6
Biology and Chemistry
Phenomena in nature do not come labeled as belonging to biology, chemistry, or physics. But scientists from these different disciplines ask different kinds of questions, and seek different kinds of answers. Part I of this report is focused on how the phenomena of life generate questions for the physics community. In that process, knowledge gathered in the biology community provides a foundation for asking new physics questions about the phenomena of life, and ultimately for discovery of new physics. This chapter is focused on the flow of knowledge back from the physics community into the biology community, and the way in which this knowledge provides a foundation for new biology. These are not crisp distinctions, but are intended to capture the spirit of interaction between the disciplines, which has been so extraordinarily productive over more than a century. Along the way are ideas and methods that could be equally well categorized as chemistry, further enriching the disciplinary mix.
TOOLS FOR DISCOVERY
Optical physicists have greatly influenced the study of living systems through their many innovations in light microscopy. Innovations in electron microscopy have been similarly influential. Notably, it was the invention of the light microscope that enabled the discovery, in 1665, of cells as the basic units of living organisms. The creation of the electron microscope was equally pivotal for the discovery of cellular organelles. Advances in microscopy have continued to propel biological discovery to the present day.
Whereas the first light microscopes used optical absorption to create image contrast, by the 1800s, dark field microscopy had emerged as a means of using light scattering to view cells. This was an important advance, as many cells are poor absorbers of light, but they can be imaged at greater contrast using photophysical processes, such as scattering, that cells enact well. Most cells also shift the phase of light to a substantial extent, and the invention of phase contrast microscopy—recognized by a Nobel Prize in 1953—enabled routine inspections of the micron-scale morphologies of many cell types that are poorly revealed by bright-field microscopy and light absorption. To this day, phase contrast imaging remains a mainstay technique in research and clinical contexts worldwide. For teaching, a phase contrast microscope equipped with a time-lapse camera also allows a compelling demonstration of how a single photon can interfere with itself. In some contexts, differential interference contrast (DIC) microscopy has replaced phase contrast imaging due to its superior sensitivity to refractive index gradients inside the cell. By comparison, polarized light microscopy remains a niche technique for viewing biological specimens that are birefringent.
Fluorescence Microscopy Becomes Dominant
Fluorescence microscopy techniques have had especially great influence on biological physics investigations, and on biology more broadly, particularly in recent decades. This impact is due in large part to the ability to mark and thereby identify specific, chosen components of biological cells with a wide range of different fluorescent dyes, genetically encoded fluorescent proteins, fluorescent nanoparticles, fluorescently labeled nucleic acids, or fluorescent markers of other kinds. The resulting ability to image select components or molecular constituents of cells and tissues, combined with the many available fluorescent labels with different targets and emission colors, has spurred innovation in a wide set of fluorescence microscopy methods.
One of the most important advances in fluorescence microscopy for biological discovery came from the isolation, cloning, and sequencing of naturally occurring fluorescent proteins such as green fluorescent protein (GFP) from the jellyfish Aqueorea victoria. Methods of molecular biology were harnessed to fuse the coding sequence of GFP to the coding sequence of almost any gene in any organism. Understanding the physics and chemistry of photon absorption and fluorescent re-emission enabled the engineering of GFP mutants in a rainbow of colors and with exceptional photophysical properties (see Figure 6.1). These advances allowed specific fluorescent “tagging” of any protein in its natural context of the living cell, enabling elucidation of that protein’s dynamics and function in situ. This revolutionized the use of fluorescence microscopy as a research tool, and was recognized by a Nobel Prize in 2008.
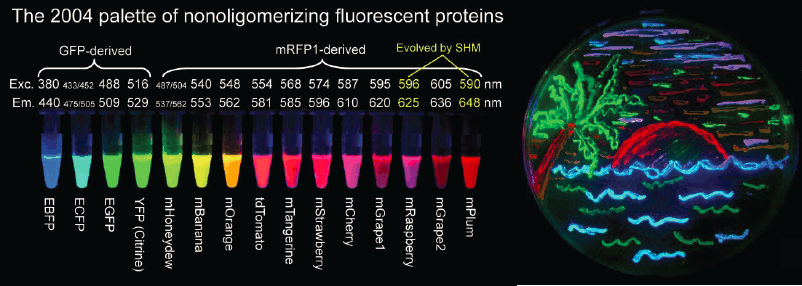
In addition to genetic encoding of fluorescent tags, the chemistry community has made important innovations in organic labels, starting with the very bright and photostable Alexa and Cy fluorophores introduced in the late 1990s and early 2000s, and the more recent development of the Janelia Fluor(R). There is an increased demand for dyes that are cell permeable and conjugate genetically encodable tags like HaloTag and SnapTag. These engineered dyes are often brighter and more photostable than fluorescent proteins, thereby providing higher signal-to-background ratios, higher localization precision, and longer trajectories when used to track individual biomolecules. These organic dyes are also engineered to expand the spectrum of available genetically encodable labels, facilitating multi-color imaging of labeled endogenous proteins which would have been difficult otherwise. These advancements in specific fluorescent labeling techniques, enabled by molecular biology, physics and chemistry, have thus propelled the tandem development of ever more sensitive, higher resolution, and faster fluorescence microscopes as biological discovery tools.
Wide-field epi-fluorescence microscopy is the simplest of these methods to implement and was the earliest to emerge, but it does not provide three-dimensional optical sectioning. One important advance from physics that revolutionized the ability of biologists to visualize the behavior of single fluorescently labeled molecules in a very thin optical section was total internal reflection fluorescence microscopy (TIRFM). By exciting fluorescence via the evanescent wave that is produced when light reflects off of a low refractive index surface at the water/microscope coverslip interface, TIRFM provides a simple form of optical sectioning, albeit limited to within a hundred or so nanometers from the
surface at which the evanescent wave emerges. This very thin plane of excitation has been exploited to excite only the small number of fluorescently labeled molecules within the evanescent wave in a dilute bulk solution of fluorescently labeled molecules, providing exquisitely high signal-to-background single molecule imaging. Together with the realization that one could literally see beyond the nominal diffraction limit (below), this enabled the dynamic tracking of motions of single molecules at the nanoscale. This has allowed the characterization of the stepping of single motor proteins along cytoskeletal filaments or DNA, statistics of single protein-protein binding interactions, and microrheological measurements of material properties, to name a few.
