 |
UNIFORMED SERVICES UNIVERSITY OF THE HEALTH SCIENCES ARMED FORCES RADIOBIOLOGY RESEARCH INSTITUTE 4301 JONES BRIDGE ROAD, BUILDING 42 BETHESDA, MARYLAND 20889-5648 www.usuhs.edu/afrri |
 |
November 15, 2021
The National Academics of Sciences Engineering and Medicine
Nuclear and Radiation Studies Board
Washington, DC 20001
Attention: Study Director
Dear Dr. Kosti Ourania,
The Armed Forces Radiobiology Research Institute (AFRRI) is pleased to answer your list of questions related to our radiation sources and facilities that are currently used or could be used to support low-dose and low-dose rate radiation research.
Please see response to respective questions below:
1. Please provide a brief description of your radiation sources and facilities including location, type of source, first year of operation and expected date for removal/decommissioning, and types of activities these sources and facilities support (e.g., research, radiobiology experiments, radioisotope production, other).
TRIGA Reactor
AFRRI’s TRIGA reactor is 1 of 66 worldwide. These types of reactors are used in university and government laboratories and medical centers for applications that include research, production of radio-isotopes for medicine and treating tumors, nondestructive testing, basic science research, education, and training. They operate at thermal power levels of <0.1–14 megawatts (MW) and may be pulsed up to 22,000 MW.
The reactor is licensed by the U.S. Nuclear Regulatory Commission (License R-84). As of 2005, it was one of 18 TRIGA reactors in the United States and the only one dedicated to applied medical radiobiology research. The AFRRI TRIGA Mark-F Nuclear Reactor Facility first went operational in 1962. It is a medium-sized unit that generates neutrons and gamma rays for radiation experiments. The reactor can produce a controlled, self-sustaining fission chain reaction in the reactor core. The core, in addition to the fuel elements and control rods (containing boron carbide), includes a neutron start-up source (americium/beryllium). It is suspended under 16 feet (~4.9 m) of water within a pool (an effective radiation shield) on a carriage assembly that allows movement of the core between two exposure rooms for experimental work with large animal or other studies (Dix, 2005). The advantages of such a movable reactor core are that the quantity and character of the radiation that reaches the exposure facilities can be controlled, and more than one exposure facility can be used during reactor operations. The reactor can operate in steady-state as well as pulse mode. It can operate from low power (100 watts) up to the maximum allowed steady-state power level of 1.0 MW. Its pulse mode can produce a short peak (from a prompt critical excursion) of up to 2,500 MW for brief periods (less than one second).
High-Level Cobalt-60 Facility
The High-Level Cobalt-60 facility at AFRRI first opened in 1969. The facility is located below ground in the AFRRI complex, with shielding provided by massive reinforced concrete and earth fill. Its panoramic irradiator is a wet-source storage unit consisting of a 450,000 Ci (at installation) Cobalt-60 source, water trench, source and storage racks, elevator mechanism, and associated equipment. The exposure room is 35ft ˘ 35ft and 25ft, 8in high (10.7m ˘ 10.7m ˘ 7.6m = 870 m3). The irradiator produces mono-energetic gamma rays at variable dose rates with flexible configurations in both unilateral and bilateral irradiation modes and may be used for acute and chronic studies of materials, biologic specimens, small and large. It has been employed in a variety of applications, including investigations of the effects of ionizing radiation exposure on cells, prognostic indicators of survival in a variety of
mammals, the efficacy of radio-protective agents and the effects of radiation on unshielded electronics in space application. The facility source was replenished in 2013, and there is currently no plan to shut down the facility.
Low-Level Cobalt Facility
The Low-Level Cobalt Facility, formerly called the Chronic Irradiation Facility, is a second Cobalt-60 radiation source facility which provides low-dose-rate gamma radiation to simulate chronic exposure. The low-level cobalt facility consists of a dry, panoramic Cobalt-60 irradiator (1.17 MeV, 1.33 MeV gamma rays) capable of providing doses to samples over a long time frame. The source is located 1.5 meters from the floor in the center of a large 6 meter square room that can accommodate biological samples of all sizes. To meet various dose-rate requirements, attenuators (2x, 4x, 8x) are available. Additional attenuators can be procured as necessary. The room is climate controlled with the proper ventilation exchange rates along with a day/night lighting system to satisfy the most demanding animal care and use requirements. Video surveillance is also available for the monitoring of animals. Personnel are available to fully support all necessary experiment protocols. The facility is licensed for up to 100 Ci of Co-60.
Linear Accelerator (LINAC)
In August 2012, AFRRI retired its 47-year-old underground linear accelerator and replaced it with an Elektra Infinity clinical accelerator that is supported by a computed tomography (CT) unit. The purchased unit has received significant upgrades to hardware and software in 2021. It is capable of providing high-energy photons from 4 MeV up to 15 MeV with variable dose rates from 0.05 to 6 Gray per minute. Imaging capabilities are provided through a portal camera and cone-beam computed tomography.
Small Animal Radiation Research Platform (SARRP)
The institute commissioned the Small Animal Radiation Research Platform (SARRP) January 2015 which provides low-energy photons up to 220kVp to achieve 0.2 to 2.6 Gy/min dose rates to targets. Imaging is provided through portal camera and cone beam computed tomography.
2. What are the dose ranges, radiation qualities, energy, and dose-rates delivered in your facilities? Is operation pulsed or steady-state? Are the facilities suitable for inhalation experiments?
All radiation facilities at AFRRI are flexible to provide a variety of dose rates and total accumulated doses to experiments through manipulation of exposure time, distance, shielding, and strength of radiation source. The TRIGA reactor is unique in the ability to provide mixed-field (gamma and neutron) radiation to experiments of varying neutron energy levels.
In addition to the ability to provide a mixed field, the TRIGA also has an accompanying hot cell with a viewing port and robotic tele-operators. Our team has considered the question of inhalation experiments, and have promising ideas of how we can provide a space for dispersed irradiated particles inside a sealed space. Table 1 details the radiation quality, energy, operation type, possible dose rates and additional notes for each facility.
Table 1: An overview of available radiation qualities and rates possible at AFRRI facilities.
| Facility | Radiation Quality | Energy | Operation | Dose Rates | NOTES |
|---|---|---|---|---|---|
| Reactor# | Mixed field, gamma/neutron | From thermal to fast | Pulse and Steady State | From less than 10 R/hr to more than 100 kR/hr | Target can be shielded for significantly lower dose |
| High Level Cobalt | Mono-energetic | 1.33. and 1.17 MeV | Dose rate can be varied by source position adjustment | From less than 1 R/hr to more than 100 kR/hr | Target can be shielded for significantly lower dose |
| Low Level Cobalt# | Mono-energetic | 1.33. and 1.17 MeV | Panoramic, with source in the middle of the room | From less than 1 mR/hr to more than 100 R/hr | Both source and target can be shielded for significantly lower dose Dose rate also adjusted by target placement in exposure room |
| LINAC | Mono-energetic | 4, 10, 15 MeV | Flexible platform that provides partial body irradiation for targets | From 0.05 to 6 Gy/min | |
| SARRP# | Mono-energetic | Up to 220kVp | Accurate and precise chamber for small animal with adjustable inserts | From 0.3 to 2.6 Gy/min | |
| # Dose and dose rates reported will go into further validation upon return to operational status. | |||||
3. For radiobiology experiments, are your facilities mostly used for exposure of cells, tissues, or animals? Please describe and if possible provide publications that describe use of your radiation facilities to facilitate radiation research.
