2
Background on Autoimmune Diseases
DEFINING AUTOIMMUNE DISEASES
The immune system comprises cellular, chemical, and soluble protein components that together protect the body against foreign substances, including infectious agents and tumor cells, while not responding to molecules that signify “self” (Chaplin, 2010; Marshall et al., 2018). Autoimmunity arises when the immune system fails to distinguish self from non-self at the level of specific regions of cell surface molecules, or epitopes, recognized by two of the major effectors of the immune system: B cells, which produce antibodies, and T cells.1 Autoimmune disease by definition, then, is autoimmunity that results over time in a pathological outcome with self-reactive, or autoreactive, T cells and autoantibodies causing tissue damage (Brent et al., 2007; Johns Hopkins University, 2022; Rose and Bona, 1993; Rosenblum et al., 2015). In 1993, Rose and Bona reevaluated Witebsky’s postulates2 defining autoimmune disease, and proposed three levels of evidence to establish that a human disease is autoimmune in origin, including direct evidence by transfer of disease with pathogenic autoantibody or autoreactive T cells, indirect evidence
___________________
1 In recent years, evidence of innate immune mechanisms that recognize and respond to damage to self-tissues has blurred self/non-self distinctions (Abbas et al., 2004; Rose and Mackay, 2014).
2 The postulates required that “an autoimmune response be recognized in the form of an autoantibody or cell-mediated immunity; that the corresponding antigen be identified, and that an analogous autoimmune response be induced in an experimental animal. Finally, the immunized animal must also develop a similar disease” (Rose and Bona, 1993).
based on reproduction of the autoimmune disease in an animal model, and circumstantial evidence from clinical data (Rose and Bona, 1993).
The terms autoimmunity and autoimmune disease originated in the 1950s, but in 1999 the term “autoinflammatory disease” emerged, which emphasizes the critical role of the innate immune system—the quickly reacting, nonspecific component of the immune system—in chronic inflammatory diseases where autoantibodies and autoreactive T cells play less of a role in mediating pathology (Abbas et al., 2004; Brent et al., 2007; Masters et al., 2009; Rose and Mackay, 2014; Stoffels and Simon, 2014). Autoinflammatory diseases are broadly considered by the scientific community to be those conditions driven predominantly by innate immune cells, such as macrophages, mediating systemic inflammation and self-tissue pathology, whereas autoimmune diseases occur when adaptive immune cells—T cells and B cells—targeting self-antigens are the dominant response causing inflammation and tissue damage.
Some diseases are clearly autoinflammatory in nature, such as familial Mediterranean fever, Behçet’s disease, and Still’s disease (Ciccarelli et al., 2014). Others are clearly autoimmune disorders, including multiple sclerosis and systemic lupus erythematosus (SLE). However, distinguishing between autoimmune and autoinflammatory disease can be difficult because activation of the innate immune system is a prerequisite for triggering an adaptive immune response (Iwasaki and Medzhitov, 2015). In fact, for most immune-mediated inflammatory diseases such as atherosclerosis, and prototypical autoimmune diseases such as multiple sclerosis, the innate and adaptive immune systems both play a role in promoting disease, leading to the notion of a spectrum or continuum of autoinflammatory-autoimmune diseases (Hedrich, 2016; McGonagle and McDermott, 2006).
Moreover, research is showing that diseases that medical science has not historically considered to be autoimmune diseases, such as atherosclerosis, Parkinson’s disease, and cancer, have autoimmune mechanisms such as autoantibodies (de Jonge et al., 2021) and autoreactive T and B cells that contribute to the pathogenesis of disease (Ketelhuth and Hansson, 2016; Lindestam Arlehamn et al., 2020). Research on Parkinson’s disease, for example, has revealed that T cell reactivity, which prompts an attack on brain cells, is associated with early and even preclinical disease (Lindestam Arlehamn et al., 2020). However, it is outside the scope of this report to consider all diseases that involve autoimmune processes, and the report focuses on diseases that have traditionally been termed autoimmune diseases, such as multiple sclerosis and SLE.
Similarly there is no consensus regarding the number of autoimmune diseases. The National Institute of Allergy and Infectious Diseases (NIAID) website states there are more than 80 diseases (NIAID, 2017), and the
Autoimmune Registry3 and the Autoimmune Association4 each list around 150 diseases (Autoimmune Association, 2021; Autoimmune Registry Inc., 2020). The National Institutes of Health’s (NIH’s) 2016–2018 triennial fiscal report to Congress discusses research applicable to autoimmune diseases in general as well as research on specific diseases, including SLE, multiple sclerosis, type 1 diabetes, myasthenia gravis, scleroderma, rheumatoid arthritis, myositis, juvenile idiopathic arthritis,5 alopecia areata, psoriasis, pemphigus vulgaris, BACH2-related immunodeficiency and autoimmunity (BRIDA), and inflammatory bowel diseases (IBD) such as Crohn’s disease and ulcerative colitis (NIH, 2016, 2019). The report notes that SLE, multiple sclerosis, type 1 diabetes, IBD, and rheumatoid arthritis are among the most common and well-known autoimmune diseases and that BRIDA is a newly described autoimmune disease.
In the medical and research community, there are two differing approaches to characterize disease. One is to characterize and name diseases according to clinical criteria such as the presence of autoantibodies for autoimmune diseases (Hargraves et al., 1948; Marshall et al., 2018). Today, medical science classifies many rheumatologic, neurologic, gastrointestinal, cutaneous, hematologic, and cardiopulmonary illnesses as “autoimmune” based on the presence of autoantibodies, for example. The emphasis on clinical presentations of disease has resulted in disease classification by clinical diagnosis and organ system. A second approach to understanding diseases is to characterize them by their biologic mechanisms—the pathways that cause, mitigate, or affect the trajectory of disease. Over the past half century, the science of autoimmunity expanded beyond the concept of autoantibodies and autoreactive T cells and led to the emergence of the understanding of the importance of the innate immune response against self and other components of inflammation as drivers of autoimmune disease (Langan et al., 2020). Studies of innate immune cells, cytokines, cell surface markers, complement, and other biological phenomena now expand the definition of “autoimmune” to include illnesses with shared immunological mechanisms (Anaya, 2012; Cho and Feldman, 2015; Gokuladhas et al., 2021). Researchers can use an
___________________
3 The Autoimmune Registry, Inc., is a nonprofit organization that serves as a hub for research, statistics, and patient data on all autoimmune disease (Autoimmune Registry Inc., 2021).
4 The Autoimmune Association (previously known as the American Association of Autoimmune Related Disorders [AARDA]) is a nonprofit organization focused on the eradication of autoimmune diseases and impact of autoimmunity (AARDA, 2017).
5 Juvenile idiopathic arthritis comprises several types of arthritis: psoriatic arthritis, ogligoarthritis, polyarthritis, enthesitis-related arthritis (spondyloarthropathy), and systemic arthritis (Still’s disease). Source: https://my.clevelandclinic.org/health/diseases/10370-juvenile-idiopathic-arthritis (accessed February 1, 2022).
understanding of biologic mechanisms to target interventions for prevention, treatment, and cure.
There is no consensus regarding boundaries of the definition of autoimmune and autoinflammatory diseases. Public stakeholders, including people living with autoimmune disease, usually do not consider autoimmune and autoinflammatory conditions together, while clinicians and investigators may consider them similar and common enough to think of them as one entity or as two closely related entities. Regardless, the criteria for diagnosing someone as having an autoimmune disease may include diagnostic or classification criteria that describe clinically identifiable phenotypes in quantitative, exclusionary, time-limited, and binary terms (American Board of Medical Specialties, n.d.; Jia et al., 2017; Lockshin et al., 2021; Thompson et al., 2018). Nonetheless, diagnostic uncertainty is common among patients with autoimmune diseases.
Use of Autoimmune Disease Definitions in Research
Both the clinical criteria and biologic mechanism approaches to disease classification play a role in research efforts to better understand autoimmune disease. Sociological and clinical research studies, including those that NIH supports, define autoimmune diseases narrowly, requiring consensus diagnostic and classification criteria. Criteria-defined diagnoses are prioritized in medical-specialty training and practice (Aggarwal et al., 2015b; American Board of Medical Specialties, n.d.) as well as in discourse by patient advocacy groups. However, criteria-based definitions of autoimmune illnesses exclude individuals who are atypical or who do not fulfill or are excluded by criteria definitions (Aggarwal et al., 2015b; Jia et al., 2017).
Biological purposes for using a diagnosis name are to support studies of mechanisms and/or phenotypes to develop interventions for individuals and to improve patient care. For these purposes, diagnosis names do not necessarily require that an individual fulfill a list of criteria. Indeed, mechanistic studies often include—and even focus on—atypical individuals whose slow illness evolution (“pre-disease”), clinical heterogeneity, and overlapping features exclude criteria-based diagnoses (Jia et al., 2017; Lockshin et al., 2015, 2019). However, clinical research protocols typically exclude individuals with autoimmune syndromes that do not meet formal classification or diagnostic criteria. These individuals are also difficult to identify in health statistics and medical billing databases. In addition, insurers may refuse to pay for tests and treatments for patients who do not fulfill criteria (Lockshin et al., 2021; Noah, 2022; Pinson, 2012).
Finding: Recent scientific advances regarding cellular and molecular mechanisms of tissue injury blur the line between autoimmune and autoinflammatory diseases, making differentiation between the two difficult.
Finding: There is no consensus on the number of autoimmune diseases.
Finding: Physicians and clinical researchers classify autoimmune diseases according to symptoms and laboratory abnormalities; basic science researchers classify autoimmune diseases according to more inclusive biological mechanisms. Lack of a consensus vocabulary impedes optimal research design and patient care.
Conclusion: To improve research, guide patient care, and coordinate communication, clinical and research communities should develop a consensus vocabulary that includes both clinically defined autoimmune diseases and autoimmune mechanisms.
Causes of Autoimmune Diseases
Autoimmune diseases may have a known genetic or environmental cause or varying degree of both. When its etiology is unknown, an illness with an identifiable clinical or biologic phenotype is considered “idiopathic.” When an illness has a known exogenous cause, such as a bacterium or toxin, the disease is classified as having been induced by the exogenous agent (Bastard et al., 2020; Woodruff et al., 2021). Examples of exogenously induced autoimmune disease include procainamideinduced lupus (Blomgren et al., 1972), gadolinium-induced scleroderma (Idée et al., 2014), and various autoimmune phenomena, especially lung and gastrointestinal disease induced by chimeric antigen receptor (CAR) T-cell CD19/CD3 (Sedykh et al., 2018), anti-CD28 monoclonal antibody (Suntharalingam et al., 2006), and checkpoint-inhibitor (Johnson et al., 2018) treatments for malignancies. As highlighted by the COVID-19 pandemic, infections can play a role in inducing or exacerbating SLE (Quaglia et al., 2021) and cause other autoimmune diseases such as myocarditis (Boehmer et al., 2021). Environmental toxicants can also cause autoimmune disease illnesses such as Spanish toxic oil syndrome (Gelpí et al., 2002), eosinophilic fasciitis triggered by L-tryptophan ingestion (Beko et al., 1993), post-9/11 sarcoidosis-like syndrome (Webber et al., 2017), and myositis-like and scleroderma syndromes found in gold miners and workers in the polyvinyl chloride industry (Haynes and Gershwin, 1982; Tager and Tikly, 1999).
Sex Differences in Autoimmune Diseases
Most autoimmune diseases are more prevalent in women than men, with conservative estimates attributing greater than 75 percent of autoimmune disease incidence to women (Desai and Brinton, 2019; Jacobson et al., 1997; Rubtsov et al., 2010). Among the exceptions are type 1 diabetes mellitus (Cartee et al., 2016) and myocarditis (Coronado et al., 2019; Fairweather et al., 2013), which occur more often in boys or men. Research suggests that sex and steroid hormones may contribute to these sex-related disparities. Sex hormones, both natural and synthetic, directly interact with cells of the immune system through receptors located on or inside immune cells (Bouman et al., 2005; Buskiewicz et al., 2016; Edwards et al., 2020). Steroid hormones, including estrogens and androgen, affect antibody production and immune cell proliferation and in this way can increase or inhibit immune response (Buskiewicz et al., 2016; Fairweather, 2014). In women, for example, estrogen is known to cause B cells to produce a greater antibody and autoantibody response compared with men (Potluri et al., 2019), while men can develop more severe inflammation in response to estrogen (Maggio et al., 2009; Tengstrand et al., 2003). Sources of hormones include external sources such as diet (e.g., soy), drugs (e.g., birth control pills), and skin care products, as well as the body, which produces steroids (Fairweather, 2014; Martin-Pozo et al., 2021; Patisaul, 2017). There is great research interest in understanding how sex hormones regulate the immune response.
Endocrine-disrupting chemicals such as phenols, parabens, and phthalates may influence sex differences in autoimmune diseases by altering sex hormone levels and/or ratios (Bruno et al., 2019; Castro-Correia et al., 2018; Edwards et al., 2018; Popescu et al., 2021). In addition, the X chromosome encodes many immune system genes, and dysregulated X-inactivation may contribute to sex differences in autoimmune diseases (Yuen, 2020). Much of our understanding of sex differences and the immune response during autoimmune disease is based on studies using animal models. In terms of prevalence and severity of disease, many animal models demonstrate a sex bias that is similar to that seen in human autoimmune diseases (Coronado et al., 2019; Nusbaum et al., 2020; Rusman et al., 2018).
There is a semantic issue associated with sex and gender that is relevant to this research. The term sex generally refers to biological sex differences between males and females—chromosomes, hormones, and reproductive organs, for example—that affect health (Springer et al., 2012), while gender refers to the differences in socially constructed roles, characteristics, and behaviors of women and men (Springer et al., 2012; WHO, 2021). Separating the two constructs can be difficult, but labeling differences that occur in human research as “gender differences” rather than sex differences is not accurate either (Morgan et al., 2021). Given the
committee’s focus on the effect of sex hormones and sex chromosomes on inflammation in relation to autoimmune disease pathogenesis, the committee chose to assume that most of the studies cited in this report involve biological sex differences. However, most of the source data did not distinguish between sex and gender differences when collecting or analyzing the data. The lack of clinical and animal studies that carefully define sex and gender in study design and that disaggregate data and conduct their analysis accordingly, rather than only controlling for sex, is a major gap in the field that future research might address. In fact, the research community has advocated for disaggregating data and analyses by sex and gender (Gebhard et al., 2020; Morgan et al., 2021).
Finding: Regarding research design, there is clinical research in which sex and gender have not been defined carefully and animal research in which sex has not been defined, thus preventing the disaggregation and analysis of data on autoimmune disease from being conducted accurately.
Changing Definitions of Autoimmune Disease
Biological mechanisms are defining characteristics of autoimmune disease as they reflect poorly regulated function of one or many parts of inflammation pathways, such as antibodies/autoantibodies; macrophages, eosinophils, and T and B cells; cytokines; genes and gene expression; microbiome; detoxification pathways; and altered endothelium, mucosa, blood–brain, and placenta barrier tissue functions. For idiopathic, exogenously induced, and genetic forms of autoimmune diseases, the faulty mechanisms may operate independently, simultaneously, or in concert.
Although this report focuses on specific “idiopathic” autoimmune disease diagnoses, changing definitional boundaries of the diagnoses, different purposes of using diagnosis names, new data defining mechanisms in exogenously induced or genetic diseases, and new ways of thinking about disease mechanisms guarantee that, in the near future, there will be a need to restructure the concept of autoimmune disease. Scientific advances in genetics and epigenetics, for example, have helped to better distinguish illnesses with phenotypic characteristics or features seen in autoimmune diseases, such as Aicardi-Goutières syndrome and vacuolesE1 enzyme-X-linked-autoinflammatory (VEXAS) syndrome (Beck et al., 2020; Crow and Rehwinkel, 2009) from autoimmune disease.
In summary, issues that make the committee’s work difficult in responding to its charge are inherent in the concept of autoimmune disease: there are no standard definitions of either autoimmunity or autoimmune disease, the diseases are heterogeneous, and they last extended
periods of time. Moreover, some stakeholders conceptualize autoimmune diseases according to clinical criteria, while other stakeholders consider them to be biological entities with overlapping borders.
OCCURRENCE AND COURSE OF AUTOIMMUNE DISEASES
Data Sources and Limitations
Two commonly used measures to calculate the occurrence of a disease are incidence and prevalence. Incidence refers to the frequency of new occurrence in a population in a specified period of time—for example, the number of people newly diagnosed with a disease per year expressed as annual new cases per 100,000 persons (Porta, 2014). Prevalence refers to the total number of people with a disease in a defined time period, and it can include people recently diagnosed as well.
Incidence and prevalence data for autoimmune diseases in the United States are limited and can be difficult to find. There is no mandatory reporting system or national registry for autoimmune diseases. Much of the available incidence and prevalence data come from countries other than the United States with health care systems covering the entire population (Eriksson et al., 2013; Munk Nielsen et al., 2019; Pasvol et al., 2020; Wei et al., 2018).
For the purpose of this report, the committee sought high-quality epidemiology data, using criteria encompassing the size and diversity of the population studied and the ability to examine potential differences in disease rates across segments of the population, the length and recency of the time period covered, and the inclusion of a validation procedure to assess the accuracy of the classification criteria used to identify specific diseases within the database being used in the study. The committee preferred studies from the past decade (i.e., studies for which the data collection period included years since 2010), but such studies were unavailable for most of the autoimmune diseases selected for this report. The committee did not consider general population studies using self-reported data that did not include a medical record review for estimates of incidence or prevalence of autoimmune diseases because the accuracy of these methods is either unknown or poor (Videm et al., 2017).
Although the committee found data sources meeting some of these criteria for all of the selected diseases, none met all of the criteria. The most robust data come from studies of SLE, type 1 diabetes, and multiple sclerosis, described below:
- Data for primary Sjögren’s disease were available from Manhattan, New York, through the Manhattan Lupus Surveillance
- For SLE, incidence and prevalence data from the early 2000s (2002 to 2004 or 2007 to 2009) are available from a network of population-based national lupus registries in Michigan (Somers et al., 2014); Georgia (Lim et al., 2014); California (Dall’Era et al., 2017); Manhattan, New York (Izmirly et al., 2017); and American Indian or Alaskan Native populations (Izmirly et al., 2021a,b). Because this effort occurred for a limited time period, it does not provide data allowing for assessing temporal trends in the incidence or prevalence of SLE. The Centers for Disease Control and Prevention (CDC) supported these studies.
- For antiphospholipid syndrome (APS), incidence data from 2001 to 2015 are available from the Rochester Epidemiology Project (Duarte-Garcia et al., 2019); these data were used to estimate prevalence in 2015. The population base for this study is relatively small and homogenous. Because of the high proportion of people with SLE who also have APS, the relative lack of representation of groups who are at higher risk for SLE in this study population would result in an underestimation of APS rates.
- For rheumatoid arthritis (Kawatkar et al., 2019), incidence and prevalence data from 2005 to 2014 are available from the Kaiser Permanente medical system of Southern California, which covers a large and diverse population with respect to sociodemographic characteristics.
- For psoriasis, prevalence data from 1996 to 2009 from the Kaiser Permanente medical system of Northern California was used (Asgari et al., 2013).
Program (2007 to 2009) (Izmirly et al., 2019). Because this effort was for a limited time period, this study does not provide data that allow assessing temporal trends in the incidence or prevalence of Sjögren’s disease. These data focus only on Sjögren’s disease (referred to as primary Sjögren’s disease) and do not include individuals with Sjögren’s disease along with other systemic rheumatic diseases such as rheumatoid arthritis and SLE. Sjögren’s disease is diagnosed in up to 30 percent of individuals with rheumatoid arthritis and up to 20 percent of individuals with SLE (Aggarwal et al., 2015a; Baer et al., 2010; Harrold et al., 2020). These rates of Sjögren’s disease co-occurrence, together with the prevalence of rheumatoid arthritis and SLE in Table 2-1, suggest that Sjögren’s disease may occur more than 20 times more frequently when co-occurring with other autoimmune diseases than when diagnosed alone. Thus, the prevalence rate data limited to primary Sjögren’s disease exclude the majority of individuals with Sjögren’s disease.
- For IBD, prevalence data are available for 1999 to 2001 from a study using a large database from nine health maintenance organizations (Herrinton et al., 2007). For more recent prevalence data, studies include a 2007 to 2016 study that used two private administrative health claims databases collectively covering approximately 62 million people annually (Ye et al., 2020), as well as studies in more limited populations (Hou et al., 2013; Shivashankar et al., 2017; Xu et al., 2021). The committee relied on the estimates of the larger study, while considering the variability in estimates and the strengths and limitations of this set of studies (see Box 2-1).
