In Chapter 5, Bridget Dooling described the statutes and regulations that determine how methadone is distributed and some of the barriers that prevent more equitable use of this life-saving treatment. Expanding on Dooling’s remarks, Corey Davis, deputy director of the Southeastern Region of the Network for Public Health Law and director of the Harm Reduction Legal Project, stated, “Law is a barrier to methadone for opioid use disorder.” But although the Controlled Substances Act prioritizes “ensuring that the wrong people aren’t using the wrong drugs for the wrong reasons,” flexibility remains regarding access to medications for opioid use disorder (OUD), he said. While both state and federal laws and regulations limit the use of methadone for OUD, he kept his comments to those at the federal level.
Matthew Lawrence, associate professor of law at Emory University School of Law, added that the federal government has tools that could be used to promote access to quality methadone treatment. He and Davis both suggested regulatory changes that could help achieve this goal. Their individual suggestions are summarized in Box 8-1 at the end of this chapter.
REGULATORY OPPORTUNITIES TO REMOVE CURRENT BARRIERS
The most important barriers to methadone access, according to Davis, are regulations that restrict dispensing of methadone to opioid treatment programs (OTPs) and the regulatory limits on who can obtain methadone maintenance treatment—only those who have had OUD for a full year and, for those under 18, two failures at detox. Regulations also require an initial in-person visit with a full physical work-up, limit initial doses, require periodic urinalysis, and set stringent criteria for take-home doses, said Davis. “And they apply regardless of the patient’s needs and clinical indications and the provider’s clinical impressions,” he said. Indeed, during the online chat discussion at the workshop Zac Talbott, president of the National Alliance for Medication Assisted Recovery (NAMA Recovery), said members and stakeholders across the country are reporting the need for higher and higher doses for stabilization. One factor that may be contributing to the need for higher initial doses of methadone is the increased use of the powerful synthetic opioid fentanyl. Mark Parrino said most newly admitted patients are using fentanyl, “whether they know it or not.”
The requirement that patients travel to OTPs to get methadone is particularly onerous, said Davis. As demonstrated by Kleinman, there are huge areas of the country where the nearest OTP is more than 2 hours away by car (Kleinman, 2020); see Figure 8-1. Therefore, even if other barriers were removed, the requirement to go to an OTP would make accessing methadone “basically impossible for people in large areas of the country,” said Davis. He noted that the Drug Enforcement Administration (DEA)
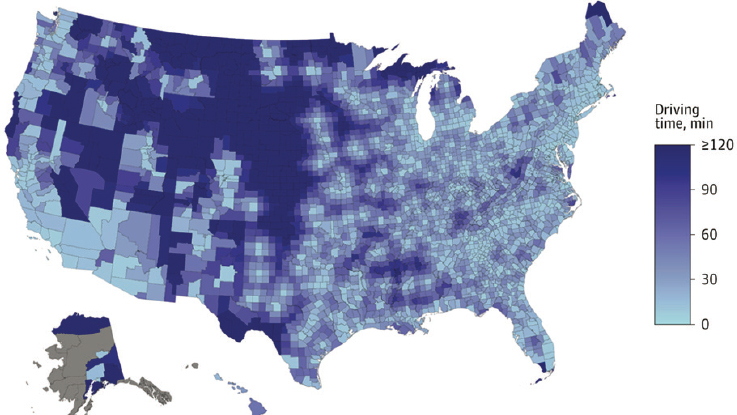
SOURCES: Presented by Corey Davis, March 4, 2022; Kleinman, 2020.
has made what he called “tweaks around the edges” allowing the delivery of methadone at satellite locations if certain criteria are met, but he maintained that most clinics lack the capacity to meet those criteria. Regulations do not permit OTPs to initiate treatment with methadone via telemedicine, even though this flexibility was extended to buprenorphine patients, who are more likely to be Whiter and are wealthier on average than methadone patients, said Davis.