By achieving a more versatile form of optical sectioning and enabling three-dimensional imaging at the sub-micron scale, confocal fluorescence microscopy revealed many new facets of cells and tissues. But this approach is limited in its ability to image deep into optically opaque tissue.
Pulsed Lasers Lead to New Microscopies
The advent of ultrashort-pulsed lasers led to the development of multiphoton fluorescence microscopy, which can penetrate far more deeply into opaque tissue, including in live animals and humans. Specifically, two-photon fluorescence microscopy has become widespread as a way to image cellular properties and dynamics in the nervous system, lymph nodes, muscles, and other scattering tissues that were previously difficult to inspect at cellular scale in intact form. In the past few years, three-photon fluorescence microscopy has begun to emerge as a means of imaging even deeper (more than 1 millimeter) into thick tissue, due to the even longer attenuation length of the illumination wavelengths used as compared to those used for two-photon imaging.
Other microscopy modalities based on nonlinear optical effects include second-harmonic generation microscopy, which reveals ordered polar polymeric structures such as those in striated muscle and collagen, and third-harmonic generation microscopy, which is suited to inspecting cell interfaces. These optical harmonic methods are not used as widely as fluorescence imaging, as they are less versatile for inspecting a broad range of tissue and cell types, but they have the key virtue of sensing intrinsic optical signatures of tissue and thus not requiring any exogenous labels. Other nonlinear optical imaging methods probe biomolecular vibrations and include Raman scattering microscopy, coherent anti-Stokes Raman microscopy, and stimulated Raman scattering microscopy. The molecular specificity of these approaches is a strong suit, although isolating particular vibrational modes within the complex biomolecular environment of a cell is often challenging.
Breaking the Optical Diffraction Limit
One of the most important microscopy advances in recent decades is the invention of super-resolution optical imaging, which achieves imaging resolution finer than the limits set by conventional diffractive considerations (see Figure I.2). Nearly all super-resolution methods rely on fluorescence contrast. The first such approach, stimulated emission depletion (STED) microscopy, emerged in the early 1990s and introduced the strategy of using an optical nonlinearity as a means of shrinking the spatial support of the optical point spread function.
In this same time period, the capacity emerged to image and probe the photophysical properties of single fluorescent molecules, which provided a powerful new approach to observe biological processes in action. An especially potent version of this relies on fluorescence resonance energy transfer (FRET) between a pair of single fluorophores, as the efficiency of energy transfer depends on the distance between the two molecules and hence provides a “molecular ruler” with the sensitivity to detect changes in macromolecular conformations of less than 10 nanometers.
From this field of single molecule biophysics emerged the stochastic localization microscopy techniques as an alternative to STED microscopy for acquiring nanoscopic information. Stochastic localization imaging methods use particular classes of fluorophores that are either photoactivatable or photoswitchable. Activating a small fraction of the flurophors converts a dense collection of molecules into a sparse array of point fluorescent sources, and these points can be localized with a precision of tens of nanometers, much better than the usual diffraction limit to image resolution. Successive rounds of activation and deactivation sample different subsets of molecules, gradually piecing together the entire image, all at a resolution of tens of nanometers, a kind of pointillism on a molecular scale.
Taken together, the super-resolution optical methods have opened entirely new avenues of research into nanoscopic properties of cells and their macromolecules. Notable examples include the discoveries of fine ultrastructure within actin filaments of the cellular cytoskeleton, recent evidence for phase condensation of RNA polymerase molecules during transcription, unprecedented insights into chromatin structure and its role in gene regulation, and the ability to characterize individual cells based on their RNA expression patterns with near single molecule sensitivity.
Microfluidics
The field of microfluidics refers to the science and technology of fluidic dynamics at the scales of microliters to femtoliters, at which surface tension and capillary effects have predominant roles. Microfluidic devices can channel, transport, sort, and mix fluids (and the specimens they may contain) from distinct sources, often using only pneumatic control mechanisms, but sometimes via other
methods such as those based on electrowetting. Microfabrication of microfluidics devices has led to integrated systems for processing, testing, combining and even performing logical operations on fluidic specimens with minute volumes. Such capabilities have had broad-ranging impact on biological physics and related fields, particularly when microfluidic devices are combined with additional approaches to characterize or manipulate specimens, such as via optical measurements or biochemical reactions. Example applications involving microfluidic control include flow microcytometry analyses of the properties of individual cells, screening of small model organisms (e.g., nematodes, fly embryos, zebrafish larvae) within microfluidic chambers, identification of optimal conditions for protein crystallization, assessments of ligand/receptor binding affinities, and amplification of genetic material.
Notably, microfluidics technology has played a crucial role in the development of high-throughput, single cell assays. For example, in systems biology, microfluidic approaches have enabled researchers to move beyond population-level characterizations of cells in bulk and instead to directly analyze large numbers of individual cells, thereby capturing the variability in cellular properties across the population. By characterizing this variability at the level of individual cells, systems biologists and biological physicists have gained key insights into cellular signaling and decision-making processes. Furthermore, such single cell measurements, particularly in the context of transcriptomic or RNA sequencing analyses, have also become central to developmental biology, where they have made it possible to disentangle biochemical signaling networks that drive specific developmental transitions.
In biotechnology, microfluidic devices have greatly reduced costs for biochemical procedures, owing to the use of extremely small sample volumes and their associated fast mixing times, which accelerate biochemical reactions and enable low-cost, sophisticated biotechnological applications. For example, a low-cost rapid antigen test based on microfluidics detects SARS-CoV-2 viral particles in one second. Microfluidic technology has also helped usher in a new era of so-called single cell “omics,” which allow genome-wide studies to be performed at the resolution of individual cells. For instance, biotechnology companies use microfluidics within single cell transcriptomic assays to identify rare populations of diseased cells, such as metastatic cancer cells circulating in the blood; for non-invasive prenatal tests using blood samples from a pregnant mother; and for immune profiling studies, such as to probe a patient’s immune system regarding its ability to neutralize viral particles of a new SARS-CoV-2 strain. Looking ahead, the miniaturized, versatile designs of microfluidic devices are enabling a new era of personalized medicine. Overall, the use of microfluidic devices and approaches has become an integral mainstay in biological physics and the biotechnology industry.
