3
Materials, Combustion, and Emissions in WUI Fires
Fires in the wildland-urban interface (WUI) differ from wildland fires. Combustible materials in the WUI have different chemical compositions and densities, and are present in different quantities, than the vegetative biomass combusted in wildland fires. The urban materials and their characteristics present in the WUI impact the combustion conditions, the chemical reaction pathways that dominate during combustion, and the emissions and residue released into the environment. Current understanding of the chemistry of WUI fires and their emissions is largely inferred based on information on wildland and urban fires.
Wildland fires are a large source of emissions and have been extensively studied. Over the last several decades, numerous laboratory studies and field efforts have sought to link the characteristics of biomass and fire behavior with the resulting emissions (Jaffe et al., 2020). These studies have made clear that emissions from biomass fires are complex and consist of many chemical species, of which only a limited number have been identified. The composition of the emissions also depends on fire conditions; however, many uncertainties remain in the details of this dependence (Coggon et al., 2019; Jaffe et al., 2020; Permar et al., 2021; Sekimoto et al., 2018).
Data on emissions from the combustion of urban materials is derived largely from enclosure fires (i.e., a fire within a room or compartment inside a building) or from laboratory test methods simulating enclosure fires (Blomqvist and Lönnermark, 2001; Stec and Hull, 2010). Both the specific material elemental composition and the amount of oxygen available for combustion strongly impact released emissions. However, very little is known about the chemical composition of urban materials and the interactions of various mixtures of these materials on combustion processes, the types of species emitted, and these species’ interactions under different fire conditions (Purser et al., 2015; Stec and Hull, 2010). The fire emissions produced (per mass of material combusted) from the combustion of human-made materials are expected to be much different than those from biomass.
This chapter summarizes what is known about the materials in the WUI environment, and the combustion conditions, dominant reaction pathways, and resulting emissions from WUI fires. The discussion of materials in the WUI environment and the unique aspects of combustion chemistry described in this chapter provide context to the information needed to describe atmospheric transformations, water and soil impacts, health effects, and measurement systems described in Chapters 4–7.
Box 3-1 defines some of the key terms used in the chapter.
MATERIALS
Many WUI fires occur in residential areas where the primary fuels are those in and around the home, which can include a diverse array of materials. Figure 3-1 shows some of these potential materials, although many others of different compositions may be present. There are numerous exterior and interior construction materials and furnishings with synthetic chemical compositions including textiles, insulations, paints and coatings, premanufactured woods, added flame retardants, antimicrobial and halogenated finishes, wall coverings, and sealants.
The quantities and compositions, cited in Figure 3-1 and elaborated on in the sections below, are illustrative examples. Structural materials may vary greatly based on the age of construction and geographic region. For example, some substances used in building materials or household goods have been banned or phased out, such as polybrominated diphenyl ether (PBDE) flame retardants, yet they may still be present in older household goods (ATSDR, 2017). Furthermore, the amount and composition of materials within the home, particularly chemicals of concern (such as flame retardants and phthlates), may be linked to race, ethnicity, and socioeconomic factors that are not well captured by the examples presented here. Black, Indigenous, and people of color and low-income groups are more likely to live in older homes in poorer condition. Given the lack of comprehensive surveys of materials in homes and how they may differ for various populations, other data sources, like chemical exposure studies, may provide some information on urban material chemical composition. Researchers have found higher exposures to some chemicals of concern (e.g., volatile organic compounds [VOCs], semi-volatile organic compounds [SVOCs], flame retardants, pesticides, lead) among these groups, demonstrating that the composition of homes and their contents likely vary by race and economic status (Adamkiewicz et al., 2011; Jacobs, 2011; Swope and Hernandez, 2019; Zota et al., 2010), although other factors such as occupational exposure may also play a role.
The unique elemental composition of the materials in the urban environment has a direct impact on the combustion chemistry and emissions described in the other sections of this chapter. Table 3-1 summarizes some of the most common building materials and their fire emissions (Blomqvist et al., 2013; Stec, 2017; Stec and Hull, 2010, 2011;
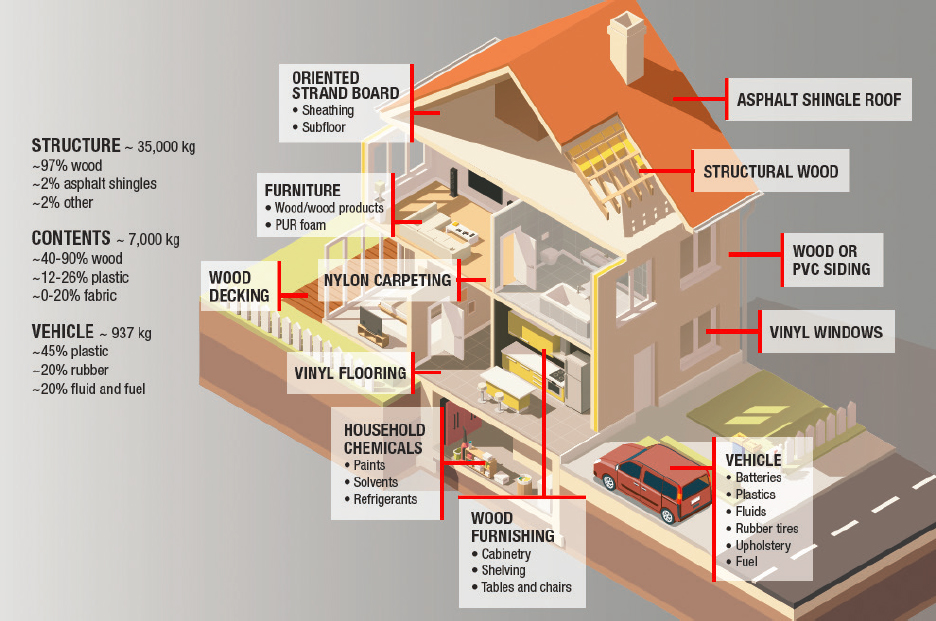
TABLE 3-1 Materials Used in Buildings and Their Common Fire Emissions
| Material | Most Commonly Released Fire Emissions |
|---|---|
| Polyurethane foam in insulation | HCN, CO, NO, NO2, NH3, HCl, H3PO4, PM, PAHs, VOCs, SVOC, TCPP, TCEP, PCDDs, PCDFs, isocyanates |
| Polyisocyanurate foam in insulation | HCN, CO, NO, NO2, NH3, HCl, H3PO4, PM, PAHs, VOCs, SVOC, TCPP, TCEP, PCDDs, PCDFs, isocyanates |
| Phenolic foam in insulation | SO2, CO, HCl, acrolein, formaldehyde, PM, PAHs, VOCs, SVOCs, TCPP, TCEP, PCDDs, PCDFs |
| Extruded polystyrene in insulation | HF, HBr, CO, PM, PAHs, VOCs, SVOCs |
| Glass wool in insulation | HCN, CO, NO2, HCl, isocyanates |
| Oriented strand board (OSB) | HCN, CO, NO2, HCl, acrolein, formaldehyde, PM, PAHs, VOCs, SVOCs, isocyanates |
| Vinyl siding and/or polyvinyl chloride (PVC) windows | HCl, CO, PCDDs, PCDFs |
| Upholstery on furniture | HCN, CO, NO, NO2, NH3, HCl, H3PO4, PM, PAHs, VOCs, SVOC, TCPP, TCEP, PCDDs, PCDFs, isocyanates |
| Vinyl carpet | HCl, CO, PCDDs, PCDFs |
| Polyamide carpet | HCN, CO, NO, NO2, NH3, PM, PAHs, VOCs, SVOCs, isocyanates |
| Electrical wiring insulation | HCl, CO, PCDD, PCDFs |
| Acrylic clothing | HCN, CO, NO, NO2, NH3, PM, PAHs, VOCs, SVOCs, isocyanates |
| Residential furniture | Benzene, toluene, formaldehyde, organophosphate flame retardants |
NOTES: PAH = polycyclic aromatic hydrocarbon; TCPP = tris(1-chloro-2-propyl) phosphate; TCEP = tris(2-chloroethyl) phosphate; PCDD = polychlorinated dibenzo-p-dioxin; PCDF = polychlorinated dibenzofuran.
Stec et al., 2013). Table 3-1 relates the materials in the urban environment to the WUI fire emissions that impact the atmosphere, water and soil, and human health as described in Chapters 4–6.
Wildland Materials and Wood Products
The natural woody biomass found in the landscape surrounding WUI structures and wood products used in building construction are of similar macromolecular composition (cellulose, hemicellulose, and lignin). Bain et al. (2003, Appendix 3) list the energy content and chemical composition for a wide range of biomass materials, including wood from numerous tree species and other vegetative types. The higher heating values of different dry woods range from 19 to 20.2 MJ/kg. The heat of combustion per unit mass of biomass material is significantly impacted by the water content, which varies between live and dead woody biomass, biomass species, and season (NWCG, 2021). Wood used in building construction has a lower moisture content (typically less than 12 percent), depending on local ambient conditions (Forest Products Laboratory, 2010).
Biomass also contains 0.1 to 0.4 percent (by mass) nitrogen, 0.01 to 0.09 percent sulfur, up to 0.05 percent chlorine, and traces of other inorganic elements (Tejada et al., 2020). In general, ash levels remaining from the complete combustion of the volatiles and char generated in the pyrolysis/burning of woods are very low (0.01 to 0.07 percent), with only a small fraction of the original sulfur and nitrogen segregated in the char (Bain et al., 2003). Wildland fire ash consists of mineral materials and charred organic components with compositions dependent upon the original fuel type, combustion completeness, and combustion temperatures that the materials experience (Bodi et al., 2014).
In addition to woody biomass (live trees and snags [standing dead trees]), other fuels contribute to the emissions from wildland fires (Atchley et al., 2021). Live foliage in the canopy, dead foliage in the leaf litter, non-woody vegetation, lichen-moss layers, duff layers, and organic soil may be consumed in wildland fires (Ottmar, 2014; Ottmar and Baker, 2007). These other fuel classes like leaf litter, grasses, and shrubs sustain fire spread and are important sources of emissions. For example, in a mixed conifer forest in Oregon, researchers estimated that 33 percent of the biomass consumed in a high-severity scenario could be attributed to the litter and duff layers
alone, and 35 percent was attributed to standing trees and snags (Campbell et al., 2007, Table 5). Additionally, duff layers and downed woody debris tend to smolder and can generate far greater emissions per unit mass consumed than litter, shrubs, and tree crowns that are consumed in more intense combustion (Urbanski, 2014).
Vegetative biomass loading in the environment is highly variable in both space and time as vegetation grows, is impacted by insect, disease, and climate conditions, and eventually decays. Biomass loadings can vary greatly among ecosystems; the highest biomass loadings are in wet forests, but only materials that are dry enough to ignite are considered available biomass and thus able to be consumed during a wildland fire. Some of the highest available biomass loadings are in dry forest ecosystems that are common in the western United States (Hessburg et al., 2019). A dry forest can have as much as 70 Mg/ha or about 28,000 kg/acre of available biomass (Ottmar, 2014). The available biomass can also be modified by land management practices, like prescribed fire. About 13 tons/ha (5,000 kg/acre) is consumed in prescribed fire in temperate forests (van Leeuwen et al., 2014).
Urban Materials
The materials in the urban environment differ greatly from the materials in the wildland environment in their amount, elemental composition, energy content, and arrangement in the landscape. Materials unique to urban areas, like polyvinyl chloride or polyurethane, contain much larger amounts of chlorine or nitrogen than biomass and these elements are known to impact combustion chemistry. Though the emissions generated from urban and wildland areas differ, in a WUI fire, they intimately comingle in the plume. In addition, the differing energy density in urban areas compared with wildlands may impact combustion conditions, modifying the timing of emissions and the physical nature of the WUI plume in comparison to the plume of a surrounding wildland fire (Trelles and Pagni, 1997).
In WUI fires, many of the structures burned are residential homes, as seen with the recent WUI fires described in Chapter 2:
- 74 percent of the approximately 18,000 structures destroyed in the Camp Fire (Wallingford, 2018)
- 99 percent of the 344 structures destroyed in the Waldo Canyon Fire (Maranghides et al., 2015)
- 99 percent of the 1,091 structures destroyed in the Marshall Fire (Boulder OEM, 2022)
Large WUI fires—those sweeping through entire communities—may also involve municipal service systems, commercial and industrial buildings, and commercial vehicles and their fuel. These commercial and industrial structures and other components of urban infrastructure likely have material loadings and compositions different from those of residential buildings. They may be composed of more noncombustible materials (e.g., concrete and steel), but may have much higher internal fuel loadings and greater amounts and different chemicals of concern. Thus, they can pose risks different from those of WUI fires confined to residential areas. Specific mappings of commercial and industrial structures and their fuel loadings and chemicals of concern are not broadly available.
Characteristics of Residential Structures
Most houses in the United States are built from wood framing and sheathing covered by an exterior of brick, stone, cement board, or vinyl siding (USCB, 2020). Sheets of fiberglass or foam installations are often added into the building cavities. The roof consists of sheets of plywood to provide the decking for exterior materials such as asphalt tiles, clay tiles, metal sheets, or even fiberglass shingles.
Limited survey data exist on the detailed material composition of residential structures, and even less information exists on elemental composition. Life-cycle analysis tools like the Greenhouse gases, Regulated Emissions, and Energy use in Technologies (GREET) life-cycle model could be used to estimate the mass and elemental composition of buildings (Cai et al., 2021). At present there is no complete accounting of both the fuel load and elemental composition of currently existing structures.
Table 3-2 provides examples of combustible materials loadings and other data for single-family homes built in different years. The committee derived the data in this table from engineering calculations; the assumptions employed in the calculations can impact the results of the analysis and are described in detail in Appendix E. The data represent a snapshot of structure characteristics for a specific location and year of construction, characteristics
TABLE 3-2 Materials Used to Construct Two Single-Family Residences
| The Fuel of Our Homes – from Building Materials to Content (Messerschmidt, 2021) | Analysis of the Lifecycle Impacts and Potential for Avoided Impacts Associated with Single Family Homes (EPA, 2016) | |||
|---|---|---|---|---|
| Year built | 2020 | 1998 | ||
| Livable area in ft2 (m2) | 2,016 (187.3) | 2,150 (153.2) | ||
| Combustible mass in kg (% total combustibles) | Energy content in GJ (% total energy) | Combustible mass in kg (% total combustibles) | Energy content in GJ (% total energy) | |
| Structural wood | 15,200 (58%) | 281.2 (49%) | 13,159 (43%) | 243.4 (42%) |
| Subfloor (OSB or particle board) | 3,844 (15%) | 71.3 (13%) | 10,115 (33%)a (OSB panels) | 187.5 (33%) |
| Sheathing | 126 (0.5%) (foam board) | 3.2 (1%) | -a | - |
| Siding | 300 (1%) (PVC siding) | 3.0 (1%) | 3,485 (12%) (wood siding) | 64.5 (11%) |
| Insulation | 990 (4%) (spray polyurethane foam) | 25.3 (4%) | 183 (1%) (fiberglass) | 5.2 (1%) |
| Roof decking (OSB) | 2,108 (8%) | 39.1 (7%) | 2,393 (8%) | 44.4 (8%) |
| Roof exterior (asphalt shingles) | 3,630 (14%) | 145.9 (26%) | 746 (2%) | 30 (5%) |
| Windows | - | 192 (1%) | 1.9 (0.3%) | |
| Total combustibles | 26,198 | 569.1 | 30,272 | 576.9 |
NOTE: OSB = oriented strand board.
a Includes sheathing.
that vary significantly over time and location. An important limitation of these examples is the lack of information on some components (e.g., air-conditioning fluids, plastic gas piping, plumbing, wire insulation, and electrical materials), which are a small fraction of the mass but may be a concentrated source of species that impact combustion or contribute to hazardous effluents.
Wood-based materials are the largest fraction of combustible mass in the examples shown here; wood and engineered wood products can be derived from a variety of species and can exist in many forms. Engineered wood products, such as oriented strand board (OSB), particle board, and plywood, contain adhesives and fillers that can account for up to 15 percent of the product by mass (Weyerhaueser, 2018). The compositions of the adhesives have changed over time; originally, they contained formaldehyde, but now most adhesives are composed of methylene diphenyl diisocyanate (Blomqvist et al., 2013; Pokhrel et al., 2021; Sandberg, 2016).
