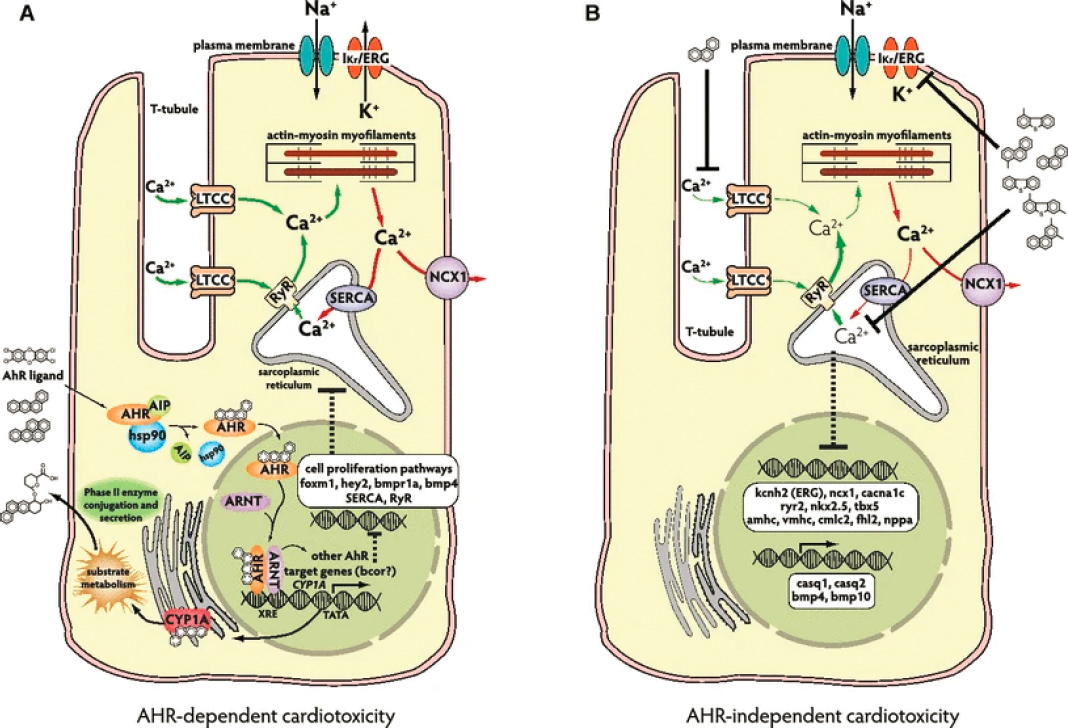
SOURCE: Incardona (2017).
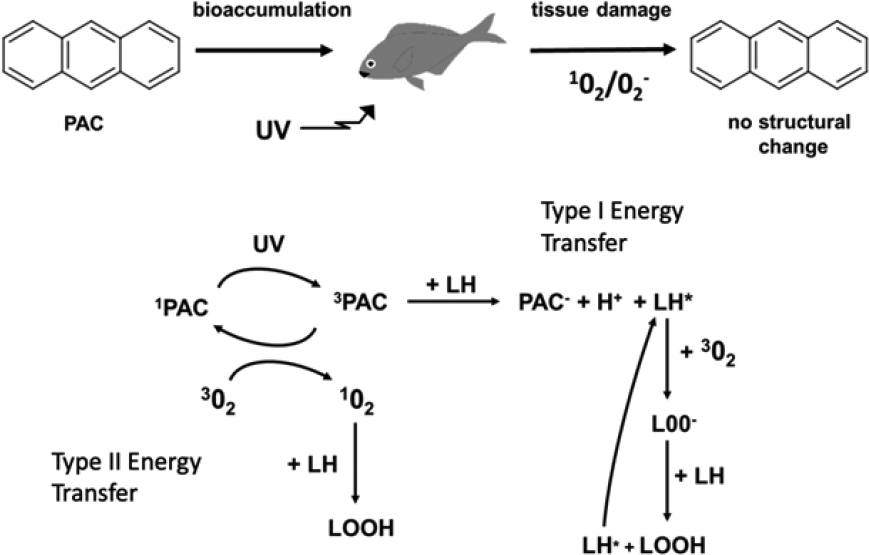
SOURCES: Derived from Landrum et al. (1987), Choi and Oris (2003), Diamond (2003), and Wang et al. (2003).
3O2 molecules and ROS, which then in effect proceed to burn tissues from the inside out, along with occasional direct binding of the PAC involved with biological substrates including proteins, RNA, and DNA, the latter of which may lead to damage to the genome.
The requirement for similar energy gaps between the ground and excited states of PACs and 3O2 limits which PACs can catalyze 1O2 production. The most active PACs include parent and alkylated variants of anthracenes, fluoranthenes, chrysenes, pyrenes, benzochrysenes, and benzopyrenes, along with the oxygen, nitrogen, and sulfur analogs of these compounds such as dibenzothiophenes and acridines (e.g., Pelletier et al., 1997; Wiegman et al., 2001; Lee, 2003). This same requirement also limits the UV wavelengths to the “UVA” component of sunlight (320–400 nm). Tissue damage from photoenhanced toxicity in organisms requires absorption of the right PAC into tissues, exposure to the UVA component of sunlight during or immediately after PAC absorption, translucent tissues at least at an organism’s epidermis, and oxygen availability. When these conditions are met, photoenhanced toxicity can be much more harmful compared with toxic effects when UVA radiation is low or absent. Depending on the PAC, such increases of toxicity range from factors of two to nearly a thousand (Willis and Oris, 2014). Consequently, small organisms with translucent bodies that inhabit the upper water column or intertidal reaches of shorelines are most vulnerable. More than 30 aquatic species, often in their earlier life stages, have been shown to be sensitive to photoenhanced toxicity effects, including crustaceans, mollusks, oligochaetes, and fish (Boese et al., 1997; Spehar et al., 1999; Barron and Ka’aihue, 2001; Barron, 2007). Conversely, large-bodied organisms, especially those with extensive pigmentation or exoskeletal armoring, or organisms that inhabit deeper waters where UVA radiation does not penetrate, are unlikely to be susceptible to effects from photoenhanced toxicity. Given the toxicity range of two to nearly a thousand, understanding of photoenhanced toxicity effects would benefit from study characterization of additional organisms and numerous life stages.
6.3.4.4 Immune System Impairment
Fish exposed to chemically enhanced water accommodated fractions (CEWAF) followed by exposure to high titers of the pathogen Vibrio anguillarm developed dermal lesions through apparent immunosuppression (Tarnecki et al., 2022; see Section 6.3.2.4).
6.3.4.5 Behavioral Effects
Laboratory tests clearly show that fish are able to detect and will avoid oil (Rice, 1973, 1977; Meinard and Weber, 1981; Martin, 2017; Claireaux et al., 2018), although repeated exposure may de-sensitize this response (Schlenker et al., 2019).
6.4 LIMITATIONS AND CHALLENGES IN INTERPRETING LABORATORY TOXICITY DATA
Laboratory toxicity tests are used for a number of reasons, first in a regulatory (environmental risk) framework to estimate the toxicity of single or mixtures of hydrocarbon constituents, oil and/or chemicals used in oil spill response (e.g., dispersants, herders). Second, they have been used with standard test species to establish relationships with and among oil types and to highlight species and/or life stages that may be more susceptible to oil exposure. The data, assuming they meet data quality requirements, can be used to populate and generate, calibrate, and/or validate toxicity tests and models to predict the impacts of oil and its constituents to various species and support decision-making during oil spill response (French-McCay et al., 2018; NASEM, 2020; see Section 6.7). Laboratory toxicity tests are also useful in establishing the specific mechanism(s) of toxic action (e.g., receptor sites and resulting effects) of oil and its constituent chemicals, and finally how oil effects can be influenced (i.e., become more or less toxic) when combined with other covariables that may occur in a field setting, such as UV light, temperature, salinity and pressure in addition to other chemical contaminants. Toxicity tests are also used following a spill event in NRDA activities and by researchers trying to establish the potential impact of a specific spill on resident species. Tests are conducted with both standard toxicity test organisms and with non-standard toxicity test species to provide a more accurate assessment of oil toxicity to exposed resident species. However, laboratory toxicity tests cannot mimic or replicate field conditions, and recently a paradigm shift was suggested in the recent National Academy dispersant report to conduct toxicity tests in such a way that the data generated would be useful in informing and validating toxicity models (NASEM, 2020).
Since the Oil in the Sea III report, new analytical molecular techniques and testing approaches have provided a wealth of new knowledge regarding drivers of toxicity (see Highlights box; NRC, 2003). These include specific chemical constituents involved in existing and new mechanisms of toxicity (e.g., cardiotoxicity in fish embryos; Incardona, 2017; Incardona et al., 2021), the influence of oil droplets (i.e., via additional exposure routes, enhanced bioavailability of dissolved constituents, or physical mechanisms) and environmental modifiers decreasing (i.e., pressure; Paquin et al., 2018) or increasing toxicity (i.e., UV light; Barron, 2017). However, how laboratory toxicity tests with complex mixtures of oil and oil/dispersants are specifically conducted influences the results obtained and hence their interpretations and in some cases decreases the utility of the data collected. Examples include inappropriate extrapolation of laboratory data to describe field conditions, over- or under-estimations of toxicity, and comparisons between oil and dispersed oil exposures based on the lack of or choice of analytical characterization metric (Bejarano et al., 2014; Redman and Parkerton, 2015; Bejarano, 2018; Hodson et al., 2019; NASEM, 2020; Mitchelmore et al., 2020a,b). Many of these issues were addressed in detail in the recent National Academy dispersant report (NASEM, 2020). This section aims to highlight some of the key points regarding the use, approaches and interpretation of laboratory toxicity tests since Oil in the Sea III. Dispersed oil and dispersant studies are not discussed in as much detail as oil-alone studies as these were recently reviewed in the National Academies dispersant report (NASEM, 2020).
6.4.1 Implications of the Variability in the Design, Execution, and Reporting of Toxicity Tests
Laboratory toxicity tests are conducted under controlled conditions and numerous standard protocols on how to conduct them exist, albeit for a relatively limited (but representative) number of standard test species (e.g., following U.S. Environmental Protection Agency [U.S. EPA] and Organisation for Economic Co-operation and Development [OECD] guidelines). However, the complexity of oil itself, and the additional use of spill response agents (e.g., chemical dispersants) combined with the influence of additional environmental variables, result in additional complications and considerations for these tests particularly concerning the variability observed and reproducibility of toxicant test exposure solutions. Standardization of protocols detailing how to prepare exposure solutions and how to conduct toxicity tests, including the use of appropriate analytical verification and choice of biological endpoints is critical. Without these standard methods, comparison across studies, data utility for inclusions and/or validation of toxicity models, or simply an accurate representation of toxicity is challenging (Redman and Parkerton, 2015; Bejarano, 2018; Hodson et al., 2019; NASEM, 2020; Mitchelmore et al., 2021a,b; Nordtug and Hansen, 2021). This need was recognized back in the 1990s and led to the development of the CROSERF Ecological Effects Forum, which standardized methods for preparing exposure media and conducting toxicity tests (Singer et al., 2000; Aurand and Coelho, 2005). Despite these guidelines, studies have continued to either not employ them or have modified them without providing all of the details required to allow toxicological interpretation. Many modified versions of the CROSERF protocol have been used since its initiation, in part due to new knowledge on processes that affect toxicity (e.g., UV light; phototoxicity) and the development of new exposure preparation methods (e.g., high energy water accommodated fraction [HEWAF]). These CROSERF protocols are now over 20 years old and new oil types and test species, an increased understanding of toxicological drivers, and new technologies for test media preparation require that these protocols be revisited and updated (see Table 6.1 for an overview and Boxes 6.3 and 6.4 for descriptions of terminologies used, an overview of exposure preparation methods, and characterization of solutions, respectively).
TABLE 6.1 Main Considerations of Parameters and Approaches That Can Lead to Different Results and Interpretations in Oil and Oil and Dispersant Toxicity Tests
| Parameter/Approach | Considerations Related to Test Media and Effects |
|---|---|
| Choice of oil type, weathering state Loading rate (oil-to-water ratio) and choice of variable loading or variable dilution Mixing regime (extent) and dispersion technique (physical or chemical) and dispersant:oil ratio (DOR) |
Differing chemical composition and entrainment of droplets (number and size), dispersant efficacy. See Figures 6.10 and 6.11 that demonstrate the differences in chemical composition between the two approaches. Will also affect coalescence of oil droplets.
Low-, mid-, and high-energy methods, with or without chemical dispersants will alter the chemical composition, oil droplet size, and oil droplet quantity. DOR will alter the efficacy and/or extent of dispersant compounds free in the water or associated with oil droplets. Affects stability of oil and coalescence of oil droplets. |
| Mixing time and settlement period | Time to reach equilibrium for dissolved hydrocarbons? Settlement time will affect stability in physically/chemically dispersed oil (i.e., longer time results in smaller droplets as larger ones have risen to the surface). |
| Type and duration of the toxicity test | Chemical and physical composition of the test media is dynamic and changes over time (e.g., loss of volatiles, photochemical reactions, coalescence of droplets). Exposure depends on test type; static, static-renewal, spiked declining, constant, etc. Effect on organisms and environmental relevance depends on exposure concentration and time. |
| Analytical chemistry | Nominal reporting (loading rate, %, etc.) unacceptable for exposure concentration. Limitations in use of bulk parameters (TPH, THC, etc.); minimum use of TPAH (50–70 individual parent/alkyl PAHs), BTEX, quantitation of dispersant components. Bulk oil via fluorescence methods. Dissolved PAH via passive sampling. Determination of dissolved and particulate fractions. |
| Physical analysis (droplet volume, number, and size) | Analysis (laser in situ scattering and transmissivity [LISST], coulter counter) of particulate phase (oil droplets) for volume, number, and size. |
| Toxicological considerations | WAF: soluble and readily bioavailable hydrocarbons. |
| Other variables to consider | What are the questions/hypotheses for conducting this test? Mixing vessel/exposure chamber types: headspace/closed or open containers? Salinity, temperature, lighting and feeding regime. Types of endpoints and frequency of assessing biological endpoints (e.g. time to death considerations for acute tests); environmental relevance, choice of species/life stage. |
NOTE: BTEX = benzene, toluene, ethylbenzene, and xylene isomers; PAH = polycyclic aromatic hydrocarbon; TPAH = total PAH; THC = total hydrocarbon content; TPH = total petroleum hydrocarbon; WAF = water-accommodated fraction of oil.
Numerous requests for further standardization of exposure test media preparation and toxicity test protocols have been made in various publications, including National Academies reports and other papers (Aurand and Coelho, 2005; NRC, 2005; Bejarano et al., 2014; Redman et al., 2015; Hodson et al., 2019; Mitchelmore et al., 2020a,b; NASEM, 2020). Key aspects of new protocols should include detailed quality assurance and quality control metrics and the reporting of key elements of the test procedures (including any deviations from the standard protocols) so that there is a minimum set of reporting requirements provided in each test, examples of which can be found in tables in the review by Mitchelmore et al. (2020a) and in Table 6.2.
As the solubility and/or partitioning behavior of oil constituents (and hence bioavailability to organisms) is heavily influenced by how exposure solutions are made up, the CROSERF standardized methods recommended in detail how to prepare exposure media. A focus for toxicity testing has been on the preparation and characterization of dissolved oil (and/or dispersant) constituent exposures, first because this fraction is considered bioavailable and second, its use in oil spill toxicity prediction models (Carls et al., 2008; MacKay et al., 2011; French-McCay et al., 2018, 2021b). The CROSERF protocols detail the type of container to use (glass aspirator bottle), the amount of headspace (in the sealed bottle), the mixing energy (i.e., low and no vortex for minimum droplet entrainment and medium, 25% vortex, when using chemical dispersants to result in oil dispersal and droplet formation) and the recommended oil to dispersant ratio when using chemical dispersants (Singer et al., 2000). Since the original method was published, additional considerations regarding how test media are prepared (such as whether to use variable dilution or loading of the oil or other preparation methods, such as passive dosing) have been discussed (NASEM, 2020; Parkerton et al., 2021).
One of the test preparation parameters that has received particular attention and discussion is that of using variable loadings of oil to prepare the concentration range versus the preparation of a high concentration stock solution from which dilutions are made to prepare each concentration. This issue has been discussed at length in previous National Academies reports (NRC, 2005; NASEM, 2020) and in reviews (Barron and Ka’aihue, 2003; Redman et al., 2015; Mitchelmore et al., 2020a) regarding each approach’s influence on the concentration and composition of the resulting test exposure solutions. The arguments for and against each method and the ramifications of each are summarized in Figures 6.10 and 6.11 and pertain to considerations of the solubility of each chemical constituent and the role of oil droplets. In the study by Forth et al. (2017a,b), WAFs prepared by the variable dilution approach presented concentrations (linear with dilution) and compositions as expected in the measured prepared exposure solutions compared with what was estimated based on chemical solubility. Furthermore, in this study the role of
microdroplets modulating WAF chemistry was highlighted emphasizing the need to differentiate between dissolved and particulate oil. Preparations of these fractions were achieved using filtration through a stacked GFF (0.3 μm) procedure resulting in a filtrate that was considered the “dissolved” fraction with the unfiltered fraction representing the “total.” Test solutions were prepared using the same initial oil loading (i.e., 1 μ/L) and although LEWAF, CEWAF, and HEWAF preparations were similar in concentrations of dissolved TPAH50, the total TPAH50 concentration and composition in each preparation type varied widely (i.e., 195, 1667, and 5325 TPAH50 μg/L for unfiltered LEWAF, CEWAF, and HEWAF, respectively). Again, these data, as highlighted also by Redman and Parkerton (2015) show the importance of choosing and using appropriate analytical methods to quantify the concentration and specific composition of oil test exposure solutions.
The importance of oil partitioning (i.e., dissolved and particulate fractions) and its characterization including oil droplet sizes, are also new considerations from the original CROSERF methods highlighted by field research from the M/V New Carissa oil spill by Payne and Driskell (2003) and subsequently discussed in detail in the National Academies dispersant report (NRC, 2005). This need has been reiterated in subsequent reviews, reports and publications (Forth et al., 2017a,b; Redman et al., 2017; Sandoval et al., 2017; Mitchelmore et al., 2020a; NASEM, 2020). Partitioning of the oil constituents can influence oil fate processes, exposure routes, bioavailability, and ultimately toxicity. Oil droplets can cause physical toxicity, increase hydrocarbon bioavailability and uptake across membranes (i.e., due to dissolution; Ramachandran et al., 2004; Sørhus et al., 2015; Sørensen et al., 2017; Laurel et al., 2019) and can be ingested/filtered by certain species (i.e., zooplankton and oysters; Payne and Driskell, 2003; Hansen et al., 2018) resulting in species-specific impacts. Despite being flagged for nearly 20 years, there still remain many data gaps hindering a full understanding of the implications and importance of oil droplets in driving toxicity; further research in this area is recommended including methods to characterize droplet concentrations and sizes.
Many environmental variables that can influence the toxicity of oil (e.g., temperature and salinity) are controlled for and measured in toxicity tests. One variable that was not usually considered during toxicity testing is the influence of UV light (quantity and spectral quality). As discussed in Section 6.3.4.3, some oil constituents are more toxic to certain organisms under UV light compared to traditional laboratory lighting. The phototoxicity of certain oil constituents has been discussed at length in previous reports, but is still a relatively understudied area (Barron et al., 2004; NRC, 2005; NASEM, 2020).
One of the most important considerations and recommendations from the CROSERF working group was the requirement for detailed analytical verification and not the use of nominal exposures (i.e., a volume or mass of oil only), such as total petroleum hydrocarbons (TPHs) or total PAHs (TPAHs; Bejarano et al., 2014; Mitchelmore et al., 2020a). The focus and specific details required of the analytical methods have since been updated in various reports to reflect new analytical capabilities and also increased understanding regarding the importance of oil exposure routes and specific chemical compositions (NRC, 2005; Forth et al., 2017a; Mitchelmore et al., 2020a,b; NASEM, 2020). Comparisons across studies using different analytical measures is difficult
TABLE 6.2 Some Considerations on How Parameters Involved in Exposure Media Preparation, Conduct of Toxicity Tests, and Analytical Verification of Exposure Solutions Can Affect Test Results and Recommendations for the Minimum Reporting Requirement for Each
| (1) Exposure Media Preparation | ||
|---|---|---|
| Parameter | Considerations/Variables for Test | Suggestions for Minimum Reporting Requirements for Future Test Protocols |
| Vessel and laboratory conditions | Type of vessel, headspace to water volume (usually 25%), how sealed (usually closed to minimize loss of volatile components) and lighting (in the dark [i.e., dark room or covered with foil]) to reduce photolysis or photochemical reactions. | Specify vessel type used, volume of water, headspace volume, sealed/unsealed, and lighting conditions. |
| Type of oil, chemical dispersant | Environmental relevance (i.e., often more appropriate to use oil weathered to some degree and concentration) Dispersant: oil ratio depends on specific surface or subsurface application. |
Detail specific oil used including weathering state and specific dispersant used (e.g., CAS #). Detail oil/water and dispersant/oil ratios used. |
| Exposure water type | Oil concentration/composition in exposure solution (dissolution/partitioning) depends on water quality (i.e., salinity, temperature, pressure, presence of potential modifying/binding substrates [i.e., organic carbon, etc.]). | Report water used (freshwater [FW], saltwater [SW]) and salinity, if filtration was used (e.g., glass fiber filter [GFF]) and for artificial seawater report salt source. If natural waters are used, ideally report water quality measures (e.g., total suspended solids [TSSs], dissolved organic carbon [DOC], particulate organic carbon [POC], etc.). |
| Test concentration preparation method (also see Figures 6.9 and 6.10) | Variable loading (i.e., each test concentration prepared individually with increasing volumes of oil) or variable dilution approach (i.e., high-concentration stock solution prepared and test concentrations are dilutions from the stock). Approach depends on specific test objectives, WAF or CEWAF, volume of test media, and cost of analytical verification. | Report the preparation method used in detail. Conduct appropriate analytical verification of test solutions (e.g., concentration and composition). Report if one (and holding times/conditions if used multiple times) or multiple preparations of test media were used. |
| Specific WAF preparation method | Report if WAF, LEWAF, MEWAF, CEWAF, HEWAF prepared and specific mixing time, mixing energy (e.g., 25% vortex) and settlement time used. The oil concentration and composition are dependent on these parameters. | Report mixing times/energy and settlement time. Report in detail how solutions are removed from the test preparation chambers, whether they are used immediately and, if not, how they are stored and methods used to resample to ensure a continued homogeneous mixture is prepared. |
| Preliminary abiotic test run | OECD test (2019) requires that for difficult to-work-with substances, investigations into test solution stability is required. | Conduct a prior test to determine the stability and reliability of the test solution preparations, report chemistry (although these can be simpler methods [such as fluorescence detection] than used for analytical verification of definitive tests). |
| (2) Toxicity Test Type Considerations | ||
| Parameter | Considerations/Variables for Test | Suggestions for Minimum Reporting Requirements for Future Test Protocols |
| Specific test used | Acute or chronic test, standard method used (or based on [e.g., EPA, OECD, ASTM]). Test duration will affect toxicity. Inclusion of appropriate negative and positive controls so that results obtained reflect the test compound rather than a problem with the health of the species under study. Include solvent and test water-only controls if a solvent is used. |
Detail test type and length of time conducted. For standard tests report method number/reference. State any and all modifications/deviations from the standard test protocol. Use a positive control to ensure reliability of the test and include two controls if a solvent is used. |
| Species and life stage used | Choice of test species and life stage critical. Some species/life-stages are more sensitive to oil constituents than others. | Detail the name, source (e.g., culture, supplier or wild-caught) and give details of field collections (location, time, etc.). Detail life stage, age, and, if possible, sex of organism. |
| Test conditions | Static (non-renewal and renewal), flow through constant exposures and spiked/declining or pulsed exposures. An organism’s exposure will depend on the test type used given the dynamic nature of oil exposure media over time. Test concentration/composition will affect toxicity. | Report how the test is conducted and if and how exposure solutions are renewed over the time course of the experiment. Detail how the analytical verification of exposure solutions ties in with this and report nominal target and measured concentrations using an appropriate sampling plan to calculate average concentrations over time. |
| (2) Toxicity Test Type Considerations | ||
| Parameter | Considerations/Variables for Test | Suggestions for Minimum Reporting Requirements for Future Test Protocols |
| Additional toxicity test details |
Specify the water used (e.g., FW, SW, filtered or non-filtered); temperature and pressure will alter oil concentration/composition.
Size of exposure chamber: exposure solution volume and number of animals per chamber (inappropriate ratios of these may result in quickly declining concentrations of oil components and/or decline in water quality parameters [e.g., dissolved oxygen, build-up of ammonia] that may end up being covariables in the organisms’ response observed). Appropriate lighting for the test species and questions being asked. Normal laboratory lighting underestimates toxicity compared to lighting mimicking natural spectral quantity/quality, as some chemical constituents are phototoxic (e.g., more toxic under UV lights); alternatively other components undergo photolysis and are degraded. Photochemical oxidation products have been measured and toxicity is largely unknown. Feeding of organisms may affect results due to binding of oil components to the food (causing co-uptake by the organisms) or sedimentation to the chamber bottom and removal from organism exposure. |
Detail specific water used and salt sources. Also type of test chamber (material), size of test chamber, and exposure volume. Report number of replicate test chambers per treatment concentration and if appropriate the number of test subjects in a test chamber.
Conduct appropriate water quality tests for the species under study (e.g., salinity, pH, conductivity, dissolved oxygen, temperature and for vertebrate species ammonia). Measure lighting (i.e., spectral quality and quantity). For water renewals, feeding, cleaning, quality/lighting parameters, etc., give details about the time of day measurements were taken; suggest keeping measurements to the same time each day. Report the day/night regime used. Report aeration and feeding regime used, how tanks are cleaned, and how cleaning (if any) occurs in relation to how solutions are renewed. Report when and if and how dead organisms are removed. For chronic tests, report how (if) test chambers are cleaned to prevent biofilm/fouling. |
| Preliminary range-finding versus definitive toxicity tests | Conduct a preliminary range-finding test using log doses over a wide range of concentrations. This will establish the approximate concentration range for effects. For a definitive test recommended to use a 2×–3× concentration range (for more accurate statistical analyses). | A preliminary test is recommended so that the concentrations used in a definitive test bracket the toxicity test thresholds appropriately. |
| Biological endpoints and statistics |
Appropriate endpoints for the type of toxicity test (acute; LC50 or chronic; EC10, NOEC, etc.). TU approach for WAF and include time endpoints (e.g., LT50).
Various statistical approaches may be taken and depend on the specific dataset. |
Report appropriate toxicity thresholds for the type of test and what the test is being used for (regulatory, model data, etc.).
Report raw data (use supplementary information in publications) and detail the specific statistical methods used. Report any problems with the dataset and why alternate methods (if any) are used. |
| (3) Analytical Approaches | ||
| Parameter | Considerations/Variables for Test | Suggestions for Minimum Reporting Requirements for Future Test Protocols |
| Analytes quantified |
The concentration and specific composition of oil has been reported in numerous ways (e.g., TPH or TPAH, using a variety of numbers of parent and/or alkylated PAHs).
What is reported may have ramifications for the ability to compare across studies and for utility in toxicity models and may ultimately over- or underestimate toxicity (see Figure 6.11). The use of nominal concentrations of oil provides limited information with respect to toxicity of the resulting exposure solutions. |
Analysis of VOCs (e.g., BTEX). Suggest to report as total PAH (50+) including alkyl homo-logues (rather than TPH). For CEWAF/HEWAFs, suggest titration (i.e., GFF) or centrifugation to determine chemical composition in the dissolved and particulate phases. If possible, also measure droplet size distribution (plus volume/quantity). The use of nominal exposures is unacceptable. |
| Oil droplet quantity and size | Oil droplets may be an exposure route for some organisms (e.g., filter feeders, incidental ingestion when drinking/feeding), some of which may feed on oil droplets depending on their size. | LISST/coulter counter analysis to determine droplet quantity (volume) and size distribution. Also important for comparison to field measurements. |
| Tissue residue and/or metabolites | Toxicity is defined by internal exposure and thus measuring TPAH in organism tissues relates internal exposure (body burden) to toxicity endpoints. In vertebrate species (e.g., fish), direct relationships between the extent of internal exposure to oil and bile metabolites have been shown in laboratory and field tests. |
Measurement will depend on the objective of the test (e.g., bioaccumulation study) and the costs involved. Determining bile metabolites also depends on the questions (e.g., validation with field specimens to estimate exposure). |
SOURCE: Modified and expanded from a table in Mitchelmore et al. (2020b).

SOURCE: Figure modified and expanded from Figure 28.2 in Mitchelmore et al. (2020b).
(e.g., using TPH versus TPAH [and how many individual analytes are measured]) and many studies have only measured the parent and not the alkylated PAHs. Expansions of this analytical effort can also include the identification and quantification of the additional unresolved hydrocarbons that can contribute to toxicity.
As recently highlighted in the National Academies dispersant report, traditional exposure metrics (i.e., TPH and TPAH) do not account for the variation in the toxicity of individual hydrocarbons. The use of toxic units (TUs) has been proposed (NASEM, 2020; see discussion of modeling in Section 6.7). Even without these new insights into the importance of specific chemical composition, CROSERF methods significantly improved the ability to compare across studies—although nominal exposures or inappropriate analytical verification continue to be used and can result in both under- and over- estimations of toxicity (see Figure 6.12).
In addition to how exposure test media are prepared and characterized, and the results interpreted, consideration must also be given to the biological endpoints that are measured and when (see Figure 6.13). For example, acute toxicity tests are often conducted for 48–96 hours depending on the species, which may not be reflective of the typical exposure time observed in an oil spill event. Two approaches to address this include shorter-duration tests or assessments at multiple earlier time points than the terminal test duration. This latter approach is useful to determine time-to-death metrics. Technological advances in molecular biology have also resulted in a new understanding of mechanisms of action and impacts to organisms. The use of additional biological endpoints, besides just the typical metrics used in risk assessments (i.e., mortality, growth, and reproduction) should be investigated further particularly with respect to their underlying assumptions and relevance to individual-, population-, and ecosystem-level impacts.
6.4.2 Challenges Regarding the Environmental Relevance and Field Applicability of Laboratory Toxicity Tests
As summarized in Figure 6.14, a variety of studies are used to study the impacts of oil on marine organisms, and all vary in their complexity, ability to control variables, and field relevance (see Box 6.5). Although laboratory tests cannot replicate or mimic field conditions during an oil spill they are an important tool in estimating potential effects. The generation of reproducible and reliable toxicity test results is critical so that implications of exposure and its relationship to toxicity can be established and compared across studies to determine potential impacts from an oil spill, identify sensitive species, and calculate the relative toxicity of different oils and/or dispersant mixtures.
SOURCE: Modified and expanded from Mitchelmore et al. (2020b).
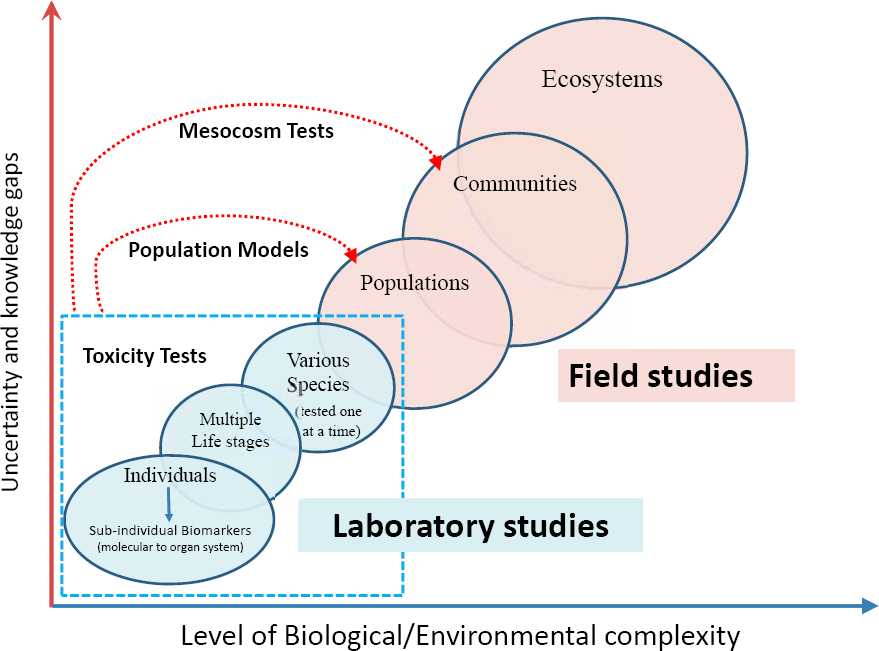
Toxicity data are used for the development, calibration, and validation of predictive toxicity models (i.e., target lipid models) to provide predictions of oil toxicity (e.g., OilTox, PETROTOX; see Section 6.7) and species sensitivity distributions. As discussed earlier, toxicity tests provide information for basic mechanistic science, if realistic exposures (concentrations/time) under pertinent and applicable environmental conditions and (if operationally relevant) chemical dispersant concentrations are used, then toxicity results can be used for oil spill response and planning and ultimately to provide tools (models) for real-world applicability in operational decisions. In summary the choice of methodological approaches used in toxicity tests has limited the utility of many studies for inclusion in toxicity models and hindered assessments of comparability across studies and accurate predictions of toxicity of petroleum hydrocarbon constituents, oil mixtures, dispersants, and dispersed oil (Bejarano, 2018; Mitchelmore et al., 2020a,b).
More generally, NRDA studies provide most of the factual basis for quantifying the environmental damage caused by oil spills. The results are used to guide oil spill response and restoration efforts, and to assess any legal liability of the responsible party (RP). The scope of these studies is usually determined collaboratively by the RP and government trustee agencies, which then use scientifically rigorous methods to address the concerns identified within the agreed scope. Advantages of this approach include study designs informed by both industry and government expertise, engagement of both government and industry personnel and resources in the conduct of these studies, and avoidance of duplicative efforts associated with adversarial approaches. Disadvantages include the sometimes lengthy process required to reach agreement on study scope, objectives, and design, which may impair identification and exploitation of ephemeral opportunities to collect critically important data for the eventually identified scoping objectives; offer scant formal capacity for recognizing evidence of environmental injury from damage pathways that are not well-understood; and use study designs that may favor consensus-point estimates of environmental damage at the expense of quantifying the associated uncertainty, and that may be prone to unquantified underestimation bias. Consequently, conclusions of some NRDA studies may not be as accurate as conclusions informed by additional data from non-NRDA sources (including data collected by non-NRDA entities before, concurrently, or after NRDA study initiation), and by differing assumptions and methods used for the data analysis.
6.5 EFFECTS ON POPULATIONS, COMMUNITIES, AND ECOSYSTEMS
While oil pollution can affect organisms in a variety of ways, as summarized in Sections 6.2 and 6.3, the likelihood and severity of these effects may vary considerably during and after a particular oil spill or other oil discharge events. The most serious effects may significantly alter population-level fitness or growth rates, which in turn may perturb the ecological communities within which these populations interact. Conversely, other effects, such as mass fish mortalities from short-term acute toxicity, occur infrequently, even after very large spills such as the Exxon Valdez or the DWH.
Often the most serious effects of oil spills and discharges are obvious, such as widespread mortalities of heavily oiled seabirds, marine mammals, or sea turtles. Less obvious effects typically require carefully designed field studies to detect them, and are necessary to quantify any effects, obvious or not. Unfortunately, while oil spills, especially large ones, present unique opportunities to evaluate the actual effects that may result from possible toxicity mechanisms, exploiting these opportunities is exceptionally challenging. Oil spills usually occur without prior notice, and opportunities to collect crucial information for damage assessment and effects research studies are ephemeral. Initial field study designs are often crafted in a crisis atmosphere, and accorded lower priority than human safety and response efforts to limit property and environmental damage. The most qualified research personnel initially available may have little familiarity with the oil pollution effects literature, so initial data collection efforts may be less than optimal, although usually any sampling immediately following an oil spill or other discharge event is preferable to not sampling at all. Yet studies of how ecosystems respond to the strong ecological perturbations caused by large marine oil spills present unparalleled opportunities to gain insights into marine ecosystem structure and function, in addition to how organisms and populations respond to the various ways that oil can cause toxicity. Research is needed that couples oil toxicity responses in organisms to populations.
Recognizing the unusual and often stressful conditions that confront oil pollution response, damage assessment, and research personnel, we begin this section with a summary of the limitations and challenges involved when designing and executing field studies to evaluate oil pollution effects (see Section 6.5.1), in the hope that this will be useful for those faced for the first time with responding to future oil spills or evaluating their consequences. Following this, we summarize effects that have been clearly established during oil spills and discharges, beginning with the most vulnerable habitats, the sea-air interface and shorelines (see Sections 6.5.2 and 6.5.3, respectively), where oil is mainly compressed into the two horizontal dimensions. Following this are coral communities (see Section 6.5.4) because of their sensitivity to oil and benthic communities (see Section 6.5.5), where oil may again be largely constrained to two horizontal dimensions, but less likely to be as heavily oiled as the sea surface. Finally, we consider effects in the water column (see Section 6.5.6), where oil contamination is usually much lower than oiling at the sea surface and along shorelines. We conclude with a summary of ecosystem-level effects, which may occur when populations of species that strongly interact with oil-affected species are themselves strongly affected through these interactions.