Irradiation facilities are configurable for live animals, cell cultures and tissues, and other inanimate projects/subjects. As an example, Table 2 below shows irradiation numbers in the HLCF from 2005 until now.
Table 2: History of Irradiation Sessions at HLCF.
| Irradiation sessions | |||||
|---|---|---|---|---|---|
| year | total | animal | cells | spores | Other |
| 2005 | 131 | 67 | 30 | 21 | 13 |
| 2006 | 163 | 67 | 35 | 43 | 18 |
| 2007 | 208 | 113 | 26 | 38 | 31 |
| 2008 | 191 | 115 | 47 | 8 | 21 |
| 2009 | 208 | 108 | 70 | 30 | |
| 2010 | 212 | 113 | 86 | 3 | 10 |
| 2011 | 206 | 123 | 81 | 2 | |
| 2012 | 170 | 117 | 49 | 4 | |
| 2013 | 151 | 108 | 37 | 6 | |
| 2014 | 201 | 113 | 67 | 21 | |
| 2015 | 158 | 90 | 65 | 3 | |
| 2016 | 122 | 79 | 43 | ||
| 2017 | 163 | 86 | 77 | ||
| 2018 | 135 | 78 | 56 | 1 | |
| 2019 | 101 | 69 | 31 | 1 | |
| 2020 | 57 | 39 | 15 | 3 | |
| 2021 | 62 | 48 | 10 | 4 | |
4. Are your facilities available for use to outside radiation researchers and other investigators?
Yes, our facilities are available to outside radiation researchers and other investigators. The proposed experiment will have to be coordinated with the Uniformed Services University of Health Sciences (USU) Vice President of Research’s office, and approvals will be required by both our animal and safety committees. Interagency Agreement, Memoranda of Understanding or coordinated requirements definition could also provide access to external investigators. Naval Support Activity Bethesda (NSAB), COVID 19 Safety protocols, and USU/AFRRI security measures must be met but do not preclude profit or non-profit organizations from utilizing the AFRRI radiation sources. Foreign nationals may require special procedures for entry into NSAB and AFRRI.
5. Is there available adjacent infrastructure that facilitates radiation research such as tissue culture and animal facilities?
USU Department of Laboratory Animal Resources provides veterinary support for animal experiments. Also, the institute is expanding its animal facilities by constructing a new state of the art vivarium, expected to be complete by summer 2022. The vivarium is adjacent and connected to AFRRI, and will provide efficient and expedited handling of animals. Each of the facilities mentioned above also has co-located animal preparation areas next to the exposure rooms. These areas are used to hold and prep animals for experiments. The radiation facilities and tissue culture or animal facilities responsibilities are not integrated under a department, but all fall under AFRRI and or USU. Biological labs are located throughout the building for in-house Principal Investigators
AFRRI appreciates the great opportunity that the National Academy of Science Committee on Developing a Long-Term Strategy for Low-Dose Radiation Research in the United States is providing by inviting us to participate in the information gathering meeting last month and requesting additional information on radiation facilities. Should you need any further technical information, please contact Lieutenant Colonel Lu Makinde at (301) 295-9246.
| Date |
Mohammad Naeem, MD, FCCP, FACR Director AFRRI |
Colorado State University Radiation Facilities for Low-Dose Rate Radiation Research
Several facilities on the CSU campus are used for low dose rate radiation research.
Low dose rate γ-ray tissue culture facility (MRB 08). This facility is housed in a shielded vault in the Molecular and Radiological Biosciences (MRB) building on the Main CSU campus. It consists of a 137Cs γ-ray source mounted above a tissue culture incubator. The incubator sits on a hydraulic lift which allows it to be raised or lowered to adjust the dose rate to cell cultures inside. Attenuators provide additional dose rate control. The dose rate is currently set for 9.3 mGy/hr but can be increased to 60 mGy/hr at the current location and increased further to about 500 mGy/ hr by adjusting the hydraulic lift. The dose rate can be decreased to near background by utilizing additional attenuators.
The facility is currently being used for radiobiology experiments, specifically continuous (weeks long) exposures of engineered human heart tissue.
Low dose rate γ-ray tissue culture facility (MRB 12). This facility is a 37° C warm room beneath which twelve 137Cs sources can be exposed. When MRB 12 was commissioned in 1997 the sources were each nominally 4 mCi (now about 2 mCi). Researchers can place their samples (typically cells in flasks) at various heights from the sources and irradiate at low dose rate for days. From a position of 56 cm to 180 cm above the sources, thirty-five stacks of T75 tissue culture flasks can be placed for different dose rates. A total 1190 T75 flasks can be irradiated simultaneously in this manner. The dose rates range from 1 mGy/h to 100 mGy/h depending on the number of sources used and the distance from sources.
Because the warm room is not a CO2 incubator, culture media must be prepared by the addition of 10-25 mM HEPES buffer to ensure pH equilibration (pH 7.2-7.4).
Low dose rate neutron tissue culture facility (MRB 02). This facility is under construction and scheduled to be operational by the end of the 2021 calendar year. An Adelphi D-T neutron generator is being mounted above a tissue culture incubator. The generator will provide a maximum yield of 2˘109 neutrons/s with an energy spectrum that is narrowly peaked at 14.1 MeV. Unscattered neutron fluences will correspond to a maximum dose rate of approximately 3.6 mGy/hr at a distance of 1 m. Neutrons will be scattered and neutron energies moderated by structural components and equipment in the room and most importantly by the shielding materials applied for radiation protection and field attenuation purposes. The neutron energies at the samples will present a spectrum and span a range of LET to be determined and can be modified further by the introduction of additional scattering material. Dose rates can be decreased by up to a factor of about 20 by reducing the generator current; additionally, the generator-incubator distance can be modified to increase or decrease the expected dose rate. The generator is expected to provide minimal background from photon radiation; photon contributions to the ambient dose rate from neutron capture reactions will be determined during the commissioning phase of the generator.
The facility will be used for low dose rate irradiation of engineered human tissues (“tissues on a chip”).
Low dose rate γ-ray vivarium (MRB 06). This facility is used to irradiate small animals continuously with 137Cs γ-rays. A panoramic J.L. Shepherd Model 81-18 irradiator is positioned opposite an arc of cage racks that can house up to 250 mice. The section of the irradiator room containing the cages is enclosed by a bio-bubble that provides HEPA filtered air. This is a fully approved vivarium and mice can be housed in it for their entire life spans. The facility has also been used to irradiate Medaka fish.