Additionally, wood products may be chemically treated to prevent rot, protect against attack by insects or microbes, or confer fire-retardant properties. The use of a treatment depends on the application, with most treatments used in exterior materials. The prevalence of these treatments in structural materials and the particular chemicals used have varied over time. For example, a combination of chromium, copper, and arsenate was commonly used in outdoor wood products (e.g., decks, playgrounds) to protect against microbial attack but was phased out and replaced with alkaline copper quaternary. Flame retardants are increasingly used in exterior and interior structural materials (Lowden and Hull, 2013), with the types of compounds evolving over time (Popescu and Pfriem, 2019). Additives can account for up to 10 percent of the mass of the treated wood, with exact amounts difficult to determine; safety data sheets provide a wide range of mass fractions. Additionally, specific compositional information is not always provided. Different treatments and/or additives can impact combustion properties and ultimate emissions into the environment. For that reason, treated wood cannot be discarded through regular municipal waste in California because of the hazardous nature of the treatments (CalRecycle, 2020).
The composition of the other components of a structure, such as roofing, siding, and insulation, can vary depending on the manufacturer of the product. The amount and type of the materials used also varies by type of home
(e.g., apartment, manufactured home), geographic location, consumer preferences, common building practices, and building codes at the time the residence was built (Box 3-2). For example, exterior finishing has varied substantially over time, with brick and wood exteriors much more common in home construction through the 1970s and 1980s, but with vinyl siding becoming the dominant exterior in new home construction after 1990 (USDOC, 2010).
These changes in consumer and builder preferences can alter the amount of combustible materials, their chemical composition, and the potential for release of chemicals of concern. For example, the transition from wood-based exteriors to asphalt roofing and polyvinyl chloride (PVC) siding changes the combustible mass, elemental composition, and energy content of homes and the nature of any combustion emissions. Similarly, the transition from vermiculite insulation (which may contain asbestos) to blow-in cellulose or polyurethane spray foam changes the amount of combustible materials that will yield emissions of chemicals of concern.
As building codes and practices change over time, the distribution of building ages results in a wide range of housing materials used across the United States, and potentially in WUI areas. Additionally, the frequency and extent of home renovations are not well known (Dixit et al., 2012; Ghattas et al., 2016). Building components like roofing or siding are periodically replaced or repaired, while other parts like the interior framing may remain the same over the lifespan of the building (EPA, 2016). The long lifetimes of houses limit the impact of building codes introduced in the last three decades that mandate the use of ignition-/fire-resistant materials to reduce the risk from wildland fires, leading to regulatory initiatives to promote retrofits for existing homes (State of California, 2019).
Characteristics of Materials in the Home
When exposed to fire, the contents of a home influence combustion conditions and emission composition. The materials inside buildings are typically characterized through fire load surveys, where fire load is the total amount of heat released from complete combustion of the contents of a structure. Physical inventories, questionnaires, and digital surveys have all been used to quantify fire load (Elhami-Khorasani et al., 2020). Fire load assessments date back to the 1940s. They have been done in various countries and do not exhibit trends by country or over time (Xie et al., 2019). However, fire load density (fire load per unit floor area) varies with residence size, with larger areas tending to have lower densities. The average fire load density for residential buildings is 645 ± 212 MJ/m2 (see Table 2 in Xie et al., 2019).
TABLE 3-3 Typical Composition of the Moveable Fire Load in Residential Buildings
| Material | Swedish Model Residences (Blomqvist and McNamee, 2009, Table 5) | Canadian Kitchen (Bwalya et al., 2010) | Canadian Bedroom (Bwalya et al., 2010) |
|---|---|---|---|
| Wood/Paper | 79.2% | 86.5% | 42.3% |
| Plastics | 12.3% | 13.5% | 26.4% |
| Fabrics | 4.3% | 0% | 31.4% |
| Other | 4.3% |
Table 3-3 summarizes the bulk composition of a typical home’s contents. Only a limited number of studies have evaluated total interior fuel loadings for residences in North America. One study found that the main bedroom has the highest fire loading, followed by the kitchen, living room, basement living space, secondary bedrooms, and dining room (Bwalya et al., 2010). The fire loading density is the greatest in the kitchen and bedrooms, driven by the large amount of wooden cabinetry, furniture, and clothing contained in relatively small spaces. Bwalya et al. (2010) found that wood flooring, cabinets, tables, and display units contributed to the wood loading. Plastics were found in carpeting, foam cushioning, and synthetic fabrics. The fabric or textile content was due to clothing.
Characteristics of Vehicles
Just as it is possible to characterize the main constituents in residential structures as shown in Table 3-3, it is also possible to do so for vehicles. Table 3-4 reports the main components of an average modern light-duty vehicle built in 2017. An average vehicle weighs 1,793 kg and is composed of ~75 percent noncombustible materials and ~25 percent combustible materials by mass. The largest contributions to the combustible mass are plastic components, fluids and lubricants, and rubber. The noncombustible mass consists primarily of various metals and a small amount of glass. The average age of light-duty vehicles in use is 11.9 years, and the combustible composition has remained nearly constant over the past several decades (Davis and Boundy, 2021). The increasing percentage of trucks in the light-duty fleet has led to a slight increase in overall vehicle mass over time (~7 percent greater in 2017 versus 1995). A slight reduction of the noncombustible mass has also occurred, from 81 percent in 1995 to 76 percent in 2017.
Although reliable data exist on the bulk material composition of vehicles, the elemental composition of these materials is not as well known but may be estimated using life-cycle analysis tools (e.g., GREET; Cai et al., 2021). Moreover, the material composition of vehicles will change as electric vehicles become more common. Hybrid electric vehicles accounted for 3.2 percent and battery electric vehicles accounted for 1.7 percent of new light-duty vehicle sales in 2020 (Davis and Boundy, 2021, Table 6.2), with both fractions of these vehicle types expected to grow.
Battery electric vehicles are generally heavier than internal combustion engine vehicles due to the additional battery and associated power systems (Wang et al., 2021). Internal combustion engine and hybrid electric vehicles have gasoline and other flammable liquids. These liquids have high energy densities and may contribute a sizable portion of energy content that is not present in a battery electric vehicle (Table 3-4).
Batteries may be a potent source of compounds of concern that could be liberated during a WUI fire or become a source of toxic combustion products. Most vehicles contain a lead acid battery, which varies from 6 to 16 kg depending on the type of vehicle and is ~70 percent lead by mass (Wang et al., 2021). The nickel metal hydride battery sometimes found in electric vehicles can weigh as much as 1,270 kg and is ~28 percent nickel by mass (Wang et al., 2021). These metals may be released into the environment during a WUI fire. Lithium-ion batteries, also used in electric vehicles, can emit fluorinated gases upon heating and during combustion (Larsson et al., 2017; Sturk et al., 2019). The composition of a lithium-ion battery varies depending on the physical design and the battery chemistry (Elgowainy et al., 2016). The most common lithium-ion battery chemistry uses a lithium cobalt oxide cathode, graphite anode, and lithium hexafluorophosphate electrolyte. However, many different types of battery chemistries are used to achieve various performance features, and it is likely that battery compositions and vehicle designs will continue to evolve, along with their overall contribution to emissions from WUI fires.
TABLE 3-4 Mass and Energy Contributions to an Average Light-Duty Internal Combustion Engine Vehicle, Model Year 2017
| Material | Mass in kg (% of combustible mass) | Energy Content in GJ (% of total energy content) |
|---|---|---|
| Regular steel | 554 | |
| High- and medium-strength steel | 347 | |
| Stainless steel | 33 | |
| Other steels | 14 | |
| Iron casting | 110 | |
| Aluminum | 189 | |
| Magnesium castings | 4 | |
| Copper and brass | 31 | |
| Lead | 17 | |
| Zinc castings | 4 | |
| Powder metal parts | 20 | |
| Other metals | 2 | |
| Glass | 43 | |
| Plastics and plastic composites | 155 (34%) | 6.7 (43%) |
| Rubber | 93 (20%) | 3.1 (19%) |
| Coatings | 13 (3%) | 0.3 (2%) |
| Textiles | 21 (5%) | 0.6 (4%) |
| Fluids and lubricants | 101 (22%) | 2.5 (16%) |
| Other materials | 42 (9%) | 1.0 (7%) |
| Fuela | 36 (8%) | 1.5 (9%) |
| Combustible portion | 461 | 15.7 |
| Total mass | 3,953 |
a Amount of gasoline assumed to be 36 kg (13 gallons), corresponding to the default value in the GREET life-cycle analysis (Cai et al., 2021).
SOURCE: Davis and Boundy, 2021.
Persson and Simonson (1998) assessed that 9 percent of the plastic material of an automobile was PVC (containing chlorine) and 17 percent was polyurethane foam (containing nitrogen). Measurements of gas-phase emissions from vehicle fires have shown that compounds with a potentially adverse health impact on humans are produced in significant concentrations: Fent and Evans (2011) carried out a series of vehicle fires and measured air concentrations of formaldehyde, acrolein, and isocyanates. They estimated that personal exposures to these compounds were nearly 10 times the acceptable levels on an additive effects basis. In addition, electric vehicles and Li-ion batteries can be the source of hydrogen chloride (HCl) and hydrogen fluoride (HF), which have more severe health effects than asphyxiant gases (Fent and Evans, 2011). Other fluorine-based compounds, such as POF3 and COF2, have not been reported in significant quantities from fire tests with batteries. Table 3-5 presents examples of fire emissions from car components (Larsson et al., 2017; Lönnermark and Blomqvist, 2006; Willstrand et al., 2020).
These studies of vehicle fires also showed that many chemicals (e.g., polycyclic aromatic hydrocarbons [PAHs] and metals) partition into the particle phase, in which form they can be inhaled deep into the lung. Table 3-6 presents an example of the elements identified in PM from a vehicle fire (Lönnermark and Blomqvist, 2006). Such data need to be carefully examined, as metal composition differs among different vehicle and battery types (Larsson et al., 2017; Lönnermark and Blomqvist, 2006; Willstrand et al., 2020).
TABLE 3-5 Fire Emissions from Car Components
| Car Component | Fire Emissions |
|---|---|
| Door panel | CO, HCl, HCN, NO, PCDDs/PCDFs, VOCs, PAHs, isocyanates |
| Ventilation system | CO, acrolein, formaldehyde, PAHs, VOCs |
| Floor material | CO, HCN, isocyanates, PAHs, VOCs |
| Dashboard | CO, HCN, NO, isocyanates, PAHs, VOCs |
| Upholstery material | CO, HCN, NO, HCl, SO2, isocyanates, PAHs, PCDDs/PCDFs, VOCs |
| Electrical wiring | CO, HCl, PAHs, PCDDs/PCDFs, VOCs |
| Tire | CO, SO2, PAHs, VOCs |
Chemicals in Consumer Products
Various data sets can provide some information on chemicals of concern in consumer products in the urban environment. These data sets have been developed for purposes like chemical exposure assessment, prioritizing of chemical toxicity screening, life-cycle analysis, or green building and design. Some data sources for building products, like Pharos (https://pharosproject.net/) and Building for Environmental and Economic Sustainability (Suh and Lippiatt, 2012), were designed to identify green alternatives or provide information for life-cycle analysis. Some data sources were designed for chemical exposure assessment, for example the Chemical/Product Categories Database (Dionisio et al., 2015) and the OrganoRelease framework (Tao et al., 2018). However, many unknowns about the composition of consumer products still exist. For example, a non-targeted analysis of consumer products by Phillips et al. (2018) measured numerous compounds in consumer products, of which over 85 percent were not listed in any chemical database. These non-targeted analyses along with surveys of consumer products and household exposure measurements demonstrate the difficulty of identifying chemicals of concern in the urban environment (Li and Suh, 2019).
In addition to chemicals of concern in current use, many chemicals have been phased out but still exist in the urban environment due to legacy use (e.g., lead, polychlorinated biphenyls) and varying abatement practices. Weschler (2009) examined major trends in consumer products and building materials since 1950 and noted that usage of chemicals of concern varied considerably over time, and that historical usage was difficult to quantify. Many factors may impact material choices in the home, but the identification of health, environmental, or fire hazards were important drivers of usage trends (Weschler, 2009; Cooper et al., 2016).
TABLE 3-6 Elements in PM Emitted from a Vehicle Fire
| Metal | Mass Concentration in PM (mg/kg) |
|---|---|
| Cadmium | 26 |
| Cobalt | 5 |
| Chromium | 59 |
| Copper | 430 |
| Nickel | 44 |
| Lead | 12,800 |
| Antimony | 230 |
| Thallium | 80 |
| Zinc | 50,300 |
| Fluorine | 510 |
| Chlorine | 39,000 |
| Bromine | 4,000 |
SOURCE: Lönnermark and Blomqvist, 2006.
Effects of Structure Density on WUI Fire Behavior
In addition to the materials that make up WUI structures, the density of the structures themselves can affect WUI fire behavior. The material loading in the landscape is a key parameter since it can impact the fire spread, fire intensity, and quantity of emissions produced. Additionally, the ratio of urban structures and vehicles to vegetative materials combusted in a WUI fire will impact the combustion chemistry and the resulting amount and type of pollutants released into the atmosphere as well as those impacting air, soil, and water quality (see Chapters 4 and 5 for further details). However, the number of houses involved in a fire can vary greatly from one fire to the next, as can the amount of biomass loading and the wildland acreage burned (CAL FIRE, 2022). And, very little is known about the number of vehicles destroyed in WUI fires, as they are not typically quantified in damage assessments (Kuligowski, 2021).
Interface WUI areas (those at the edge of a large area of wildland) can have a high density of houses, and when these areas are exposed to wildland fire, large numbers of structures can burn and may have fire behavior and combustion emissions very different from WUI fires with less structures. The Marshall Fire in 2021 (estimated 1,081 structures and approximately 6,200 acres burned) and the Tunnel Fire in 1991 (estimated 2,900 structures and 2,000 acres burned; CAL FIRE, 2022) are two such examples, where relatively small grassland fires moved into densely populated areas, and the loading of urban fuels greatly exceeded the loading of wildland vegetation.
Researchers have used models and WUI fire observations to better understand how a high structure density in interface WUI areas impacts the rate of spread and the heat release rate of a fire in an urban area in comparison to the surrounding wildland fire. For example, the high density of combustible materials in a structure in comparison to surrounding wildlands increases the duration of the fire and may influence the spread of the fire (Maranghides et al., 2013, 2015). Burning structures can also generate fire-induced winds that impact the rate of spread, as observed in the Tunnel Fire, where the spread rate slowed when the number of structures involved in the fire increased (Trelles and Pagni, 1997). These examples contrast with WUI fires in intermix areas (alternating areas of housing and wildland), where the structure fuel loading is much lower (it may be comparable with that of vegetative fuels) and may have less of an impact on fire behavior.
Rate of spread is often assessed for WUI fires, but few studies have specifically looked at the impact of structure fuel loading, although it is likely an important factor in fire spread (Masoudvaziri et al., 2021). Additionally, the heat release rate from WUI fires has not been comprehensively evaluated but will likely vary with the differing fuel composition and density in WUI fires compared to wildland fires (Simeoni et al., 2012). Rate of spread and heat release rate can impact combustion conditions, the timing of emissions, and the plume dispersion in the atmosphere. It is currently not known how the loading of urban fuels will impact these characteristics in WUI fires.
Factors Impacting Structure Destruction in a WUI Fire
The number of structures that will burn when exposed to wildland fire is highly variable. Many factors can impact the likelihood of destruction, and the significance of each factor, or combination of factors, is an active area of research.
Environmental conditions coupled with the topography of a WUI area can drive extreme fire behaviors that result in destructive WUI fires (Keeley and Syphard, 2019). The most destructive fires have occurred in the western United States during dry, downslope wind events (e.g., the Santa Ana or Diablo winds) and in terrain that channels fire-induced winds, increasing the probability of spot fires igniting ahead of the main fire front (Keeley and Syphard, 2019). Strong winds and dry conditions are common features of WUI fires across the United States (e.g., Maranghides and McNamara, 2016; the example fires described in Chapter 2).
The number of structures present and their arrangement in the landscape are important factors (Syphard et al., 2019). Areas with a high density of combustible structures may experience greater structure-to-structure spread (Syphard et al., 2021, and references therein), and the increased combustible load may lead to more extreme fire behavior (Keeley and Syphard, 2019). However, fire spread can be mitigated in communities using fire-resistant materials and reduced combustible vegetation around the home. In areas where WUI fires are more frequent, regulations have been implemented to prevent the spread of fire (Philson et al., 2021).
Finally, defensive action by firefighters can reduce destruction and potentially limit the spread of a fire within the urban area. However, it is difficult to quantify the impact of such defensive actions and how important they may be in relation to the other characteristics of WUI structures and their surroundings.