As described earlier in the workshop, other flexibilities have also been introduced by DEA and the Substance Abuse and Mental Health Services Administration (SAMHSA) in response to the pandemic, such as mobile delivery of methadone and the availability of take-homes. While SAMHSA has proposed to extend the take-home flexibility after the COVID public health emergency ends, Davis noted that these extensions are currently slated to come with onerous requirements, such as total adherence to a treatment plan and negative toxicology tests for 60 days. Moreover, he said, none of these restrictions are required by law. “This is, in my mind, an example of the agency defaulting to the idea that our primary concern needs to be diversion and social control, not how to make sure that people who need this medication are getting it,” he said.
Lawrence agreed, noting that DEA, with its focus on diversion, reports to the Attorney General and has veto power over the Department of Health and Human Services (HHS), which focuses on patient health. “So it’s kind
of a structural loading of the dice in favor of diversion as against other values,” he said.
Ayana Jordan added, “The current situation is not working. People are dying at unprecedented rates” because of a shortsighted focus on diversion, which results in limited access. Available data show that when access to medication is increased, diversion goes down, she said.
Davis suggested that flexibilities could be extended by tying them to the opioid emergency declaration that has been in place since October 2017, similar to how they are currently tied to the COVID-19 emergency declaration. Both DEA and SAMHSA have the authority to grant exemptions to many methadone regulations and nearly unlimited enforcement discretion, he said, and they could relax enforcement immediately. Longer term, all of these onerous restrictions could be modified or removed through the regulatory process without congressional action, said Davis. Statutes require only that practitioners obtain a separate registration to deliver OUD treatments, he said.
The federal government could also take actions to improve the accessibility of methadone, said Davis. For example, Medicare funds nearly all medical residency positions in the United States and could require that to maintain funding, residents would have to be trained to deliver medications for opioid use disorder (MOUD). Similarly, funding of programs in the criminal legal system could also be tied to making sure people who want methadone are able to access it, he said.
REGULATORY INCENTIVES TO FACILITATE ACCESS TO QUALITY TREATMENT
Lawrence authored a commissioned paper (available in Appendix C) on federal administrative pathways the government could use to promote access to quality methadone treatment. In his workshop remarks, he focused primarily on regulations regarding permission and payments, likening them to sticks and carrots (see Figure 8-2): permissions being the sticks that regulatory authorities use to forbid doctors from prescribing or dispensing medications to patients, and carrots being the payments and reimbursements that attract providers into this space and enable them to continue and expand their operations. “We’ve spent a lot of time talking about the sticks, the limitations,” he said. “But even if you take away the limitations, you’re not going to see a lot of adoption if you don’t have reimbursements.”
Earlier in these proceedings, Dooling and Davis outlined the sticks side of this equation—the permissions required by the Controlled Substances Act and the broad standard-setting authority granted to the secretary of HHS, who delegates responsibility to SAMHSA, and the waiver authority granted to the attorney general, who delegates authority to DEA.
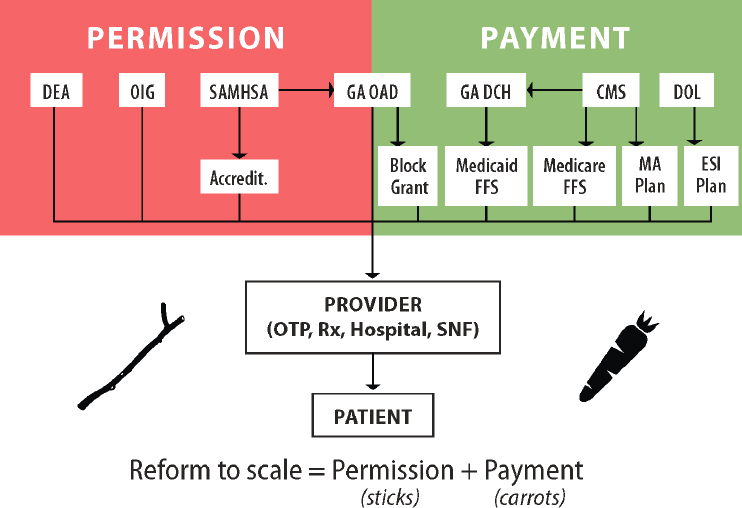
NOTE: CMS = Centers for Medicare & Medicaid Services; DEA = Drug Enforcement Administration; DCH = Department of Community Health; DOL = Department of Labor; ESI = employer-sponsored insurance; FFS = fee-for-services; GA = Georgia; MA = Medicare Advantage; OAD = Office of Addictive Diseases; OIG = Office of Inspector General, U.S. Department of Health and Human Services; OTP = opioid treatment program; SAMHSA = Substance Abuse and Mental Health Services Administration; SNF = skilled nursing facility.