MRB 06 was set up to provide a dose rate of 10 cGy per day. A day consists of 22 hours with the source exposed during which time the mice are irradiated and 2 hours with the source shielded so personnel can enter for animal husbandry. The dose rate can be adjusted by bringing the cages closer to the source and by adding or removing attenuators. A higher dose rate of 0.41 cGy/min was used in a radiation countermeasure study done by Cleveland BioLabs in the facility.
Low dose rate neutron vivarium. The neutron irradiator/vivarium is on the CSU Foothills Campus which is located in Fort Collins about 5 miles west of the Main campus. The facility is described in detail in Borak et al 2021 (see the reference section below). The irradiator building is a standalone structure built in 1964 consisting of an irradiator room, a control room, and two storage/equipment rooms. It was decommissioned as an irradiator facility in the 1970s. In 2016/2017, the building was renovated as a small animal vivarium housing a panoramic 252Cf neutron irradiator. Cage racks capable of housing 900 mice and 60 rats were positioned in a circle around the irradiator. Animal irradiation experiments were initiated there September 2017. The target dose rate is 1 mGy/day. Initially, a day was 16 hours with the source exposed and the animals being irradiated followed by 8 hours with the source shielded so personnel could enter the facility for animal care and maintenance. Because 252Cf decays (t½ ~ 2.6 years), the daily exposure times must be steadily increased to deliver a 1 mGy dose. After about 2 years, a day becomes 22 hours of exposure followed by 2 hours with the source shielded. Two hours is the minimum needed for animal care, so at that point the source must be replaced or the dose rate reduced.
A scheduled replacement of the source was put on hold due to the COVID-19 pandemic. However, a new source has now been ordered and should be installed this calendar year (2021).
Availability
All of the described facilities are available to extramural investigators. We have previously provided access to CSU irradiators on a fee for service or collaborative basis. In the case of the NASA funded neutron irradiator/vivarium facility, five other extramural NASA funded research groups used the facility beginning on the first day it was operational. Some of these groups worked on site and we assisted them with finding accommodations, securing animal care and use protocols, and getting access to CSU computing networks and key cards.
None of the facilities is currently set up for inhalation work. However, we have considerable experience in this field. CSU used to conduct large inhalation studies, most notably on Beagles,
in the past. The current Director of the Irradiation Services Laboratory has more than twenty years of experience in internal dosimetry. Recent work by his graduate students includes the measurement and modeling of the biokinetics of injected radionuclides utilizing murine and canine models at the CSU Veterinary Teaching Hospital.
There are no plans to decommission any of these facilities.
Additional infrastructure
Several other irradiators are available on the CSU campus, they are not listed here because they are used for treatment or for research involving acute exposures, including for large animals. Since acute exposure controls may be needed for some low dose rate experiments, note that a 137Cs γ-ray panoramic irradiator is available in the MRB building. It is used to irradiate cells and mice. Using the standard distance to the source, the current dose rate is 0.77 Gy/min; this dose rate can be reduced by up to a factor of about 10. Also, using a tray immediately adjacent to the source allows small samples to be irradiated at 5.3 Gy/min.
Tissue culture and molecular biology laboratories are located on the 4th floor of the MRB building.
Small animal vivariums with procedure rooms are available on both the Main and Foothills campuses. CSU has a college of veterinary medicine. Large animals are housed on the Foothills and South campuses (South campus is 1 mile south of the Main campus). Veterinary residents have been recruited to assist in some experiments run by extramural investigators (e.g., slit lamp exams for cataracts in irradiated mice).
An additional irradiator building, the RSDF building, is located on the Foothills campus. It was constructed in 1967 for experiments requiring chronic low dose rate exposures of animals and is currently decommissioned. The RSDF contains two large irradiator rooms, both similar to the one in the low dose rate neutron irradiator/vivarium. This building may be available for renovation and recommissioning if additional facilities are needed.
Publications
MRB 08 Low dose rate tissue culture
Kato TA, Nagasawa H, Weil MM, Little JB, Bedford JS. Levels of gamma-H2AX Foci after low-dose-rate irradiation reveal a DNA DSB rejoining defect in cells from human ATM heterozygotes in two at families and in another apparently normal individual. Radiat Res. 2006 Sep;166(3):44353. doi: 10.1667/RR3604.1. PMID: 16953663.
MRB 12 Low dose rate tissue culture
Amdur, R.J. and J.S. Bedford. Dose rate effects between 0.3 and 30 Gy/h in a normal and a malignant human cell line. Int. J. Radiat. Biol. 30:83-90, 1994.
Bedford, Joel S. The radiobiology of low dose-rate and fractionated irradiation. In: Principles and Practice of Brachytherapy (Joslin, Flynn, Hall, eds.), Arnold, London, 2001, pp. 161-179.
Huang, Q., Li, F., Liu, X., Li, W., Shi, W., Liu, F-F., O’Sullivan, B., He, Z., Peng, Y., Tan, A-C., Zhou, L., Chen, J., Han, G., Wang, X-J., Thorburn, J., Thorburn, A., Jimeno, A., Raben, D., Bedford, J.S., and Li, Chuan-Yuan, Caspase-mediated paracrine signaling from dying cells potently stimulates tumor cell repopulation during cancer radiotherapy. Nature Medicine 17, 860-866 (2011)
Kato, T. A., H. Nagasawa, M. M. Weil, J. B. Little and J. S. Bedford, Levels of gamma-H2AX Foci after low-dose-rate irradiation reveal a DNA DSB rejoining defect in cells from human ATM heterozygotes in two at families and in another apparently normal individual. Radiat. Res. 166, 443-453, 2006.
Kato TA, Nagasawa H, Weil MM, Genik PC, Little JB, Bedford JS. Gamma-H2AX foci after low-dose-rate irradiation reveal Atm haploinsufficiency in mice. Radiat Res. 2006 Jul;166(1 Pt 1):47-54. doi: 10.1667/RR3587.1.
Kato, T.A., P.F. Wilson, H. Nagasawa, M.M. Fitzek, M.M. Weil, J.B. Little, and J.S. Bedford, A defect in DNA double strand break processing in cells from unaffected parents of retinoblastoma patients and other apparently normal humans. DNA Repair (Amst.) 6, 818-829, 2007.
Lin, J.Y-D, M.C. Muhlmann-Diaz, M.A. Stackhouse, J.F. Robinson, G.E. Taccioli, D.J. Chen, and J.S. Bedford. An ionizing radiation-sensitive CHO mutant cell line: irs-20. IV. Genetic complementation, V(D)J recombination and the scid phenotype. Radiat. Res. 147:166-171, 1997.
Lin, J.Y-D. and J.S. Bedford. Regional gene mapping using mixed radiation hybrids and reverse chromosome painting. Radiat. Res. 148:405-412, 1997.
Ochola, D, Sharif R, Bedford JS, Keefe, T., Kato, T., Fallgren, C., Demant, P., Costes, S., and Weil, M., Persistence of Gamma-H2AX Foci in Bronchial Cells Correlates with Susceptibility to Radiation Associated Lung Cancer in Mice. Radiat Res 191.. 67-75 (2019).
Peng Y, Nagasawa H, Warner C, Bedford JS. Genetic susceptibility: radiation effects relevant to space travel. Health Phys. 103, 607-620 (2012).