Finding: Limited data exist on the composition of residential building materials and the materials within residences; the composition of these potential WUI fuels will be difficult to ascertain from existing fuel loading records, especially for important species such as halogens, phosphorous, and metals. WUI fire fuel loadings are complex and depend on the density of structures and landscape vegetation, the use of fire-resistant building materials, the number and type of vehicles left behind by evacuees, and defensive actions taken during the fire.
Research need: More information is needed on the amount, type, and chemical composition of consumer products in residences at the WUI, including legacy materials and how these may vary with the age and condition of the home. Data are also needed on the amount and arrangement of urban structures, vehicles, and their surroundings.
Changing Materials at the WUI
As experience with WUI fires grows, some jurisdictions are changing building codes, which in turn will change the composition of structures and the wildland at the WUI. Table 3-7 describes actions that are currently used or proposed for use in WUI areas. For example, California building codes such as Chapter 7A of the California State Building Code (State of California, 2016b) or the National Fire Protection Association Standard for Reducing Structure Ignition from Wildland Fire (NFPA, 2018) describe building practices such as hardening of the home using Class A ignition-resistant roofs, enclosed eaves, multipane glass, and the use of noncombustible materials or ignition-resistant materials on the structure exterior. Many areas also have requirements to maintain defensible space by limiting the amount of combustible materials (e.g., landscape vegetation, leaf litter, wood stacks) near the home and reducing the horizontal and vertical distribution of fuels in areas surrounding the house that may promote the spread of a fire.
TABLE 3-7 Current and Potential Approaches to Modify Materials to Reduce Risk of WUI Fire
| Applicable Area | Actions |
|---|---|
| Wildlands |
|
| Community |
|
| External to the structure |
|
| Inside the structure |
|
In addition to state and local regulations, initiatives like the Firewise Communities (NFPA https://www.nfpa.org/Public-Education/Fire-causes-and-risks/Wildfire/Firewise-USA) have been established to help WUI communities identify mitigation strategies and encourage residents to take actions to protect their homes. Additionally, organizations such as the National Fire Protection Association and the Insurance Institute for Building Home and Safety have developed policy recommendations aimed at reducing risk (https://www.nfpa.org/outthinkwildfire and https://ibhs.org/wp-content/uploads/2019/05/wildfire-public-policy.pdf, respectively). A summary of the recommended policies and mitigation steps from these resources and others described in subsequent text is provided in Table 3-7.
Prescribed fire is widely used in many parts of the United States, often for ecosystem health as well as reducing the loading of fine combustible biomass (grasses and shrubs). Grazing in shrub- or grassland-dominated areas reduces the fine biomass load and thus fire severity (Colantoni et al., 2020; Davies et al., 2015). Close to urban areas, mechanical treatments like tree and brush removal can reduce the combustibles immediately adjacent to developments. These approaches may have limited effectiveness, especially in shrub and grassland regions where vegetation regrows quickly, so additional actions are needed to reduce WUI fire risk (Schoennagel et al., 2017).
A recent literature survey and compilation of recommendations in California (Moritz and Butsic, 2020) highlighted community-scale risk reduction measures to help protect homes and lives from WUI fires in new developments. One example of a measure that may or may not reduce collective fire risk is clustering structures. The design, maintenance, and use of defensible space for fire protection is improved when neighborhoods are developed more densely and are built with stringent fire-resistant building codes; however, clustering may contribute to high fuel loadings. Wildland fire–resistant construction techniques to harden all the homes in a community can be used to minimize risk and protect homes from wildland fires with minimal additional cost (Quarles and Pohl, 2018); after a community or subdivision has been developed, the responsibility to mitigate risk by modifying the environment falls on homeowners, whose resources for retrofitting structures and maintenance of defensible space may be limited.
In existing communities, the approaches to mitigate risk from WUI fires focus on using ignition-resistant building materials, reducing fuels next to structures, and maintaining defensible space. In some areas, state laws or local ordinances are used to ensure that property owners maintain defensible space around structures. Changes to the building structure are implemented largely through renovations and replacements with ignition-resistant materials to align with current building codes. Proposed California legislation would more widely encourage low-cost retrofits, require disclosure of fire safety retrofits during the sale of existing homes, and provide financial assistance for retrofits in an attempt to protect vulnerable communities that may not otherwise have the means to implement mitigation measures (State of California, 2019).
The effectiveness of any of these mitigation actions at preventing structure loss during a WUI fire has yet to be fully understood, and assessments of previous WUI fires show that many factors may play a role in structure survival (Syphard et al., 2021). A survey of some severe WUI fires in California showed that the impact of mitigation actions was only modest (Syphard and Keeley, 2019) and no single category explained more than 25 percent of the likelihood of survival (Syphard et al., 2021). The most effective actions were using covered eaves, multipaned windows, and screened vents to reduce ignition risk (Syphard and Keeley, 2019). Maintaining defensible space was less effective, showing the significance of ember ignitions, since embers can travel as far as 1 km in wind-driven fires (Keeley and Syphard, 2019).
Mitigation measures to the structure and its surroundings may not be sufficient to reduce risk in some fire-prone landscapes. Keeley and Syphard (2019) noted landscape features that were common in some destructive fires. For example, developments on the edges of canyons or within a wind corridor may be particularly vulnerable. Additionally, some California coastal areas, despite repeated fire, have rapid regrowth of vegetation and seasonal meteorology that create hazardous conditions (e.g., dry fuels and strong winds) that lead to destructive fires. Some planning ordinances require the siting of developments to avoid areas of high fire risk, although this approach to mitigate risk is rarely used (Moritz and Butsic, 2020).
Finding: A variety of measures, from managing the combustible materials in the urban environment and surrounding landscape to using fire-resistant materials and structural designs, have been pursued to mitigate WUI fire ignition and spread. The collective effectiveness of the approaches and their associated costs remain uncertain.
COMBUSTION
As a result of the fuel loading and material composition of urban dwellings and infrastructure, intermixed with fuel loadings and compositions from wildlands, the chemistry of fires at the WUI is more complex than that of wildland or urban fires. However, the physics of WUI fires, wildland fires, and urban fires remain similar. For solid combustibles, all progress through slow and fast decomposition stages to emit volatile, semi-volatile, and particulate combustibles; wildland, urban, and WUI fires can all experience a variety of different types of combustion conditions, and leave residual char and noncombustible materials. Once in the gas phase, combustible materials can further react in flaming combustion and/or in hot regions without flames that support chemical oxidation reactions producing soot precursors, PAHs, and carbon-containing particulates. Depending on the chemical composition of the materials, collectively and through various reactive extinction and quenching effects, these processes lead to emissions of VOCs, species containing halogens, nitrogen, sulfur, flame-retardant species, organometallics, metals and metal compounds, and carbon-containing particulates.
The spatial and temporal distribution of ignition, evolution, and propagation steps of all large-scale fires depends on the spatial distribution of combustible materials, meteorology, and interactions with fire-induced (i.e., buoyancy-induced) convection and terrain features. Ignition processes and fire spread result from local radiative and convective heat transfer, as well as the spot fires produced by the projection of firebrands (i.e., “glowing” combustion fragments that can drift through the air and cause flaming combustion when they encounter more fuel). Firebrands carried by turbulent fluid dynamics can ignite combustible materials, starting spot fires that are as much as 5 km or more ahead of the major propagation line (e.g., Koo et al., 2010; Maranghides et al., 2021). Predicting and mitigating spot fire ignition from firebrands remains a subject of continuing research (Manzello et al., 2020; Suzuki and Manzello, 2021). The overall heat release and interactions of urban fire emissions with those from surrounding vegetation (which include in-soil, ground level, mid-height, and canopy contributions) will influence the fire plume injection altitude and composition. The fire’s burning rate together with these plume injection parameters will affect the downstream plume aging and secondary pollutant generation (Atchley et al., 2021; Hallquist et al., 2009), as discussed in detail in Chapter 4.
This section describes the following:
- The nature of premixed and mixing-limited combustion behavior, which determines the temperatures, chemical kinetics, and heat release associated with fires fueled by condensed phase materials (biomass and human-made products) and liquid combustibles
- The chemical pathways and radical species that dominate combustion chemistry, and how these pathways are influenced by the presence of human-made materials, particularly halogen-, nitrogen-, and metal-containing species
- The factors impacting the interaction of the fire plume with surrounding ambient air, eventually leading to buoyant plume rise and longer characteristic reaction times, changing plume composition and plume injection characteristics.
Premixed and Mixing-Limited Combustion
Flaming combustion occurs under two distinctly different limiting regimes: (1) fuel and air fully mix prior to combustion, termed “premixed” combustion, and (2) fuel and air are initially separate, but react in a region where they simultaneously mix, termed “mixing-limited” or diffusively limited combustion (diffusion flames). Mixing-limited combustion is characteristic of burning solids and liquid fuel sprays that vaporize as they burn, but understanding premixed combustion is important for describing the gas-phase combustion behaviors involved in ignition processes and the “partially premixed” turbulent flame structures typical of fire plumes (Tieszen and Gritzo, 2008).
Mixing-limited flames occur in both wildland and WUI fires. An example is a stabilized, mixing-limited flame held in the air flow around a small-diameter tree branch, from which flammable fuel concentrations are produced as a result of radiative heating from the surrounding fire and surface reactions. Another example is a stabilized, open-air, turbulent, mixing-limited flame generated by the rapid mixing of flammable gases and aerosol exiting
from an enclosure fire, such as through a broken window of a burning structure. Premixed and partially premixed flames also occur in both wildland and WUI fires, at times in complex spatial relationships with mixing-limited flames, such as a burning, convecting cloud within a rising fire plume.
For both premixed and mixing-limited combustion, the chemistry will be impacted by the amount of oxidizing species. The fuel-to-oxidizer ratio of any unreacted fuel and oxygen can be referenced against that which is theoretically required to fully oxidize the fuel. The equivalence ratio, ϕ, is defined as
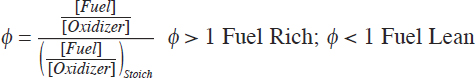
where the brackets denote molar concentration units. In any flame, the combustion heat release and therefore the maximum theoretical flame temperature correspond to stoichiometric conditions (i.e., ϕ = 1).
For premixed flames, the maximum theoretical flame temperature is defined by the premixed value of ϕ. Lean and rich flammability-limit values of ϕ exist that bracket the values for which premixed flames can exist. The lean limit for laminar premixed hydrocarbon flames is typically between 0.5 and 0.55 for air, but the rich limits vary substantially with the fuel’s chemical composition (Glassman et al., 2014). Chemical reactions can proceed outside these flammability limits, if the gas temperatures are sufficiently high, but no flames will be present. For mixing-limited flames, the mixing of fuel and air as burning occurs results in a flame structure with a range of equivalence ratios spanning from very rich values to very lean values. The peak mixing-limited flame temperature occurs where fuel and oxidizer meet in stoichiometric proportions, and the rate of overall reaction within the flame depends on the rate of fuel/oxidizer mixing.
Flame temperatures achieved in premixed and diffusion flames, in turn, have a complex impact on rates of combustion reactions and on species that result when a quenching or extinction of a flame structure occurs. In premixed flames, dilution of the oxidizer by nitrogen (air) and/or by the potential recirculation of combustion products into the flaming region reduce the peak flame temperature and affect flammability limits. Significant dilution or fluid dynamic interactions of premixed as well as diffusion flames lead to quenching/extinction, resulting in incomplete conversion to final combustion products.
A common belief is that overall chemical oxidation timescales for flammables containing hydrogen, carbon, and oxygen in the gas phase, whether aerosols or vapor, decrease exponentially with increasing temperature. Often this is not the case, especially for species with a large carbon number (carbon number > 4 for alkane structures; carbon number > 8 for aromatic structures). In these cases, the overall rate of oxidation is controlled by the complex interactions of numerous elementary reactions. These reactions each have unique chemical thermodynamic and elementary rate parameters, which in concert change the governing reaction pathways, the likely intermediate species, and the most impactful active radicals (e.g., see Curran, 2019; Kohse-Hoinghaus, 2021; Wang et al., 2019). As a result, the global oxidation reaction rate may increase or decrease with increasing temperature.
Typically, the characteristic reaction times at temperatures below ~500 K are too long to be relevant to the gas phase of the flame and near-field plume but may be important in terms of condensed phase processes (for example, spontaneous heating to ignition; Jones et al., 2015). The overall rate of oxidation increases with increasing temperature from the usual values found in the troposphere (~193 to ~298 K) to around 600 K. As temperature increases through this range, the overall reaction rate eventually “turns over” and decreases with increasing temperature. The decreasing trend continues with increasing temperature until the overall rate abruptly begins increasing with increasing reaction temperature once again. The range of temperatures over which the rate decreases with increasing temperature is termed the “negative-temperature-coefficient regime.”
The abrupt transition from the negative-temperature-coefficient regime behavior, frequently termed “hot ignition” in the kinetic literature, results from the rapid thermal decomposition of hydrogen peroxide (H2O2). The fast decomposition of this intermediate species, which is much more stable at lower temperatures, leads to the production of OH radicals and autothermal acceleration of the overall rate by the production of water from reactions with fuel species. With further increases in reaction temperature, the thermal decomposition of large hydrocarbon radicals becomes rapid, and eventually chemical chain branching (i.e., reactions for which the consumption of
a reactive radical results in the generation of more than one reactive radical) ensues. The turnover temperature, hot ignition temperature, and transition to chemical chain branching behavior are all determined by fuel species, oxygen concentration, and pressure.
Complex behaviors are driven by the range and spatial distributions of temperature found in all fires involving hydrocarbons or oxygenated hydrocarbons with large carbon numbers. The fundamental kinetic details for the oxidation of hydrocarbon and oxygenated hydrocarbon species at temperatures below the transition to chemical chain branching remain a very active area of combustion-related research (Ju, 2021; Ju et al., 2019; Wang et al., 2019). The significance of these kinetic behaviors in the various stages of smoldering combustion and in large-scale free-burning fires has not been explored. However, some of the large-carbon-number hydrocarbon and oxygenated species other than aromatics, known to be present, are likely to react within the fire environment and the plume at temperatures below 800–900 K but above the surrounding ambient temperatures by several hundred degrees Kelvin.
Chemical Pathways and Radical Species
The hydroxyl radical (OH) is a key active species in all oxidation processes occurring in low-, intermediate-, and high-temperature kinetic regimes. Two primary types of reactions involving OH and fuel species occur. Hydroxyl radicals can abstract an H atom directly from a C-H bond site, or OH can add to a molecule, forming an “adduct.” In both cases, the result eventually produces other products.
The abstraction reaction can be written generically as RiH + OH ⇒ Ri + H2O, where Ri represents a hydrocarbon or oxygenated hydrocarbon radical with a H atom removed from a C-H bond site. The radical Ri generally proceeds to react with molecular oxygen (i.e., Ri + O2 ⇒ RiO2); or if Ri is a radical with a large carbon number, it may thermally decompose to form additional radicals (Ri ⇒ Ri′, Ri″ + . . . + olefinic species; i.e., species containing carbon double bonds). Reactive radicals such as OH or HO2 will be regenerated only after a number of additional reactions involving further reactions of R, R′, R″, the olefinic species, and intermediates formed from them with oxygen. Many radical and molecular oxygen addition reactions are also possible.
The addition reactions of OH with molecular hydrogen and with carbon monoxide (CO) are unique in that each reaction results in a stable species and a highly reactive H atom (i.e., H2 + OH ⇒ H + H2O and CO + OH ⇒ HOCO ⇒ CO2 + H). The adduct HOCO is typically short lived at the reaction temperatures of interest in fires and hot fire plumes. The OH addition reactions represent a class of reactions referred to as “chain carrying” reactions, in which the consumption of a reactive radical leads to the formation of a product and another reactive radical. As noted earlier, both OH and H are highly reactive radicals.
Which type of reaction occurs depends on the reacting species and temperature. Especially at lower temperatures and for olefinic or aromatic species, the addition reaction RiH + OH ⇒ HORiH is favored. For ethylene and propene, species often found in wood’s oxidative pyrolysis products, the OH addition reaction is also favored over abstraction below ~900 K, but becomes disfavored above ~900 K; for acetylene, addition remains prevalent to temperatures approaching 1050 K (Khaled et al., 2019). Understanding these fundamentals of OH radical reactions is essential to understanding the effects of the relative rates of OH reaction with hydrocarbons and oxygenated hydrocarbons in comparison to OH reaction with CO and hydrogen, at intermediate and high temperatures.