6.5.1 Limitations and Challenges
One message from the Oil in the Sea III (NRC, 2003) report, which was not necessarily new then or now, is that it becomes increasingly difficult to identify effects or recovery or both in a progression of individuals to population to community levels within an ecosystem (see Figure 6.1). For example, the clearly identified toxic effects of oil from the DWH oil spill on marsh nekton in some field studies and most laboratory experiments (Whitehead et al., 2012) were not necessarily reflected in adverse negative effects on populations of these same fish within the marsh habitat (Fodrie et al., 2014; Able et al., 2015), when populations of marsh and other coastal fishes increased 1–2 years after the spill (Schaefer et al., 2016). Similarly, sea grass nekton were as abundant or increased in abundance within 1 year of the spill in oiled and unoiled sites, with no change in fish assemblages (Fodrie and Heck, 2011). It is even more difficult to extrapolate to ecosystems that will be identified as “stressed” or “recovered” after 5–10 years of potential recovery. Similar effects on changes in complex aquatic communities and ecosystems are confounded by other changes over time that may or may not be related to the initial hydrocarbon exposure (e.g., multiple stressors several years from the initial exposure).
6.5.1.1 Multiple Stressors
The inputs of petroleum into marine environments occurs with other environmental stressors, so the impact of oil will depend on the interactions of oil and influences of various potential co-stressors as a complex multiple stressor event. As an example, the 2010 DWH oil spill in 1500 m water depth off the Mississippi River delta occurred against a background of various other stressors, which cause chronic hydrocarbon contamination for the northern Gulf of Mexico. These stressors included natural deepwater oil and gas seeps, a heavily industrialized oil and gas production and transport system, coastal petroleum activity onshore, regular marine transportation of petroleum and non-petroleum products (Garcia-Pineda et al., 2014), and a recurring oil leakage from a damaged well (MacFayden et al., 2014). The area of the DWH oil spill is influenced by freshwater discharge, nutrient loads, and sediment flux from a major river, the Mississippi, high rates of sea-level rise, shoreline erosion, loss of coastal habitats, and coastal systems perennially exposed to petroleum from oil and gas production and transportation (Boesch, 2014). Laboratory experiments usually conclude in mortality, genetic impairments, or indirect effects, such as the inability to locate food. The ability to transfer laboratory experimental results to populations, communities, or ecosystems is not straight forward. Extrapolation of these experimental and field effects does not necessarily lead to accurate estimates or calculation of impacts on higher trophic levels or complexity of organismal organization. The ability to disentangle multiple stressors from petroleum impacts may be difficult (e.g., Turner et al., 2016).
6.5.1.2 Baseline and Long-Term Data
It is challenging to identify or acquire population-, community-, or ecosystem-level characterizations and ancillary chemical data prior to an oil spill, as well as to identify appropriate existing baseline data from existing databases for comparison to an ongoing spill or exposure to other oil sources.
Baseline data include:
- numerical data, such as existing concentrations of petroleum hydrocarbons and their reaction products, and the density and diversity of potentially affected populations, prior to oiling, and
- knowledge of biological, chemical, physical and geological ecosystem processes relevant to the responses to oil spills (see Chapter 4), and the fates and effects of oil inputs, including those relevant to human health (see Chapters 5 and 6).
Several U.S. agencies developed programs to collect baseline data prior to Outer Continental Shelf oil and gas drilling on continental shelves (e.g., the U.S. Department of the Interior, first Bureau of Land Management, Minerals Management Service, now Bureau of Ocean Energy Management) (e.g., Flint and Rabalais, 1981; Murray, 1998). There were appropriate long-term data sets from the Louisiana shelf low oxygen area that provided a comparison of low oxygen
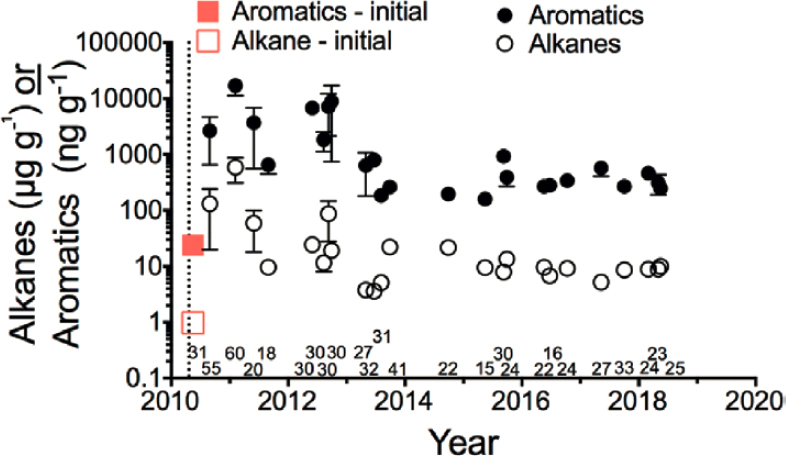
NOTES: The red squares are the samples taken before oil invaded the marsh. The vertical dotted line represents the start of the DWH oil spill offshore. The sample numbers averaged for each sampling trip are below each symbol (along the x-axis) and are the same for alkanes and aromatics. (The targeted 28 alkanes and 43 polycyclic aromatic hydrocarbons and their respective alkyl homologs [18 parent PAHs and 25 alkyl homolog groups] are provided in Tables 1 and 3 of the Supplemental Information.)
SOURCE: Turner et al. (2019), CC BY NC ND.
conditions in the spring and summer of the DWH oil spill (Rabalais et al., 2018). Appropriate historical hydrocarbon data were available from Gulf of Mexico coastal ecosystems (e.g., Wade et al., 1988). The U.S. EPA’s Environmental Monitoring and Assessment Program3 followed later by the U.S. EPA’s Aquatic Resource Surveys,4 collected some appropriate baseline data for assessing general environmental quality. Prior to the Exxon Valdez spill, baseline data documented regional background concentrations of hydrocarbons (Karinen et al., 1993). Additionally, the National Institute of Standards and Technology (NIST) maintains a bank of frozen marine mammal tissues collected from “natural” stranded animals throughout the United States such that comparative studies can be undertaken after an oil spill.
Baseline, and especially the term benchmark that was used at one time in the Outer Continental Shelf (OCS) programs, invoke interpretations involving a static situation. It is important that the concept of an evolving baseline of data and knowledge be adopted relevant to inputs, fates, and effects of oil inputs to the sea and to inform oil spill response and damage assessment activities. It is known that numerous ecosystems in coastal and continental margin waters are in a state of change as a result of changing climate; human activities, such as commercial and recreational fishing and waste disposal to coastal waters; and invasive species. These programs have evolved with time, in part in response to reviews and suggestions of the programs’ Scientific Advisory Committees and also in response to reviews and recommendations set forth in reports of committees of the National Research Council (see, e.g., NRC, 1993).
Appropriate baseline data can be acquired prior to the interface of spilled oil within an environment, given enough response time and reasonable model predictions of where the oil may reach a shoreline. In anticipation of oil from the DWH spill reaching Louisiana marshes, a group of researchers collected a suite of samples from several salt marsh sites in southeastern Louisiana for typical marsh populations and hydrocarbon composition (Turner et al., 2019). Data for marsh sediment hydrocarbons provided a historical context, a measure of peak oil exposure, the peak sediment concentrations when DWH oil residue reached the marsh sediments, and a post-oiling period of reduced contamination (see Figure 6.15). The return to “pre-spill” hydrocarbon conditions, however, remained elevated for at least 8 years (i.e., a new baseline).
6.5.1.3 Inability to Achieve Appropriate Experimental Designs
An oil spill does not affect coastal and offshore waters and intertidal coastlines uniformly; there are differences in the amount of oiling (heavily oiled versus lightly oiled) and concentrations of various constituents and levels of
___________________
3 See https://archive.epa.gov/emap/archive-emap/web/html/index.html.
4 See https://www.epa.gov/national-aquatic-resource-surveys.
toxicity of the encroaching oil residue. Also, the duration, frequency, and mechanisms of exposure (see Section 6.3) will generate variable levels of contamination. There is also the complication of presumed exposure on communities such as phytoplankton and zooplankton near, or even within, an area of an oil spill, without adequate exposure data for the hydrocarbon residues. Just because an oil spill traversed a body of water at one time does not imply similar exposure or contamination of the plankton and nekton communities in the same or nearby locations or predicted new location (based on circulation, known or modeled). This understanding is critical for determining effects, potential effects, or no effects. The ability to reference long-term data may help alleviate some of these problems (Rabalais et al., 2018).
The lack of comparable habitats, other than oil exposure, which is not uniform, is a daunting complication for experimental design. There is seldom an adequate “control” for habitat comparisons following exposure to oiling. Placing a control site in a rocky intertidal habitat that was more quiescent with regard to waves or was less exposed compared to a high-energy rocky intertidal habitat that was heavily oiled is inappropriate (see the Environmental Sensitivity Index in Appendix G). A similar situation would exist with oiling of a salt marsh margin in a highly eroding environment subject to more frequent and higher wave characteristics compared to an opposite shoreline that is more sheltered with regard to meteorological conditions and has a lower erosion rate. These challenges can be mitigated by matching reference and oiled sites for important environmental factors, and by randomizing pairings between reference and oiled sites that have closely similar factors. In addition, sampling designs of field studies should ensure that there is adequate statistical power to detect effects that actually occur, because conclusions of no effects based on sampling designs that have low power to detect those effects may be misleading (e.g., Peterson et al., 2001).
6.5.1.4 An “Open” Ecosystem
Communities and ecosystems change substantially over time, and may not remain the same before and after exposure to an oil spill. There is also a notably high variability in physical and biological characteristics against which to define a “difference,” even if the best data on exposure are available.
Motile organisms, such as marsh nekton, are difficult to monitor relative to effects of exposure to oil in high-exposure field conditions that lead to an observed decline in densities or change in the composition of the nekton community (Fodrie et al., 2014). They can move among marsh microhabitats such as marsh tidal creeks, marsh surface, or marsh ponds, depending on inundation levels, and their marsh microhabitats may not be related to the presence of oil or its residues. Other dynamics that might hide negative population impacts are high spatiotemporal variability, behavioral avoidance, compensatory pathways, and temporal lags. However, “positive” density responses can also be a warning of disruption if changes in population age structure releases juveniles from competition with or predation from older and larger individuals and species, such as appeared to have occurred with Gulf menhaden following the DWH oil spill (Short et al., 2017). In addition, results from subtle, long-term effects to ecosystem damage caused by loss of habitat and elevated oil contamination in sediments for decades may not yet be recognizable (Zengel et al., 2021).
Less mobile organisms, such as attached epifauna or benthic infauna, truly are exposed to hydrocarbon constituents of known or unknown toxicity. This is why they are often considered the “canary in the coal mine,” because they cannot escape the exposure. Benthic infaunal communities are notoriously variable in their composition and relative abundance and require numerous time-consuming replications to detect statistical differences. In other instances, visual analysis of photographs or videos can capture these differences (see Box 6.6).
6.5.1.5 Before-and-After Controlled Experiments Versus Inferential Observations
A clear-cut experimental design that defines the effects of an oil spill on organisms or habitat based on conditions before and after the exposure, or in a control-versus-exposure comparison, is preferred over less definitive results or inferential observations. The latter are more likely than the former in the case of an oil spill under field conditions. Many efforts to detect environmental damage do not provide results with statistical significance to reject a null hypothesis of no difference—even when it is false—nor can these studies make the connection between observed decreases in densities following anthropogenic disturbance and a specific event.
The difficulty in identifying effects on higher organization of biological structure—populations to ecosystems—continues. Longer-term funded studies of populations, communities, and ecosystems that combine ecosystem-level processes and interactions over years to decades are necessary to better understand those relationships.
6.5.2 Air-Sea Interface
Some of the most immediate and serious effects of oil discharges occur when oil spreads across the sea surface as a sheen or oil slick. Seabirds are frequently the most vulnerable, as attested by the photographs of oiled birds that often accompany media reports of even small oil spills. Other vulnerable groups include marine mammals, sea turtles, fish that gulp air at the sea surface to inflate or maintain pressure in their swim bladders, and floating marine vegetation. Buoyant eggs released by some species of fish and other marine organisms that spawn at sea may rise to the surface, bringing them into contact with surface oil slicks when present.
Contact with oil released in large oil spills can cause mass mortalities of seabirds, marine mammals and sea turtles. The Exxon Valdez incident is a stark example of this: up to 375,000 seabirds and 5,500 sea otters may have died from acute exposure to oil (Bodkin and Udevitz, 1994; Ford et al., 1996). More recently, sea turtles were regularly observed trapped in the oily “mousse” entrained in the windrows of the Gulf of Mexico during the DWH oil spill (Stacy, 2012), and more than 400,000 surface-pelagic juveniles were estimated to have been oiled in that spill (McDonald et al., 2017). NRDA studies following the DWH oil spill estimated that up to 7,600 large juvenile/adult sea turtles and 166,000 small juvenile sea turtles were killed (see Box 6.7), 1,141 dolphins died from March 2010 through July 2014, and hundreds of thousands of birds across 93 species were killed by the spill (Haney et al., 2014a,b; DWH NRDA Injury Assessment, 2015). Although during an oil spill response efforts are made to capture, rehabilitate and document the overlay between the presence and distribution of wildlife and oil, direct observations of all potentially exposed and impacted organisms are limited both spatially and temporally. To address this limitation following the DWH incident, DWH trustees employed a number of different techniques to best estimate overall impact to the environment—including novel model-based approaches (DWH Natural Resource Damage Assessment Trustees, 2016). These injury determination efforts extended for many affected taxa, with the mission to
quantify exposure and injuries to resources where direct measurement was infeasible given the scope of the incident. For example, numerical modeling was employed to quantify injuries to nearshore resources based on exposure to toxic concentrations of oil, and to quantify injuries to marsh fauna such as flounder and shrimp.
For higher vertebrates (e.g., birds, marine mammals, and sea turtles), very different approaches were employed for each. In birds, a combination of observational data, laboratory models, field studies, and three models (Shoreline Deposition, Offshore Exposure, and Live Oiled Bird models) were blended to get the best estimate possible for the “impact” in the various species in the Gulf. For marine mammals, historical distribution data were combined with stranding records and an extensive case-control study for dolphins in Barataria Bay to establish estimates of injury (see Section 6.3.3 for detailed findings). Due to the extent of these assessments, specific details of each cannot be adequately covered in a review such as this, and the reader is directed to the full DWH Final Programmatic Restoration Plan for details. However, as an example of the detailed modeling efforts that can be undertaken as a consequence of SONS-level incidents (and how those then inform scientific knowledge), Box 6.7 details the modeling accomplished for sea turtles, a taxa of particular concern in this region due to difficulty ascertaining observable injury coupled with their conservation value, especially in species like turtles (Shigenaka and Milton, 2003).
Although such large-scale petroleum releases can be catastrophic, it should also be noted that chronic, comparatively low-level releases into the marine environment can take a significant toll on wildlife populations. Wiese and Robertson (2004) estimated that, between 1998 and 2000, the illegal discharges of oil from ships caused an average of 315,000 ±65,000 alcid deaths annually in southeastern Newfoundland. Similarly, the S.S. Jacob Luckenbach (see Box 3.5), which sank off of San Francisco, California, in 1953 with 457,000 gallons of bunker fuel, was found to be leaking sporadically over the years and to have killed 51,569 birds and 8 sea otters between 1990 and 2003 (Luckenbach Trustee Council, 2006).
Impaired ability of seabirds, marine mammals, and sea turtles to find and capture food or to avoid predators resulting from sub-lethal effects of exposure to oil (see Section 6.3) may result in delayed mortalities, and these may lead to underestimates of population losses. Afflicted individuals may not die until months or, for long-lived marine mammals or sea turtles, years following oil exposure. These individuals may move considerable distances between exposure and death, so the place where they die may be far removed from the oil-contaminated region, which increases the likelihood that the death will not be attributed to oiling. More generally, attributing the cause of death in oil-exposed seabirds, marine mammals, and sea turtles is complicated by the possibility of several mechanisms of toxicity contributing to eventual mortality. These complications may also apply to fish such as herring and other physostomous fish that may ingest slick oil while gulping air at the sea surface (e.g., Price and Mager, 2020). Widespread mortalities of seabirds, marine mammals and sea turtles may have population- and community-level effects (see Section 6.7); understanding of these effects could be improved by conducting studies focused on better estimation of these mortalities during future spills of opportunity.
The pelagic brown alga Sargassum spp. forms biogenically structured habitat for high biodiversity (including endangered and threatened species) and productivity in surface waters of the western Atlantic Ocean, the Caribbean Sea, and the northern Gulf of Mexico. A large section of the Gulf of Mexico’s floating (S. natans and S. fluitans, considered a single complex) was exposed to the immense pool of oil from the DWH oil spill. Theses oiled Sargassum mats were treated with aerially applied Corexit 9500 A dispersants. Aerial surveys each over the same 3,100 km2 of ocean surface from the panhandle of Florida to the Chandeleur Islands, Louisiana, were completed in 2010; these documented extensive co-occurrence of oil/dispersant and Sargassum (Powers et al., 2013). The surveys documented that Sargassum abundance was less during the oil spill in 2010 compared to a 4-fold increase in abundance of Sargassum in 2011 and 2012. A delta-lognormal approach for aerial data from fishery-independent data was applied to determine abundance (Powers et al., 2013) from the aerial survey data, which take into account non-zero observations with positive sightings (Lo et al., 1992; Maunder and Punt, 2004).
Buoyant eggs of fish and other marine organisms may directly contact surface oil slicks, promoting uptake of PACs dissolved in seawater immediately beneath the slick, or directly from the surface slick oil. Because fish embryos are extremely vulnerable to adverse cardiotoxic effects following exposure to dissolved PAC concentrations of less than 1 μg/L (see Section 6.3.4.2), even brief exposure may have lethal consequences for developing fish embryos.
Confirming evidence from field studies regarding the effects of surface oil ingested by fish or of cardiotoxic effects of PACs from surface oil on developing fish embryos is lacking; future spills of opportunity could be used to conduct such studies.
6.5.3 Shorelines
Regardless of whether an oil spill impinges on an intertidal shoreline from a surface spill or a submerged spill, these areas will show different hydrocarbon composition of the oil, depending on the weathering of the oil from its source to the shoreline. Weathering of the oil will vary with the length of trajectory times from a subsurface oil input to a surface expression, through the many fates outlined in Chapter 5 or through mitigation measures. Similarly, weathering of the oil at the surface, time to deposition at a shoreline, subsequent degradation in the exposed shoreline, and additional mitigation efforts will change the composition of the oil. Thus,
there are many intertidal shorelines that may be affected by different contaminant compositions and levels of exposure.
There is an abundance of historical documentation of the effects of oil spills on coastal areas across a range of latitudes. Some garnered a high level of both fates and effects studies (e.g., West Falmouth, Massachusetts), because there were scientists with expertise, technical support, and scientific curiosity associated with the nearby Woods Hole Oceanographic Institution (see Box 6.8). Others were much larger in volume, but also located near a research institution, such as the Galeta oil spill near the Smithsonian Tropical Research Institution in Panama. Two were within the top five accidental marine oil spills in volume and also well funded for research: the Exxon Valdez tanker grounding in Prince William Sound, Alaska, in 1989; and the DWH catastrophic explosion and subsequent expulsion of oil from the uncapped wellhead in 2010.
The National Oceanic and Atmospheric Administration Office of Response and Restoration (NOAA, revised 2019) developed an Environmental Sensitivity Index (ESI) (see Section 4.2.1.3 and Appendix G). A rank of “1” indicates shorelines with the least susceptibility to damage by oiling. Such as steep, exposed rocky cliffs and banks. Waves and tidal action will quickly wash the oil off the rocks; the oil cannot penetrate into rocks. A rank of “10” indicates shorelines most likely to be damaged by oiling. These include protected, vegetated wetlands, such as mangrove swamps and salt marshes.
6.5.3.1 Salt Marshes (ESI 10)
The DWH oil spill represents the largest accidental marine oil spill in volume (McNutt et al., 2011) and as measured by shoreline length (Nixon et al., 2016). The broad scope of research, length of continued study, and funding by multiple agencies and industry (BP and other operators) generated immediate data on the effects of oil on coastal marshes, as well as longer-term observations. The National Science Foundation Rapid Response Research program and initial funding to the five Gulf states from BP placed researchers in the field to collect pre-spill salt marsh data before the oil came ashore. The Gulf of Mexico Research Initiative (GoMRI) developed and guided a 10-year, $500 million research program on the DWH oil spill (Zimmermann et al., 2021).
The oil residue-water mixture began coming ashore in mid May 2021 (Turner et al., 2019) after a 1-month period in offshore surface waters. “Fresh” oil continued to be deposited in salt marshes through September 2010, as seen in the sharp rise in total target aromatics concentrations in marsh sediments within Barataria Bay, compared to the previous three months (see Figure 6.15).
Several features of the marsh oiling from the DWH were that (1) pre-spill petroleum concentrations in sediment samples were determined and followed at the same locations for 9 years (see Figure 6.15), (2) consistent gas chromatography-mass spectrometry (GC-MS) analytical chemistry with the same total targeted aromatics and total targeted alkanes was applied to all marsh sediment samples (at least for the research conducted by the Coastal Waters Consortium, GoMRI [Turner et al., 2014]), (3) tropical storm activity resuspended and redistributed the oil, especially during Hurricane Isaac (Turner et al., 2014) (see Figure 6.20), and (4) recovery occurred at different intervals for different marsh and marsh community features, and some were not considered recovered even after 9 years (Zengel et al., 2021).
Researchers have been following the fate of No. 2 fuel oil from the grounded tanker barge Florida since 1969 (see Box 6.8). Study of the long-term fate of the oil, and elucidation of continuing effects, was undertaken 30 years after the spill. As with the original studies of the West Falmouth spill, these latter studies combined the latest in analytical chemistry methodology with the latest in biological/ecological studies.
6.5.3.1.1 Effects on Marsh Vegetation and Stability
Early assessments for the DWH oil spill in two southeast Louisiana salt marshes (Barataria Bay and Terrebonne Bay) in July 2010 found no signs of living vegetation, only dead stems above the exposed oiled sediments, which clearly documented the dieback of all marsh vegetation in heavily oiled marsh (Lin and Mendelssohn, 2012; Silliman et al. 2012; Zengel et al., 2015) (see Figure 6.22). Vegetation cover after 7 to 16 months continued at much lower levels than in the control sites (Silliman et al., 2012; Lin and Mendelssohn, 2012, respectively). The average live aboveground biomass combined of Spartina alterniflora (smooth cordgrass) and Juncus romaeriaus (black needle rush)—dominant species in the marsh—was significantly lower, almost none, in the heavily oiled marsh compared to reference marshes. Moderately oiled marshes fared better, with no significant difference between the combined biomass by weight of the dominant species with that in the reference marsh. However, the live aboveground biomass and stem density of Spartina was about 10 times as much as it was for Juncus in the moderately oiled marsh.
All metrics indicated initial impacts from oiling and most showed recovery time frames of several years or more for Spartina. Spartina stem density was the exception, with more rapid recovery due to possible stimulation by unoccupied space and perhaps residual oiling (inference); however, increased stem density was not leading to comparable increases in cover or biomass (Lin and Mendelssohn, 2012). In contrast to Spartina, Juncus was affected to a greater degree, with much slower or lack of recovery. In comparison to the marsh edge, the oiled marsh interior tended to have a lesser degree of impacts, at least initially. Impacts from oil were eventually detected in the interior, which saw similar recovery to the marsh edge. Complete vegetation recovery was not observed after 9 years, especially for marshes with a Juncus component and for belowground biomass (Zengel et al., 2021).


SOURCE: Photos from Coastal Waters Consortium, Gulf of Mexico Research Initiative.
The common reed, Phragmites australis, is the dominant vegetation in the Mississippi River bird-foot delta and was exposed to DWH oil in 2010 (Hester and Willis, 2011; Shapiro et al., 2016). Field assessments were minimal and there was little exposure data other than qualitative categorization by shoreline cleanup assessment technique (SCAT). Shapiro et al. (2016) examined AVIRIS data for Phragmites marsh for 2010, prior to the oil spill and in 2011 post-spill. They documented minimal change in percent coverage of photosynthetic vegetation (an increase of 2.6% from 2010). In contrast, Zhu et al. (2013) indicated that there were detrimental effects to reed communities related to increasing hydrocarbon concentrations in an area of high oil production in the Yellow River delta. In greenhouse mesocosm experiments with application of weathered and emulsified Macondo oil (DWH) to Phragmites aboveground shoots, Judy et al. (2014) demonstrated that across a range of oil exposures and repeated shoot oiling, there was no major impact on overall plant growth. A factor in the lack of response is that the plants developed side branches that compensated for any vegetative stress. When the oil treatments were applied to the soils in the mesocosms, the total belowground biomass was highest in the controls and decreased with increasing oil dosage. However, there were no oil effects on dead root biomass, live rhizome, or live above- and belowground total biomass. At least for marsh vegetation in southeastern Louisiana exposed to DWH oil, there is a higher to lower sensitivity—from Phragmites to Juncus to Spartina.
6.5.3.1.2 Shoreline Erosion
Southeastern Louisiana is a location for high conversion rates of coastal land to open water, with the average land loss rate of 42.9 km2 y–1 from 1985 to 2010 (Couvillion et al., 2011), although higher in prior decades. Marsh shoreline erosion is a significant factor in “recovery” following an oil spill because it results in emergent vegetation seldom being reestablished after oiling (Silliman et al., 2012; McClenachan et al., 2013; Zengel et al., 2015; Rangoonwala et al., 2016; Turner et al., 2016).
McClenachan et al. (2013) identified 30 sites along a north shoreline in Bay Batiste of the northern Barataria Bay, Louisiana, estuary that ranged from “low” oil (<200 μg kg–1 PAHs, most without the Macondo oil chemical signature) to “high” oil (>20,000 μg kg–1, all with the Macondo oil chemical signature). The location of these sites along a similar shoreline negated the differential effects of wind fetch and erosion potential that might affect erosion rates. Measurements of shoreline erosion, soil strength, percent vegetation cover, sediment PAH concentrations, and marsh overhang (distance from marsh edge under which the sediment has sloughed off into the adjacent water) taken in November 2010 to assess shoreline health. High oil sites showed significantly higher marsh overhang than observed at the low oil sites, with the exception of one location. The upper 50 cm of both high and low oil sights showed similar oil shear strength, but the shear strength in the high oil sites below 60 cm was much lower than the low oil sites. Soil shear strength demonstrates a decomposition of root structure in anaerobic, oiled sediments that would leave the marsh structure less strong and more likely to be eroded by wave energy (Turner et al., 2016). Although the marsh platform appeared healthy, it was falling apart below ground. During four of the five sampling time frames the percent of Sporobolus alterniflora vegetation cover showed no significant differences. After this period, the promontories at the low oil sites, produced by erosion of neighboring oiled sites, began to erode because of exposure to wave action. Moreover, the erosion rates attributed to DWH oil averaged greater than the long-term average for the area.
Turner et al. (2016) focused on the erosion rates at distinct oiled and unoiled islands in three estuaries of southeast Louisiana. In the first 6 months after oiling, erosion at oiled islands increased by 275% and for the first 2.5 years after oiling, were 200% of that of the unoiled islands. These erosion rates were 12 times as high as the average land loss in the deltaic plain of 0.4% per year from 1988 to 2011 (Turner et al., 2016).
Assessments of oil spill damage to marshes typically include percent cover by plants, species of vegetation, aboveground live biomass of vegetation by species, canopy height, and sometimes below ground living/dead vegetation biomass. This suite of measurements, along with hydrocarbon concentrations, may document damage and recovery of the dominant vegetation of marshes that were impaired by an oil spill (e.g., Lin and Mendelssohn, 2012; Silliman et al., 2012; Zengel et al., 2015). These measurements, however, may not reveal the overall health of the marsh. Live belowground biomass is an indicator of the structural support for the aboveground marsh biomass (Turner et al., 2011, 2016) that prevents the breakup of the marsh where erosion rates can be high in anaerobic sediments, due to waterlogging or an oil spill that results in marsh loss. Determining belowground live biomass is a tedious, time-consuming exercise. An alternative method of measuring the strength of the living belowground root structure is shear vane resistance in a vertical soil profile. In a study of oiled marsh shoreline erosion, Turner et al. (2016) demonstrated that while the soil strength within the upper layer of the marsh soil down to 50 cm was similar in both highly oiled and more lightly oiled salt marshes, the soil below 60 cm was less strong, indicating that the shoreline was susceptible to erosion—and it eventually did fall apart. The protocols of natural resource damage assessment for marshes following exposure to an oil spill should incorporate additional measures of the “health” of marshes, including their structural integrity. Research into other measures or development of technological advances (e.g., portable photosynthesis systems for gas exchange and chlorophyll fluorescence measurements in plants) may also generate more universal representative indicators.
Marsh-Dwelling Invertebrates
The obvious marsh inhabitants, such as fiddler crabs, marsh periwinkles, mussels, oysters, blue crabs, shrimp, and small fish (nekton), are often the foci of oil spill impacts. The same was true for the DWH, but the expanded research funding
provided many opportunities to examine aspects outside of natural resource assessments. Fiddler crabs are easy to enumerate if one accepts the assumptions that (1) the number of burrows within a known area approximates their abundance, and (2) the width of their burrow approximates their size. Burrow densities were reduced by 39% in oiled sites, with effects of oiling and only partial recovery observed over 2010–2014 (Zengel et al., 2016). A return to the “reference” abundances was not complete by 2014. However, burrow diameters (~crab size) recovered by 2012, after being reduced from 2010–2011. Following oil deposition on the marsh surface, the proportion of Uca spinicarpa surpassed that of Uca longisignalis because of increase in largely unvegetated areas with a residual oil crust over the sediment surface (Deis et al., 2017). A reduction in burrow size initially and then an increase may be related to the altered species composition, with U. longisignalis being larger in carapace width and size of male claw compared to U. spinicarpa (Crane, 1975). Zengel et al. (2016) proposed that a return to species composition would likely follow the revegetation of Spartina.
The common salt marsh gastropod is the periwinkle (Littoraria irrorata), which feeds on benthic microalgae and algae on the stems of Spartina, both microhabitats subject to oiling. Post-spill surveys indicated significant losses of periwinkles in oiled habitat and a continuing slow recovery in both their abundance and size distribution attributed to habitat recovery (Zengel et al., 2016b). With longer-term data, neither density nor population size structure of periwinkles recovered at heavily oiled sites after 9 years, where snails were smaller and more variable in size structure. Likely linked to the lower total aboveground live plant biomass and stem density remaining over time in heavily oiled marshes. Periwinkle population rebound in moderately and heavily oiled sites may take one to two decades after the oil spill, respectively (Deis et al., 2020).
Benthic infauna (small animals living in the marsh sediments) are often used as indicators of pollution (in this case, an oil spill), because they cannot move away. In heavily oiled areas, total petroleum hydrocarbon (TPH) concentrations ranged from 50 mg TPH per gram sediment to 500 mg TPH per gram sediment compared to reference marsh levels of ~0.3 mg TPH g–1 sediment. These levels caused severe damage among meiofauna (animals > 63 μm but < 0.3 to 0.5 mm) similar to that of Spartina in heavily oiled areas. Over time, TPH degraded and Meiofauna began to recover following similar time courses of Spartina recovery, with considerable recovery of many organisms within 36 months of the spill. But, certain organisms such as, polychaetes, ostracods, and kinorhynchs, had not recovered to background levels in reference marshes 48 months post spill (Fleeger et al., 2015).
After 6.5 years, one community of 12 abundant taxa of meiofauna and juvenile macroinfauna had not fully recovered despite beginning to rebound from oiling in less than 2 years. The rate and speed of recovery of nematodes, copepods, most polychaetes, tanaids, juvenile bivalves, and amphipods were significantly positively linked to the recovery of Spartina and benthic microalgae (phytoplankton living on the sediment surface) (Fleeger et al., 2020). However, over time TPH concentrations remained high similar to the aromatics and alkanes, and live belowground plant biomass, bulk density, dead aboveground plant biomass, and live aboveground biomass of Juncus were not resilient. These conditions suppressed recovery of a kinorhynch, a polychaete, ostracods, and juvenile gastropods (Fleeger et al., 2020).
Overall abundances of the terrestrial arthropod insect community in oiled and unoiled Spartina marshes exposed to DWH oil were diminished by 50% at oiled sites in 2010, but by 2011 had largely recovered. Additionally, subguilds of predators, sucking herbivores, stem-boring herbivores, parasitoids, and detritivores all appeared to be suppressed at oiled sites by 25% to 50% in 2010 and recovered by 2011 (McCall and Pennings, 2012).
Studies of the greenhead horse fly (a top predator insect in marsh food webs of south Louisiana) were done, with biweekly monitoring in oiled and un-oiled areas from June 2010 through October 2011 (Husseneder et al., 2016). The population of horse flies crashed in oiled areas in 2010. The genetic makeup of six of seven oiled populations compared to six “pristine” sites indicated 10 polymorphic loci that identified genetic bottlenecks caused by fewer breeding parents, reduced effective population size, less family clusters and fewer migrants amid communities. The beauty of the experimental design was that it ranged from genetics to population levels on a keystone insect species with consistent oiled and unoiled results. Follow-up studies 4–5 years later in 2015 and 2016 by the same researchers (Husseneder et al., 2018) demonstrated signs of recovery of populations of the greenhead horse fly in formerly oiled areas, and previously detected genetic bottlenecks in oiled populations no longer exist. Husseneder et al. (2018) postulated that the greenhead horse fly larvae and adults followed, in succession, the regrowth of Spartina and recovery of 90% of the meiofauna, as documented by Fleeger et al. (2015).
Marsh-Dwelling Terrestrial Vertebrates
A dominant terrestrial vertebrate in Louisiana coastal salt marshes is the resident seaside sparrow (Ammospiza maritima). Those living in oiled marshes were potentially exposed to DWH oil directly, through inhalation and ingestion during preening and feeding while potentially experiencing reduced prey, especially insects (Pennings and McCall, 2014). Sparrows in areas with higher PAHs in sediments had elevated CYP1A gene expression, indicating metabolism of PAHs in oil. Carbon isotopic evidence further indicated DWH oil incorporated into seaside sparrow tissues as lower levels of 14C were found in feathers and crop contents in sparrows in oiled versus unoiled sites (Bonisoli-Alquati et al., 2020). Comparing the transcriptomic response in the livers of seaside sparrows exposed to DWH oil with birds from a control site found 295 differentially expressed genes. These genes are critical in basic physiological attributes, such as energy homeostasis, including carbohydrate metabolism and gluconeogenesis, and the biosynthesis, transport and metabolism of lipids.

SOURCE: Phillip C Stouffer, Louisiana State University.
Furthermore, these genetic analyses offer molecular explanations for the long-standing observation of hepatic hypertrophy and altered lipid biosynthesis and transport in birds exposed to crude oil (Bonisoli-Alquati et al., 2020). Further research on visibly oiled seaside sparrows and those where UV light was used to determine oiling indicated that small amounts of external exposure to oil were associated with hemolytic anemia (Fallon et al., 2018, 2020). The multiple pathways for DNA damage and cell death support the observations of reproductive failures in seaside sparrows. Data from 2012 and 2013 (2 and 3 years after marsh oiling) of seaside sparrow nesting indicated that nests on unoiled sites were considerably more likely to fledge than those on oiled sites (Bergeon Burns et al., 2014).
The nest failure results were re-examined with further studies of the seaside sparrow in the same salt marshes through 2017 (Hart et al., 2021). The authors suggested that oil exposure may have initially reduced nesting success and that with further degradation of the oil nesting success would increase. Multiple factors affecting the salt marsh ecosystem were indicated in the follow-on research, including (1) redistribution of DWH oil during Hurricane Isaac in 2012 and continued oil degradation, (2) loss of salt marsh habitat across southeastern Louisiana, (3) loss of insect prey, (4) population dynamics of predatory marsh rice rats, (5) nest placement in microhabitats of high marsh grass stem density at nest height, and (6) location of some nest sites in areas of greater exposure to higher wind and wave energy and subsequent higher water levels. Hart et al. (2021) found that overall (2012–2017) nest survival was low (24%) and the majority of nests (76%) failed due to depredation (see Figure 6.23). There was no definitive effect of initial oiling or oiled sediment, or estimated predator abundance for years with those data. Nest success was greater in areas of less dense marsh grass, a factor that may have reduced depredation. More dense marsh grass could possibly provide small mammals with refugia or easier climbing access to nests. Predators identified (via deployed video cameras) were primarily the marsh rice rat and the American mink, and a sequence of the squareback marsh crab followed by a rice rat.
Although the effects of oiling on individuals was clear, the proposal that these would translate to population-level effects on reproductive success (Bergeon Burns et al., 2014) was not supported by the additional research (Hart et al., 2021). However, there was a decidedly better ecosystem-level understanding of interacting factors.