- For celiac disease, serology (tissue transglutaminase and endomysial IgA antibodies) data from the 2009 to 2012 National Health and Nutrition Examination Survey6 are available (Mardini et al., 2015). Although this study provides data on the prevalence of these antibodies in a representative sample of the U.S. population, it does not include symptom data or other details allowing for assessing disease status based on a full clinical evaluation.
- For primary biliary cholangitis (PBC), prevalence data from 2003 to 2014 are available from the Fibrotic Liver Disease Consortium (Lu et al., 2018), a large network of health care systems drawing patients from across the United States.
- For multiple sclerosis, prevalence data from 2008 to 2010 are available from a study using three public (Veterans Administration, Medicare, and Medicaid) and three private administrative health claims databases collectively covering 125 million U.S. adults (Culpepper et al., 2019; Nelson et al., 2019; Wallin et al., 2019). The data were weighted to reflect the source of insurance coverage in the U.S. population, and the study used a validation procedure to assess the sensitivity and specificity of the classification criteria. However, the design of this study did not allow for assessing incidence rates or differences in prevalence among racial or ethnic groups, or assessment of temporal trends in incidence or prevalence. The National Multiple Sclerosis Society initiated and supported this study.
- For type 1 diabetes, incidence data from 2002 to 2012 and prevalence data from 2001 to 2009 are available from the SEARCH for Diabetes in Youth study, a large population-based study conducted at five centers across the United States (Dabelea et al.,
___________________
6 CDC conducts the National Health and Nutrition Examination Survey, which uses health interview, physical examination, and biospecimen data to assess the health and nutritional status of U.S. adults and children (NHANES, 2017).
- The committee did not find any recent (year 2000 or later) studies of incidence or prevalence of autoimmune thyroid diseases in the United States.
2014; Mayer-Davis et al., 2017). CDC and the National Institute of Diabetes and Digestive and Kidney Diseases initiated and supported this study.
In contrast, the Surveillance, Epidemiology, and End Results (SEER) database (NCI, 2021b) developed by the National Cancer Institute, provides easily accessible, verified data on incidence, prevalence, and mortality rates for the U.S. population in total, for males, females, as well as for five race and ethnicity groups, for all cancers, and for more than 30 individual types of cancers. It also provides trends in these rates over the past 20 years. This kind of resource is not available for autoimmune diseases.
The committee did take special note of the usefulness of the Olmsted County, Minnesota, Rochester Epidemiology Project (St. Sauver et al., 2011) conducted by the Mayo Clinic. This resource has provided epidemiologic data for rheumatoid arthritis, SLE, IBD, and many other autoimmune (and other) diseases since the 1960s, and is one of the few sources of long-term data on trends in the occurrence of these diseases. It is also a resource used for studies of prognosis, concomitant illnesses, and autoimmune-related mechanisms in other diseases. An important limitation, however, is that it covers a relatively small and homogenous population in terms of sociodemographic background,7 and the sample size is small for many of these diseases. NIH continues to support the Rochester Epidemiology Project.
Finding: There is no mandatory reporting system or national population-based data-collection program for autoimmune diseases, as there is for cancer through the National Cancer Institute’s SEER system.
There are also difficulties with respect to obtaining accurate data on mortality relating to autoimmune diseases. Death certificates can provide population-level data on mortality from autoimmune diseases, but they are not a good source of data pertaining to occurrence of chronic conditions that are not associated with acute mortality risks, such as most autoimmune diseases. Death certificates include information on immediate and underlying causes of death, with an additional field for other significant conditions contributing to the death. However, the completeness
___________________
7 U.S. census data for Olmsted County, Minnesota, in 2000 reported a population size of 124,277 with 90.3 percent of residents identifying as White and 6.4 of residents reporting incomes below the poverty level (Rochester Epidemiology Project, 2012).
and accuracy of this assessment, particularly for deaths relating to autoimmune diseases, is low: studies have demonstrated considerable under-reporting of autoimmune diseases as a cause of death, with no mention of an underlying condition in 40 to 80 percent of deaths of people enrolled in clinical cohort studies of SLE and rheumatoid arthritis (Calvo-Alén et al., 2005; Molina et al., 2015). It may be difficult to quantify mortality in most autoimmune diseases since death more commonly results from—and is recorded as being the result of—complications such as cardiovascular disease, infection, or malignancy rather than specific disease causes such as lethal hemorrhage resulting from thrombocytopenia. Studies of the age at death of persons with diagnosed autoimmune diseases constitute an indirect measure of mortality. A recent Dutch study that used death
certificate data concluded that “Systemic autoimmune diseases constitute a rare group of causes of death, but contribute to mortality through multiple comorbidities. Classification systems could be adapted to better encompass these diseases as a category” (Mitratza et al., 2021).
The committee also noted challenges in conducting and interpreting some clinic-based studies of mortality risk among people with specific autoimmune diseases. For example, studies based in tertiary care center(s) limit the generalizability of the findings. It is also important to distinguish between incidence and prevalence in analysis of risk over time, to report absolute risk in addition to relative risk, to address loss to follow-up, and to include a sample large enough to be able to examine the experiences of specific sociodemographic groups within the patient population.
Finding: There is a lack of accurate data on mortality associated with autoimmune diseases. Death certificates may provide incomplete information on an underlying autoimmune disease that contributed to the cause of death.
Additional Data Challenges
Studies using International Classification of Diseases (ICD) 9 or ICD 10 codes in electronic medical records to identify potential cases of autoimmune diseases may be inadequate (Moores and Sathe, 2013). Other information, such as number of visits with specific codes, medication use, and results of specific types of laboratory tests can improve the accuracy of the case ascertainment methods (Barnado et al., 2017; Liao et al., 2010). Researchers can conduct a complete medical record review to evaluate a case ascertainment algorithm’s sensitivity and specificity within the database being used (Carroll et al., 2012). Insurance-based algorithms used in the United States present an additional difficulty in terms of determining initial diagnosis for incidence studies, as medical records may be incomplete because of changes in coverage or providers. The pattern of remissions and flares seen in some autoimmune diseases presents additional challenges to determining disease prevalence. Prevalence studies based on current medication use or a specified frequency of medical visits may undercount patients experiencing prolonged periods of remission.
Estimates of Overall Prevalence of Autoimmune Diseases
In 1997, a compilation and analysis of studies reporting incidence or prevalence data for diseases estimated that at least one autoimmune disease would occur in approximately 8.5 million U.S. residents, or 3.2 percent of the population (Jacobson et al., 1997). This was the first attempt to estimate the overall burden of autoimmune diseases as a class of diseases, and it used a literature-survey approach, collecting studies published since 1965. A subsequent analysis building on this work expanded the number of autoimmune diseases to 31, used country-specific (Denmark) hospitalization data to better estimate more current disease patterns, and accounted for co-occurrence of diseases, resulting in an estimate of overall prevalence of greater than 5 percent (Eaton et al., 2007). This analysis may underestimate diseases that generally do not require hospitalizations or clinic visits in a specific time period. For example, prevalence rates for alopecia, psoriasis, and hypothyroidism were three to five times lower in this study compared with other studies from European populations. Accounting for this deficiency resulted in an overall estimate of 7.6 to 9.4 percent for a set of 29 autoimmune diseases (Cooper et al., 2009).
These estimates are all based on the prevalence of autoimmune diseases, rather than the prevalence of people with autoimmune diseases. Because many people have more than one autoimmune disease, the number of people with any autoimmune disease is less than the number suggested by summing the prevalence of individual autoimmune diseases. The sum of the prevalence of individual diseases will be larger than the sum of individuals with any autoimmune disease, with the difference reflecting the degree of over-counting resulting from multiple diseases occurring within an individual. A study in seven provinces in Canada estimated the combined prevalence of systemic autoimmune rheumatic disease (SLE, scleroderma, primary Sjögren’s disease, and polymyositis/dermatomyositis) and counted individuals with one or more of these diseases. The estimated prevalence of this group of diseases was 200 to 500 per 100,000 (Broten et al., 2014). This is likely a low estimate for the systemic rheumatic diseases, given the low sensitivity of the disease classification algorithms used and the exclusion of rheumatoid arthritis from this analysis.
Another way to estimate total burden of a group of diseases is by cumulative incidence or lifetime risk. For example, the estimate of the lifetime risk of all cancers combined, based on 2016 to 2018 SEER data was 39.2 percent (NCI, 2021a), with individual risks for colon, lung, and breast cancer each being greater than 4 percent. In an analysis of lifetime risk of systemic rheumatic diseases (rheumatoid arthritis, SLE, psoriatic arthritis, polymyalgia rheumatica, giant cell arteritis, ankylosing spondylitis, primary Sjögren’s disease) in Olmsted County, Minnesota, the lifetime risk was 8.42 percent in women and 5.13 percent in men (Crowson et al., 2011). Notably, this analysis does not include multiple sclerosis, thyroid diseases, type 1 diabetes, or other autoimmune diseases, which would most likely increase these estimates several fold.
Epidemiology of Select Autoimmune Diseases
Prevalence Rates
Among the most common autoimmune diseases are celiac disease, rheumatoid arthritis, and psoriasis, with prevalence rates approximately 790 to 939 per 100,000 and approximately 2.3 to 2.5 million U.S. residents living with each of these diseases; IBD, with a prevalence rate of approximately 500 per 100,000; multiple sclerosis, with a prevalence rate of approximately 300 per 100,000; and type 1 diabetes, with a prevalence rate of approximately 190 per 100,000 (Table 2-1). The available data pertaining to Sjögren’s disease are limited to primary Sjögren’s disease,
which does not occur in conjunction with SLE, rheumatoid arthritis, or scleroderma, and so underestimates the overall burden of this condition.
Table 2-1 does not include the autoimmune thyroid diseases Graves’ disease (hyperthyroidism) and Hashimoto’s thyroiditis (hypothyroidism) because of a lack of U.S. data covering the past 20 years. A study of Graves’ disease from 2008 to 2013 in Sheffield, United Kingdom, reported an annual incidence of 24.8 cases per 100,000 persons, with a median age at diagnosis of 44 (33 to 56) years; 80 percent of patients were women (Hussain et al., 2017). A large cohort study from the Netherlands examined thyroid medication use and thyroid hormone levels to classify overt and subclinical thyroid diseases; 3.1 percent of participants reported levothyroxine use, and 9.4 percent of the people who were not taking thyroid medications had subclinical hypothyroidism (thyroid stimulating hormone levels of 4.01–10.0 milli-International Units per liter) (Wouters et al., 2020).
Trends in Incidence and Prevalence
Trends in disease incidence are important indicators of changes in the underlying risk factors for the disease, such as an increasing or decreasing level of an environmental exposure that contributes to the development of the disease. These rates can best be ascertained from studies applying the same case ascertainment methods over time within a specific population. Trend data from U.S. studies spanning periods within the past 20 years and meeting these criteria were not available for psoriasis, multiple sclerosis, or the autoimmune thyroid diseases; for psoriasis and multiple sclerosis, the committee has included data from Canada in the following summary.
Only one study, of psoriasis incidence in Ontario, Canada, reported decreasing incidence for time periods covering 2000 to 2015 (Table 2-2). Studies of Sjögren’s disease, rheumatoid arthritis, PBC, and in some studies of IBD (ulcerative colitis and Crohn’s disease) and type 1 diabetes, found increasing incidence rates compared with pre-2000 years or during the 2000s. There was little or no trend observed in the studies of APS and multiple sclerosis.
Trends in disease prevalence can reflect changes in the incidence of disease, in the age distribution of a population, or in survival of people with the disease. Multiple sclerosis (Rotstein et al., 2018) and PBC (Lu et al., 2018) are examples of diseases for which studies have demonstrated that prevalence increased despite little change in incidence; reductions in mortality rates were also observed among people with these diseases.
In children and adolescents, the incidence or prevalence of two of the most common autoimmune diseases, IBD and type 1 diabetes, appears to have increased in the United States since 2000 (Table 2-2). The pediatric
TABLE 2-1 Prevalence Rates of Selected Autoimmune Diseases, United States
| Disease | Study Design and Data Source | Area, Time Period | Age | Prevalence Rate (per 100,000) | Estimated U.S. Total in 2020a | ||
|---|---|---|---|---|---|---|---|
| Total | Females | Males | |||||
| Sjögren’s disease (primary)b | Population-based study using Manhattan Lupus Surveillance Program Registry | Manhattan, NY 2007 | ≥ 18 | 13.1 | 21.1 | 3.5 | 35,370 |
| Systemic lupus erythematosus | Population-based study using data from 4 CDC SLE registries and Indian Health Service | GA, MI: 2002–2004 CA, Manhattan, NY, and Indian Health Service: 2007–2009 |
All ages | 72.8 | 128.7 | 14.6 | 206,000 |
| Antiphospholipid syndrome | Population-based study, Rochester Epidemiology Project | MN 2001–2015 | ≥ 18 | 50 | 51 | 48 | 123,000 |
| Rheumatoid arthritis | Population-based, Kaiser Permanente, patient electronic records | Southern, CA 2014 | ≥ 18 | 890.0 | 1326.0 | 387.0 | 2,403,000 |
| Disease | Study Design and Data Source | Area, Time Period | Age | Prevalence Rate (per 100,000) | Estimated U.S. Total in 2020a | ||
|---|---|---|---|---|---|---|---|
| Total | Females | Males | |||||
| Psoriasis | Population-based study, Kaiser Permanente, patient electronic, computerized records | Northern CA, 2009 | ≥ 18 | 939.0 | No sex difference reported | No sex difference reported | 2,535,000 |
| Population-based study, Truven Health, patient electronic, computerized records | U.S. 2015 | < 18 | 128.0 | 146.0 | 110.0 | 94,768 | |
| Inflammatory bowel diseasec | |||||||
| Ulcerative colitis | Retrospective cross-sectional study using two health claims databases | U.S. 2016 | ≥ 18 2–17 |
181.1 21.6 | NA | NA | 464,000 16,400 |
| Crohn’s disease | Retrospective cross-sectional study using two health claims databases | U.S. 2016 | ≥ 18 2–17 |
197.7 45.9 | NA | NA | 504,000 35,000 |
| Total IBDd | Retrospective cross-sectional study using two health claims databases | U.S. 2016 | ≥ 18 2–17 |
478.4 77.0 | NAe | NAf | 1,217,000 58,000 |
| Celiac disease (based on serology) | Population-based, representative U.S. population sample, NHANES | U.S. 2009–2012 | > 5 | 790.0 | NA | NA | 2,346,000 |
| Primary biliary cholangitis | Fibrotic Liver Disease Consortium data from 11 health systems | U.S. 2014 | All ages | 39.2 | 57.8 | 15.4 | 129,000 |
| Retrospective, cross-sectional population-based study using three public and three private administrative health claims databases | U.S. 2008–2010 | ≥ 18 | 309.2g | 450.1 | 159.7 | 775,000 | |
| Type 1 diabetes | Population-based study, SEARCH data from five participating centers in CA, CO, OH, SC, WA and select American Indian reservations | U.S. 2009 | < 20 | 193.0 | 193.0 | 193.0 | 178,000 |
a U.S. total population estimates for Sjögren’s disease, rheumatoid arthritis, psoriasis, and primary biliary cholangitis were calculated by applying total prevalence rate to 2020 U.S. census population estimates (330 million all ages; 270 million age ≥ 18; 297 million > age 5; 92.4 million < age 20). The SLE estimate was calculated by extrapolating the estimate from (Izmirly et al., 2021b) based on 2018 U.S. census data to 2020 using the percentage increase in total population from 2018 to 2020. The APS estimate was calculated by extrapolating the 2015 estimate provided by (Duarte-Garcia et al., 2019) to 2020 U.S. census population estimates. Inflammatory bowel disease estimates were calculated by extrapolating the 2016 estimates provided by (Ye et al., 2020) to 2020 U.S. census population estimates. The multiple sclerosis estimate was calculated by extrapolating the 2010 estimate provided by (Wallin et al., 2019) to 2020 U.S. census population estimates.
b Sjögren’s disease commonly co-occurs with other systemic rheumatic diseases (including up to 30% of individuals with rheumatoid arthritis and up to 20% of individuals with SLE (Aggarwal et al., 2015a; Baer et al., 2010; Harrold et al., 2020)), but this was not included in the studies of primary Sjögren’s disease. If these individuals are included, prevalence of all Sjögren’s disease would be estimated to be on the order of 800,000 in the United States in 2020. However, this figure does not account for individuals with Sjögren’s disease and other systemic autoimmune diseases (e.g., dermatomyositis, systemic sclerosis) or for children.
c See Box 2-1 for further discussion of variability in inflammatory bowel disease estimates.
d Includes ulcerative colitis, Crohn’s disease, and unspecified inflammatory bowel disease.
e Reported 54.5 percent of adults in pooled databases were female.
f Reported 55.0 percent of pediatric patients in pooled databases were male.
g Estimates reported are adjusted 10-year cumulative prevalence per 100,000.
NOTES: CA, California; CDC, Centers for Disease Control and Prevention; GA, Georgia; CO, Colorado; IBD, inflammatory bowel disease; MI, Michigan; MN, Minnesota; NHANES, National Health and Nutrition Examination Survey; NY, New York; OH, Ohio; SC, South Carolina; SEARCH, SEARCH for Diabetes in Youth study; SLE, systemic lupus erythematosus; U.S., United States; WA, Washington.
SOURCES: Sjögren’s disease (primary) (Izmirly et al., 2019); systemic lupus erythematosus (Izmirly et al., 2021b); antiphospholid syndrome (Duarte-Garcia et al., 2019); rheumatoid arthritis (Kawatkar et al., 2019); psoriasis ≥ 18(Asgari et al., 2013), < 18 (Paller et al., 2018); inflammatory bowel disease: ulcerative colitis, Crohn’s disease, and total IBD (Ye et al., 2020); celiac disease (Mardini et al., 2015); primary biliary cholangitis (Lu et al., 2018); multiple sclerosis (Wallin et al., 2019); type 1 diabetes, (Dabelea et al., 2014).
TABLE 2-2 Trends in Incidence or Prevalence of Autoimmune Diseases in the United States and Canada
| Disease | Study Design and Data Source | Area, Time Period | Age | Incidence | Prevalence |
|---|---|---|---|---|---|
| Sjögren’s disease (primary) | Population-based study using Rochester Epidemiology Project data | Olmstead County, MN 1976–2015 |
≥ 18 | Fluctuating (wavelike) rates, with higher values around 1990, 2005, and 2015, with overall increasing trend (p=0.005) | — |
| Antiphospholipid syndrome | Population-based study using Rochester Epidemiology Project data | Olmstead County, MN 2001–2015 |
≥ 18 | No trends observed | — |
| Rheumatoid arthritis | Population-based study using Rochester Epidemiology Project data | Olmstead County, MN 1995–2007 |
≥ 18 | Increased in women from 1995 to 2007 by about 2.5 percent per year | — |
| Population-based study using Kaiser Permanente, patient electronic records | Southern CA 1995–2014 |
≥ 18 | Average annual increase from 1995 to 2014 was 3 percent (95 percent CI, -4 percent to 10 percent), relatively steady from 2005 to 2014 | — |
| Disease | Study Design and Data Source | Area, Time Period | Age | Incidence | Prevalence |
|---|---|---|---|---|---|
| Psoriasis | Population-based study using health administrative data | Ontario, Canada 2000–2015 |
≥ 20 | Decreased from 111.0 to 69.0 per 100,000 per year | Increased from 1,740 to 2,320 per 100,000 |
| Inflammatory bowel disease | |||||
| Ulcerative colitis | Population-based study using Rochester Epidemiology Project data | Olmstead County, MN 1970–2010 |
All ages | Increased from 9.2 to 12.2 per 100,000 per year (p=0.06) | Increased from 241.0 to 286.3 per 100,000 |
| Population-based study, Kaiser Permanente, patient electronic records | Northern CA 1996–2002 |
0–17 | Increased from 1.8 to 4.9 per 100,000 per year (p<0.001) | — | |
| Crohn’s disease | Population-based study using Rochester Epidemiology Project data | Olmstead County, MN 1970–2010 |
All ages | Increased from 6.9 to 10.7 per 100,000 per year (p=0.003) | Increased 174.0 to 246.7 per 100,000 |
| Population-based study, Kaiser Permanente, patient electronic, computerized records | Northern CA 1996–2006 |
0–17 | Increased from 2.2 to 4.3 per 100,000 per year (p=0.09) | — | |
| All IBD* | Retrospective cross-sectional study using two health claims databases | U.S. 2007–2016 |
≥ 18 | — | Increased from 214.9 to 478.4 per 100,000 |
| 2–17 | — | Increased from 33.0 to 77.0 per 100,000 | |||
| Primary biliary cholangitis | Population-based study | 24 zip-code areas in Midwestern WI 1992–2011 |
≥ 18 | The overall age- and sex-standardized annual incidence rate increased, though not significantly | — |
| Increased in females from 6.9 cases per 100,000 per year 1992–1996 to 11.3 cases per 100,000 per year 2002–2006; rates steady from 2002 to 2011 | |||||
| Fibrotic Liver Disease Consortium data from 11 health systems | 2006–2014 | All ages | Increased from 4.2 per 100,000 per year (2006) to 4.3 per 100,000 per year (2014) | From 2006 to 2014, increased from 33.5 to 57.8 per 100,000 in women; from 7.2 to 15.4 per 100,000 in men; total rate change, from 21.7 to 39.2 per 100,000 | |
| Multiple sclerosis | Population-based study using Province of Ontario administrative health data | Ontario, Canada 1996–2013 |
≥ 20 | Generally stable except for short-term increase 2010–2013 | Increased from 157 to 265 per 100,000 |
| Disease | Study Design and Data Source | Area, Time Period | Age | Incidence | Prevalence |
|---|---|---|---|---|---|
| Type 1 diabetes Incidence: | Population-based study, SEARCH data from five participating centers in CA, CO, OH, SC, WA, and select American Indian reservations | SEARCH 2002–2012 |
< 20 | Increased from 19.5 per 100,000 per year in 2002–2003 to 21.7 per 100,000 per year in 2011–2012; annual increase, 1.4 percent; p=0.03) | — |
| Population-based study, SEARCH data from five participating centers in CA, CO, OH, SC, WA, and select American Indian reservations | SEARCH 2001–2009 |
< 20 | — | Increased from 148 to 193 per 100,000 | |
| Population-based study using Rochester Epidemiology Project data | Olmstead, MN 1994–2010 |
All ages | Average annual incidence rate was 9.2 per 100,000 per year, little variation or trend | — | |
| Population-based study using MarketScan Multi-State Medicaid Claims Database | U.S. (Medicaid population) 2002–2016 |
< 18 | — | Increased from 129 to 234 per 100,000 |
* Includes ulcerative colitis, Crohn’s disease, and undifferentiated IBD.
