5
Bioaccumulation and Measured Concentrations of UV Filters in Biota
Human activities have altered natural concentrations of multiple elements in the environment and added new chemicals. These chemicals can be taken up by organisms through various routes of exposure, potentially impacting the chemical concentration in biological tissue. Since chemicals can be integrated into the tissue of organisms, and predators of those organisms may consume the contaminants secondarily, the chemicals may persist in the food web and ecosystem. The stored chemicals may also be subject to indirect chemical, physical, or biochemical degradation. Whether an organism will accumulate chemicals will depend on the properties described in Chapter 4. This chapter describes methods for estimating bioaccumulation and measured concentrations of UV (ultraviolet) filters in biota, including estimates for bioaccumulation, bioconcentration, and biomagnification factors.
HOW BIOACCUMULATION INFORMATION IS USED IN ECOLOGICAL RISK ASSESSMENT
Understanding bioaccumulative potential of chemicals is an essential element of both classification and labeling of chemicals (e.g., Safety Data Sheets, U.S. Department of Transportation compliance, international import/export) as well as risk assessment. More hydrophobic/lipophilic compounds have a potential to accumulate in the tissues of organisms and, therefore, more emphasis on longer-term, chronic exposure versus shorter-term, acute exposure is important. While it is most common for responses to chemical exposures in toxicity tests to be expressed as external concentration (e.g., mg/L in aqueous media or mg/kg in sediment/soil), the real need is to understand concentrations of chemicals at the site of toxic action. This concept is known variously as critical body burden (CBB), lethal body burden, and critical body residue analysis (McCarty and Mackay, 1993). Thus, toxicity and bioaccumulation can be directly linked. It has been shown that CBB can assist ecotoxicologists to address mixture effects by allowing the organism to “sort out” chemicals with different hydrophobicities, metabolic activities, toxicity modifying factors (organic carbon, hardness, pH, etc.) and external concentrations by focusing on concentrations in the target tissues (McCarty and Borgert, 2006; Versteeg and Rawlings, 2003). Last, understanding bioaccumulation improves assessment of the potential for trophic transfer in food webs, or via consumption of invertebrates and fish by people. Irrespective of whether a substance bioaccumulates to a certain level in an aquatic species and fails to reach a concentration that elicits a response by an environmental species, it still may be at a level unhealthy for human consumption resulting in restrictions on recommended dietary intake.
For many chemicals released in the environment, the U.S. Environmental Protection Agency (EPA) develops such recommendations in cooperation with other state and federal agencies.1
BIOACCUMULATION OF UV FILTERS
Overview of Bioaccumulation, Bioconcentration, and Biomagnification Concepts
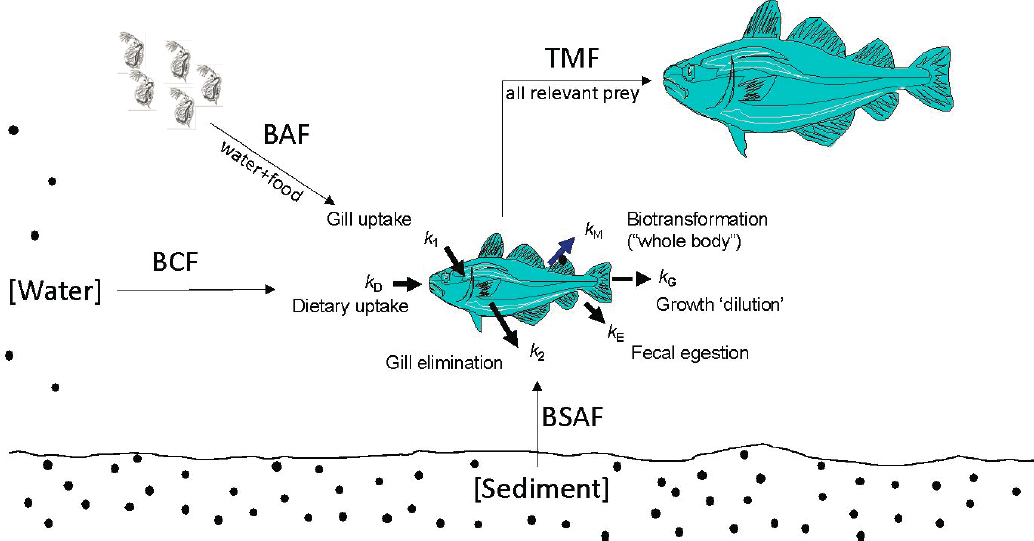
Bioaccumulation refers to the accumulation of a chemical into an organism via all routes of exposure (Figure 5.1). The process occurs through the uptake of contaminated food, sediment, air, or water. Bioconcentration is a process where the concentration of a chemical accumulates in tissues of an organism at levels higher than the chemical concentration in the medium (air, water, soil sediment) to which it is exposed. While bioaccumulation and bioconcentration occur within an organism, biomagnification (also called trophic magnification) is the increase in concentration of a chemical as it proceeds from one trophic level to the next. Biotransformation is the process by which chemicals that enter an organism are changed to facilitate their elimination; the goal is to produce less toxic metabolites, but some may result in more toxic metabolites.