The longer-term study with multiple integrated features of the salt marsh ecosystem points to the need for these types of studies to integrate the multiple aspects of contamination, salt marsh ecology, trophic structure, predator–prey dynamics, understanding of microhabitats within the marsh, and an integrated approach ranging from genetics and enzymatic responses to ecosystem-level effects.
Marsh Nekton
Marsh killifish, or minnows, are ubiquitous across the temperate salt marsh platform, in tidal creeks, marsh ponds, and adjacent waterways across 1,000 km and are considered resident (movements of tagged fish within 100 m) (Fodrie et al., 2014). As expected, early in the arrival of DWH oil to salt marshes in Louisiana, the in situ observations by Whitehead et al. (2012) of killifish in lower Barataria Bay, Louisiana, near marshes that had been heavily oiled had elevated CYP1A gene
expression, indicating a response to PAHs in their livers; this was not so for killifish in Mississippi and Alabama. Experimental results ranged across populations present in Gulf of Mexico marshes before oil landfall (May 1–9, 2010), during the peak of oil landfall (June 28–30, 2010), and after much of the surface oil was no longer apparent two months later (August 30–September 1, 2010). Whitehead et al. (2012) found that controlled laboratory exposures of developing killifish to water collected from heavily oiled salt marshes in lower Barataria Bay on June 28 and August 30, 2010, prompted CYP1A protein expression in larval fish. This response matched the location and timing of oil contamination, and showed that the remaining oil components dissolved at very low concentrations in lower Barataria Bay after landfall and were bioavailable and bioactive to developing fish.
Later field collections of marsh nekton from oiled and unoiled sites did not reveal any effects of oil exposure on population dynamics. These collections did not occur until 2012–2013, and hydrocarbon degradation may have rendered remaining DWH oil less toxic than that in the initial oiling. The mostly consistent results showed little indication of any effect from potential DWH oil exposure (Fodrie and Heck, 2011; Fodrie et al., 2014; Able et al., 2015). Another study compared the total fishes caught on seagrass beds from the Chandeleur Islands, Louisiana, to St. Joseph’s Bay, Florida (all along oiled shorelines) in June–September of 2006–2009 with peak oiling in 2010. The total fishes caught across geographic areas between the 4 pre-spill years and the year of the spill showed no statistical difference (Fodrie and Heck, 2011; Moody et al., 2013).
These results leave a gap between toxicity of the initial oiling and physiological performance of killifish and population dynamics following the immediate time of the oil spill in the salt marshes (Fodrie et al., 2014). There is a possibility that gulf killifish distributions reflect extremely local conditions (<100 m). Meaning, this species can act as a site-specific indicator of disruption, however only individuals collected within the same year as the event were likely directly exposed (Jensen et al., 2019).
Marsh nekton distribution did not differ in oiled vs unoiled marshes after 2 years of oil spill exposure, because of many species-specific characteristics, population dynamics, and trophic dynamics (see Figure 6.19). Population responses did not reflect the immediate demise of heavily oiled killifish because of many of the characteristics described in Figure 6.24
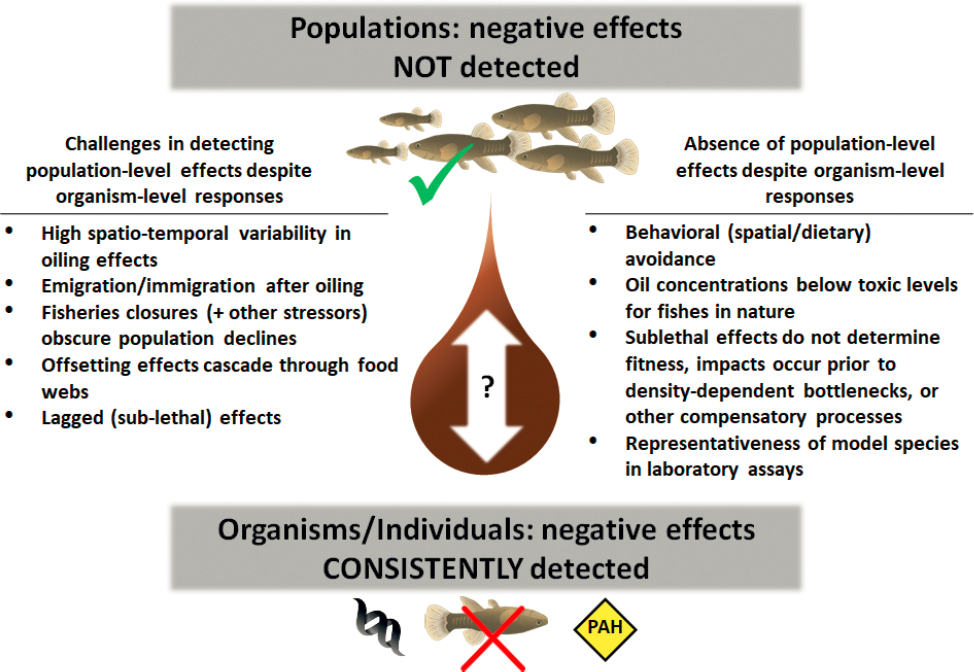
NOTES: The list on the left highlights logistical and structural challenges in detecting population-level effects following large-scale disturbances. The list on the right concentrates on why oil pollution may not have affected estuarine fishes at the population (or community) level, despite known responses at the organismal level. PAH = polycyclic aromatic hydrocarbons.
SOURCES: The symbols are courtesy of the Integration and Application Network, University of Maryland Center for Environmental Science (http://ian.umces.edu/symbols). Figure modified by J. Fodrie, from Fodrie et al. (2014); permission for use granted by BioScience.
(Fodrie et al., 2014). Another possibility is that reduced mortality of these fishes as a result of the deaths of avian predators by direct oiling overshadowed fish mortalities from the directly toxic effects of oil exposure (Short et al., 2017).
Salt Marsh Recovery
For many spills, marsh recovery occurs within one to two growing seasons, even in the absence of any treatment (Michel and Rutherford, 2014), but that time period is often the length of a research program.
A meta-analysis by Zengel et al. (2021) of DWH-oiled marshes in southeastern Louisiana over 7 years post-spill concluded that the DWH oil spill effects were multi-year and that full recovery would likely exceed 10 years, especially in heavily oiled marshes where erosion may prevent full recovery or there is no recovery. In other words, there are no effects to be documented when the salt marsh no longer exists. All metrics (plant cover, stem density, vegetation height, and above- and belowground biomass) were tracked in 10 studies and 255 sampling sites. All plant metrics pointed to impacts from oiling, with 20–100% maximum reductions depending on oiling level and distance from the marsh edge. Heavily oiled sites at the marsh edge showed peak reductions of ~70–90% in total plant cover, total aboveground biomass, and belowground biomass, with Juncus roemerianus affected more than Spartina alterniflora. Most plant recovery metrics ranged from 3 years to at least 7 years post-spill (the length of the research programs). Belowground biomass reductions were particularly concerning, because the declines over time were longer than recovery of aboveground vegetation. The loss of belowground biomass and root structure as indicated by soil shear strength (Turner et al., 2016) over time was a strong indicator of continuing impact, limited recovery, and impaired resilience.
In experimental mitigation efforts within heavily oiled salt marshes covered by the DWH oil, manual treatment of oiled marshes lead to greater vegetation cover than mechanical treatment and no treatment, through 1 year (Zengel et al., 2015). The percentage of vegetation cover in both treated and untreated marshes did not reach the level of reference marshes for more than 2 years after the initial oiling. Planting allowed for quicker vegetation recovery and reduced shoreline retreat compared with areas of no planting.
Michel and Rutherford (2014) combined their assessment of marsh oil spills and known or predicted recovery rates (see Figure 6.25) with similar syntheses by Hoff (1996) and Sell et al. (1995) and provided the following summary.
Spills in the following conditions display the longest recovery time (example of spills):
- Cold environments (e.g., Metula, Arrow, Amoco Cadiz)
- Sheltered/protected locations (e.g., Metula, Arrow, Gulf War, Nairn pipeline, Mill River)
- Thick oil on the marsh surface (e.g., Metula, Amoco Cadiz, Gulf War)
- Light refined products with heavy loading (e.g., Florida, Bouchard-65, Exxon Bayway)
- Heavy fuel oils that formed persistent thick residues (e.g., Arrow)
- Intensive treatment (e.g., Aransas Pass, Amoco Cadiz, Golden Robin)
Spills in the following conditions display the shortest recovery:
- Warm environments (e.g., many spills in Louisiana and Texas)
- Light to heavy oiling of the vegetation only
- Medium crude oils
- Less-intensive treatment
6.5.3.2 Mangrove Communities (ESI 10)
Mangroves as intertidal flora range from short bushes (usually the black mangrove, Avecinnia germinans, ~1 m high) to more substantial genera, such as red mangroves (Rhizophora mangle) or white mangroves (Laguncularia racemose) that reach 6 m and 15 m, respectively. Mangroves are not a majority part of the intertidal shoreline habitats in the primary study area of this report, inclusive of Canada and the Mexico/Caribbean region, with the exception of the subtropical and tropical areas. The intricate structure of the mangals (mangrove swamps) provides habitat to a high diversity of organisms and stability to sediments. Mangroves as a shoreline habitat are much more developed in the southern Gulf of Mexico and throughout the Caribbean than in the northern Gulf of Mexico. Mangroves are affected variably by oil spills, depending on the type of oil and toxicity, the amount of oil, and the duration of weathering. Mechanisms of effects are via direct toxicity to the plants, via the prop roots and the pneumatophores through smothering and reducing the uptake of oxygen as avoidance to low oxygen conditions in the sediments, and via penetration of the oil into burrows of associated animals.
One of the more obvious indicators of the effects of oil on mangrove communities is the albinism of the plant leaves when exposed to xenobiotics, which is related to the translocation of aromatic hydrocarbons into their tissues (Getter et al., 1985). Trees heterozygous for chlorophyll-deficient alleles are fairly easy to distinguish in the mangrove species Rhizophora mangle (Klekowski et al., 1994) and are associated with albinism. This genetic mutation is often seen in red mangroves exposed to xenobiotics, and albinism in mangroves is recognized as an indicator of pollutant stress.
Getter et al. (1981) summarized the effects of oil spills at five sites in the Gulf of Mexico and the Caribbean Sea. Light, refined oils, such as No. 2 fuel oil, often penetrate the substrate through animal burrows and can be took up by the tree roots, with mortality in 24 to 48 hours in red mangroves
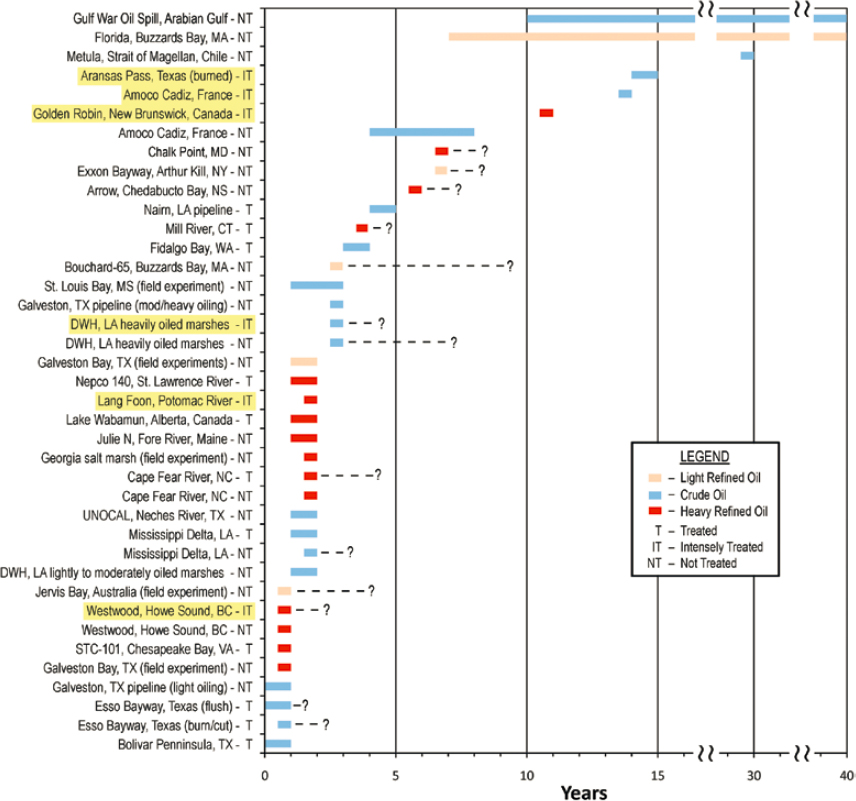
NOTES: Recovery is defined as re-establishment of natural biota within the range of dominance, diversity, abundance, and zonation expected for the affected habitat (Sell et al., 1995), or return to comparable values for important ecological parameters for affected and nearby similar control habitats (Hoff, 1996). Yellow highlighting identifies spills with intensive treatment. Question marks represent potential time to recovery based on the results of the most recent data.
SOURCE: Used with permission of the authors.
and black mangroves. Alternately, crude oils and heavy refined products such as Bunker C coat the prop roots and pneumatophores, limiting the ability of the tree to exchange gases (see Figure 6.26). Crude oil contamination—both in the presence and in absence of dispersant in the water—causes a significant reduction in water flux through the red mangrove root tissues (Tansel et al., 2015). The root samples that were in the solutions contaminated with Louisiana crude oil showed dehydration and drying and separation between the epidermis and endodermis both in the absence and presence of dispersant after 7 days (see Figure 6.27). Heavy oils will have long-term persistence, especially with heavy accumulations resulting in leaf loss and possibly death. Oiled areas may also see reduced recruitment of seedlings (Getter et al., 1981).
In oil spills with massive and thick oil coverage on the roots and pneumatophores (Galeta spill in Panama), the epibiont and sessile invertebrate communities die off to < 5% of complete coverage (Burns et al., 1993). All groups of epibionts were present after 2 years, but corals, anemones, and tunicates were still scarcer at oiled versus unoiled sites. Oiled and unoiled sites continued to show significant differences through the 5-year post-spill study.
In August 2006, the tanker SOLAR 1 sank in the Philippines’ Guimaras Strait, spilling a substantial part of her 2,100-tonne cargo of intermediate fuel oil (Yender et al., 2008). The area is rich in mangroves, coral reefs, and seagrass beds, with a rugged shoreline of sand, pebble, and cobble beaches, into which seeped the oil. Cleanup focused on the shoreline with little cleanup in oiled mangroves. Mangrove prop roots and pneumatophores have lenticels, large pores for gas exchange, which if coated with oil lose the ability for gas exchange. After weeks of the oil spill the root surface remained oiled, however, these roots had increased the shedding of cells from lenticels, freeing them up for gas

NOTES: The photo depicts oil adhered to the mangrove roots 3–4 weeks after the spill (January 2014). Some mangrove leaves turned from green to yellow in color, an indication of sublethal effects. The strings of pom-poms were deployed to prevent re-oiling.
SOURCE: With permission from Paul Schuler, Oil Spill Response Limited.
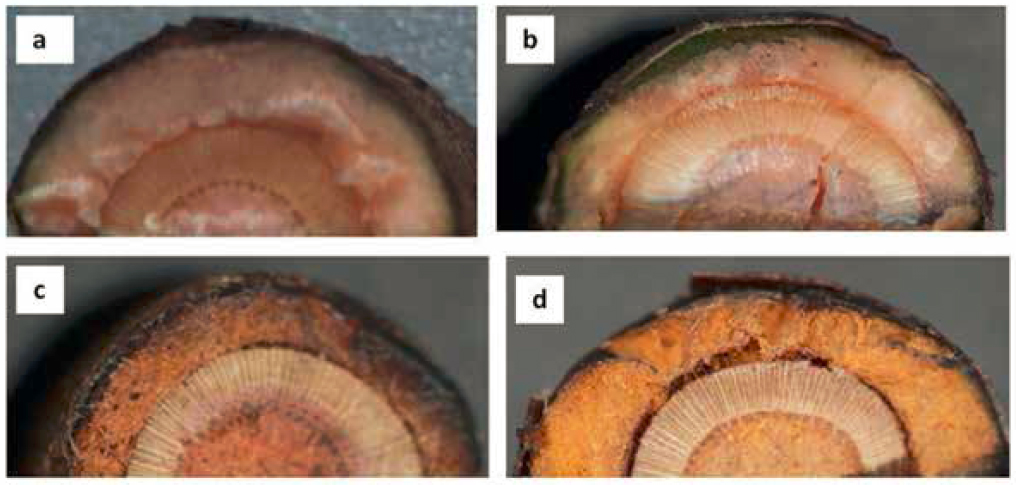
SOURCE: Tansel et al. (2015).
exchange. Severely affected mangrove trees displayed signs of stress, including yellowing of the leaves, partial defoliation, and occasionally the spread of roots and trunk sprouts, among other symptoms. Areas where the oil remained trapped in sediments for several weeks or months after the spill saw highest adult tree mortality. Observations 1 year after the spill showed that oiled mangroves were recovering naturally and suffered only minor mortality.
Depending on the severity, results from oil exposure can range from initial defoliation and eventual recovery to mass mortality and complete loss of habitat. Many follow-up studies of mangrove recovery do not last long enough to determine the time to recovery. A spill from the Era tanker in southern Australia in September 1992 resulted in the oiling of approximately 100 ha of a monospecific stand of Avicenna marina mangroves (Connoly et al., 2020). Lightly oiled trees saw a full recovery; however heavily oiled trees experienced mass defoliation and death over several months after the oiling event. Aerial imagery analysis indicated that heavily oiled areas did not recover for 10 years following the oiling event. Seedlings had become established and canopy cover increased to 35% of pre-oiling cover within heavily oiled areas after between 10 and 25 years. Predictive modeling estimates based on trajectories of vegetation cover change over periods with adequate aerial imagery were used to model complete recovery of mangroves to pre-oiling cover, which was estimated to take 55 years from oiling (Connolly et al., 2020).
6.5.3.3 Sheltered Rocky Shores (ESI 8)
In 1967, the tanker Torrey Canyon wrecked while carrying 117,000 tons of crude oil off the southwest coast of the United Kingdom. Experts from the Plymouth Laboratory of the Marine Biological Association (MBA) of the United Kingdom mobilized in response to this environmental catastrophe. Staff from the MBA, A.J. and E.C. Southward (1978) studied the rocky shores affected by the spill and unaffected control sites; charting fluctuations of rocky shore fauna (particularly barnacles) and flora from the early 1950s, in relation to climate. This study provided a baseline to judge recovery of rocky shoreline from the beached oil and application of toxic engine room degreasers not designed for used in the marine environment. Subsequent follow-up studies in the 1980s and 1990s found that recovery took up to 15 years along the shore at Porthleven, which was subject to the most severe degreaser application. In contrast, at Godrevy where degreasers were not applied over the concerns about their impact on seals, recovery occurred in 2–3 years. The degreasers killed the dominant grazer, limpets of the genus Patella, which allowed a massive colonization by seaweeds. The resulting canopy of macroalgae (“rockweed” or “wrack”) fostered a dense population of limpets, which then completely grazed the seaweeds until the starving limpets largely died off after migrating across the shore in search of food. The die off of limpets and now reduced grazing pressure prompted another bloom of algae. By the mid-1980s through 2016, normal levels of spatial and temporal variation of seaweeds and limpets fluctuations were charted at Porthleven. Lessons learned from observations over 60 years, both before and after the spill, for rocky shore monitoring highlighted the need for broad-scale and long-term monitoring to differentiate local impacts (such as oil spills) from global climate-driven change (Hawkins et al., 2017).
In the reports following the natural recovery of Mearns Rock after the Exxon Valdez oil spill in 1989 in Snug Harbor on Knight Island, Alaska, more than 30 years of observations have memorialized huge annual variations on the attached flora and fauna. Those crafting definitions of “fully recovered” needs to bear in mind these annual/seasonal fluctuations (see Box 6.6).
Spilled oil that contacts rocky armored shorelines presents a smothering hazard for diverse intertidal communities that inhabit the surface as well as subsurface sediments, which may require years to recover. Efforts to remove this oil may compound adverse effects by causing additional mortalities. For example, high-pressure washing of poorly sorted gravel beaches after oiling by the Exxon Valdez oil spill transported finer-grained sediments from the upper to the lower intertidal, and appears to have resulted in high mortalities of clams that survived the initial oiling. In fact, this may have reduced clam recruitment by removing finer-grained sediments and organic matter from these shorelines (Houghton et al., 1996). Seabirds, sea otters, and predatory invertebrates such as starfish that forage in the intertidal are especially vulnerable to adverse (and often lethal) effects of contact with oil. After the Exxon Valdez, population recovery and habitat reoccupancy of intertidally foraging seabirds and sea otters took well over a decade (Esler et al., 2018). Infauna that consume sediments are susceptible to ingesting oil, as are filter- and suspension-feeding invertebrates such as clams, mussels, and other organisms, which may be important subsistence food items for Indigenous communities.
Oil that contaminates hypoxic zones of porous, armored shorelines may persist for decades with little weathering beyond any that occurred prior to deposition, forming a long-term reservoir of oil that can contaminate aquatic biota (Nixon and Michel, 2018). After the 1989 Exxon Valdez spill, sea otters, sea ducks, and other species that excavate intertidal sediments in search of prey occasionally encountered subsurface shoreline oil for more than a decade following the spill (Short et al., 2006), oiling their pelage, feathers, or epidermal tissues and thereby often providing an oil ingestion pathway.
6.5.3.4. Seagrass Communities (no ESI category)
Healthy seagrass beds are usually located between supratidal habitats, such as mangroves or salt marshes, and coastal subtidal areas. The health of seagrasses depends on shallow, calm waters with low turbidity and lack of toxic pollutants and excess nutrients. During an oil spill, exposure may occur via coating of seagrass blades and seepage into sediments (Zieman
et al., 1984). Seagrass habitats are often less studied than the more obvious salt marshes, mangroves, and coral reefs.
Kenworthy et al. (2017) documented shoreline oiling following the DWH oil spill via aerial and satellite imagery on the shallow back barrier portions of the Chandeleur Islands where seagrass communities existed in a patchy mosaic among unvegetated and vegetated areas of subtidal sediments. The method used by Kenworthy et al. (2017) was aerial photography assessment of the seagrasses in May 2010 before the spill and post-spill in October 2010, 2011, and 2012, where DWH oil exposure was confirmed. The investigation conservatively assessed a seagrass loss of 104.22 acres (42.18 ha). An estimated loss of 271 acres of seagrasses were lost due to immediate or delayed oil exposure and from outboard motor propeller scarring associated with spill response activities (Kenworthy et al., 2017). Oil exposure did not result in a catastrophe for seagrasses in this area. Similar results were found for the seagrass beds along the mainland coastline of eastern Mississippi Sound, with no short-term declines in their growth or abundance following the DWH oil spill (Cho et al., 2017). Results from other oil spills affecting seagrass beds demonstrate more negative effects, depending on the intertidal environment and relative differences in oil exposure. The most serious known effects of oil on seagrasses have been observed on communities that are intertidal or marginally subtidal and subject to occasional exposure, unlike seagrass beds that remain submerged during oil exposure (reviewed by Zieman et al., 1984).
One of the better-documented oil spills was that from the Amoco Cadiz, which occurred near Roscoff, on the Brittany coast of France, in March 1978. A massive oil slick washed ashore and portions of it covered a Zostera marina bed that had been studied continuously since 1976 by den Hartog and Jacobs (1980). Two seagrass beds at different tidal levels were studied: the upper one was exposed at nearly every low tide, whereas the lower station was about 0.5 m lower and located in an enormous tidepool that retained several centimeters of water during every low tide. The Zostera was “almost unaffected by the oil spill” (den Hartog and Jacobs, 1980). During the few weeks following the oiling, numerous leaves turned black and were lost, but this seemed to be only an acceleration of the normal leaf loss, and no other short-term damage to the plants was observed. den Hartog and Jacobs (1980) concluded that there were minimal effects on the seagrasses. Other baseline data on the fauna associated with the Zostera beds beginning the year before the oil spill provided comparative data for post-spill effects (Boucher, 1985; Dauvin, 1988). The influence of oiling left some animal groups unaffected, some with rapid recovery, but there was little re-establishment of a diverse amphipod community that was replaced by only two species of amphipods.
Fonseca et al. (2017) added other measurements of effect to oiled eelgrass in their study of oiling impacts to eelgrass (Zostera marina) from the 2007 Cosco Busan event in San Francisco Bay. At sites with pre-spill records, data collections included the shoot densities, reproductive status, and rhizome elongation of Z. marina, and post-spill measurements captured eelgrass photosynthetic efficiency. SCAT oiling categories identified during shoreline cleanup assessments show no consistent relationship with variations in shoot densities and percent elongation of rhizome internodes formed after the oil spill. In addition, changes in seagrass photosynthetic efficiency were also not consistent with SCAT oiling categories. A comparison of SCAT oiling categories with chemical characterization of hydrocarbons would benefit subsequent field studies and comparison of effects results based primarily on the four-tier SCAT categories.
6.5.3.5 Tidal Flats (ESI 7 and 9)
Heavy fuel oil was accidentally spilled in a drainage ditch near the Kaomei Wetland, south of the Tachia River estuary, Taiwan, in June 2010. Semi-diurnal tides of three to four meters quickly spread about eight metric tonnes of the oil over 300 ha of the reserve, including extensive tidal flats. The area is subtropical with average temperatures of 29°C in July and August. There were baseline data for the area, which had been monitored since 2008 for benthic communities and microbial processes (Lee and Lin, 2013). One site was selected for the polluted area; a similar tidal flat less than 400 m from the polluted site was established as a control site, which had not been oiled but likely was exposed to dispersed oil in the water column. CO2 fluxes at the air-sediment interface during emersion at low tide were determined by benthic chambers. All parameters were measured five times within a 38-day period following the oil spill. Sediment bacteria increased in abundance and microalgal biomass decreased, indicating that the ecological functioning of the flats was compromised with the oil suppressing or stopping gross community production and increasing community respiration. Net community production became negative within 5 days, indicating that the community had switched to heterotrophy with suppressed microalgal photosynthesis. Net community production also became negative after 8 days at the control site, indicating that the oil had been dispersed by the tides from the polluted area. The contamination at the control site was not as great as at the polluted site because the gross community production remained positive. Macroinfaunal abundance was reduced shortly after the spill, and different fauna recovered at varying rates. Crabs and bivalves were observed after five days in the polluted site, but no macroinfauna were observed eight days post-spill, thereafter recolonizing at different times. Polychaetes and amphipods were observed only at the “control” site, but polychaetes reappeared at the polluted site only 38 days after the oil spills.
Paranaguá Bay in southern Brazil is one of the largest subtropical estuaries and best preserved in the southern hemisphere, but susceptible to oil operations that process mostly diesel fuel oil. Intertidal flats dominate in this estuary and in confined low-energy areas along the southeastern and southern Brazilian coasts. An experimental in situ diesel fuel oil spill was conducted in unvegetated tidal flats along the Cotinga Channel, a sub-estuary of Paranaguá Bay (Gonzalez Egres et al., 2012). The experiment was a multivariate before and after/
control and impact (M-BACI) model, with three levels of treatment contrasted with controls in 14 successive periods before and after the oil spill covering a total of 147 days for the full experiment. The monitored response was the composition of the benthic infauna. An acute effect was recorded immediately after the impact, but the recovery to pre-disturbance population levels occurred mostly complete within 30 days (i.e., within long-term variability of the community). There was an increase in the total density of the benthic community of small snails, oligochaetes, and ostracods after the disturbance, which was ascribed to background variability.
Nwipie et al. (2019) followed a series of oil spills in Bobo Creek (Niger delta) and related low-intertidal, soft-bottom infaunal macrobenthic invertebrates for 7 years after spills and compared changes in the benthic community after initial observations from Zabbey and Uyi (2014). Initially, the two major spills reduced macroinfauna by 81%, with two stations not supporting any benthos. Subsequent sampling on a bimonthly basis (spring and neap low tides) for 2 years indicated an initial increase in an opportunistic polychaete, then a polychaete-dominated but more diverse benthic community in 3 and 5 years after the spill. A recovery rate of only 9.7% of the benthic fauna abundance after 7 years (Nwipie et al., 2019) reflected remaining high levels of hydrocarbons, continued oiling from polluted tidal creeks, low dissolved oxygen and high biological oxygen demand from decomposing flora and fauna. There was no increase in an endemic characteristic chemosymbiotic lucinid bivalve population for at least 7 years post-spill.
6.5.3.6 Sandy Beaches (ESI 3 and 4)
Beach oiling can occur when oil is deposited by waves and coastal currents onto the shoreface of barrier islands. Subsequently the oil can be incorporated into the beach sands by tides and waves. With weathering of the oil residue and mixing by waves, the oil may be incorporated into subsurface oil mats along exposed sandy beaches, within burrows of organisms (Amos et al., 1983), or as tar balls (see Chapter 5, Section 5.3.2.1).
Oil spills on sandy beaches are seldom often thought about past the effects on tourism and the health of oil spill cleanup workers (see Section 5.2.5.2). However, the beach intertidal sands are home to many sediment-dwelling organisms that form the food base for shorebirds in residence and at important periods of migration cycles. Although sandy beach shoreline habitats are often the first affected by an oil spill coming ashore, the effects on their living inhabitants and communities are seldom studied at the same level of detail as are salt marshes, mangroves, and rocky shorelines. Analyses of the abundance and community composition of interstitial sediment dwellers are seldom accompanied by the makeup and concentrations of the oil residue hydrocarbons. Bejarano and Michel (2016, and references therein) synthesized the peer-reviewed literature and reported on the impacts of oil spills on sand beaches (63-μm to 1-mm grain size) with a focus on intertidal invertebrate communities and their recovery. Several complications exist in comparing results among beach intertidal infauna studies:
- Different ranges of sizes of organism categories (e.g., meiofauna are usually considered >63 μm and less than 0.5 mm, but are often described as >45 μm and less than 0.5 mm; macrofauna are usually those retained on a 0.5-mm sieve size, but some researchers put the lower limit at 0.3-mm sieve size for retention of macrofauna, especially juveniles).
- Inconsistent level of taxonomic enumeration (e.g., grouping of oligochaetes or nematodes versus specific identification of harpacticoid copepods, or specific identification of polychaetes versus counting as larvae versus adults).
- The groupings of invertebrates are often considered residents within positions along the intertidal interface of the beach between low- and high-water levels, for which there is overlap between the upper, middle, and lower intertidal communities.
- Infaunal beach communities shift in position on the beach forefront as water levels increase with tides or wind-induced water submergence of the beach and move to lower elevations of the beach with receding tides. Supratidal zones maintain more “permanent” residents such as ghost crabs (Ocypode quadrata).
Despite the complications already listed and limited publications that document effects of oil spills on sand beaches, Bejarano and Michel (2016), identified
(1) an impact phase, where the associated invertebrate community experiences a measurable reduction in abundance and species diversity caused mostly by mortality and oil fouling; and (2) a recovery phase, where there is an increase in dominance of opportunistic species, followed by the return of species characteristic of the assemblage signaling the start of the recovery.
These findings are similar to those for other oiled habitats, with the exception that the organisms are mostly small infaunal meiofauna and macrofauna. Loss of abundance of beach infaunal organisms can be well over 50% and up to 100%. Groups with direct development, such as harpacticoid copepods and amphipods, are more affected than polychaetes with larval recruitment from ambient waters. Recovery may be rapid, such as within 1 year, or much longer, taking place over several years.
6.5.4 Coral Reefs (ESI 4)
Corals are found in almost all of the world’s oceans: there are the shallow coral reefs that are restricted to tropical, warm, clear waters, but also deep or cold-water corals in temperate to Arctic locations. In tropical shallow-water
locations, reef-building corals are symbiotic with photosynthetic zooxanthellae (e.g., Acropora spp.), but the common cold-water deepsea stony coral, Lophelia pertusa, does not contain these algal symbionts. Intertidal and subtidal shallow coral reefs in tropical regions of the world are often close to areas of oil extraction and tanker routes, and there have been a number of examples of acute oil spill events and chronic exposures from operational discharges or natural seeps (summarized in NRC, 2003). In a Net Environmental Benefit Analysis (NEBA), corals are often one of the organisms highest at risk and valued ecosystems listed for protection. This assessment was largely based on the results from one of the longest-lasting (currently over three decades) oil release field experiments (the 1984 TROPICS study, see Box 6.9; Renegar et al., 2017, 2021, 2022), which was conducted to determine if the use of a chemical dispersant would reduce the overall impact of oil to a coral reef and result in a quicker recovery (Ballou et al. 1987; Dodge et al., 1995). As coral reproduction and early life stages are especially sensitive to oil the timing of when an oil spill occurs is critical (see Nordborg et al., 2020, 2021). Furthermore, these early life stages of coral (i.e., gametes, embryos, and planula larvae) often reside in the ocean’s surface where oil concentrations are typically higher. The effects may also be compounded by environmental factors and co-stressors such as ultraviolet radiation (UVR), elevated temperature, and specific phototoxicity reactions that enhance or exacerbate oil toxicity (see Section 6.3.4.3; also Kegler et al. 2015; Nordborg et al., 2018, 2020, 2021). The impact of oil and/or chemical dispersants on corals has also been investigated using laboratory toxicity tests, although much fewer than for standard toxicity test organisms given the complexities and difficulties with working with corals. Coral toxicity records (all chemical contaminants) in the CAFE database (see Section 6.7.2) represent only 0.1% of the total records across all taxonomic groups (Bejarano et al., 2016). Toxicity studies have been conducted exposing numerous coral species and life stages (i.e., adult fragments, gametes, and larvae) to single hydrocarbons, hydrocarbon mixtures, various fuels and oils, and chemical dispersants.
Although the vast majority of studies have focused on intertidal and subtidal species, the DWH incident highlighted the significant knowledge gaps regarding the toxicity of oil and chemical dispersants to deep-sea coral species, including whether these coral ecosystems would be more sensitive than their shallow-water counterparts. This led to a new research focus on understanding the exposure to and effects of oil on mesotrophic and deep-sea coral species, not just in the Gulf of Mexico but also in other areas of the world, as discussed later in this chapter (e.g., White et al., 2012; Silva et al., 2016; Girard et al., 2019; Bytingsvik et al., 2020).
Unlike for many other marine species, there are currently no standard toxicity test organisms or protocols for corals, which are notoriously challenging to obtain, sustain, and maintain health in laboratory settings (Mitchelmore et al., 2021). Typical metrics used in toxicity tests (i.e., mortality, growth and reproduction) are difficult to assess or achieve in typical testing time frames. Determining mortality in a coral is difficult given that polyps retract into the skeleton, and accurate assessments for many species may only be completed after assessment in control exposure media during recovery as discussed by Bytingsvik et al. (2020). Many corals are also slow-growing and hard to maintain in laboratory culture for extended periods making chronic toxicity studies challenging. There are, however, some species for which growth differences are possible in a suitable time frame (see Renegar et al., 2019; Renegar and Turner, 2021). Offsetting these limitations has been the inclusion of additional nontraditional acute and chronic endpoints, directed at both the host coral and its algal symbionts (if present). For example, Bytingsvik et al. (2020) used polyp retraction instead of mortality to calculate acute EC50s. However, the relevance of polyp behavior and other alternate non-traditional toxicity test biological endpoints to population-level consequences is not well established, and further investigation is needed (Mitchelmore et al., 2021).
This section discusses the implications of oil spills to both shallow-water and deep-sea coral reefs, highlighting studies that were not discussed or occurred after the Oil in the Sea III report (NRC, 2003). It includes results from field studies following oil spill events, in situ experiments and also laboratory toxicity studies.
6.5.4.1 Intertidal and Subtidal Coral Reefs
Intertidal reef flats are potentially at risk of higher impact from oil than subtidal reefs, as oil can coat surfaces in addition to the water-soluble fraction exposures typical for both reef types. The 1985 report on oil in the sea (NRC, 1985) focused on describing the effects to corals from a number of oil spills that had occurred in tropical habitats. The Oil in the Sea III (NRC, 2003) study highlighted the extensive additional field, in situ and laboratory studies that were done in the subsequent 20 years. The 1986 Galeta oil spill (Bahia las Minas, Panama) was highlighted, as long-term effects to corals were observed due to chronic oil exposure from the surrounding mangroves. In addition to the previous National Academies reports, many recent comprehensive reviews have summarized the effects of petroleum hydrocarbons on coral reefs, including field exposures during oil-spill incidents, planned field studies (in situ exposures), and the wealth of data stemming from laboratory exposures using single PAH compounds, mixtures of PAHs, and various fuels and oils (e.g., Shigenaka, 2001; NRC, 2005; van Dam et al., 2011; Renegar et al., 2017; Turner and Renegar, 2017; Kroon et al., 2020; NASEM, 2020; Negri et al., 2021). Studies have also included investigations of response options (e.g., chemical dispersants; Negri et al., 2018), covariables, and multiple stressors, including elevated UV light and temperature (Nordborg et al., 2018, 2020, 2021).