NOTE: CA, California; CI, confidence interval; CO, Colorado; IBD, inflammatory bowel syndrome; MN, Minnesota; OH, Ohio; SC, South Carolina; U.S., United States; WA, Washington; WI, Wisconsin.
SOURCES: Sjögren’s disease (primary) incidence (Maciel et al., 2017a); antiphospholipid syndrome (Duarte-Garcia et al., 2019); rheumatoid arthritis (1) (Myasoedova et al., 2010), (2) (Kawatkar et al., 2019); psoriasis (Eder et al., 2019); IBD: ucerative colitis all ages (Shivashankar et al., 2017), 0–17 (Abramson et al., 2010); Crohn’s disease all ages (Shivashankar et al., 2017), 0–17 (Abramson et al., 2010); all IBD (Ye et al., 2020); primary biliary cholangitis ≥ 18 (Kanth et al., 2017), all ages (Lu et al., 2018); multiple sclerosis (Rotstein et al., 2018); type 1 diabetes: incidence < 20 (Mayer-Davis et al., 2017), prevalence < 20 (Dabelea et al., 2014), incidence all ages (Cartee et al., 2016), prevalence < 18 (Chen et al., 2019).
prevalence of IBD in the United States increased between 2007 to 2016 from 33.0 to 77.0 per 100,000, with Crohn’s disease being twice as prevalent as ulcerative colitis (45.9 versus 21.6) (Ye et al., 2020). Type 1 diabetes has also increased. From 2001 to 2009, a large U.S. study showed an increase in type 1 diabetes prevalence from 148 per 100,000 to 193 per 100,000 (Dabelea et al., 2014). A subsequent study of the U.S. Medicaid pediatric population during the period 2002 to 2016 showed an increase in annual type 1 diabetes prevalence from 129 to 234 per 100,000 (Chen et al., 2019). This trend was not seen, however, in a study spanning 1994 to 2010 in Olmsted County, Minnesota (Cartee et al., 2016).
Demographic Patterns with Respect to Disease Risks
Many, but not all, autoimmune diseases affect women predominantly (Figure 2-1). The diseases with the most marked sex difference in occurrence, with female-to-male ratios greater than 5:1 are SLE (Izmirly et al.,
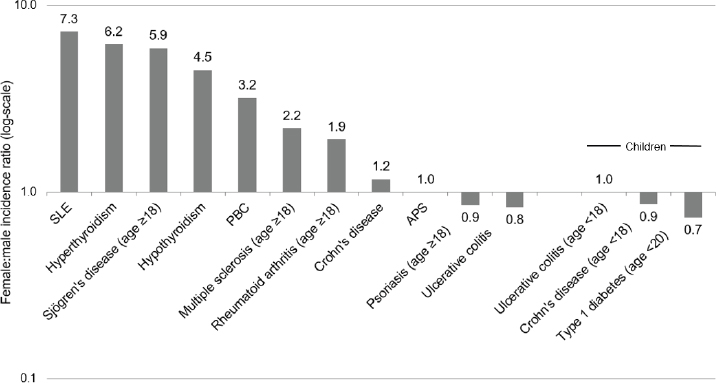
NOTES: Age ranges provided when available.
APS, antiphospholipid syndrome; PBC, primary biliary cholangitis; SLE, systemic lupus erythematosus.
SOURCES: Data drawn from the following studies that provided sex-specific incidence rates. SLE (Izmirly et al., 2021a); Sjögren’s disease (Maciel et al., 2017a); hyperthyroidism (Leese et al., 2008); PBC (Lu et al., 2018); hypothyroidism (Leese et al., 2008); rheumatoid arthritis (Myasoedova et al., 2010); Crohn’s disease (Herrinton et al., 2008); APS (Duarte-Garcia et al., 2019); psoriasis (Icen et al., 2009); ulcerative colitis (Herrinton et al., 2008); multiple sclerosis (Langer-Gould et al., 2013); ulcerative colitis, children (Abramson et al., 2010); Crohn’s disease, children (Abramson et al., 2010); type 1 diabetes (Cartee et al., 2016).
2021a,b), hyper- and hypothyroidism (Leese et al., 2008), and Sjögren’s disease (Maciel et al., 2017a, 2017b) The female-to-male ratio is lower, between 4:1 and 2:1, for multiple sclerosis (Langer-Gould et al., 2013), PBC (Lu et al., 2018), systemic sclerosis (Mayes et al., 2003), and rheumatoid arthritis (Myasoedova et al., 2010). However, for other autoimmune diseases, such as psoriasis (Icen et al., 2009), the female-to-male incidence ratio is close to or less than 1.0 and also influenced by age. Myocarditis, for example, occurs more frequently in males before age 50 (Coronado et al., 2019). Although the female-to-male ratio for APS is close to 1.0 in the only population-based study available (Duarte-Garcia et al., 2019), this ratio may be higher in populations that include a greater proportion of patients with SLE.
Regarding disease incidence in children, one study of juvenile idiopathic arthritis found an increased incidence in females compared with males in each of the age groups studied (ages 0 to 5, 6 to 10, and 11 to 15 years) (Harrold et al., 2013). Other autoimmune diseases do not show a female predominance in children, and some diseases, such as type 1 diabetes (Cartee et al., 2016), occur more frequently in boys than girls.
Although most autoimmune diseases can occur at any age, different diseases present different patterns with respect to age at onset or diagnosis. Figure 2-2 shows four patterns, all based on recent studies from Olmsted County, Minnesota. Type 1 diabetes most often occurred between 5 and 15 years of age, but incidence extended through ages 30 to 39, and rates were higher for males compared with females beginning around age 10. Incidence rates for primary Sjögren’s disease increased steadily throughout adulthood, reaching the highest rates around ages 65 to 74, with higher incidence seen in women (Maciel et al., 2017a). The peak age at diagnosis of Crohn’s disease and ulcerative colitis was 20 to 29 years, but the study also saw new cases through early and late adulthood. There was little difference in rates between males and females for Crohn’s disease, but for ulcerative colitis, rates in males were somewhat higher than in females.
Health Disparities in Autoimmune Diseases
There are known disparities in the incidence, prevalence, severity, prognosis, outcomes, and care related to autoimmune diseases. These disparities adversely affect groups that are socially disadvantaged and marginalized based on race and ethnicity, socioeconomic status, and geographic region. In addition, these factors may interact with one another to cause, accentuate, and perpetuate health disparities at the individual patient, community, and societal levels (Reifsnider et al., 2005). While studies may report race and ethnicity as a biologic proxy for genetic origin, the committee acknowledges
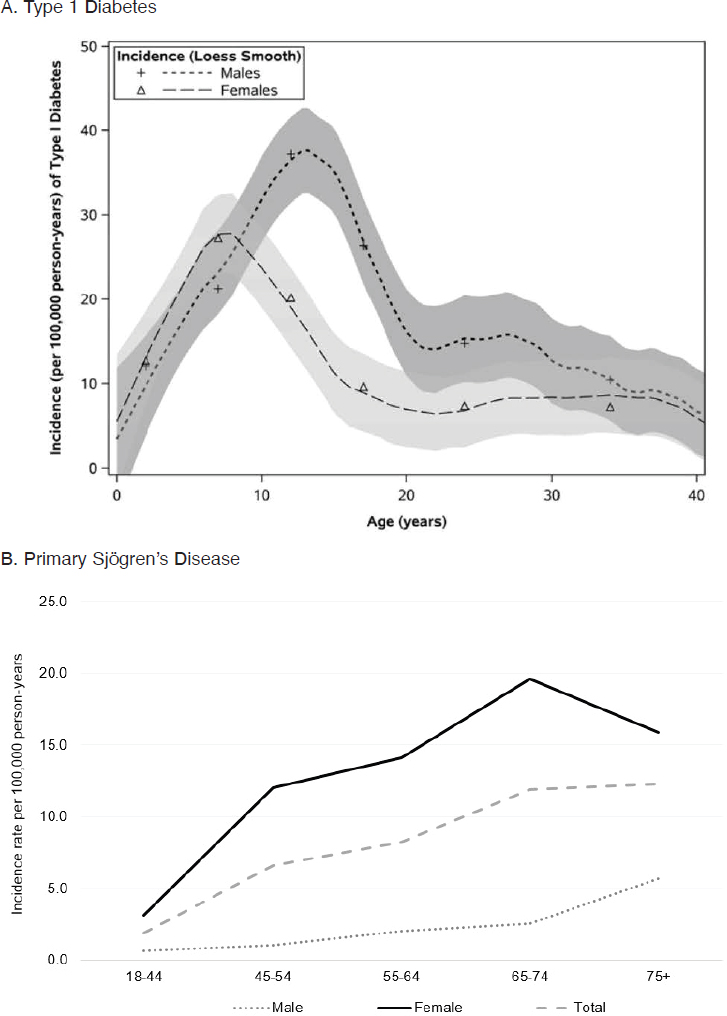
NOTE: Per 100,000 person-years = annually per 100,000 persons.
SOURCES: A. Cartee et al., 2016; B. Figure created using data from Maciel et al., 2017a; C. and D. Shivashankar et al., 2017.
that the terms “race” and “ethnicity” denote the full breadth of experiences and exposures of a population, rather than a limited lens focused on genetic variability among populations (see Box 2-2).
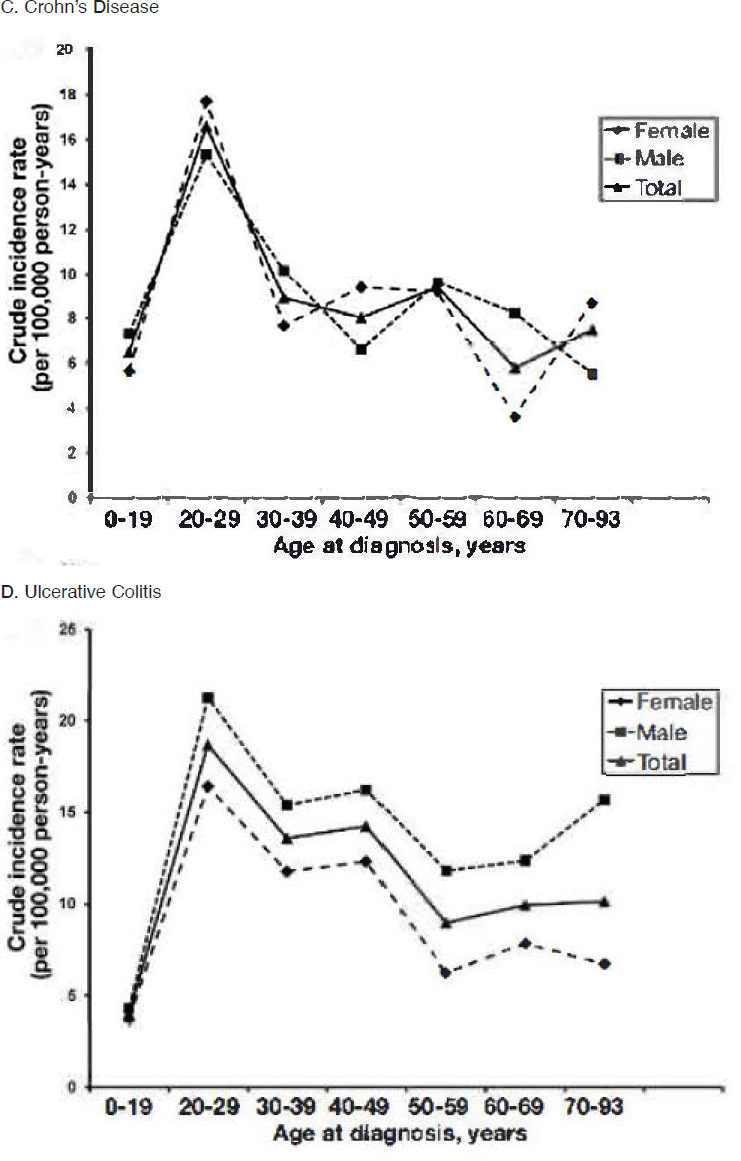
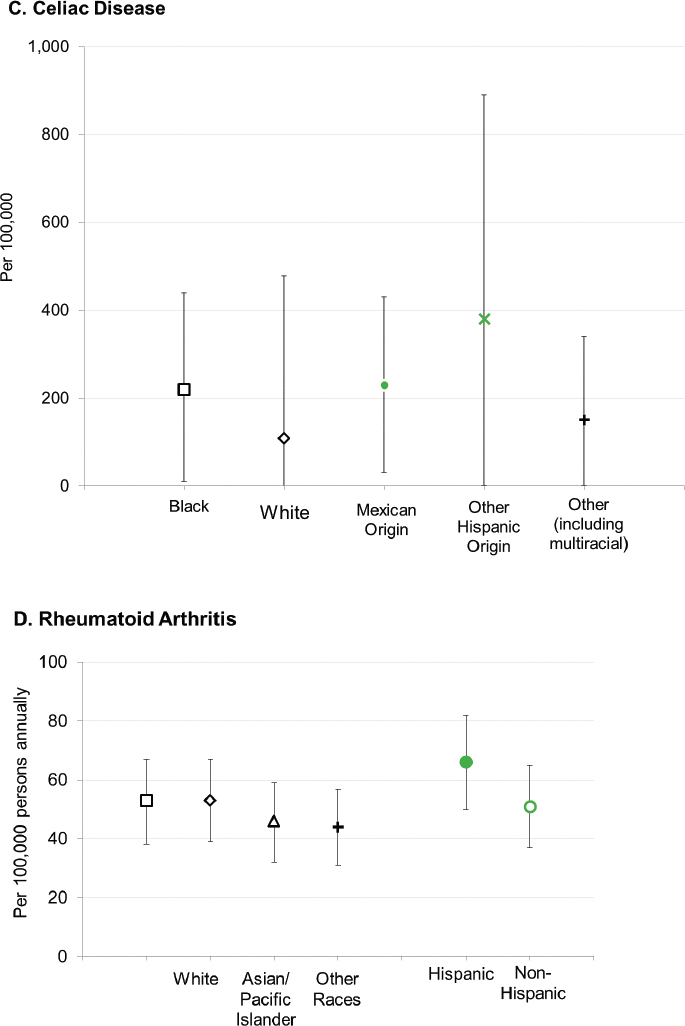
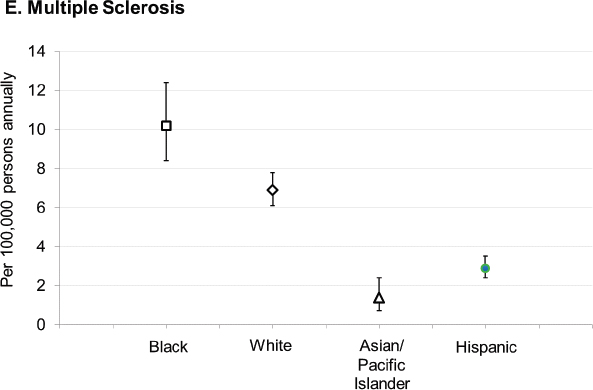
With respect to racial and ethnic disparities in incidence or prevalence of disease, it is important to note that no single pattern describes the patterns seen among the autoimmune diseases; for some diseases, the highest rates occur among Black individuals, while for other diseases, the highest rates occur in White individuals (Figure 2-3). Studies have reported increased rates of juvenile idiopathic arthritis, autoimmune liver disease, and SLE in Indigenous peoples in the United States and Canada (Barnabe et al., 2012; Mauldin et al., 2004; Yoshida et al., 2006). These studies reinforce the need for more directed research into autoimmune disease risk in American Indian and Alaska Native populations.
Studies have also found disparities in the severity and prognosis of many diseases according to race and ethnicity (Barton et al., 2011; Lim et al., 2019; Ventura et al., 2017). Barriers to diagnosis, access to specialist care or to enrollment in clinical trials, and affordability of treatments are all issues that can affect people with autoimmune diseases (Bailey et al.,
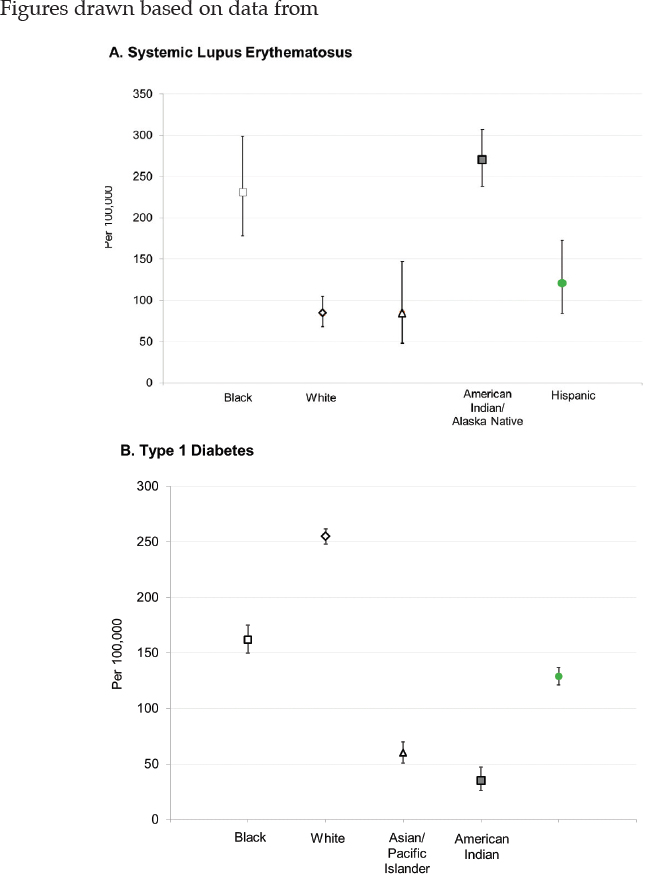
NOTE: Per 100,000 person-years = annually per 100,000 persons.
SOURCES: Figure A. created based on data drawn from Izmirly et al., 2021b; Figure B. created based on data drawn from Dabelea et al., 2014; Figure C. created based on data drawn from Mardini et al., 2015; Figure D. created based on data drawn from Kawatkar et al., 2019; Figure E. created based on data drawn from Langer-Gould et al., 2013.
2021; Sankar et al., 2004). These barriers can be geographic (e.g., availability of services in rural areas), economic, and rooted in sociocultural experiences that can result in mistrust of medicine or medical services.