An important characteristic of the homogeneous, gas-phase oxidation of a mixture of RiH species is that the CO that forms is oxidized to CO2 (>98 percent) almost entirely through only one reaction pathway, CO + OH ⇒ CO2 + H. The oxidation reactions of CO with molecular oxygen, oxygen atoms, or hydroperoxyl radicals (HO2) are all much, much slower than reaction with OH. In comparison to the overall rate constant, the rate constants for all hydrogen abstraction reactions by OH from C-H bonds are typically more than an order of magnitude larger (Atkinson, 2003; Han et al., 2018). This holds for all homogeneous, gas-phase oxidations of mixtures of RiH species found in wildland and WUI fires.
Phenomenologically, this disparity in rate constants for OH reactions with CO and RiH leads to a sequential, overall oxidative progress: (1) first hydrocarbons and/or oxygenated hydrocarbons concurrently convert to
species with smaller carbon numbers, such as formaldehyde (CH2O), methane (CH4), ethylene (C2H4), acetylene (C2H2), and eventually CO and H2O, and (2) the oxidation of CO to CO2 follows (Glassman et al., 2014), only after most of the hydrocarbon species have been substantially depleted. For example, the rate constant for CH2O (formaldehyde) + OH ⇒ HCO + H2O is larger than that for CH4 + OH ⇒ CH3 + H2O, and more than an order of magnitude larger than that for CO + OH. Thus, for mixtures of equal concentrations of CH2O or CH4 and CO, the oxidation of CO by OH will be strongly inhibited by the competitive reactions of OH with CH2O and CH4. Only after the hydrocarbon species are substantially depleted can CO successfully complete for OH radicals. This means that CO can be used as an indicator species for the overall extent of oxidation of hydrocarbons in plumes, as discussed later in this chapter. On the other hand, however, the addition reactions of OH with C2H4 and C2H2 do not compete favorably with CO + OH. In fact, for ethylene and acetylene, their co-presence with CO actually promotes CO oxidation (Yetter and Dryer, 1992).
In general, in wildland and WUI fires and their hot plumes, where intermediate and high temperatures are present, the gas-phase oxidation of CO to CO2 will be negligibly slow in mixtures of larger-carbon-number hydrocarbons, oxygenated hydrocarbons, and CO, as long as the following is true:
([CO] × kCO,OH) « Σj([RiH] × ki,OH)j
The brackets denote concentration units consistent with the units used in specifying the rate constants, k. The relationship described by the equation has implications in terms of the relative [RiH]/[CO] ratios found for all the species present, since the OH in the plume is typically the most reactive species with VOCs.
The Effect of Halogens
The introduction of human-made materials as fuels alters the radical concentrations and dominant pathways. Halogens, particularly Cl and Br, play significant roles (Hastie, 1973). The inhibitory effects of halogens, phosphorous, and antimony have inspired their use in formulating flame retardants (Gann and Gilman, 2003; Morgan and Gilman, 2013). Conversely, and as discussed in Chapter 4, chlorine radicals can accelerate the reactions of hydrocarbons under ambient tropospheric conditions (Tanaka, 2003). The gaseous emissions produced in fires with halogens present (whether from flame retardants or the oxidative decomposition of human-made materials containing halogens) have differing compositions and yields than the combustion emissions of materials without halogens (Senkan, 2000). The presence of Cl, which yields HCl (a stable product) during the oxidation process, enhances the rate of radical termination through a number of elementary reactions:
Cl + HO2 ⇒ HCl + O2
CO + Cl + M ⇔ COCl + M
COCl + Cl ⇒ CO + Cl2
Cl2 + H ⇒ HCl + Cl,
The net change produced by these reactions is
H + Cl ⇒ HCl.
Other Cl reactions that yield stable products are
HCl + OH ⇒ H2O +Cl
H + Cl + M ⇒ HCl +M.
And, at high flame temperatures, the reactions are
Cl + Cl + M ⇔ Cl2 + M
H + Cl + M ⇔ HCl + M.
In addition to quenching combustion reactions by enhancing radical termination, halogen chemistry can have a variety of other effects, including the stong inhibition of CO oxidation by CH3Cl, another species found in emissions from the combustion of plastics containing Cl.
The impacts of halogen chemistry can also vary between premixed and mixing-limited flames. In premixed flames (Babushok et al., 2014), chlorine-containing inhibitors scavenge radicals. Other halogens also enhance termination reactions, with the effectiveness of the inhibition cycles of all the halogens scaling in the order F < Cl < Br ≈ I, in both laminar premixed flames and perfectly stirred reactor conditions (Schefer and Brown, 1982).
For mixing-limited flames, the impacts are more complex. Halogens have different effects depending on whether the halogenated species is added to the fuel side or to the oxidant side of the mixing-limited flames. Simmons and Wolfhard (1956) noted in their early work on the inhibition of coaxial mixing-limited flames of propane and air by CH3Br that the inhibitor was much more effective when added to the fuel side rather than the oxidizer side of the flame. Later, Creitz (1961) investigated the inhibition of axial mixing-limited flames of six different fuels with air. Contrary to the work of Simmons and Wolfhard, when adding CH3Br and CF3Br to the fuel or oxidizer side, the volume percentage required to extinguish the flame was much greater when the species was added to the fuel side for all fuels studied, except carbon monoxide.
Pitz and Sawyer (1978) investigated the combustion of flammable vapors produced by polyethylene or PVC using an opposed-flow reactor, which combines the flammable vapors with oxidant under mixing-limited conditions. (PVC is essentially polyethylene with one hydrogen atom replaced by one Cl atom.) Pitz (1982) followed this initial work with more refined experiments using chlorinated polyethylene as the solid fuel and numerical modeling of the results. Similar to Simmons and Wolfhard, all the data suggested that halogen inhibits more strongly when added to the fuel side rather than the oxidizer-rich side of mixing-limited flames.
The disparate nature of the work of Pitz with that of Creitz can be to some extent resolved by studies reported by Linteris (1997) on the amount of acid gases generated near the extinction of propane flames in two coaxial diffusion configurations. A wide range of molecular inhibitor candidates containing halogens were compared, each having different chemical structure elemental ratios of carbon/hydrogen/halogen (Cl, Br, F). For the same inhibitor, the amounts of the inhibitor species required for flame extinction in the two configurations were different. In each configuration, the amount also differed whether the inhibitor was added to the fuel or oxidizer side of the flame. The amount of acid gases emitted at extinction was also different for each inhibitor and configuration.
For solid materials burning diffusively, the material C/H/halogen elemental composition is defined, and the flame structure will be one in which the halogen is present on the fuel-rich side. However, once acid gases and other species from the diffusive-limited combustion are produced, these species may be present on both the oxidizer and fuel sides in downstream, partially premixed, turbulent flames, for example, in the case of enclosure fire emissions burning in external, open-flame structures of a building.
The contrast of these studies indicates the complexity of considering the influence of halogens in mixing-limited combustion under different flame configurations, and with chlorinated species of different carbon numbers and halogen contents. In WUI fires, halogens will be present on the fuel side as a halogen-containing solid such as PVC is burned. On the other hand, the HCl and other chlorinated species produced as products may enter other partially premixed and mixing-limited flame structures mixed with air. Interactions of halogens in partially premixed turbulent flames, as are present in wildland and WUI fires, need further investigation. The unknown extent of halogen chemistry in WUI fire plumes limits the applicability to WUI fire plumes of empirical results relating emissions to CO concentrations that are based on wildland fire plumes.
The Effect of Nitrogen Species
Nitrogen species in flames can be produced at relatively low temperatures from the oxidation of organically bound nitrogen species such as nylon, and from the oxidation of plastics (Chaos et al., 2009). At temperatures from about 600 to 1150 K, the presence of even small amounts of NO or NO2 can provide a rapid conversion
of HO2, a much less reactive radical, to a reactive OH radical. This increase in OH production occurs through a collection of reactions involving very small concentrations of NO and NO2 present in an oxidizing mixture of RiH:
NO + HO2 ⇒ NO2 + OH
NO2 + H ⇒ NO + OH
H + O2 + M ⇒ HO2 + M.
In combination, these elementary reactions yield the overall reaction result
2 H + O2 ⇒ 2 OH,
thereby transforming the pseudo-termination species HO2 into reactive OH radicals. A substantial amount of published literature shows the enhancement of oxidation reactions of hydrocarbons and hydrocarbon oxygenates by the presence of NO and/or NO2 at temperatures from around 600 to 1050 K. Studies also consider other reactions involving NO2 as an oxidant of hydrocarbon radicals (Alam et al., 2017; Ano and Dryer, 1998; Glarborg et al., 2018; Marrodán et al., 2019). The role of CO2 and H2O on NO formation during char-bound N oxidation has also been noted in the literature (Karlström et al., 2020).
As in the case of halogens, interactions of nitrogen species in flames present in wildland, urban, and WUI fires need further investigation. The unknown extent of nitrogen chemistry limits the applicability to WUI fire plumes of empirical results relating emissions to CO concentrations that are based on wildland fire plumes. Finally, both halogenated and nitrogen-containing WUI plume species that evolve from the combustion of synthetic materials are important to initializing subsequent secondary aerosols and emissions generated in plume dispersion and transport (Cai and Griffin, 2006; Choi et al., 2020; Wang and Ruiz, 2017).
All of this discussion is based on chemistries that occur in the presence of oxygen and at relatively high temperatures. Pyrolysis chemistry and smoldering combustion will also be important in some fire environments, and the nature of these chemistries in WUI fires is expected to be different than pyrolysis and smoldering combustion chemistry in wildland and urban fires for many of the same reasons discussed here.
Finding: Both the mass of fuel and the elemental composition of human-made fuel materials are important in assessing the effects of halogens, nitrogen, and other elements that have potential to alter the chemistry and affect the composition of the species found in WUI fire plumes, both in the near field and as the plumes age. Temperatures experienced by these human-made materials during the combustion process will also influence the evolution of WUI fire chemistry by impacting the evolution of key radical species.
Finding: Comprehensive, detailed kinetic models are not available for assessing the individual or combined effects of halogen-, sulfur-, metal-, and nitrogen-containing species, found in biomass or human-made materials, on combustion emissions associated with wildland and WUI fires.
The relative importance of various pathways depends on temperature as well as concentrations of reactants, intermediate products, and products of the combustion chemistry. These conditions are not well understood for combustion at the WUI.
EMISSIONS
The chemistry of WUI plumes evolves in complex ways as temperatures change and as the chemical species in the plume encounter different radical populations. Defining emissions associated with a plume implicitly requires a definition of the point at which a chemically evolving plume is injected into the atmosphere as an emission. There are different ways to define emissions in different technical disciplines. For example, in the area of fire toxicity, the commonly used term “fire emissions” is replaced by the term “fire effluents,” which include all gases and
aerosols, including suspended particles, created by combustion or pyrolysis (ISO, 2017). In regulatory emissions reporting, the emissions sometimes are defined at the point of release (e.g., in an exhaust stack) and sometimes after some mixing with the ambient atmosphere (e.g., as for flares). There is not yet a precise and widely accepted definition of the term “emission” for WUI fires and, therefore, variable definitions are in use for when and how an emission is injected into a surrounding air mass.
Approaches used to characterize emissions utilize emission factors (EFs) and emission ratios (ERs). Different approaches are employed for wildland fires and enclosure fires, but most approaches use empirical relationships between EFs or ERs and some measure of combustion efficiency.
Wildland Fire Emissions
Andreae and Merlet (2001) summarize a framework of using EFs and the early history of developing methodologies to quantify the impact of biomass burning on air pollution (Akagi et al., 2011; Andreae, 2019; Urbanski, 2013). An EF is typically defined as the mass of a compound emitted per kilogram of dry fuel combusted. Numerous studies on emissions from wildland fires over the past several decades have resulted in a large database of EFs for a wide range of ecosystems and biomass species. Large databases of average EFs and their variations over a wide number of fires, derived from plume data, have been generated. Andreae (2019) recently expanded and updated prior efforts, to include additional data and analyses with a global context, and Prichard et al. (2020) have compiled EFs with a North American focus.
EFs are typically defined using the “carbon balance method” as
![]()
where EFi is the mass of compound i (in grams) emitted per kilogram of dry fuel burned, FC is the mass fraction of carbon in the fuel (typically assumed to be 0.45–0.5; Andreae, 2019), MWi is the molecular weight of compound i, and Ci/CT is the number of moles of compound i emitted, divided by the total number of moles of carbon emitted. The mass fraction of carbon in the fuel varies little for biomass, but in the case of human-made materials, the value may vary substantially. The value of Ci/CT is typically determined from an individual fire-averaged measurement, calculated from the averaged ERs using
![]()
where NCj is the number of carbon atoms in compound j, summed over all carbon-containing compounds, and ∆Ci/∆CCO2 is the fire-averaged ER of compound i to CO2:
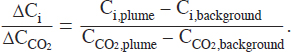
This “carbon balance method” is most accurate when all of the burnt carbon has been volatilized and quantified. Ignoring particulate carbon content and undetermined volatile species typically leads to at most a 10 percent underestimation of the total carbon, even for full-scale fire conditions (Yokelson et al., 1999). Enhanced ERs or “normalized excess ERs” correct for background concentrations and may sometimes use other compounds conserved in the plume other than CO2. The reference compound chosen is generally selected such that its background concentration in the atmosphere is low and exhibits minimal variation (for example, CO). In this case, the normalized excess ER, EnRi/CO, for compound i is defined as
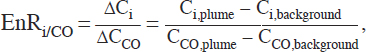
where ∆Ci is the background-subtracted concentration of species i, Ci,plume is the concentration of species i in the plume, and Ci,background is the concentration of species i in the background air; similarly, ∆CCO is the background-subtracted CO concentration, CCO,plume is the CO concentration in the plume, and CCO,background is the concentration of CO in the background air.
EFs will depend on the combustion characteristics under which the emissions are generated, and these conditions are frequently characterized by a combustion efficiency (CE) in the plume. The CE determined from plume measurements yields a reasonable estimate of the total carbon consumed in the burning process as well as the relative contributions of smoldering and flaming combustion to the composition of species injected into the plume. The CE is derived from
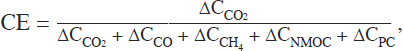
where the denominator represents all sources of carbon added to the atmosphere by the plume and the subscripts designate CO2, CO, CH4, non-methane organic carbon (NMOC), and particulate carbon (PC). The term ∆CNMOC and the term ∆CPC can sometimes be made using direct measurements, for example, methane and total non-methane organic carbon can be estimated using an instrument that is sensitive to total carbon content, although the accuracy of these types of measurements depends on the species being analyzed. Similarly, ∆CPC can be measured using instruments sensitive to total carbon in the particle phase. However, these measurements are often not available, and modified combustion efficiency (MCE) is frequently used as an approximation, ignoring all but CO and CO2 carbon emissions, as
![]()
Well-ventilated flaming (low smoldering contributions) produces a MCE near 0.99, while the MCE of smoldering itself may vary substantially (~0.65–0.85) but is typically found to be near 0.8. Thus, an overall, fire-integrated, time-averaged MCE near 0.9 might be expected to imply roughly equal contributions from flaming and smoldering. However, some of the emissions from smoldering are likely to be further processed in high-temperature, flaming regions, depending on if the smoldering was initial, concurrent with, or subsequent to the flaming of the wildland fire.
As described in the previous section, the oxidation of CO in WUI fires depends on the temperature history of the fire and the materials combusted in the fire. Historically, the CE and MCE have been used to indicate the contributions of smoldering and flaming combustion in wildfire plumes. CE and MCE might also indicate the contributions of smoldering and flaming combustion in WUI plumes; however, the precise relationship between smoldering and flaming combustion and CE/MCE is uncertain.
The correlation of enhanced ERs for species i as a function of MCE would perhaps allow the determination of a more robust description of the composition of species injected into the plume. MCE is also likely to be time dependent for large-scale fires, resulting in variability of the EFi values from the averaged value frequently reported over many different fires (Wiggins et al., 2021). Historically, “fresh” EFs from both laboratory and field studies for wildland fires compare favorably for some species with conditions generating the same MCE. Attempts to devolve the averaged measurements in a meaningful manner to obtain a time history of plume composition are complex and contribute to uncertainties in quantifying plume properties. Nonetheless, determining ERs and EFs from plume sampling, along with CE and MCE from plume measurements, has progressed immensely over the last decade.
Data from Recent Measurement Campaigns
Several intense field campaigns and emerging analyses of the resulting data show an improved ability to define the composition of wildland fire and WUI fire plumes. For example, the National Science Foundation and National Center for Atmospheric Research used a C-130 research aircraft during the 2018 Western Wildfire Experiment for
Cloud Chemistry, Aerosol Absorption, and Nitrogen (WE-CAN) field campaign (NCAR-UCAR, 2018) to take comprehensive smoke-characterization measurements; the aircraft traversed fire plumes and sampled and analyzed their compositions using a custom-built proton-transfer-reaction time-of-flight mass spectrometry (PTR-ToF-MS) instrument and other instrumentation (Permar et al., 2021).