Responses to and the effect of oil and/or hydrocarbon exposures to corals in laboratory tests and from field and/or in situ studies vary from no impact to reproductive failures, increased mortality, reductions in coral cover and health, growth or skeletal differences, and injury, including bacterial infections. An array of sublethal effects have also been reported, many of which have shown correlations to and implications for individual or coral ecosystem health (Turner and Renegar, 2017 [summary tables detailing oil spills, in situ and ex situ exposures]). This wide array of impacts is due in part to the diversity of oil (and dispersant) types, specific environmental conditions including exposure concentration and duration, the specific coral species (and life stage), and other potentially confounding environmental variables. For example, chronic crude oil pollution in the Red Sea increased mortality and reduced coral reproduction, but a short-term exposure of dispersed oil showed little residual effect on growth (Rinkevich, 1977). Field surveys of chronically oiled sites in the Caribbean Sea showed a decline in coral cover (survival) and suggested that coral recruitment was impaired (Bak, 1987). In contrast, monitoring in 1992–1994 of the Gulf War oil spill of 1991 showed no detectable longer-term impacts to corals despite the large volumes of oil released (Vogt, 1995).
A number of oil spills have occurred around coral reefs globally, with the most well studied being the 1986 Bahia las Minas spill on the Caribbean coast of Panama (in 1986) and the DWH spill in the Gulf of Mexico (in 2010). Coral mortality was observed following the Bahia las Minas crude oil spill and reductions in coral cover and growth rates and other sublethal changes were observed 2 years post-spill, with reproduction and fecundity still affected 5 years after the spill, although longer-term (>10 years) chronic impacts could not be assessed due to confounding influences of additional stressors (summaries in Shigenaka et al., 2001; NRC, 2003, 2005; Turner and Renegar, 2017; Guzman et al., 2020). However, studies of other spill events have shown no detectable impacts to corals (see Turner and Renegar, 2017).
The most extensive and longest studies of oil and dispersant impacts on corals come from the Panama TROPICS study (in 1984) and the Arabian Gulf (LeGore et al., 1989) study, where controlled in situ oil spills (with and without the use of chemical dispersants) were conducted (see Renegar et al., 2017, 2021, 2022; see also Box 6.9). In the TROPICS experiment Acropora cervicornis and three other coral species showed a limited impact at the crude oil alone site but coral cover declined and growth was reduced at the chemically dispersed crude oil site during the first 2 years (Ballou et al., 1987). No residual effects of crude or dispersed crude oil were observed 10 years after the study, although mangroves continued to be impacted, even after 10 years at the oil-only site (Dodge et al., 1995; Ward et al., 2003). In contrast, in the Arabian Gulf experiment, no impact on Acropora spp. growth was observed following exposure to Arabian light crude oil and Corexit 9527 (LeGore et al., 1989). The greater short-term effects resulting from the 1986 Bahia las Minas spill compared to the 1984 TROPICS experiment that were observed in corals may be partially explained by species sensitivities to oil exposure, as the more sensitive Acropora palmata was largely absent at the TROPICS site, which was dominated instead by the more resilient Porites furcata species. A species-specific differential impact of oil was also noted in Bak (1987), where the branching Acropora spp. was found to be more sensitive than the massive species, Montastrea spp. Recent laboratory studies with single PAHs have also noted differences in coral species sensitivities, as discussed later in this section (Renegar and Turner, 2021).
Laboratory exposure studies have highlighted specific mechanisms of action of oil constituents and/or chemical dispersants and assessed exposure routes, toxicity thresholds, and species sensitivities in a number of species and multiple life stages from adult, juvenile, and larval stages. As with the field studies, a variety of biological repercussions have been demonstrated (see Shigenaka et al., 2001; Turner and Renegar, 2017). It is important to recognize that in many of these studies, the exposure concentration and durations used may be much higher than those expected and/or measured following oil spill events. Thus, they may not be environmentally relevant, and/or have limitations due to media preparation and/or validation, toxicity test designs or reporting (Bejarano, 2018; Mitchelmore et al., 2020a,b) as discussed in Section 6.4.
A number of studies have investigated the impact of hydrocarbons on coral reproduction, either gamete fertilization success or larval settlement, metamorphosis, and development. For example, exposure of Pocillopora damicornis to WAFs of natural gas condensate resulted in larval expulsion during early embryogenesis and early release of larvae in late embryogenesis (Villanueva et al., 2011). This is similar to the results of earlier studies, where corals showed reproductive failure as they aborted/released planula larvae following oil contact (Loya and Rinkevich, 1979). More recently, oil-contaminated seawater was shown to reduce settlement of Orbicella faveolata and Agaricia humilis (Hartmann et al., 2015). A number of studies have shown that crude oil inhibited fertilization (Negri and Heyward, 2000) and also metamorphosis (Te, 1991). A concentration-dependent reduction in settlement and survival occurred with larvae exposed to weathered oil, chemical dispersant, and chemically dispersed oil in P. damicornis and O. faveolate (Goodbody-Gringley et al., 2013). The use of dispersants has exacerbated reproductive damage (Epstein et al., 2000; Negri and Hayward, 2000).
Numerous other endpoints have also been assessed in both the coral host and algal symbiont, and presented either as individual measures (e.g., visual coloration, mucus production, polyp retraction/extension, tissue thinning/swelling, algal or chlorophyll a content, or photosynthetic efficiency) or summations of a number of endpoints to reflect a corals condition index (e.g., Renegar et al., 2021). Polyp behavior has been a common endpoint reported in numerous coral
studies with other chemical contaminants, but the implication of this response, especially at the population level, is unknown and further studies should investigate the long-term repercussions of this to fully understand its utility in determining impact (Mitchelmore et al., 2021). Polyp retraction is also sensitive to changes in water flow/handling, time of day and lighting and the presence of food, so measurements must be conducted considering these variables (e.g., at set times of the day) (Bytinsvik et al., 2020; May et al., 2020). Coral exposed to HEWAF in both 96-hr static and pulsed exposures showed tissue regeneration and polyp behavior to be sensitive endpoints (May et al., 2020). Polyp retraction was also the metric used in a deep-sea coral study and highlighted to provide results suitable for an acute assessment (see following text and Bytingsvik et al., 2020).
Corals also represent a variety of species and forms (i.e., branching, massive) and life stages, all of which can significantly influence the toxicity thresholds reported (Shigenaka et al., 2001). Although differences in coral species sensitivity was highlighted by Bak (1987), very few studies have actually investigated this. One method to investigate a species’ sensitivity to a chemical is to use the toxicity thresholds reported for that chemical in multiple species, producing a cumulative distribution which is termed a species sensitivity distribution or SSD (see Section 6.7 and Bejarano, 2016).
Species-sensitivity distributions for five Atlantic scleractinian coral species’ exposure to 1-methylnaphthalene demonstrated that, similar to the Bak (1987) observation, Acropora spp. (in this case Acropora cervicornis) was the most sensitive species (Renegar and Turner, 2021). Interestingly, recent research using passive-dosing exposures to MC 252 surrogate oil and single hydrocarbons has indicated the relative resilience of some corals, likely due to their ability to produce excessive protective mucus, at least over short-term exposure periods (Renegar et al., 2017, 2019; Renegar and Turner, 2021). Corals have typically been thought of as one of the most sensitive marine species; however, in ranking coral toxicity endpoints with those of other marine species the corals were not the most sensitive, but considerably more resilient to this single hydrocarbon dissolved phase exposure (Renegar and Turner, 2021; also see Section 6.7 on SSDs). Of eight tropical species tested in acute exposures to weathered Ichthys condensate, Acropora millepora larvae were not the most sensitive species (a Porifera sponge larvae was), although they were second (Negri et al., 2021). The least sensitive species was adult fragments of Acropora muricata which were not significantly affected by the condensate WAF at the highest concentration used (2,031 μg/L TAH or 100% WAF; Negri et al., 2021). In comparing the toxicity of chemical dispersants in five coral species compared to other aquatic species, Bejarano (2018) highlighted that “[a]lthough it is commonly assumed that corals are among the most sensitive taxa, the sensitivity of five coral species fell within the lower to middle percentiles and were not clustered toward the lower percentiles.”
Corals are exposed to many other natural and anthropogenic stressors, so hydrocarbon pollution often does not act in isolation. Many studies have shown that exposure to additional stressors results in elevated impacts from hydrocarbons, although this remains a relatively understudied area. Furthermore, many environmental conditions typical of coral reefs (i.e., high UV light, temperature stress) are often overlooked and rarely considered in coral oil toxicity tests and risk assessments. For example, impacts of diesel exposure in Pocillopora verrucosa were only apparent with co-exposure to elevated temperature (Kegler et al., 2015). One of the most studied co-stressors has been UV light due to observations of elevated PAH toxicity due to phototoxicity reactions (as discussed in Section 6.3) via photosensitization or photomodification/photo-oxidation reactions (Barron, 2017; Nordborg et al., 2018, 2020). For example, enhanced phototoxicity in low-dose, short-term exposures of fluoranthene to Porites spp. in natural sunlight was calculated to result in a 14× increase in toxicity by comparing upper and lower sides of the coral fragments. However, reduced effects were seen in replicate exposures kept under laboratory lighting, thereby highlighting the importance of using appropriate spectral quantity and quality in laboratory tests so that they do not underestimate toxicity in the field (Martinez et al., 2007). The inhibition of metamorphosis in Acropora tenuis larvae exposed to low concentrations of crude oil WAF (103 μg/L TPAH; suggested to be similar to levels that would be found following a spill) resulted in a 40% increased sensitivity of the larvae when co-exposed to UV light (Negri et al., 2016). Similarly, exacerbation of toxicity of a heavy fuel oil was observed in several early life stages of Acropora millepora following co-exposure with UVR, resulting on average in a 1.3-fold reduction of toxicity thresholds across life stages and endpoints (Nordborg et al., 2021). Co-exposure of two common marine fuels and UVR resulted in decreased larval settlement success in Acropora tenuis compared exposure to the fuels alone (Nordborg et al., 2018).
A review of all coral literature suggested UVR exposure could account for increases in toxicity up to 7.2-fold, leading the authors to conclude that UVR co-exposure should be accounted for in all future coral oil toxicity studies so that reliable toxicity thresholds can be determined for use in the development of credible oil spill risk models (Nordborg et al., 2020; see Figure 6.28). Although there are fewer data available to assess the influences of increased temperature or low pH on oil toxicity, increases, although more modest, were observed (i.e., 3- and 1.3-fold, respectively). Further research is needed to assess the impacts that tropical environmental and climate change co-factors have on the impact of oil pollution in shallow reef ecosystems (see Figure 6.28).
Other confounding stressors that may influence the impact of oil on corals are the health and disease status of the corals. Colonies of the reef-building coral Orbicella faveolata affected with Caribbean yellow band disease (CYBD) were
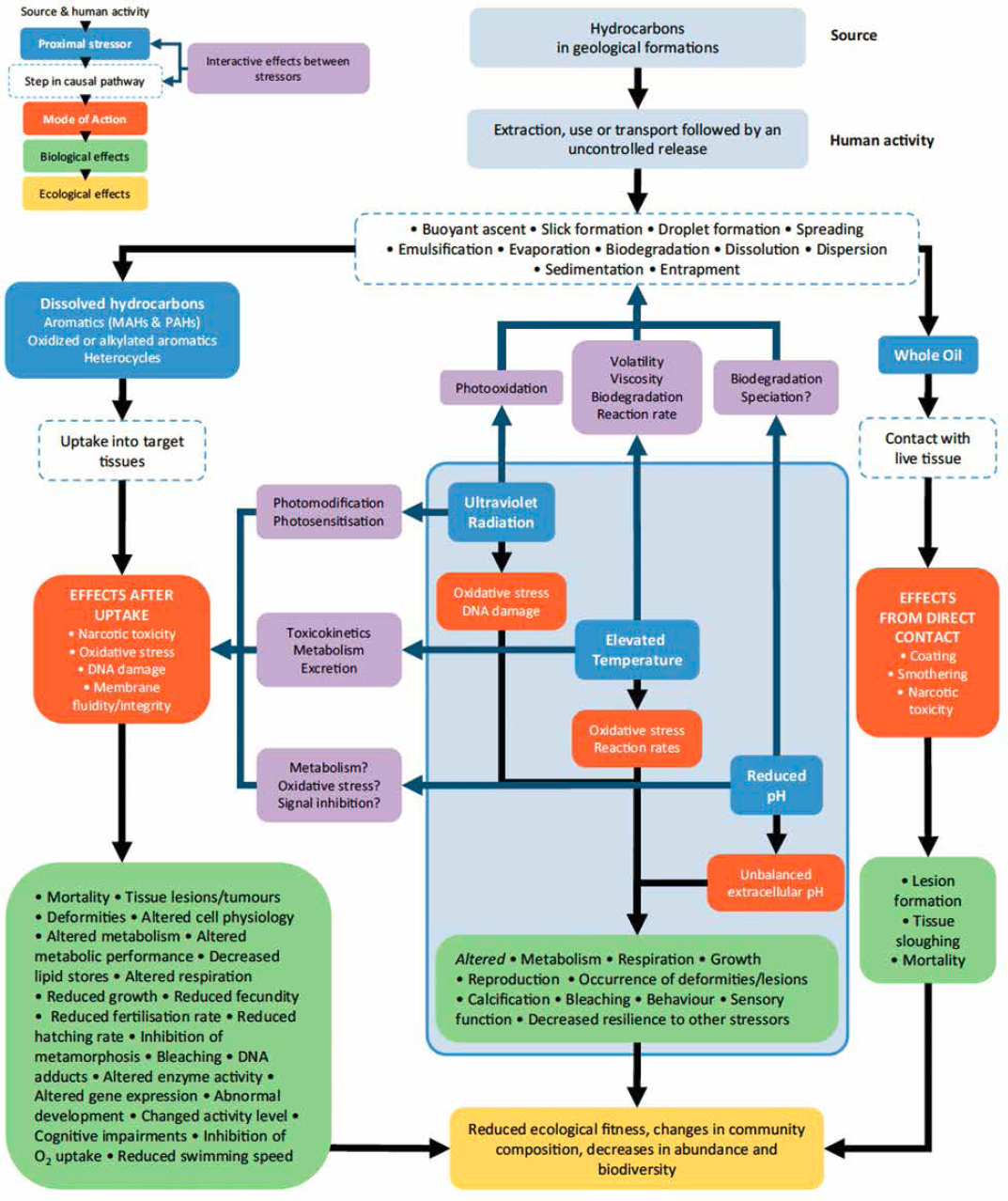
NOTE: Oil toxicity can be affected by environmental co-factors (purple boxes) through direct co-exposure, and resulting interactions, or by affecting oil weathering, dissolution and reaction rates prior to or during uptake into target tissues.
SOURCE: Nordborg et al. (2020).
found to be more vulnerable to the effects of anthracene than healthy colonies, due to a compromised anti-xenobiotic response (Montilla et al., 2016). Alternatively, corals exposed to petroleum hydrocarbons may be less resilient to subsequent infections and levels of disease could increase. Therefore, numerous environmental and coral health co-stressors, together with additional investigations of species and life-stage sensitivities, must be explored further to understand the impact of oil on these important coral reef ecosystems.
6.5.4.2 Mesophotic and Deep-Sea, Cold-Water Corals
As highlighted earlier, deep-sea, cold-water corals are found globally over a wide range of latitudes and depths, and are some of the longest-lived deep-water organisms (i.e., 100 to >1,000 years old; Watling et al., 2011). This high longevity, along with their low metabolic rates, slow growth, and low recruitment rates, makes them particularly vulnerable and slow to recover from anthropogenic impacts (Risk et al., 2002; Girard et al., 2019). Like tropical inter- and sub-tidal corals, these deep-sea species are ecologically and economically important, harboring a high density and diversity of organisms, providing habitat and essential ecosystem services for many, including commercially important species (Cordes et al., 2016). However, compared to their tropical counterparts, the impact of oil and dispersant on cold water species is a significant data gap, recently highlighted by the DWH oil spill which affected abundant and diverse deep sea coral ecosystems in the Northern Gulf of Mexico—which contains 285 deep-sea coral species (Etnoyer and Cairns, 2017). Very little is known about the impact of oil and/or dispersants on these species. Are they more or less sensitive than shallow-water coral species? Are any differences due to environmental variables, such as increased pressure or decreased temperatures, influencing either the fate, uptake, or toxicity of petroleum constituents? Bytinsvik et al. (2000) concluded that, based on their results and the current literature, deep-sea species were similar in their acute toxicity sensitivities to hydrocarbons, oil, and dispersant compared with shallow-water species.
During the DWH oil spill, deep sea coral ecosystems were exposed to oil plumes, dispersed oil, and dispersant, and were also affected by sinking of oil-contaminated marine snow (Camilli et al., 2010; Passow et al., 2017). An impacted coral community covered in brown flocculant material containing DWH oil and dispersant constituents was discovered at 1370 m by the U.S. Bureau of Ocean Energy Management (BOEM; White et al., 2012, 2014). Corals, primarily the octocoral Paramuricea biscaya, showed visual signs of stress, including abnormal skeletal development, increased mucus production, tissue sloughing, and death (White et al., 2012). Subsequent studies have highlighted sublethal impacts and health declines and long-term impacts (7+ years), including branch loss, reduced growth, and hydroid overgrowth (indirect impact from oil/dispersant exposure), suggesting that decades would be needed for recovery to original status (see Hsing et al., 2013; Girard and Fisher, 2018; Girard et al., 2019). Girard and Fisher (2018) highlighted that the ongoing effect (branch loss) observed in 2016–2017 in DWH oil-injured corals could ultimately result in delayed mortality. Similar to other deepsea ecosystems, the NRDA process highlighted that there was a lack of baseline information on the eco-toxicological vulnerability of these deep-sea species (Peterson et al., 2012).
Similar to deep-sea species, injury to mesophotic coral reefs (at depths of 65–75 m) was quantified in more than 400 colonies using pathological assessments. Commonly reported was a biofilm with a clumped or flake-like appearance, with more extensive injuries showing broken and loss of branches and bare skeletons (Etnoyer et al., 2016; Silva et al., 2016). Many of the injured gorgonian octocorals highlighted in 2012 had declined further in condition by 2014 (Etnoyer et al., 2016; see Figure 6.29). The presence of elevated tissue TPAH levels led the authors to conclude that the injuries observed in 2011 may have resulted from an acute event (Silva et al., 2016).
The observation of DWH-impacted deep-sea corals spurred laboratory toxicity experiments with oil and dispersant using octocorals to provide estimates of toxicity for comparison with other species and mechanistic information on responses at the physiological and molecular levels (see DeLeo et al., 2016; Frometa et al., 2017; Ruiz-Ramos et al., 2017; DeLeo et al., 2018, 2021). Many of the gene expression responses were common to those observed in shallow water coral species, including up-regulation of metabolic, immune, wound-repair and oxidative stress responses. However, opposite to typical responses, a reduction in cytochrome-P450 expression was observed following oil exposure (DeLeo et al., 2018). To answer the question of whether an Arctic cold-water coral species (Lophelia pertusa) was more sensitive than other species, acute toxicity tests with individual hydrocarbons were conducted and fit to the target lipid model to generate predictive models and determine species sensitivity (Bytingsvik et al., 2020). Although it appeared that the deepsea coral was more sensitive to 1-methylnaphthalene than the tropical shallow-water Porites spp., this was attributed to differences in the biological endpoints each study used, as observations of narcotic effects were in agreement between the two studies (Renegar et al., 2017b; Bytingsvik et al., 2020). Responses of the deep-sea and tropical corals in these studies were also similar with both 2-methylnaphthalene and phenanthrene. A potential limitation of this work was that toxicity tests were conducted at ambient rather than elevated pressures, although (as discussed in Section 6.7) modeling efforts have shown that elevated pressures result in reductions in hydrocarbon toxicity (Paquin et al., 2018). Therefore, these results are probably conservative, although more empirical data are needed to confirm the acute toxicity of oil and dispersants to deep-sea species and to investigate delayed and chronic impacts.
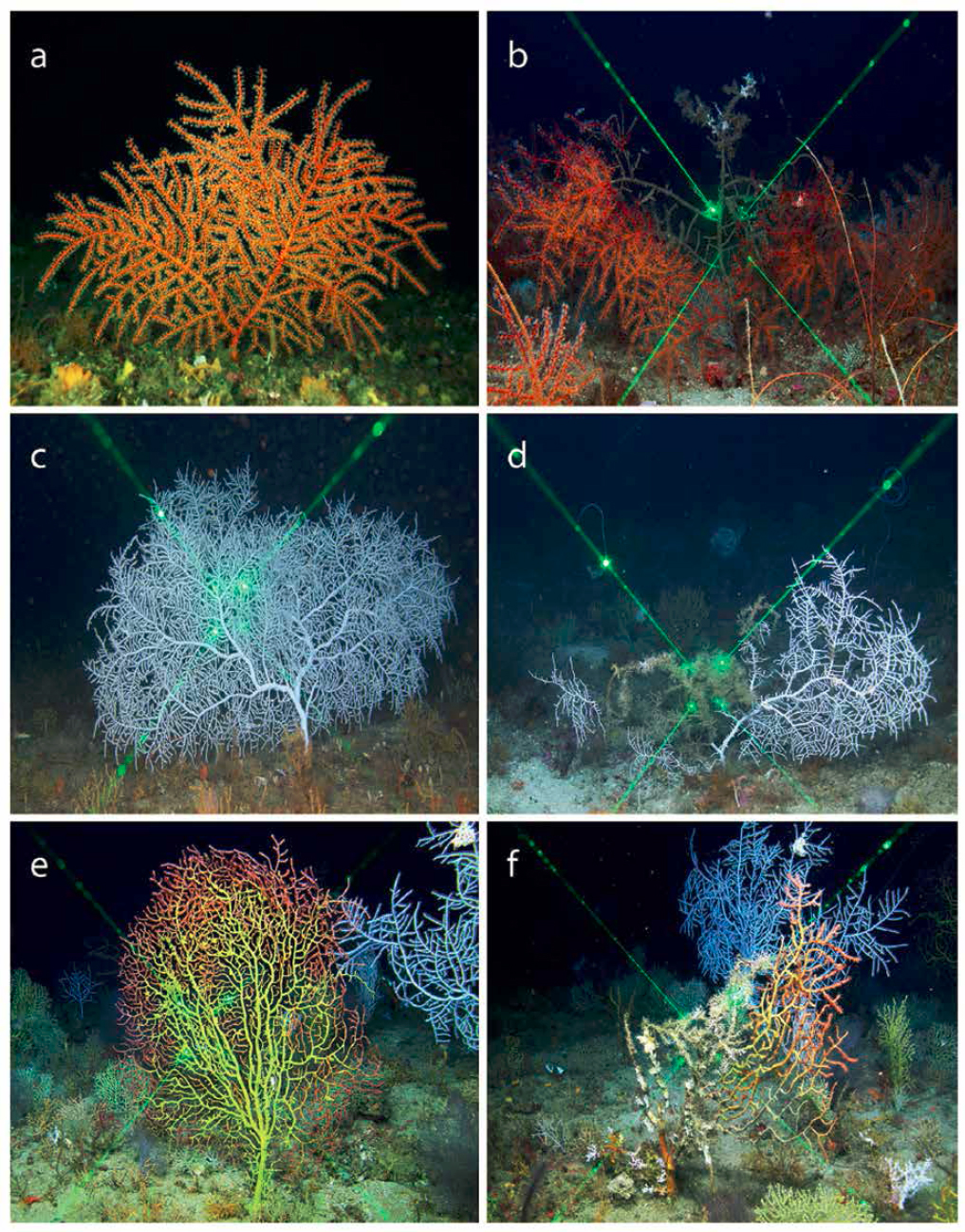
SOURCE: Etinoyer et al. (2016).
A number of studies have investigated the toxicity of Corexit 9500 on deep-sea corals (i.e., Arctic and Gulf of Mexico species), and found similar acute toxicities across the five species despite differences in the biological endpoints used (DeLeo et al., 2016; Frometa et al., 2017; Bytingsvik et al., 2020): 34.8 mg/L for L. pertusa, 7.9–35 mg/L range for Paramuricea type B3, Callogoria delta, and Leiopathes glaberrima and 70.3 mg/L for Swiftia exserta. These ranges are typical compared to other aquatic species tested, falling around the middle of the SSDs reported, and so we may conclude that these deep-sea species are not any more sensitive than other species.
These studies in mesophotic and deep-sea corals highlight the need for prior baseline studies of the health of benthic ecosystems, together with long-term follow-up studies on recovery, delayed mortality, and continued
declines in health in these species, particularly given their slow growth and lower recruitment compared to other marine species and hence potential for a protracted recovery period.
6.5.5 Benthic Communities
Benthos (adj. benthic) are the flora and fauna associated with the bottom sediments of an aquatic system. Benthos are often characterized by position related to the sediment surface (e.g., infauna [subsurface] or epifauna [above the sediment]); by size (e.g., meiofauna or macroinfauna), depending on the sieve size on which they are captured or pass through. Benthic organisms above the sediment surface may be sedentary, such as deep-sea corals (epifauna); stationary benthos below the sediment surface (macroinfauna, meiofauna); or mobile benthic organisms dependent on the bottom (demersal), such as shrimp or crabs with limited ability to move up in the water column or horizontally. Benthic organisms, because of their limited mobility, are monitored in response to pollutants, including petroleum hydrocarbons. The typical response of a benthic community to a continuous stress is a change from a deep-burrowing, larger, and more diverse fauna (not polluted) to high abundance of small organisms that are usually opportunistic surface deposit feeders in response to organic loading in polluted conditions (see Figure 6.31). The nematode:harpacticoid copepod ratio for meiofauna increases with concentration of contaminants in either chronic or acute oiling. Within a given habitat certain species of nematodes are typically most tolerant to stress variables, whereas crustacean meiofauna often are least tolerant (Wetzel et al., 2001). The petroleum inputs may be chronic (drilling or production activities from a platform) or acute (from an oil spill).
The benthos in this section are restricted to open-water habitats, such as estuarine, continental shelf and slope, and deep-sea sediments. Other benthos associated with shoreline habitats will be discussed in those sections (see Section 6.5.3 and others), as relevant.
6.5.5.1 Continental Shelf Soft Sediments
Continental shelf benthos can be affected by the sinking of oil or exposure to production fluids. An example of an oil spill negatively impacting benthic communities is the Amoco Cadiz spill of 1978 in the Bay of Moraix in the western end of the English Channel. A benthic study of a biologically diverse macroinfaunal community was dominated in biomass by Arba alba (bivalve) but the highest abundance among 25 species was composed of three species of Ampelisca amphipods (Dauvin, 1998). The same species of Ampelisca were absent from the community immediately after the oil spill for at least 2 years. These crustaceans lack pelagic larvae, and recruitment to prior population levels would take longer than for the polychaete populations that broadcast numerous larvae to the water column. The Bay of Moraix benthic community was repopulated by one species of Ampelisca after 3 years, but not all three of the originally dominant Ampelisca spp.
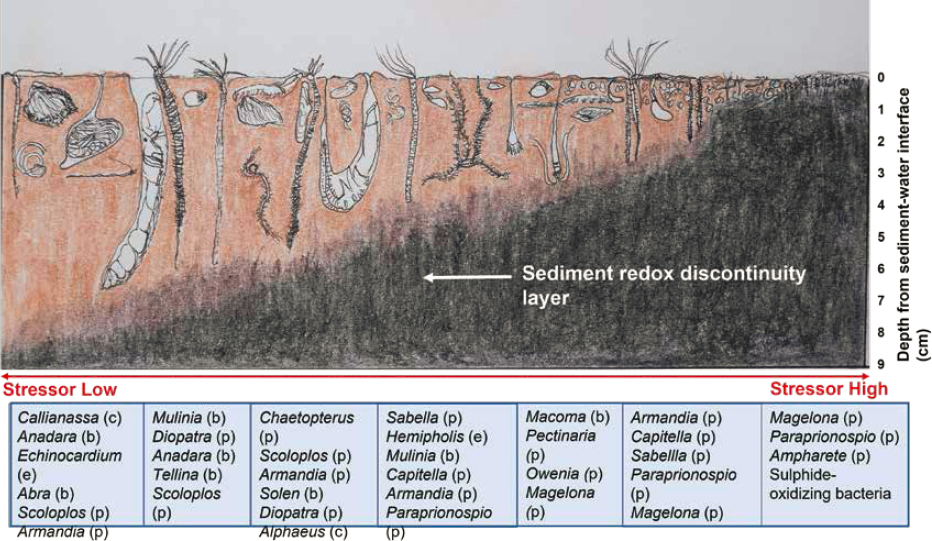
SOURCE: N.N. Rabalais, modified from Pearson and Rosenberg (1978).
until 13 years after the oil spill through the total period of the study (18 years). At the same time, similar proportions of other infauna returned to similar pre-spill proportions.
Chronic exposure of benthic communities to petroleum hydrocarbons was examined in meiofaunal and macroinfaunal communities around three gas platforms on the continental shelf (29–157 m water depths) in the Gulf of Mexico associated with long-term production (Montagna and Harper, 1996). Effects only extended to the local area 100 m from the platforms. Total polychaete and nonselective deposit-feeding nematode density increased near platforms, which could be a response to organic enrichment. Amphipod and harpacticoid profusion and diversity and harpacticoid reproductive success declined near platforms, consistent with other studies of these organisms having lower recruitment because of the lack of pelagic larvae.
6.5.5.2 Deep-Sea Soft Sediments
Meiofauna (size > 63 but < 300 μm) and macrofauna (size retained on a 300 μm sieve) were used to examine the effects of the DWH oil spill of 2010 (Montagna et al., 2013). Ryerson et al. (2012) estimated that up to 35% of the hydrocarbons were trapped and transported in continuous deep sea plumes. Direct sinking of oil from the deepwater plume transported it to deepwater sediments, adsorption of small oil droplets onto particles in marine snow, among others; and sinking of oil-mud complexes resulting from the injection of drilling muds during top-kill operations (reviewed by Mongagna et al., 2013). Meiofaunal and macrofaunal samples were collected 2–3 months after the well was capped in water depths ranging from 76 to 2,767 m along a gradient of suspected oil contamination. The benthic communities experienced severe and moderate damage in 58 samples within an area of 148 km2. Impacts included low diversity, low evenness, and low taxonomic richness, correlated with high levels of total petroleum hydrocarbons (TPHs) and polycyclic aromatic hydrocarbons (PAHs). Additionally, barium levels near the wellhead were very high. High nematode-to-harpacticoid copepod ratios corroborated the severe disturbance of meiofauna communities. For macroinfauna, the impacts were loss of biodiversity and low abundance of amphipods.
An additional 58 station samples were analyzed to enhance the resolution of the original Montagna et al. (2013) assessment and determine if impacts occurred further afield (Reuscher et al., 2020). These samples indicated that an area covering about 24 km2, extending 3 km in all directions from the wellhead, displayed the most severe relative reduction of faunal abundance and diversity. Moderate impacts were observed up to 17 km toward the southwest and 8.5 km toward the northeast of the wellhead. The samples also correlated benthic effects to total petroleum hydrocarbons, polycyclic aromatic hydrocarbons, barium concentrations, and distance to the wellhead. Hydrocarbon seeps located 100 km to the east and 240 km to the southwest of the Macondo wellhead were not implicated in the effects. The work of Reuscher et al. (2020) calculated the affected area to be 78% higher than original estimates, covering approximately 263 km2 around the wellhead. Adding new sampling stations extended the benthic footprint map to about twice as large as Montagna et al. (2013) originally estimated and improved the resolution of the spatial interpolation.
Initial impacts to benthic infauna were greater in spatial extent than defined by resource assessment sampling. This sentiment was echoed in the Trustee Council’s Final Programmatic Restoration Plan (2016).
In the future, understanding of impacts on benthic infauna could be improved by increasing the spatial and temporal extent of sampling when designing assessment studies. Such an expansion is applicable to many other assessment indicators for impacts of and recovery from oil spills.
There was one sample in 76-m water depth designated as “moderately affected” by the DWH oil spill among all other sites in >300-m water depth for the deep-water study of Montagna et al. (2013), but this categorization was inconsistent for less contamination and fewer impacts on the fauna in stations in closest distance and in a northwest-to-southeast transect from the wellhead. This station was closest to the birdfoot delta and subject to high sediment and organic loading that could be detrimental to the formation of a typical continental shelf area. Another station in the extended analyses of samples by Reuscher et al. (2020), located 12 km offshore of Grand Isle in the Mississippi Bight, was characterized by faunal indicators that would indicate a highly stressed faunal community but no sign of hydrocarbon contamination. The station had the highest nematode-to-harpacticoid copepod ratio and low values for macrofauna and meiofauna diversity and evenness among the additional stations. The disturbance of the infauna was likely caused by hypoxic bottom-water conditions, which are common along the Louisiana coast during summer months (Rabalais and Turner, 2019). Hypoxia causes local extinction of sensitive organisms, while tolerant taxa may thrive (Baustian and Rabalais, 2009). These two atypical continental shelf stations amid a study of deep-water benthos exposed to DWH oil spill contaminants emphasizes the need to know the potential multiple stressors that may be affecting a habitat (see Section 6.5.5.1).
The above-identified benthic work for the DWH oil spill was coupled with other data in a synthesis of benthic faunal impacts (Schwing et al., 2020b) including microbes, foraminifera, macrofauna, meiofauna, megafauna (invertebrates), corals (see Section 6.5.4.2 on Mesophotic and Deep-Sea Cold-Water Corals), and demersal fishes. Where there were adequate baseline data from before the oil spill, compilations were able to identify impacts of shifts in community structure and changes in diversity and abundance. Time to return to a pre-DWH oil spill condition was (1) within 2 years for the microbial community, (2) within 5 years following
a decrease in density and diversity for foraminiferans, (3) not within 4 years for meiofaunal taxa richness in the impacted area, (4) also not for decreased macrofaunal taxa richness as of 4 years post-spill, (5) inconclusive for megafauna due to lack of suitable data for comparison, and (6) a progression of some recovery for deep-sea corals after 7 years and within 10 years, but remaining predicted impact for as much as 50 for some species while other recoveries are predicted to extend to 100 years.
6.5.5.3 Hydrocarbon Seeps
Hydrocarbon and methane seeps are most abundant and most prolific in the central and western regions of the northern Gulf of Mexico in depths of 300 to 1,500 m (Garcia-Pineda et al., 2014), and also in the Bay of Campeche, southern Gulf of Mexico (MacDonald et al., 2015). Hydrocarbon seeps are also a feature off southern California, but at shallower depths (16–18 m) in the Santa Barbara Channel between Coal Oil Point and Goleta Point off Santa Barbara, California (Spies and Davis, 1979). The volume of inputs, composition, and fates of the hydrocarbons are covered in Sections 3.2 and 5.4.1.
Because of their slow seepage through the marine sediments before release, which allows solute-equilibrium with the subsurface water, liquid oil is not expected to contain significant fractions of light components. Furthermore, natural seeps have limited benthic footprint of impact because most of the seep oil is weathered and rises to the ocean surface in droplets when it releases from the sea floor (Sassen et al., 1999; MacDonald et al., 2002).
The nature of sediment organic matter enrichment owing to increased bacterial biomass of hydrocarbon-degrading bacteria is similar to that in shallow-water petroleum seeps. The organic enrichment is coupled with a response of increased meiofaunal and macrofaunal abundance and biomass compared to non-seep sediments (Montagna et al., 1987). Rather than a toxic and negative impact on benthic communities, there is a response of higher abundance of nematodes (meiofauna) but not harpacticoid copepods, which were similar in abundance in non-oiled seep areas. The macroinfauna, which are dominated by deposit-feeding polychaetes, were more abundant at the seep than at a nearby non-seep station (Spies and Davis, 1979). Mats of the sulfide-oxidizing bacteria Beggiatoa spp. are a common feature of oil seeps, as they are with deep-water seeps. In both situations, the seeped oil is not as toxic as a fresh oil because of the slow release from the formation, but supports an enriched organic sediment for higher abundances of organisms that feed on the bacteria.
Associated with Gulf of Mexico deep-water hydrocarbon seeps are low-temperature complex chemosynthetic communities (tube worms, methanotrophic mussels, clams, and various other fauna) that derive energy from reduced carbon, mainly methane, and bacterial H2S (MacDonald et al., 1994; Sassen et al., 1999). Later research confirmed that a number of complex chemosynthetic communities were spatially associated with gas hydrates on the continental slope. At seeps below the photic zone (>200 m), where food sources are limited, the consistent supply of oil and gas supports dense aggregations of sessile invertebrates and associated fish and crustaceans (MacDonald et al., 1994). The oil and gas seeps and hydrate communities host chemoautotrophic symbionts that are unique biogenic communities with a high diversity of organisms, including bacteria consortia that support numerous, as yet unknown, biogeochemical processes. Metazoan invertebrates such as tubeworms, clams, and mussels host chemoautotrophic symbionts that are able to fix new carbon using H2S and methane as energy sources (Fisher, 1990). These organisms irrigate seep sediments and further promote degradation of hydrocarbons (Cordes et al., 2016). The Gulf of Mexico deep-sea gas and hydrate seeps are not unique in the ocean; similar ecological adaptations occur worldwide (Dubilier et al., 2008). As relatively understudied habitats, research is needed to increase understanding of these communities, especially in deeper sea locations, to identify novel species/biochemical pathways and chemosynthesis, and to identify bacteria that may be useful in oil spill response (i.e., oil degraders).