Priestley, A., H.J. Beamish, D. Gell, A.G. Amatucci, M.C. Muhlmann-Diaz, B.K. Singleton, G.C. Smith, T. Blunt, L.C. Schalkwyk, J.S. Bedford, S.P. Jackson, P.A. Jeggo, and G.E. Taccioli. Molecular and biochemical characterization of DNA-dependent protein kinase-defective rodent mutant irs-20. Nucleic Acids Res. 26:1965-1973, 1998.
Stackhouse, M.A. and J.S. Bedford. IRS 20: An ionizing radiation sensitive mutant of CHO cells: irs cells. I. Isolation and Initial Characterization. Radiat. Res. 136: 241-249, 1993.
Stackhouse, M.A. and J.S. Bedford. IRS 20: An ionizing radiation sensitive mutant of CHO cells: irs cells. II. Dose-rate effects and cellular recovery process. Radiat. Res. 136: 250-254, 1993.
Stackhouse, M.A. and J.S. Bedford. An ionizing radiation sensitive mutant of CHO cells: irs-20. III. Chromosome aberrations DNA breaks and mitotic delay. Int. J. Radiat. Biol. 65(5):571-582, 1994.
Ulsh, B.A., F.W. Whicker, T.G. Hinton, J.D. Congdon and J.S. Bedford. Chromosome Translocations in T. scripta : The dose-rate effect and in vivo lymphocyte radiation response. Radiat. Res. 155: 63-73, 2001.
Ulsh, B.A., M.C. Mühlmann-Diaz, F.W. Whicker, T.G. Hinton, J.D. Congdon, and J.S. Bedford. Chromosome Translocations in Turtles: A biomarker in a sentinel animal for ecological dosimetry. Radiat. Res. 153:752-759, 2000.
Wilson, P.F., and Bedford, J.S., Radiobiological Principles. Chapter 1 (In Textbook of Radiation Oncology, 3rd Edition, S.A. Leibel, and T.L. Phillips, eds), Elsevier, Philadelphia. (In Press 2009).
Wilson PF, Nagasawa H, Warner CL, Fitzek MM, Little JB, Bedford JS. Radiation sensitivity of primary fibroblasts from hereditary retinoblastoma family members and some apparently normal controls: colony formation ability during continuous low-dose-rate gamma irradiation. Radiat Res. 2008 May;169(5):483-94. doi: 10.1667/RR1333.1.PMID: 18439048
MRB06 Low Dose Rate Gamma Ray Irradiator/Vivarium
Shakhov AN, Singh VK, Bone F, Cheney A, Kononov Y, Krasnov P, Bratanova-Toshkova TK, Shakhova VV, Young J, Weil MM, Panoskaltsis-Mortari A, Orschell CM, Baker PS, Gudkov A, Feinstein E. Prevention and mitigation of acute radiation syndrome in mice by synthetic lipopeptide agonists of Toll-like receptor 2 (TLR2). PLoS One. 2012;7(3):e33044. doi: 10.1371/journal.pone.0033044. Epub 2012 Mar 27. PMID: 22479357; PMCID: PMC3314012.
Ochola DO, Sharif R, Bedford JS, Keefe TJ, Kato TA, Fallgren CM, Demant P, Costes SV, Weil MM. Persistence of Gamma-H2AX Foci in Bronchial Cells Correlates with Susceptibility to Radiation Associated Lung Cancer in Mice. Radiat Res. 2019 Jan;191(1):67-75. doi: 10.1667/RR14979.1. Epub 2018 Nov 6. PMID: 30398394.
Foothills Campus Low Dose Rate Neutron Irradiator/Vivarium
Borak TB, Heilbronn LH, Krumland N, Weil MM. Design and dosimetry of a facility to study health effects following exposures to fission neutrons at low dose rates for long durations. Int J Radiat Biol. 2021;97(8):1063-1076. doi: 10.1080/09553002.2019.1688884. PMID: 31687872.
Perez RE, Younger S, Bertheau E, Fallgren CM, Weil MM, Raber J. Effects of chronic exposure to a mixed field of neutrons and photons on behavioral and cognitive performance in mice.
Behav Brain Res. 2020 Feb 3;379:112377. doi: 10.1016/j.bbr.2019.112377. Epub 2019 Nov 22. PMID: 31765722.
Acharya MM, Baulch JE, Klein PM, Baddour AAD, Apodaca LA, Kramár EA, Alikhani L, Garcia C Jr, Angulo MC, Batra RS, Fallgren CM, Borak TB, Stark CEL, Wood MA, Britten RA, Soltesz I, Limoli CL. New Concerns for Neurocognitive Function during Deep Space Exposures to Chronic, Low Dose-Rate, Neutron Radiation. eNeuro. 2019 Aug 22;6(4):ENEURO.0094-19.2019. doi: 10.1523/ENEURO.0094-19.2019. Erratum in: eNeuro. 2019 Oct 18;6(5): PMID: 31383727; PMCID: PMC6709229.
Loma Linda University
James M. Slater, M.D. Proton Treatment and Research Center
Radiation Biology Facilities and Logistic Support Report

Marcelo E. Vazquez M.D., Ph.D.
October 25, 2021
LOCATION:
The James M. Slater, M.D. Proton Treatment and Research Center is located at Loma Linda University Medical Center (LLUMC) in the city of Loma Linda, located approximately 60 miles east of Los Angeles in San Bernardino County, California. Loma Linda is home to Loma Linda University (LLU). LLUMC and its Children’s Hospital contain the largest neonatal intensive care unit in California, the Proton Accelerator Cancer Treatment Center, and the infant heart and multiple organ transplant center. Over 900 physicians are on the University and Medical Center staff.
The James M. Slater Proton Treatment and Research Center, operated and staffed by the Department of Radiation Medicine (LLURM), was the first proton facility in the world designed for patient treatment and related research in a hospital setting. Staff at the facility have treated more than 25,000 patients with protons since 1990. Protons have been used for patients with various tumors involving the prostate, brain, base of skull, eye, head and neck, spine, lung, liver, and various other sites. A significant number of patients are children. LLURM also provides other comprehensive radiation therapy services, including x-ray IMRT complementary to proton therapy. Patients treated with protons are followed for life and all events such as local or distal failure, side effects, and secondary cancers are recorded in computerized databases by dedicated LLURM clinical research staff.
FACILITIES
Proton Radiation Facilities
The LLURM synchrotron-based proton facility started operation in 1990. This facility supports patient treatments, clinical research, and radiobiological research. At present there are no plans/dates for the removal or decommissioning of this facility. The proton treatment center has three clinical gantry treatment rooms and two clinical horizontal fixed beamlines. In addition, the center has a research room equipped with three horizontal beam lines featuring semi-permanent experimental setups for physics and biological studies. Currently, biological experiments are conducted using one of the clinical gantries (G2) and one of the fixed horizontal beam lines (HBL). Research beam lines are dedicated to physics and technological development studies. However, these beam lines can be commissioned for biological studies as needed.