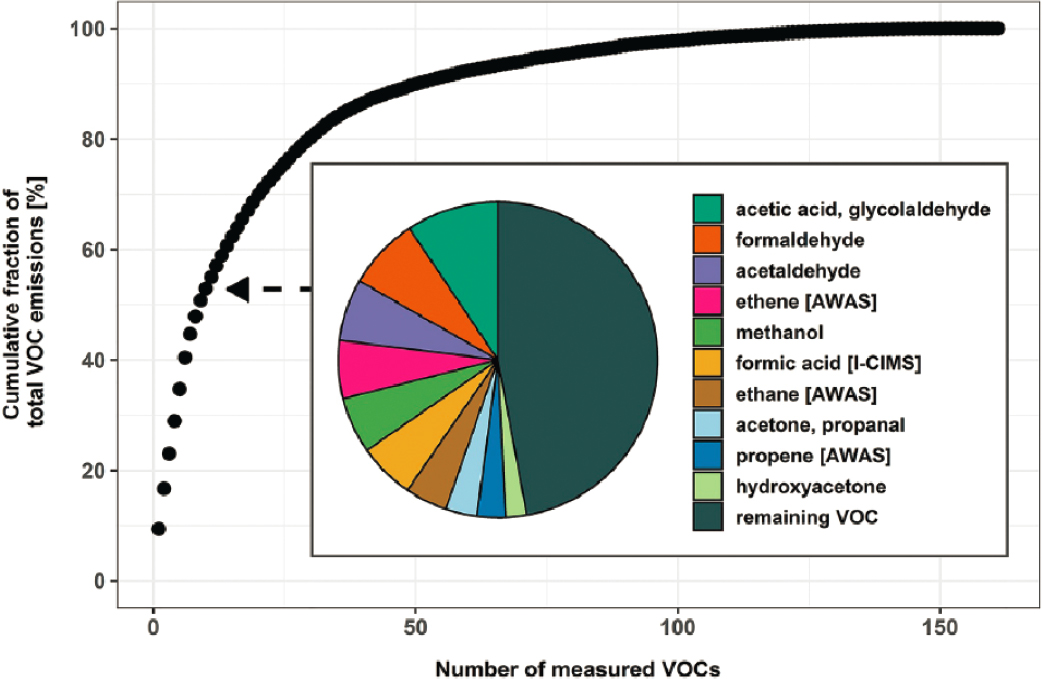
Researchers identified and quantified ERs and EFs for 161 VOCs in 31 near-plume flight transects of 24 wildland fires. As shown in Figure 3-2, 10 species accounted for about 53 percent of the measured mass.
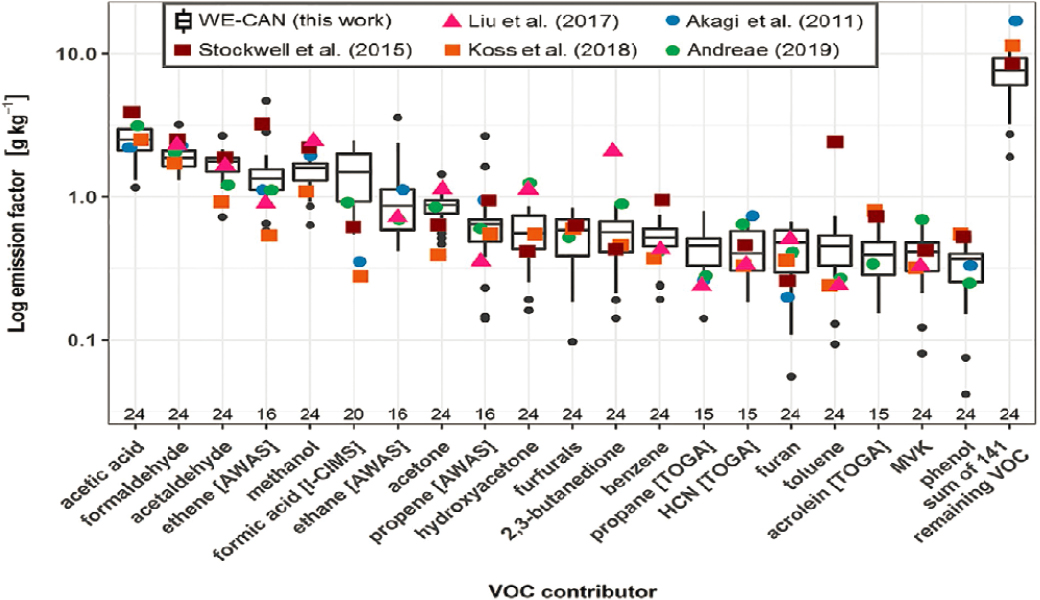
Figure 3-3 plots EFs (Permar et al., 2021) for the 20 most abundant emitted VOC species, by mass, as measured in a number of field experiments. Overall, the WE-CAN data campaign increased the number of species that have been identified in wildland fire plumes, found that the emissions of only 98 species accounted for 76 percent of the average total measured VOC mass, and found that the EFs had statistically significant and negative dependences on MCE. VOC mass fractions showed much less MCE dependence, with significant overlap within the observed MCE range, suggesting that a single speciation profile might be able to describe VOC emissions for the western US coniferous forest wildland fires sampled during WE-CAN.
Additional field studies, such as Fire Influence on Regional to Global Environments and Air Quality (FIREX-AQ; Sekimoto et al., 2018), have suggested alternatives to CE and MCE for characterizing combustion consitions (e.g., high- and low-temperature combustion), and have examined the contributions that fire emissions can make to regional photochemistry as the plumes evolve (see Chapter 4). These types of field programs undertaken for wildland fires demonstrate the types of advances that can be made in developing emissions estimates; however, data for WUI fires similar to the wildland fire data from WE-CAN and FIREX-AQ are
not available. EFs for WUI fires would be expected to contain contributions from halogenated compounds, nitrogen-containing compounds, and other species and would likely need to characterize not only combustion characteristics, but also the relative magnitudes and compositions of urban and wildland fuels consumed by the fires.
PM Emissions
Particulate matter (PM), consisting of species contained in a liquid or solid atmospheric aerosol, is one of the most important pollutants associated with fires. While PM accounts for only about 2 percent of the carbon emissions from wildland fires (Urbanski, 2014), its health impacts can be significant (see Chapter 6). Wildland fire PM is primarily composed of organic carbonaceous species, with minor contributions from elemental or “black” carbon (5–10 percent) and less than 5 percent from inorganic compounds (e.g., potassium, chlorine, sulfate), which depend upon the original concentration in the biomass (Jaffe et al., 2020).
Nearly 3,000 different compounds have been detected in PM emitted by fires, but only about half have been identified (Jen et al., 2019). Major classes of organic compounds included sugars, noncyclic aliphatics, organic nitrogen compounds, methoxyphenols, and substituted phenols. Researchers identified a small percentage of emissions from the burning of woody debris that, despite burning under very different conditions (MCE 0.98 vs 0.78), emitted similar compounds, demonstrating that fuel type can be a more important factor than combustion conditions in determining organic speciation (Jen et al., 2019). These organic compounds also span a range of volatilities, with many having the potential to partition between gas and particle phases, depending on the local concentration and temperature (May et al., 2013; Hatch et al., 2018). No comparable analyses of PM generated by WUI fires is available.
Urban Fire Emissions
Emission estimates for WUI fires must involve some parameterization of urban emissions that add to and potentially modify emissions estimates for wildland fires. Current understanding of urban emissions is based on laboratory experiments using configurations and conditions relevant to enclosure fires, (i.e., fires within buildings). The configurations emphasize the pyrolysis, oxidative pyrolysis, and boundary layer conditions that determine the intermediate reaction products (i.e., as the condensed phase reacts), which may be flammable gas-phase species, which can then ignite. In enclosure fires, where heating of the condensed phase is augmented by radiative/convective heat transfer from adjacent regions, the heating leads to the production of excess pyrolyzate in regions of oxidizer deficiency (i.e., incomplete combustion produces prolific amounts of flammable, gas-phase species). In larger enclosures, if ventilation air is abruptly supplied, the pyrolyzate can rapidly ignite and propagate gas-phase combustion via a combination of premixed and mixing-limited flames, referred to as a “flashover” event within the enclosure (Pitts, 1995; Stec and Hull, 2010). Researchers have developed several experimental configurations to characterize the evolution of pyrolyzate species relevant to enclosure fires, from early flaming to flashover.
Full-scale fire simulations reporting comprehensive EFs are limited for urban fires. RISE (Research Institutes of Sweden) has carried out a very limited number of large-scale urban fire simulations to develop emissions data for use in inventories of the fire life cycle (Amon et al., 2019). These studies provide some EFs for a mixture of materials. Table 3-8 provides the EFs of some of the most commonly emitted fire emissions, as measured in these studies.
The EFs in Table 3-8 vary widely, most by more than one order of magnitude. Some EFs vary from two to four orders of magnitude, with the most extreme variation observed in the polychlorinated dibenzo-p-dioxin (PCDD) and polychlorinated dibenzofuran (PCDF) EFs. This is because the full-scale experiments described here had a variety of materials and combustion conditions, with some tests where the ventilation was not controlled, and others in simulated rooms with ventilated, under-ventilated, and flashover conditions. Additionally, there may be even larger variability in the composition of structures, structure density, and combustion conditions both external and internal to the structures. Collectively, the variability in WUI fire emissions may be very large, and effective methods to parameterize emissions given these uncertainties are needed. Nonetheless these data show important distinctions in urban fire emissions from wildland fire emissions that should be evaluated for WUI fires.
Ventilation conditions and the chemical composition of the fuel likely play an important role in the formation of some pollutants like HCl, HF, SOx, and metals, as these species are emitted only if their precursors are present in the original material consumed in the fire. Additionally, persistent organic pollutants such as PCDD/PCDF are emitted in much greater amounts when Cl is present in the materials and the combustion conditions are favorable for dioxin formation (Gullett et al., 2000; Stec et al., 2013). As shown in fire toxicity testing, the combustion conditions greatly impact the emissions for many different pollutants, and this variation is not consistent across single-component materials.
Full-scale tests with multiple types of materials and their mixtures can provide emissions data and improve upon the methodologies. However, data are limited and smaller-scale apparatuses can be better at controlling the fire conditions, probing the impact of materials on emissions under controlled combustion conditions. The steady-state tube-furnace test (ISO, 2016) produces EFs per unit mass consumption of material that are relevant to the behavior of enclosure fires and immediate human exposure, as a function of the equivalence ratio. Figure 3-4 is a schematic of the ISO/TS 19700 experimental configuration (Stec and Hull, 2010).
As an engineering tool for quantitative assessment of toxic product yields (or EFs), any furnace temperature or ventilation condition can be selected. However, certain conditions relate to ISO fire stages (ISO, 2011), using specified furnace temperatures and primary air flows that approximate those of enclosure fires. A typical analysis may use experiments conducted at three furnace temperatures (650°C, 923 K; 750°C, 1023 K; and 850°C, 1123 K) and stoichiometric, fuel-lean, and fuel-rich equivalence ratios; if necessary, furnace temperatures are increased or decreased to obtain the specified condition.
Most bench-scale fire test methods were developed to assess flammability and burning behavior, and therefore use a well-ventilated fire scenario. However, EFs frequently increase by factors of 10 to 50 in the transition from well-ventilated to under-ventilated flaming. Test apparatuses that are unable to force combustion to occur in oxygen-depleted atmospheres are unsuited to the generation of EFs for enclosure fires. Currently, most existing
TABLE 3-8 EFs from Large-Scale Urban Fire Simulations
| Material | CO (g/kg) | NOx/NO (g/kg) | HCN (g/kg) | HCl (g/kg) | PM (g/kg) | C6H6 (g/kg) | CH2O (g/kg) | Pb (mg/kg) | Total PCDD (μg/kg) | Total PCDF (μg/kg) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tires (heap or pile) | 48.8–58.9 | 0.41–0.42 | 16–149 | 0.009–0.01 | 0.013–0.07 | Lönnermark and Blomqvist, 2005 | |||||
| Scrap tire material | 114 | 2.2 | 0.47 | Lemieux and Ryan, 1993 | |||||||
| Vehicle (medium-class 1998 car) | 63 | 1.6 | 13 | 64 | 3.0 | 1.1 | 820 | 2.1 | 56 | Lönnermark and Blomqvist, 2006 | |
| Electrical wiring | 2.3–22.5 | 48–53 | 0.06–1.47 | 0.1–0.6 | 0.02–2.28 | 0.19–60.94 | Andersson et al., 2004, 2005; Simonson et al., 2001 | ||||
| Simulated room contents | 32–48 | 1.3–2.2 | 0.5–1.3 | 0.17–1.09 | 0.04–0.20 | 0.36–1.37 | Blomqvist et al., 2004 | ||||
| Sofa with flame retardant in room | 24–41 | 0.9–4.5 | 0.9–1.4 | 0.10–1.34 | 0.5 | 2.6–25 | 0.003–1.02 | 0.2–13.3 | Andersson et al., 2003 | ||
| Simulated room with sofa | 14–51 | 3.5–15 | 6–18 | 45–282 | Gann et al., 2010 | ||||||
| Chemicals in storage room | 8–293 | 0.1–36.3 | 0.8–44.7 | 77.8–141 | 7–76 | 0.04–7.1 | Månsson et al., 1996 |
bench-scale test methods do not represent a particular fire stage; some represent several fire stages separately, while others represent the progress of a fire through an indeterminate number of stages. Furthermore, most test methods produce data that are a function of both the flammability of the specimen and the yield of toxic products. Other methods provide toxic product yield data that are independent of the flammability. Table 3-9 summarizes the attributes of various bench-scale apparatuses. A recent study by RISE evaluated the suitability of four of these five test methods for quantifying the smoke toxicity of polymethylmethacrylate and 11 building insulation products under non-flaming, well-ventilated, and under-ventilated conditions (Blomqvist and Sandinge, 2018, 2021). The study concluded that significant differences in results when testing the same products could be attributed to the differences in the test methods.
Despite this variability, these test methods allow the collection of data that examine the dependence of EFs for commonly used materials, and mixtures of commonly used materials, on combustion conditions. These data can serve as a starting point for more extensive examination of WUI fire emissions under realistic combustion conditions.
Fire Retardants
ISO defines a fire retardant as a substance added, or a treatment applied, to a material in order to delay ignition or to reduce the rate of combustion (ISO, 2017). Fire retardants, which act in the gas phase and interfere with flame reactions, are frequently applied to insulation foams, electrical equipment, and upholstered furniture. The presence or absence of specific flame retardants can dramatically change the emissions associated with a particular material or structure.
A fire retardant should inhibit or even completely suppress the combustion process. Depending on their nature, fire retardants can act chemically and/or physically in the solid, liquid, or gas phase. They interfere with combustion during a particular stage of the combustion process, for example, during heating, decomposition, ignition, or flame spread (Purser, 2009). Several ways exist in which the combustion process can be retarded. It can be done by physical actions (cooling, formation of a protective layer, dilution) or chemical actions, in the condensed or gas phase, as shown in Figure 3-5.