6.5.6 Water Column
The effects of oil spills are obvious as surface-water oil slicks impinge on shorelines or as larger organisms such as marine mammals, sea turtles, diving ducks, and piscivorous birds encounter the oil at the sea surface. The oil in the water column below the surface slick, in contrast, affects different pelagic communities. The naturally dispersed oil below the surface can affect the 1 to 1.5 m of the water column below the surface (personal observations, diving operations, N.N. Rabalais, Louisiana State University) (see Figure 1 in Peterson et al., 2012), and may extend to depths of 25 m or more (Short and Harris, 1996). This dispersed oil mixture with water and finely dispersed droplets of weathered surface oil may affect organisms in the pelagic water column, in particular phytoplankton and zooplankton, in upper water column communities.
6.5.6.1 Bacterial Communities
The stimulation of oil-degrading bacteria was evident in the deep-water dispersed hydrocarbon plume from the DWH spill, with the peak at approximately 1,200 m water depth (Hazen et al., 2010). Cell densities within the plume were twice those detected outside the plume. The presence of these aerobic oil-degrading bacteria resulted in oxygen saturations of 59% within the plume compared to 67% outside the plume. These saturation levels were well above the critical saturation level of 30%, below which fishes, crabs, and shrimp are negatively affected in the coastal Gulf of Mexico oxygen-depleted waters (Rabalais and Turner, 2019).
Associated flocculants such as those detected within oil contaminated waters of the DWH oil spill (Achberger et al., 2021) eventually lead to the sedimentation of oil as marine oil snow (MOS; see Section 5.3.2.2). In an experiment to understand the role of aggregates in hydrocarbon degradation and transport, a MOS sedimentation event was produced using Gulf of Mexico coastal waters amended with oil or oil plus dispersant. Results showed smaller micrometer-scale (10- to 150-μm) microbial aggregate formation in addition to MOS. These microaggregates were most abundant in the oil-amended treatments and commonly associated with oil droplets, connecting their formation to the presence of oil. The maximum observations of the microaggregates overlapped with the maximum rates of biological hydrocarbon oxidation estimated by the mineralization of 14C-labeled hexadecane and naphthalene. To clarify the prospect of microaggregates serving as hot spots for degradation, Achberger et al. (2021) categorized the free-living and aggregate hydrocarbon associated microbial collections using 16S rRNA gene sequencing. The study found the microaggregate population dominated by bacteria and enriched with supposed hydrocarbon-degrading taxa. Using catalyzed reporter deposition fluorescence in situ hybridization (CARD-FISH) (Pernthaler et al., 2002; Pernthaler and Pernthaler, 2007) for observation of these taxa confirmed higher amounts within microaggregates compared to the surrounding seawater (Achberger et al., 2021). Metagenomic sequencing of these bacteria-oil microaggregates (BOMAs) further revealed their community’s ability to use various hydrocarbon compounds. These data lead to the fact that BOMAs are intrinsic features in the biological response to oil spills. They are also possibly important hot spots for hydrocarbon oxidation in the water column. These natural biological responses to oil in the environment from the DWH spill produced vast quantities of oil-associated marine snow (MOS). However, ambiguity remains about the forces controlling MOS formation and how it influences the environment.
There is detailed information on microbial communities in Chapter 5 (see Sections 5.2.8.2 and 5.3.2) concerning microbial community composition related to shifts that occur after oil spills and those associated with MOS. Furthermore, there is a conclusion in Section 5.4.5, Fates of Oil in Coastal Ecosystems: Monitoring PAH Profiles in Sediments and Bivalves, stating that
[t]he development and application of ‘omics has revolutionized microbial ecology and understanding of the microbes that respond to and biodegrade oil in the sea. . . . ‘Omics techniques provide insight into the composition of microbial communities, their succession patterns, and both individual and composite biochemical activities which, together, influence the fate of oil in the sea.
Observations found part of the natural biological response to the DWH drilling oil spill resulted in vast quantities of oil-associated MOS forming throughout the water. However, the mechanisms controlling MOS formation and its impact on the environment remain largely unknown. Continued research is needed on the formation of MOS, influences on the processes of oil degradation and eventual hydrocarbon fates, as well as ecological consequences.
6.5.6.2 Phytoplankton Communities
Phytoplankton inhabit near-surface waters where they are most likely to be exposed to dispersed oil in the upper water column mixed layer. The conditions in these waters reduce the sunlight necessary for phytoplankton primary production, and the water may contain toxic hydrocarbons. Zooplankton vertically migrate, usually to take advantage of phytoplankton prey during the dark, so they are exposed to oil dispersed throughout the upper water column.
Studies of the effects of oil on phytoplankton often use single algal species lab cultures in laboratory microcosms (reviewed by Ozhan et al., 2014), and sometimes “natural” phytoplankton communities collected from the field (e.g., González et al., 2009). The field-collected phytoplankton studies examining multiple effects may be held at in situ environmental conditions, but the communities remain confined to microcosms within simulated environmental conditions or incubated within the environment from which the samples were collected (Ren et al., 2009; Gilde and Pinckney, 2012, respectively). Determining shifts in phytoplankton community composition, abundance, and biomass over time within an area that experiences an oil spill is complicated by lack of a suitable reference area, unknown exposure level and history, and other environmental factors (see Section 6.5.1).
Phytoplankton communities differ by season, physical variables, and nutrient availability (Parsons et al., 2021), which complicates the determination of the effects of an oil spill. The DWH oil spill and an existing long-term data set enabled a comparison of pre-, during-, and post-spill phytoplankton composition (Parsons et al., 2015). The study period encompassed spring and summer of 1990–2009, 2020, and 2011. The baseline data were collected as part of the Rabalais and Turner (2019) Gulf of Mexico hypoxia legacy data and represented monthly samples between 1989 and 2009 and monthly samples during the spill period of May–October 2010 (Parsons et al., 2015). The results of the CLUSTER and SIMPROF analyses (with PRIMER 7) indicated that the years 1994, 1996, 1998, 2001, 2003, and 2008 were not statistically different (p > 0.05) from 2010 in terms of the environmental parameters between the months of May and October, thus removing potential seasonal effects from consideration. Additionally, the CLUSTER and SIMPROF results on the baseline-averaged monthly phytoplankton data versus the 2010 monthly phytoplankton data indicated that the phytoplankton assemblage was different in 2010 compared to the baseline data and that the overall abundance was 22% lower following the DWH oil spill (p <0.05). The cyanobacteria, autotrophic
ciliates, cryptomonads, and chlorophytes accounted for the majority of the decrease. Diatoms and euglenophytes were more abundant in 2010, suggesting a possible stimulation from the oil or a relaxation in grazing pressure. Similarly, some individual phytoplankton species increased in abundance (e.g., small centric diatoms and Cerataulina pelagica), whereas others decreased (e.g., Thalassionema nitzschioides and Mesodinium rubrum) during the oil spill.
For comparison of phytoplankton communities potentially affected by the DWH oil spill, there were increases and decreases in the abundances of phytoplankton east and west of the Mississippi River delta (Quigg et al., 2021b). Small temporal and spatial scale variability of phytoplankton community dynamics precluded any inferences of petroleum exposure related to their composition. Phytoplankton studies to the east of the Mississippi River were limited and lacked good long-term data.
6.5.6.3 Zooplankton Communities
Most zooplankton do not occupy the upper water column during the day, in order to avoid their predators. The result is exposure in the surface layer at night and what might be encountered subsurface in day light. Daly et al. (2021) assessed the zooplankton community in the area from the DWH well head northeastward through the DeSoto Canyon to nearshore. Parameters included abundance, biomass, spatial distribution, species composition, and diversity indices in spring, summer, and winter, May 2010 to August 2014. SEAMAP (Southeast Area Monitoring and Assessment Program, National Marine Fisheries) samples collected between spring and summer 2005–2009 were analyzed as a baseline against which supplemental studies during the oil spill may be compared. The results of Daly et al. (2021) demonstrated that zooplankton community dynamics are strongly governed by environmental variability and riverine processes. The oil spill in spring 2010 did not significantly affect the Zooplankton abundances compared with those from spring 2011 and 2012. Over the period from 2005 to 2014, the summer 2010 zooplankton abundances were the highest observed and correlated with a high river discharge, high chlorophyll, and aggregation in eddies.
Carassou et al. (2014) examined zooplankton communities from the Alabama continental shelf (north central Gulf of Mexico) before and during (2005–2009) the DWH oil spill (for the months of May–August). They observed shifts in assemblage structure in May and June 2010, but these differences were no longer significant by July 2010. ANOSIM testing confirmed weakly significant, differences in mesozooplankton population composition during the oil spill years, when compared to historic years. Mesozooplankton assemblages were different during the oil spill at both sites when all months were combined together (p <0.2).
Many taxa had higher densities during the oil spill year (e.g., calanoid and cyclopoid copepods, ostracods, bivalve larvae and cladocerans, and echinoderm larvae) but the differences were inconsistent among stations. Daly et al. (2021) cited environmental variables as important factors affecting the zooplankton communities in their study, but similar environmental factors were dismissed by Carassou et al. (2014), and the differences were attributed to the DWH oil spill. In neither case were data for potential exposure to petroleum hydrocarbons provided.
6.5.6.4 Kelp Beds
Kelp is a macroalga, attached to the seabed with gas-filled structures at the base of the blades to hold the kelp blades close to the water’s surface. Thus, its oil exposure may be in the surface sheen or in the subsurface water column. Results are mixed concerning kelp communities exposed to an oil spill. The World Prodigy tanker released approximately 922 tons of No. 2 fuel oil on Brenton Reef, Rhode Island, and into surrounding coastal waters in 1989. Peckol et al. (1990) investigated the effects of oiling on the subtidal kelps Laminaria saccharina and L. digitata. Data on conditions of kelp at the same site, during pre-spill conditions (1984–1987) were compared with post-spill kelp condition, growth rates with depth, and pigment acclimation. The data indicated no evidence of detrimental effects by oiling on the kelps. There were no necrotic or bleached tissues on any kelps in an oiled cove. Both kelp species continued growth rates within the range of previous years’ data and pigment acclimation was similar for all years. A significant brown tide in 1985 caused the lowest growth rates. This study and other data suggest that Narragansett Bay avoided harmful effects of the oil spill because little fuel oil mixed into the water column and therefore subtidal vegetation was spared.
A massive outflow of oil from an offshore drilling accident occurred near Santa Barbara, California, in January 1969. A part of the offshore-through-intertidal transitions of vegetation, algae, and invertebrate communities exposed to oil were offshore kelp communities. Some direct observations close in time to the spill (Foster et al., 1971) indicated that the kelp, consisting almost entirely of Macrocystis angustifolia, received the first dose of incoming oil. The floating fronds initially held large quantities of oil, especially during low tides, and the brown color of the beds turned black. Most of the oil in the offshore fringes of the kelp was dispersed by winds, currents, and tides, then moved shoreward. Oil did not adhere to healthy kelp fronds. Furthermore, invertebrate organisms associated with the oiled kelp beds did not differ from those in communities associated with non-oiled kelp.
6.5.6.5 Fish and Other Water-Column Inhabitants
Although widespread mortalities of fish, crustaceans, and other megafauna inhabiting the marine water column are infrequent, high winds during or immediately after spills that promote and entrain dispersion of oil droplets into
seawater increase deleterious effects on these organisms. The 1996 North Cape spill provides an example of serious effects attributed to oil naturally dispersed into the air and water. Amid a winter storm near the coast of Rhode Island the barge North Cape discharged ~3,000 tonnes of No. 2 fuel oil. Storm winds above 100 km/h and breaking waves >5 m spread the oil along the coast and into inshore salt ponds, and dispersed the oil throughout the water column (Reddy and Quinn, 2001). Concentrations of 26 PAC (polycyclic aromatic compounds)5 and of total petroleum hydrocarbons, existing as droplets of dispersed oil and dissolved compounds, in the water column reached 115 and 3,940 μg/L, respectively. These measurements are some of the highest concentrations of PAHs in the water column ever recorded after an oil spill (Reddy and Quinn, 2001), causing substantial mortality to aquatic organisms. Estimated deaths include 2,292 birds, 312,000 kg of lobsters, nearly 1 million kg of shellfish, and 116,000 kg of fish (NOAA et al., 1999).
Detection of photoenhanced toxicity effects in the open marine water column has not been clearly established. Studied of natural seawater polluted with oil from the North Cape showed a significant increase in the toxic effects of the oil to embryos of dwarf surf clams (Mulinea lateralis; Ho et al., 1999) after photoenhancement (Arfsten et al., 1996). However, photoenhanced toxicity effects of oil from the 2002 Prestige oil spill were not detected in embryogenesis bioassays involving mussels (Mytilus galloprovincialis) or sea urchins (Paracentrotus lividus), nor in the copepod Acartia tonsa or the fish Cyprinodon variegatus (Saco-Álvarez et al., 2008). Photoenhanced toxicity effects in the sea water column were also not reported after the DWH oil spill, but that may have been a consequence of lack of sampling effort.
Cardiotoxic effects appeared in Pacific herring (Clupea pallasii) embryos reared in subtidal cages near shorelines oiled by the 2007 Cosco Busan spill (Incardona et al., 2012). Though cardiotoxic effects probably affected developing embryos of fish and perhaps other organisms exposed to PACs dissolved from Macondo 252 oil following the DWH spill, clear evidence supporting this has not been presented.
6.5.6.6 Produced Water Discharges
Another type of dispersed petroleum hydrocarbon results from the disposal of produced waters, a by-product of oil and gas exploration and production with contamination by elevated salinity (usually), petroleum hydrocarbons, trace metals, and radionuclides (see Sections 3.4.1.2 and 5.2.5). Produced waters are usually discharged mid-water but sometimes onto the water surface or near the seabed (Neff et al., 2011). Produced waters are the primary source of hydrocarbons from these activities, with minor contributions from deck washing and drilling fluids and cuttings. The constituents of produced water and their relative proportions vary widely according to the petroleum reservoir (Neff et al., 2011). The consensus of several reviews (Holdway, 2002; Neff et al., 2011; International Association of Oil & Gas Producers, 2020) is that the produced water pollutants disperse quickly into the water column, and laboratory studies of toxicity and field surveys suggest that the overall risk of produced water discharge inducing adverse impacts in populations of pelagic organisms is low.
The Canadian Environmental Research Studies Funds supported monitoring of juvenile fish that were exposed to hydrocarbon discharges from three oil and gas production platforms on the Grand Banks and a reference area at least 2 km from the production platforms (LGL Limited and Ocean LTD, 2018). Four species of fish (American plaice, Atlantic cod, capelin, and sand lance) were collected in both bottom trawls and mid-water trawls at least 1 km from the discharges. Comparisons of ethoxyresorufin-O-deethylase (EROD) activity as indicators of increased enzymatic responses and metabolites in fish tissues and bile were inconsistent with relationship to a fish type, fish size, discharge platform or distance from it. There were no clear relationships of produced water discharges with metabolic indicators in the fish (LGL Limited and Ocean LTD, 2018). Potential reasons for this were the mobility of the fish collected at least 1 km from the discharge, and the uniqueness of produced water chemical constituents by platform.
Produced water also contains substantial concentrations of alkyl-phenols, some of which are estrogen-mimic endocrine disruptors (Boitsov et al., 2007; Meier et al., 2007). Discharge in hypersaline produced waters may transport these compounds to the seafloor. Adverse effects of these compounds appear to be limited to 1–2 km from the point of discharge (Bakke et al., 2013). Examination of organisms in situ that are exposed to produced water discharges include studies of kelp and deployed mussels. Giant kelp (Macrocystis pyrifera) recruitment near a produced water diffuser off the Santa Barbara, California, coast was affected only within 50 m of the outfall, most likely related to gametophyte survival (Reed et al., 1994). Osenberg et al. (1992) deployed mussels (Mytilus edulis and M. californianus) down-plume from the same discharge off Santa Barbara and found distance-from-source sublethal effects in shell growth and condition. Osenberg et al. (1992) noted that it was difficult to separate differences in mussel recruitment from planktonic larvae and local production of propagules.
Although not water column organisms, the produced water discharge affected benthic communities with nematodes being more abundant closer to the diffuser off Santa Barbara, but there were reduced abundances of most carnivorous groups, including nemerteans and several families of polychaetes (Osenberg et al., 1992). Similarly, Rabalais et al. (1991, 1992) documented adverse effects on benthic communities in the northern Gulf of Mexico, such as
___________________
5 Although PACS and PAHs are sometimes used interchangeably in the literature, PAHs containing only carbon and hydrogen are a subset of PACs. See Section 2.1.3 for more details.
mortality, lower abundance, and dominance by a few species of opportunistic polychaetes. These effects were within 500 m of the discharge. Discharges closer to the seabed (in this case, at 19 m in a 20-m water column) contained higher concentrations of petroleum compounds than discharges in mid water, because the produced waters were entrained in the hypersaline plume at the sea bed and not dispersed.
Determining effects on pelagic communities is difficult, primarily because of unknown exposure, wide dispersal of oil hydrocarbons, multiple environmental factors in this water habitat, and the ephemeral nature of these communities. To fill this gap, in situ sensors could be developed for petroleum hydrocarbon detection and image analysis for plankton, as well as using autonomous underwater vehicles (AUVs) for determination of water column effects. However, these communities are ephemeral.
6.5.7 Ecosystem Effects
Oil in the Sea III (NRC, 2003) noted the difficulty of translating individual-level effects of an oil spill to the level of population effects. Extrapolation of population-level effects to the level of ecosystem effects is more difficult. Reasons for the difficulty include the high variability of temporal and spatial characteristics of the habitat, along with the variability in population structure and dynamics, environmental forcing factors, and unknown community structure and trophic interactions. Within the context of open ecosystems, the successful recruitment of individuals is essential for the recovery of populations and subsequent community interactions.
6.5.7.1 Trophic Transfer
Oil pollution may become incorporated into marine food webs through trophic transfer of toxic compounds via ingestion of PAHs from the primary producer level to higher organism levels. Evidence of trophic transfer is found in studies where the source of hydrocarbons contained in prey organisms was clearly linked to oil components ingested or absorbed by those organisms, and the transfer of those hydrocarbons from prey to their predators is also clear. These hydrocarbons are bioaccumulated when ingestion leads to incorporation into the somatic tissues of the organisms that consume them. Biomagnification occurs when organisms are unable to efficiently excrete accumulated contaminants, resulting in contaminant concentrations that increase in species occupying higher trophic levels. The enzymatic pathways of CYP1A induce metabolism of PAHs in vertebrates, but there is no biomagnification of these contaminants, as in growing-body burdens of mercury. Oil-contaminated plants and animals, whether through contact with or ingestion of oil, provide a route of additional contamination of species that consume them. Animals that consume oil-contaminated prey may incorporate various components of the ingested oil into their tissues. These oil components tend to migrate into the most lipid-rich tissues because of the higher solubility of oil components in natural oils compared with water. The persistence of oil components in tissues depends mainly on how fast the organism turns over its lipid reserves, and whether the organism is able to biochemically degrade the accumulated oil components.
Most invertebrates are cold-blooded and hence have relatively slow metabolic rates, and also have less well-developed biochemical pathways to degrade the more persistent and toxic oil components such as aromatic hydrocarbons, so half-lives of accumulated oil components may range from several days to months. However, fish and most other vertebrates, as well as some invertebrates, can produce enzymes that rapidly degrade aromatic hydrocarbons into more water-soluble products that are readily excreted. These pathways may be activated within an hour of initial exposure, and can rapidly degrade most ingested aromatic hydrocarbons within 1 day or so.
Vertebrates constitute most of the species that occupy the higher trophic levels of marine food webs, and their ability to rapidly degrade aromatic hydrocarbons precludes biomagnification of these compounds in marine food webs. Although organisms may readily bioaccumulate oil and oil-derived compounds through ingestion or absorption, these compounds rarely biomagnify in marine food webs. Finfish and most arthropods are able to metabolize PACs relatively rapidly (Meador et al., 1995), and consequently are considerably less likely to be detectably tainted by petroleum-derived compounds after a spill in comparison with filter- or suspension-feeding shellfish. Filter- and suspension-feeding shellfish can efficiently accumulate dispersed organic particles, including oil droplets, from seawater, and their depuration rate of these compounds is relatively slow. For example, blue mussels (Mytilus edulis) ingest food particles ranging from 1–35 μm (Strohmeier et al., 2012), and oysters (Crassostrea virginica) ingest particles as large as 400 μm (Tamburri and Zimmer-Faust, 1996). Once ingested petroleum compounds may remain detectable for months (Meador et al., 1995) following surface oil spills that generate widespread dispersions of oil microdroplets into the water column near the sea surface.
6.5.7.2 Community Effects
Damage inflicted by large oil spills on populations and habitats can perturb ecological communities, with consequences that are long term and possibly permanent. For example, effects may result from widespread initial mortalities of keystone species that subsequently alter populations of other species and communities that have strong links with them. Avoidance of persistent oil-contaminated habitats by organisms, aquatic and terrestrial, can alter community interactions, including permanent alteration following local
extirpation of one or more aquatic or terrestrial species. Community-level effects remain among the least well-understood consequences of oil spills. Potential pitfalls for lack of evidence are identified in Section 6.5.1. This section examines community-level effects of major oil spills and makes appropriate suggestions of altered ecological processes. Conclusions may not be definitive and remain inconclusive, but may outline suitable rationale for an “effect” or no “effect” call.
6.5.7.3 Indirect Effects and Trophic Cascades
Community responses to strong environmental perturbations provide invaluable insights regarding ecological structure, linkages, and functioning, especially in marine ecosystems at large spatial scales that are not readily amenable to direct experimental manipulation and composed of species populations that are usually difficult to monitor. The appropriate standards of evidence for evaluating hypotheses in these cases are similar to those often applicable in geology, where ancient processes that are not directly observable must be inferred from available observational data in the present. In such cases, acceptance of proposed hypotheses depends on the ability of a candidate hypothesis to account for an extensive body of widely ranging qualitative and quantitative observations, along with the absence of any conclusively contradictory evidence.
Large marine oil spills have the potential to cause strong ecological perturbations if they result in widespread mortalities of major component species. These indirect effects have often been suspected after major spills, but have rarely been clearly documented. The most compelling examples of such indirect effects are associated with the DWH and Exxon Valdez oil spills, and even for these the available evidence remains merely suggestive. Nevertheless, these are summarized here in recognition that appropriate appreciation for the weight of this evidence provides guidance for more careful evaluation of similar effects following future oil spills or other perturbations of marine ecosystems. Substantial evidence suggests that the extensive mortalities of piscivorous seabirds following the DWH oil spill (Haney et al., 2014a,b) released juvenile Gulf menhaden from predation, triggering a trophic cascade response (see Figure 6.32). Gulf menhaden abundance and biomass reached record-breaking levels in 2011 and 2012, which were associated with the poorest body condition on record as well (Short et al., 2017, 2021), suggesting that the population had outstripped its available supply of phyto- and zooplankton food. As an important forage fish in the coastal waters that were contaminated by oil from the DWH spill, the poor body condition of Gulf menhaden in 2011 and 2012 made them less nutritious for the many species that consume them. This is an example of a community-level response that may have gone undetected in prior oil spills such as the Exxon Valdez oil spill.
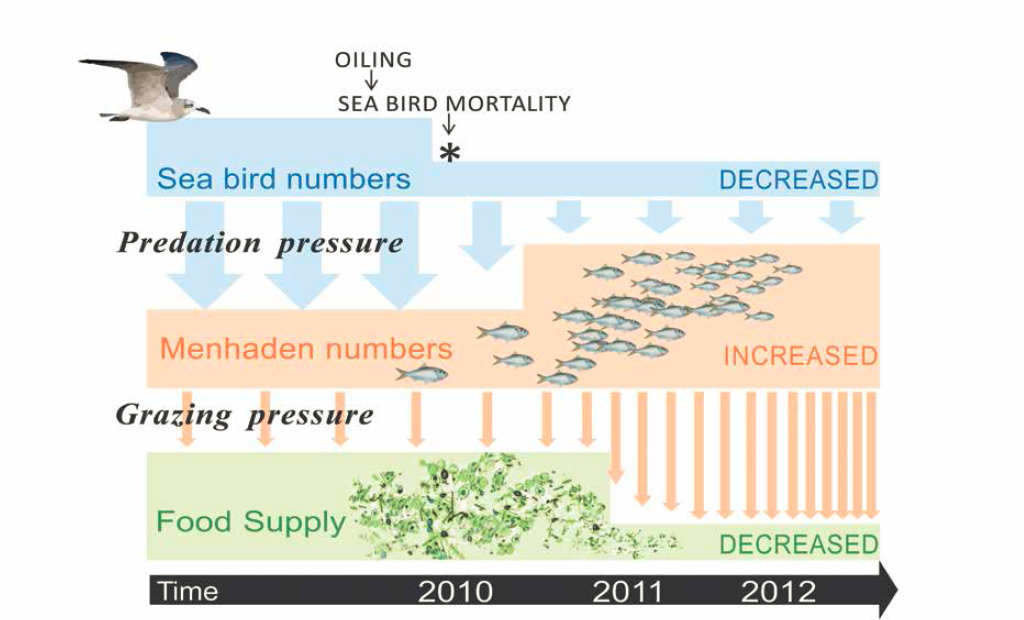
SOURCE: Short et al. (2017). CC BY.
Initial loss of cover by the biogenic habitat—the rockweed Fucus gardneri—following the Exxon Valdez oil spill triggered a cascade of indirect impacts that lasted for a decade or more. Studies that distinguished effects from natural variability at un-oiled locations, from shoreline treatment efforts, and from oil exposure (reviewed by Peterson, 2001) showed oil-associated reductions in Fucus and important predatory limpets and gastropods, increased space on rocks for blooms of ephemeral green algae in 1989 and 1990, and for an opportunistic barnacle (Chthamalus dalli) in 1991. Loss of the structure of the Fucus itself led to declines in associated invertebrates and inhibited recovery of the Fucus. Fucus plants that subsequently settled on tests of C. dalli became dislodged during storms because of the structural instability of the attachment of this opportunistic barnacle. After apparent recovery of Fucus, previously oiled shores exhibited mass rockweed mortality in 1994, which is a cyclic senility and resulting instability (see Box 6.5). The general sequence of succession on rocky intertidal shores is of rapid recovery of short general times of intertidal plants and animals (Menge, 1995). Expectations of rapid recovery based on short generation times of most intertidal plants and animals are not supported by the data. The general sequence on rocky intertidal shores after the Exxon Valdez spill was a delayed set of indirect effects over a decade or longer (Peterson et al., 2003).
Indirect interactions are not restricted to trophic cascades or to intertidal benthos. Interaction cascades defined broadly include loss of key individuals in complexly organized populations, which then suffer subsequently enhanced mortality or depressed reproduction. The most compelling example of a trophic cascade radically modifying a marine community comes from the Gulf of Alaska kelp ecosystem (Estes and Duggins, 1995). Sea otters control sea urchin populations, preventing them from overgrazing kelp and other macroalgae, and thereby retaining structural habitat for fishes and invertebrates. If sea otters were eliminated from an area by an oil spill, the otter-urchin-kelp cascade is put at risk. However, a ~50% reduction of sea otters was apparently not sufficient to induce this cascade following the Exxon Valdez oil spill (Dean et al., 2000).
6.5.7.4 Ecosystem-Level Effects
Media statements regarding the effect of DWH hydrocarbon on the 2010 continental shelf “dead zone” were dire because they extrapolated nominal reductions in deep waters (approximately 1,200 m) by bacterial respiration (Hazen et al., 2010). However, there was no indication that DWH oil residues influenced the seasonal occurrence of bottom-water hypoxia in 2010. There were indications of different carbon signatures near the Mississippi River, but these stations also demonstrated characteristics of high freshwater inputs onto the continental shelf (Hu et al., 2016). The combination of oil and dispersants mixed in the water column from the DWH oil spill combined with the annual development of oxygen-depleted bottom waters on the Louisiana continental shelf. It was unknown whether the oil and dispersants from the spill might affect the seasonal hypoxic area formation by either worsening the extent or severity. In May, measurements of the surface and bottom water hydrocarbons were higher than levels observed in June and July. The dissolved oxygen concentrations in bottom water were higher in May and June than in July. It was unknown the level of oil degradation in the water column or sediment. Statistical analysis of the progression of hypoxia development in May, June, and July 2010, and an analysis of conditions in July compared background levels collected over a 27-year period, indicated no difference in oxygen concentrations for May, June, or July 2010, with or without oil data included. The analysis also did not find any difference in July 2010 compared to the background years. Findings instead showed, throughout the dataset, that, the hypoxic area increased with higher river discharge, higher nitrate-N load, an easterly (westward) wind, and reduced wind speed. The analyses could not determine whether the oil spill affected, or did not affect, the size of the 2010 hypoxic zone, but there were signals that the 2010 hypoxia season was similar to the long-term record (Rabalais et al., 2018).
6.5.7.5 Ecosystem Services
The effects of an oil spill on ecosystem services is not an area of coverage for this report, but it is important to note that ecosystem services are curtailed with negative impacts on ecosystems or their components. Intertidal biogenic habitats, such as salt marshes and mangrove swamps, provide niches for high biodiversity. They also accumulate sediments and serve as a means of their cohesion against sediment resuspension and erosion. They serve as nursery habitat for larval, post-larval, and juvenile organisms in estuarine-coastal water networks. Salt marshes and mangroves also provide a defense against high tides and waves during tropical storms and hurricanes. Salt marshes filter nutrients and pollutants from the water column. Deep-sea corals and their associated invertebrate and fish communities are not only biodiversity hotspots but also unique among marine habitats. Intertidal and subtidal coral reefs also host high biodiversity. Ecosystem services can be converted to economic value. Should an ecosystem be negatively affected by an oil spill, the loss of ecosystem functions will convert to an economic loss for otherwise societal and environmental benefits. The National Research Council provided a report on ecosystem services related to the DWH oil spill and recommended several avenues of research that would more fully address this issue and future evaluation of effects (NRC, 2013).
Ecosystem-level effect conclusions remain elusive; the addition of longer-term observations and experiments that include higher organization-level components and trophic interactions could shed light on these effects.
6.6 EFFECTS IN ARCTIC ENVIRONMENTS
Decline by nearly one-third of the summertime minimum sea ice cover in the Arctic since the early 1980s (NASA)6 has opened up more of the region’s coastal waters to exploration for oil and gas, commercial fishing, trans-continental shipping, and commercial tourism. As described in Box 3.1, these activities bring greater risks of accidental oil discharges in a region with scant infrastructure, a challenging climate, highly variable weather that can turn treacherous with little warning, and often the presence of sea ice. These conditions also make response efforts to accidental oil discharges, as well as efforts to study the Arctic marine ecosystem and evaluate environmental damage from spills and other oil discharges, considerably more difficult than in more temperate waters. Consequently, marine ecosystem structure and functioning in the Arctic remain poorly understood, and the functioning of these ecosystems is changing rapidly in response to accelerating warming of the Arctic Ocean. The challenges to understanding Arctic marine ecosystems and the effects of oil on vulnerable ecosystems are briefly summarized in this section.
6.6.1 Marine Ecosystems in the North American Arctic
The annual marine production cycle in the North American Arctic is strongly modulated by ice. Complete ice cover during winter and most of spring results in nearly all of the solar radiation prior to the summer solstice impinging on ice which, with an albedo of 0.5 to 0.7, reflects more than half of it back into the atmosphere. Open sea water absorbs more than 90% of incident solar radiation, and open water is most widespread during summer. Primary production rates are consequently highest during summer, but considerable primary production occurs during spring as well despite the ice cover. This is for two reasons. First, most of the ice cover in the Arctic Ocean is relatively thin first-year ice, usually 1–2 m thick (Renner et al., 2013), and can transmit much of the incident photosynthetically active radiation (PAR) to the lower sea ice surface interface with sea water. The lower ice surface provides habitat that keeps attached marine algae exposed to PAR, and upwelled inorganic nutrients from the continental shelf break current in the Bering Sea are transported through the Bering Strait into the Chukchi Sea, fueling rapid growth of these epontic algal communities (Springer et al., 1996).
Second, and more importantly, as sea ice melts during the spring and especially summer retreating phase, the meltwater stabilizes the water column, allowing dispersal of the epontic algae into the mixed layer that often extends to a shallow seafloor where, along with other algal species, very rapid growth is supported by the presence of considerably higher PAR intensities present throughout most of the day, and the steady supply of inorganic nutrients. These rapid, intense algal blooms typically follow the retreating sea ice edge, and saturate the capacity of zooplanktonic grazers to consume them (see Figure 6.33).
Annual primary productivity in the western Arctic Ocean can be quite high, reaching several hundred grams carbon per m2 of sea surface per year (Springer et al., 1996; Smith et al., 2017). Some measurements exceeded 800 g C/m2-yr north of the Bering Strait (Sapozhnikov et al., 1993). Most of
NOTE: MIZ = Marginal Ice Zone.
SOURCE: Perette et al. (2011).
___________________
the algal production falls unconsumed to the seafloor, where it sustains high secondary production of the benthic invertebrate community, including dense accumulations of numerous species of crabs, starfish, and various marine worms (Smith et al., 2017).
Marine consumer species inhabiting the Arctic Ocean year-round must develop bioenergetic strategies to survive the fall, winter, and early spring months, when primary production is low or negligible. Fish, especially young-of-the-year, must accumulate sufficient lipid reserves during the brief season of abundant availability of phyto- and zooplankton prey to survive until the following spring, something relatively few species such as saffron cod and Arctic cod manage. Another strategy is to specialize in consuming live or dead animals in the benthic community, because this food supply is available throughout the year. Crabs, starfish, bivalve and other mollusks, and other species that can feed on living organisms or decaying detrital matter produced by the benthic community adopt this strategy. So do most species of marine mammals that reside in the Arctic Ocean year round, as well as several marine mammal species that migrate seasonally to the Arctic Ocean.
6.6.2 Arctic Marine Organisms Vulnerable to Oil Pollution
As elsewhere, seabirds and marine mammals are particularly vulnerable to adverse effects of oil pollution in the western Arctic Ocean. This being said, challenges associated with maintaining core body temperatures in the face of external oiling for these taxa become even more challenging than in temperate climates, even in the face of light oiling. Several species of marine mammals excavate and maintain breathing holes in first-year sea ice throughout the ice-cover seasons (Smith et al., 2010). Accidental releases of oil would tend to accumulate in these holes, exposing these mammals to oil through inhalation and possibly ingestion. They may also expose polar bears to oil, because these bears often wait for marine mammals to surface in these holes to attack them. Similarly, oil released in broken sea ice will be herded by the ice patches into the channels separating them, presenting a similar contact and inhalation hazard to marine mammals. Most seabirds in the Bering and Chukchi Seas follow the retreating sea ice edge northward in spring, feeding on fish and invertebrates associated with the high primary production associated with the ice edge, and concentrations of seabirds in large numbers occur along the northwest Alaska coast during summer (Smith et al., 2017). An accidental oil discharge near the retreating ice edge could cause widespread mortalities of seabirds. In the water column, acute toxicity tests indicate that aquatic organisms inhabiting Arctic waters are about as sensitive as comparable organisms living in more temperate waters, although adverse effects of exposure to oil may take longer to manifest in Arctic organisms.7
An accidental subsurface oil release would tend to spread horizontally at the seawater–sea-ice interface (see Section 5.3.5), where it could contaminate the epontic communities associated with the ice, and also be drawn into the ice by capillary action into brine channels (Faksness and Brandvik, 2008). Brine channels form as sea ice accumulates through exclusion of salt by the ice crystals, which increases the concentration of salt in the receiving boundary layers of seawater. This lowers the freezing temperature of the seawater, which can create microchannels within ice floes.
Accidentally discharged oil that becomes encapsulated in growing sea ice during fall may be transported several km per day (Nansen, 1902; Kwok et al., 2013). If oil leaking from a future pipeline or other source in the Arctic Ocean during fall went undetected, it could reappear as a “mystery spill” 100 or more km distant from the point of initial release during the subsequent spring thaw.
6.7 OIL EFFECTS MODELING
6.7.1 Overview of Fate, Exposure, and Toxicity Models
Modeling efforts have been directed toward understanding the past, current, and future trajectories of oil location, fate, exposure, and impacts of oil to organisms. These tools (see Section 4.2.1.4) have been essential components in contingency planning and risk assessments and in oil-spill response decisions (NASEM, 2020). Oil spill models have been used to support oil spill response decisions as part of NEBA and its refinements (i.e., spill impact mitigation assessments [SIMAs]) (NASEM, 2020). For example, oil spill models have been used to estimate habitats exposed to oil concentrations above certain thresholds for certain periods of time in comparative risk assessments (CRAs; a SIMA-type of approach) that allow for tradeoff decisions on potentially affected resources (French-McCay et al., 2018b). They are also essential in NRDA and hindcasting efforts.