The clinical treatment rooms allow for image guidance and treatment planning support. The accelerator is available for research outside regular patient treatment hours and on weekends. Limited access during regular patient treatment hours is available. During the week, typically, short runs (30 minutes to 1 hour.) can be scheduled between patient treatments or at the end of the day after scheduled patient treatments are completed. Extended runs (of several hours) can be scheduled during weekends. Protons are delivered via a dedicated control system; accelerator and engineering personnel are available 24 hours/7 days per week. Engineering support staff are on site to maintain the facility and related proton research equipment.
The LLURM proton facility (maintained by Optivus Proton Therapy, Inc.) produces pulsed stream of proton radiation with variable energies (50 MeV – 250 MeV) and variable dose rates (standard setup: 100-200 cGy/min). The facility can provide very low dose rates settings if a non-clinical setup is arranged. Previous and current studies demonstrated that the facility could produce dose rates as low as 1 cGy/min and even lower with a dedicated beam tuning. Therefore, lower dose rates can be produced as needed requiring special allocated time. Higher dose rates (up to about 300 cGy/min) can also be produced for small radiation fields.
Passive scattered beam delivery is accomplished in conventional ways: field size (G2 max: 18 cm diameter circular field, HBL max: 14 cm diameter circular field) is achieved by means of scattering foils; spreading out the Bragg peak (SOBP) to irradiate different target volumes (from cells to animals) is done by a modulator wheel; absorber materials are used in addition to some electronic manipulations to control the depth of the Bragg peak; shaping the beam in the first two dimensions (height and width) is accomplished by Cerrobend or
brass cut-outs; and beam shaping in the third dimension is achieved by compensators milled on a computer-controlled machine. Samples alignment is accomplished by a light field and multiple laser beams.
Conventional Radiation Facilities
One clinical TrueBeam linear accelerator (Varian Medical System, Palo Alto, CA) is available for clinical and research use at LLURM. This accelerator provides a range of X-ray energies, image guidance, lasers for alignment, and treatment planning support. The unit is used clinically for IMRT. For research purposes, the accelerator provides a vertical beam that can be used for in vitro and in vivo experiments as well as multidirectional beams for more complex animal studies. The maximum field size achievable is a square beam spot of 20 × 20 cm, while a light field and multiple laser beams can be used to aid in sample alignment. The linear accelerator provides an energy range from 6 to 22 MeV, with a dose rate range of 5 to 600 cGy/min. Access to this facility is limited, but short runs (30 minutes) can be scheduled between patient treatments during the week, or at the end of the day after scheduled patient treatment are completed.
Radiation Sources Characteristics Summary
| Protons | Photons | ||
|---|---|---|---|
| G2 | HBL | LINAC | |
| Machine/Brand | synchrotron based (Optivus) |
synchrotron based (Optivus) |
LINAC (TrueBeam, Varian) |
| Energies (MeV) | 50 to 250 | 50 to 250 | 2 to 22 |
| Dose Rates (cGy/min) | <1 to 300 | <1 to 300 | 5 to 600 |
| Beam Spot | 18 cm dia. (circular) | 14 cm dia. (circular) | 20 X 20 cm (square) |
| Beam direction | Vertical (any direction) | Horizontal (fixed) | Vertical (any direction) |
| Availability (hrs.): | |||
|
0.5 to 1 6 + |
NA 6 + |
0.5 to 1 NA |
| Experimental set up: | |||
|
Yes | Yes | Yes |
|
No | Yes | No |
| Inhalation setup | Yes | Yes | Yes |
| Study type capability: | |||
|
Yes | Yes | Yes |
|
TBD | Yes | TBD |
LLURM Research and Development Team:
The LLURM’s research and management activities are performed by the LLURM Research and Development Team. This team headed by Dr. Vazquez is a very active team focusing on studying mechanisms of biological effects of ionizing radiation on animals, tissues, and cells. Research projects currently underway at LLURM include: 1) proton radiobiology basic mechanisms; 2) combined therapies to improve clinical outcomes for aggressive cancers; 3) develop and validate new technologies in proton therapy; 4) CNS neurotoxicity induced by radiation; and 5) hypo- and hyper-fractionated studies. Laboratory space assigned to radiobiologic investigations is equipped to support tissue culture activities, cytogenetic research, and animal studies. The goal of this laboratory is development of technology and biological knowledge which can be rapidly translated into clinical practice. The LLURM research and development team coordinates the basic and translational research and proton beam planning activities for intra- and extra-mural scientists.
Core Research Laboratories:
The Department of Radiation Medicine at LLUMC has a well-equipped 6,700-square-feet core radiation research laboratory dedicated to physics, radiobiology, and translational research. In support of this mission the core laboratory facility is subdivided in modular areas with assigned benches and shared laboratory support rooms. Also available is a conference room equipped with a video conferencing system in addition to office space for researchers and administrative support personnel.
Collectively these facilities have their own Principal Investigators and staff and serve as resources for our investigators and external users. The laboratories’ fundamental objective is to provide equipment and expertise that investigators may employ as needed to conduct research in photon/proton radiobiology and physics. Specifically, each component of the core laboratory research facility will provide the following resources:
Radiobiology Laboratories: These laboratories focus on providing a center for radiobiology translational research supporting cell and tissue culture activities and biochemistry for investigators conducting biological studies. The laboratories have specific equipment in support of this mission including:
➢ Biochemistry Laboratory:
- Image Capture and Analysis:
- Fluorescence microscopy (Olympus BX95 with Cell Sense imaging software).
- Protein Determination (Western blot station, spectrophotometer)
- Cytochemistry workstation
- Cold storage capability: 4°, -20° and -80°C refrigerators and freezers.
- Bench top centrifuges
- 3 chemical hoods.
- Waste disposal capability
➢ Cell Culture Laboratory:
- Four CO2 incubators for cell culture activities.
- Two laminar flow hoods for sterile operations.
- Inverted microscope (TCM4000 BIOMED) with image analysis system
- Cell counter (Countess® II)
- Liquid nitrogen storage
- Ancillary Equipment: water baths, refrigerators/freezers, etc.
➢ Animal Studies:
The LLURM radiation facilities are adjacent to the LLUMC Animal Care Facility, which provides assistance in acquiring, housing, feeding, preparing and treating vertebrate animals. Researchers have access to animals and can use equipment and surgical rooms to perform research within the facility. Research support services, such as CTs and MRIs, are available as well. In addition to animal husbandry areas, the LLUMC Animal Care Facility contains five surgical suites and an intensive care/recovery room for postoperative care. The facility also has fully equipped and staffed clinical pathology and microbiology laboratories and an animal necropsy laboratory. The diagnostic laboratories can perform hematology, clinical chemistry, urinalysis, cytology, coagulation profiles, parasitology examinations, blood gas examinations, and microbiologic and serological procedures.
LLURM’s facilities and staff have extensive capabilities and experience in designing, planning, and running experiments using small (rodents, ferrets, flies, worms, and medium size animals (mini pigs)) for intra- and extra-mural investigators (NASA, NSBRI, etc.). Irradiation facilities can provide anesthesia and monitoring capabilities for in vivo studies.