Modern fire retardants prevent fuel molecules from escaping to the gas phase, often by formation of a protective barrier layer, and by inhibiting the flow of fuel and oxygen. This makes the fuel molecules less likely to encounter conditions that could ignite them. While the formulations required for char promotion and intumescence are generally specific to a particular polymer, halogenated flame retardants tend to be nonspecific in their action, and one flame retardant can be incorporated into many polymers. Unfortunately, this ease of use is matched by
TABLE 3-9 Attributes of Five Bench-Scale Testing Apparatuses
| Name | The Smoke Density Chamber (ISO 5659) | Controlled Atmosphere Cone Calorimeter | The Non-dynamic Tube Furnace | Steady State Tube Furnace (BS7990 & ISO/TS 19700) | Fire Propagation Apparatus (ISO 12136 & ASTM E-2058) |
|---|---|---|---|---|---|
| Heat regimes | 25, 50 kW/m2 | 25, 35, 50 kW/m2 | 400, 600, 800°C | 350, 650, 825°C (15 to 80 kW/m2) | Up to 100 kW/m2 |
| Design | Closed box | Flow-through | Flow-through | Flow-through | Flow-through |
| Mass loss rate | Variable, sample dependent | Variable, sample dependent | Variable, sample dependent | Fixed | Variable, sample dependent |
| Sample requirement | 75 × 75 mm2 results only valid for thickness tested | 100 × 100 mm2 results only valid for thickness tested | 0.1 to 1.0 g | 800 × 25 × 25 mm3 (maximum size) | 100 × 100 mm2 results only valid for thickness tested |
| Smoldering | Y | Y | Y | Y | Y |
| Well-ventilated | Y | Y | Y | Y | Y |
| Under-ventilated | N | N | ? | Y | Y |
| Post-flashover | N | N | ? | Y | Y |
| Advantages | Widely available; currently used for toxicity assessment in mass transport industries | Modification to widely used standard test; measures flammability as well as toxicity | Simple and low cost | Designed for smoke toxicity assessment; ideal for linear or homogeneous products; correlates with large-scale tests for all conditions | Able to replicate high CO yields for under-ventilated flaming |
| Disadvantages | Poor interlaboratory reproducibility; unable to force under-ventilated flaming; fire stage unknown; sample probe can miss toxic plume | No standard equipment exists; unable to go above 50 kW/m2; toxic product yields do not correlate to large-scale fire tests; equivalence ratio only known after test | Conditions not related to fire stages; does not distinguish between flaming and non-flaming; limited volume of effluent for analysis | Interlaboratory reproducibility not proven for layered materials; separate assessment of flammability required | Expensive; not widely used; capabilities insufficiently demonstrated |
SOURCE: Stec and Hull, 2010. ASTM = American Society for Testing and Materials.
an ease of release, particularly when operating at elevated temperatures (Hull et al., 2014). Many halogenated flame retardants are persistent, bioaccumulative, and ubiquitous throughout the built and natural environment (Hull et al., 2014).
Halogenated flame retardants, which act in the gas phase, interfere with the free-radical reactions responsible for flaming combustion, as discussed in the earlier section on combustion (and summarized in Babushok et al., 2014). In terms of fire emissions, the presence of hydrogen bromide (HBr) or hydrogen chloride (HCl), produced by flame-retardant combustion, typically produces more carbon monoxide, smoke, and other products of incomplete combustion (e.g., acrolein and formaldehyde), as well as larger cyclic molecules such as PAHs and soot particulates (Molyneux et al., 2014; Schnipper et al., 1995).
Many other types of fire retardants present in condensed media reduce fuel release to the gas phase, frequently by increasing the formation of the surface char layer, decreasing both net radiative heat transfer to the surface and conductive heat transfer into the media. The char may inhibit diffusion of oxygen to the surface and fuel release from it, depending on its porosity. Such barriers have also been deployed in intumescent systems, where gas is released within the molten polymer, causing significant swelling and so increasing the effectiveness of the thermal and physical barrier. Mineral filler flame retardants such as aluminum hydroxide or magnesium hydroxide, char formers based on inorganic phosphates and borates, and intumescents all fall into this category. By keeping the fuel in the condensed phase, they reduce gaseous emissions and simultaneously lower the flammability.
Finding: Limited data exist on emission factors and the sources of uncertainty for WUI fires; no studies of single- or multiple-dwelling, full-scale structures, with realistic contents and realistic WUI fire conditions (e.g., strong winds), have reported emission factors or yields.
Finding: WUI fire emission factors are expected to be different from emission factors for wildland fires and urban fires and to vary widely, depending on structure density and composition, combustion conditions, and other factors.
Finding: Current experimental methods do not capture the dynamic nature of WUI fires; large information gaps lead to large uncertainties in predicting plume composition and structure. Nevertheless, bench-scale reference methods that produce results such as toxic product yields or emission factors are effective tools for applied engineering analyses, material selection, and toxicological evaluations. These methods also allow investigators to control many of the parameters affecting the combustion efficiency and emission factors, often allowing a wider exploration of parameters than possible with enclosure fire tests. These bench-scale analyses could be complemented by field measurements of WUI fire plume composition and structure.
Finding: Halogenated species found in human-made materials and in gas-phase flame retardants change the yields and the types of a wide variety of products of incomplete combustion.
Research need: The interactions between human-made fuels with different elemental compositions (e.g., containing halogens, phosphorous, sulfur, nitrogen, metal salts, and organometallics) and biomass (particularly timber) and other wildland fire fuels as they generate fire plumes need to be modeled mechanistically and explored experimentally at bench, laboratory, and larger scales.
Research need: Experimental and modeling investigations of WUI fire plume compositions should be used to develop emission factors and chemical fingerprints for WUI fire emissions.
REFERENCES
Adamkiewicz, G., A. R. Zota, and M. P. Fabian. 2011. “Moving Environmental Justice Indoors: Understanding Structural Influences on Residential Exposure Patterns in Low-Income Communities.” AJPH. https://doi.org/10.2105/AJPH.2011.300119.
Akagi, S. K., R. J. Yokelson, C. Wiedinmyer, M. J. Alvarado, J. S. Reid, T. Karl, J. D. Crounse, and P. O. Wennberg. 2011. “Emission Factors for Open and Domestic Biomass Burning for Use in Atmospheric Models.” Atmospheric Chemistry and Physics 11 (9): 4039–4072. https://doi.org/10.5194/acp-11-4039-2011.
Alam, F. E., F. M. Haas, T. I. Farouk, and F. L. Dryer. 2017. “Influence of Trace Nitrogen Oxides on Natural Gas Oxidation: Flow Reactor Measurements and Kinetic Modeling.” Energy Fuels 31. https://doi.org/10.1021/acs.energyfuels.6b02369.
Amon, F., J. Gehandler, R. McNamee, M. McNamee, and A. Vilic. 2019. Measuring the Impact of Fire on the Environment. Fire Impact Tool, version 1. Gothenburg, Sweden: Research Institutes of Sweden.
Andersson, P., M. Simonson, and L. Rosell. 2003. Fire-LCA Model: Furniture Study. SP Report 2003:22. Gothenburg, Sweden: Research Institutes of Sweden.
Andersson, P., L. Rosell, and M. Simonson. 2004. “Small and Large Scale Fire Experiments with Electric Cables under Well-Ventilated and Vitiated Conditions.” Fire Technology 40 (3). https://doi.org/10.1023/B:FIRE.0000026879.07753.86.
Andersson, P., M. Simonson, L. Rosell, P. Blomqvist, and H. Stripple. 2005. Fire-LCA Model: Cable Case Study II – NHXMH and NHMH Cable. Gothenburg, Sweden: Research Institutes of Sweden.
Andreae, M. O. 2019. “Emission of Trace Gases and Aerosols from Biomass Burning – An Updated Assessment.” Atmospheric Chemistry and Physics 19 (13): 8523–8546. https://doi.org/10.5194/acp-19-8523-2019.
Andreae, M. O., and P. Merlet. 2001. “Emission of Trace Gases and Aerosols from Biomass Burning.” Global Biogeochemical Cycles 15 (4): 955–966. https://doi.org/10.1029/2000gb001382.
Ano, T. A., and F. L. Dryer. 1998. “Effect of Dimethyl Ether, NOx, and Ethane on CH4 Oxidation: High Pressure, Intermediate-Temperature Experiments and Modeling.” Symposium (International) on Combustion 27 (1). https://doi.org/10.1016/S0082-0784(98)80428-1.
Atchley, A. L., R. Linn, and A. Jonko. 2021. “Effects of Fuel Spatial Distribution on Wildland Fire Behaviour.” International Journal of Wildland Fire 30 (3). https://doi.org/10.1071/WF20096.
Atkinson, R. 2003. “Kinetics of the Gas-Phase Reactions of OH Radicals with Alkanes and Cycloalkanes.” Atmospheric Chemistry and Physics 3: 2233–2307. https://doi.org/10.5194/acp-3-2233-2003.
Babushok, V. I., G. T. Linteris, and O. C. Meier. 2014. “Flame Inhibition by CF3CHCl2 (HCFC-123).” Combustion Science and Technology 186 (6). https://doi.org/10.1080/00102202.2013.878709.
Bain, R. L., W. A. Amos, M. Downing, and R. L. Perlack. 2003. Biopower Technical Assessment: State of the Industry and Technology. Golden, CO: National Renewable Energy Laboratory. https://www.nrel.gov/docs/fy03osti/33123.pdf.
Blomqvist, P., and A. Lönnermark. 2001. “Characterization of the Combustion Products in Large-Scale Fire Tests: Comparison of Three Experimental Configurations.” Fire and Materials 25. https://doi.org/10.1006/fam.761.
Blomqvist, P., and M. S. McNamee. 2009. Estimation of CO2-Emissions from Fires in Dwellings, Schools and Cars in the Nordic Countries. SP Technical Note 2009:13. Borås, Sweden: SP Technical Research Institute of Sweden.
Blomqvist, P., and A. Sandinge. 2018. Experimental Evaluation of Fire Toxicity Test Methods. Gothenburg, Sweden: Research Institutes of Sweden.
Blomqvist, P., and A. Sandinge. 2021. “An Experimental Evaluation of the Equivalence Ratios in Tests Apparatus Used for Fire Effluent Toxicity Studies.” Fire and Materials 45 (8): 1085–1095. https://doi.org/10.1002/fam.2995.
Blomqvist, P., L. Rosell, and M. Simonson. 2004. “Emissions from Fires Part II: Simulated Room Fires.” Fire Technology 40: 59–73.
Blomqvist, P., M. S. McNamee, A. A. Stec, D. Gylestam, and D. Karlsson. 2013. “Detailed Study of Distribution Patterns of Polycyclic Aromatic Hydrocarbons and Isocyanates under Different Fire Conditions.” Fire and Materials 38 (1): 125–144. https://doi.org/10.1002/fam.2173.
Bodí, M. B., D. A. Martin, V. N. Balfour, C. Santín, S. H. Doerr, P. Pereira, A. Cerdà, and J. Mataix-Solera. 2014. “Wildland Fire Ash: Production, Composition and Eco-hydro-geomorphic Effects.” Earth-Science Reviews 130: 103–127. https://doi.org/10.1016/j.earscirev.2013.12.007.
Boulder OEM (Office of Emergency Management). 2022. “Boulder County Releases Updated List of Structures Damaged and Destroyed in the Marshall Fire.” https://www.bouldercounty.org/news/boulder-county-releases-updated-list-of-structures-damaged-and-destroyed-in-the-marshall-fire/.
Bwalya, A., G. Lougheed, A. Kashef, and H. Saber. 2010. “Survey Results of Combustible Contents and Floor Areas in Canadian Multi-Family Dwellings.” Fire Technology 47 (4): 1121–1140. https://doi.org/10.1007/s10694-009-0130-8.
Cai, H., X. Wang, and J. Kelly. 2021. Building Life-Cycle Analysis with the GREET Building Module: Methodology, Data, and Case Studies. Lemont, IL: Argonne National Laboratory.
Cai, X., and R. J. Griffin. 2006. “Secondary Aerosol Formation from the Oxidation of Biogenic Hydrocarbons by Chlorine Atoms. Journal of Geophysical Research: Atmospheres 111. https://doi.org/10.1029/2005JD006857.
CAL FIRE (California Department of Forestry and Fire Protection). n.d. Wildfire is Coming. Are You . . . Ready? https://www.readyforwildfire.org/wp-content/uploads/calfire_ready_brochure_LINOweb.pdf (accessed April 1, 2022).
CAL FIRE. 2022. Stats and Events. https://www.fire.ca.gov/stats-events/.
CalRecycle. 2020. Urban Wood Waste. https://www.calrecycle.ca.gov/ConDemo/Wood/.
Campbell, J., D. Donato, D. Azuma, and B. Law. 2007. “Pyrogenic Carbon Emission from a Large Wildfire in Oregon, United States.” Journal of Geophysical Research: Biogeosciences 112 (G4). https://doi.org/10.1029/2007jg000451.
CDLA (Colorado Department of Local Affairs). 2016. “Wildland-Urban Interface Code (WUI Code).” In Planning for Hazards: Land Use Solutions for Colorado. https://planningforhazards.com/wildland-urban-interface-code-wui-code (accessed March 28, 2022).
Chaos, M., M. P. Burke, Y. Ju, and F. L. Dryer. 2009. “Syngas Chemical Kinetics and Reaction Mechanisms.” In Synthesis Gas Combustion: Fundamentals and Applications Chapter 2. Edited by Tim C. Lieuwen, Vigor Yang, and Richard Yetter. Boca Raton, FL: CRC Press.
Choi, M. S., X. Qiu, and J. Zhang. 2020. “Study of Secondary Organic Aerosol Formation from Chlorine Radical-Initiated Oxidation of Volatile Organic Compounds in a Polluted Atmosphere Using a 3D Chemical Transport Model.” Environmental Science & Technology 54 (21). https://doi.org/10.1021/acs.est.0c02958.
Coggon, M. M., C. Y. Lim, A. R. Koss, K. Sekimoto, B. Yuan, J. B. Gilman, D. H. Hagan, V. Selimovic, K. J. Zarzana, S. S. Brown, J. M. Roberts, M. Müller, R. Yokelson, A. Wisthaler, J. E. Krechmer, J. L. Jimenez, C. Cappa, J. H. Kroll, J. de Gouw, and C. Warneke. 2019. “OH Chemistry of Non-methane Organic Gases (NMOGs) Emitted from Laboratory and Ambient Biomass Burning Smoke: Evaluating the Influence of Furans and Oxygenated Aromatics on Ozone and Secondary NMOG Formation.” Atmospheric Chemistry and Physics 19 (23): 14875–14899. https://doi.org/10.5194/acp-19-14875-2019.
Colantoni, A., G. Egidi, G. Quaranta, R. D’Alessandro, S. Vinci, R. Turco, and L. Salvati. 2020. “Sustainable Land Management, Wildfire Risk and the Role of Grazing in Mediterranean Urban-Rural Interfaces: A Regional Approach from Greece.” Land 9 (1): 21. https://doi.org/10.3390/land9010021.
Cooper, E. M., G. Kroeger, K. Davis, C. R. Clark, P. L. Ferguson, and H. M. Stapleton. 2016. “Results from Screening Polyurethane Foam Based Consumer Products for Flame Retardant Chemicals: Assessing Impacts on the Change in the Furniture Flammability Standards.” Environmental Science & Technology 50 (19): 10653–10660. https://doi.org/10.1021/acs.est.6b01602.
Creitz, E. C. 1961. “Inhibition of Diffusion Flames by Methyl Bromide and Trifluoromethyl Bromide Applied to the Fuel and Oxygen Sides of the Reaction Zone.” Journal of Research of the National Bureau of Standards A. Physics and Chemistry 65A (4): 389–400. https://nvlpubs.nist.gov/nistpubs/jres/65A/jresv65An4p389_A1b.pdf.
Curran, H. J. 2019. “Developing Detailed Chemical Kinetic Mechanisms for Fuel Combustion.” Proceedings of the Combustion Institute 37 (1). https://doi.org/10.1016/j.proci.2018.06.054.
Davies, K. W., C. S. Boyd, J. D. Bates, and A. Hulet. 2015. “Winter Grazing Can Reduce Wildfire Size, Intensity and Behaviour in a Shrub-Grassland.” International Journal of Wildland Fire 25: 191–199. https://doi.org/10.1071/WF15055.
Davis, S. C., and R. G. Boundy. 2021. Transportation Energy Data Book. ORNL/TM-2020/1770 (Edition 39 of ORNL-5198). Oak Ridge, TN: Oak Ridge National Laboratory. https://tedb.ornl.gov/wp-content/uploads/2021/02/TEDB_Ed_39.pdf.
Dionisio, K. L., A. M. Frame, M. R. Goldsmith, J. F. Wambaugh, A. Liddell, T. Cathey, D. Smith, J. Vail, A. S. Ernstoff, P. Fantke, O. Jolliet, and R. S. Judson. 2015. “Exploring Consumer Exposure Pathways and Patterns of Use for Chemicals in the Environment.” Toxicology Reports 2: 228–237. https://doi.org/10.1016/j.toxrep.2014.12.009.
Dixit, M. K., J. L. Fernandez-Solis, S. Lavy, and C. H. Culp. 2012. “Need for an Embodied Energy Measurement Protocol for Buildings: A Review Paper.” Renewable and Sustainable Energy Reviews 16 (6). https://doi.org/10.1016/j.rser.2012.03.021.
Elgowainy, A., J. Han, and J. Ward. 2016. Cradle-to-Grave Lifecycle Analysis of US Light-Duty Vehicle-Fuel Pathways: A Greenhouse Gas Emissions and Economic Assessment of Current (2015) and Future (2025-2030) Technologies. Oak Ridge, TN: US Department of Energy, Office of Scientific and Technical Information. https://doi.org/10.2172/1254857.
Elhami-Khorasani, N., J. G. Salado Castillo, E. Saula, T. Josephs, G. Nurlybekova, and T. Gernay. 2020. “Application of a Digitized Fuel Load Surveying Methodology to Office Buildings.” Fire Technology 57 (1): 101–122. https://doi.org/10.1007/s10694-020-00990-2.