Since Oil in the Sea III (NRC, 2003), new models have been developed, and existing ones have been updated and refined based on new knowledge. For impacts to occur, organisms need to be exposed to oil, and field sampling cannot fully quantify—spatially or temporally—the details of concentration and hydrocarbon composition that would be required. Therefore, models have been directed at understanding the fate (e.g., location, concentration, specific chemical constituents, among other features) of oil; these were previously discussed in Chapters 3 and 4 and thus are only briefly mentioned in this chapter. Oil trajectory models provide information regarding where the oil may go, providing estimates of surface or subsurface oil exposure (Boufadel and Geng, 2014; Ji et al., 2020; Keramea et al., 2021; Nordtug and Hansen, 2021). Oil fate and exposure models employ multiple steps and provide estimates on the composition, concentration, and partitioning of oil constituents both temporally and spatially and can also include the distribution,
___________________
movements, and behaviors of aquatic organisms to estimate their exposure to oil (see reviews by McCay et al., 2018a,b, 2021a).
Integrated trajectory, fate, and effects models also show the evolution of the spill, including oil component concentrations, which can be used for planning response options, response optimization in drills and aid in responding to oil spill events. Oil trajectory models have been expanded in recent years (NRDA) for use in quantifying the extent of oil impact (French-McCay, 2004, 2009) and in forecasting oil droplet distributions and oil constituents, and improved for use in Arctic locations (Boufadel and Geng, 2014; Nelson and Grubesic, 2018; Nordam et al., 2019; Ji et al., 2020; Keramea et al., 2021). These models have been used to identify and predict the resources of concern for impacts although specific impacts are not identified given that chemical toxicity depends on the concentration, specific type, and weathering state of the oil unique to each spill event.
Data derived from laboratory toxicity tests have been used in developing and also validating models to predict biological effects, using mechanistic studies of single hydrocarbons, which are then validated using both hydrocarbon mixture and whole oil exposure tests (e.g., French-McCay, 2002). Laboratory toxicity test data suitable for model inclusion (see Section 6.4 regarding laboratory toxicity test data limitations) have also been collected. Approaches used allow a comparison of species to identify those that may be more sensitive than others (e.g., see Section 6.7.2.5 on CAFE) or are able to predict the toxicity of oil and its constituents to species that have not yet been studied (e.g., see Section 6.7.2.6 on ICE). Numerous issues have been highlighted regarding the utility of toxicity tests, especially in terms of representing field conditions for dynamic complex mixtures (see Section 6.4). Indeed, a recommendation made in the recent oil spill dispersant report (NASEM, 2020) was that toxicity tests should not be developed to represent or replicate field-exposure conditions (which they cannot do), but rather for use to further develop and refine models so that integrated fate, exposure and effects models can support the decision-making process during an oil spill response (i.e., by comparing all of the options; NASEM, 2020).
The comparative advantages of specific exposure metrics (e.g., chemistry reporting) have also been discussed in detail in the National Academies oil spill dispersant report and will not be repeated here (see NASEM, 2020). Briefly, models use chemistry concentration inputs as a number of pseudo-components, hydrocarbon groups or specific individual hydrocarbons and assume equal additive toxicity effects, or they input specific hydrocarbons and weight their individual toxic effects using a toxicity unit (TU) approach. Ultimately, the type of exposure metric chosen (in addition to specific analytical approaches used) may under- or over-represent the toxicity reported, and this should be considered when the choice is made. Two models often used that use boiling cuts (soluble and insoluble divisions) to reflect around eight pseudo-components are SIMAP (Spill Impact MAPping; see French-McCay, 2004; French-McCay et al., 2018a,b, 2021a) and also OSCAR (Oil Spill Contingency and Response; Reed, 2004; Stephansen et al., 2021). The SIMAP model has been modified continually and incorporates exposure to oil droplets, the influence of temperature and light, and the movements of organisms to provide a biological effects model to which specific aquatic toxicity models are then applied (e.g., OilToxEx; French McCay, 2002; see Section 6.7.2).
Trajectory models have been used in quantifying impacts to shoreline habitats, birds, mammals and reptiles. Using satellite-derived surface oil distributions and direct observations of oiled turtles a spatio-temporally explicit model was developed during the NRDA process in the DWH oil spill to statistically estimate the number of turtles that may have been exposed to oil (Wallace et al., 2017). These turtle numbers were later used to provide estimates of the number of turtles affected by chemical exposure and toxicity mechanisms (Mitchelmore et al., 2017; see Section 6.7.2). Specific advances discussed in Chapters 3 and 4, that are pertinent to this chapter include transport, fate and exposure models that estimate the concentration and composition of oil components, which are particularly focused on dissolved phase concentration and composition estimations. Since Oil in the Sea III, models have also been used to refine estimations for particular oil components (e.g., photo-oxidation products, MOS), predicting droplet size/quantity and particulate phase components and including environmental covariables that may alter fate and exposure (see NASEM, 2020).
6.7.2 Models to Estimate Aquatic Toxicity to Individuals
Numerous approaches and specific models have been used to provide information at multiple scales and levels of complexity regarding the adverse effects that may result from oil exposure, including future projections of population losses. Some of these modeling efforts provide predictive estimations of toxicity and can include considerations of specific environmental conditions (co-variables such as temperature, light, and pressure) (French-McCay, 2002, 2004, 2009; McGrath and Di Toro, 2009; Bejarano and Mearns, 2015; Marzooghi and Di Toro, 2017; Carroll et al., 2018; French-McCay et al., 2018b; Gallaway et al., 2019; Bejarano and Wheeler, 2020; Colvin et al., 2020; Stephansen et al., 2021). Initial models used representative oil chemical compositions, but are now more sophisticated and include mixture-based approaches, such as summations of effects of constituents using a TU approach. The validation of many models has been performed using hindcasts after a spill event and the use of data derived from laboratory toxicity test studies. These models are briefly described here, with pros and cons of each approach highlighted using examples of their application in understanding the impacts of oil in the environment. As described later, these models have been
combined with physical fate and biological effects models to quantify impacts (e.g., OilToxEx in SIMAP, PETROTOX).
Regarding aquatic organisms, models have been used to estimate oil entrainment and dissolution into the water column. These models predict the concentrations of oil components that have been a focus of toxicological impacts: namely, the more soluble lower- and intermediate-molecular weight monoaromatic hydrocarbons (MAHs) and the PAHs (e.g., OilToxEx; French-McCay, 2002). These are predictive models that have over time been modified to incorporate evolving knowledge. Additional models include the target lipid model (TLM; McGrath and Di Toro, 2009) and associated PETROTOX model (Redman et al., 2012, 2017). Since Oil in the Sea III was published, other components in oil that also contribute to toxicity (e.g., heterocyclics) have been identified, in addition to covariates (e.g., UV light and phototoxicity) that modify toxicity. Thus, models have been refined and further developed based on these advances (Marzooghi and Di Toro, 2017). Furthermore, models traditionally focused on acute toxicity whereas refinements of models have included chronic toxicity endpoints (e.g., McGrath et al., 2018). A third and more recent type of model developed for oil toxicity has been directed toward hazard assessments. These models, which use laboratory toxicity data, were developed to predict the impact of oil constituents on standard or non-standard species (e.g., Interspecies Correlation Estimation or ICE models) or to estimate concentrations that are protective for 95% of the organisms (e.g., preparing species sensitivity distributions [SSDs] using CAFE).
6.7.2.1 Narcosis Target Lipid Models
Target lipid models quantify toxicity based on a relationship between octanol-water partition coefficient (Kow) and are applicable to dissolved oil components on the assumption that such components result in narcosis effects directly proportional to the extent of accumulation in the tissues. This also assumes that each chemical results in an additive effect (Di Toro et al., 2000). The methodology is intended to be applicable to 95% of species (i.e., HC5), particularly for early life stages. McGrath and Di Toro (2009) developed the concept for aromatic hydrocarbons, using TUs for hydrocarbon mixtures (for a description on the use of toxic units, see NASEM, 2020). Essentially, this increases the complexity, because although individual components are still additive, their toxicity is not equal and each component provides its own proportion into the toxicity calculation and the specific composition and concentration of each chemical results in an estimation of toxicity. This is very different from the approach of reporting toxicity based on concentrations of mixtures using units of TPH or TPAH (see NASEM, 2020). Briefly, TUs for mortality are calculated from each of the pseudo-component chemical constituents’ dissolved exposure concentrations divided by the L(E)C50, with each constituent considered additive. This provides an estimate of the mixture’s LC50 (i.e., sum of TUs = 1) at a certain concentration. The L(E)C50s for this model were calculated using quantitative structure activity relationship (QSAR) models (McGrath et al., 2018).
The original concept is that 95% of species would be protected using this modeling approach. With increased understanding of toxicity and exposure mechanisms, the model has been updated (McGrath et al., 2018). Based on toxicity work during the T/V Prestige oil spill, Barata et al.’s (2005) work with copepods agrees with the concept of octanol-water partitioning being successful as they found the toxic effects of components to be additive. An early additive toxicity model, OilToxEx, was developed by French-McCay (2002) and used the TLM and TU approach to predict oil toxicity based on the effects from the dissolved chemical composition. Additive toxicity models are simple in terms of adding the individual toxicities from a mixture and are incorporated into the SIMAP model (French-McCay, 2003). These models are generalized by adding a coefficient to scale the model with observations. These models have been updated (e.g., PETROTOX) from the first models developed (French-McCay, 2002).
Given that toxicity data typically are provided for temperate standard test species, both TLM and experimental approaches were recently compared to assess the protection for tropical species exposed to gas condensate oils (Negri et al., 2021). Although several of the tropical species were among the most sensitive to be included in the TLM database, the comparison of the experimental and modeled efforts demonstrated the utility of the model for tropical species. The TLM-modeled HC5 was found to be more conservative than the experimentally derived hazard concentration after testing eight diverse tropical taxa (i.e., 78 and 167 μg/L, respectively; Negri et al., 2021). The authors did note that further research should be conducted to validate this with additional oil types, covariables (e.g., UV light), and especially with other keystone species, including additional coral species.
6.7.2.2 PETROTOX Model
PETROTOX can predict the toxicity of any oil, providing that its detailed oil composition is known. Equilibrium calculations are made on the distribution of the hydrocarbons based on the physical properties such as octanol-water partitioning. The most recent input type is based on GC×GC-FID. The higher-resolution version has 16 hydrocarbon classes based on hydrocarbon number for input. The model was developed to predict the aquatic toxicity of water in contact with oil as described in Redman et al. (2012). It employs the TLM to predict individual oil component toxicities and the TU mixture model to predict the whole toxicity of the oil mixture. The acute and chronic HC5 critical body burdens used in the calculations are from McGrath et al. (2018). So, PETROTOX is used to predict oil toxicity but also is used to examine the utility of TPAH/TPH for oil constituent
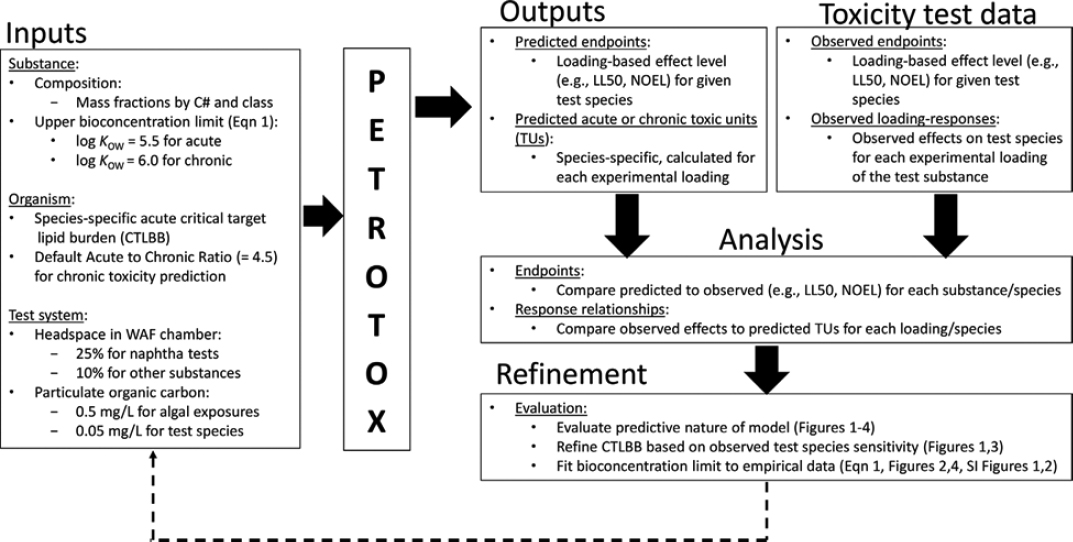
NOTE: CTLBB = critical target lipid body burden; LL = lethal loading; NOEL = no observed effect loading; WAF = water-accommodated fraction.
SOURCE: Redman et al. (2017).
concentrations and evaluate how toxicities vary with different oil types, oil loading, and oil droplet concentrations (see NRC, 2020). The latest advancement in the PETROTOX model is a recalibration of two parameters, discussed in Redman et al. (2017) demonstrating the utility of the TLM-TU model in PETROTOX to reproduce observed chronic toxicity (see Figure 6.34).
The effect of UV in sunlight on oil can add oxygen molecules to the hydrocarbons as well as interact with polycyclic aromatic compounds (PACs). This can greatly increase toxicity by up to three orders of magnitude (see Barron, 2017b). A number of models of PAH phototoxicity have been developed (see Table 3.3 in NASEM, 2020). Marzooghi et al. (2017, 2018) recently developed the Phototoxic Target Lipid Model (PTLM) as found in the PETROTOX model (Marzooghi and Di Toro, 2018). This model calculates the ratio of the phototoxic LC50 (PLC50) to the TLM LC50 as a function of PAH spectral absorbance and the spectral distribution of the incident light exposure (NASEM, 2020).
6.7.2.3 De Minimiz Risk Models
This model by Sellin Jeffries et al. (2013) used ultraviolet A (UVA) data to calculate the depth of PAH phototoxicity for young herring during the T/V Exxon Valdez oil spill. The results were a worst case estimate of 2-m depth, which correlated well with experimental data, and where 1% of the population would be located. The model compares well with data using median lethal times (LT50).
6.7.2.4 The Dispersant and Chemically Dispersed Oil Toxicity Database (DTox)
Similar to the CAFE database, the DTox database provides a publicly accessible compilation of carefully selected available dispersant and chemically dispersed oil toxicity data (Bejarano et al., 2013). Data for inclusion had to meet a number of criteria (see details in Bejarano et al., 2013a), including a minimum requirement for method descriptions and chemical analysis and various quality assurance/quality control (QA/QC) evaluations. DTox is a user-friendly search tool and can provide the data in the form of SSD plots. It provides a centralized repository useful not just to the scientific community but also to oil spill responders in making decisions regarding the use (or not) of various response options (e.g., use of oil spill chemical dispersants and comparisons between specific ones). The database can also be used to compare the relative toxicity of chemically (CEWAF) versus physically dispersed oils (WAF) using a combined number of petroleum hydrocarbon products. Even despite the caveats discussed in Section 6.3 and NASEM (2020) regarding differential interpretations of toxicity based on the choice of analytical chemistry used, DTox database queries were consistent in demonstrating the lack of evidence that CEWAFs were more toxic than WAFs (NRC, 2005; Bejarano et al., 2013a). This was evidenced by comparing CEWAF versus WAF data for fish and crustacean species and also alternatively using all WAF and CEWAF data for a single species. The comparison
showed that data were interspersed over the curve and that CEWAF data points did not cluster at the lower end of the curve indicative of higher toxicity. The dataset is also able to compare acute versus chronic endpoints, the toxicity for different species (e.g., temperate versus Arctic) and different oil types. For example, SSDs were similar irrespective of use of LC or EC50 data and showed that polar species demonstrated similar responses to temperate species (see Figure 6.35A); thus temperate species could be suitable surrogates for understudied Arctic species. The dataset also highlighted that Venezuelan crude oil was much more toxic than two Alaskan oils (i.e., Prudhoe Bay and Alaska North Slope) (Bejarano et al., 2013a; see Figure 6.35B).
As demonstrated, DTox allows for queries to investigate and compare the toxicity of different oils, oil preparation types, and species and can also be informative for response decisions regarding the use of dispersants. This DTox database was ultimately incorporated into a newly developed database, NOAA’s CAFE model as described in Section 6.7.2.5.
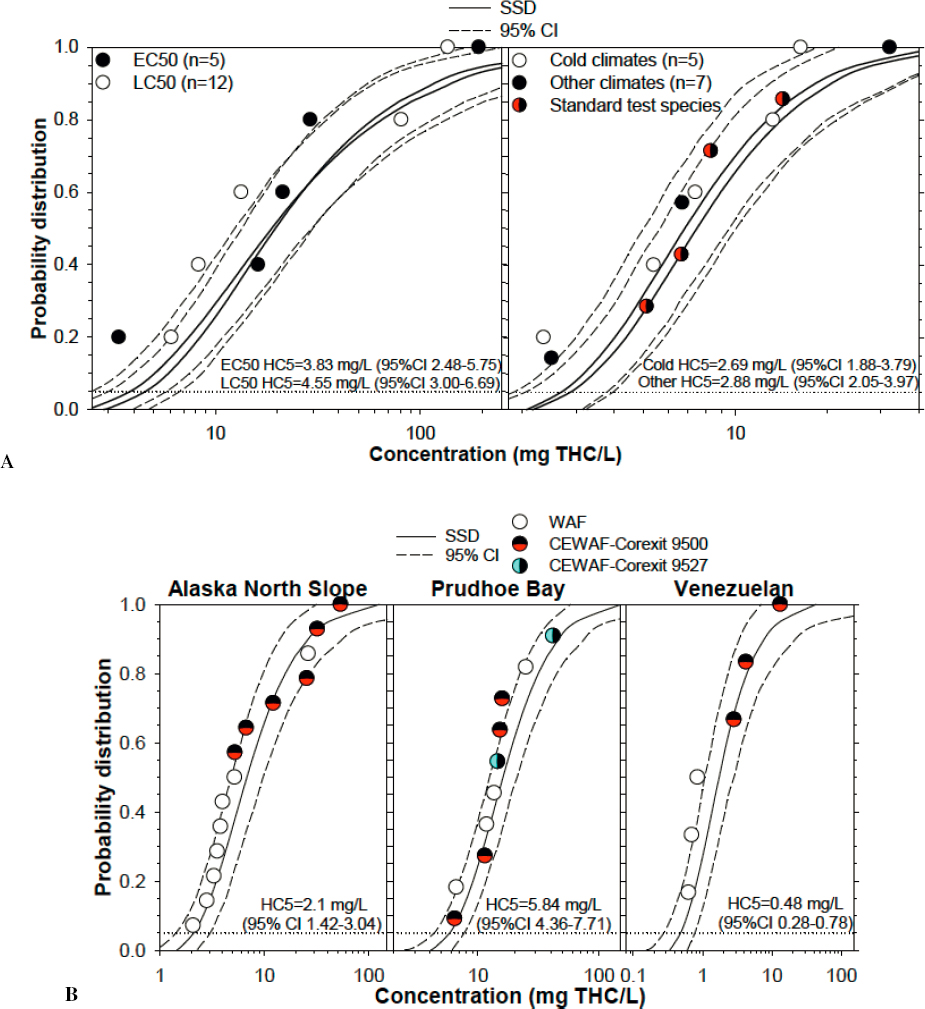
NOTE: 96-hour LC50/EC50 data for fish and crustacean from flow-through contact and studies reporting toxicity based on measured THC made up SSDs.
SOURCE: Bejarano et al. (2013a).
6.7.2.5 CAFE and Species-Sensitivity Distributions
In environmental risk assessments (ERAs), a number of approaches are used to determine the risk of chemical contaminants to resident species and estimate the acceptable concentration of a chemical in the environment: that is, when predicted no effect concentrations (PNECs) are lower than the predicted environmental concentration (PEC; see Box 6.10). Species sensitivity distribution curves (SSDs) are an important technique used in risk assessments; they estimate the first and fifth percentile hazard concentrations (HCs), which are the concentrations considered protective of 99% and 95% of the species, respectively. HC estimates are used as levels of concern protective to a broad array of aquatic species, as they inform of potential impacts and are used to derive PNECs because they are a community-level threshold making full use of the available data on the toxicity of a chemical to standard and non-standard toxicity test species and so relate concentration to ecological impacts (i.e., the proportion of species at risk). This is in contrast to the alternate (traditional) approach, in which hazard is predicted using concentration-effect data from the most sensitive single-species laboratory test data combined with an application (or uncertainty) factor (see Belanger et al., 2017). The application factor (AF) applied to SSDs is also much lower (i.e., 1–5) compared to those applied using the alternate traditional individual sensitive species approach (see Box 6.10). SSDs also reduce the need to study every species with every oil, although some sensitive taxa may still have to be investigated. Although the SSD approach is becoming the more common approach, because it estimates potential hazard to communities, SSDs do not address ecosystem processes or interspecific interactions (e.g., competition or predation). An additional advantage is that often the shape and form of the SSD can inform about some specific modes of action (e.g., narcosis) (Belanger et al., 2017).
The NOAA Chemical Aquatic Fate and Effects (CAFE) database, developed by NOAA’s Emergency Response Division (ERD), includes fate and effect data compilations that provide a quantitative basis on which to assess hazard concentrations for hundreds of chemicals (see Bejarano and Mearns, 2015; Bejarano et al., 2016b, 2017; NOAA ERD, 2015; see Figure 6.36). This database was developed to facilitate access to fate and toxicity data following an oil spill and allows for SSD development to improve hazard estimates during oil spill response planning and activities. In CAFE SSDs summarize the aquatic toxicity data and provide estimates of the first and fifth percentile HCs.
Although there is a significant amount of toxicity data available regarding the impact of oil, its individual chemical constituents, and oil spill response chemicals (e.g., dispersants), often data are limited regarding the number of species, so SSDs cannot be developed (SSDs typically require between four to eight species; CAFE requires five [Bejarano et al., 2017]). To address this data gap without conducting an extensive number of laboratory toxicity tests, further modeling approaches have been developed to predict toxicity; for example, the Interspecies Correlation Estimation (ICE) models described in Section 6.7.2.6.
6.7.2.6 Interspecies Correlation Estimation Models
ICE models are a type of interspecies correlation model (ICM). ICE models extrapolate the known toxicity data from surrogate species to generate median lethal or effect concentration (L/EC50) toxicity predictions for one or several new species (Bejarano and Barron, 2014; Bejarano, 2019) The advantage of this method lies in leveraging older or broader toxicity results as a baseline for prediction of new LC50 curves for new or related species. Bejarano and Barron (2014) developed the first ICMs for the toxicity of oils and chemical dispersants (also see Bejarano and Wheeler, 2020). The most recent integration of ICE models into CAFE addresses, at least partially, utility for many chemicals for which aquatic toxicity data are limited. ICE predicted values enhance species diversity in CAFE and allow the derivation of SSDs, both improving the capabilities of this tool and ultimately increasing confidence in environmental assessments related to accidental chemical releases in aquatic environments. Also, broader access to ICE models available to date facilitate their use and further refinement. Numerous studies have now shown the utility and validity of this approach, by demonstrating that SSDs developed using ICE-predicted values have HC estimates similar (i.e., less than 3-fold difference) to those SSDs calculated using measured toxicity data from the most sensitive laboratory test species (Dyer et al., 2006, 2008; Awkerman et al., 2014; Bejarano and Barron, 2014; Sorgog and Kamo, 2019). They are often more conservative; in less than 10% of cases was toxicity underpredicted compared with measured values (Bejarano et al., 2017). The main advantage of the ICE approach is that it increases the number of species that can be included in an SSD (i.e., by an average of 34 species), which generally reduces the uncertainty around the HC values generated and reduces the need for additional toxicity tests. Therefore, the integration of ICE-predicted values into the CAFE database will increase the confidence in environmental risk assessments (Bejarano et al., 2017).
The use of ICE/CAFE also has demonstrated that we do have a significant amount of data regarding the toxicity of oil, its constituents, and chemical dispersants and that research efforts toward conducting further toxicity tests (especially acute and standard test species) may not be the most useful allocation of resources. However, ICE models could be developed even further with the inclusion of aquatic toxicity data directed toward sensitive taxa (Bejarano et al., 2017). A related recommendation made in the oil spill dispersant report (NASEM, 2000) is to rethink how toxicity tests are used, moving away from trying to replicate field exposure scenarios (which is not possible) to providing metrics that
SOURCE: Bejarano et al., 2016.
can be used in fate, exposure, and mechanistic mixture-based oil toxicity determinations. Employing these integrated modeling approaches supports response decision-making by allowing predictions of exposure and toxicity using various spill response agents to be compared.
6.7.3 Limitations and Challenges of Modeling Approaches
The main weakness in modeling still revolves around gaps in our knowledge of the impacts of oil spills, although the models have been improved and verified using extensive datasets collected from the DWH incident. Limitations in toxicity models derived from laboratory data include lack of appropriate experimental design. Underpinning predictive models of impact are laboratory toxicity tests, the utility of which depend on how exposure solutions were made and reported and toxicological thresholds calculated. Unfortunately, even despite standard method guidelines outlined more than 20 years ago (CROSERF; Singer et al., 2000), the toxicological implications of many studies cannot be established and/or used in model development/validation. Recent reviews have addressed this issue in detail (e.g., Bejarano, 2018; Hodson et al., 2019; Murawski et al., 2020) including the recent report titled Use of Dispersants in Marine Oil Spill Response (NASEM, 2020). In this report a recommendation was made that toxicity tests should be used, not to attempt to reproduce field exposures, but to develop a consistent means of using toxicity metrics, such as HC5 and LC50 for toxicity models used with fate and transport models to compare the exposure and toxicity of various response options, including dispersants (NASEM, 2020).
The toxicity models described earlier are also based on acute toxicity and a narcotic mode of action, although (as this chapter highlights) there are many other mechanisms of action and sublethal effects that have implications at the individual, population, and ecosystem levels as a result of both acute (not lethal and also including delayed responses) and chronic longer-term exposures. Accounting for all of these potential effects in a model would be extremely challenging, so current approaches to estimate chronic
longer-term impacts have applied the acute toxicity data to predict chronic toxicity by calculating acute to chronic ratios (ACRs). An additional limitation of the narcosis models is that they represent a specific group of chemicals, and new research has highlighted the importance of other components in driving acute and chronic toxicity in aquatic organisms. Further research is needed to identify and characterize other toxic components and degradation/metabolite products (e.g., photo-oxidized constituents) (see Hodson et al., 2019).
Although new models have been developed and existing ones refined, particularly to include the influence of oil droplets and also co-variables that modify exposure and/or toxicity (such as, temperature, UV light, and pressure), there are still a number of data gaps for improvement and refinements of the existing models, including validation with tropical and other non-standard test species (Negri et al., 2021) and with new oil types (e.g., very low sulfur fuel oils, diluted bitumen; NASEM, 2016a; Ruberg et al., 2021). One modifying toxicity factor investigated recently as a result of the DWH spill has been that of pressure. Paquin et al. (2018) accounted for pressure in a modified target lipid model and demonstrated that increased pressure resulted in lower toxicity, although further toxicity data are needed to validate this. Exposure time is also a critical variable component, as typically toxicity tests follow standard guidelines of typically 48–96 hours for acute tests, whereas most acute oil spill exposure scenarios may last only a few hours. This potential disconnect could be easily addressed by reporting additional times when conducting a toxicity test (e.g., providing time-to-death estimates and also LC50s at multiple time points) or conducting tests of shorter duration.
As highlighted in Section 6.7.3, there are many models for assessing individual toxicity to organisms, but more research is needed directed toward studying the impacts of oil and response options on populations. The use of population models is common practice in conservation biology and a tool frequently used by resource managers for managing wildlife, but has yet to be broadly used within the context of oil spills. Indeed, the NRC (2013) urged the inclusion of population models for assessing pesticide risks to threatened and endangered species, which could also prove useful for setting a framework applicable to the implementation of these models in oil spill assessments. However, there are examples (mainly with fish) of the integration of dose-response toxicity data with species-specific and spatially explicit information resulting in models potentially useful for assessing population risks (e.g., Awkerman et al., 2016; Gallaway et al., 2017, 2019).
6.8 HUMAN HEALTH EFFECTS
The first two committees formed by the National Academy of Sciences to consider oil in the sea (NAS, 1975, 1985) included short sections in their reports discussing the potential for human health effects, primarily focusing on polycyclic aromatic hydrocarbons. The introductory chapter to Oil in the Sea III (NRC, 2003), in a section titled “Scope of the Present Study” describing how the goals of the study were determined, contains a parenthetical sentence: “It was agreed early on that inclusion of an examination of potential effects on human populations, while undoubtedly of interest, would overly complicate an already daunting task” (NRC, 2003).
Human health also was not part of the original conceptualization of the present Oil in the Sea IV study. However, discussion at the first committee meeting led to the recognition that human health, including community health, would fit within the formal charge. As a result, this section on the human health effects of oil spills was added.
The approach taken to the human health aspects of Oil in the Sea IV follows a dictum stated by Savitz and Engler (2010) in a perspective providing advice on the health research approach to the then-ongoing DWH oil spill work: “Studies should focus on the health effects of the oil spill rather than solely on the health effects of the oil.”
The ability to do so in the current document is based largely on the marked increase in depth and breadth of the published information available from recent spills, including the DWH. Valuable information about the human health impact of oil spills has also been obtained from recent studies of the Hebei Spirit and Prestige oil spills, which led the South Korean and Spanish governments to support ongoing follow up studies. Extensive reviews of the available information, from these and other studies of oil spill health effects, are now available (Aguilera et al., 2010; Solomon and Janssen, 2010; Goldstein et al., 2011; Zock et al., 2011; Laffon et al., 2016; Rusiecki et al., 2018; Park et al., 2019; Partyka et al., 2021; Sandifer et al., 2021). Particularly notable have been studies evaluating the indirect health effects of oil spills on communities (e.g., mental and behavioral effects; see Section 6.8.8), as well as greater understanding of the linkages between human health and the well-being of other species. Evidence that human health and well-being are dependent on interaction with nature has grown, as has the linkage between healthy ecosystems and healthy humans (Rabinowitz and Conti, 2013; Sandifer and Walker, 2018; Bratman et al., 2019).
Humans are an integral part of marine ecosystems in much of the world. We interact with marine environments as workers, as nearby residents, and for recreation. Our complex interactions with other marine species include serving as direct and indirect predators and despoilers as well as stewards and protectors. Historically, many communities have developed close to the shore. Their health and well-being have depended on successful interaction of human communities with the marine environment—an interaction that can be put at risk by oil in the sea. The U.S. Census Bureau (2019) reported that 94.7 million Americans (almost 30% of the U.S. population) live in shoreline regions, that this number is growing rapidly, and that many of these regions are characterized by great ethnic diversity. Globally, the United Nations
Environmental Programme (UNEP) estimates that 40% of the world population lives within 100 km of the sea.8
Just as every oil spill has different effects on ecosystems, so they also differ greatly in their direct and indirect effects on humans. Our focus here is on the relatively recent DWH oil spill, for a number of reasons. Increasing occurrence of disasters has led to a larger research community interested in the human health impact of disasters and better able to respond with focused studies. The recurring disasters in the Gulf Coast area had led to baseline information which obviated some of the shortcomings of post-disaster studies. Perhaps of greatest importance was that following the DWH oil spill, much more funding was made available for health research, in part because of a rapid response by the National Institute of Environmental Health Sciences (NIEHS) and other U.S. governmental organizations and because of the willingness of BP to be responsive, thus ameliorating, however incompletely, many of the litigation related issues that have complicated the study of other oil spill responses in the United States and elsewhere (Shore et al., 1986; Picou, 2011; Ritchie et al., 2018; Park et al., 2019). Also helpful was the coordination of federal health research at the level of the Assistant Secretary of Health and Human Services (HHS) for Preparedness and Response, an office that had been formed in 2006 as a result of problems in responding to Hurricane Katrina. The NIEHS funded a variety of programs (NIEHS, n.d.). A joint intramural/extramural program, the Gulf Long-term Follow-up Study (GuLF) is performing a 10-year follow-up study of more than 30,000 response workers which led to 40 peer-reviewed publications by early 2021 (Kwok et al., 2017b; NIEHS, 2021a,b). A 5-year program of university–community partnerships was competitively funded from which a consortium developed that performed research responsive to community needs. Working with the National Library of Medicine, a National Institutes of Health Disaster Response Program was developed (DR2, n.d.). The National Toxicology Program formed a program to further the study of the toxicology of PAHs; and, working with other agencies, the NIEHS Worker Training Program expanded its on-site training program for cleanup workers (NIEHS, 2012).
Another important federally funded program was the DWH Oil Spill Coast Guard Cohort study. This study compared 8,696 responders with 44,823 non-responders, all of whom were members of the U.S. Coast Guard (Rusiecki et al., 2018). The study design allowed for comparison of responders with a similar population of non-responders, as well as providing a comparison between those responders who were oil-exposed and those who were not.
Also unique for a major oil spill, BP, the principal responsible party, was a relatively rapid source of direct and indirect hands-off funding that produced significant research efforts. The Gulf of Mexico Research Initiative (GoMRI), formed with a distinguished leadership group, received $500 million of BP funding for a 10-year period. One of its five research themes was public health, including behavioral and socioeconomic impacts; however, the public health portion of the research portfolio was not as pronounced as others, for many reasons (Eklund et al., 2019; Goldstein, 2020).
Another source of published research was indirectly funded by BP through a set-aside of $105 million of the $7.6 billion medical settlement to form the Gulf Region Health Outreach Program (GRHOP). Its goal was to improve local health in specified counties in four states most affected by the oil spill, with a particular focus on mental and behavioral health and on environmental health. Overseen by the court, GRHOP included an academic program primarily focused on environmental health, and four programs working on mental and behavioral health and social and community issues at state universities in the four affected states. The latter resulted in the Gulf Coast Resilience Coalition (Hansel et al., 2015). Each program worked with community organizations (Buckner et al., 2017). Although not formally a research program, GRHOP produced a large number of peer-reviewed publications, primarily focused on mental and behavioral health, including a special supplement to the Journal of Public Health Management and Practice (2017). That supplement included consideration of environmental justice issues as well as the training of community health workers (Hansel et al., 2017; Nicholls et al., 2017). GRHOP also was part of a groundbreaking effort in enterprise evaluation, an approach increasingly needed to gauge the success of broad multidisciplinary efforts (NIEHS, 2018; Sherman et al., 2019). More recently, the National Academies created the Gulf Research Program (GRP) with $500 million received through the U.S. Department of Justice from the criminal settlement of the DWH oil spill for activities related to the prevention of and response to future oil disasters. Human health research has already become a significant part of its broad portfolio. With its 30-year time frame (ending in 2043), and a mandate that extends to U.S. coastal waters beyond the Gulf Coast, the GRP has the opportunity for much needed longer-term integrated investigations of the impact of oil spills (NASEM, n.d.).
In this section we address the components of crude oil and their derivatives that may affect human health directly or indirectly, the pathways of human exposure, the direct and indirect health effects of concern, and the modulating effect of factors such as individual and community susceptibility and resilience; and we touch on the potential health impacts of the different cleanup responses to oil in the sea, such as burning of surface oil and the use of dispersants. Safety issues, including fire and explosions and the safety of response workers, are considered briefly. In keeping with the recommendations of a 2010 Institute of Medicine Letter Report titled Research Priorities for Assessing Health Effects from the Gulf of Mexico Oil Spill, particular attention will be paid to psychological and behavioral effects, exposure
___________________
8 See https://www.unep.org/explore-topics/oceans-seas/what-we-do/working-regional-seas/coastal-zone-management.
assessment, and seafood safety (IOM, 2010a,b). We also focus on studies considering the potential linkage between the economic effects of an oil spill disaster and a disaster’s mental and behavioral effects.
6.8.1 Components and Derivatives of Crude Oil of Known Importance to Human Health Effects
Crude oil is a mixture of hydrocarbons which vary according to source. As with other species, two of the major factors determining the human health effects of oil in the sea are the chemical and physical composition and properties of the oil, and the products of chemical and physical transformations of oil components within the environment (see Chapter 2). Following release into the sea, crude oil changes its physicochemical properties, with different components undergoing different pathways and fates. Lighter weight components, such as BTEX, tend to volatilize at rates depending on water and air temperature and on sea surface turbulence. Heavier crude oil components are particularly affected by the process known as “weathering.” This leads to greater oil viscosity, which can allow the formation of “tar balls” that can be brought to the shore or sink to the sea bottom. Little information is available about the potential human health effects of weathered oil or other oil spill degradation products (Black et al., 2016; NASEM, 2020). Weathered crude oil has been evaluated in other species with findings that may be relevant to humans (see Chapter 6).
The major components known to have human health impacts are the monocyclic and polycyclic aromatic hydrocarbons, which are considered in more detail later in this section. Microbial degradation and photo-oxidation participate in changes in the chemical structure of oil products with different resultant degradation products (Garrett et al., 1998). Crude oils also vary in their composition of trace elements such as arsenic.