Bioaccumulation is a key hazard-based parameter in the assessment and regulation of chemicals in commerce. From a regulatory perspective, bioaccumulation, bioconcentration, and trophic magnification are expressed as ratios of tissue concentrations to exposure medium concentrations (Table 5.1). For example, bioconcentration factor (BCF) would be the ratio of concentration in organism tissue to concentration in water. Other expressions of bioaccumulation potential are important, including the biological half-life of the substance (i.e., how long the substance is retained in the organism once the exposure is removed). This is typically done by experimentally moving the organism to a “clean” environment.
___________________
1 See https://www.epa.gov/choose-fish-and-shellfish-wisely/fish-and-shellfish-advisories-and-safe-eating-guidelines.
TABLE 5.1 Summary of Key Bioaccumulation Assessment Endpoints, Metrics, and Standard Calculations
| Bioaccumulation endpoint | Bioaccumulation metric (units) | Calculation |
|---|---|---|
| BCF | BCFWD/LW (L-water/kg-lipid) | CB-LW/CWD |
| BAF | BAFWD/LW (L-water/kg-lipid) | CB-LW/CWD |
| BSAF | BSAFOC/LW (kg-organic carbon/kg-lipid) | CB-LW/CS-OC |
| BSSAF | BSSAFOC/LW (kg-organic carbon/kg-lipid) | CB-LW/CSS-OC |
| BMF | BMFLW/LW (kg-lipid/kg-lipid) | CB-LW/CD-LW |
| TMF | TMF (unitless) | em or 10m |
BAF = bioaccumulation factor; BCF=bioconcentration factor; BMF = biomagnification factor; BSAF = biota-sediment accumulation factor; BSSAF = biota-suspended sediment accumulation factor; TMF=trophic magnification factor. SOURCE: Burkhard et al., 2012a.
As sediment, water, air, and food may contribute to bioaccumulation potential, it can be challenging to isolate each contributor experimentally. These challenges can be exacerbated by processes like biotransformation, which has recently been found to be a significant modifier in lowering bioaccumulation (and also biomagnification) potential of some organic substances (Nichols et al., 2018; OECD, 2018a,b). However, as bioaccumulation behavior is also a consequence of physico-chemical properties, routes of uptake, and elimination processes, it can be predicted by models based primarily on other indicators, including octanol–water partition coefficients2 and organism metabolism/biotransformation (Nichols et al., 2018; Weisbrod et al., 2009).
New methods that reliably estimate biological functions have been developed and applied to improve the accuracy of current models. For example, methods with the ability to estimate liver intrinsic clearance rate have been developed and can be used as input to physiologically based toxicokinetic models (PBTK) for fish bioaccumulation assessment (Stadnicka-Michalak, et al., 2014) or to extrapolate to whole body (in vivo) biotransformation rate constants (Nichols et al., 2018). The latter can then be applied in in vitro to in vivo (IVIVE) extrapolation models to estimate bioaccumulation.
Standard approaches are available to measure bioaccumulation potential of compounds including OECD 305 (OECD, 2012), OECD 319a and 319b (OECD, 2018), and EPA (2016b), which are representative of standard test guidelines addressing bioaccumulation potential. In most regulatory environments, critical threshold values important to regulatory action are 1,000 to 10,000 and 1,000 to 100,000 for the BCF-BAF (bioaccumulation factor) and Kow, respectively (ECHA, 2017; Gobas et al., 2009). As described in Chapter 4, Kow measures a chemical’s hydrophobicity—its tendency to partition out of water. The critical threshold values are used to indicate potential for trophic magnification through the food web and potentially leading to consequences for human health or environmental organisms. The levels are also used to prioritize chemicals for testing and/or regulatory action before they impart harm.