Increasing Data Rates
Experimentalists in the biological physics community are well positioned to create basic technologies that enable rapid biological exploration. Such technologies are necessary to increase the rate at which theoretical predictions are tested, to make new discoveries, and to contribute applied technologies to society.
The speed of collecting data imposes limits on our exploration of life, and many advances have been enabled by the ability to gather data more quickly. In neuroscience, the biological physics community developed a microtome capable of cutting extremely thin slices of tissue (tens of nanometers thick) in an electron microscope. This advance made it possible to automate the collection of three-dimensional, nanoscale imaging to map out the network of synaptic connections between neurons (the connectome, Chapter 3), pushing the field forward. The entire field of genomics now relies on the ability to sequence single DNA molecules rapidly. The first methods of single DNA molecule sequencing came out of the biological physics community, which has continued to play a key role in the development of nanolithography methods and nanopore technologies that significantly speed up the process.
Such tools are needed to carry out high-quality experiments capable of generating large amounts of high-quality data. Similar new technology is needed in other areas, such as protein and organelle purification, cell and animal care, and the development of transgenic organisms. These frontiers of measurement often are explored by the biological physics community in response to physicist’s questions about the phenomena of life, but the resulting methods are transferred to the larger biology community at ever increasing rates.
MOLECULAR AND STRUCTURAL BIOLOGY
One of the most important results of the interactions between physics and biology in the 20th century is the understanding of life’s basic mechanisms as being the results of specific interactions among a set of identifiable molecules. This view of life as molecular machinery is the defining feature of modern molecular biology. Structural biology is the study of the molecular structure and dynamics of these molecules, typically proteins and nucleic acids, which provide the working machinery of the cell. The underlying goal of determining these structures is to understand their function and interactions in the complex environment inside the cell. While much of this effort is in support of basic scientific inquiry, structural biology is also a critical contributor in the discovery and development of new pharmaceutical therapeutics, which are almost always targeted to a specific macromolecule.
The dominant techniques used to determine these structures have been X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and more recently, cryogenic electron microscopy (cryoEM). Macromolecular crystallography (MX) is a mature technique with very high throughput (capable of characterizing
many thousands of structures per day) but requires crystallization of samples. NMR is a particularly useful technique for determining the dynamics of macromolecules in solution as well as membrane proteins in a solid state. Following a dramatic expansion in the early 2010s dubbed the “resolution revolution,” cryoEM has now been firmly established alongside MX and NMR as an essential structural biology technique. These efforts were recognized by a Nobel Prize in 2017.
In particular, cryoEM can provide structures of proteins that were previously intractable by other methods, including large protein complexes like the ribosome, integral membrane proteins, and highly heterogeneous or conformationally dynamic systems; an example in Figure 6.2 is the very recent structure of the flagellar motor (Chapter 1). These three principal approaches are supported by a wealth of other methods such as small angle scattering (SAS), hydrogen deuterium exchange mass spectrometry (HDX-MS), electron paramagnetic resonance (EPR), FRET, and
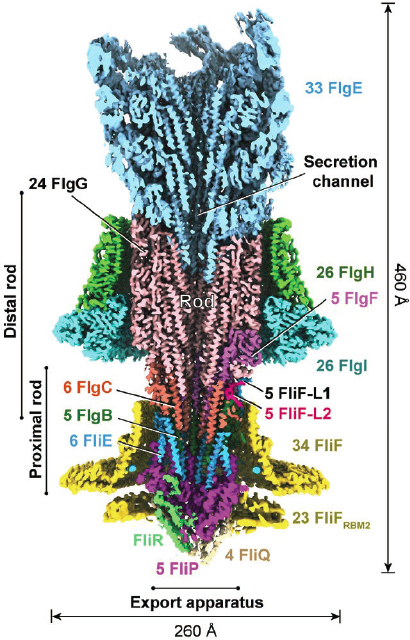
others. Increasingly, an integrated approach is required for a complete understanding of the structure and function of these complex macromolecular machines.
One way to measure the success of structural biology is through the growth of the Protein Data Bank (PDB), an open-access, public archive of the atomic coordinates of molecular structures. Launched in 1971 with just seven entries, the archive now holds roughly 170,000 structures and serves as a critical resource for both academic researchers and drug developers. The number of structures in the PDB continues to grow rapidly. At the same time, protein folding prediction algorithms, accelerated by machine learning, hold promise for a purely computational approach to providing structures that would be much faster and cheaper than traditional experimental methods.
Almost all of the protein structures in the PDB were determined from proteins in a purified form, separated out from their natural cellular environment. A major frontier in structural biology concerns the precise interactions of these macromolecules in the context of the cell—in particular, how and where macromolecules come together to form short-lived, transient but functional complexes. A complete understanding of the cell at atomic resolution will not be possible in the absence of this understanding. Achieving this will require a combination of techniques including cryo light microscopy (cryoLM), milling cells and tissues using focused ion beams (FIBs), and cryo electron tomography (cryoET). These three methods, currently undergoing rapid development, allow molecular complexes of interest to be located in the context of the cell (cryoLM), reduce bulk cells to thin lamella (FIB) suitable for high-resolution imaging by electron microscopy, and enable collection of a tilted series of images of the thin lamella in the transmission electron microscope (cryoET) that can then be converted to a three-dimensional volume using mathematical methods similar to those used in X-ray computed tomography or magnetic resonance imaging. A remaining challenge is then to identify individual molecules in the three-dimensional volumes, which have a very low signal-to-noise ratio and are also closely packed and crowded within the complex machinery of the cell.
While all these methods have their origins in physics, especially in the biological physics community, there has been a substantial effort to export these methods to a wider range of biologists. This has sped up, enormously, the exploration of the molecular structures relevant to the mechanisms of life. While this export was a slow process for X-ray crystallography, it was a bit faster for NMR and faster still for cryoEM. The result is that quite advanced methods of structural biology are broadly accessible. Nonetheless, from the standpoint of biology one can identify challenges that require solutions from the physics community.
Macromolecular crystallography.