As noted in Section 5.2.5, there is increasing information about the photo-oxidation process and its products. Volatile hydrocarbons, along with oxides of nitrogen (NOx) and sunlight, underlie the photochemical process that leads to tropospheric ozone pollution. Middlebrook et al. (2012), in an extensive analysis of the air pollution resulting from the DWH oil spill, attributed ozone formation to NOx from oil spill response activities, including emissions from the many ships involved and the flaring of natural gas. Burning of oil at sea contributed to production of NOx as well as particulates (Middlebrook et al., 2012). Particulates and ozone are air pollutants of particular concern as causes of cardiovascular and lung disease which could potentially affect community members and cleanup workers. Coastal areas with drilling sites, refineries, and other major sources of hydrocarbons such as South Texas, appear to be particularly at risk for exceedances of the health-based ozone standard from offshore oil spills (Sanchez et al., 2008). Atmospheric photo-oxidation also produces potentially toxic carbonyl compounds such as acrolein and peroxyacyl nitrates (Middlebrook et al., 2012; Weitekamp et al., 2020). Impetus for further study of the potential human health effects of photo-oxidation also comes from their persistence in degradation products that may come in contact with humans. Black et al. (2016) noted that weathered oil oxidative by-products were present in beach sand 5 years after the DWH oil spill, and that the lack of toxicological data on such products (as well as dispersants) was a limitation of their risk assessment for children exposed to beach sands affected by the DWH oil spill.
An extensive literature exists on the role of free radicals and active species of oxygen in human disease, including cancer (Klaunig, 2018). While externally produced short-lived reactive species such as hydroxyl radicals, superoxide anion radical, and singlet oxygen all decay much too rapidly to enter the human body, photo-oxidation results in a variety of longer lived potentially active compounds, including peroxides, which once ingested or inhaled could conceivably have sufficiently long lifetimes in vivo to cause adverse effects. This could occur through their further release of active oxygen species or through the mutagenic action of carbonyl derivatives from peroxidized lipid leading to their attachment to DNA, including producing DNA-DNA or DNA-protein crosslinks (Mukai and Goldstein, 1976; Lu et al., 2010). The presence of oxidized forms of PAHs in air may be toxicologically important (Lammel et al., 2020). Further study is warranted of the formation, persistence, and potential adverse human health effects of photodegradation products of oil in the sea.
Various metals are present in trace amounts in crude oil. An association of these metals with endocrine and genotoxic effects among Prestige spill cleanup workers who remove the heavy fuel oil has been reported (Perez-Cadahia et al., 2008). Gohlke et al. (2011) discussed the evidence for bioaccumulation of metals in marine organisms following oil spills and criticized seafood safety determinations for failure to include analysis of metals. Black et al. (2016) noted that the levels of arsenic, vanadium, and barium, as well as four PAH components, exceeded guideline levels.
Black et al. (2016) followed up on their finds of guideline exceedances for metals and PAHs by performing a formal risk assessment for a child playing on the beach. They found overall low levels of risk, consistent with the findings of other investigators who performed risk assessments following oil spills. Two prior risk assessments related to beach activities following the Erika heavy fuel oil spill in France also reported low or limited risk depending on the scenario employed (Baars, 2002; Dor et al., 2003). Dor et al. (2003) suggested concerns about exposure to relatively high levels of PAHs for the scenario of a pregnant woman walking on a rocky shore from which it is difficult to remove oil. Another approach to estimating risk was taken by Afshar-Mujaher et al. (2019), who measured the air emissions from a tank containing crude oil with or without dispersants which
was subjected to a horizontal wave simulator. They found airborne levels of VOCs that on an exploratory analysis were within ranges of concern depending on the estimated time of exposure.
6.8.2 Pathways of Human Exposure to Oil in the Sea
The DWH oil spill, and other spills affecting human populations, conform to the five elements of a complete exposure pathway: sources of contaminants; readily identifiable environmental media, including air, water, and soil; points of exposure for both response workers and the public; routes of exposure including inhalation, ingestion, and absorption through the skin; and a wide variety of receptor populations encompassing the diversity of the Gulf area and of the response workforce (Goldstein et al., 2011).
Similar to marine and shore biota, the major factor determining the extent of human exposure to oil in the sea and its derivatives is location in relation to human habitation and activities. Seafood gatherers, other maritime workers, and those employed in the offshore oil industry are more likely to be exposed at work. Again, similar to many marine species, the location of humans often depends on the stage of life. Relatively placid water areas with sandy beaches are where children may be found, as are their mothers, who are more likely to be pregnant with another child than are other women. Areas of high surf in which breaking waves might lead to more off-gassing of volatile components tend to attract teenagers (Afshar-Mohajer et al., 2018). Individuals on chartered fishing boats tend to be older and male. Beachcombers may encounter weathered oil on the beach. Children, with their curiosity and hand to mouth habits, may be particularly at risk from touching and ingesting tar balls. Local weather is important in determining both the direction of movement of the oil components in air or water, and the activities of humans that result in exposure. These all factor into decisions by authorities restricting public access to contaminated shorelines until cleanup activities have been completed.
Human exposure pathways will differ greatly for different crude oil components. Those living or working on or near to the shoreline will generally have relatively larger exposures to airborne volatiles, such as BTEX, particularly when oil releases are near the shore. The off-shore location of the DWH spill contributed to the lack of evidence of significant levels of benzene reaching shore. Factors affecting the extent of human exposure to BTEX include their chemical degradation rates, the amount of BTEX in the crude, the usage of subsea dispersant injection, the length of time for the crude to travel toward the shore, water temperature, wind direction, tidal direction, water turbulence, the proximity of humans to the shore, their activities (including breathing rate), and (if they are indoors) air exchanges and flow rates. Skin absorption of BTEX also occurs (Gorman et al., 2019). Theoretically, skin absorption could be enhanced by the presence of dispersants in the same mixture, although exposure to such a mixture is unlikely. BTEX components degrade relatively rapidly, with a half-life usually less than 1 week. Sunlight hastens degradation in air, and microbes hasten degradation in water or soil.
The major pathway for exposure to PAHs is through ingestion, either of seafood or, particularly in children, during hand-to-mouth activities. Inhalation of PAHs also occurs, as does direct skin contact, particularly among response workers and curious children. In humans and higher predators, active loss through metabolism occurs, with differing rates in different food species.
Idiosyncratic factors related to release of oil components into the sea and the vagaries of human activities can make a significant difference in the extent of exposure. For example, fishing is said to be relatively common around abandoned drilling sites at which greater volatile hydrocarbon (VHC) release might be anticipated.
Furthermore, response to oil spills may reduce the extent of volatilization, as occurs with dispersants, or may lead to the secondary production of PAHs and benzene by offshore burning (NASEM, 2020). Transdermal exposure also can occur. Aerosols of oil-containing particles have been suggested to have potential for inhalation or dermal exposure (Middlebrook et al., 2012; Ehrenhauser et al., 2014; Afshar-Mojafer et al., 2019).
6.8.3 Methodological Approaches to Estimating Human Exposure
The quantitative estimation of risk requires understanding of both the intrinsic hazard of the agent and the exposure dose. Dose is itself dependent on two factors: the pollutant level and the duration or frequency of exposure. Quantitative estimation of individual exposure levels is often challenging. Many of the oil in the sea scenarios provide at best an indirect estimate of actual exposure, usually through data from measuring sites in proximity to exposed individuals, or at an intermediary site between the source and the potential receptor. Such factors as wind or current speed and direction, and whether the individual was outdoors or using personal protective equipment (PPE) may also be considered. For inhaled agents, the respiration rate is an important determinant of personal exposure.
For most epidemiological studies of oil spills, estimation of whether the release of oil caused adverse health effects has depended on qualitative metrics of exposure, such as comparing those who worked in the spill response with those who did not, or time series studies comparing symptoms or findings in responders during and subsequent to exposure. Rarely is information available beforehand. Particularly problematic has been the use of self-reporting of exposure, which is subject to recall bias. Recall bias is the well-documented tendency for those with adverse consequences to give a positive response to questions about the extent of exposure to potential causes; for example, a mother with a
child who is born with a congenital malformation is much more likely to remember a mild respiratory infection that occurred during the first trimester than the mother whose baby was born with no malformations (Thorpe et al., 2015; Crump, 2020).
Among the reasons for major advances in the science of environmental epidemiology has been the development of valid biological markers of exposure suitable for linking relatively low-level human exposures to adverse outcomes or to valid biomarkers of effect. For organic compounds that undergo metabolism, such as almost all crude oil components, the use of biological markers of exposure is limited by the length of time that the agent or its products persist in the human body. Although biological markers of exposure are available for crude oil components of particular interest, such as PAHs and benzene, most studies of the DWH oil spill began well after those components’ expected persistence. For example, although there are some relatively long-lasting metabolites of benzene, the most sensitive and reproducible measurements of exposure to low levels of benzene in the workplace are best obtained at the end of the work shift (Loomis et al., 2017).
Techniques to improve measurement of individual exposure suitable for use in epidemiological studies have been developed, and do provide a better opportunity to accurately relate dose to effects. These include the use of personal monitors which can be worn by potentially exposed individuals. Personal monitors which are sensitive to 1 ppb are available for benzene (as a comparison, the Occupational Safety and Health Administration [OSHA] standard is 1,000 ppb as an 8-hr average), and for total hydrocarbons. Notable in response to the DWH spill was the development of a sophisticated Job Exposure Matrix (JEM), which allowed estimation of exposure levels for each specific task (Stewart et al., 2018). JEMs also have been developed for offshore petroleum workers (Stenehjem et al., 2021). JEMs are refinements of classic occupational epidemiology in which assignment of workers to different exposure levels, qualitatively or quantitatively, allows assessment of whether the outcome of concern followed expected dose-response relationships, where those more highly exposed to the agent of concern had a higher risk of an adverse outcome.
The pathways by which a disaster, such as a major oil spill, produces social and behavioral effects in communities and community members are discussed in Section 6.8.8.
6.8.4 Human Susceptibility Factors Related to the Potential Toxicity of Oil in the Sea
Human susceptibility, also known as vulnerability, plays a major role in health. Individual, family, and community choices related to potential exposure constitutes the major susceptibility factor related to oil in the sea, including location of home, work, and recreation. Because of the centrality of susceptibility factors to human medicine, and the generally greater emphasis given to protecting and ministering to the health of individuals, much is generally known about what potentially might make different humans inherently more or less susceptible to chemicals. As many of the chemical components of particular interest require metabolism for their toxicity, enzyme polymorphisms or other causes of individual variation in metabolism can be important. (See discussion of benzene later in chapter.)
Resilience can be defined as the opposite of vulnerability. Differences in vulnerability are mediated by different levels of individual and community resilience in responding to disasters. Sandifer and Walker (2018) have extensively reviewed the relation between disaster resilience and stress-related health impacts. They and others have made multiple recommendations to improve individual and community resilience (Morris et al., 2013; Buckner et al., 2017; Nicholls et al., 2017; Sandifer and Walker, 2018; Fuchs et al., 2021). Evidence that repetitive disasters, as has occurred on the Gulf Coast, create a cumulative effect on mental and behavioral health, has been among the factors leading to efforts to improve individual and community resilience (Hansel et al., 2015; Harville et al., 2018; Lowe et al., 2019a,b)
As discussed in more detail in Section 6.8.8.2, economic well-being is a major factor in resilience, particularly in relation to mental and behavioral health of individuals and social disruption of communities. Poverty, and other issues potentially reflecting environmental injustice, limits choices in responding to the economic losses caused by the loss of jobs directly or indirectly due to the closure of seafood gathering. Functional disruption of a community and the loss of social capital means there is less of a safety net for individual community members.
6.8.5 Human Health Effects Potentially Due to Oil in the Sea: Direct Toxicity of Crude Oil and Its Degradation Products
6.8.5.1 Acute Effects
Acute effects of crude oil include skin and eye irritation, dizziness and other neurotoxic effects, and effects on the respiratory tract including throat irritation and cough. These have been reported in response workers (e.g., Meo et al., 2009; Gwack et al., 2012; Ha et al., 2012; Rusiecki et al., 2018) as well as in community members when the spills were near shore (e.g., the Tasman Spirit oil spill in the port area of Karachi [Janjua et al., 2006], the Hebei Spirit oil spill in Korea [Park et al., 2019] and the MV Braer oil spill near the Shetland Islands (Campbell et al., 1993, 1994). Skin and eye irritation and respiratory difficulties are generally ascribed to the complex crude oil mixture and its immediate derivatives, with inhalation effects occurring particularly with freshly spilled oil. More recently, the fouling of Israel’s beaches by a tanker apparently intentionally releasing oil reportedly led to the initial response workers suffering respiratory difficulties
that required supplemental oxygen (Rasgon, 2021), and a refinery oil spill affecting beaches in Peru was said to cause early responders to pass out from breathing the fumes (Taj, 2022). The DWH oil spill Coast Guard Cohort Study noted an increase in gastrointestinal and genitourinary effects (Rusiecki, 2018), perhaps related to the heat stress. An increase in deaths due to heart attacks has been reported in relation to some disasters, usually ascribed to stress (Kario et al., 2003; Yousuf et al., 2020). Mice exposed to particulate matter obtained from the burning of surface oil during the DWH disaster have developed pulmonary inflammation as well as an alteration in immune response (Jaligama et al., 2015).
6.8.5.2 Cancer
The known human carcinogens present in crude oil are benzene and the class of compounds known as PAHs. These are discussed separately later in the chapter. In this section we consider studies of cancer incidence or genotoxicity related to crude oil in aggregate rather than to the individual components.
One of the largest prospective cancer studies of workers exposed to crude oil directly related to oil in the sea is the Norwegian Offshore Petroleum Workers (NOPW) cohort. It began in 1998 based on questionnaires returned by 27,917 workers, about 10% of whom were females (Stenehjem et al., 2021). Excluded from their cohort were those who were unlikely to have had offshore exposure to petroleum. The country-wide Norwegian Registry of Employers and Employees was utilized to cross-check data and compare findings. Exposure was estimated based on a series of job-exposure matrices. Data collection about workplace activities was done only at the beginning of the study, thereby limiting the use of the data in estimating the effects of subsequent exposures. The major finding has been a statistically significant increase in the standardized incident ratios (SIRs) of all cancers in males, SIR 1.07 (95% confidence interval [CI] 1.04–1.11) and females SIR 1.13 (95% CI 1.01–1.36). Not surprisingly, statistically significant increases in hematological and skin cancer types were noted. For skin cancer, the extent of exposure to sun or artificial tanning equipment are potential confounders (Stenehjem et al., 2017). Unexpected was an increase in breast cancer both in males and females, although in females it was not statistically significant. A study of cancer incidence in communities exposed to the Hebei Spirit oil spill 7 years before and 7 years after the spill reported an increase in prostate cancer (Choi et al., 2018).
Studies have evaluated indicators of genotoxicity in those exposed during oil spills. Following the MV Braer incident, tests used to assess genotoxicity and mutagenesis were both negative (Cole et al., 1997). However, subsequently a number of studies have been suggestive of genotoxic effects (see Aguilera et al., 2010, for an extensive review of these studies). Using different genotoxicity assays, two different studies after the Prestige oil spill reported findings indicative of DNA damage (Rodriguez-Trigo et al., 2010). In the long-term follow-up of the Prestige oil spill volunteer cohort, persistent DNA damage was observed after 2 years but not after 7 years following exposure (Laffon et al., 2016). In a separate study of local fishermen who participated in the Prestige oil spill cleanup and were assumed to be more heavily exposed, persistent chromosomal damage continued to be observed 6 years after the initial exposure. Transient decreases in DNA repair activity were also noted. The authors report on chromosome bands that appear to be prone to breakage and that are said to be associated with hematological cancers. Genotoxicity and some alteration in DNA repair activity were noted in rats inhaling fuel oil similar to that released from the Prestige (Valdiglesias et al., 2012).
Two urinary measures of oxidative stress in heavily exposed community members, including 8-hydroxydeoxyguanosine, were reported to be increased as late as 6 years following the Hebei Spirit crude oil spill (Kim et al., 2017), although the mechanism for this delayed response is not fully apparent.
6.8.5.3 Other Longer-Term Effects
Reports of longer-term respiratory tract effects have been noted by a number of investigators Community studies include the finding of asthma, respiratory tract allergies, and deficits in pulmonary function in children after the Hebei Spirit oil spill (Park et al., 2019). One year after the Tasman Spirit oil spill a decline in pulmonary function was observed in healthy response workers, though this improved over time (Meo et al., 2008).
Intensive studies after the Prestige oil spill included a long-term study by the Spanish Society of Respiratory Medicine of a cohort of fishermen who were involved in the cleanup efforts and who for the most part did not use PPE during the initial response period (Zock, 2011). At a little more than 1 year after the oil spill, these relatively heavily exposed workers were found to have a higher prevalence of upper and lower respiratory tract symptoms (Zock et al., 2007). A subsequent study at 2 years post-spill found similar symptomatic differences from a control group of lesser-exposed fishermen but no significant difference in pulmonary function as measured by spirometry was observed (Rodriguez-Trigo et al., 2010). The latter study also found increases in levels of biomarkers consistent with respiratory tract damage and oxidative stress. A subsequent study of a sample of this cohort 6 years following the Prestige oil spill showed no clear differences between the exposed and control groups (Zock et al., 2012). As pointed out by the authors, the study was limited to non-smokers and may have missed an effect in those whose lungs were compromised by smoking.
In the NIEHS GuLF study, suggestion of a reduction in lung function among those involved with handling oily plants
or dead animals was observed in participants tested from 1–3 years following the spill, but lung function was unrelated to the extent of estimated THC exposure. It was suggested that lung function was affected by the stressful nature of the cleanup job (Gam et al., 2018). However, lung function was not affected by working in those jobs for which Kwok et al. (2017a) had found the highest levels of mental and behavioral effects. A return to normal lung function was seen during the 4–6-year time frame with greatest improvement in those with the highest exposure (Lawrence et al., 2020).
Longer-term cardiovascular effects have also been suggested. The researchers conducting the NIEHS GuLF study follow-up of 24,375 workers have published two studies evaluating heart attacks (Strelitz et al., 2018, 2019). During the first 3 years of follow-up, the authors noted a suggestive association of heart attacks with individual exposures to total hydrocarbons (THCs) while working as responders (Strelitz et al., 2018). Their subsequent study extended the follow-up period to 5 years and reported that when maximum THC levels were above 3.00 ppm, there was a statistically significant 1.8-fold increase in heart attack risk as compared to those with a maximum THC level of less than 0.30 ppm. They point out that particulate air pollution has been associated with cardiovascular disease but they do not appear to have considered this as a possible confounder, although evidence of increased particulate levels was reported by Middlebrook et al. (2012), who noted that burning of oil was a significant cause. This was recently confirmed by Pratt et al. (2020) of the GuLF program, who estimated that major exceedances of the particulate air standard would result from in situ burning. Strelitz et al. (2018, 2019) also do not review whether there was an increase in heart attack rates in the many previous studies of workers in the petroleum industry or other industries whose THC exposure levels were presumably far higher than those of the DWH response workers. However, in comparing the two studies by Strelitz et al. (2018, 2019), one finds that in the earlier publication the authors consider that their preliminary findings of an increased risk of heart attacks may be due to psychosocial stress and anxiety associated with those response jobs that had higher exposure to THCs. In the second study, although the authors expertly adjusted for many possible confounders, they did not consider psychosocial stress factors. Furthermore, current understanding of the toxicological mechanism of action of THC components would not readily explain the observed higher association with maximum THC levels than median THC levels, particularly as there does not appear to be an attenuation of the effect during the 5-year follow up. The authors do cite a study associating benzene exposure with cardiac risk among 210 clinic patients with cardiovascular risk (Abplanalp et al., 2017). However, this study has only a single measurement in each subject of a urinary metabolite were by itself is inadequate to determine long term benzene effects even if the metabolite was not also produced by agents other than benzene. Nevertheless, whether THCs are a causative factor, or a proxy for another cause related to responding to oil spills, these observations deserve careful follow-up.
In view of the large number of workers, and the extent of long-term occupational surveillance of workers directly exposed to relatively high levels of crude oil in the past, it would seem unlikely that unrecognized long-term hazards exist. But such workforces were composed primarily of relatively healthy males. Toxicological evidence suggests the possibility of reproductive and developmental toxicity from crude oil components (Feuston, 1997; Diamante, 2017), particularly PAHs. Epidemiological studies of the relation of PAHs to birth defects have not been conclusive. For example, case control studies using the National Birth Defects Prevention Database to investigate neural or cardiac birth defects in babies whose mothers had been occupationally exposed to PAHs found some evidence of an association of PAH exposure with spina bifida but not other neural tube defects, and not with congenital heart disease (Langlois et al., 2012; Lupo et al., 2012). Though not a consistent finding, studies suggest that disasters affecting pregnant women may have a behavioral impact on their infants (Zhang et al., 2018).
Further evaluation of potential reproductive and developmental toxicity of crude oil mixtures and components is warranted. The recent finding in Norwegian offshore workers of an increase in breast cancer in both sexes (although not statistically significant in females), if confirmed, could be related to an endocrine effect (Stenehjem et al., 2021).
6.8.6 Toxicity of Crude Oil Components of Particular Concern
6.8.6.1 Polycyclic Aromatic Hydrocarbons
PAHs are well-studied known human carcinogens that are present naturally in crude oil in varying total amounts, and with varying distributions of the individual PAH components. A major source of human exposure is through inhalation of products of hydrocarbon combustion, although the resultant mixture of pyrogenic PAHs differs somewhat from the petrogenic PAH mixtures found in crude oil (Straif et al., 2005). PAHs also are formed in nature from terpenes and other related ring structures.
PAHs are likely to be responsible for one of the earliest recognized occupational causes of human cancer, that of scrotal cancer observed in chimney sweeps and reported by Sir Percival Pott in 1775. Although the individual PAH compounds differ widely in their toxicity and carcinogenicity, when present as a mixture, such as in soot or cigarette smoke, the overall mixture is carcinogenic. Current evidence indicates a direct relation between PAHs and cancer of the skin, bladder and lung, with lung cancer primarily due to inhalation of PAHs in cigarette smoke. Benzo(a)pyrene, a common
PAH, was the first thoroughly studied carcinogen in laboratory animals. As with other PAHs, it acts primarily through metabolic activation leading to products that bind to DNA (Mumtaz et al., 1996).
A major direct and indirect impact of oil in the sea is the uptake of PAHs into seafood which is then eaten by humans. PAHs are relatively persistent in the environment as compared to volatile hydrocarbon components. As discussed in Chapter 2 of this report, the different chemical structures result in different physical properties that affect their liquid/vapor phase distribution and environmental persistence, and thus the extent of human exposure. Furthermore, the PAHs vary greatly in the extent of their mutagenicity and presumed human carcinogenicity, a fact that has complicated the risk assessment for determining levels of PAHs in seafood pertinent to recommendations concerning reopening of seafood gathering (see discussion in Section 6.8.11).
6.8.6.2 Benzene and Alkyl Benzenes
BTEX are ubiquitous components of our petrochemical era. Benzene is a well-studied known chemical carcinogen, as demonstrated in epidemiological evaluation of large workforces relatively closely and followed for many decades in many different countries. All of the BTEX components, in common with most other volatile hydrocarbons, have properties similar to anesthetics at high concentrations. Measurements of BTEX during the DWH oil spill were variously reported. Huynh et al. (2022) estimated BTEX levels on supporting vessels as being in the part per billion (ppb) range. In contrast, the on-scene coordinator reported that levels of benzene above 200 ppm (i.e., 200,000 ppb) were observed on vessels drilling relief wells, and that on these vessels the overall levels of volatile hydrocarbons raised concerns about fire (Tavares, 2011).
Central Nervous System Effects of Benzene and Alkyl Benzenes
At petrochemical industry workplaces, a feared event is buildup of gas concentrations from BTEX and other petroleum hydrocarbons to lethal levels in enclosed spaces. At sufficient concentrations BTEX compounds all have effects on the central nervous system. The effects are believed to be additive (Wilbur and Bosch, 2004). At lower levels, drowsiness and lack of coordination can contribute to safety incidents. This concern is supported by a U.S. EPA study in rats equating relatively low levels of toluene with the effects of ethanol on coordination (Benignus et al., 2011). The adverse central nervous system effects of toluene are among the most thoroughly studied of the alkyl benzenes, due in part to toluene being intentionally inhaled at high concentrations for intoxication purposes (e.g., glue sniffing).
n-hexane is also a known neurotoxin. It causes peripheral nerve damage through a metabolite that cross-links nervous tissue (Spencer, 2020). As with other aliphatic components of petroleum, it also contributes to the anesthetic-like effects of crude oil fractions.
The U.S. Coast Guard study found an association between exposure to crude oil and headaches, lightheadedness, difficulty concentrating, numbness/tingling sensation, blurred vision, and memory loss/confusion (Krishnamurthy et al., 2019). Similar results were found with inhalation or skin exposure. For all neurological symptoms, the results were of greater magnitude for those reported to also have dispersant exposure than for those exposed to oil alone, perhaps indicative of recall bias.
In the GuLF study, longer-term evaluation of neurobehavioral effects in response workers 4–6 years following the DWH event found modest decreases in neurobehavioral function, especially attention, memory, and executive function (Quist et al., 2019). It is unclear how these longer-term effects would occur. While present understanding of the mechanism of action of anesthetics appears to be moving from older concepts of lipid solubility to more specific effects on membrane function, neither appears to be a mechanism for longer-term effects.
Werder et al. (2019) found a relationship between blood levels of individual BTEX components, particularly benzene and to some extent toluene, and the results of a questionnaire about potential central nervous system or peripheral nervous system self-reported symptoms (e.g., dizziness, stumbling). The study population was 690 Gulf area residents who had previously been exposed to the DWH oil spill as residents or responders. However, the investigators point out that the blood levels of BTEX generally reflect exposure within the past 24 hours, and the DWH exposure occurred years previously. Only one blood level was obtained, and the blood BTEX levels were not different from those found in the National Health and Nutrition Examination Survey—which used the same laboratory to measure blood benzene as did this study—so presumably the blood benzene levels were representative of the general population. Nor was there information about when during the day the blood was drawn beyond it usually being the morning to facilitate overnight shipping of samples to the lab (Engel et al., 2017), nor the length of time before the blood draw that the individual had been at home where benzene levels are generally much higher than outdoor levels. While cigarette smoking and environmental tobacco smoke were considered in the analysis, no questions appear to have been asked about other household sources of benzene. As just one example, the World Health Organization’s review of indoor benzene exposures states that 40–60% of indoor benzene may be attributable to an attached garage (Harrison et al., 2010). Finally, as with many of the other DWH-related studies attributing effects to relatively low BTEX levels, the authors have not provided a rationale for why this outcome was not recognized in previous comprehensive studies of workers exposed to
perhaps three orders of magnitude higher levels of benzene or toluene.
Hematological Effects of Benzene
Benzene is the only hematotoxic BTEX component. At high doses one or more of its phase 1 metabolites destroys hematological stem cells and can cause death due to the failure to produce red blood cells, white blood cells, and platelets. Lesser loss of stem cells leads to pancytopenia, a decrease in all of the blood cell types. Subtle changes in the number of these cells in the circulation have been reported in Chinese workers chronically exposed to levels as low as 1 ppm (Lan et al., 2004). In comparison, the OSHA benzene standard is 1 ppm for an 8-hr time-weighted average (TWA); the exposure level recommended by the National Institute for Occupational Safety and Health (NIOSH) is 0.1 ppm for a 10-hr TWA; outdoor levels in the United States are usually about 1 ppb; and indoor levels can be as high as about 10–12 ppb depending on indoor sources, such as the extent of cigarette smoking, the presence of an attached garage or other benzene source, and indoor/outdoor air exchange.
Benzene itself does not directly affect blood cells. It causes hematological effects through its active metabolites that produce mutations in stem cells. These mutations may cause hematological neoplasms, including leukemia and lymphoma.
Contrary to the appropriate concerns that mixtures of chemicals may enhance the toxicity of any one component, the evidence indicates that relatively high concentrations of toluene actually protect against benzene hematotoxicity. This is not surprising in that both undergo oxidative metabolism by Cytochrome P450 2E1 which in the case of benzene, but not toluene, produces hematotoxic metabolites. Usually about half of inhaled benzene is exhaled harmlessly. If there is sufficient toluene to competitively inhibit benzene metabolism such that more is exhaled unmetabolized, this would decrease benzene toxicity. Note that, except in heavily exposed workers, the relatively lower levels of benzene and toluene inhaled by most of those exposed to oil in the sea makes an inhibitory interaction unlikely, as sufficient metabolic machinery appears to exist to metabolize both. Similarly, variations in the rate of benzene metabolism, including due to genetic polymorphisms, affect susceptibility to hematological effects (Rothman et al., 1997; Hosgood et al., 2009).
Many of these same issues about the utility of a single blood draw to measure a rapidly changing parameter such as blood benzene, and potential confounding by indoor benzene sources other than smoking and environmental tobacco smoke, are pertinent to the GuLF-related study of Doherty et al. (2017). The investigators report an inverse relation between blood benzene levels and a number of red blood cell parameters such as hemoglobin. Particularly problematic, as recognized by the investigators, is that benzene’s well-studied impact on circulating red blood cells is through benzene’s attack on nucleated stem cells. Circulating red blood cells obtained in a blood draw have lost their nucleus, and in normal individuals will survive 120 days after leaving the bone marrow. In essence, the investigators have compared endpoints that integrate what has happened in the bone marrow over the past 4 months with the level of benzene obtained from an evanescent exposure measure which primarily reflects about 24 hours of benzene exposure, and that itself may vary greatly depending on such factors as whether a window happened to be open. Furthermore, the effect estimates for toluene were in the same range or even higher than for benzene, despite toluene not having any known hematological effects at exposure levels three or four orders of magnitude higher.
Hematologic effects were also reported by Park et al. (2019) in Hebei Spirit oil spill residents and responders at two time frames following the oil spill. In this case, though, the hematocrit went up from an average of 39.3% to 39.5%, a miniscule change that was said to be statistically significant. This is well within the range of instrument variability due to slight recalibration. The white blood count also increased, which is contrary to the usual effect of benzene.
Other Effects of Alkyl Benzenes
In addition to BTEX there are many other alkyl benzenes present in crude oil. One of the few that has been studied in some detail is cumene (isopropyl benzene) which is usually present in crude oil at concentrations of 0.1–1.0%. Based on genotoxicity and cancer studies in laboratory animals, cumene is classified by the National Toxicology Program as “Reasonably Anticipated to Be a Human Carcinogen.”
The xylene isomers are also irritants, particularly of the eyes and upper respiratory tract, as to a lesser extent are toluene and ethylbenzene.
A number of recent studies have associated relatively low levels of benzene or BTEX with asthma or with endocrine disruption (Bolden et al., 2015). In the absence of toxicological mechanistic support, and the previous lack of such observations in relatively heavily exposed workforces, these observations require further rigorous validation (Bolden and Kwiatkowski, 2016; Lynch, 2016).
6.8.7 Dispersants
Dispersants are used to prevent or reduce oil from subsea releases from reaching the surface and forming slicks, or to disperse surface slicks already formed to prevent their direct contact with birds and marine mammals, including humans (e.g., response workers on vessels). Dispersing an offshore slick also prevents oil from contaminating shoreline where it could cause exposure to response workers and to the general public. Intended operational best practices lessen the likelihood of direct contact of the general public
with dispersants or dispersed oil. Dispersant application procedures also are designed to limit the opportunity for response workers to interact with dispersants directly. See Chapters 4 and 5 for detailed discussion on dispersant use and fate of dispersed oil.
In 2020, a National Academies committee fully reviewed the issues associated with the unprecedented amount of dispersants used in response to the DWH oil spill (NASEM, 2020). Both the U.S. Coast Guard and NIEHS studies reported that response workers who believed they were exposed to dispersants had higher levels of reported adverse respiratory effects (McGowan et al., 2017; Alexander et al., 2018). However, in the absence of biological markers and more specific information about when and where dispersants were used in relation to much of the workforce, it was not possible to verify personal reports of dispersant exposure. The paucity of information and the lack of a biological marker made it not possible to add dispersant exposure to the JEM. Accordingly, recall bias likely was a strong potential confounder in both studies. Also, no distinction was made between the personal protective equipment used whether or not dispersant was present, thus making it harder for the individual responder to ascertain whether dispersant was present (NASEM, 2020).
The well-publicized debate about the use of an unprecedented amount of dispersants added to the concern of families affected by job loss related to the oil spill (Osofsky and Osofsky, 2021). Also adding to public concern was the initial failure to release total information about the chemical composition of the dispersant, leading to publicity about a secret ingredient (Goldstein, 2020). Lack of transparency contributes to what is called the “social amplification of risk,” which itself is related to the trust of the information source (Kasperson et al., 1988). Avoidance of unnecessary secrecy about dispersants was recommended by the National Academies report, The Use of Dispersants in Marine Oil Spill Response (NASEM, 2020).
6.8.8 Human Health Effects Due to Oil Spilled in the Sea: Mental and Behavioral Effects
6.8.8.1 Evidence of Disasters as a Cause of Mental and Behavioral Health Effects
An extensive body of literature unequivocally documents mental and behavioral effects in those directly involved in disasters, such as military experiences, the London King’s Cross tube fire (Rosser et al., 1991); the Buffalo Creek, West Virginia, dam failure (Titchener and Kapp, 1976), and the World Trade Center disaster (Diab et al., 2020). The failure to fully consider the importance of mental and behavioral effects in disasters is believed to have exerted a major toll in lack of preparedness and responsiveness. For example, Murthy (2014) argued that the relative lack of consideration of community mental and behavioral effects following the Bhopal disaster, in which more than 3,000 residents died, left a wide range of unaddressed adverse effects that could have been ameliorated by psychological support for community members. Natural disasters, such as hurricanes, are believed to produce mental and behavioral effects in more individuals than are affected by the physical force of the hurricane (Espinel et al., 2019).
McCoy and Salerno, in summarizing an Institute of Medicine workshop on “Assessing the Effects of the Gulf of Mexico Oil Spill on Human Health,” held 2 months after the DWH explosion, noted the need for evaluation of psychological and socioeconomic health. This has been more than confirmed in the many studies that followed. The three most common findings have been relatively high levels of posttraumatic stress disorder (PTSD), depression, and anxiety (IOM, 2010a,b).
Further indication of the heightened prominence of mental and behavioral health in disaster response was a “Perspective” article in the New England Journal of Medicine titled “Moving Mental Health into the Disaster-Preparedness Spotlight” (Yun et al., 2010). It appeared in September 2010, soon after the DWH oil source was finally capped, and was co-authored by Nicole Lurie, the Assistant Secretary for Preparedness and Response of HHS. The Perspective began with the statement: “As the Deepwater Horizon oil disaster enters its next phase, consensus is emerging that among its most profound immediate health effects are those on the emotional and psychosocial health of Gulf Coast communities.”
The authors added: “If one bright spot emerges from this catastrophe, it will be incorporation of mental health-related emergency response into the core competencies for disaster preparedness” (Yun et al., 2010).
The evidence gathered from studies of the DWH and other oil spills fully supports this emerging consensus. Of particular pertinence is the growing evidence that major oil spills produce mental and behavioral effects unrelated to direct contact of community members with crude oil or its components. This is distinct from the earlier reported direct effects on mental and behavioral health such as following the 1990 explosion on the Piper Alpha oil rig in the North Sea, in which there were 125 deaths (Alexander, 1991). Psychiatric intervention was needed for survivors on the oil rig, as well as for police officers handling the charred bodies. While not stated, presumably interventions would also have been helpful for family members and close friends of those who were directly affected.
The committee does not at all belittle the impact of the DWH disaster on affected workers and their families, colleagues, and friends. For example, the psychological impact on the survivors of the Piper Alpha explosion were still apparent 10 years after the event (Hull et al., 2002). Rather, we wish to extend this review of health effects due to oil in the sea to include the broader effects of oil spills on the psychosocial health of community members.
Studies of Alaskan coastal communities affected by the Exxon Valdez oil spill provided significant evidence that community disruption impacted the mental and behavioral effects of individual community members (Picou et al., 1992; Palinkas et al., 1993; Gill and Picou, 1998; Arata et al., 2000). Comparison of the impact of the Exxon Valdez oil spill on Native Alaskan communities with others involved in the oil spill reported differences in the extent and type of the mental and behavioral impacts (Palinkas et al., 2004; see also Section 6.6.6).
Building on these earlier studies of the Exxon Valdez and other disasters, more recent work, and particularly studies of the impact of the DWH oil spill, have provided the evidence base to strongly confirm the relation between a major oil spill and the disruption and mental and behavioral factors.
Before the DWH oil spill, the mental and behavioral impact of oil spills on community members was usually determined in one or at most a few studies. The extent of this literature now has been dwarfed by numerous studies of different investigators using different approaches on different populations which show mental and behavioral effects in response workers and community members following the DWH spill. Comparing the studies is difficult as they usually depend on questionnaires of different length and different levels of standardization. However, as reviewed here, the published evidence relatively consistently finds that populations affected by the DWH oil spill show an increase in mental and behavioral health effects.