Testing of engineered nanomaterials (ENMs) with respect to bioaccumulation is difficult (Petersen et al., 2019). One of the most important conclusions of the review by Petersen et al. (2019) is the critical need for further analytical method development to identify and quantify ENMs in complex matrices. Inorganic UV filters provide especially difficult complications due to the fact that the metals of interest are naturally occurring. Background concentrations and nutritional essentiality of zinc are particularly vexing for exposure and accumulation assessments of zinc (Van Sprang et al., 2009). Bioaccumulation for essential elements, such as zinc, is difficult because homeostatic control mechanisms of organisms modify accumulation potential. McGeer et al. (2003) reviewed the evidence on bioconcentration and bioaccumulation of zinc as a function of exposure concentration in a number of taxonomic groups (algae, molluscs, arthropods, annelids, salmonid fish, cyprinid fish, and other fish). The data clearly illustrated that internal zinc content is well regulated and unlike bioaccumulative compounds, zinc tissue
___________________
2 Due to octanol being a comparable surrogate phase for lipids in biological organisms and the tendency for fat-soluble bioaccumulative chemicals to reside in lipid-rich areas.
concentrations show an inverse relationship to exposure concentrations due to lack of accumulation at higher exposures.
Bioaccumulation Potential Based on Molecular Weight, Log Kow, and Ionic Charge for UV Filters
Of the 15 organic UV filters on the U.S. market, 13 have measured log Kow values and six of these have log Kow values above 6, indicating a high potential for bioaccumulation (Table 5.2). Bioaccumulation models are not suitable for estimating the bioaccumulative potential of the two inorganic UV filters. At this time, there are no mathematical prediction models for inorganic bioaccumulation, and the fact that some inorganics (certain metals particularly) are also essential elements for nutrition confounds bioaccumulation measurements. This latter point is evident in the European Union REACH (Registration, Evaluation, Authorization and restriction of Chemicals) system where bioaccumulation for metals tied to essential nutrition have bioaccumulation endpoints waived for registration purposes. The BCF-BAF modeling program within the EPA EPI Suite™ was used to perform estimations of BCF. Measured log Kow values were used when available; otherwise, log Kow values were first estimated using EPA ECOSAR (V 2.11). This was the case for UV filters not registered for use in the European Union (i.e., cinoxate, dioxybenzone, meradimate, and trolamine salicylate). Measured values were exclusively from European Union (i.e., REACH) chemicals registrations on the compounds. Molecular weight (MW) is unlikely to be a factor in bioaccumulation of UV filters since all 15 UV filters are well below typical cutoff values for size exclusion (generally 700–1,000 MW). Ionic charge is known to affect bioaccumulation (Arnot et al., 2010). Only one of the
TABLE 5.2 Estimated BCFs for UV Filters
| Common Name | CAS # | MW | Log Kow | Ready Biodegradable | Estimated BCF (L/kg WW)b | |
|---|---|---|---|---|---|---|
| Measured | QSAR | |||||
| Aminobenzoic acid | 150-13-0 | 137.14 | 0.96a | Yes, measured | 0.500 | |
| Avobenzone | 70356-09-1 | 310.39 | 6.1a | No, measured | 1,278 | |
| Homosalate | 118-56-9 | 262.34 | > 6a | 6.16a | Yes, measured | 5,387 |
| Octocrylene | 6197-30-4 | 361.5 | 6.1a | No, measured | 4,918 | |
| Octinoxate | 83834-59-7 and 5466-77-3 | 290.4 | > 6a | 5.8c | Yes, measured | 3,128 |
| Octisalate | 118-60-5 | 250.33 | 6.36a | Yes, measured | 7,300 | |
| Oxybenzone | 131-57-7 | 228.24 | 3.45a | Yes, measured | 22.81 | |
| Padimate O | 21245-02-3 | 277.4 | > 6.2a | Yes, measured | 5,725 | |
| Ecamsule | 92761-26-7 | 562.7 | 3.83a | 0.078a | No, measured | 350.4 |
| Ensulizole | 27503-81-7 | 274.3 | 0.038a | No, measured | 3.162 | |
| Sulisobenzone | 4065-45-6 | 308.31 | 0.515a | Yes, measured | 3.162 | |
| Trolamine salicylate | 2174-16-5 | 287.31 | 2.26 (TEA), 1.22 (salicylate)c | 2.245 (TEA), 2.476 (salicylate)c | Not available | 3.162 |
| Cinoxate | 104-28-9 | 250.28 | — | 2.6546c | Not available | 26.2 |
| Meradimate | 134-09-8 | 275.4 | — | 6.2783c | Not available | 6,448 |