Physics Laboratory:
This laboratory focusses on supporting medical physics research and additionally provides physics and dosimetry support for investigators conducting biological studies. The laboratory has specific equipment in support of this mission, including:
- Geant4 Monte Carlo Workstations
- A range of small-field dosimetry apparatuses, including ionization chambers, diodes and diamond detectors
- Gafchromic film imaging suite, including film scanner and dedicated software
- Treatment Planning workstations for proton and X-ray treatment planning
➢ High-Powered Computing Center:
This center supports Monte Carlo, treatment planning, and medical imaging development, while also providing data storage and handling for these activities. Specific facilities available include:
- 88 Tb Raid 6 storage server
- Fiver servers with 64 virtual nodes, each giving a total of 320 nodes for computer simulation support. Three of these servers have 512 Gb of RAM each to provide sufficient memory for intensive image-based research.
- A 19 quad core node cluster (providing 76 virtual processors) for computer simulation support.
- Battery backup power supplies to ensure uptime.
Utilization path
Proton and LINAC beam time are limited but free of charge for interested users. Scientists can access the LLURM radiation sources via collaborative agreement with the LLURM Research and Development team. Collaborative agreements and beam utilization are coordinated by Dr. Vazquez and review/approval are accomplished by an internal research committee (feasibility review), led by the Chair of the Department of Radiation Medicine.
Point of Contact:
Marcelo E. Vazquez, M.D., Ph.D.
Associate Professor
Director, Research and Development, Dept. of Radiation Medicine
James M. Slater, MD, Proton Treatment and Research Center
Department of Basic Sciences, School of Medicine
Loma Linda University Medical Center, B125
11234 Anderson Street
Loma Linda, CA 92354
Phone: 909 558 9490
Cell: 516 512 2032
E-mail: mvazquez@llu.edu
Other Resources:
LLU provides a unique environment for conducting scientific research. In addition to LLURM, LLU also houses the following core facilities available to intra- and extra-mural investigators:
- Center for Genomics (https://lluh.org/cancer-center/research/shared-resources/institute-genetics-translational-genomics)
- Mass Spectrometry Core Facility (https://medicine.llu.edu/research/core-facilities/mass-spectrometry-facility)
- Flow Cytometry Core (https://lluh.org/cancer-center/research/shared-resources/flow-cytometry-core)
- Advanced Imaging Microscopy (https://lluh.org/cancer-center/research/shared-resources/advanced-imaging-microscopy-core)
- Animal Care Facility (https://lluh.org/cancer-center/research/shared-resources/animal-care-facility-core)
- Imaging (https://lluh.org/cancer-center/research/shared-resources/center-imaging-research)
- PDX core (https://lluh.org/cancer-center/research/shared-resources/patient-derived-xenograft-core)
REFERENCES
a) M. Katerji, A. Bertucci, V. Filippov, M. Vazquez; P. Duerksen-Hughes; Ionizing radiation-induced DNA damage promotes integration of foreign plasmid DNA and HPV16 episome into human genome, (In preparation), 2021.
b) Unternaehrer-Hamm J, Chirshev E, Hojo N; Bertucci A, Sanderman L, Nguyen N, Wang H, Suzuki T, Brito E, Martinez S, Castañón C, Mirshahidi S, Vazquez ME, Oberg K and Ioffe Y; Epithelial/mesenchymal heterogeneity of high-grade serous ovarian carcinoma samples correlates with let-7 levels and predicts tumor growth and metastasis. Molecular Oncology, Vol. 14 (11): 2796-2813, November 2020.
c) E. Pariset; A. Bertucci; M.Petay; S. Malkani; A. Lopez Macha; I. G. Paulino Lima; V. Gomez Gonzalez; A. S. Tin; J. Tang; I. Plante; E. Cekanaviciute; M.Vazquez; S.V. Costes; DNA damage baseline predicts space radiation and radio-therapeutic resilience, Cell Report Cell Rep. 2020 Dec 8;33(10):108434, 2020.
d) Boyle KE, Boger DL, Wroe A, Vazquez M; Duocarmycin SA, a potent antitumor antibiotic, sensitizes glioblastoma to proton radiation. Bio. & Med. Chem. Letters Sep 1; 28(16):2688-2692, 2018.
e) Mao XW, Nishiyama NC, Pecaut MJ, Campbell-Beachler M, Gifford P, Haynes KE, Becronis C, Gridley DS. Simulated Microgravity and Low-Dose/Low-Dose-Rate Radiation Induces Oxidative Damage in the Mouse Brain. Radiat Res. 2016 Jun;185(6):647-57. doi: 10.1667/RR14267.1. Epub 2016 May 31.
f) Mao XW, Nishiyama NC, Campbell-Beachler M, Gifford P, Haynes KE, Gridley DS & Pecaut MJ. Role of NADPH oxidase as a mediator of oxidative damage in low-dose radiated and hindlimb unloaded mice. Radiat Rad. 2017 Aug 1. doi: 10.1667/RR14754.1.
g) Slater JD: Clinical applications of proton radiation treatment at Loma Linda University: review of a fifteen-year experience. Technology in Cancer Research and Treatment,5(2):81-89, 2006.
KNOWLEDGMENTS
I would like to thank Dr. Abiel Ghebremedhin, PhD, Baldev Patyal, PhD and Jerry D, Slater, MD for their support in preparing this report.
NASA Space Radiation Laboratory at Brookhaven National Laboratory
Date: October 25, 2021
Response to Letter dated Sept. 30, 2021: “National Academies’ Study on Developing a Long-Term Strategy for Low-Dose Radiation Research: Request for Information on Radiation Sources and Facilities at the NASA Space Radiation Lab.”
The committee would appreciate receiving responses/comments to the following questions:
- Please provide a brief description of your radiation sources and facilities including location, type of source, first year of operation and expected date for removal/decommissioning, and types of activities these sources and facilities support (e.g., research, radiobiology experiments, radioisotope production, other).
NSRL Commissioning: first year of operations was 2003; expected decommissioning date >2040
Activities: Radiobiology research and electronics testing
Location: NSRL is located at DOE Brookhaven National Laboratory, Upton, New York on Long Island
Radiation sources: Accelerator-based ions and gamma;
- What are the dose ranges, radiation qualities, energy, and dose-rates delivered in your facilities? Is operation pulsed or steady-state? Are the facilities suitable for inhalation experiments?
Dose Rates: Typical dose rates of approximately 10 Gy/min and approximately 0.5 Gy/min are available for the 20 ˘ 20 cm2 and the 60 ˘ 60 cm2 configurations, respectively.
As demonstrated in our dose rate studies, individual beam fractions as low as 0.1 to 0.2 mGy can be reliably measured and delivered at the NSRL.
The spill structure during most radiobiology exposures has a 4 second repetition time. During the 4 second period, the ions are extracted more or less uniformly in time during a 0.3-0.4 second spill, followed by a ~3.6 second beam-off time. For protons, the maximum beam intensity is delivered when using the LINAC as the ion source. If using the Tandem as the ion source instead, the maximum proton beam intensity is 2.5 × 1011 protons per spill.