SOURCE: Presented by Matthew Lawrence, March 4, 2022.
On the carrots side is a fragmented payment system, with different payers having different coverage requirements, said Lawrence. For example, traditional Medicare provides a payment bundle to OTPs for methadone. “The payment formula for that is threatening to cut rates for OTPs. CMS is considering ways to avoid those cuts, or maybe increase the generosity now, and that is an important source of flexibility that can really matter,” he said. There is currently no reimbursement model (e.g., as through Medicare Part D) for non-OTP options, but Lawrence suggested that the Centers for Medicare & Medicaid Services (CMS) could create more flexible reimbursement options.
Medicare Advantage plans, or “privatized Medicare” as Lawrence put it, employs use management approaches, which often include prior authorization, copays, and step therapy to determine coverage. Whether these plans provide adequate coverage for methadone treatment is unclear, he said. “We just don’t know what’s happening in that black box.”
Lawrence noted that the Center for Medicare and Medicaid Innovation (CMMI) has broad authority to test different payment models and roll them out nationwide if they are successful. For example, they tested a diabetes prevention program in a few geographic areas and found it successful before rolling it out. “That’s kind of a wide-open lane to test experiments with improving access to methadone,” said Lawrence.
Medicaid managed care is also a black box, said Lawrence. But he noted that Medicaid has broad authority to increase payments to states through section 1115 waivers and could exercise that authority to promote access to methadone through state Medicaid programs, also potentially wrapping in housing, transportation, and other services.
Employer-sponsored insurance plans are required by federal law—the Mental Health Parity and Addiction Equity Act of 2008 and amendments to the Affordable Care Act—to provide benefits for mental health or substance use disorder that are similar to medical and surgical benefits. However, Lawrence said enforcement of this law by the Department of Labor has been limited and there are reports of employer group plans wrongly denying coverage for methadone.
Given that these regulatory pathways exist to expand flexibilities for take-homes and telehealth, Lawrence asked why it took a global pandemic to implement changes. Racism and stigma may be partially responsible, but he suggested that another important factor is ossification. The high costs of regulatory change create a strong bias for maintaining the status quo, even when everyone agrees it is not working, he said. In terms of the regulatory environment for methadone, Lawrence identified several contributors to ossification: (1) horizontal fragmentation among different agencies within the federal government with authority over methadone regulation; (2) extensive vertical fragmentation from federal and state agencies as well as institutional players and individual providers; (3) an organized expert industry in the form of OTPs that are constituents of this regulatory environment; and (4) disempowered regulatory beneficiaries—patients and potential patients who are inherently disempowered as a result of their disease as well as other marginalizing factors.
Lawrence outlined potential solutions to each of these contributors to ossification:
- To address horizontal fragmentation, what is needed, he said, is energetic executive leadership through HHS or the Office of
- National Drug Control Policy (ONDCP) with agenda-setting authority, which could launch a methadone “moonshot” similar to President Biden’s cancer moonshot. Horizontal fragmentation could also be simplified by pooling authorities, said Lawrence.
- Vertical fragmentation might be addressed by having federal authorities encourage state alignment, for example, by having CMS issue a guidance and invite states to apply for waivers to test different models for providing methadone to patients.
- To empower regulatory beneficiaries, Lawrence said options are available to expand petitions for rulemaking that allow beneficiaries to be the ones who set the agendas for consideration of regulatory changes. He also suggested that agencies could better publicize and respond to complaints from patients.