| Titanium dioxide | 13463-67-7 | 79.87 | — | — | Not applicable | — |
| Zinc oxide | 1314-13-2 | 81.4 | — | — | Not applicable | — |
bBCFBAF 3.01 (EPI Suite™ version 2012)
cECOSAR (v 2.11)
UV filters, trolamine salicylate, is a salt composed of a triethanolamine cation and salicylate anion. Triethanolamine is low in toxicity and bioaccumulation potential. The salicylate anion is freely dissolved in solution and is expected to drive the bioaccumulation potential of the complex overall. In general, the acid dissociation constants (i.e., pKas) for many of the organic UV filters are unknown (see also Chapter 4, Physico-Chemical Parameters of Organic UV Filters Impacting Fate, Table 4.1). For those that are known and indicate the organic UV filters will be present as anions at environmental pH values, bioaccumulation estimations using quantitative structure–activity relationships (QSARs) will likely carry significant uncertainties, as bioaccumulation models for charged compounds are less well-developed (Armitage et al., 2017).
Bioaccumulation of Organic UV Filters
Several of the UV filters have measured or estimated log Kow values above 6 (avobenzone, homosalate, octocrylene, octinoxate, octisalate, padimate O) and can be considered as likely to bioaccumulate above threshold values for regulation in the absence of definitive bioaccumulation data from well conducted laboratory studies or evidence of high rates of metabolism. It is therefore unsurprising that these compounds are also studied in laboratory environments to provide experimental log Kow and bioconcentration data (see section on Laboratory-based Tissue Concentration Studies). It is also important to consider biodegradation potential because it will reduce how much of a compound bioaccumulates regardless of its Kow. Biodegradability is a key component of understanding dispersion and fate of chemicals in the environment. Ready biodegradability (OECD, 1992) is a screening hazard assessment indicator of microbial metabolism in nearly every form of regulatory assessment. While it is not a guarantee of metabolism by higher organisms, it can provide a quick indication of the possibility of metabolism at higher trophic levels (which is more complex given differences in overall biochemical pathways in nonmicrobial biota). Octinoxate, homosalate, octisalate, and padimate O are ready biodegradable (a conclusion based on > 60 percent mineralization to CO2 and water in 28 days) whereas avobenzone and octocrylene are not biodegradable (see Chapter 3). From this, one could hypothesize that avobenzone and octocrylene have the greatest potential to bioaccumulate in the absence of experimental data.
Several of the UV filters (dioxybenzone, oxybenzone, ensulizole, sulisobenzone, trolamine salicylate, cinoxate) have low to very low log Kow values and are also highly soluble. It is highly unlikely that these chemicals will bioaccumulate, even if they are found not to be biodegradable.
Role of Metabolism in Bioaccumulation of UV Filters
Metabolism of UV filters likely varies with species, life stages, and environmental variables such as water quality. However, few formal studies on metabolism of UV filters are available in the literature and no systematic assessment of metabolism by aquatic biota has been performed to date. Saunders et al. (2020) performed formal bioconcentration studies of octocrylene and octinoxate using rainbow trout under controlled laboratory conditions. Using measured whole body depuration rate constants, combined with rate constants derived from reference chemicals, growth dilution, egestion, and measured exposures, biotransformation rate constants (KMET) useful in BCF models were determined for both compounds. The whole body depuration rate constants (kBT) calculated for fish exposed to octinoxate were 0.473 ± 0.078 to 0.680 ± 0.195/d (mean ± SE) for all treatment levels. The kBT values for fish exposed to octocrylene were 0.102 ± 0.023/d to 0.134 ± 0.020/d for all treatment levels. Metabolism was independent of exposure concentration for both compounds; therefore, predicted rate constants are useful and independent of exposure concentration. This study provides evidence for the metabolic biotransformation of both octinoxate and octocrylene. It should be noted, though, that this study does not identify metabolic by-products, only that parent compounds were metabolized.