The previous discussion has highlighted diseases with moderate to strong predominance in women and diseases with increased risk in racial and ethnic minority populations. Within the full spectrum of autoimmune diseases, however, many other autoimmune illnesses do not reflect this pattern. Differences in the incidence or severity of diseases across demographic groups may have important implications for diagnosis and management of disease and may implicate the importance of genetic susceptibility in conjunction with environmental factors in disease pathogenesis. The committee believes that these striking variations in sex, race, and age patterns among autoimmune diseases are worthy of further research.
Finding: There is a lack of population-based data from diverse populations to accurately assess the incidence, prevalence, lifetime risk, epidemiologic trends, and the extent of the impact of autoimmune diseases on the U.S. population. In addition, existing data may not distinguish sex and gender. The best available long-term data are from a relatively racially and socioeconomically homogenous population, thereby limiting its application for understanding the variation
in genetic susceptibility and the variety of exposures, experiences, and socioeconomic drivers of autoimmune diseases.
Conclusion: There is a need for population-based epidemiology studies that can provide the basis for studying numerous autoimmune diseases over at least a 20-year period. This resource could collectively provide a picture of the impact of these diseases in all groups representative of the U.S. population, and investigators could use it to facilitate the kind of population-based research needed to fully understand the development and prognosis of these diseases.
Coexisting Autoimmune Diseases
Historically, medical management occurring in subspecialties according to the organ system involved may have obscured associations between autoimmune diseases. However, shared features among certain autoimmune diseases is so well recognized, particularly within rheumatology, that nomenclature distinguishes between disease that is primary (standalone) or secondary to another autoimmune condition. In one study, a second diagnosable autoimmune disease occurred in 52 percent of those diagnosed with Sjögren’s disease, 43 percent of those with antiphospholipid syndrome, 38 percent of those with SLE, and 30 percent of those with rheumatoid arthritis (Lockshin et al., 2015). The terms “primary” and “secondary” are misleading, however, since no evidence exists to support the hypotheses that one autoimmune disease precedes, causes, or dominates the other, or even that they are independently diagnosable illnesses. The appropriateness of such terminology was recently reviewed for Sjögren’s disease (Kollert and Fisher, 2020), but the concepts discussed apply to other autoimmune diseases as well. Thus, the designation of “primary” or “secondary” has largely fallen out of favor.
In the medical literature, definitions of terms such as “comorbidity” and “complication” can be fluid and overlapping (Valderas et al., 2009). That much remains unknown about autoimmune diseases further complicates the use of precise definitions. For the purpose of this report, the committee uses the definitions provided in Box 2-3.
Abundant anecdotal evidence and case series of autoimmune disease clustering, beyond the classically described “overlap” conditions, has prompted research addressing whether co-occurrence of selected autoimmune diseases occurs within individuals and families at higher rates than expected by chance (Somers et al., 2006). Investigators have not studied all combinations of autoimmune diseases at the population level, and key studies on autoimmune coexistence within individuals have focused largely on multiple sclerosis, rheumatoid arthritis, autoimmune
thyroiditis (Hashimoto’s thyroiditis), type 1 diabetes, IBD, and vitiligo as index conditions (Cooper et al., 2009). Overall, data support the idea that intra-person co-occurrence does occur at greater than expected rates for several combinations of autoimmune disease. For example, two large studies—one examining Kaiser Permanente Health Plan records and another examining two U.S. medical claim data sets—found that persons diagnosed with IBD had a significantly increased risk of developing multiple sclerosis, psoriasis or psoriatic arthritis, and rheumatoid arthritis compared with persons without an IBD diagnosis; moreover, the second study also found an increased risk of developing ankylosing spondylitis (Cohen et al., 2008; Weng et al., 2007).
A major epidemiologic study in the United Kingdom investigated the co-occurrence of four autoimmune conditions within individuals—multiple sclerosis, rheumatoid arthritis, type 1 diabetes, and autoimmune thyroiditis (Somers et al., 2009). After controlling for age and calendar year, this study documented increased co-occurrence between rheumatoid arthritis, autoimmune thyroiditis, and type 1 diabetes compared with population expected rates, regardless of diagnostic sequence; sex-specific results were consistent with the overall findings (Figure 2-4). The magnitude of association was most prominent for autoimmune thyroiditis among patients with type 1 diabetes, in which risk was more than four times that expected. A notable exception to the premise of clustering was between multiple sclerosis and rheumatoid arthritis, where findings suggested an inverse association. Importantly, this study predated the availability in the United Kingdom of tumor necrosis factor inhibitors, used today to treat some autoimmune diseases, which otherwise could have confounded results given reports of multiple sclerosis in association
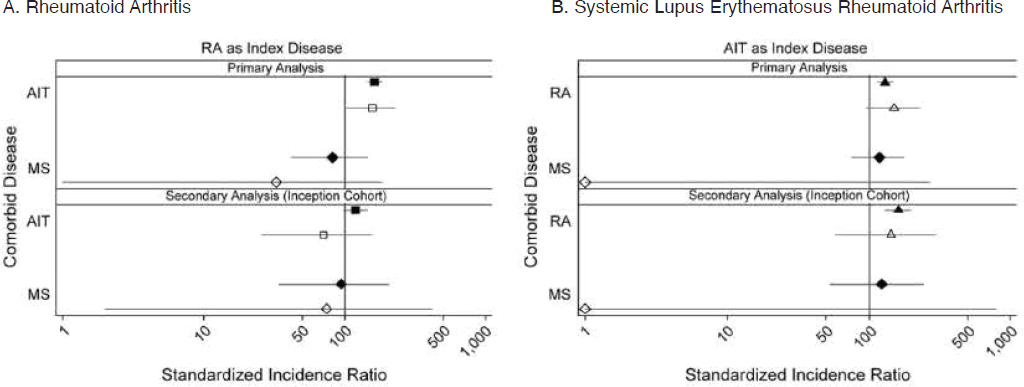
 ): females; hollow symbols (
): females; hollow symbols ( ): males. Coexisting autoimmune diseases: (
): males. Coexisting autoimmune diseases: ( /
/ ): rheumatoid arthritis; (
): rheumatoid arthritis; ( /
/ ): autoimmune thyroiditis; (
): autoimmune thyroiditis; ( /
/ ): multiple sclerosis.
): multiple sclerosis.NOTE: AIT, autoimmune thyroid thyroiditis; ANCA, anti-neutrophil cytoplasmic autoantibody; IDDM, insulin-dependent diabetes mellitus; MS, multiple sclerosis; RA, rheumatoid arthritis.
SOURCE: Somers et al., 2009.
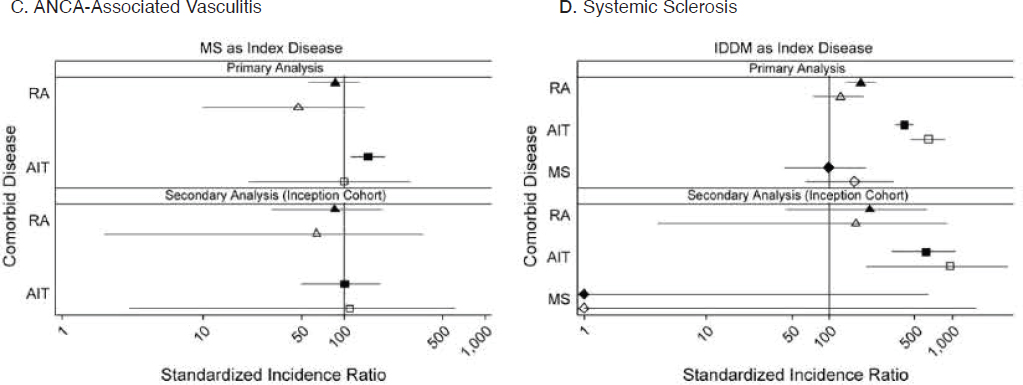
with such treatment. An inverse association between multiple sclerosis and rheumatoid arthritis was also supported by a pair of Danish studies of individuals and families (Eaton et al., 2007; Nielsen et al., 2008) and within families based on a systematic review (Somers et al., 2006). A broader implication is that characterization of baseline rates of coexistence provides important context for interpreting pharmacovigilance data as immunotherapy options continue to expand (Somers et al., 2009).
Family studies have also reported increased occurrence of autoimmune diseases among first degree relatives of case versus control individuals. Aside from the combinations of autoimmune diseases detected within individuals described above, additional disease associations reported in family studies include polyarteritis nodosa, Addison’s disease, Crohn’s disease, and “autoimmune diseases in general” in relatives of individuals with multiple sclerosis; autoimmune thyroid diseases and “autoimmune diseases in general” for SLE; “autoimmune diseases in general” for Sjögren’s disease; and autoimmune thyroid diseases and “autoimmune diseases in general” for idiopathic inflammatory myopathy (Cooper et al., 2009).
Finding: Limited data exist on the presence of co-occurring autoimmune diseases in individuals, and the studies have concentrated on comparatively few autoimmune diseases when measured against the large number of diseases generally accepted as being autoimmune diseases.
Conclusion: Additional research is needed to identify the patterns of a broad range of co-occurring autoimmune diseases as this could provide important context for interpreting pharmacovigilance data and provide insight into underlying biologic mechanisms that could inform strategies for the prevention, early diagnosis, and treatment of subsequent autoimmune diseases in individuals with autoimmune disease.
Morbidity, Mortality, and Quality of Life
The manifestations and consequences of autoimmune diseases vary depending on the target organs or systems. It is important to view the effects of autoimmune diseases not just through a lens directed at physical health, but through one that also views elements such as normal social interaction and development, mental health, the ability to gain an education and pursue employment, and the capacity to have children, all of which can affect quality of life (Figure 2-5).
For some autoimmune diseases, acute effects can be severe. Undiagnosed or inadequately controlled type 1 diabetes, for example, can lead to
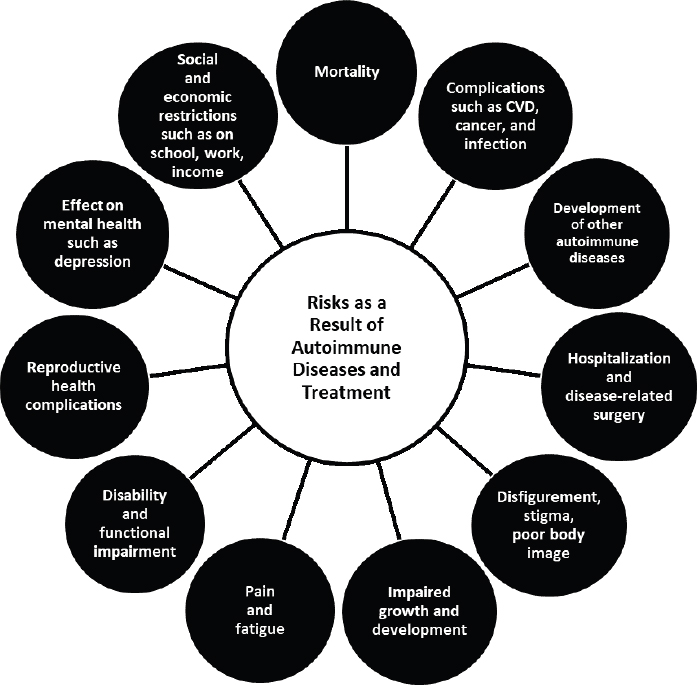
NOTE: CVD, cardiovascular disease.
diabetic ketoacidosis, coma, and death. SLE has a relatively high mortality rate and a significant racial disparity in mortality, and mortality risk is significantly elevated compared with expected rates (standardized mortality ratios, 2 to 5 times higher) (Gianfrancesco et al., 2021; Jorge et al., 2018). Ten-year mortality rates in an incidence cohort of SLE in Georgia were 28 percent in Black persons and 9 percent in White persons (Lim et al., 2019). In other autoimmune diseases, absolute and relative mortality risks are lower. A meta-analysis of 35 studies of IBD reported a standardized mortality ratio of 1.08 (95 percent confidence interval 0.97–1.21) in inception cohorts of ulcerative colitis and 1.34 (95 percent confidence interval 1.15–1.56) in inception cohorts of Crohn’s disease (Bewtra et al., 2013).
For many autoimmune conditions, the underlying disease process, including a chronic inflammatory response and/or the long-term use of immunosuppressant drugs, results in increased risks of infectious disease
(bacterial, viral, mycobacterial, and fungal) as well as cardiovascular disease. For example, an elevated risk of cardiovascular disease, including coronary revascularization procedures, myocardial infarction, peripheral vascular disease, and cardiovascular deaths, occurs for rheumatoid arthritis, systemic sclerosis, and SLE (Kremers et al., 2008; Kurmann et al., 2020; Man et al., 2013; McMahon et al., 2011). The concept of accelerated atherosclerosis associated with autoimmune disease is key to understanding the increased risk of cardiovascular disease seen even at relatively young ages (under 45 years of age), and the evaluation and control of cardiovascular risk factors is a vital component of clinical care for autoimmune diseases (Durante and Bronzato, 2015).
Autoimmune diseases are also associated with specific types of cancer, and there is a need for a better understanding of pathogenic mechanisms in these individuals as well as optimal patient care. One study using the SEER database found that Sjögren’s disease, rheumatoid arthritis, SLE, and autoimmune hemolytic anemia were associated with a 1.5- to 2-fold increased risk of three or more types of lymphomas, while psoriasis, pemphigus, and discoid lupus erythematosus were associated with a 3- to 6-fold increased risk of T-cell non-Hodgkin lymphoma (Anderson et al., 2009; Chiesa Fuxench et al., 2016).
Some autoimmune diseases are also associated with increased cancer risk at specific sites related to the disease. For example, people with IBD have an increased incidence of colorectal and other gastrointestinal cancers (Axelrad et al., 2016). The role of chronic inflammation, prolonged use of immunosuppressant agents, and common risk factors—as in the case of rheumatoid arthritis and lung cancer (Simon et al., 2015)—are important avenues for future research into cancer risk and autoimmune disease. In addition, much remains to be learned about how best to treat patients with autoimmune disease and cancer. Until recently, for example, patients with autoimmune disease and cancer were excluded from clinical trials of immunotherapy, which increases the immune system’s capability to detect and kill tumor cells, because of concerns that the drugs might increase autoimmunity and possibly cause severe and life-threatening complications (NLM, 2021).
Flare-ups of symptoms or disease activity, often requiring hospitalization, are common in many autoimmune diseases, including multiple sclerosis, rheumatoid arthritis, SLE, and IBD (Morales-Tisnes et al., 2021; Panopalis et al., 2012). The remitting-relapsing course of these diseases can be challenging to manage from a medical as well as a social and psychological perspective. In addition, the damage caused by some autoimmune diseases, such as vasculitis, SLE, and PBC, may require organ transplantation (Albuquerque et al., 2019; Carey et al., 2015; Jain et al., 2021).
Pain is a common symptom in many autoimmune diseases although the etiology varies. For example, owing to nerve involvement or damage in multiple sclerosis, individuals with the disease often experience spasticity with stiffness, flexor spasms, and uncontrollable muscle contractions as well as the burning sensations of dysesthesias and facial pain due to the severe stabbing-like tic douloureux (IOM, 2001). The chronic inflammation in rheumatoid arthritis causes progressive joint deterioration and secondary osteoarthritis, leading to pain and stiffness; while up to 90.4 percent of patients with the disease seek health care for severe pain, pain-management options remain limited (Sanchez-Florez et al., 2021). Persons with IBD often experience joint and spinal pain from inflammatory arthritis, in addition to abdominal and pelvic pain; in those with perianal disease from fistulae, pain can be severe. Moreover, pain is a risk factor for depression, anxiety, and disability in individuals with IBD (van der Valk et al., 2014a).
One of the most common complaints among individuals with autoimmune diseases is fatigue, which in this context is particularly complex and variable because it is likely linked to differing causal mechanisms. Multiple physiological processes may contribute to fatigue in autoimmune diseases including inflammatory activation of the immune system that affects the peripheral and central nervous systems (Lee and Giuliani, 2019b; Morris et al., 2015). Fatigue can be debilitating, impede performance of even simple daily tasks, and contribute to mental health problems such as depressed mood (Zielinski et al., 2019). When fatigue prevents people from fulfilling normal social roles or holding a job, it can cause isolation, impose financial burdens on them and their families, and decrease quality of life. There are currently no long-lasting interventions to effectively treat fatigue in persons with autoimmune diseases (Zielinski et al., 2019).
Autoimmune disease can have impacts on physical function. Neurological effects in multiple sclerosis include vision impairment and problems with balance and mobility, some being severe, and these effects are highly important to people living with this condition (Heesen et al., 2008). Blindness resulting from retinopathy is one of the most common complications of type 1 diabetes (James et al., 2014). In addition, the lesions and scarring that can result from skin manifestations of some diseases, such as psoriasis, can result in disfigurement and stigmatization (van Beugen et al., 2017). All of these effects can act to isolate a person physically and/or psychologically, impede social relationships, and restrict access to education and employment. Measures focusing on health care utilization may, in fact, overlook the significant effects of autoimmune diseases.
Children with autoimmune diseases are at risk for adverse impacts on growth and development as a result of the diseases and their treatment.
Growth failure, as indicated by height velocity and final height that rank below age- and sex-expected percentile norms, can be caused by the effects on bone growth of chronic systemic inflammation as well as glucocorticoid therapy. Inflammation may also cause delayed puberty, which may adversely impact peak bone mass and linear growth (Kao et al., 2019). Studies in individuals with childhood-onset SLE have found that about 15 percent experience low height velocity (Bandeira et al., 2006; Gutierrez-Suarez et al., 2006), that the average final height is lower than target height, a calculation based on the heights of the parents (Heshin-Bekenstein et al., 2018), and that these effects are most marked in those experiencing pre-menarche disease onset (Sontichai et al., 2020). A recent large cohort study found that 10 percent of children with the systemic form of juvenile idiopathic arthritis had short stature (Guzman et al., 2017). However, some children with autoimmune disease experience a period of catch-up growth when their disease responds to treatment and/or glucocorticoid therapy is reduced (Gutierrez-Suarez et al., 2006; Guzman et al., 2017). Growth hormone therapy also may mitigate adverse growth effects (Simon and Bechtold, 2009). Development of new effective glucocorticoid-sparing medications may lessen adverse effects on growth and development for children with autoimmune diseases.
In young adults and during the reproductive years, autoimmune diseases can generate additional risks and complications (Sammaritano et al., 2020). Pregnancy or the post-partum period may worsen the course of autoimmune diseases, or the disease itself may result in increased risk of adverse pregnancy outcomes, including spontaneous abortion. This can lead to the need to make difficult choices regarding the use of disease-modifying medications during pregnancy. Those wishing to have children may have to consider the potential effects of treatments on fertility or carrying a pregnancy to term, and consider options for oocyte preservation.
Another effect of autoimmune diseases, particularly during the young and middle-aged adult years, is employment-related disability. Studies of people with IBD, multiple sclerosis, psoriasis, rheumatoid arthritis, systemic sclerosis, and SLE report partial or full work disability, with one cohort study of persons with early rheumatoid arthritis observing a work disability rate of 28 percent at study start and 44 percent 15 years later (Eberhardt et al., 2007; Orbai et al., 2021; Raggi et al., 2016; Scofield et al., 2008; Sharif et al., 2011; van der Valk et al., 2014b). The relapsing-remitting nature of these diseases, as well as some of the features that contribute to disability such as fatigue and pain, may result in additional challenges to receiving disability benefits (Scofield et al., 2008). The indirect costs of lost productivity represent approximately 30 percent of the estimated total costs of rheumatoid arthritis (Birnbaum et al., 2010) and psoriasis (Vanderpuye-Orgle et al., 2015) in the United States, and approximately
50 percent of the total costs of IBD during the first year after diagnosis among working-age people in Sweden (Khalili et al., 2020).
Within the sphere of mental health, depression or depressive symptoms are common among people living with autoimmune diseases and can contribute to the risk of reduced employment (Eckert et al., 2017; Kurd et al., 2010; Moustafa et al., 2020; Vanderpuye-Orgle et al., 2015). In some diseases, research has not ruled out the possibility that the direct central nervous system effects of rheumatoid arthritis, SLE, and multiple sclerosis may play a role in inducing depression (Vallerand et al., 2018, 2019). In addition, difficulties with mobility, the challenges of working while coping with disease flares, and the physical effects of specific diseases that produce visible damage to skin or joints are consequences of autoimmune diseases that can significantly contribute to social isolation and affect quality of life. The directionality of effects of inflammation, depression, fatigue, and disease expression is an area requiring additional research (Lee and Giuliani, 2019a; Vallerand et al., 2018).
Finding: Autoimmune diseases are associated with an increased risk of cancer.