Understanding biological dynamics across many time scales is an exciting new frontier. These dynamics will be explored in existing and next generation synchrotrons as well as X-ray Free Electron Lasers (XFEL), which provide capabilities for serial femtosecond X-ray crystallography. These
approaches will generate important new structures that will help to understand chemical reaction mechanisms catalyzed by enzymes and the molecular motions underpinning biochemical phenomena in living systems. Physics will undoubtedly continue to play an important role in moving the fundamental technology forward as well as in interpreting the results. New sources create opportunities that can be realized only with new detectors. There also is much to be done to support sample preparation for both XFEL and synchrotrons that will require fundamental physics insights, especially for data collection and dynamics at physiological temperatures.
Nuclear magnetic resonance.
Biological molecules are not static, rigid objects. Flexibility is essential for function, and few interactions are truly lock and key. The unique analytic powers of NMR have been applied in structural biology and biological physics to clarify these dynamical mechanisms, including identifying allosteric effects in molecular recognition, and especially elucidating the role of conformational exchange in protein function. As such, NMR can provide the crucial missing link between static structures and functional insights for drug discovery. The major challenge for NMR achieving this impact is its low throughput, high required amount of expert intervention, and limited (though growing) ability to analyze very large molecules. A number of broad technical efforts will make NMR a more routine tool for complex biological systems. These include rendering NMR analysis tools suitable for large complex biopolymers (e.g., proteins of 100 kDa and larger) through development of higher magnetic fields and associated hardware at a price and volume that can serve numerous investigators; and rendering the tool conveniently accessible to non-specialists through improvements in the efficiency and automation of the crucial project steps (sample preparation, data collection, and data analysis).
Cryogenic electron microscopy.
To further mature cryoEM techniques, advances are needed in sample preparation, instrumentation, and analysis. A deeper understanding of how macromolecules interact with substrates and the air-water interface would inform improvements to sample preparation. In terms of instrumentation, a large gap persists between the physical estimates of the number of macromolecules required to reconstruct a high-resolution, three-dimensional map, as well as the size of the macromolecules for which such reconstructions are possible, and what can currently be achieved in practice. Improvements may come from the development of laser-based phase plates, aberration corrections for electro-magnetic lenses, improvements to the electron detectors (cameras), and reductions in signal loss due to radiation damage and specimen movement during imaging. In terms of analysis, many macromolecules may exist in a continuum of conformational states; recent approaches using manifold embedding have the potential to uncover the work-cycle of a molecular machine as it passes through a continuum of states and map out its free-energy landscape.
CryoLM/FIB/CryoET.
Major challenges remain in the use of these technologies, some of which will be addressed by further engineering, technical, and computa-
tional developments. There are also fundamental physical obstacles, for example the need to precisely target macromolecules of interest in three dimensions within the cell. Currently cryoLM is used for this targeting, but it lacks the resolution required to ensure that the milled lamella will contain the region of interest.
An important complement to methods of structure determination is the ability to simulate the dynamics of these molecules. Conceptually simple, simulation is both subtle and computationally demanding. Motions of atoms in these large molecules are largely classical, but the forces are determined by quantum mechanics of the electrons. It is not feasible to solve the full quantum mechanics problem, so molecular dynamics depends on semi-empirical models for the forces between atoms in a protein, and between these atoms and the surrounding water molecules. These models have improved over decades, as have simulation methods themselves, so that one can now expect reasonably accurate estimates of equilibrium structures, binding free energies, and other quantities of functional importance. The long path to this level of precision was recognized by a Nobel Prize in 2013.
But brute force simulation is not enough. A typical protein molecule has thousands of atoms, and the output of a simulation is a sample trajectory through this enormously high-dimensional space. Theoretical ideas from biological physics play a central role in analyzing these simulations by extracting simplified descriptions of the dynamics.
Solving the structures of many different proteins has provided a scaffolding on which to build a more precise understanding of life’s basic mechanisms. But the emergence of these structures from interactions among many amino acids is itself a profound problem. Proteins are unusual in that many of them fold into compact and nearly unique structures. This raises deep questions about how the amino acid sequences of real proteins avoid the competing interactions that would frustrate a typical random sequence’s search for a well-defined equilibrium conformation. Put another way, what are the physical principles that distinguish functional proteins from all possible polymers of amino acids? As explained in Chapter 3, this problem has been a focus of interest in the biological physics community. Out of this work has come ideas about the funnel-like structure of the energy landscape for folding (see Figure 3.4), the many-to-one nature of the mapping from sequences to structure, and a statistical mechanics in sequence space describing the evolution of protein families. All of this has resulted in a coherent theory of protein folding.
A more practical formulation of the protein problem is concerned not with the general question of why proteins fold, but with the prediction of the folded structure for particular amino acid sequences. This prediction remains out of reach for direct molecular dynamics simulations, except in very special cases. On the other hand, the energy landscape picture suggests that the folding process has some hidden simplicity. There have long been efforts to learn the mapping from short sequences to local structures, such as helices and sheets, through a combina-
tion of simulation and generalization from known structures. Most recently, there has been an extraordinarily successful effort to learn the sequence/structure mapping more globally, using deep networks to generalize from the large number of examples now available. While this is an exciting step forward, there is still a long way to go in understanding the full repertoire of protein structures.
Most of the work on protein folding has been focused on the ability of proteins to self-assemble while not interacting with any other molecules—that is, in the dilute limit. In the cell, however, proteins are not free polymers. The situation is much more complex, and protein folding often begins as the protein is being synthesized on the ribosome. There are chaperone molecules that help to guarantee successful folding, and in such a dense environment there is a serious danger of aggregation being thermodynamically competitive with folding. Misfolding and aggregation are triggers for diseases, notably the prion diseases, and cells have significant machinery devoted to avoiding these errors. Understanding all these problems is part of a more general transition from thinking of biological molecules in isolation, as has been traditional in structural biology, to thinking of them as an interacting system, forming the underpinnings of molecular biology.
Molecular biology examines the molecular basis of biological processes in and between cells. The view of the biological cell has come a long way from the picture of the cell as a featureless vessel filled with chemical species undergoing reactions through random collision. Approaches such as cryoEM, fluorescence correlation spectroscopy, and dynamical rheology have indicated that the actual picture is much more interesting, involving cytoskeletal networks, membrane-bound and membrane-less organelles, ribosomes, proteins, nucleic acids, and small molecules—all of which are in a state of constant flux that can be dynamically modulated by the cell in response to changing conditions. While not so long ago characterization of a single protein was viewed as a major breakthrough, it now is appreciated that each protein is generally linked together with many others into complex “molecular machines” that carry out specialized and coordinated tasks. Approaches from the biological physics community have been helpful, and often necessary, in advancing understanding of the behavior of this molecular machinery and establishing the principles of its function. By abstracting and simplifying the highly complex inner workings of living systems, these approaches enable the application of simple models that have predictive value.