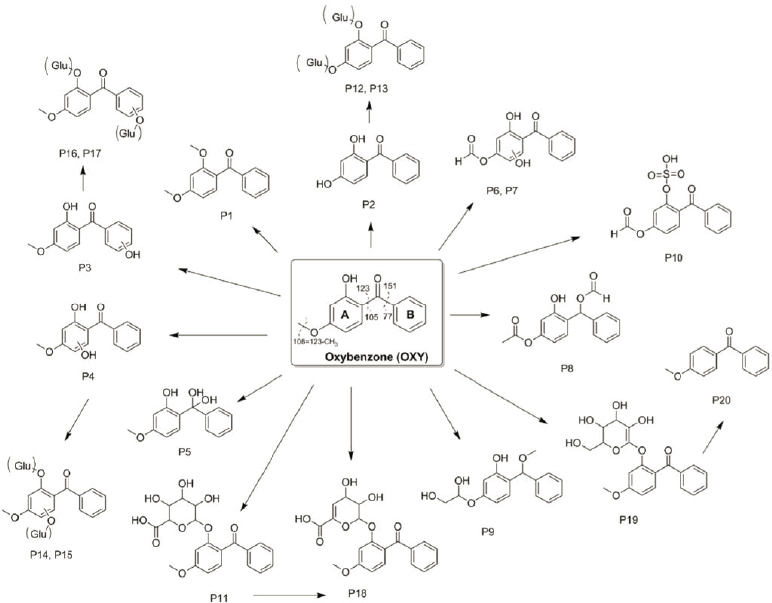
The existence of a wide range of metabolites indicate biotransformation and reductions in parent compounds. Many of the studies on the metabolic pathway of UV filters involve detailed development of new and novel chemical isolation and detection techniques for the target analytes. Ziarrusta et al. (2018a) used HPLC-MS/MS (high performance liquid chromatography–tandem mass spectrometry) to identify 21 different metabolic degradation products of oxybenzone in gilt sea bream by identification of presence in liver, bile, plasma, muscle, and gills. There
was evidence of o-methylation, o-demethylation, monohydroxylation, and hydration with glucuronide and Phase II derivatives, all at varying abundances and presence across the five tissues (Figure 5.2). There was no evidence of ring opening (often the rate-limiting step in metabolism). Lu et al. (2018) studied metabolism during tissue accumulation studies of padimate O (2-ethylhexyl-4-dimethylaminobenzoate) in crucian carp (Carrasius carrasius). Padimate O significantly increased the activities of cytochrome P450 (CYP) 1A, CYP3A and glutathione S-transferase in the fish liver after only 3 days exposure and it remained at similar levels for the 28-day exposure. Zhang et al. (2016) found significant upregulation of esr1 and cyp19b genes in zebrafish (Danio rerio) gonads, suggesting metabolism of octocrylene in laboratory exposures, although metabolites were not measured directly.
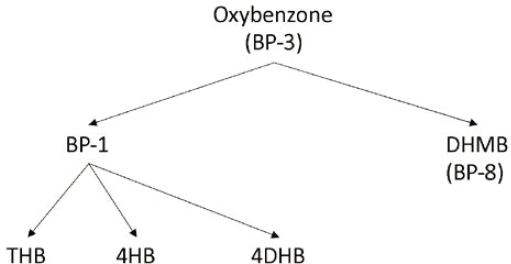
Several studies address the presence of metabolites of oxybenzone in various species of aquatic biota to varying degrees. Figure 5.3 shows the relationships of several oxybenzone metabolites known from mammalian studies. Some or all of these metabolites have been found in jellyfish (Mastigias papua etpisoni; Bell et al., 2017), mussels (species unspecified; Sang and Leung, 2016) and fish (cod, mackerel, monkfish, plaice, tuna; Cuhna et al., 2018) during studies of tissue concentrations from natural seawater environments. While oxybenzone metabolism has been demonstrated, it does not always yield detectable levels of metabolites when studied. Gago-Ferraro et al. (2015; 12 different fish species from Spanish rivers), Yang et al. (2020; snails, shrimp, zooplankton, phytoplankton, mussels, and carp), and Emnet et al. (2015; clams, sea urchins, and fish) all found detectable levels of oxybenzone in various species at all trophic levels but did not detect benzophenone-1 or other benzophenone metabolites.