Conclusion: Additional research is needed to characterize the roles of chronic inflammation, prolonged use of immunosuppressant agents, and common risk factors in increased cancer risk in persons with autoimmune disease. In addition, there is a need for research to obtain a greater understanding of how best to treat patients with autoimmune disease and cancer.
Finding: Fatigue and depression or depressive symptoms commonly occur in individuals with autoimmune diseases; their etiology is unclear. There are no long-lasting treatments for fatigue. Both can greatly affect quality of life.
Conclusion: Additional research is needed to understand the directionality of effects of inflammation, depression, fatigue, and disease expression in persons with autoimmune diseases.
Estimates of Economic Impact
Few studies have estimated the direct and indirect costs of specific autoimmune diseases in the United States, and the committee is not aware of any studies attempting to estimate the overall costs of these conditions. The limited information that is available on the economic impact of specific autoimmune diseases is discussed above and in Chapter 3. The available evidence, while limited in scope, supports the notion that the
direct and indirect costs of autoimmune diseases can be high (Adelman et al., 2013; Birnbaum et al., 2010; Vanderpuye-Orgle et al., 2015).
Finding: There are little data on the direct and indirect costs in the United States of specific autoimmune diseases and of autoimmune diseases overall.
THE LIFE-COURSE FRAMEWORK AND AUTOIMMUNE DISEASES
The life-course framework for health research is highly relevant for the study of autoimmune diseases. This framework emphasizes understanding how early life exposures—factors such as environmental toxicants or psychological stressors that are associated with an outcome—and timing of these exposures influence health along the life stages (Ben-Shlomo and Kuh, 2002; Jacob et al., 2017; Liu et al., 2010; Lynch and Smith, 2005), both at a population and individual patient level. This approach highlights the importance of conceptualizing autoimmune diseases as a result of social and environmental factors, or exposures, experienced at different points, from the in utero period, through childhood and adolescence, and on to young, mid-, and older adulthood (Figure 2-6).
Examples of environmental exposures are toxicants and viruses, and examples of social exposures are stressors such as poverty, abuse, and discrimination. The multidisciplinary life-course framework enables examination of how these social and environmental determinants of health throughout the life stages can differentially affect biological processes to influence the development and the course of chronic diseases. For
SOURCE: Adapted with permission from National Institute of Environmental Health Sciences.
example, data from the longitudinal World Trade Center Health Registry show an increased risk for developing systemic autoimmune disease following exposure to dust and traumatic experiences from the September 11, 2001, terrorist attack (Miller-Archie et al., 2020). Additionally, data from the Nurses’ Health Study II, a large longitudinal cohort of U.S. female nurses, show an increased risk of developing SLE in those with exposure to childhood physical and emotional abuse (Feldman et al., 2019).
The epidemiologic study of autoimmune diseases using a life-course framework emphasizes a longitudinal approach inclusive of all life stages, linking a multiplicity of exposures across the life course to later health outcomes. It also emphasizes attention to the inter-relationships of these exposures and vulnerable developmental time periods. For example, findings from the National Institute of Environmental Health Sciences-funded Sister Study Cohort (Parks et al., 2016) showed that the early life factors of preterm birth and childhood pesticide exposure were associated with development of SLE in adulthood. Additional aspects for chronic autoimmune diseases in particular include the fluctuation of disease activity over periods of flare and remission, as well as the accumulation of permanent organ damage over time resulting from inflammation. Accounting for these complexities often presents methodological challenges for modeling repeat observations, hierarchical data, latent exposures, and multiple interactive effects (Kuh et al., 2003). Researchers have used complex analytic models, such as multi-level models, latent growth models, and Markov models in studying chronic diseases, and they are highly useful for understanding complex autoimmune diseases.
While researchers have applied the life-course framework to the epidemiologic study of populations, it is also useful for studying disease at the individual level (Halfon and Hochstein, 2002). Measuring the effect of socio-environmental factors on individuals with autoimmune disease across time and critical developmental periods increases the understanding of disease mechanisms, trajectories, and heterogeneity. This is of particular relevance to studying autoimmune diseases because they typically evolve over time, and a given disease can manifest differently from patient to patient. To understand the mechanisms underlying development and course of autoimmune diseases, it is important to characterize changes in biologic structure and function across the lifespan, and how genetic, social, and environmental factors cause deviation in expected biologic trajectories of typical development (Hardy and O’Neill, 2020). These trajectories may be non-linear, reflecting critical exposure periods, differential impacts across time and biologic structures, and the biologic impact of cumulative exposures. To increase knowledge of autoimmune disease and develop treatments, long-term systems for data collection
and complex analytic approaches are necessary to model these aspects of disease mechanisms and trajectories within and across individuals and to elucidate the heterogeneity present within diseases.
Several implications arise from incorporating a life-course approach for studying autoimmune diseases:
- This approach requires longitudinal studies that incorporate children and adult populations, with particular attention to critical life periods for the effects of inflammation and disease damage.
- Methodological expertise is critical for modeling the issues of time, timing, and inter-relationship of exposures in relation to disease mechanisms and outcomes.
- A team science approach is key to bringing together multidisciplinary expertise in genetics and biology, social and environmental factors, life stages, and other relevant knowledge areas.
- Although the life-course approach involves epidemiology at the population level, it is also relevant to understanding disease processes at the individual patient level. It therefore provides understanding of opportunities for development of clinical treatments and behavioral interventions, as well as policy changes aimed at both prevention and provision of interventions.
Finding: A life-course approach to the epidemiological study of autoimmune disease could involve longitudinal studies that incorporate children and adult populations, enable modeling of the issues of time, timing, and inter-relationship of exposures in relation to disease mechanisms and outcomes, and engage multidisciplinary expertise in genetics and biology, social and environmental factors, life stages, and other relevant knowledge areas. Such an approach could better characterize the disease processes of autoimmune diseases, conditions that can affect individuals at any age and that are chronic, heterogeneous, and may involve flare and remission.
SUMMARY AND RESEARCH IMPLICATIONS
Autoimmunity arises when the immune system fails to distinguish self from non-self. This can result, over time, in disease conditions involving pathological tissue damage caused by autoreactive T cells and autoantibodies. Both the innate and adaptive immune systems are involved in promoting autoimmune disease, and genetics, social and environmental exposures, and sex differences may contribute to the development or exacerbation of autoimmune diseases.
There is no consensus definition of autoimmune disease. For more than 50 years, clinical criteria such as specific autoantibodies and symptoms have been used to characterize and classify autoimmune diseases. More recently, insights from research on biologic mechanisms have revealed commonalities between autoimmune diseases and autoinflammatory diseases, as well as other diseases that are not considered to be autoimmune diseases, making the definition less clear-cut. The two different approaches to characterize and understand autoimmune diseases each have advantages. Defining autoimmune diseases according to biological mechanisms may be useful in developing interventions and treatment, particularly when the mechanisms are shared across diseases. Characterizing autoimmune diseases by clinical phenotype alone, however, can impact patient care and impede research. An atypical presentation of an autoimmune disease, for example, could slow its diagnosis and treatment, or result in the person with the disease being excluded from a clinical trial when their inclusion might provide insight into the disease.
Incidence and prevalence data for autoimmune diseases in the United States are limited and not easily accessible. The last study that considered the prevalence of autoimmune diseases as a whole in the United States estimated a 7.6 to 9.4 percent prevalence of these conditions, but the study focused on just 29 autoimmune diseases and was published more than a decade ago (Cooper et al., 2009). Among the most common autoimmune diseases are celiac disease, rheumatoid arthritis, psoriasis, IBD, and type 1 diabetes. A critical limitation is the lack of population-based data from diverse populations that would enable researchers to accurately assess the incidence, prevalence, lifetime risk, and epidemiologic trends of autoimmune diseases in the U.S. population as well as extent of their impact.
Many, but not all, autoimmune diseases predominantly affect women, with some female-to-male incidence ratios equaling 6:1 or greater. Exceptions include type 1 diabetes and myocarditis, which occur more often in males (Coronado et al., 2019; Fairweather et al., 2013). No single pattern of racial and ethnic disparities is seen in incidence or prevalence among autoimmune diseases; for some conditions, the highest rates occur among Black, American Indian, and Alaska Native populations, while for others, the highest rates occur in White populations (Izmirly et al., 2021b). Researchers have also observed disparities in the severity and prognosis of autoimmune diseases according to race and ethnicity (Barton et al., 2011; Lim et al., 2019; Ventura et al., 2017). Research is needed to identify the reasons for these and other disparities. Autoimmune diseases display varied age-of-onset patterns, with some occurring primarily in children, teens, and young adults, such as type 1 diabetes, and others occurring more commonly through adulthood or reaching the highest incidence after age 60, such as primary Sjögren’s disease.
Autoimmune diseases are heterogeneous and long-lasting conditions. The impact on physical health—which can include pain and fatigue, an increased risk of developing a wide variety of other conditions, impaired growth and development, disfigurement, disability, functional impairment, and death—is substantial. Having an autoimmune disease may carry an increased risk of cancer (Anderson et al., 2009; Axelrad et al., 2016; Chiesa Fuxench et al., 2016); research is needed to establish how best to treat the patient. Autoimmune diseases can have adverse effects on social interaction and development, mental health, the capacity to have children, and education and employment.
Economic-impact research is inadequate, but available data indicate that the direct and indirect costs of autoimmune diseases can be high. Accurate mortality data are also lacking, one reason being that death certificates may not provide complete information on the autoimmune disease that contributed to death (Calvo-Alén et al., 2005; Molina et al., 2015).
The study of co-occurring autoimmune diseases in individuals has focused largely on multiple sclerosis, rheumatoid arthritis, autoimmune thyroiditis, type 1 diabetes, and IBD, with several combinations occurring at greater than expected rates. A broader understanding of the patterns of coexisting autoimmune diseases, as well as of their underlying biologic mechanisms, could inform strategies for the prevention, early diagnosis, and treatment of subsequent autoimmune diseases in individuals with one autoimmune disease.
Finally, applying a life-course, multidisciplinary framework to longitudinal epidemiologic studies that include children and adults could provide insight into issues involving exposures and timing that play a role in the etiology, mechanisms, and course and outcomes of these highly variable diseases.
REFERENCES
AARDA (American Autoimmune Related Diseases Association). 2017. AARDA survey study. Eastpointe, MI: American Autoimmune Related Diseases Association. https://www.aarda.org/wp-content/uploads/5yr-Survey-Study-REPORT-updated-2017.pdf (accessed July 8, 2021).
Abbas, A. K., J. Lohr, B. Knoechel, and V. Nagabhushanam. 2004. T cell tolerance and autoimmunity. Autoimmunity Reviews 3(7-8):471–475. https://doi.org/10.1016/j.autrev.2004.07.004.
Abramson, O., M. Durant, W. Mow, A. Finley, P. Kodali, A. Wong, V. Tavares, E. McCroskey, L. Liu, J. D. Lewis, J. E. Allison, N. Flowers, S. Hutfless, F. S. Velayos, G. S. Perry, R. Cannon, and L. J. Herrinton. 2010. Incidence, prevalence, and time trends of pediatric inflammatory bowel disease in northern California, 1996 to 2006. Journal of Pediatrics 157(2):233-239.e231. https://doi.org/10.1016/j.jpeds.2010.02.024.
Adelman, G., S. G. Rane, and K. F. Villa. 2013. The cost burden of multiple sclerosis in the United States: A systematic review of the literature. Journal of Medical Economics 16(5):639–647. https://doi.org/10.3111/13696998.2013.778268.
Aggarwal, R., J.-M. Anaya, K. A. Koelsch, B. T. Kurien, and R. H. Scofield. 2015a. Association between secondary and primary Sjögren’s syndrome in a large collection of lupus families. Autoimmune Diseases 2015:298506. https://doi.org/10.1155/2015/298506.
Aggarwal, R., S. Ringold, D. Khanna, T. Neogi, S. R. Johnson, A. Miller, H. I. Brunner, R. Ogawa, D. Felson, A. Ogdie, D. Aletaha, and B. M. Feldman. 2015b. Distinctions between diagnostic and classification criteria? Arthritis Care & Research 67(7):891–897. https://doi.org/10.1002/acr.22583.
Albuquerque, B. C., V. B. Salles, R. D. d. P. Tajra, and C. E. M. Rodrigues. 2019. Outcome and prognosis of patients with lupus nephritis submitted to renal transplantation. Scientific Reports 9(1):11611. https://doi.org/10.1038/s41598-019-48070-y.
American Board of Medical Specialties. n.d. Specialty and subspecialty certificates. https://www.abms.org/member-boards/specialty-subspecialty-certificates (accessed January 26, 2022).
American College of Rheumatology. Criteria. https://www.rheumatology.org/Practice-Quality/Clinical-Support/Criteria (accessed January 26, 2022).
Anaya, J. M. 2012. Common mechanisms of autoimmune diseases (the autoimmune tautology). Autoimmunity Reviews 11(11):781–784. https://doi.org/10.1016/j.autrev.2012.02.002.
Anderson, L. A., S. Gadalla, L. M. Morton, O. Landgren, R. Pfeiffer, J. L. Warren, S. I. Berndt, W. Ricker, R. Parsons, and E. A. Engels. 2009. Population-based study of autoimmune conditions and the risk of specific lymphoid malignancies. International Journal of Cancer 125(2):398–405. https://doi.org/10.1002/ijc.24287.
Asgari, M. M., J. J. Wu, J. M. Gelfand, C. Salman, J. R. Curtis, L. R. Harrold, and L. J. Herrinton. 2013. Validity of diagnostic codes and prevalence of psoriasis and psoriatic arthritis in a managed care population, 1996-2009. Pharmacoepidemiology and Drug Safety 22(8):842–849. https://doi.org/10.1002/pds.3447.
Autoimmune Association. 2021. Autoimmune disease list. https://autoimmune.org/about-us (accessed November 29, 2021).
Autoimmune Registry Inc. 2020. The autoimmune diseases. https://www.autoimmuneregistry.org/autoimmune-diseases (accessed April 12, 2021).
Autoimmune Registry, Inc. 2021. About Autoimmune Registry, Inc. https://www.autoimmuneregistry.org/about-us (accessed June 22, 2021).
Axelrad, J. E., S. Lichtiger, and V. Yajnik. 2016. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World Journal of Gastroenterology 22(20):4794–4801. https://doi.org/10.3748/wjg.v22.i20.4794.
Baer, A. N., J. W. Maynard, F. Shaikh, L. S. Magder, and M. Petri. 2010. Secondary Sjögren’s syndrome in systemic lupus erythematosus defines a distinct disease subset. Journal of Rheumatology 37(6):1143–1149. https://doi.org/10.3899/jrheum.090804.
Bailey, Z. D., J. M. Feldman, and M. T. Bassett. 2021. How structural racism works - racist policies as a root cause of U.S. racial health inequities. New England Journal of Medicine 384(8):768–773. https://doi.org/10.1056/NEJMms2025396.
Bandeira, M., S. Buratti, M. Bartoli, C. Gasparini, L. Breda, A. Pistorio, S. Grassi, M. G. Alpigiani, G. Barbano, L. L. Janz-Junior, A. Martini, and A. Ravelli. 2006. Relationship between damage accrual, disease flares and cumulative drug therapies in juvenile-onset systemic lupus erythematosus. Lupus 15(8):515–520. https://doi.org/10.1191/0961203306lu2316oa.
Barnabe, C., L. Joseph, P. Belisle, J. Labrecque, S. Edworthy, S. G. Barr, M. Fritzler, L. W. Svenson, B. Hemmelgarn, and S. Bernatsky. 2012. Prevalence of systemic lupus erythematosus and systemic sclerosis in the first nations population of Alberta, Canada. Arthritis Care & Research 64(1):138–143. https://doi.org/10.1002/acr.20656.
Barnado, A., C. Casey, R. J. Carroll, L. Wheless, J. C. Denny, and L. J. Crofford. 2017. Developing electronic health record algorithms that accurately identify patients with systemic lupus erythematosus. Arthritis Care & Research 69(5):687–693. https://doi.org/10.1002/acr.22989.
Barton, J. L., L. Trupin, D. Schillinger, S. A. Gansky, C. Tonner, M. Margaretten, V. Chernitskiy, J. Graf, J. Imboden, and E. Yelin. 2011. Racial and ethnic disparities in disease activity and function among persons with rheumatoid arthritis from university-affiliated clinics. Arthritis Care & Research 63(9):1238–1246. https://doi.org/10.1002/acr.20525.
Bastard, P., L. B. Rosen, Q. Zhang, E. Michailidis, H. H. Hoffmann, Y. Zhang, K. Dorgham, Q. Philippot, J. Rosain, et al. 2020. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370(6515):eabd4585.
Beck, D. B., M. A. Ferrada, K. A. Sikora, A. K. Ombrello, J. C. Collins, W. Pei, N. Balanda, D. L. Ross, D. Ospina Cardona, Z. Wu, B. Patel, K. Manthiram, E. M. Groarke, F. Gutierrez-Rodrigues, P. Hoffmann, S. Rosenzweig, S. Nakabo, L. W. Dillon, C. S. Hourigan, W. L. Tsai, S. Gupta, C. Carmona-Rivera, A. J. Asmar, L. Xu, H. Oda, W. Goodspeed, K. S. Barron, M. Nehrebecky, A. Jones, R. S. Laird, N. Deuitch, D. Rowczenio, E. Rominger, K. V. Wells, C. R. Lee, W. Wang, M. Trick, J. Mullikin, G. Wigerblad, S. Brooks, S. Dell’Orso, Z. Deng, J. J. Chae, A. Dulau-Florea, M. C. V. Malicdan, D. Novacic, R. A. Colbert, M. J. Kaplan, M. Gadina, S. Savic, H. J. Lachmann, M. Abu-Asab, B. D. Solomon, K. Retterer, W. A. Gahl, S. M. Burgess, I. Aksentijevich, N. S. Young, K. R. Calvo, A. Werner, D. L. Kastner, and P. C. Grayson. 2020. Somatic mutations in uba1 and severe adult-onset autoinflammatory disease. New England Journal of Medicine 383(27):2628–2638. https://doi.org/10.1056/NEJMoa2026834.
Beko, E., S. Pervaiz, V. Nanda, and S. Dhawan. 1993. Long-term follow-up of patients with diffuse fasciitis and eosinophilia associated with l-tryptophan ingestion. Cutis 51(4):266–270.
Ben-Shlomo, Y., and D. Kuh. 2002. A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology 31(2):285–293. https://doi.org/10.1093/ije/31.2.285.
Bewtra, M., L. M. Kaiser, T. TenHave, and J. D. Lewis. 2013. Crohn’s disease and ulcerative colitis are associated with elevated standardized mortality ratios: A meta-analysis. Inflammatory Bowel Diseases 19(3):599–613. https://doi.org/10.1097/MIB.0b013e31827f27ae.
Birnbaum, H., C. Pike, R. Kaufman, M. Maynchenko, Y. Kidolezi, and M. Cifaldi. 2010. Societal cost of rheumatoid arthritis patients in the US. Current Medical Research and Opinion 26(1):77–90. https://doi.org/10.1185/03007990903422307.
Blomgren, S. E., J. J. Condemi, and J. H. Vaughan. 1972. Procainamide-induced lupus erythematosus. The American Journal of Medicine 52(3):338-348. https://doi.org/10.1016/0002-9343(72)90021-6.
Boehmer, T. K., L. Kompaniyets, A. M. Lavery, J. Hsu, J. Y. Ko, H. Yusuf, S. D. Romano, A. V. Gundlapalli, M. E. Oster, A. M. Harris. 2021. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. Morbidity and Mortality Weekly Report 70(35):1228–1232.
Brent, L., I. R. Cohen, P. C. Doherty, M. Feldmann, P. Matzinger, L. Ghost, S. T. Holgate, P. Lachmann, N. A. Mitchison, G. Nossal, N. R. Rose, and R. Zinkernagel. 2007. Crystal-ball gazing--the future of immunological research viewed from the cutting edge. Clinical & Experimental Immunology 147(1):1–10. https://doi.org/10.1111/j.1365-2249.2006.03234.x.
Broten, L., J. A. Aviña-Zubieta, D. Lacaille, L. Joseph, J. G. Hanly, L. Lix, S. O’Donnell, C. Barnabe, P. R. Fortin, M. Hudson, S. Jean, C. Peschken, S. M. Edworthy, L. Svenson, C. A. Pineau, A. E. Clarke, M. Smith, P. Bélisle, E. M. Badley, L. Bergeron, and S. Bernatsky. 2014. Systemic autoimmune rheumatic disease prevalence in Canada: Updated analyses across 7 provinces. Journal of Rheumatology 41(4):673–679.