The NSRL is not suitable for inhalation studies.
| Ion Species [1] | Max Energy [2] (MeV/n) |
LET in H2O at Max Energy (keV/micron) |
Peak LET (keV/micron) |
Range in H2O (mm) |
Maximum Intensity [3] (ions per spill) |
|---|---|---|---|---|---|
| H1 | 2500 | 0.206 | 84.3 | 10490 | 2.2 × 1011 |
| He4 | 1500 | 0.84 | 237 | 5550 | 0.3 × 10^10 |
| C12 | 1500 | 7.55 | 922 | 1856 | 1.2 × 1010 |
| O16 | 1500 | 13.4 | 1306 | 1391 | 0.4 × 1010 |
| Ne20 | 1000 | 21.9 | 1637 | 657 | 0.10 × 1010 |
| Si28 | 1000 | 43.4 | 2519 | 463 | 0.3 × 1010 |
| Ar40 | 1000 | 74.2 | 3268 | 387 | 0.02 × 1010 |
| Ti48 | 1000 | 105.6 | 3924 | 327 | 0.08 × 1010 |
| Fe56 | 1000 | 147 | 4706 | 274 | 02 × 1010 |
| Kr84 | 721 | 314 | 6221 | 132 | 2.0 × 107 |
| Nb93 | 520 | 594 | 6690 | 70 | 1 × 107 |
| Ag107 | 575 | 576 | 8470 | 70.7 | 3.5 × 106 |
| Xe129 | 589 | 761 | 9788 | 68.3 | 5.0 × 107 |
| Ta181 | 475 | 1449 | 12300 | 39.2 | 5.0 × 107 |
| Au197 | 400 | 1865 | 13140 | 27.7 | 1 × 108 |
| Bi209 | 359 | 21.8 | 138.7 | 22.6 | 7.0 × 107 |
| Sequential Field | Various | Various | Various | Various | Various |
| Solar Particle Event [5] | Various | Various | Various | Various | Various |
| GCR Simulation | Various | Various | Various | Various | Various |
https://www.bnl.gov/nsrl/userguide/beam-ion-species-and-energies.php
- For radiobiology experiments, are your facilities mostly used for exposure of cells, tissues, or animals? Please describe and if possible provide publications that describe use of your radiation facilities to facilitate radiation research.
Facilities are used for all three - animal experiments accounts for over ~50% of work performed. Two relevant publications:
- Simonsen, L.C.; Slaba, T.C.; Guida, P., and Rusek, A. “NASA’s first ground-based Galactic Cosmic Ray Simulator: Enabling a new era in space radiobiology research,” PLOS Biology. Published May 19, 2020. https://doi.org/10.1371/journal.pbio.3000669
- La Tessa C, Sivertz M, Chiang IH, Lowenstein D, Rusek A. Overview of the NASA Space Radiation Laboratory. Life Sci Space Res (Amst), 11, 2016, pp. 18–23. https://doi.org/10.1016/j.lssr.2016.10.002
- Are your facilities available for use to outside radiation researchers and other investigators?
Yes, Brookhaven uses a Strategic Partnership Project (SPP) agreement when a partner seeks research and development to complete a project but does not intend to perform the work jointly. The partner fully covers the costs of the work to be performed. This is the mechanism typically used by non-NASA users at the NSRL. Please see: https://www.bnl.gov/techtransfer/partnerships.php - Is there available adjacent infrastructure that facilitates radiation research such as tissue culture and animal facilities?
Yes, both tissue culture and animal facilities are available.
For more information please visit:
The Columbia University Radiological Research Accelerator Facility
RARAF: www.raraf.org
The Radiological Research Accelerator Facility (RARAF) provides the radiation research community advanced irradiation techniques using charged particle and neutron beams. In particular, a number of the RARAF beams have been specifically designed to facilitate studies of radiobiological effects at very low doses. For example, the RARAF microbeam beamline is designed to allow delivery of the ultimate low dose of exactly one particle to targeted cells, and has been extensively used to facilitate studies of the effects of domestic radon exposures – the largest single source of background radiation exposure.
During the more than 50 years that RARAF has been in operation, experiments have been performed for over 50 different research groups from more than 40 institutions including universities, national laboratories, cancer centers, and private corporations. These experiments, performed with radiations such as protons, alpha particles, and neutrons, have resulted in more than 200 publications in refereed journals, proceedings, and books. Research has been conducted in the fields of radiation biology, radiological physics, radiation chemistry, health physics, and medicine.
All irradiations are supported by NIST-traceable doismetry where appropriate, and state-ofthe-art biology labs are available less than a 1-minute walk from the irradiation facilities.
Marino, S. A. (2017). 50 Years of the Radiological Research Accelerator Facility (RARAF). Radiation Research 187(4): 413-423.
1. Ion beams: Broad beams, Single-Particle Beams, and Microbeams
Our Singletron particle accelerator generates ion beams, including protons, helium ions (alpha particles), lithium ions, boron ions and carbon ions. These ions have ranges sufficient to irradiate cellular monolayers, if required targeting cellular or sub-cellular target (microbeams), or they can be used to irradiate thin tissues.
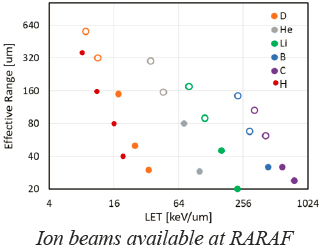
Specifically, these ion beams allow broad area irradiations or a focused sub-micron microbeam, with a range of dose rates spanning orders of magnitude from less than a single particle per cell (simulating low dose exposures from domestic radon) to hundreds of Gy.
The recent installation of a linac booster allows irradiation of thicker samples beams enabling irradiation of thin tissue samples in addition to cell cultures.
Miller RC, Randers-Pehrson G, Geard CR, Hall EJ, Brenner DJ (1999). The oncogenic transforming potential of the passage of single alpha particles through mammalian cell nuclei. Proc Natl Acad Sci US A. 96:19-22
Randers-Pehrson, G., et al. (2009). The Columbia University sub-micron charged particle beam”. Nuclear Instruments and Methods in Physics Research A: 609: 294-9
2. Very Low Dose Rates
The VAriable Dose-rate External 137Cs irradiatoR (VADER) allows modeling of low dose rate 137Cs exposures in mice and other samples using 137Cs brachytherapy seeds to generate very low dose rates (0.1 to 1 Gy/day), mimicking fallout and ingestion exposures. Within the VADER, up to 15 mice can be housed in an IACUC approved “mouse hotel” for several weeks. A custom incubator is available for performing ex-vivo blood (or other tissue) irradiations.
Garty, G., et al. (2020). VADER: a variable dose-rate external 137Cs irradiator for internal emitter and low dose rate studies. Scientific Reports10(1): 19899.
3. Ultra High Dose Rates
The prompt exposure from nuclear device such as at Hiroshima or Nagasaki, or from an Improvised Nuclear Device (IND), will be delivered in less than 1 microscond. Thus it is important to have facilities to investigate the effects of different doses of radiation delivered at ultra-high doses rates.