Emrath, P. October 7, 2010. “Characteristics of Single-Family Homes Started in 2009.” https://www.nahbclassic.org/generic.aspx?genericContentID=145984 (accessed February 8, 2022).
EPA (US Environmental Protection Agency). 2016. Analysis of the Lifecycle Impacts and Potential for Avoided Impacts Associated with Single Family Homes. EPA Report 530-R13-004. https://www.epa.gov/smm/analysis-lifecycle-impacts-and-potential-avoided-impacts-associated-single-family-homes (accessed February 16, 2022).
Fent, K. W., and D. E. Evans. 2011. “Assessing the Risk to Firefighters from Chemical Vapors and Gases during Vehicle Fire Suppression.” Journal of Environmental Monitoring 13 (3): 536–543. https://doi.org/10.1039/c0em00591f.
Forest Products Laboratory. 2010. Wood Handbook—Wood as an Engineering Material. General Technical Report FPL-GTR-190. Madison, WI: US Department of Agriculture, Forest Service.
Gann, R. G., and J. W. Gilman. 2003. “Flame Retardants: An Overview.” Kirk-Othmer Encyclopedia of Chemical Technology. Hoboken, NJ: John Wiley & Sons Inc. https://doi.org/10.1002/0471238961.1522051807011414.a01.pub2.
Gann, R. G., J. D. Averill, E. L. Johnsson, M. R. Nyden, and R. D. Peacock. 2010. “Fire Effluent Component Yields from Room-Scale Fire Tests.” Fire and Materials 34 (6): 285–314. https://doi.org/10.1002/fam.1024.
Ghattas, R., J. Gregory, M. Noori, T. R. Miller, E. Olivetti, and S. Greene. 2016. Life Cycle Assessment for Residential Buildings: A Literature Review and Gap Analysis, Rev. 1. Cambridge, MA: Massachusetts Institute of Technology Concrete Sustainability Hub. http://hdl.handle.net/1721.1/104794.
Glarborg, P., J. A. Miller, B. Ruscic, and S. J. Klippenstein. 2018. “Modeling Nitrogen Chemistry in Combustion.” Progress in Energy and Combustion Science 67: 31–68. https://doi.org/10.1016/j.pecs.2018.01.002.
Glassman, I., R. A. Yetter, and N. G. Glumac. 2014. Combustion 5th edition. New York, NY: Elsevier.
Gullett, B. K., A. F. Sarofim, K. A. Smith, and C. Procaccini. 2000. “The Role of Chlorine in Dioxin Formation.” Process Safety and Environmental Protection 78 (1): 47–52. https://doi.org/10.1205/095758200530448.
Hallquist, M., J. C. Wenger, and U. Baltensperger. 2009. “The Formation, Properties and Impact of Secondary Organic Aerosol: Current and Emerging Issues.” Atmospheric Chemistry and Physics 9 (14): 5155–5236. https://doi.org/10.5194/acp-9-5155-2009.
Han, L., F. Siekmann, and C. Zetzsch. 2018. “Rate Constants for the Reaction of OH Radicals with Hydrocarbons in a Smog Chamber at Low Atmospheric Temperatures.” Atmosphere 9 (8). https://doi.org/10.3390/atmos9080320.
Hastie, J. W. 1973. “Molecular Basis of Flame Inhibition.” Journal of Research of the National Bureau of Standards A. Physics and Chemistry 77A (6): 733–754. https://doi.org/10.6028/jres.077A.045.
Hatch, L. E., A. Rivas-Ubach, C. N. Jen, M. Lipton, A. H. Goldstein, and K. C. Barsanti. 2018. “Measurements of I/SVOCs in Biomass-Burning Smoke Using Solid-Phase Extraction Disks and Two-Dimensional Gas Chromatography.” Atmospheric Chemistry and Physics 18 (24): 17801–17817. https://doi.org/10.5194/acp-18-17801-2018.
Hessburg, P. F., C. L. Miller, S. A. Parks, N. A. Povak, A. H. Taylor, P. E. Higuera, S. J. Prichard, M. P. North, B. M. Collins, M. D. Hurteau, A. J. Larson, C. D. Allen, S. L. Stephens, H. Rivera-Huerta, C. S. Stevens-Rumann, L. D. Daniels, Z. e. Gedalof, R. W. Gray, V. R. Kane, D. J. Churchill, R. K. Hagmann, T. A. Spies, C. A. Cansler, R. T. Belote, T. T. Veblen, M. A. Battaglia, C. Hoffman, C. N. Skinner, H. D. Safford, and R. B. Salter. 2019. “Climate, Environment, and Disturbance History Govern Resilience of Western North American Forests.” Frontiers in Ecology and Evolution 7. https://doi.org/10.3389/fevo.2019.00239.
Hull, T. R., R. J. Law, and A. Bergman. 2014. “Environmental Drivers for Replacement of Halogenated Flame Retardants.” In Polymer Green Flame Retardants Chapter 4. Edited by C. D. Papaspyrides and P. Kiliaris. New York, NY: Elsevier. https://doi.org/10.1016/B978-0-444-53808-6.00004-4.
IFSTA (International Fire Service Training Association). 2016. “Interior Finishes and Passive Fire Protection.” In Building Construction Related to the Fire Service 4th edition, Chapter 5. https://www.ifsta.org/sites/default/files/Chapter%205_BC_4th_edition.pdf (accessed April 1, 2022).
ISO (International Organization for Standardization). 2011. Guidelines for Assessing the Fire Threat to People. ISO 19706:2011. Edition 2. https://www.iso.org/standard/56864.html.
ISO. 2016. Controlled Equivalence Ratio Method for the Determination of Hazardous Components of Fire Effluents—Steady-State Tube Furnace. ISO/TS 19700:2016. Edition 2. https://www.iso.org/standard/70630.html.
ISO. 2017. Fire Safety—Vocabulary. ISO 13943:2017. Edition 3. https://www.iso.org/standard/63321.html.
Jacobs, D. E. 2011. “Environmental Health Disparities in Housing.” American Journal of Public Health 101 (S1): S115–S122. https://doi.org/10.2105/AJPH.2010.300058.
Jaffe, D. A., S. M. O’Neill, N. K. Larkin, A. L. Holder, D. L. Peterson, J. E. Halofsky, and A. G. Rappold. 2020. “Wildfire and Prescribed Burning Impacts on Air Quality in the United States.” Journal of the Air & Waste Management Association 70 (6): 583–615. https://doi.org/10.1080/10962247.2020.1749731.
Jen, C. N., L. E. Hatch, V. Selimovic, R. J. Yokelson, R. Weber, A. E. Fernandez, N. M. Kreisberg, K. C. Barsanti, and A. H. Goldstein. 2019. “Speciated and Total Emission Factors of Particulate Organics from Burning Western US Wildland Fuels and Their Dependence on Combustion Efficiency.” Atmospheric Chemistry and Physics 19 (2): 1013–1026. https://doi.org/10.5194/acp-19-1013-2019.
Jones, J. M., A. Saddawi, B. Dooley, E. J. S. Mitchell, J. Werner, D. J. Waldron, S. Weatherstone, and A. Williams. 2015. “Low Temperature Ignition of Biomass.” Fuel Processing Technology 134: 372–377. https://doi.org/10.1016/j.fuproc.2015.02.019.
Ju, Y. 2021. “Understanding Cool Flames and Warm Flames.” Proceedings of the Combustion Institute 38 (1): 83–119. https://doi.org/10.1016/j.proci.2020.09.019.
Ju, Y., C. B. Reuter, O. R. Yehia, and T. I. Farouk. 2019. “Dynamics of Cool Flames.” Progress in Energy and Combustion Science 75. https://doi.org/10.1016/j.pecs.2019.100787.
Karlström, O., D. Schmid, M. Hupa, and A. Brink. 2020. “Role of CO2 and H2O on NO Formation during Biomass Char Oxidation.” Energy & Fuels 35 (9): 7058–7064. https://doi.org/10.1021/acs.energyfuels.0c03471.
Keeley, J. E., and A. D. Syphard. 2019. “Twenty-First Century California, USA, Wildfires: Fuel-Dominated vs. Wind-Dominated Fires.” Fire Ecology 15 (1). https://doi.org/10.1186/s42408-019-0041-0.
Khaled, F., B. R. Giri, and A. Farooq. 2019. “On the Reaction of OH Radicals with C2 Hydrocarbons.” Proceedings of the Combustion Institute 37 (1): 213–219. https://doi.org/10.1016/j.proci.2018.06.052.
Kohse-Hoinghaus, K. 2021. “Combustion in the Future: The Importance of Chemistry.” Proceedings of the Combustion Institute 38 (1): 1–56. https://doi.org/10.1016/j.proci.2020.06.375.
Koo, E., P. J. Pagni, D. R. Weise, and J. P. Woycheese. 2010. “Firebrands and Spotting Ignition in Large-Scale Fires.” International Journal of Wildland Fire 19 (7): 818–843. https://doi.org/10.1071/WF07119.
Kuligowski, E. 2021. “Evacuation Decision-Making and Behavior in Wildfires: Past Research, Current Challenges and a Future Research Agenda.” Fire Safety Journal 120. https://doi.org/10.1016/j.firesaf.2020.103129.
Larsson, F., P. Andersson, P. Blomqvist, and B. E. Mellander. 2017. “Toxic Fluoride Gas Emissions from Lithium-Ion Battery Fires.” Scientific Reports 7 (1): 10018. https://doi.org/10.1038/s41598-017-09784-z.
Lemieux, P. M., and J. V. Ryan. 1993. “Characterization of Air Pollutants Emitted from a Simulated Scrap Tire Fire.” Air & Waste 43 (8): 1106–1115. https://doi.org/10.1080/1073161x.1993.10467189.
Li, D., and S. Suh. 2019. “Health Risks of Chemicals in Consumer Products: A Review.” Environment International 123: 580–587. https://doi.org/10.1016/j.envint.2018.12.033.
Linteris, G. T. 1997. “Acid Gas Production in Inhibited Propane—Air Diffusion Flames.” In Halon Replacements Chapter 19. Edited by A. W. Miziolek and W. Tsang. Washington, DC: American Chemical Society. https://doi.org/10.1021/bk-1995-0611.ch019.
Lönnermark, A., and P. Blomqvist. 2005. Emissons from Tyre Fires. SP Report 2005:43. Borås, Sweden: SP Swedish National Testing and Research Institute. https://www.diva-portal.org/smash/get/diva2:962334/FULLTEXT01.pdf.
Lönnermark, A., and P. Blomqvist. 2006. “Emissions from an Automobile Fire.” Chemosphere 62 (7): 1043–1056. https://doi.org/10.1016/j.chemosphere.2005.05.002.
Lowden, L. A., and T. R. Hull. 2013. “Flammability Behaviour of Wood and a Review of the Methods for Its Reduction.” Fire Science Reviews 2: 4. https://doi.org/10.1186/2193-0414-2-4.
Månsson, M., A. Lönnermark, P. Blomqvist, H. Persson, and V. Babrauskas. 1996. TOXFIRE – Fire Characteristics and Smoke Gas Analyses in Under-Ventilated Large-Scale Combustion Experiments. SP Report 1996:44. Borås, Sweden: SP Swedish National Testing and Research Institute. https://www.diva-portal.org/smash/get/diva2:962010/FULLTEXT01.pdf.
Manzello, S. L., S. Suzuki, M. J. Gollner, and A. C. Fernandez-Pello. 2020. “Role of Firebrand Combustion in Large Outdoor Fire Spread.” Progress in Energy and Combustion Science 76. https://doi.org/10.1016/j.pecs.2019.100801.
Maranghides, A., and D. McNamara. 2016. 2011 Wildland Urban Interface Amarillo Fires Report #2 – Assessment of Fire Behavior and WUI Measurement Science. NIST Technical Note 1909. Gaithersburg, MD: NIST. https://doi.org/10.6028/NIST.TN.1909.
Maranghides, A., D. McNamara, W. Mell, J. Trook, and B. Toman. 2013. A Case Study of a Community Affected by the Witch and Guejito Fires Report #2 – Evaluating the Effects of Hazard Mitigation Actions on Structure Ignitions. NIST Technical Note 1796. Gaithersburg, MD: NIST. https://doi.org/10.6028/NIST.TN.1796.
Maranghides, A., D. McNamara, R. Vihnanek, J. Restaino, and C. Leland. 2015. A Case Study of a Community Affected by the Waldo Fire – Event Timeline and Defensive Actions. NIST Technical Note 1910. Gaithersburg, MD: NIST. https://doi.org/10.6028/NIST.TN.1910.
Maranghides, A., E. Link, W. R. Mell, S. Hawks, M. Wilson, W. Brewer, C. Brown, B. Vihnaneck, and W. D. Walton. 2021. A Case Study of the Camp Fire – Fire Progression Timeline. NIST Technical Note 2135. Gaithersburg, MD: NIST. https://doi.org/10.6028/nist.Tn.2135.
Marrodán, L., Y. Song, M. Lubrano Lavadera, O. Herbinet, M. De Joannon, Y. Ju, M. U. Alzueta, and F. Battin-Leclerc. 2019. “Effects of Bath Gas and NOx Addition on n-Pentane Low-Temperature Oxidation in a Jet-Stirred Reactor.” Energy & Fuels 33 (6): 5655–5663. https://doi.org/10.1021/acs.energyfuels.9b00536.
Masoudvaziri, N., F. S. Bardales, and O. K. Keskin. 2021. “Streamlined Wildland-Urban Interface Fire Tracing (SWUIFT): Modeling Wildfire Spread in Communities.” Environmental Modelling & Software 143. https://doi.org/10.1016/j.envsoft.2021.105097.
May, A. A., E. J. T. Levin, C. J. Hennigan, I. Riipinen, T. Lee, J. L. Collett Jr., J. L. Jimenez, S. M. Kreidenweis, and A. L. Robinson. 2013. “Gas-Particle Partitioning of Primary Organic Aerosol Emissions: 3. Biomass Burning.” Journal of Geophysical Research: Atmospheres 118 (19): 11327–11338. https://doi.org/10.1002/jgrd.50828.
Messerschmidt, B. 2021. “The Fuel of Our Homes – From Building Materials to Content.” Presented at The Chemistry of Urban Wildfires: An Information-Gathering Workshop on June 8, 2021, National Academies of Sciences, Engineering, and Medicine, Washington, DC.
Molyneux, S., A. A. Stec, and T. R. Hull. 2014. “The Effect of Gas Phase Flame Retardants on Fire Effluent Toxicity.” Polymer Degradation and Stability 106: 36–46. https://doi.org/10.1016/j.polymdegradstab.2013.09.013.
Morgan, A. B., and J. W. Gilman. 2013. “An Overview of Flame Retardancy of Polymeric Materials: Application, Technology, and Future Directions.” Fire and Materials 37 (4).
Moritz, M., and V. Butsic. 2020. Building to Coexist with Fire: Community Risk Reduction Measures for New Development in California. Publication 8680. Davis, CA: University of California, Agriculture and Natural Resources. https://doi.org/10.3733/ucanr.8680.
NCAR-UCAR (National Center for Atmospheric Research–University Corporation for Atmospheric Research). 2018. WE-CAN: Western Wildfire Experiment for Cloud Chemistry, Aerosol Absorption and Nitrogen. https://www.eol.ucar.edu/field_projects/we-can.
NFPA (National Fire Protection Association). 2018. Standard for Reducing Structure Ignition Hazards from Wildland Fire. NFPA 1144. Quincy, MA: National Fire Protection Association.
NWCG (National Wildfire Coordinating Group). 2021. “Live Fuel Moisture Content.” In Fire Behavior Field Reference Guide. PMS 437. https://www.nwcg.gov/publications/pms437/fuel-moisture/live-fuel-moisture-content.
Ottmar, R. D. 2014. “Wildland Fire Emissions, Carbon, and Climate: Modeling Fuel Consumption.” Forest Ecology and Management 317. https://doi.org/10.1016/j.foreco.2013.06.010.
Ottmar, R. D., and S. P. Baker. 2007. Forest Floor Consumption and Smoke Characterization in Boreal Forested Fuelbed Types of Alaska. Boise, ID: Joint Fire Science Program. https://www.fs.fed.us/pnw/fera/research/smoke/jfsp_boreal_final_report.pdf.
Permar, W., Q. Wang, and V. Selimovic. 2021. “Emissions of Trace Orgnic Gases From Western US Wildfires Based on WE-CAN Aircraft Measurements.” JGR Atmospheres 126 (11).