Bouman, A., M. J. Heineman, M. M. Faas. 2005. Sex hormones and the immune response in humans. Human Reproduction Update 11(4):411–423.
Bruno, K. A., J. E. Mathews, A. L. Yang, J. A. Frisancho, A. J. Scott, H. D. Greyner, F. A. Molina, M. S. Greenaway, G. M. Cooper, A. Bucek, A. C. Morales-Lara, A. R. Hill, A. A. Mease, D. N. Di Florio, J. M. Sousou, A. C. Coronado, A. R. Stafford, and D. Fairweather. 2019. BPA alters estrogen receptor expression in the heart after viral infection activating cardiac mast cells and T cells leading to perimyocarditis and fibrosis. Frontiers in Endocrinology 10:598. https://doi.org/10.3389/fendo.2019.00598.
Buskiewicz, I. A., S. A. Huber, and D. Fairweather. 2016. Sex hormone receptor expression in the immune system. In Sex differences in physiology, edited by G. Neigh and M. Mitzelfelt. London, UK: Academic Press. Pp. 45–60.
Calvo-Alén, J., G. S. Alarcón, R. Campbell, Jr., M. Fernández, J. D. Reveille, and G. S. Cooper. 2005. Lack of recording of systemic lupus erythematosus in the death certificates of lupus patients. Rheumatology (Oxford, England) 44(9):1186–1189. https://doi.org/10.1093/rheumatology/keh717.
Carey, E. J., A. H. Ali, and K. D. Lindor. 2015. Primary biliary cirrhosis. Lancet 386:1565–1575. https://doi.org/10.1016/S0140-6736(15)00154-3.
Carroll, R. J., W. K. Thompson, A. E. Eyler, A. M. Mandelin, T. Cai, R. M. Zink, J. A. Pacheco, C. S. Boomershine, T. A. Lasko, H. Xu, E. W. Karlson, R. G. Perez, V. S. Gainer, S. N. Murphy, E. M. Ruderman, R. M. Pope, R. M. Plenge, A. N. Kho, K. P. Liao, and J. C. Denny. 2012. Portability of an algorithm to identify rheumatoid arthritis in electronic health records. Journal of the American Medical Informatics Association 19(e1):e162–169. https://doi.org/10.1136/amiajnl-2011-000583.
Cartee, A. K., L. A. Owens, B. D. Lahr, B. P. Yawn, J. A. Murray, and Y. C. Kudva. 2016. Incidence of type 1 diabetes is not increasing in a population-based cohort in Olmsted County, Minnesota, USA. Mayo Clinic Proceedings 91(8):1066–1073. https://doi.org/10.1016/j.mayocp.2016.05.019.
Castro-Correia, C., L. Correia-Sa, S. Norberto, C. Delerue-Matos, V. Domingues, C. Costa-Santos, M. Fontoura, and C. Calhau. 2018. Phthalates and type 1 diabetes: Is there any link? Environmental Science and Pollution Research International 25(18):17915–17919. https://doi.org/10.1007/s11356-018-1997-z.
Chae, D. H., C. D. Martz, T. E. Fuller-Rowell, E. C. Spears, T. T. G. Smith, E. A. Hunter, C. Drenkard, and S. S. Lim. 2019. Racial discrimination, disease activity, and organ damage: The Black women’s experiences living with lupus (BeWELL) study. American Journal of Epidemiology 188(8):1434–1443. https://doi.org/10.1093/aje/kwz105.
Chaplin, D. D. 2010. Overview of the immune response. Journal of Allergy and Clinical Immunology 125(2 Suppl 2):S3–23. https://doi.org/10.1016/j.jaci.2009.12.980.
Chen, Y., T. Wang, X. Liu, and R. R. Shankar. 2019. Prevalence of type 1 and type 2 diabetes among US pediatric population in the MarketScan Multi-State Database, 2002 to 2016. Pediatric Diabetes 20(5):523–529. https://doi.org/10.1111/pedi.12842.
Chiesa Fuxench, Z. C., D. B. Shin, A. Ogdie Beatty, and J. M. Gelfand. 2016. The risk of cancer in patients with psoriasis: A population-based cohort study in the health improvement network. JAMA Dermatology 152(3):282–290. https://doi.org/10.1001/jamadermatol.2015.4847.
Cho, J. H., and M. Feldman. 2015. Heterogeneity of autoimmune diseases: Pathophysiologic insights from genetics and implications for new therapies. Nature Medicine 21(7):730–738. https://doi.org/10.1038/nm.3897.
Ciccarelli, F., M. De Martinis, and L. Ginaldi. 2014. An update on autoinflammatory diseases. Current Medicinal Chemistry 21(3):261–269. https://doi.org/10.2174/09298673113206660303.
Cohen, R., D. Robinson, Jr., C. Paramore, K. Fraeman, K. Renahan, and M. Bala. 2008. Autoimmune disease concomitance among inflammatory bowel disease patients in the United States, 2001-2002. Inflammatory Bowel Diseases 14(6):738–743. https://doi.org/10.1002/ibd.20406.
Cooper, G. S., M. L. K. Bynum, and E. C. Somers. 2009. Recent insights in the epidemiology of autoimmune diseases: Improved prevalence estimates and understanding of clustering of diseases. Journal of Autoimmunity 33(3–4):197–207. https://doi.org/10.1016/j.jaut.2009.09.008.
Coronado, M. J., K. A. Bruno, L. A. Blauwet, C. Tschöpe, M. W. Cunningham, S. Pankuweit, S. van Linthout, E.-S. Jeon, D. M. McNamara, J. Krejčí, J. Bienertová-Vašků, E. J. Douglass, E. D. Abston, A. Bucek, J. A. Frisancho, M. S. Greenaway, A. R. Hill, H.-P. Schultheiss, L. T. Cooper, Jr., and D. Fairweather. 2019. Elevated sera sST2 is associated with heart failure in men ≤50 years old with myocarditis. Journal of the American Heart Association 8(2):e008968. https://doi.org/10.1161/jaha.118.008968.
Crow, Y. J., and J. Rehwinkel. 2009. Aicardi-Goutieres syndrome and related phenotypes: Linking nucleic acid metabolism with autoimmunity. Human Molecular Genetics 18(R2):R130–R136. https://doi.org/10.1093/hmg/ddp293.
Crowson, C. S., E. L. Matteson, E. Myasoedova, C. J. Michet, F. C. Ernste, K. J. Warrington, J. M. Davis, 3rd, G. G. Hunder, T. M. Therneau, and S. E. Gabriel. 2011. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis and Rheumatism 63(3):633–639.
Culpepper, W. J., R. A. Marrie, A. Langer-Gould, M. T. Wallin, J. D. Campbell, L. M. Nelson, W. E. Kaye, L. Wagner, H. Tremlett, L. H. Chen, S. Leung, C. Evans, S. Yao, N. G. LaRocca, and on behalf of the United States Multiple Sclerosis Prevalence Workgroup (MSPWG). 2019. Validation of an algorithm for identifying MS cases in administrative health claims datasets. Neurology 92(10):e1016–e1028. https://doi.org/10.1212/WNL.0000000000007043.
Dabelea, D., E. J. Mayer-Davis, S. Saydah, G. Imperatore, B. Linder, J. Divers, R. Bell, A. Badaru, J. W. Talton, T. Crume, A. D. Liese, A. T. Merchant, J. M. Lawrence, K. Reynolds, L. Dolan, L. L. Liu, R. F. Hamman, and Search for Diabetes in Youth Study. 2014. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 311(17):1778–1786. https://doi.org/10.1001/jama.2014.3201.
Dahlhamer, J. M., E. P. Zammitti, B. W. Ward, A. G. Wheaton, and J. B. Croft. 2016. Prevalence of inflammatory bowel disease among adults aged ≥18 years—United States, 2015. MMWR: Morbidity and Mortality Weekly Report 65(42):1166–1169.
Dall’Era, M., M. G. Cisternas, K. Snipes, L. J. Herrinton, C. Gordon, and C. G. Helmick. 2017. The incidence and prevalence of systemic lupus erythematosus in San Francisco County, California: The California Lupus Surveillance Project. Arthritis & Rheumatology 69(10):1996–2005.
de Jonge, H., L. Iamele, M. Maggi, G. Pessino, and C. Scotti. 2021. Anti-cancer auto-antibodies: Roles, applications and open issues. Cancers 13(4). https://doi.org/10.3390/cancers13040813.
Desai, M. K., and R. D. Brinton. 2019. Autoimmune disease in women: Endocrine transition and risk across the lifespan. Frontiers in Endocrinology 10:265. https://doi.org/10.3389/fendo.2019.00265.
Duarte-Garcia, A., M. M. Pham, C. S. Crowson, S. Amin, K. G. Moder, R. K. Pruthi, K. J. Warrington, and E. L. Matteson. 2019. The epidemiology of antiphospholipid syndrome: A population-based study. Arthritis & Rheumatology 71(9):1545–1552. https://doi.org/10.1002/art.40901.
Durante, A., and S. Bronzato. 2015. The increased cardiovascular risk in patients affected by autoimmune diseases: Review of the various manifestations. Journal of Clinical Medicine Research 7(6):379–384. https://doi.org/10.14740/jocmr2122w.
Eaton, W. W., N. R. Rose, A. Kalaydjian, M. G. Pedersen, and P. B. Mortensen. 2007. Epidemiology of autoimmune diseases in Denmark. Journal of Autoimmunity 29(1):1–9. https://doi.org/10.1016/j.jaut.2007.05.002.
Eberhardt, K., B.-M. Larsson, K. Nived, and E. Lindqvist. 2007. Work disability in rheumatoid arthritis—development over 15 years and evaluation of predictive factors over time. Journal of Rheumatology 34(3):481–487.
Eckert, L., S. Gupta, C. Amand, A. Gadkari, P. Mahajan, and J. M. Gelfand. 2017. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: An analysis using the national health and wellness survey. Journal of the American Academy of Dermatology 77(2):274–279.e273. https://doi.org/10.1016/j.jaad.2017.04.019.
Eder, L., J. Widdifield, C. F. Rosen, R. Cook, K.-A. Lee, R. Alhusayen, M. J. Paterson, S. Y. Cheng, S. Jabbari, W. Campbell, S. Bernatsky, D. D. Gladman, and K. Tu. 2019. Trends in the prevalence and incidence of psoriasis and psoriatic arthritis in Ontario, Canada: A population-based study. Arthritis Care & Research 71(8):1084–1091.
Edwards, M., R. Dai, and S. A. Ahmed. 2018. Our environment shapes us: The importance of environment and sex differences in regulation of autoantibody production. Frontiers in Immunology 9:478. https://doi.org/10.3389/fimmu.2018.00478.
Edwards, M. R., R. Dai, B. Heid, C. Cowan, S. R. Werre, T. Cecere, and S. A. Ahmed. 2020. Low-dose 17α-ethinyl estradiol (EE) exposure exacerbates lupus renal disease and modulates immune responses to TLR7/9 agonists in genetically autoimmune-prone mice. Scientific Reports 10(1):5210. https://doi.org/10.1038/s41598-020-62124-6.
Eriksson, J. K., M. Neovius, S. Ernestam, S. Lindblad, J. F. Simard, and J. Askling. 2013. Incidence of rheumatoid arthritis in Sweden: A nationwide population-based assessment of incidence, its determinants, and treatment penetration. Arthritis Care & Research 65(6):870–878. https://doi.org/10.1002/acr.21900.
Fagundes, C. P., R. Glaser, and J. K. Kiecolt-Glaser. 2013. Stressful early life experiences and immune dysregulation across the lifespan. Brain, Behavior, and Immunity 27(1):8–12. https://doi.org/10.1016/j.bbi.2012.06.014.
Fairweather, D. 2014. Sex differences in inflammation during atherosclerosis. Clinical Medicine Insights: Cardiology 8(Suppl 3):49–59. https://doi.org/10.4137/CMC.S17068.
Fairweather, D., L. T. Cooper, Jr., and L. A. Blauwet. 2013. Sex and gender differences in myocarditis and dilated cardiomyopathy. Current Problems in Cardiology 38(1):7–46. https://doi.org/10.1016/j.cpcardiol.2012.07.003.
Feldman, C. H., S. Malspeis, C. Leatherwood, L. Kubzansky, K. H. Costenbader, and A. L. Roberts. 2019. Association of childhood abuse with incident systemic lupus erythematosus in adulthood in a longitudinal cohort of women. Journal of Rheumatology 46(12):1589–1596. https://doi.org/10.3899/jrheum.190009.
Gebhard, C., V. Regitz-Zagrosek, H. K. Neuhauser, R. Morgan, and S. L. Klein. 2020. Impact of sex and gender on COVID-19 outcomes in Europe. Biology of Sex Differences 11(1):29. https://doi.org/10.1186/s13293-020-00304-9.
Gelpí, E., M. P. de la Paz, B. Terracini, I. Abaitua, A. G. de la Cámara, E. M. Kilbourne, C. Lahoz, B. Nemery, R. M. Philen, L. Soldevilla, S. Tarkowski, and WHO/CISAT Scientific Committee for the Toxic Oil Syndrome. 2002. The Spanish toxic oil syndrome 20 years after its onset: A multidisciplinary review of scientific knowledge. Environmental Health Perspectives 110(5):457–464. https://doi.org/10.1289/ehp.110-1240833.
Gianfrancesco, M. A., M. Dall’Era, L. B. Murphy, C. G. Helmick, J. Li, S. Rush, L. Trupin, and J. Yazdany. 2021. Mortality among minority populations with systemic lupus erythematosus, including Asian and Hispanic/Latino persons—California, 2007–2017. MMWR: Morbidity and Mortality Weekly Report 70(7):236–239. https://doi.org/10.15585/mmwr.mm7007a2.
Gokuladhas, S., W. Schierding, E. Golovina, T. Fadason, and J. O’Sullivan. 2021. Unravelling the shared genetic mechanisms underlying 18 autoimmune diseases using a systems approach. Frontiers in Immunology 12:693142. https://doi.org/10.3389/fimmu.2021.693142.
Gutierrez-Suarez, R., N. Ruperto, R. Gastaldi, A. Pistorio, E. Felici, R. Burgos-Vargas, A. Martini, and A. Ravelli. 2006. A proposal for a pediatric version of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index based on the analysis of 1,015 patients with juvenile-onset systemic lupus erythematosus. Arthritis and Rheumatism 54(9):2989–2996.
Guzman, J., T. Kerr, L. M. Ward, J. Ma, K. Oen, A. M. Rosenberg, B. M. Feldman, G. Boire, K. Houghton, P. Dancey, R. Scuccimarri, A. Bruns, A. M. Huber, K. Watanabe Duffy, N. J. Shiff, R. A. Berard, D. M. Levy, E. Stringer, K. Morishita, N. Johnson, D. A. Cabral, M. Larche, R. E. Petty, R. M. Laxer, E. Silverman, P. Miettunen, A. L. Chetaille, E. Haddad, L. Spiegel, S. E. Turvey, H. Schmeling, B. Lang, J. Ellsworth, S. E. Ramsey, J. Roth, S. Campillo, S. Benseler, G. Chedeville, R. Schneider, S. M. L. Tse, R. Bolaria, K. Gross, D. Feldman, B. Cameron, R. Jurencak, J. Dorval, C. LeBlanc, C. St Cyr, M. Gibbon, R. S. M. Yeung, C. M. Duffy, and L. B. Tucker. 2017. Growth and weight gain in children with juvenile idiopathic arthritis: Results from the reACCh-Out cohort. Pediatric Rheumatology Online Journal 15(1):68. https://doi.org/10.1186/s12969-017-0196-7.
Halfon, N., and M. Hochstein. 2002. Life course health development: An integrated framework for developing health, policy, and research. Milbank Quarterly 80(3):433–479. https://doi.org/10.1111/1468-0009.00019.
Hardy, R., and D. O’Neill. 2020. Life course biological trajectories: Maximising the value of longitudinal studies. Annals of Human Biology 47(2):227–228. https://doi.org/10.1080/03014460.2020.1737732.
Hargraves, M. M., H. Richmond, and R. Morton. 1948. Presentation of two bone marrow elements; the tart cell and the l.E. Cell. Proceedings of the Staff Meetings of the Mayo Clinic 23(2):25–28.
Harrold, L. R., C. Salman, S. Shoor, J. R. Curtis, M. M. Asgari, J. M. Gelfand, J. J. Wu, and L. J. Herrinton. 2013. Incidence and prevalence of juvenile idiopathic arthritis among children in a managed care population, 1996–2009. Journal of Rheumatology 40(7):1218–1225. https://doi.org/10.3899/jrheum.120661.
Harrold, L. R., Y. Shan, S. Rebello, N. Kramer, S. E. Connolly, E. Alemao, S. Kelly, J. M. Kremer, and E. D. Rosenstein. 2020. Prevalence of Sjögren’s syndrome associated with rheumatoid arthritis in the USA: An observational study from the Corrona registry. Clinical Rheumatology 39(6):1899–1905. https://doi.org/10.1007/s10067-020-05004-8.
Haynes, D. C., and M. E. Gershwin. 1982. The immunopathology of progressive systemic sclerosis (PSS). Seminars in Arthritis and Rheumatism 11(3):331–351.
Hedrich, C. M. 2016. Shaping the spectrum—from autoinflammation to autoimmunity. Clinical Immunology 165:21–28.
Heesen, C., J. Böhm, C. Reich, J. Kasper, M. Goebel, and S. M. Gold. 2008. Patient perception of bodily functions in multiple sclerosis: Gait and visual function are the most valuable. Multiple Sclerosis 14(7):988–991. https://doi.org/10.1177/1352458508088916.
Herrinton, L. J., L. Liu, J. E. Lafata, J. E. Allison, S. E. Andrade, E. J. Korner, K. A. Chan, R. Platt, D. Hiatt, and S. O’Connor. 2007. Estimation of the period prevalence of inflammatory bowel disease among nine health plans using computerized diagnoses and outpatient pharmacy dispensings. Inflammatory Bowel Diseases 13(4):451–461. https://doi.org/10.1002/ibd.20021.
Herrinton, L. J., L. Liu, J. D. Lewis, P. M. Griffin, and J. Allison. 2008. Incidence and prevalence of inflammatory bowel disease in a northern California managed care organization, 1996–2002. The American Journal of Gastroenterology 103(8):1998–2006. https://doi.org/10.1111/j.1572-0241.2008.01960.x.
Heshin-Bekenstein, M., L. Perl, A. O. Hersh, E. von Scheven, E. Yelin, L. Trupin, J. Yazdany, and E. F. Lawson. 2018. Final adult height of patients with childhood-onset systemic lupus erythematosus: A cross sectional analysis. Pediatric Rheumatology Online Journal 16(1):30. https://doi.org/10.1186/s12969-018-0239-8.
Hou, J. K., J. R. Kramer, P. Richardson, M. Mei, and H. B. El-Serag. 2013. The incidence and prevalence of inflammatory bowel disease among U.S. veterans: A national cohort study. Inflammatory Bowel Diseases 19(5):1059–1064. https://doi.org/10.1097/MIB.0b013e31828028ca.
Hussain, Y. S., J. C. Hookham, A. Allahabadia, and S. P. Balasubramanian. 2017. Epidemiology, management and outcomes of Graves’ disease-real life data. Endocrine 56(3):568–578. https://doi.org/10.1007/s12020-017-1306-5.
Icen, M., C. S. Crowson, M. T. McEvoy, F. J. Dann, S. E. Gabriel, and H. Maradit Kremers. 2009. Trends in incidence of adult-onset psoriasis over three decades: A population-based study. Journal of the American Academy of Dermatology 60(3):394–401. https://doi.org/10.1016/j.jaad.2008.10.062.
Idée, J.-M., N. Fretellier, C. Robic, and C. Corot. 2014. The role of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: A critical update. Critical Reviews in Toxicology 44(10):895–913. https://doi.org/10.3109/10408444.2014.955568.
IOM (Institute of Medicine). 2001. Multiple sclerosis: Current status and strategies for the future Washington, DC: National Academies Press.
Iwasaki, A., and R. Medzhitov. 2015. Control of adaptive immunity by the innate immune system. Nature Immunology 16(4):343–353. https://doi.org/10.1038/ni.3123.
Izmirly, P. M., I. Wan, S. Sahl, J. P. Buyon, H. M. Belmont, J. E. Salmon, A. Askanase, J. M. Bathon, L. Geraldino-Pardilla, Y. Ali, E. M. Ginzler, C. Putterman, C. Gordon, C. G. Helmick, and H. Parton. 2017. The incidence and prevalence of systemic lupus erythematosus in New York County (Manhattan), New York: The Manhattan Lupus Surveillance Program. Arthritis & Rheumatology 69(10):2006–2017. https://doi.org/10.1002/art.40192.