The capacity of biological macromolecules to act as sophisticated and highly efficient cellular machines—switches, assembly factors, pumps, or motors—is realized through their conformational transitions, that is, their folding into distinct shapes and selective binding to other molecules. As the number of molecular structures in the protein database grew, there was a clear need for methods to characterize the conformational dynamics of these structures. For decades, scientists could only investigate biochemical processes on a bulk level. The forces that molecules
exert on each other and the displacements that they undergo in the course of reactions were not directly measurable. Two different approaches have been developed to follow the time histories of individual molecules. In single-molecule manipulation methods (as in Chapter 1), the dynamics of the individual molecules are measured by attaching them to an external probe, which is used to exert defined forces on the molecules in order to characterize their mechanical properties or induce conformational changes. In single-molecule detection methods, the molecule is tagged with a fluorescence label, or a pair of labels that can undergo FRET, and the dynamics of the molecule are followed in real time from a change in the intensity of the fluorescence of the single probe or from the change in the FRET signal. These approaches have made it possible to follow, for the first time, the dynamics of individual molecules as they interact and undergo transformations, and to control and even alter the fate of these transformations.
Single-molecule methods elucidate details that are typically lost to ensemble averaging or asynchrony when studied by traditional “bulk” methods of biochemistry. The resulting time trajectories contain a wealth of unaveraged information that is directly amenable to mechanistic interpretation. However, the analysis and interpretation of these trajectories to reveal the kinetic barriers and time scales of the associated biological process required reformulation of many of the traditional concepts of thermodynamics and kinetics. Approaches rooted in non-equilibrium statistical mechanics have produced general theories of force-induced conformational dynamics, which enable the extraction of intrinsic kinetic rates and activation free energy barriers from single-molecule data. Single-molecule manipulation methods also present a natural approach to test some of the fundamental relationships in statistical mechanics (Chapter 5).
GENES, GENOMES, AND EVOLUTION
Genetics and genomics are at their heart the study of heredity, and describe how genetic variation leads to phenotypic differences between individuals. The tools of genetics also provide powerful methods to understand cellular and molecular biology, by exploiting genetic perturbations and patterns of inheritance to dissect the physiological basis of important traits. Much of this work has traditionally viewed genetic networks as isolated systems that affect specific traits according to essentially digital rules of logic. However, in recent years the field of systems biology has recognized that the functioning of a cell depends on the quantitative details of physical interactions between biological components across space and time. This has led to efforts to describe genetic networks not only as logical structures but as stochastic dynamical systems. In the biological physics community, this perspective has been pursued in many directions—to examine the formation of patterns of gene expression in space and time (Chapter 1), the flow of information through
genetic networks (Chapter 2), and the possibility of collective or emergent behaviors in these networks (Chapter 3).
In exploring genetic networks, biological physicists have developed methods that now are being adopted more widely by the biological community. An important example is provided by tools to interrogate the expression levels of many genes simultaneously, in single cells. There are two broad strategies. In the first, single cells are routed through microfluidic devices in which their mRNA molecules are extracted, encapsulated in droplets, and then amplified and sequenced (see above). In the second, cells are fixed and treated so that fluorescently tagged sequences can diffuse in and bind to complementary sequences of mRNA; these molecules can then be counted, one at a time, and successive rounds of washing and labeling with different sequences allows surveying of many different genes (see Figure 2.6). These methods have been used to provide objective definitions of cell types based on clusters in the high-dimensional space of expression levels, to identify very rare populations of cells, and more.
Our understanding of genetics has also been revolutionized in recent years by advances in molecular biology and in sequencing technology that makes it possible to analyze and manipulate genetic and phenotypic variation on a genome-wide scale. For example, systematic large-scale screens of CRISPR mutant libraries, transposon mutagenesis libraries, and knockout collections allow us to comprehensively survey the effects of every gene in model organisms or human cell lines, and the interactions between them. Many exciting experiments today bring these core biological methods together with physics-based methods for measuring gene expression, chromatin dynamics (Chapter 3), and other aspects of cell state.
Our understanding of genetics has also been enhanced by viewing sequence variation and corresponding phenotypic changes in their evolutionary context. This broad field of evolutionary and quantitative genetics aims to draw inferences from variation observed in natural populations. In quantitative genetics, the goal is to find statistical associations between genetic and phenotypic changes among closely related organisms, and to use these signatures to map the genetic basis of diseases, behaviors, and other important traits. Essentially, this work attempts to infer the structure of the genotype-phenotype map. Research in biological physics has played a role in developing methods for inference and for analyzing the structure of the resulting genotype–phenotype maps. For example, methods from statistical physics have helped to categorize types of epistasis and analyze how evolution across genotype–phenotype maps with a given statistical structure will tend to lead to regions of the landscape with specific properties. For more than a century, evolution was an observational science. Early efforts to bring evolution into the laboratory in the 1950s were led by physicists and chemists. A huge step forward came with the launch of the long-term evolution experiment in 1988. This experiment now has followed a dozen replicate populations of the bacterium
Escherichia coli over more than 75,000 generations, keeping a “fossil record” along the way. As described in Chapter 4, the biological physics community has contributed to understanding the data that emerge from this experiment, and to taking experimental evolution in new directions.
CELL AND DEVELOPMENTAL BIOLOGY
Cell biology is concerned with elucidating the structure and function of the cell, the “basic unit of life.” This field aims to determine how biomolecules self-assemble into functioning organelles, or subcellular compartments, that perform specific functions necessary for energy production, waste removal, or self-propagation; how systems of organelles mediate whole-cell functions such as motility or phagocytosis; and how cells interact with each other and their microenvironment to mediate tissue-scale physiological functions such as muscle contraction or glandular secretion. The biological physics community has been interested in all these phenomena, and has produced ideas and methods that have spread into the larger cell biology community.