Just prior to the DWH oil spill, Harville and her colleagues (2010) had reviewed the impact of disasters on perinatal health, exemplifying how the greater extent of disaster research prepared investigators to evaluate the health effects of the DWH incident. Similarly, studies of children and adolescents following the DWH oil spill were able to use the baseline of prior studies of children of the same age group, often in the same group (Osofsky et al., 2014).
A number of studies have shown additive effects of exposure to multiple disasters, including exposure to both Hurricane Katrina and the DWH spill (Osofsky et al., 2011). Harville et al. (2018), in a long-term study of 1,366 women of childbearing age, specifically looked for interactive effects of exposure to up to four Gulf Coast hurricanes and the DWH spill. Their findings were most consistent with a cumulative effect for PTSD and depression. When the experiences were particularly severe, some evidence was observed for sensitization, in which exposure to one disaster would increase the extent of effects from a second disaster. But there was no evidence in this population of habituation, in which exposure to one disaster might lessen the impact of a second disaster, as was seen for PTSD in a study of Gulf residents affected by repetitive natural disasters (Hu et al., 2021).
As a result of the oil spill, particularly in tandem with the effects of Hurricane Katrina and other natural disasters, teenagers seemed to be at particular risk for mental and behavioral effects and such outcomes as substance abuse (Fuchs et al., 2021). The authors suggest that psychological resilience protects the individual teenager. Ha et al. (2013) noted the sensitivity of children to depression following the Hebei Spirit oil spill.
Taking into account the importance of developmental factors in children and adolescents in their response to disaster and their resilience has been stressed (Osofsky and Osofsky, 2021).
The importance of a baseline for the study of the mental health of disasters was illustrated when two floods occurred during an ongoing longitudinal study of older adult mental health in a relatively impoverished area of eastern Kentucky in 1981 and 1984 (Phifer and Norris, 1989). Personal loss was found to be particularly associated with shorter-term increases in mental and behavioral effects; community disruption led to longer-term effects, and the intensity of the flood was associated with greater effects.
6.8.8.2 Evidence for the Role of Economic Impacts on the Mental and Behavioral Effects and Social Effects of Oil Spills
Communities in the area of the Gulf of Mexico benefit substantially from the economic value of the Gulf ecosystem. Shepard et al. (2013) have estimated that the 2010 revenues from provisioning ecosystem goods and services from the five states bordering the Gulf of Mexico amounted to more than $2 trillion.
As described in Section 6.8.8.1, the evidence now strongly supports the contention that virtually any major disaster, including an oil spill, causes mental and behavioral effects. However, the Oil in the Sea IV committee has also explored a slightly different question: whether there is evidence that those who lose income as a result of an oil spill are more likely to have mental and behavioral effects than those who do not. If so, then oil spill prevention and response efforts should consider not only community economic health but also what would appear to be inevitable community mental and behavioral health effects due to the oil spill causing economic loss. In making such a determination, associated factors such as resilience and community social capital would have to be taken into account (Clay and Abramson, 2021).
A related question is whether the duration of economic impact is also a factor in the extent of mental and behavioral effects. The duration of closure of seafood gathering is not only an economic issue but also one related to human mental and behavioral health. This issue was considered by the National Academies’ Committee on the Use of Dispersants in Marine Oil Response (NASEM, 2020). As summarized here, the DWH oil spill substantially added to the evidence base that both the extent of economic loss and the duration of time until reopening of seafood gathering are significant factors in the causation of human mental and behavioral health effects.
Evidence that spill-related income loss appears to cause greater psychological distress than the presence of shoreline oil comes from studies comparing two fishing communities, one directly affected by the DWH oil spill (Baldwin County, Alabama), and the other (Franklin County, Florida) having economic consequences from loss of fisheries and tourism, but no fouling of its shoreline. Grattan et al. (2011) found no difference in the measures of psychological distress between residents of these two communities, but within both communities those who had spill-related income loss had much more psychological distress than those with stable incomes. This finding persisted in a follow-up study 1 year after the oil spill (Morris et al., 2013). The authors conclude that spill-related income loss may have greater psychological effects than the presence of shoreline oil.
Choi et al. (2021), in their ongoing study of local residents 9 years following the Hebei Spirit oil spill, observed persistent effects that were more common among those with lower income (Choi et al., 2021). Loss of income from the oil spill also appeared to be a factor. In the Hu et al. (2021) study cited earlier, the severity of property or crop loss was associated with anxiety, depression, and PTSD.
Using the lens of the relative weight of psychological risk factors associated with disasters, shorter term economic loss figured heavily in a tabulation of those risk factors that were “Present and Prominent,” as compared to “Absent or Minimal” (Shultz et al., 2015). The authors point out that the psychological impact of the DWH oil spill was less than many feared, despite the multiple-impact effect of Hurricane Katrina, which they attribute to coastal residents generally not experiencing significant injury or mortality, disruption of vital services, or population displacements, as well as to the relatively rapid infusion of economic support.
Lowe et al. (2015) noted that workers enrolled in the GuLF program were relatively well paid but yet had an increased incidence of major depression, PTSD, and generalized anxiety in which a greater level of physical effects played a major role. Even within this group of relatively well-paid workers, they found evidence that greater income mitigated mental depression and generalized anxiety disorder. Lowe et al. (2015) also analyzed the factors that led members of this cohort to seek mental health support.
Osofsky and Osofsky (2021) considered the lessons from both Hurricane Katrina and the DWH oil spill, and noted the prolonged mental health impact on those having fewer resources and who received less help to recover. Other studies include those of Drescher et al. (2014), who, in a study of 1,119 coastal Mississippi residents receiving mental and behavioral health services, reported that the chronic problems of life affecting Gulf coast communities were significantly associated with psychological distress, with those having income below the poverty line reporting higher levels of distress related to the DWH oil spill. Similarly, in a telephone survey of 812 adults, those with spill-related economic impact had more symptoms consistent with depression and PTSD (Shenesy and Langhinrichsen-Rohling, 2015). The authors also found an association between an individual’s perceived resilience and lower levels of psychological symptoms. Gould et al. (2015) attributed the rapid distribution of funds from various sources, including the BP tort settlement, for the findings of relatively minor impact on substance abuse in two federal surveys. Similarly, the unusually rapid distribution of funding support to local residents in Spanish communities affected by the Prestige oil spill was posited to play a role in the lack of a statistically significant effect on measures of health quality of life, including mental health (Carrasco et al., 2007).
As part of the Women and Their Children Health Study (WaTCH), which followed a cohort of women from southern Louisiana, Rung et al. (2017) reported that the impact of economic loss on causing depression was largely mediated by the concomitant loss of social capital (Rung et al., 2017). Gaston et al. (2016), in a study based on the same WaTCH cohort, reported that the three stressors of economic problems, physical/environmental exposure, and signs of neighborhood decline were independent predictors of depressive symptoms. Evaluating PTSD symptoms, Nugent et al. (2019) found that wealthier women were more likely to be in their low-symptom category.
Johnson et al. (2020) recently pointed out the semantic ambiguity involved in many of the approaches to understanding why natural disasters have different impacts on different communities. Using factor analysis, the authors have attempted to tease out the commonalities among the 130 variables used in various indices that have been developed to measure community vulnerability and resilience. They report that 50 of these variables fit within five factors: wealth, poverty, agencies per capita, elderly populations, and non-English speaking populations. Two of these five factors, wealth and poverty, reflect the role of economics in the vulnerability and resilience of communities to natural disasters. A similar analysis for type-specific technological disasters, such as oil spills, could be helpful.
Economic factors also play a major role in social vulnerability to environmental disasters. In assessing the dimensions of community social vulnerability, Cutter et al. (2003) found that personal wealth ranked first in explaining the observed variance. The association of community attachment with health impacts and with employment in seafood gathering or the oil industry was studied in a repeated telephone survey of Louisiana coastal households (Cope et al., 2013). The first survey was done in June 2010 while the oil was still flowing, and subsequent studies were in June 2010 and April 2011. Major findings were that both mental and physical impacts were highest initially but persisted after the oil was contained; that higher levels of community attachment were associated with lower levels of negative mental and physical health impacts; and that fishing households had significantly higher adverse mental health impacts than others. The greater impact on seafood gatherers than other
community members had previously been noted following the Exxon Valdez oil spill (Picou et al., 1992; Picou and Gill, 1997; Arata et al., 2000).
The economic impact of an oil spill disaster similar to that of the DWH would seem to be dependent on the duration of closure of seafood gathering and other maritime-related activities. Berren et al. (1980) listed the duration of a disaster as one of five factors in their suggested typology of disasters. Evidence that the duration of a disaster is accepted as a factor in the extent of mental and behavioral effects includes a study of Dutch survivors of an Asian tsunami which found that duration of threat to life was a major determinant of ongoing physiological and psychological health problems (Marres et al., 2011); and a study by Caramello et al. (2019), who reported less mental and behavioral impact in responders to a mass casualty event in Turin, Italy, which they ascribed in part to its brief duration. Lowe et al. (2015), in their study of GuLF cleanup workers, found a complex relation of duration of work to greater physical effects also leading to more mental and behavioral health problems, which was further complicated by greater income appearing to lead to lesser mental and behavioral problems. Choi et al. (2021), in their follow up study 9 years after the Hebei Spirit oil spill, found that duration of cleanup work was related to the extent of persistent mental health effects. Continued studies of this cohort will be helpful.
The many studies following the DWH oil spill amply confirm that a major impact of oil spills affecting shore communities is on their mental and behavioral health, and that economic impacts of the oil spill play a significant role, as does the vulnerability and resilience of the affected communities. One potential ameliorating response is to provide greater prominence to preventing mental and behavioral effects in the decision process responding to oil spills (Goldstein, 2020). The Incident Command System (ICS) currently plays a major role in responding to oil spills (refer to Section 4.2). The ICS, under the direction of the U.S. Coast Guard for offshore spills and the U.S. EPA for inland spills within the United States, has been a positive development in disaster response. Its strategic priorities initially focus on human health, which is interpreted as preventing acute safety hazards such as explosion and fire. This is followed by responding to the need to protect ecosystems, which is assisted by a relatively formal science-based evaluation (see Chapter 4 for consideration of NEBA and similar ecological approaches of value for ICS decision-making).
The relatively low priority given to health concerns beyond immediate safety considerations is apparent from a review of the post-DWH spill On Scene Coordinator report (Tavares, 2011). Human health issues are subsumed under the heading of “Safety.” The on-scene coordinator (OSC) report contains an organizational chart for the ICS Houston Headquarters (see Figure 3.1, Organizational Chart Task Forces). It shows four operating branches. The first three are Procedures, Source Control, and Dispersant. The fourth operating branch has a catch-all title of “Safety, Human Resources, Information, Legal, Security.” Chapter 4 of the OSC report is on safety. It is positive about the relatively good safety record related to the health of response workers, and goes into detail about seafood safety and the decision process concerning closures of seafood gathering. Mental health concerns, which led to the specific request for HHS involvement, are described as follows:
Although not covered in the National Contingency Plan, the combined effects of the spill on a population that had only five years earlier endured Hurricane Katrina raised concerns about impacts on the mental health of the people living near oil-impacted areas. (Tavares, 2011)
Using the find function on this 244-page report reveals three other instances in which human health is mentioned. Two are related to the handling of oiled wastes, and the third concerns a report on potential post-cleanup risks to humans and ecosystems due to residual oil. In contrast, “turtle” elicits 149 entries and “bird” 71 entries.
Also instructive is a review of the U.S. EPA’s Handbook on Area Contingency Planning (2018), which includes detailed consideration of ecosystems, including endangered species, historic preservation, tradeoffs using NEBA, and collaborative efforts with the U.S. Fish and Wildlife Service, NOAA, and other agencies—but not HHS. The Handbook does provide an overview of EPA’s legal authority to consider public health. However, it contains no planning tools to identify population health risks, vulnerabilities, evacuation routes, or other standard public health approaches to minimizing disaster risks.
In view of the increasing evidence of mental and behavioral effects, additional focus on the role of the ICS in preventing mental and behavioral effects of an oil spill would seem to be deserved. However, achieving the level of information needed to inform ICS decisions would require mental and behavioral health scientists to focus on further developing and validating standardized measures of community mental and behavioral health equivalent to those now provided for decisions about ecosystems. Approaches to measure and improve community resilience are also needed to include human health within National and Area Contingency Planning.
6.8.9 Dinoflagellate Toxin Poisoning
Dinoflagellates are a class of primarily unicellular organisms which when present in seafood (particularly shellfish), produce toxins that are responsible for a variety of effects including neurotoxicity, diarrheal diseases and ciguatera fish poisoning. In the United States, deaths are uncommonly reported but may be underestimated due to physicians’ lack of familiarity with dinoflagellate toxicity. The linkage of dinoflagellate blooms to oil in the sea has been controversial, although recent studies have suggested that toxicity of oil to dinoflagellates with or without dispersants is less than to
their main grazers (ciliates and heterotrophic). The resulting higher abundance of dinoflagellates may result more from the release from predation than from stimulation by the oil. The disruption of the bottom-up limiting processes may allow blooms of dinoflagellates, including harmful ones, to develop (Almeda et al., 2018). In another study by Park et al. (2020), the combination of bacterial communities exposed to oil-contaminated sediment from the Texas City, Texas “Y” oil spill stimulated the growth of the dinoflagellate Prorocentrum texanum. Furthermore, when isolates of oil-degrading bacteria were co-cultured with the dinoflagellates, dinoflagellate growth was stimulated by unidentified bacterially generated substances.
6.8.10 Other Effects on Human Health: Workers Health and Safety
Much of the earlier information about the health effects of oil spills has come from study of cleanup workers, both professionals and citizen volunteers. This is reflected in the discussion earlier in this section. As described in the various health-based sections, much of the information about effects of oil in the sea comes from studies of response workers. This includes mental and behavioral health problems, so it is not surprising that such findings were also noted in DWH responders (Fullerton et al., 2004; Goldmann and Galea, 2014; Lowe et al., 2016, 2017; Quist et al., 2019).
Worker safety issues and the safety culture in the oil industry are entwined in the subject of oil in the sea. Failure to adhere to worker safety rules arguably was a significant causative factor in the Exxon Valdez oil spill and a contributor to the system failure that led to the DWH oil spill. Eleven rig workers died in the latter (NRC, 2012). Many of the worker safety issues are an amalgam of those related to the sea and those common to the petroleum industry on shore, including drilling for and transporting flammable and explosive materials. Response activities can be more intense or at variance from standard industry activities (e.g., dealing with the carcasses of oiled birds or mammals, or spending much more time cleaning ships and gear). Response workers in particularly stressful situations and those exposed to high amounts of total hydrocarbons have an increased risk of mental health effects such as PTSD or depression (Kwok et al., 2017b). Performing research related to oil spills and seeps can lead to risks specific to deep sea diving, although humans are largely being replaced by controlled submersibles.
In recent decades, coastal fouling from major oil spills has led to complex response operations by petroleum company employees and governmental organizations such as the U.S. Coast Guard. In addition, there has often been a major influx of volunteers and of paid workers to help clean the shore and the oiled birds and mammals. Significant concerns about the well-being of these cleanup workers have included standard safety issues, mental health concerns, an overlay of local climate issues such as sunburn and dehydration on Gulf beaches, and a concern that the new responders are not sufficiently trained or have unreported pre-existing medical conditions that might add to their risks.
6.8.11 Seafood Safety
The complexities of seafood safety determinations after an oil spill have been relatively well explained for public understanding by NOAA and in a video by the Sea Grant Program (NOAA, 2021d) as well as in the Report of the On Scene Coordinator (Tavares, 2011). Many steps are taken to avoid contaminated seafood reaching the public. Seafood gathering is not allowed if there is visual oil on the water. Once the sheen of oil is no longer visible, the next step is sensory testing in which trained observers test gathered or cooked seafood for the smell of oil. Finally, a chemical determination is made to ensure that seafood PAH levels are within allowable standards. However, despite these lucid explanations, Simon-Friedt et al. (2016), in a 3-year survey of residents of Southeastern Louisiana, found that almost 50% reported not having sufficient information to determine whether they should eat local seafood. Further transparency about the seafood safety risk determination would be helpful.
As discussed earlier in this report, there are thousands of PAHs whose different chemical structures result in different physical properties that affect their liquid/vapor phase distribution and environmental persistence, and thus the extent of seafood and human exposure (Allan et al., 2012; see also more detailed discussion of PAH chemistry, toxicology, and analysis in Section 2.1.3, including Figure 2.8; Section 2.1.7.7; and Section 3.3.2). Factors that influence the extent of seafood contamination following an oil spill include the quantity and composition of the oil; its proximity to seafood gathering areas; weather and other seasonal factors; and the specifics of the ecosystem that affect uptake into seafood, including the potential for bioaccumulation and depuration. For example, vertebrates can catabolically excrete accumulated oil contaminants much more rapidly than most invertebrates.
PAHs vary greatly in the extent of their bioavailability, persistence, mutagenicity, and presumed human carcinogenicity. As has been done with other toxicologically relevant large classes of compounds having similar properties, such as polychlorinated biphenyls (PCBs), the allowable risk-based standard for seafood PAH levels is developed by first assigning an equivalent risk level for each of the PAHs whose toxicity and usual relative concentration are most likely to cause adverse effects. Based on the measured amounts of these selected PAHs, multiplied by the equivalency factor for each, a total PAH risk is determined which is used as the basis for decisions about seafood safety.
Although this is a useful approach, it inherently requires updating to accommodate additional information that becomes available about the toxicity of different PAHs alone and
in a mixture. For example, Farrington (2020) reviewed information on the PAH compositions in (petrogenic) petroleum compared to pyrogenic sources that had been obtained during the several decades since the adoption of the specific PAHs included in the U.S. EPA’s Priority Pollutant listing. The author advocated reevaluating human health risk protocols for seafood because the level of alkylated compounds and their potential toxicity may have been underestimated. Similarly, as offshore burning is a potential response to oil released to the sea, an issue particularly relevant to oil spills is the potential toxicological differences between petrogenic (petroleum) PAHs, which would be decreased by burning the oil, and pyrogenic PAHs, which are formed when oil is burned.
Gohlke et al. (2011) provide an overview of the various federal and state agencies involved and their different jurisdictions (see also Yender et al., 2002; NASEM, 2020). Gohlke et al. (2011) noted inconsistencies in the risk methodology used to close or reopen seafood gathering in the past. Further studies by this group include finding higher seafood consumption among children living close to the Gulf shore as compared to those living farther inland, although in all cases the intake by children of PAHs, metals, or a dispersant component were below the level of concern (Sathiakumar et al., 2017); and that testing of fish caught by commercial fishing folk found detectable levels of PAH in only 2 of 92 fish, both of which were far below the level of concern (Fitzgerald and Gohlke, 2014). Both Ylitalo et al. (2012) and Gohlke et al. (2011) reported that all of the officially measured PAH levels in seafood were below the level of concern. These were fish that were tested beginning after there was no longer a sheen in the water and the trained observers could detect no smell. Similarly, Wickliffe et al. (2018) reported that consumption of fish and shrimp from Southeast Louisiana posed no unacceptable lifetime cancer risk. (See also NASEM [2020] for discussion of seafood safety issues after the DWH oil spill.)
The uncertainties in the PAH risk methodology are evident from a critical published review of the U.S. Food and Drug Administration’s (FDA’s) approach to estimating the risk of PAHs in seafood which particularly emphasized concerns about pregnant women and children (Rotkin-Ellman et al., 2012). The response by Robert Dickey of the FDA (Dickey, 2012), argued that the conservative approach used by FDA likely overestimated actual risk, including by assuming a 5-year consumption period of the contaminated seafood. He also contrasted the 1/100,000 risk level for PAHs used to determine seafood safety with the much higher PAH background levels in the general U.S. population and the value of fish consumption to human health. This was responded to by two of the original authors who again highlighted the uncertainties in the risk approaches used for vulnerable populations (Rotkin-Ellman and Solomon, 2012).
Given that the economic and social consequences of the inability to gather seafood are a significant cause of the mental and behavioral health effects and community disruption caused by major oil spills (see preceding discussion), much more information is needed to narrow these risk-related scientific uncertainties. The tradeoff between the public health risks of PAHs in seafood and the public health impact of seafood closures on community well-being also warrants attention by policy makers.
6.8.12 Vulnerability of Humans to Oil Pollution in the Arctic
Indigenous cultures who have lived along the Arctic coast of Alaska and western Canada for many thousands of years depend heavily on stable ecosystems for their food security through subsistence harvests from the sea. Because participation in subsistence harvests are essential for the intergenerational transmission and maintenance of their cultures, members of these communities are uniquely vulnerable to adverse effects of accidental oil discharges. Members of these communities harvest, on average, more than 300 kg of marine mammals per person per year (based on data from the Alaska Department of Fish and Game), providing their primary source of calories as well as protein. Many marine mammals can be harvested throughout the year, and it is their abundance and availability that make life, and these ancient cultures, possible. These community sites have been occupied for as long as 6,000 years or more, and are the longest continuously occupied sites in North America (Giddings, 1962). This long and intimate relation between these subsistence-based cultures and the marine mammals (as well as all the other resources) that they harvest has led to development of extensive and intricate methods for making use of nearly every part of the animals and plants harvested.
Subsistence harvesting in Indigenous communities of the Alaskan and western Canadian Arctic also provides the foundation for maintenance and transmission of their cultures. Subsistence harvests are community efforts, with members of both sexes engaged in various aspects of harvesting, processing, storing, and utilizing harvested plants and animals from an early age. These activities provide the means through which art, technology, hunting skills and techniques, and cultural history are transmitted across generations. Consequently, any disruption of these subsistence food-gathering activities poses a serious threat to the integrity and maintenance of these cultures.
Following the 1989 Exxon Valdez oil spill, members of Indigenous communities in the spill-affected region became deeply concerned about the safety and continued availability of their subsistence food supplies. As noted by Fall (1999), after personal observations of erratic effects of oil on fish, wildlife and their habitats,
[T]he following questions became very important for the people of the villages: Are our subsistence foods still safe to eat? If some beaches, waters, and animals were oiled and other, seemingly unoiled animals are inexplicably dying, are any resources safe to use?
After hearing assurances from health officials that absence of an oily taste or smell probably indicated that consumption of subsistence-gathered foods was safe, Fall (1999) noted that “villagers responded with skepticism and disbelief” and quoted one villager as saying “We saw too much oil, and we didn’t want nothing to do with [the fish]. . . . We don’t want to eat them until we find out what’s really going on.” Consequently, “subsistence harvests in many villages virtually ceased soon after the spill,” and had not fully recovered 5 years later.
Indigenous communities had no cultural experience that deals with the effects of oil spills, in contrast with firmly rooted cultural approaches to dealing with natural disasters. This, along with overwhelming numbers of strangers having little familiarity with Native culture or institutions implementing oil spill response and remediation over which Natives had little or no influence, led to profoundly adverse psychological and social effects (Fall et al., 1999), exacerbated by the fact that oil spills are technological and not natural disasters (Picou et al., 1997).
Once interrupted, re-establishing traditional subsistence lifestyles can be difficult or impossible. Members of younger generations may abandon their villages and turn to cash economies in urban settings in search of food and other necessities. If widespread and sustained, this can seriously jeopardize the vitality of the Native cultures affected (Gill and Picou, 1997).
6.9 CONCLUSIONS AND RESEARCH NEEDS
The following conclusions and research needs are aimed at filling in important research gaps in understanding the effects of oil in the marine environment so that the environment may be better protected in the future.
Conclusion—Evolving Baseline Knowledge and Data: There are numerous and diverse sources of data and knowledge that can be reviewed and assembled into evolving baseline knowledge and data for marine ecosystems in North American marine waters to inform response, damage assessment, and fates and effects of oil spills.
Conclusion—Long-Term Effects: The committee noted increasing evidence suggesting significant longer-term effects on multiple aquatic and shoreline species and communities, including humans, than previously estimated. Short-term assessments do not cover enough time or provide opportunities for holistic studies to recognize changes in environmental conditions or resources in current time frames, particularly in relation to potentially changing baseline conditions.
Conclusion—Rapid Scientific Response, Communication, and Coordination: During and after an oil spill, there is often an extended time before scientists become engaged in field data collection resulting in missed opportunities to assess critical issues, including establishing environmental baselines and determining initial impacts. Rapid decision-making, funding, and deployment of scientists into the field provided critical information in both the Exxon Valdez and DWH oil spills, though there is still room for improvement.
Conclusion—Protection of Key Foodweb Components and Endangered Species: Mounting evidence suggests that widespread adverse effects on species that are endangered or are major components of marine food webs, such as seabirds and marine mammals, may have substantial repercussions on other species, operating through strong trophic linkages or cascades. Furthermore, adverse health effects on marine mammals and sea turtles exposed at the air-sea interface, primarily on those with rapid gas exchange and high air flow (cetaceans) or prolonged exposure (sea otters) manifest in short- and long-term morbidity and mortality. Lack of avoidance of oil, especially in calm waters, leads to significant risk in these species.
Conclusion—Natural Seeps: Not all effects of oil in the sea are negative. Natural seeps contain areas of (often unique) biological communities that have evolved to use oil as an energy and nutrient source. The oil and gas seeps and hydrate communities host chemoautotrophic symbionts that are unique biogenic communities, particularly in deep-sea locations with a high diversity of organisms, including bacteria consortia (including oil degraders) that support numerous, as yet unknown, biogeochemical processes (including oil degradation), particularly in deep-sea locations.
Conclusion—Marine Oil Snow: Observations found part of the natural biological response to the DWH drilling oil spill resulted in vast quantities of oil-associated marine oil snow (MOS) forming throughout the water. However, the mechanisms controlling MOS formation and its impact on the environment remain largely unknown.
Conclusion—Shoreline Oiling Characterization: The SCAT program supports response efforts by conducting rapid assessment of shoreline oiling, recommending shoreline treatment techniques, and identifying cleanup endpoints. An ancillary sampling program focused on gathering more detailed information on hydrocarbon composition, concentration, and distribution over time would complement SCAT-collected data and provide valuable information for the environmental impact analysis and monitoring of restoration progress.
Conclusion—Behavioral Effects of Oil: Recent research has demonstrated complex effects of oil on olfaction in fish that altered behavioral responses to oil exposure. These findings suggest that these and possibly other effects of oil on organismal behavior may reveal adverse effects of oil that are currently unrecognized. Further research focused on behavioral responses to oil exposure is needed.
Conclusion—Toxicity Studies and Models: Laboratory toxicity studies focus extensively on water column and acute toxicity effects of relatively fresh oil, for which a large amount of data is already available, and models have been developed that can predict the acute toxicity to additional non-standard test species. Limited data are available for chronic, delayed and multi-generational impacts in addition to field related effects and the influence of multiple stressors and environmental covariables.
Despite previous efforts (i.e., CROSERF) to standardize laboratory toxicity testing approaches, many laboratory toxicity studies are still conducted/reported in such a way that results cannot be used in models or implications of the findings understood and many may under- or over-represent toxicity. Furthermore, new knowledge and/or technologies have been developed since the original standard methods were published.
Inaccurate predictions of toxicity can occur based on how exposure media are prepared and characterized, and the choice of the concentration metric used. Depending on the specific species/life-stage and associated exposure pathways, oil mixtures containing droplets (i.e., high-energy preparations or those containing dispersants) will result in under- or over-estimations of toxicity if oil partitioning (dissolved/particulate) and/or combined chemical metrics (e.g., total petroleum hydrocarbons or total polycyclic aromatic hydrocarbons) are used.
Models of fate, exposure, and effects have been developed and refined and are useful for informing response options and for predicting effects for NRDA. Models have been refined to be inclusive of new information regarding the role of environmental variables, including temperature, light and pressure and the partitioning of oil constituents; thus, they now include oil droplets in addition to dissolved phase components, although many models require validation by laboratory and/or field data.
There are understudied exposure pathways that may be critical for the health of some marine species, for example, exposure to volatile oil constituents in air-breathing marine mammals and turtles based on studies of DWH-affected dolphins. Furthermore, the role of understudied and novel toxicological mechanisms has been highlighted in recent studies, including toxicity and toxic mechanisms of photo-oxidation products, impacts to the adrenal and immune systems, and the importance of oil inducing microbiome changes and ultimate health consequences. In some instances, no appropriate laboratory models exist to better understand these pathways (e.g., effects on HPA axis for dolphins). The development of various ‘omics techniques have elucidated the potential role and impact of oil on many new toxicological pathways, although many require further development and investigation to resolve consequences at the individual and ultimately population levels.
Conclusion—Seafood Safety: Standard U.S. Food and Drug Administration designations of seafood safety do not adequately describe potential toxicity effects on humans. The basis for re-opening fishery resources is in part related to the risk assessment of the U.S. EPA priority-list PAHs established several decades ago. There are additional PAH and reaction product analytes that may be important to consider. Further evaluations are needed of these and the originally listed PAHs, including the implications of this information to the mechanism of carcinogenesis, to understand whether the current risk is over- or under-estimated.
Indigenous communities along coastlines rely heavily on subsistence harvests of marine food sources. Following a major oil exposure, members of these communities will have concerns regarding additional contamination of these food sources by the discharged oil.
The major economic impact of the DWH oil spill, and others, has been the closing of seafood gathering based on protection of human health. There is a need for improved understanding of existing uncertainties in the risk basis for reopening of seafood gathering in ocean areas that had been oiled and for transparent communication of this information. New information available about the chemistry and toxicology of the mixtures of PAHs, as well as increasing recognition of the impact of the closing of seafood gathering on community health effects, indicate a need to reassess risk to humans related to ingesting seafood following an oil spill. (The committee notes that further research to narrow uncertainties could provide the scientific basis to make the criteria more or less stringent.)
Conclusion—Photo-Oxidation: UV light has been shown to influence (often increase) the toxicity of oil constituents, especially to certain life stages and species in the photic zone. This includes photoenhanced toxicity mechanisms and the production of photo-oxidation products.
Conclusion—Arctic Industrialization: The Arctic is an extraordinarily challenging and difficult place to live and work, which increases risks of industrial accidents associated with oil and gas exploration and production, and commercial fishing, tourism cruises, and container ships. Within the circumboreal continental shelves, the region with the greatest potential for oil and gas exploration and development includes the Alaskan and western Canadian Arctic coasts, placing this region at the highest risk of a major oil spill should such development progress. If a spill were to occur, the likelihood of effective oil discharge response and remediation efforts beyond “natural recovery” is low given the scant availability of support infrastructure and the high frequency of inclement weather conditions.
Conclusion—Risk to Coastal Communities: North American coastal communities have unique social and cultural characteristics which differentiate the effect of an oil spill and can make these communities particularly vulnerable to natural
disasters and the effects of oil spills, as seen following the Exxon Valdez and DWH oil spills.
Conclusion—Indirect Human Health Effects: The ICS has proven to be a very effective approach to decision-making following an oil spill. The ICS ranks human health as its highest priority, but human health is primarily conceptualized as safety from direct oil spill effects (including, but not limited to, inhalation, contact, and the risk of fire and explosion). As has been seen as a consequence of the DWH and other oil spills, oil spills have the potential to indirectly affect human health; studies of the DWH oil spill have confirmed and further demonstrated the major impact of oil spills on community mental and behavioral health, as well as the role of economic impacts from oil spills.
RESEARCH NEEDS
Continued research to better understand and model effects of oil on the marine ecosystem, including humans, is encouraged. More specifically, the research included in Table 6.3 would continue to advance this important component of oil spill science.
TABLE 6.3 Research Recommended to Advance Understanding of the Effects of Oil in the Sea on the Marine Environment
| 6.1 | Natural Seeps: As relatively understudied habitats, research is needed to increase our understanding of unique chemosynthetic communities near natural seeps, especially deeper sea locations, to identify novel species/biochemical pathways and chemosynthesis, and to identify bacteria that may be useful in oil spill response (i.e., oil degraders), and to understand how organisms and communities respond to the presence of oil. |
| 6.2 | Marine Oil Snow: Continuing research on the formation of marine oil snow (MOS) is needed with respect to influences on processes of oil degradation and eventual hydrocarbon fates, such as flux through the water column, interactions with water column organisms, and short- and long-term deep-water biogenic communities. |
| 6.3 | Assessment Techniques: Potential research areas would be the development of sensors for petroleum hydrocarbons and image analysis for plankton in situ, and autonomous underwater vehicles (AUVs) for determination of water column effects. |
| 6.4 |
Marsh Ecosystem Health: The protocols of natural resource damage assessment for marshes following exposure to an oil spill should incorporate additional measures of the health of marshes, including their structural integrity. Research into other measures or development of technological advances (e.g., portable photosynthesis systems for gas exchange and chlorophyll fluorescence measurements in plants) may also generate more universal representative indicators.
The longer-term study with multiple integrated features of the salt marsh ecosystem points to the need for these types of studies to integrate the multiple aspects of contamination, salt marsh ecology, trophic structure, predator-prey dynamics, understanding of microhabitats within the marsh, and an integrated approach from genetics and enzymatic responses to ecosystem-level effects. |
| 6.5 | Marine Vertebrates: Studies focused on better estimation of mortalities of seabirds, marine mammals, and sea turtles during future spills of opportunity are needed. These studies should include sampling methods that permit estimation of statistical confidence intervals in addition to point estimates of, for example, numbers of animals killed. |
| 6.6 | Corals: The numerous environmental and coral health co-stressors should be studied together with additional investigations of species and life stage sensitivities to better understand the impact of oil on these important coral reef ecosystems. Studies of mesophotic and deep-sea corals highlight the need for prior baseline studies of the health of benthic ecosystems, together with long-term follow-up studies on recovery, delayed mortality, and continued declines in health in these species, particularly given their slow growth and lower recruitment compared to other marine species and hence potential for a protracted recovery period. |
| 6.7 |
Ecosystem-Level Effects: Better understanding of trophic structure in marine systems could be accomplished with experimental design that incorporates populations, the community trophic interactions, multiple stressors, and interrelationships that could anticipate indirect or cascading effects of an oil spill. Field studies that incorporate all these features may not be able to reproduce the complexity of a marine ecosystem, but models could provide a basis for further exploration.
The addition of longer-term observations and experiments that include higher organization level components and trophic interactions should be funded by the appropriate agencies and responsible parties. To these ends, appropriate agencies should encourage and support efforts to develop ecological atlases of marine resources that identify especially important ecological areas and habitats of threatened or endangered species, similar to those developed by Audubon, Oceana, and other environmental nongovernmental organizations (NGOs) for the Alaskan Arctic, which may serve to extend to offshore waters the Environmental Sensitivity Index approach currently used for shorelines. |
| 6.8 | Shoreline Oiling Characterization: A comparison of Shoreline Cleanup and Assessment Technique (SCAT) oiling categories with chemical characterization of hydrocarbons would benefit subsequent field studies and comparison of effects results based primarily on the four-tier SCAT categories. Also, the use of statistically-based sampling methods to estimate upper and lower bounds of the extent and intensity of shoreline oiling, including estimates of subsurface oil, would permit meaningful comparisons among sampling periods to estimate temporal trends of oil persistence. |
| 6.9 |
Photo-Oxidation: The toxicity of photo-oxidized products of compounds that dissolve from oil into seawater should be evaluated to determine how much they contribute to acute and chronic toxicity effects on test organisms. Further studies are needed to characterize the array of photo-oxidation products produced from various oils and assess their persistence, bioaccumulation, and toxicity to standard toxicity test organisms. Additional organisms and numerous life stages should be included in these studies.
Further research on UV radiation, elevated temperature, and decreased pH is needed to fully assess the effects of these co-factors and changing climate conditions on toxicity and the impact of oil pollution in exposed ecosystems. |
| 6.10 | Behavioral Effects of Oil: As a relatively understudied effect, research is needed to more fully evaluate the effects of exposure to oil on organismal behavioral responses. |
| 6.11 |
Toxicity Studies and Models:
|
| 6.12 |
Seafood Safety:
|
| 6.13 | Coastal Community Response: Programs to improve community resilience in response to future oil spills must be tailored to the individual communities at risk. Support is needed for social science research initiatives that will incorporate understanding of the broad range of local factors as a basis for preparation for the next oil spill or other disasters. Existing and new findings should be incorporated by EPA and other agencies into disaster planning (e.g., the EPA Handbook on Area Contingency Planning, which at present does not consider community health). |
| 6.14 | Follow up of Epidemiological Studies: Studies of response workers have noted a longer-term association between cardiovascular and central nervous system effects at levels of petroleum hydrocarbons exposure that are orders of magnitude below currently allowable worker exposures. This association seems unlikely to be causative in the absence of such findings in well-studied workforces exposed to the higher levels for longer periods of time, but should not be discounted without further rigorous follow-up. |
| 6.15 | Maternal and Child Health: The relative absence of female workers in the petroleum industry in the past has limited the availability of information about potential maternal and child health impacts. Longer-term follow-up of cohorts of women pregnant during the DWH oil spill and of community children should be performed. An increase in the limited amount of toxicological information on the potential reproductive and developmental effects of crude oil derivatives possibly reaching shore communities would also be useful. |


































































