Izmirly, P. M., J. P. Buyon, I. Wan, H. M. Belmont, S. Sahl, J. E. Salmon, A. Askanase, J. M. Bathon, L. Geraldino-Pardilla, Y. Ali, E. M. Ginzler, C. Putterman, C. Gordon, C. G. Helmick, and H. Parton. 2019. The incidence and prevalence of adult primary Sjögren’s syndrome in New York county. Arthritis Care & Research 71(7):949–960. https://doi.org/10.1002/acr.23707.
Izmirly, P. M., E. D. Ferucci, E. C. Somers, L. Wang, S. S. Lim, C. Drenkard, M. Dall’Era, W. J. McCune, C. Gordon, C. Helmick, and H. Parton. 2021a. Incidence rates of systemic lupus erythematosus in the USA: Estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries. Lupus Science & Medicine 8(1). https://doi.org/10.1136/lupus-2021-000614.
Izmirly, P. M., H. Parton, L. Wang, W. J. McCune, S. S. Lim, C. Drenkard, E. D. Ferucci, M. Dall’Era, C. Gordon, C. G. Helmick, and E. C. Somers. 2021b. Prevalence of systemic lupus erythematosus in the United States: Estimates from a meta-analysis of the Centers for Disease Control and Prevention National Lupus Registries. Arthritis & Rheumatology 73(6):991–996. https://doi.org/10.1002/art.41632.
Jacob, C. M., J. Baird, M. Barker, C. Cooper, and M. Hanson. 2017. The importance of a life course approach to health: Chronic disease risk from preconception through adolescence and adulthood. Geneva, Switzerland: World Health Organization. https://www.who.int/life-course/publications/life-course-approach-to-health.pdf (accessed February 23, 2022).
Jacobson, D. L., S. J. Gange, N. R. Rose, and N. M. H. Graham. 1997. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clinical Immunology and Immunopathology 84(3):223–243. https://doi.org/10.1006/clin.1997.4412.
Jain, K., P. Jawa, V. K. Derebail, and R. J. Falk. 2021. Treatment updates in antineutrophil cytoplasmic autoantibodies (anca) vasculitis. Kidney360 2(4):763–770. https://doi.org/10.34067/kid.0007142020.
James, S., R. Gallagher, J. Dunbabin, and L. Perry. 2014. Prevalence of vascular complications and factors predictive of their development in young adults with type 1 diabetes: Systematic literature review. BMC Research Notes 7(1):593. https://doi.org/10.1186/1756-0500-7-593.
Jia, L., A. B. Levine, and M. D. Lockshin. 2017. American College of Rheumatology criteria for systemic lupus erythematosus exclude half of all systemic lupus erythematosus patients. Arthritis & Rheumatology 69(7):1502–1503. https://doi.org/10.1002/art.40120.
Johns Hopkins University. 2022. Definition of autoimmunity & autoimmune disease. https://pathology.jhu.edu/autoimmune/definitions/ (accessed January 18, 2022).
Johnson, D. B., S. Chandra, and J. A. Sosman. 2018. Immune checkpoint inhibitor toxicity in 2018. JAMA 320(16):1702–1703. https://doi.org/10.1001/jama.2018.13995.
Jorge, A. M., N. Lu, Y. Zhang, S. K. Rai, and H. K. Choi. 2018. Unchanging premature mortality trends in systemic lupus erythematosus: A general population-based study (1999–2014). Rheumatology (Oxford) 57(2):337–344.
Kanth, R., R. B. Shrestha, I. Rai, J. J. VanWormer, and P. K. Roy. 2017. Incidence of primary biliary cholangitis in a rural midwestern population. Clinical Medicine & Research 15(1–2):13–18. https://doi.org/10.3121/cmr.2017.1351.
Kao, K. T., M. Denker, M. Zacharin, and S. C. Wong. 2019. Pubertal abnormalities in adolescents with chronic disease. Best Practice & Research Clinical Endocrinology & Metabolism 33(3):101275.
Kawatkar, A. A., S. E. Gabriel, and S. J. Jacobsen. 2019. Secular trends in the incidence and prevalence of rheumatoid arthritis within members of an integrated health care delivery system. Rheumatology International 39(3):541–549.
Ketelhuth, D. F., and G. K. Hansson. 2016. Adaptive response of T and B cells in atherosclerosis. Circulation Research 118(4):668–678.
Khalili, H., A. H. Everhov, J. Halfvarson, J. F. Ludvigsson, J. Askling, P. Myrelid, J. Söderling, O. Olen, M. Neovius, and SWIBREG Group. 2020. Healthcare use, work loss and total costs in incident and prevalent Crohn’s disease and ulcerative colitis: Results from a nationwide study in Sweden. Alimentary Pharmacology and Therapeutics 52(4):655–668.
Kollert, F., and B. A. Fisher. 2020. Equal rights in autoimmunity: Is Sjögren’s syndrome ever ‘secondary’? Rheumatology (Oxford) 59(6):1218–1225.
Kremers, H. M., C. S. Crowson, T. M. Therneau, V. L. Roger, and S. E. Gabriel. 2008. High ten-year risk of cardiovascular disease in newly diagnosed rheumatoid arthritis patients: A population-based cohort study. Arthritis and Rheumatism 58(8):2268–2274.
Kuh, D., Y. Ben-Shlomo, J. Lynch, J. Hallqvist, and C. Power. 2003. Life course epidemiology. Journal of Epidemiology and Community Health 57(10):778–783. https://doi.org/10.1136/jech.57.10.778.
Kurd, S. K., A. B. Troxel, P. Crits-Christoph, and J. M. Gelfand. 2010. The risk of depression, anxiety and suicidality in patients with psoriasis: A population-based cohort study. Archives of Dermatology 146(8):891–895. https://doi.org/10.1001/archdermatol.2010.186.
Kurmann, R. D., A. S. Sandhu, C. S. Crowson, E. L. Matteson, T. G. Osborn, K. J. Warrington, R. Mankad, and A. Makol. 2020. Cardiovascular risk factors and atherosclerotic cardiovascular events among incident cases of systemic sclerosis: Results from a population-based cohort (1980–2016). Mayo Clinic Proceedings 95(7):1369–1378. https://doi.org/10.1016/j.mayocp.2019.12.015.
Langan, D., N. R. Rose, and K. D. Moudgil. 2020. Common innate pathways to autoimmune disease. Clinical Immunology 212:108361. https://doi.org/10.1016/j.clim.2020.108361.
Langer-Gould, A., S. M. Brara, B. E. Beaber, and J. L. Zhang. 2013. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology 80(19):1734–1739. https://doi.org/10.1212/WNL.0b013e3182918cc2.
Lee, C.-H., and F. Giuliani. 2019a. The role of inflammation in depression and fatigue. Frontiers in Immunology 10:1696. https://doi.org/10.3389/fimmu.2019.01696.
Lee, C. H., and F. Giuliani. 2019b. The role of inflammation in depression and fatigue. Frontiers in Immunology 10:1696. https://doi.org/10.3389/fimmu.2019.01696.
Leese, G. P., R. V. Flynn, R. T. Jung, T. M. Macdonald, M. J. Murphy, and A. D. Morris. 2008. Increasing prevalence and incidence of thyroid disease in Tayside, Scotland: The thyroid epidemiology audit and research study (tears). Clinical Endocrinology 68(2):311–316. https://doi.org/10.1111/j.1365-2265.2007.03051.x.
Liao, K. P., T. Cai, V. Gainer, S. Goryachev, Q. Zeng-treitler, S. Raychaudhuri, P. Szolovits, S. Churchill, S. Murphy, I. Kohane, E. W. Karlson, and R. M. Plenge. 2010. Electronic medical records for discovery research in rheumatoid arthritis. Arthritis Care & Research 62(8):1120–1127. https://doi.org/10.1002/acr.20184.
Lim, S. S., A. R. Bayakly, C. G. Helmick, C. Gordon, K. A. Easley, and C. Drenkard. 2014. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: The Georgia Lupus Registry. Arthritis & Rheumatology 66(2):357–368. https://doi.org/10.1002/art.38239.
Lim, S. S., C. G. Helmick, G. Bao, J. Hootman, R. Bayakly, C. Gordon, and C. Drenkard. 2019. Racial disparities in mortality associated with systemic lupus erythematosus - Fulton and DeKalb Counties, Georgia, 2002-2016. MMWR: Morbidity and Mortality Weekly Report 68(18):419–422. https://doi.org/10.15585/mmwr.mm6818a4.
Lindestam Arlehamn, C. S., R. Dhanwani, J. Pham, R. Kuan, A. Frazier, J. Rezende Dutra, E. Phillips, S. Mallal, M. Roederer, K. S. Marder, A. W. Amara, D. G. Standaert, J. G. Goldman, I. Litvan, B. Peters, D. Sulzer, and A. Sette. 2020. Α-synuclein-specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nature Communications 11(1):1875. https://doi.org/10.1038/s41467-020-15626-w.
Liu, S., R. N. Jones, and M. M. Glymour. 2010. Implications of lifecourse epidemiology for research on determinants of adult disease. Public Health Reviews 32(2):489–511. https://doi.org/10.1007/BF03391613.
Lockshin, M. D., A. B. Levine, and D. Erkan. 2015. Patients with overlap autoimmune disease differ from those with ‘pure’ disease. Lupus Science & Medicine 2(1):e000084. https://doi.org/10.1136/lupus-2015-000084.
Lockshin, M. D., M. Barbhaiya, P. Izmirly, J. P. Buyon, and M. K. Crow. 2019. SLE: Reconciling heterogeneity. Lupus Science & Medicine 6(1):e000280. https://doi.org/10.1136/lupus-2018-000280.
Lockshin, M. D., M. K. Crow, and M. Barbhaiya. 2021. When a diagnosis has no name: Uncertainty and opportunity. ACR Open Rheumatology 2021 Nov. 21. https://doi.org/10.1002/acr2.11368.
Lu, M., Y. Zhou, I. V. Haller, R. J. Romanelli, J. J. VanWormer, C. V. Rodriguez, H. Anderson, J. A. Boscarino, M. A. Schmidt, Y. G. Daida, A. Sahota, J. Vincent, C. L. Bowlus, K. Lindor, T. Zhang, S. Trudeau, J. Li, L. B. Rupp, S. C. Gordon, and for the Fibrotic Liver Disease Consortium Investigators. 2018. Increasing prevalence of primary biliary cholangitis and reduced mortality with treatment. Clinical Gastroenterology and Hepatology 16(8):1342–1350.e1341 https://doi.org/10.1016/j.cgh.2017.12.033.
Lynch, J., and G. D. Smith. 2005. A life course approach to chronic disease epidemiology. Annual Review of Public Health 26:1–35. https://doi.org/10.1146/annurev.publhealth.26.021304.144505.
Maciel, G., C. S. Crowson, E. L. Matteson, and D. Cornec. 2017a. Incidence and mortality of physician-diagnosed primary Sjögren’s syndrome: Time trends over a 40-year period in a population-based cohort in the United States. Mayo Clinic Proceedings 92(5):734–743. https://doi.org/10.1016/j.mayocp.2017.01.020.
Maciel, G., C. S. Crowson, E. L. Matteson, and D. Cornec. 2017b. Prevalence of primary Sjögren’s syndrome in a population-based cohort in the United States. Arthritis Care & Research 69(10):1612–1616. https://doi.org/10.1002/acr.23173.
Maggio, M., G. P. Ceda, F. Lauretani, S. Bandinelli, E. J. Metter, A. Artoni, E. Gatti, C. Ruggiero, J. M. Guralnik, G. Valenti, S. M. Ling, S. Basaria, and L. Ferrucci. 2009. Estradiol and inflammatory markers in older men. Journal of Clinical Endocrinology & Metabolism 94(2):518–522. https://doi.org/10.1210/jc.2008-0940.
Man, A., Y. Zhu, Y. Zhang, M. Dubreuil, Y. H. Rho, C. Peloquin, R. W. Simms, and H. K. Choi. 2013. The risk of cardiovascular disease in systemic sclerosis: A population-based cohort study. Annals of the Rheumatic Diseases 72(7):1188–1193. https://doi.org/10.1136/annrheumdis-2012-202007.
Mardini, H. E., P. Westgate, and A. Y. Grigorian. 2015. Racial differences in the prevalence of celiac disease in the US population: National Health and Nutrition Examination Survey (NHANES) 2009–2012. Digestive Diseases and Sciences 60(6):1738–1742. https://doi.org/10.1007/s10620-014-3514-7.
Marshall, J. S., R. Warrington, W. Watson, and H. L. Kim. 2018. An introduction to immunology and immunopathology. Allergy, Asthma, and Clinical Immunology 14(Suppl 2):49. https://doi.org/10.1186/s13223-018-0278-1.
Martin-Pozo, L., M. D. C. Gomez-Regalado, I. Moscoso-Ruiz, and A. Zafra-Gomez. 2021. Analytical methods for the determination of endocrine disrupting chemicals in cosmetics and personal care products: A review. Talanta 234:122642. https://doi.org/10.1016/j.talanta.2021.122642.
Martz, C. D., A. M. Allen, T. E. Fuller-Rowell, E. C. Spears, S. S. Lim, C. Drenkard, K. Chung, E. A. Hunter, and D. H. Chae. 2019. Vicarious racism stress and disease activity: The Black women’s experiences living with lupus (BeWELL) study. Journal of Racial and Ethnic Health Disparities 6(5):1044–1051. https://doi.org/10.1007/s40615-019-00606-8.
Masters, S. L., A. Simon, I. Aksentijevich, and D. L. Kastner. 2009. Horror autoinflammaticus: The molecular pathophysiology of autoinflammatory disease. Annual Review of Immunology 27:621–668. https://doi.org/10.1146/annurev.immunol.25.022106.141627.
Mauldin, J., H. D. Cameron, D. Jeanotte, G. Solomon, and J. N. Jarvis. 2004. Chronic arthritis in children and adolescents in two Indian Health Service user populations. BMC Musculoskeletal Disorders 5(1):30. https://doi.org/10.1186/1471-2474-5-30.
Mayer-Davis, E. J., D. Dabelea, and J. M. Lawrence. 2017. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. The New England Journal of Medicine 376(15):1419–1429. https://doi.org/doi:10.1056/NEJMoa1610187.
Mayes, M. D., J. V. Lacey, Jr., J. Beebe-Dimmer, B. W. Gillespie, B. Cooper, T. J. Laing, and D. Schottenfeld. 2003. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis and Rheumatism 48(8):2246–2255. https://doi.org/10.1002/art.11073.
McGonagle, D., and M. F. McDermott. 2006. A proposed classification of the immunological diseases. PLOS Medicine 3(8):e297. https://doi.org/10.1371/journal.pmed.0030297.
McMahon, M., B. H. Hahn, and B. J. Skaggs. 2011. Systemic lupus erythematosus and cardiovascular disease: Prediction and potential for therapeutic intervention. Expert Review of Clinical Immunology 7(2):227–241. https://doi.org/10.1586/eci.10.98.
Mersha, T. B., and T. Abebe. 2015. Self-reported race/ethnicity in the age of genomic research: Its potential impact on understanding health disparities. Human Genomics 9(1):1. https://doi.org/10.1186/s40246-014-0023-x.
Miller-Archie, S. A., P. M. Izmirly, J. R. Berman, J. Brite, D. J. Walker, R. C. Dasilva, L. J. Petrsoric, and J. E. Cone. 2020. Systemic autoimmune disease among adults exposed to the September 11, 2001 terrorist attack. Arthritis & Rheumatology 72(5):849–859. https://doi.org/10.1002/art.41175.
Miller, G. E., E. Chen, and K. J. Parker. 2011. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin 137(6):959–997. https://doi.org/10.1037/a0024768.
Mitratza, M., B. Klijs, A. E. Hak, J. W. P. F. Kardaun, and A. E. Kunst. 2021. Systemic autoimmune disease as a cause of death: Mortality burden and comorbidities. Rheumatology (Oxford) 60(3):1321–1330. https://doi.org/10.1093/rheumatology/keaa537.
Molina, E., I. del Rincon, J. F. Restrepo, D. F. Battafarano, and A. Escalante. 2015. Mortality in rheumatoid arthritis (RA): Factors associated with recording RA on death certificates. BMC Musculoskeletal Disorders 16:277. https://doi.org/10.1186/s12891-015-0727-7.
Moores, K. G., and N. A. Sathe. 2013. A systematic review of validated methods for identifying systemic lupus erythematosus (SLE) using administrative or claims data. Vaccine 31 Suppl 10:K62–73. https://doi.org/10.1016/j.vaccine.2013.06.104.
Morales-Tisnes, T., L. Quintero-Ortiz, E. Quintero-Munoz, F. Sierra-Matamoros, J. AriasAponte, and A. Rojas-Villarraga. 2021. Prevalence of hospital readmissions and related factors in patients with autoimmune diseases. Journal of Translational Autoimmunity 4:100121. https://doi.org/10.1016/j.jtauto.2021.100121.
Morgan, R., P. Baker, D. M. Griffith, S. L. Klein, C. H. Logie, A. A. Mwiine, A. I. Scheim, J. R. Shapiro, J. Smith, C. Wenham, and A. White. 2021. Beyond a zero-sum game: How does the impact of COVID-19 vary by gender? Frontiers in Sociology 6(126):650729. https://doi.org/10.3389/fsoc.2021.650729.
Morris, G., M. Berk, K. Walder, and M. Maes. 2015. Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Medicine 13(28):28. https://doi.org/10.1186/s12916-014-0259-2.
Moustafa, A. T., M. Moazzami, L. Engel, E. Bangert, M. Hassanein, S. Marzouk, M. Kravtsenyuk, W. Fung, L. Eder, J. Su, J. E. Wither, and Z. Touma. 2020. Prevalence and metric of depression and anxiety in systemic lupus erythematosus: A systematic review and meta-analysis. Seminars in Arthritis and Rheumatism 50(1):84–94. https://doi.org/10.1016/j.semarthrit.2019.06.017.
Munk Nielsen, N., G. Corn, M. Frisch, E. Stenager, N. Koch-Henriksen, J. Wohlfahrt, M. Magyari, and M. Melbye. 2019. Multiple sclerosis among first- and second-generation immigrants in Denmark: A population-based cohort study. Brain 142(6):1587–1597. https://doi.org/10.1093/brain/awz088.
Myasoedova, E., C. S. Crowson, H. M. Kremers, T. M. Therneau, and S. E. Gabriel. 2010. Is the incidence of rheumatoid arthritis rising?: Results from Olmsted County, Minnesota, 1955–2007. Arthritis and Rheumatism 62(6):1576–1582. https://doi.org/10.1002/art.27425.
NCI (National Cancer Institute). 2021a. Cancer stat facts: Cancer of any site. https://seer.cancer.gov/statfacts/html/all.html (accessed March 17, 2022).
NCI. 2021b. SEER is an authoritative source for cancer statistics in the United States. https://seer.cancer.gov/ (accessed August 16, 2021).
Nelson, L. M., M. T. Wallin, R. A. Marrie, W. J. Culpepper, A. Langer-Gould, J. Campbell, S. Buka, H. Tremlett, G. Cutter, W. Kaye, L. Wagner, N. G. Larocca, and United States Multiple Sclerosis Prevalence Workgroup. 2019. A new way to estimate neurologic disease prevalence in the United States: Illustrated with MS. Neurology 92(10):469–480. https://doi.org/10.1212/WNL.0000000000007044.
NHANES (National Health and Nutrition Examination Suvey). 2017. About NHANES. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed July 1, 2021).
NIAID (National Institute of Allergy and Infectious Diseases). 2017. Autoimmune diseases. https://www.niaid.nih.gov/diseases-conditions/autoimmune-diseases (accessed January 18, 2021).
Nielsen, N. M., M. Frisch, K. Rostgaard, J. Wohlfahrt, H. Hjalgrim, N. Koch-Henriksen, M. Melbye, and T. Westergaard. 2008. Autoimmune diseases in patients with multiple sclerosis and their first-degree relatives: A nationwide cohort study in Denmark. Multiple Sclerosis 14(6):823–829. https://doi.org/10.1177/1352458508088936.
NIH (National Institutes of Health). 2016. Report of the director—National Institutes of Health: Fiscal years 2014 and 2015. Bethesda, MD: National Institutes of Health.
NIH. 2019. Report of the director—National Institutes of Health: Fiscal years 2016, 2017, and 2018. Bethesda, MD: National Institutes of Health.