To mimic prompt exposures from an IND, we have adapted a clinical accelerator to deliver ultra high dose rates high dose rates. Using 9 MeV electrons, samples can be irradiated inside the Clinac head at average dose rates of up to 600 Gy/sec (3 Gy per 0.5 µsec pulse, 180 pulses per sec). In this mode multiple pulses are required for most irradiations. By modulating pulse repetition rate, dose rates of 1 Gy/sec to <1 Gy/min can be achieved at the isocenter, allowing comparison of very high dose rates vs. conventional irradiations with the same beam.
Using 6 MeV electrons, samples can be irradiated at instantaneous dose rates of up to 300 MGy/sec (0.2-150 Gy per 0.5 µsec pulse, 360 pulses per sec) and most irradiations can be performed with a single very high dose rate pulse.
In addition, an ultra-high dose rate (4 kGy/sec) 4.5 MeV proton beam is also available for irradiation of thin samples.
Grilj, V., et al. (2020), “Proton Irradiation Platforms for Preclinical Studies of High-DoseRate (FLASH) Effects at RARAF”. Radiat Res, 194:646-55
4. Neutrons:
The Columbia IND Neutron Facility (CINF), is a novel accelerator-based neutron source with an energy spectrum modeled on the Hiroshima atomic bomb spectrum at 1.5 km from the epicenter. Beams are generated by impinging a mixed proton/deuteron beam on a beryllium target generating a broad spectrum neutron beam peaked around 1 MeV. Mice and tissue samples can be irradiated at a dose rate of up to 3 Gy/h. The neutron energy spectrum obtained is also a good model for space radiations such as lunar albedo neutrons., as well as simulating the LET distribution from high-LET galactic cosmic rays.
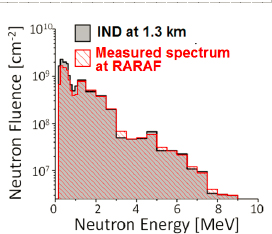
Xu, Y., et al. (2015). Accelerator-Based Biological Irradiation Facility Simulating Neutron Exposure from an Improvised Nuclear Device. Radiation Research184: 404-10.
Lab Facilities
All facilities are available for use by outside users. On-site fully equipped biology labs and a mouse housing facility are available to support experiments, less than 1-minute walk from the irradiation facilities.
Summary
| Neutrons | Ion beams | FLASH | VADER | |
|---|---|---|---|---|
| Location | RARAF, Irvington, NY | RARAF, Irvington, NY | RARAF, Irvington, NY | CUIMC, New York, NY |
| Start year | 2015 | 1980 <=150 keV/µm (H+, D+, He+2) 2022 >150 keV/µm (Li+3, B+5, C+6) |
2020 | 2017 |
| Decommissioning | Not planned | Not planned | Not planned | Not planned |
| Activities | radiobiology experiments | radiobiology experiments | radiobiology experiments | radiobiology experiments |
| Dose range (max) (min) |
Up to 10 Gy No lower limit |
Up to 100 Gy Single particle |
Up to 100 Gy .01 Gy |
1 Gy/day 0.1 Gy/day |
| Radiation type | Neutrons | Ion beams (H+, D+, He+2, C+6, Li+3, B+5) LET=8-1000 keV/µm |
6 or 9 MeV electrons | Cs137 gammas |
| Dose rate 150 |
Up to 3 Gy/h | Up to Gy/usec | 0.1-1 Gy/day | |
| Spot size | 10 cm | 1 µm - 3cm | 1-10 cm | 35 cm |
| Pulsed | DC | DC or pulsed (10 Hz) | Pulsed (180-360 Hz) |
DC |
| Inhalation experiments | No | No | No | No |
| Used for | Mice Tissue Cell culture |
Cell culture Thin tissues |
Mice Tissue Cell culture |
Mice Cell culture |
| Contact: | |
| Guy Garty, PhD RARAF, Nevis Labs 136 S. Broadway, Irvington NY 10533 |
gyg2101@cumc.columbia.edu +1(914) 591 9244 |
List of recent publications using RARAF facilities:
Neutrons:
- Klein, P.M., et al., Acute, Low-Dose Neutron Exposures Adversely Impact Central Nervous System Function. International Journal of Molecular Sciences, 2021. 22(16): p. 9020.
- Oster, L., et al., Demonstration of the Potential and Difficulties of Combined TL and OSL Measurements of TLD-600 and TLD-700 for the Determination of the Dose Components in Complex Neutron-Gamma Radiation Fields. Radiat Prot Dosimetry, 2020. 188(3): p. 383-388.
- Mukherjee, S., et al., Human Transcriptomic Response to Mixed Neutron-Photon Exposures Relevant to an Improvised Nuclear Device. Radiat Res, 2019. 192(2): p. 189-199.
- Broustas, C.G., et al., Identification of differentially expressed genes and pathways in mice exposed to mixed field neutron/photon radiation. BMC Genomics, 2018. 19(1): p. 504.
- Laiakis, E.C., et al., Metabolic Dysregulation after Neutron Exposures Expected from an Improvised Nuclear Device. Radiat Res, 2017. 188(1): p. 21-34.
- Broustas, C.G., et al., Impact of Neutron Exposure on Global Gene Expression in a Human Peripheral Blood Model. Radiation Research, 2017. 187(4): p. 443-450.
- Broustas, C.G., et al., Comparison of gene expression response to neutron and x-ray irradiation using mouse blood. BMC Genomics, 2017. 18: p. 2.
Low dose rate
- Wang, Q., et al., DNA damage response in peripheral mouse blood leukocytes in vivo after variable, low-dose rate exposure. Radiation and Environmental Biophysics, 2020. 59(1): p. 89-98.
- Pannkuk, E.L., et al., Biofluid Metabolomics of Mice Exposed to External Low-Dose Rate Radiation in a Novel Irradiation System, the Variable Dose-Rate External (137)Cs Irradiator. J Proteome Res, 2021.
High dose rate
- Grilj, V., et al., Proton Irradiation Platforms for Preclinical Studies of High-Dose-Rate (FLASH) Effects at RARAF. Radiation Research, 2020. 194(6): p. 646-655.
- Buonanno, M., V. Grilj, and D.J. Brenner, Biological effects in normal cells exposed to FLASH dose rate protons. Radiotherapy and Oncology, 2019. 139: p. 51-55.
Ion beams
- Buonanno, M., et al., A Mouse Ear Model for Bystander Studies Induced by Microbeam Irradiation. Radiation Research, 2015. 184(2): p. 219-225.
- Wu, J., et al., Cytoplasmic Irradiation Induces Metabolic Shift in Human Small Airway Epithelial Cells via Activation of Pim-1 Kinase. Radiation Research, 2017. 187(4): p. 451-463.
- Wu, J., et al., Targeted cytoplasmic irradiation and autophagy. Mutat Res, 2017. 806: p. 88-97.
- Miller RC, Randers-Pehrson G, Geard CR, Hall EJ, Brenner DJ. The oncogenic transforming potential of the passage of single alpha particles through mammalian cell nuclei. Proc Natl Acad Sci U S A. 1999 Jan 5;96(1):19-22.
