Developmental biology is the study of how a single cell becomes a complex organism, and in particular how cells make decisions that determine their fates and identities during this process. It has long been known that signals driving these decisions are carried by the concentrations of particular molecules, called morphogens. Now classical biological studies have identified many of these morphogens, and the biological physics community has pushed to understand how much information these molecules carry, how that information flows through the genetic networks for which they provide input (Chapter 2), and how these interactions lead to spatial patterns (Chapter 1). Beyond morphogens, the field has come to appreciate that physical forces influence all stages of development, from the early embryo, to gastrulation and establishment of the body plan, to organogenesis. The field now seeks to understand how developmental morphogenesis is influenced by intrinsic force generation and tuning of tissue stiffness and fluidity as well as extrinsic factors including microenvironmental mechanics, pressure, and fluid flow. In the past decade, advances in animal models, live-imaging approaches, and biophysical measurements have shed light on how the physics of cells and tissues mediate morphogenesis.
The folding of proteins and nucleic acids has been a major focus of the biological physics community, as described in Chapter 3, and this line of inquiry continues in the exploration of self-assembly of cellular structures such as the cytoskeleton and cell membranes. As an example, studies of lipid–lipid and lipid–protein interactions in vitro and later applied to studies of lipid and protein dynamics in living cells have led to fundamental and well-accepted concepts about the structure and function of cellular lipid bilayer membranes such as the fluid mosaic model, the
notion of “lipid rafts” as cell signaling platforms, and the fission and fusion of membranes that mediate processes such as endo and exocytosis and cell division. There continue to be exciting questions at the frontier of this subject.
Another important contribution of physics to cell biology is in the areas of cell motility and mechanobiology. These subfields are aimed at elucidating how cells generate and react to forces and material properties; thus, by their very definition they require physics. The biological physics community has contributed to understanding across a range of scales. At the smallest scale, research is aimed at understanding how individual proteins generate and respond to force. The study of force- and motion-generating motor proteins that use ATP and the cytoskeleton or DNA as substrates grew out of the 1950s studies of muscle contraction and is an expansive subfield of biological physics. This field was aided early on by biophysicists applying methods of diffraction to give rise to models such as the sliding filament theory of muscle contraction. Investigations of motor proteins at the atomic level are aimed at elucidating the intramolecular rearrangements that take place in response to substrate binding interactions and energy released by ATP hydrolysis to drive conformational changes that generate force and motion or changes in protein-protein binding affinity. Insights into the structural basis of motor protein force generation or cell adhesion molecule bond strength have been aided by crystallography methods with roots in the physics of diffraction. More recently, as in Figure 1.3, single-molecule approaches utilizing optical and magnetic traps and microfluidics systems that were directly developed by physicists and adapted by biologists have allowed quantitative characterization of force generation by single motor proteins, catch bond behavior by individual cell adhesion molecules, and force-induced unmasking of protein-protein interaction sites.
At the scale of cells, soft matter and polymer physicists are advancing a quantitative and predictive understanding of how ensembles of mechanoactive proteins and biopolymers give rise to emergent properties of organelles or whole cells (Chapter 5). For example, this approach has been applied to understand the formation of the mitotic spindle or the waveforms of flagellar motility, wildly different processes that are both generated by the same basic elements of microtubules, motor proteins, and microtubule crosslinkers. These subfields of physics have also made important contributions to understanding the theoretical basis for how systems of cytoskeletal filaments give rise to cell and tissue material properties of stiffness and viscoelasticity. This has had an important impact, for example, on understanding the mechanoactive behavior of lung tissue that must expand and contract on time scales that are orders of magnitude longer than the motion generated by a motor protein.
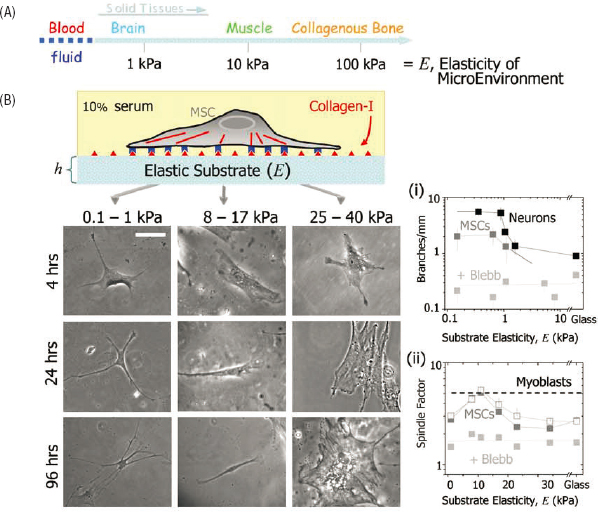
In stem cell biology, the seminal discovery that fate determination could be controlled by the physical cue of tissue stiffness, led by a physicist, has revolutionized the field. The cellular response to these cues can be seen in macroscopic
morphology, as in Figure 6.3, and also in the patterns of gene expression inside the cells. This discovery, and the cell biological mechanisms that have been pursued as a result, now form the basis for applications in tissue engineering and regenerative medicine in which physical properties of tissue scaffolds are carefully constructed to control cell fate for engineered tissue implants.
The notion that cells sense, respond to, and modulate tissue stiffness and viscoelasticity has also had a major impact on cancer cell biology and cancer research more generally. The vast majority of the research effort in cancer has historically been devoted to uncovering the biochemical mechanisms and genomics underly-
ing cancer. That situation has changed over the past decade with an explosion of research on the physics of cancer.
Physical mechanisms and properties can strongly influence tumor progression. The field of mechanobiology, which considers mechanical effects on biological processes such as mechanical changes of nuclei, cells, and tissues, encompasses an important class of physical mechanisms. Tissue stiffness is an example of an important mechanobiological property. Dysmorphic nuclei are common in cancer, as are changes in chromosome number, and further changes in nuclear properties are emerging. Tumor architecture is likewise abnormal compared to normal tissue, and it is clear that some solid tumors such as breast tumors are palpably stiffer than surrounding normal tissue. It has also been noted that stiffer tissues tend to exhibit more mutations. Changes in tissue stiffness can occur long before cancer is detectable; for example, liver stiffness is an excellent predictor of liver cancer. A proposed mechanism for how tissue stiffness might promote DNA damage is illustrative because it involves coupling between the mechanical process of cell migration and the biochemical process of DNA repair: Cell migration through pores that are more constrictive in stiffer tissue can promote segregation of fluid, including DNA repair factors, from the chromosome in the nucleus, thereby suppressing the repair of double-stranded DNA breaks. The coupling between mechanics and biochemistry in cancer is also illustrated in gene expression, which depends on the spatial structure of chromatin and how it varies over time. As a third example, the epithelial-to-mesenchymal transition marks the transition from a state of epithelial tissue in which cells do not change their relative positions (what in physics would be called a solid) to one in which the cells migrate and invade normal tissue (a fluid state).