Persson, B., and M. Simonson. 1998. “Fire Emissions into the Atmosphere.” Fire Technology 34.
Phillips, K. A., A. Yau, K. A. Favela, K. K. Isaacs, A. McEachran, C. Grulke, A. M. Richard, A. J. Williams, J. R. Sobus, R. S. Thomas, and J. F. Wambaugh. 2018. “Suspect Screening Analysis of Chemicals in Consumer Products.” Environmental Science & Technology 52 (5): 3125–3135. https://doi.org/10.1021/acs.est.7b04781.
Philson, C. S., L. Wagner, and R. Nawathe. 2021. “Mitigating California Wildfire Impact Through Zoning and Housing Policy.” Journal of Science Policy & Governance 18 (1). https://doi.org/10.38126/JSPG180112.
Pitts, W. M. 1995. “The Global Equivalence Ratio Concept and the Formation Mechanisms of Carbon Monoxide in Enclosure Fires.” Progress in Energy and Combustion Science 21 (3).
Pitz, W. J. 1982. Structure, Inhibition and Extinction of Polymer Diffusion Flames. Report number LBL-15825. Berkeley, CA: Lawrence Berkeley National Laboratory. https://escholarship.org/uc/item/1046z3g2.
Pitz, W. J., and R. F. Sawyer. 1978. “Inhibition Effects on Extinction of Polymer Burning.” Presented at Western States Section, The Combustion Institute Spring Meeting, Boulder, CO.
Pokhrel, G., D. J. Gardner, and Y. Han. 2021. “Properties of Wood-Plastic Composites Manufactured from Two Different Wood Feedstocks: Wood Flour and Wood Pellets.” Polymers (Basel) 13 (16). https://doi.org/10.3390/polym13162769.
Popescu, C. M., and A. Pfriem. 2019. “Treatments and Modification to Improve the Reaction to Fire of Wood and Wood Based Products—An Overview.” Fire and Materials 44 (1): 100–111. https://doi.org/10.1002/fam.2779.
Prichard, S. J., S. M. O’Neill, and P. Eagle. 2020. “Wildland Fire Emission Factors in North America: Synthesis of Existing Data, Measurement Needs and Management Applications.” International Journal of Wildland Fire 29 (2). https://doi.org/10.1071/WF19066.
Purser, D. 2009. “Influence of Fire Retardants on Toxic and Environmental Hazards from Fires.” In Fire Retardancy of Polymers: New Strategies and Mechanisms Chapter 24. London, UK: The Royal Society of Chemistry.
Purser, D. A., R. L. Maynard, and J. C. Wakefield (editors). 2015. Toxicology, Survival and Health Hazards of Combustion Products. London: The Royal Society of Chemistry. https://doi.org/10.1039/9781849737487.
Quarles, S. L., and K. Pohl. 2018. Building a Wildfire-Resistant Home: Codes and Costs. https://headwaterseconomics.org/wildfire/homes-risk/building-costs-codes/.
Sandberg, D. 2016. “Additives in Wood Products—Today and Future Development.” In Environmental Impacts of Traditional and Innovative Forest-Based Bioproducts Chapter 4. Edited by A. Kutnar and S. S. Muthu. Singapore: Springer Singapore. https://doi.org/10.1007/978-981-10-0655-5.
Schefer, R. W., and N. J. Brown. 1982. “A Comparative Study of HCl and HBr Combustion Inhibition.” Combustion Science and Technology 29. https://doi.org/10.1080/00102208208923593.
Schnipper, A., L. Smith-Hansen, and E. S. Thomsen. 1995. “Reduced Combustion Efficiency of Chlorinated Compounds, Resulting in Higher Yields of Carbon Monoxide.” Fire and Materials 19 (2).
Schoennagel, T., J. K. Balch, and H. Brenkert-Smith. 2017. “Adapt to More Wildfire in Western North American Forests as Climate Changes.” Proceedings of the National Academy of Sciences 114 (18).
Sekimoto, K., A. R. Koss, J. B. Gilman, V. Selimovic, M. M. Coggon, K. J. Zarzana, B. Yuan, B. M. Lerner, S. S. Brown, C. Warneke, R. J. Yokelson, J. M. Roberts, and J. de Gouw. 2018. “High- and Low-Temperature Pyrolysis Profiles Describe Volatile Organic Compound Emissions from Western US Wildfire Fuels.” Atmospheric Chemistry and Physics 18 (13): 9263–9281. https://doi.org/10.5194/acp-18-9263-2018.
Senkan, S. M. 2000. “Combustion of Chlorinated Hydrocarbons.” Pollutants from Combustion 547.
Simeoni, A., J. C. Thomas, and P. Bartoli. 2012. “Flammability Studies for Wildland and Wildland–Urban Interface Fires Applied to Pine Needles and Solid Polymers.” Fire Safety Journal 54. https://doi.org/10.1016/j.firesaf.2012.08.005.
Simmons, R. F., and H. G. Wolfhard. 1956. “The Influence of Methyl Bromide on Flames. Part 2.—Diffusion Flames.” Transactions of the Faraday Society 52: 53–59.
Simonson, M., P. Andersson, L. Rosell, V. Emanuelsson, and H. Stripple. 2001. Fire-LCA Model: Cables Case Study. SP Report 2001:22. Borås, Sweden: SP Swedish National Testing and Research Institute. https://www.diva-portal.org/smash/get/diva2:962180/FULLTEXT01.pdf.
State of California. 2016a. “Exterior Windows, Skylights and Doors.” California Building Code Section 708A. https://up.codes/viewer/california/ca-building-code-2016/chapter/7A/sfm-materials-and-construction-methods-for-exterior-wildfire-exposure#708A (accessed February 16, 2022).
State of California. 2016b. “Materials and Construction Methods for Exterior Wildfire Exposure.” California Building Code — Matrix Adoption Table Chapter 7A. https://www.hcd.ca.gov/building-standards/state-housing-law/wildland-urban-interface/docs/2010-part-2-cbc-ch7a.pdf (accessed April 1, 2022).
State of California. 2019. Fire Safety: Low-Cost Retrofits: Regional Capacity Review: Wildfire Mitigation. Assembly Bill 38.
State of California. 2020. Fire Prevention: Wildfire Risk: Defensible Space: Ember-Resistant Zones. Assembly Bill 3074. https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200AB3074 (accessed April 1, 2022).
Stec, A. A. 2017. “Fire Toxicity – The Elephant in the Room?” Fire Safety Journal 91: 79–90. https://doi.org/10.1016/j.firesaf.2017.05.003.
Stec, A. A., and T. R. Hull. 2010. Fire Toxicity. Sawston, UK: Woodhead Publishing.
Stec, A. A., and T. R. Hull. 2011. “Assessment of the Fire Toxicity of Building Insulation Materials.” Energy and Buildings 43 (2–3): 498–506. https://doi.org/10.1016/j.enbuild.2010.10.015.
Stec, A. A., J. Readman, P. Blomqvist, D. Gylestam, D. Karlsson, D. Wojtalewicz, and B. Z. Dlugogorski. 2013. “Analysis of Toxic Effluents Released from PVC Carpet under Different Fire Conditions.” Chemosphere 90 (1): 65–71. https://doi.org/10.1016/j.chemosphere.2012.07.037.
Sturk, D., L. Rosell, P. Blomqvist, and A. Ahlberg Tidblad. 2019. “Analysis of Li-Ion Battery Gases Vented in an Inert Atmosphere Thermal Test Chamber.” Batteries 5 (3). https://doi.org/10.3390/batteries5030061.
Suh, S., and B. C. Lippiatt. 2012. “Framework for Hybrid Life Cycle Inventory Databases: A Case Study on the Building for Environmental and Economic Sustainability (BEES) Database.” International Journal of Life Cycle Assessment 17.
Suzuki, S., and S. L. Manzello. 2021. “Firebrands Generated in Shurijo Castle Fire on October 30th, 2019.” Fire Technology. https://doi.org/10.1007/s10694-021-01176-0.
Swope, C. B., and D. Hernandez. 2019. “Housing as a Determinant of Health Equity: A Conceptual Model.” Social Science & Medicine 243.
Syphard, A., and J. Keeley. 2019. “Factors Associated with Structure Loss in the 2013–2018 California Wildfires.” Fire 2 (3). https://doi.org/10.3390/fire2030049.
Syphard, A. D., H. Rustigian-Romsos, M. Mann, E. Conlisk, M. A. Moritz, and D. Ackerly. 2019. “The Relative Influence of Climate and Housing Development on Current and Projected Future Fire Patterns and Structure Loss across Three California Landscapes.” Global Environmental Change 56: 41–55. https://doi.org/10.1016/j.gloenvcha.2019.03.007.
Syphard, A. D., H. Rustigian-Romsos, and J. E. Keeley. 2021. “Multiple-Scale Relationships between Vegetation, the Wildland–Urban Interface, and Structure Loss to Wildfire in California.” Fire 4 (1). https://doi.org/10.3390/fire4010012.
Tanaka, P. L. 2003. “Development of a Chlorine Mechanism for Use in the Carbon Bond IV Chemistry Model.” Journal of Geophysical Research 108 (D4). https://doi.org/10.1029/2002jd002432.
Tao, M., D. Li, R. Song, S. Suh, and A. A. Keller. 2018. “OrganoRelease – A Framework for Modeling the Release of Organic Chemicals from the Use and Post-use of Consumer Products.” Environmental Pollution 234: 751–761. https://doi.org/10.1016/j.envpol.2017.11.058.
Tejada, J., J. Wiedenmann, and B. Gall. 2020. “Trace Element Behavior in Wood-Fueled Heat and Power Stations in Terms of an Urban Mining Perspective.” Fuel 267.
Tieszen, S. R., and L. A. Gritzo. 2008. “Transport Phenomena That Affect Heat Transfer in Fully Turbulent Fires.” In WIT Transactions on State of the Art in Science and Engineering. Ashurst, Southampton, UK: WIT Press. https://doi.org/10.2495/978-1-84564-160-3/02.
Trelles, J., and P. Pagni. 1997. “Fire-Induced Winds in the 20 October 1991 Oakland Hills Fire.” Fire Safety Science. https://doi.org/10.3801/IAFSS.FSS.5-911.
Urbanski, S. 2014. “Wildland Fire Emissions, Carbon, and Climate: Emission Factors.” Forest Ecology and Management 317. https://doi.org/10.1016/j.foreco.2013.05.045.
Urbanski, S. P. 2013. “Combustion Efficiency and Emission Factors for Wildfire-Season Fires in Mixed Conifer Forests of the Northern Rocky Mountains, US.” Atmospheric Chemistry and Physics 13 (14): 7241–7262. https://doi.org/10.5194/acp-13-7241-2013.
USCB (US Census Bureau). 2020. Characteristics of New Housing. https://www.census.gov/construction/chars/.
USDOC (US Department of Commerce). 2010. 2010 Characteristics of New Housing: Single-Family Houses Completed, Units in Multifamily Buildings Completed, Multifamily Buildings Completed, Single-Family Houses Sold, Contractor-Built Houses Started. https://www2.census.gov/programs-surveys/soc/tables/time-series/chars/c25ann2010.pdf.
USFS (US Forest Service). 2022. Fuel Treatments. https://wildfirerisk.org/reduce-risk/fuel-treatments/ (accessed April 1, 2022).
van Leeuwen, T. T., G. R. van der Werf, A. A. Hoffmann, R. G. Detmers, G. Rücker, N. H. F. French, S. Archibald, J. A. Carvalho Jr., G. D. Cook, W. J. de Groot, C. Hély, E. S. Kasischke, S. Kloster, J. L. McCarty, M. L. Pettinari, P. Savadogo, E. C. Alvarado, L. Boschetti, S. Manuri, C. P. Meyer, F. Siegert, L. A. Trollope, and W. S. W. Trollope. 2014. “Biomass Burning Fuel Consumption Rates: A Field Measurement Database.” Biogeosciences 11 (24): 7305–7329. https://doi.org/10.5194/bg-11-7305-2014.
Wallingford, N. 2018. Camp Incident Damage Inspection Report, CABTU 016737. https://www.nist.gov/system/files/documents/2020/11/16/2018%20Camp%20Incident%20DINS%20Final%20Report.pdf.
Wang, D. S., and L. H. Ruiz. 2017. “Secondary Organic Aerosol from Chlorine-Initiated Oxidation of Isoprene.” Atmospheric Chemistry and Physics 17. https://doi.org/10.5194/acp-17-13491-2017.
Wang, M., A. Elgowainy, Z. Lu, A. Bafana, S. Banerjee, P. T. Benavides, P. Bobba, A. Burnham, H. Cai, U. Gracida, T. R. Hawkins, R. K. Iyer, J. C. Kelly, T. Kim, K. Kingsbury, H. Kwon, U. Lee, Y. Li, X. Liu, L. Ou, N. Siddique, P. Sun, P. Vyawahare, O. Winjobi, M. Wu, H. Xu, E. Yoo, G. G. Zaimes, and G. Zang. 2021. Greenhouse Gases, Regulated Emissions, and Energy Use in Technologies Model. Computer software. Washington, DC: US Department of Energy, Office of Energy Efficiency and Renewable Energy. https://doi.org/10.11578/GREET-Net-2021/dc.20210903.1.
Wang, Z., O. Herbinet, N. Hansen, and F. Battin-Leclerc. 2019. “Exploring Hydroperoxides in Combustion: History, Recent Advances and Perspectives.” Progress in Energy and Combustion Science 73: 132–181. https://doi.org/10.1016/j.pecs.2019.02.003.
Weschler, C. J. 2009. “Changes in Indoor Pollutants Since the 1950s.” Atmospheric Environment 43 (1).
Weyerhaueser. 2018. Safety Data Sheet (SDS): Weyerhaeuser Oriented Strand Board (OSB) Products including: Sheathing, Edge™, Edge Gold™, RBS™ (Radiant Barrier Sheathing), Rim Board, SturdiStep™. Longview, WA: Weyerhaueser.
Wiggins, E. B., A. Andrews, C. Sweeney, J. B. Miller, C. E. Miller, S. Veraverbeke, R. Commane, S. Wofsy, J. M. Henderson, and J. T. Randerson. 2021. “Boreal Forest Fire CO and CH4 Emission Factors Derived from Tower Observations in Alaska during the Extreme Fire Season of 2015.” Atmospheric Chemistry and Physics 21 (11): 8557–8574. https://doi.org/10.5194/acp-21-8557-2021.
Willstrand, O., R. Bisschop, P. Blomqvist, A. Temple, and J. Anderson. 2020. Toxic Gases from Fire in Electric Vehicles. RISE Report 2020:90. Gothenburg, Sweden: Research Institutes of Sweden. http://ri.diva-portal.org/smash/get/diva2:1522149/FULLTEXT01.pdf.
Xie, Q., J. Xiao, P. Gardoni, and K. Hu. 2019. “Probabilistic Analysis of Building Fire Severity Based on Fire Load Density Models.” Fire Technology 55 (4): 1349–1375. https://doi.org/10.1007/s10694-018-0716-0.
Yetter, R. A., and F. L. Dryer. 1992. “Inhibition of Moist Carbon Monoxide Oxidation by Trace Amounts of Hydrocarbons.” Presented at 24th International Symposium on Combustion, The University of Sydney, Sydney, Australia. https://doi.org/10.1016/S0082-0784(06)80093-7.
Yokelson, R. J., J. G. Goode, D. E. Ward, R. A. Susott, R. E. Babbitt, D. D. Wade, I. Bertschi, D. W. T. Griffith, and W. M. Hao. 1999. “Emissions of Formaldehyde, Acetic Acid, Methanol, and Other Trace Gases from Biomass Fires in North Carolina Measured by Airborne Fourier Transform Infrared Spectroscopy.” Journal of Geophysical Research: Atmospheres 104 (D23): 30109–30125. https://doi.org/10.1029/1999jd900817.
Zammarano, M., M. S. Hoehler, J. R. Shields, A. L. Thompson, I. Kim, I. T. Leventon, and M. F. Bundy. 2020. Full-Scale Experiments to Demonstrate Flammability Risk of Residential Upholstered Furniture and Mitigation Using Barrier Fabric. NIST Technical Note 2129. Gaithersburg, MD: NIST. https://doi.org/10.6028/NIST.TN.2129.
Zota, A. R., G. Adamkiewicz, and R. A. Morello-Frosch. 2010. “Are PBDEs an Environmental Equity Concern? Exposure Disparities by Socioeconomic Status.” Environmental Science and Technology 44 (15): 5691–5692. https://doi.org/10.1021/es101723d.
This page intentionally left blank.






