NLM (National Library of Medicine). 2021. Nivolumab in treating patients with autoimmune disorders and advanced, metastatic, or unresectable cancer. https://clinicaltrials.gov/ct2/show/NCT03816345 (accessed October 8, 2021).
Noah, L. 2022. Confronting the inevitability of diagnostic uncertainty across multiple legal domains. In Diagnoses without names: Challenges for medical care, research, and policy, edited by M. Lockshin, M. K. Crow, and M. Barbhaiya. Switzerland: Springer Nature.
Nusbaum, J. S., I. Mirza, J. Shum, R. W. Freilich, R. E. Cohen, M. H. Pillinger, P. M. Izmirly, and J. P. Buyon. 2020. Sex differences in systemic lupus erythematosus: Epidemiology, clinical considerations, and disease pathogenesis. Mayo Clinic Proceedings 95(2):384–394. https://doi.org/10.1016/j.mayocp.2019.09.012.
Orbai, A. M., S. M. Reddy, N. Dennis, R. Villacorta, S. Peterson, L. Mesana, S. D. Chakravarty, I. Lin, C. S. Karyekar, Y. Wang, M. Pacou, and J. Walsh. 2021. Work absenteeism and disability associated with psoriasis and psoriatic arthritis in the USA-a retrospective study of claims data from 2009 to 2020. Clinical Rheumatology 40(12):4933–4942. https://doi.org/10.1007/s10067-021-05839-9.
Paller, A. S., R. Singh, M. Cloutier, M. Gauthier-Loiselle, B. Emond, A. Guérin, and A. Ganguli. 2018. Prevalence of psoriasis in children and adolescents in the United States: A claims-based analysis. Journal of Drugs in Dermatology 17(2):187–194.
Panopalis, P., A. E. Clarke, and E. Yelin. 2012. The economic burden of systemic lupus erythematosus. Best Practice & Research: Clinical Rheumatology 26(5):695–704. https://doi.org/10.1016/j.berh.2012.08.006.
Parks, C. G., A. A. D’Aloisio, and D. P. Sandler. 2016. Early life factors associated with adult-onset systemic lupus erythematosus in women. Frontiers in Immunology 7:103. https://doi.org/10.3389/fimmu.2016.00103.
Pasvol, T. J., L. Horsfall, S. Bloom, A. W. Segal, C. Sabin, N. Field, and G. Rait. 2020. Incidence and prevalence of inflammatory bowel disease in UK primary care: A population-based cohort study. BMJ Open 10(7):e036584. https://doi.org/10.1136/bmjopen-2019-036584.
Patisaul, H. B. 2017. Endocrine disruption by dietary phyto-oestrogens: Impact on dimorphic sexual systems and behaviours. Proceedings of the Nutrition Society 76(2):130–144. https://doi.org/10.1017/S0029665116000677.
Pinson, R. D. 2012. Properly coding an uncertain diagnosis. The Hospitalist (August 28, 2012). https://www.the-hospitalist.org/hospitalist/article/125124/properly-coding-uncertain-diagnosis (accessed January 3, 2022).
Popescu, M., T. B. Feldman, and T. Chitnis. 2021. Interplay between endocrine disruptors and immunity: Implications for diseases of autoreactive etiology. Frontiers in Pharmacology 12:626107. https://doi.org/10.3389/fphar.2021.626107.
Porta, M. 2014. A dictionary of epidemiology (draft). Oxford: Oxford University Press.
Potluri, T., A. L. Fink, K. E. Sylvia, S. Dhakal, M. S. Vermillion, L. Vom Steeg, S. Deshpande, H. Narasimhan, and S. L. Klein. 2019. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines 4:29. https://doi.org/10.1038/s41541-019-0124-6.
Quaglia, M., G. Merlotti, M. De Andrea, C. Borgogna, and V. Cantaluppi. 2021. Viral infections and systemic lupus erythematosus: New players in an old story. Viruses 13(2):277.
Raggi, A., V. Covelli, S. Schiavolin, C. Scaratti, M. Leonardi, and M. Willems. 2016. Work-related problems in multiple sclerosis: A literature review on its associates and determinants. Disability and Rehabilitation 38(10):936–944. https://doi.org/10.3109/09638288.2015.1070295.
Reifsnider, E., M. Gallagher, and B. Forgione. 2005. Using ecological models in research on health disparities. Journal of Professional Nursing 21(4):216–222. https://doi.org/10.1016/j.profnurs.2005.05.006.
Rochester Epidemiology Project. 2012. https://rochesterproject.org/wp-content/uploads/2014/03/summary_tables_dec_2012.pdf (accessed July 9, 2021).
Rose, N. R., and C. Bona. 1993. Defining criteria for autoimmune diseases (Witebsky’s postulates revisited). Immunology Today 14(9):426–430. https://doi.org/10.1016/0167-5699(93)90244-F.
Rose, N. R., and I. R. Mackay. 2014. Autoimmune disease. In The autoimmune diseases, 5th ed., edited by N. R. Rose and I. R. Mackay. San Diego, CA: Elsevier Inc. Pp. 3–9.
Rosenblum, M. D., K. A. Remedios, and A. K. Abbas. 2015. Mechanisms of human autoimmunity. Journal of Clinical Investigation 125(6):2228–2233. https://doi.org/10.1172/JCI78088.
Rotstein, D. L., H. Chen, A. S. Wilton, J. C. Kwong, R. A. Marrie, P. Gozdyra, K. M. Krysko, A. Kopp, R. Copes, and K. Tu. 2018. Temporal trends in multiple sclerosis prevalence and incidence in a large population. Neurology 90(16):e1435–e1441. https://doi.org/10.1212/WNL.0000000000005331.
Rubtsov, A. V., K. Rubtsova, J. W. Kappler, and P. Marrack. 2010. Genetic and hormonal factors in female-biased autoimmunity. Autoimmunity Reviews 9(7):494–498. https://doi.org/10.1016/j.autrev.2010.02.008.
Rusman, T., R. F. van Vollenhoven, and I. E. van der Horst-Bruinsma. 2018. Gender differences in axial spondyloarthritis: Women are not so lucky. Current Rheumatology Reports 20(6):35. https://doi.org/10.1007/s11926-018-0744-2.
Sammaritano, L. R., B. L. Bermas, E. E. Chakravarty, C. Chambers, M. E. B. Clowse, M. D. Lockshin, W. Marder, G. Guyatt, D. W. Branch, J. Buyon, L. Christopher-Stine, R. Crow-Hercher, J. Cush, M. Druzin, A. Kavanaugh, C. A. Laskin, L. Plante, J. Salmon, J. Simard, E. C. Somers, V. Steen, S. K. Tedeschi, E. Vinet, C. W. White, J. Yazdany, M. Barbhaiya, B. Bettendorf, A. Eudy, A. Jayatilleke, A. A. Shah, N. Sullivan, L. L. Tarter, M. Birru Talabi, M. Turgunbaev, A. Turner, and K. E. D’Anci. 2020. 2020 American College of Rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Care & Research 72(4):461–488. https://doi.org/10.1002/acr.24130.
Sánchez-Flórez, J. C., D. Seija-Butnaru, E. G. Valero, C. D. P. Acosta Acosta, and S. Amaya. 2021. Pain management strategies in rheumatoid arthritis: A narrative review. Journal of Pain & Palliative Care Pharmacotherapy Oct. 8:1–9. https://doi.org/10.1080/15360288.2021.1973647.
Sankar, P., M. K. Cho, C. M. Condit, L. M. Hunt, B. Koenig, P. Marshall, S. S. Lee, and P. Spicer. 2004. Genetic research and health disparities. JAMA 291(24):2985–2989. https://doi.org/10.1001/jama.291.24.2985.
Scofield, L., L. Reinlib, G. S. Alarcón, and G. S. Cooper. 2008. Employment and disability issues in systemic lupus erythematosus: A review. Arthritis and Rheumatism 59(10):1475–1479. https://doi.org/10.1002/art.24113.
Sedykh, S. E., V. V. Prinz, V. N. Buneva, and G. A. Nevinsky. 2018. Bispecific antibodies: Design, therapy, perspectives. Drug Design, Development and Therapy 12:195–208. https://doi.org/10.2147/DDDT.S151282.
Sharif, R., M. D. Mayes, P. M. Nicassio, E. B. Gonzalez, H. Draeger, T. A. McNearney, R. M. Estrada-Y-Martin, D. K. Nair, J. D. Reveille, F. C. Arnett, S. Assassi, and the GENISOS Study Group. 2011. Determinants of work disability in patients with systemic sclerosis: A longitudinal study of the GENISOS cohort. Seminars in Arthritis and Rheumatism 41(1):38–47. https://doi.org/10.1016/j.semarthrit.2011.01.002.
Shivashankar, R., W. J. Tremaine, W. S. Harmsen, and E. V. Loftus, Jr. 2017. Incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clinical Gastroenterology and Hepatology 15(6):857–863. https://doi.org/10.1016/j.cgh.2016.10.039.
Simon, D., and S. Bechtold. 2009. Effects of growth hormone treatment on growth in children with juvenile idiopathic arthritis. Hormone Research 72 Suppl 1:55–59. https://doi.org/10.1159/000229765.
Simon, T. A., A. Thompson, K. K. Gandhi, M. C. Hochberg, and S. Suissa. 2015. Incidence of malignancy in adult patients with rheumatoid arthritis: A meta-analysis. Arthritis Research & Therapy 17(1):212. https://doi.org/10.1186/s13075-015-0728-9.
Somers, E. C., S. L. Thomas, L. Smeeth, and A. J. Hall. 2006. Autoimmune diseases co-occurring within individuals and within families: A systematic review. Epidemiology 17(2):202–217. https://doi.org/10.1097/01.ede.0000193605.93416.df.
Somers, E. C., S. L. Thomas, L. Smeeth, and A. J. Hall. 2009. Are individuals with an autoimmune disease at higher risk of a second autoimmune disorder? American Journal of Epidemiology 169(6):749–755. https://doi.org/10.1093/aje/kwn408.
Somers, E. C., W. Marder, P. Cagnoli, E. E. Lewis, P. DeGuire, C. Gordon, C. G. Helmick, L. Wang, J. J. Wing, J. P. Dhar, J. Leisen, D. Shaltis, and W. J. McCune. 2014. Population-based incidence and prevalence of systemic lupus erythematosus: The Michigan Lupus Epidemiology and Surveillance Program. Arthritis & Rheumatology 66(2):369–378. https://doi.org/10.1002/art.38238.
Sontichai, W., F. Liao, D. Dominguez, D. M. Levy, M. Al Mutairi, L. Ng, F. Silverio, E. D. Silverman, J. D. Wasserman, and L. T. Hiraki. 2020. Timing of childhood-onset systemic lupus erythematosus diagnosis relative to menarche impacts final height. Arthritis Care & Research 74(2):199–207. https://doi.org/10.1002/acr.24461.
Springer, K. W., J. Mager Stellman, and R. M. Jordan-Young. 2012. Beyond a catalogue of differences: A theoretical frame and good practice guidelines for researching sex/gender in human health. Social Science and Medicine 74(11):1817–1824. https://doi.org/10.1016/j.socscimed.2011.05.033.
St. Sauver, J. L., B. R. Grossardt, B. P. Yawn, L. J. Melton, 3rd, and W. A. Rocca. 2011. Use of a medical records linkage system to enumerate a dynamic population over time: The Rochester Epidemiology Project. American Journal of Epidemiology 173(9):1059–1068.
Stoffels, M., and A. Simon. 2014. The concept of autoinflammatory diseases. In The autoimmune diseases, 5th ed., edited by N. R. Rose and I. R. Mackay. Cambridge, MA: Elsevier Inc. Pp. 39-50.
Suntharalingam, G., M. R. Perry, S. Ward, S. J. Brett, A. Castello-Cortes, M. D. Brunner, and N. Panoskaltsis. 2006. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. The New England Journal of Medicine 355(10):1018–1028. https://doi.org/10.1056/nejmoa063842.
Tager, R. E., and M. Tikly. 1999. Clinical and laboratory manifestations of systemic sclerosis (scleroderma) in Black South Africans. Rheumatology (Oxford) 38(5):397–400. https://doi.org/10.1093/rheumatology/38.5.397.
Tengstrand, B., K. Carlström, L. Felländer-Tsai, and I. Hafström. 2003. Abnormal levels of serum dehydroepiandrosterone, estrone, and estradiol in men with rheumatoid arthritis: High correlation between serum estradiol and current degree of inflammation. Journal of Rheumatology 30(11):2338–2343.
Thompson, A. J., B. L. Banwell, F. Barkhof, W. M. Carroll, T. Coetzee, G. Comi, J. Correale, F. Fazekas, M. Filippi, M. S. Freedman, K. Fujihara, S. L. Galetta, H. P. Hartung, L. Kappos, F. D. Lublin, R. A. Marrie, A. E. Miller, D. H. Miller, X. Montalban, E. M. Mowry, P. S. Sorensen, M. Tintoré, A. L. Traboulsee, M. Trojano, B. M. J. Uitdehaag, S. Vuku-sic, E. Waubant, B. G. Weinshenker, S. C. Reingold, and J. A. Cohen. 2018. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. The Lancet Neurology 17(2):162–173. https://doi.org/10.1016/S1474-4422(17)30470-2.
Valderas, J. M., B. Starfield, B. Sibbald, C. Salisbury, and M. Roland. 2009. Defining comorbidity: Implications for understanding health and health services. Annals of Family Medicine 7(4):357–363. https://doi.org/10.1370/afm.983.
Vallerand, I. A., R. T. Lewinson, A. D. Frolkis, M. W. Lowerison, G. G. Kaplan, M. G. Swain, A. G. M. Bulloch, S. B. Patten, and C. Barnabe. 2018. Depression as a risk factor for the development of rheumatoid arthritis: A population-based cohort study. RMD Open 4(2):e000670. https://doi.org/10.1136/rmdopen-2018-000670.
Vallerand, I. A., S. B. Patten, and C. Barnabe. 2019. Depression and the risk of rheumatoid arthritis. Current Opinion in Rheumatology 31(3):279–284. https://doi.org/10.1097/bor.0000000000000597.
van Beugen, S., H. van Middendorp, M. Ferwerda, J. V. Smit, M. E. J. Zeeuwen-Franssen, E. B. M. Kroft, E. M. G. J. de Jong, A. R. T. Donders, P. C. M. van de Kerkhof, and A. W. M. Evers. 2017. Predictors of perceived stigmatization in patients with psoriasis. British Journal of Dermatology 176(3):687–694. https://doi.org/https://doi.org/10.1111/bjd.14875.
van der Valk, M., M. Mangen, M. Leenders, G. Dijkstra, A. van Bodegraven, H. Fidder, D. de Jong, M. Pierik, v. d. W. CJ, R.-C. MJ, C. CH, J. JM, M. N, v. d. M. PC, v. d. M.-d. J. AE, P. CY, B. CJ, V. JR, S. PD, v. O. MG, and O. B. 2014a. Coin study group; Dutch initiative on Crohn and colitis. Journal of Crohn’s & Colitis 8:590–597.
van der Valk, M., M. Mangen, M. Leenders, G. Dijkstra, A. A. van Bodegraven, H. H. Fidder, D. J. de Jong, M. Pierik, C. Janneke van der Woude, M. J. L. Romberg-Camps, C. H. M. Clemens, J. M. Jansen, N. Mahmmod, P. C. van de Meeberg, A. E. van der Meulen-de Jong, C. Y. Ponsioen, C. J. M. Bolwerk, J. Reinoud Vermeijden, P. D. Siersema, M. G. H. van Oijen, and B. Oldenburg. 2014a. Risk factors of work disability in patients with inflammatory bowel disease—A Dutch nationwide web-based survey. Journal of Crohn’s & Colitis 8:590–597. https://doi.org/10.1016/j.crohns.2013.11.019.
Vanderpuye-Orgle, J., Y. Zhao, J. Lu, A. Shrestha, A. Sexton, S. Seabury, and M. Lebwohl. 2015. Evaluating the economic burden of psoriasis in the United States. Journal of the American Academy of Dermatology 72(6):961–967.e965. https://doi.org/10.1016/j.jaad.2015.02.1099.
Ventura, R. E., A. O. Antezana, T. Bacon, and I. Kister. 2017. Hispanic Americans and African Americans with multiple sclerosis have more severe disease course than Caucasian Americans. Multiple Sclerosis 23(11):1554–1557. https://doi.org/10.1177/1352458516679894.
Videm, V., R. Thomas, M. A. Brown, and M. Hoff. 2017. Self-reported diagnosis of rheumatoid arthritis or ankylosing spondylitis has low accuracy: Data from the Nord-Trøndelag Health Study. Journal of Rheumatology 44(8):1134–1141. https://doi.org/10.3899/jrheum.161396.
Wallin, M. T., W. J. Culpepper, J. D. Campbell, L. M. Nelson, A. Langer-Gould, R. A. Marrie, G. R. Cutter, W. E. Kaye, L. Wagner, H. Tremlett, S. L. Buka, P. Dilokthornsakul, B. Topol, L. H. Chen, N. G. LaRocca, and US Multiple Sclerosis Prevalence Workgroup. 2019. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology 92(10):e1029–e1040.
Webber, M. P., J. Yip, R. Zeig-Owens, W. Moir, P. Ungprasert, C. S. Crowson, C. B. Hall, N. Jaber, M. D. Weiden, E. L. Matteson, and D. J. Prezant. 2017. Post-9/11 sarcoidosis in WTC-exposed firefighters and emergency medical service workers. Respiratory Medicine 132:232–237. https://doi.org/10.1016/j.rmed.2017.06.004.
Wei, J. C., L. H. Shi, J. Y. Huang, X. F. Wu, R. Wu, and J. Y. Chiou. 2018. Epidemiology and medication pattern change of psoriatic diseases in Taiwan from 2000 to 2013: A nationwide, population-based cohort study. Journal of Rheumatology 45(3):385–392. https://doi.org/10.3899/jrheum.170516.
Weng, X., L. Liu, L. F. Barcellos, J. E. Allison, and L. J. Herrinton. 2007. Clustering of inflammatory bowel disease with immune mediated diseases among members of a northern California-managed care organization. American Journal of Gastroenterology 102(7):1429–1435. https://doi.org/10.1111/j.1572-0241.2007.01215.x.
WHO (World Health Organization). 2021. Gender and health. https://www.who.int/health-topics/gender#tab=tab_1 (accessed October 29, 2021).
Woodruff, M. C., R. P. Ramonell, A. S. Saini, N. S. Haddad, F. A. Anam, M. E. Rudolph, R. Bugrovsky, J. Hom, K. S. Cashman, D. C. Nguyen, S. Kyu, M. Piazza, C. M. Tipton, S. A. Jenks, F. E. Lee, and I. Sanz. 2021. Relaxed peripheral tolerance drives broad de novo autoreactivity in severe COVID-19. medRxiv medRxiv [Preprint]. Jul 27:2020. https://doi.org/10.1101/2020.10.21.20216192.
Wouters, H. J. C. M., S. N. Slagter, A. C. Muller Kobold, M. M. van der Klauw, and B. H. R. Wolffenbuttel. 2020. Epidemiology of thyroid disorders in the Lifelines Cohort Study (the Netherlands). PLOS ONE 15(11):e0242795. https://doi.org/10.1371/journal.pone.0242795.
Xu, F., S. A. Carlson, Y. Liu, and K. J. Greenlund. 2021. Prevalence of inflammatory bowel disease among Medicare fee-for-service beneficiaries — United States, 2001−2018. Morbidity and Mortality Weekly Report 70(19).
Ye, Y., S. Manne, W. R. Treem, and D. Bennett. 2020. Prevalence of inflammatory bowel disease in pediatric and adult populations: Recent estimates from large national databases in the United States, 2007–2016. Inflammatory Bowel Diseases 26(4):619–625. https://doi.org/10.1093/ibd/izz182.
Yoshida, E. M., M. Riley, and L. T. Arbour. 2006. Autoimmune liver disease and the Canadian First Nations Aboriginal communities of British Columbia’s Pacific Northwest. World Journal of Gastroenterology 12(23):3625–3627. http://dx.doi.org/10.3748/wjg.v12.i23.3625.
Yuen, G. J. 2020. Autoimmunity in women: An examination of existing models. Clinical Immunology 210:108270. https://doi.org/10.1016/j.clim.2019.108270.
Zielinski, M. R., D. M. Systrom, and N. R. Rose. 2019. Fatigue, sleep, and autoimmune and related disorders. Frontiers in Immunology 10:1827. https://doi.org/10.3389/fimmu.2019.01827.
































