More generally, the coupling of physical and biochemical processes is likely important to many aspects of cancer as well as many other biological processes. The local environment around a tumor—its microenvironment—interacts constantly with a tumor and affects its progression. Physical aspects of the tumor microenvironment are known hallmarks of cancers, and there is evidence that they are functionally linked to metabolism, the immune cell interactions with tumors, drug transport into tumors, the proliferation of cells, the ability of cancer cells to develop drug resistance, the ability of cells or clusters of cells to migrate out of the tumor to cause metastasis, and the plasticity of cancer stem cells. The heterogeneous physical properties of the tumor microenvironment can change significantly in space and time due to changes in local blood flow and oxygenation, or due to chemotherapy or other treatments. Thus, the tumor and its microenvironment can be viewed as a system of many components that interact and evolve via coupled physical and biochemical processes.
From a very broad perspective, understanding how microscopic changes lead to collective behavior in highly complex, adaptive many-body systems lies at the
core of the mission of biological physics. Cancer is a disease of altered genes and gene expression. This genetic information is transferred within cells and between cells and across a range of scales in space and time, eventually leading to collective behavior—symptoms of disease—at the system level. More specifically, a fitness landscape links genotypes and phenotypes to reproductive success and depends on local environmental conditions. Mutations and changes in gene expression thus evolve within the fitness landscape. The question of how subtle changes at the microscopic level disrupt processes in many-component systems to cascade into the collective phenomenon of cancer is a fascinating and impactful area of focus for biological physics.
Another recent and major breakthrough in cell biology that came from the biological physics community was based on understanding of the principles of phase separation (Chapter 3). For nearly 150 years, cell biologists had thought the only way to spatially sequester biochemical reactions to subcellular compartments is to encapsulate them inside a membrane or immobilize them on a scaffold. The notion that specific biomolecules could coalesce into discrete, non-membrane-bounded droplets within the cell cytoplasm or nucleoplasm by phase separation amounted to the first discovery of a novel principle of subcellular protein organization in more than a century. This has revolutionized thinking in cell biology about the organization and function of signal transduction, the genome, and protein processing. The therapeutic potential for this discovery is being explored as evidence gathers that liquid-liquid phase separation or its misregulation may be at the root of protein aggregation diseases such as Alzheimer’s disease, Parkinson’s disease, or amyotrophic lateral sclerosis.
A major thrust in the application of physics to developmental biology centers on how systems of motor proteins and cytoskeletal filaments in cells couple to cell-cell and cell-extracellular matrix (ECM) adhesion to generate the forces required for cells to pull on their neighbors or the ECM scaffold to drive tissue movement and morophogenesis. Actomyosin contractions can locally constrict parts of the cell, as in apical constriction, in order to change cell shape or allow a cell to exert traction in order to move over a surface, as in cell migration. These active forces at the level of single cells can be translated into dramatic changes in shape at the tissue level. For example, forces generated by actomyosin networks drive cell shape changes required for transforming early vertebrate embryos from a spherical mass of cells (the morula) into a fluid-filled blastocyst with an inner cell mass and an outer layer of cells called the trophoblast. Researchers are actively working to elucidate how morphogenesis in this and other tissues and organs is mediated by the spatial and temporal dynamics and magnitude of force generation at the single cell level.
In addition to generating forces, developing tissues are subject to a variety of extrinsic physical constraints, including compression by the ECM, attachment
to neighboring tissue, and sculpting by surrounding contractile tissues. Physical constraints can prevent or direct tissue growth, as well as lead to mechanical instabilities or tissue buckling. For example, growing tissues subject to compressive forces from the ECM, contraction, or cell growth in neighboring tissues can fold to relieve the resultant strains, as observed in the developing eye and intestine of the chick embryo. Cells also can sense physical cues and transform them into biochemical signals that inform cell behavior and fate decisions in a process termed “mechanosensation.” For example, airway epithelial cells cultured in wide tubes to simulate proximal airways or narrow tubes to simulate distal airways experience curvature-dependent tension that dictates the expression of either proximal or distal fate markers. The mechanism of such cellular mechanosensation from the atomic to the tissue scale is a major thrust in the fields of cell and developmental biology.
Physicists have applied the concepts of fluid dynamics to the study of developmental cell migration and tissue movement during morphogenesis, similarly to how fluid dynamics has been applied to the epithelial-to-mesenchymal transition (EMT) in cancer cell biology. This has provided accurate predictors of whether cells migrate independently or collectively (or somewhere in between). In fluid-like tissues, cells elongate, rearrange, and move past their neighbors, whereas in solid-like tissues, neighbors are maintained, cell shape remains essentially the same, and rearrangements are minimal. The transition between these two states, the jamming transition, is a concept from soft condensed matter physics (Chapter 5) and can theoretically be caused by changes in cell contractility or adhesion. In epithelial cell sheets, the jamming transition is predicted to occur when a single cell shape parameter reaches a specific value; hexagonal cells correspond to solid tissue and deviations from hexagonal are associated with fluidity. An example of a solid-to-fluid transition during development is the onset of neural crest cell migration, which requires a reduction in cell-cell adhesion mediated by internalization of cadherin cell-cell adhesion molecules. This allows neural crest cells to fluidize and thus migrate collectively to establish the neural tube and eventual spinal cord.
Fluid-filled lumens play diverse mechanical and biochemical roles during development, and several experimental techniques have been devised to quantify pressure within them. Lumens form when cells release solutes and generate an osmotic gradient that directs fluid flow. The fluid in the lumen is contained by cell-cell adhesions in the tissue surrounding them, and its expansion generates hydrostatic pressure. Pressure in lumens has recently been shown to play a role early on in blastocyst development, as well as later in development, such as when the neural tube becomes filled with cerebrospinal fluid that exerts pressure on the neuroepithelium and during lung development where pressure drives formation of complex tissue architecture and influences cell differentiation. Cells that line fluid-filled lumens can be subjected not only to pressure, but also to fluid flow. Flow
generates shear forces along the walls of the lumen. A wide range of fluid velocities are present throughout the embryo, from weak flows generated by beating cilia to fast flows generated by the beating heart. It is well established that embryonic left-right patterning is established by fluid flow generated by motile cilia, while midway through embryogenesis, once the heart has formed and started beating, blood flow shapes the development of the vascular network.
The development and application of methods to measure and manipulate force, pressure, stiffness, and flow in developing organisms will be key to uncovering their full role in developmental biology. On the flip side, the developing organism provides a rich platform for the discovery of new principles of physics and material science in dynamic living tissues.
FROM NEUROSCIENCE TO PSYCHOLOGY
Our modern understanding of voltage and charge has its origins in the discovery of “animal electricity” in the late 1700s. All living cells have a voltage difference across their membrane, and the dynamics of voltage changes are crucial to the functioning of muscles, the nervous system, and the heart. As explained in Chapter 2, the elucidation of the basic mechanisms of this electrical signaling, through a combination of theory and quantitative experiment, forms a classical chapter in the emergence of biological physics. The path from quantitative phenomenology to the identification and structure of the ion channels responsible for the electrical activity of cells has been recognized by three Nobel Prizes.
The understanding of ion channels has had broad implications for biology. Many organisms, including humans, have genes for more than 100 different kinds of channels. Individual neurons in the brain use a handful of these, and the decision processes through which cells decide on the correct mix of channels are an important part of brain development. As with many genes in complex organisms, the genes encoding ion channel proteins also come in separate pieces along the chromosome, and the splicing together of these pieces is an important example of how this mechanism can fine-tune cellular functions. The different channels have a wide range of structures, selectivities, kinetics, and sensitivities to drugs. As a result, ion channels are central to modern drug discovery in cardiology, nephrology, neurology, and psychiatry. More subtly, it has gradually been appreciated that ion channels play key roles even in non-neuronal cells.
Throughout the brain, neurons communicate through synapses, where electrical signals in neurons are transduced into a chemical form and then back into electrical signals. The same dynamics occur at the junctions between nerves and muscles. This report has explored the dynamics of synaptic transmission as part of the problem of processing single photon signals in the retina (Chapter 1), and as the substrate for connections in neural networks (Chapter 3). As with ion chan-
nels, the modern picture of synaptic transmission has its roots in work from the biological physics community, has been recognized by multiple Nobel Prizes, and has implications far beyond the examples presented here. As an example, molecular components responsible for synaptic transmission are shared by all cells that release vesicles, which contain everything from hormones to waste products. In a very different direction, the processes of synaptic transmission are the targets of the most widely prescribed drugs for mood disorders that affect the lives of tens of millions of people in the United States alone. There is a continuous path from work in the biological physics community, through the broader application of these results in neurobiology, to an impact on mental health.
Neuroscience as a field was actively constructed from multiple more well-established biological disciplines—physiology and pharmacology, anatomy and cell biology, biochemistry, and more. Perhaps because of this history, there has been a relatively rapid absorption of ideas from the biological physics community into the mainstream of neuroscience. This has happened with new experimental methods, with new theoretical ideas, and with new approaches to data analysis, resulting in a continuum of activity from theoretical physics to experimental neurobiology. It is a remarkable feature of this larger community that some of the most sophisticated physics-based methods are driving developments in our understanding of the human brain, reaching from neuroscience to psychology and neurology.
Functional magnetic resonance imaging is the most widely used method for visualizing human brain activity. Much of what is known about the dynamics of information processing in the human brain now has come from fMRI studies. Today, virtually all leading academic psychology departments have researchers who conduct fMRI studies in human subjects, using behavioral paradigms that probe sensory perception, diverse modes of cognition and decision-making, language processing, social interactions, motor control, and more.
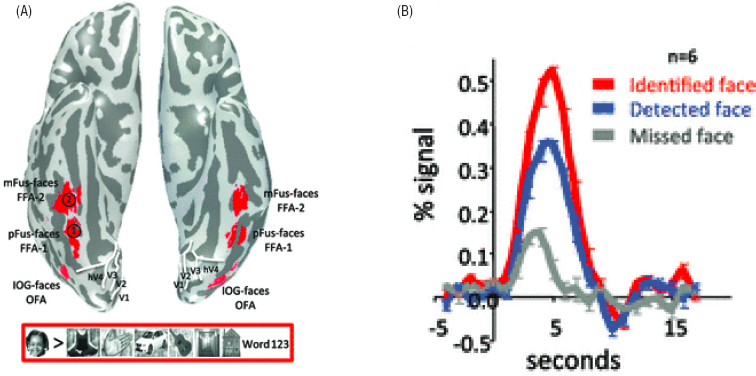
Our ability to image the human brain in action is a direct outgrowth of work in the biological physics community. The electrical activity of neurons is supported by their metabolism, and oxygen is essential. Oxygen is carried throughout the body by hemoglobin in the blood, which is a paradigmatic protein—one of the first to have its structure solved by X-ray diffraction. Binding and unbinding of oxygen to hemoglobin changes the optical absorption spectrum of the protein, so that blood in our veins is a different color from blood flowing through our arteries, and the fact that oxygen binds directly to an iron atom at the active site of the protein provides opportunities for even more spectroscopic tools. NMR in particular was used intensively to probe protein structural changes in response to oxygen binding. At some point it was realized that the oxygen binding, and the changing spin state of the iron atom, affects not only the NMR spectrum of the protein, but the relaxation dynamics of proton spins in the surrounding water. Thus, measurements of proton spin relaxation can generate a map of blood oxygenation, and indirectly
neural activity, in humans, in real time; an example of these experiments is shown in Figure 6.4. It is hard to overstate the impact that these developments have had on cognitive science and psychology.
In many ways, the use of physics-based techniques to explore the human brain closes a circle. In the 19th century, scientists routinely crossed boundaries among subjects now distinguished as physics, chemistry, biology, and psychology. The physics community’s interest in human perception was especially strong, and productive. It is not only that physicists were interested in the mechanics of the ear or the optics of the eye, they also were interested in the inferences that the brain draws from these raw sense data. Some went so far as to wonder if someday we might be able to unify not just physics and biology, but the natural sciences and aesthetics—if we really understand our perceptions, we should understand why we find some things to be beautiful.