Oxybenzone is one of the most well studied of the UV filters and its degradation pathway is well hypothesized and described (Figure 5.3), and thus it is used here as an example for evaluation of bioaccumulation of metabolites. Assessment of bioaccumulation potential normally begins with evaluation of the parent compound because phase I and II metabolism and conjugation leading to excretion reduces the internal metabolic burden of foreign compounds (Schlenk et al., 2008). Cleavage or modification of functional groups of the parent compound generally results in lower hydrophobicity (lowered lipophilicity). In turn, BCFs and acute toxicity of the degradants are also lowered. Using the metabolites in Figure 5.3, oxybenzone and its metabolites were compared with respect to log Kow, modeled BCF, and modeled acute toxicity to fish using EPIWIN v 4 and ECOSAR v 2 (Table 5.3). With the exception of dioxybenzone, all metabolites have lower log Kows, BCFs, and acute fish toxicities than the parent compound, in line with conventional understanding. Furthermore, BCFs are far below regulatory triggers for concern. Focus on the parent compound seems justified in many cases. Recently, Vuckovic et al. (2022) present information which adds caution to the focus on parent as photooxidation of oxybenzone to a stronger phototoxic oxybenzone–glucisode conjugate in both anemone (Aiptasia sp.) and mushroom coral (Discosoma sp.). More needs to be learned regarding understudied metabolic pathways, especially those in marine invertebrates.
Two separate studies evaluated the metabolism of octocrylene in species relevant to coastal environments but without quantification of parent compounds in tissues. Lucas et al. (2020) exposed clownfish (Amphiprion ocellaris) to an artificial dried food dosed at 10 μg/g octocrylene for 60 days. Clownfish were not impaired with respect to standard metabolic rate, active metabolic rate, or aerobic metabolic scope. Octocrylene fatty acid conjugates were detected in muscle and liver tissues, which likely improves excretion of the parent compound. Stien et al. (2019) investigated octocrylene metabolism in corals (Pocillopora damicornis). Similar to Lucas et al. (2020),
TABLE 5.3 Relationship Among Oxybenzone and Its Metabolites with Respect to Hydrophobicity (log Kow), Predicted BCF, and Predicted Acute Toxicity to Fish
| Compound/Metabolite | Log Kow | BCF (L/kg) | Acute Toxicity (mg/L) |
|---|---|---|---|
| Oxybenzone | 3.52 | 38.24 | 2.78 |
| Dioxybenzone | 3.82 | 40.12 | 1.08 |
| Benzophenone-1 | 2.96 | 10.91 | 3.06 |
| 4,4'-Dihydroxybenzophenone | 2.19 | 3.347 | 8.88 |
| 2,4,4'-Trihydroxybenzophenone | 2.48 | 5.524 | 6.35 |
| 4-Hydroxybenzophenone | 2.67 | 1.107 | 9.40 |
octocrylene was transformed into fatty acid conjugates via oxidation of the ethylhexyl chain, yielding lipophilic octocrylene analogs that accumulated in coral tissues. Reminiscent of Ziarrusta et al. (2018a), approximately 20 metabolites were identified and complexity of metabolite profiles increased with increasing concentration (above 50 μg/L). Mitochondrial dysfunction occurred at higher octocrylene exposures. As these concentrations are high relative to environmental exposures, they still provide an indication of metabolic capacity.
As seen in the studies described, there is experimental and modeling evidence that octinoxate and octocrylene biotransformation occurs in fish under controlled settings in the laboratory. Thus, it is likely that BCF or BAF estimates based solely on log Kow will overestimate bioaccumulation potential, which can be confirmed in controlled laboratory studies. A wide range of oxybenzone metabolites are known from a nonstandard laboratory study in fish (i.e., Ziarrusta et al., 2018a) and this is supported by measured tissue concentrations of benzophenone-1 in a wide range of wild-sampled organisms including mussels, jellyfish, and fish (i.e., Bell et al., 2017; Cuhna et al., 2018; and Yang et al., 2020). Other UV filters have not been investigated fully (or even partially) for evidence of biotransformation of the parent molecule. The significance of metabolites to aquatic biota health is unknown, although in general, metabolites are less bioaccumulative, more easily excreted, and lower in toxicity (Schlenk et al., 2008).
Standard Tests of UV Filter Bioaccumulation Potential: Kinetic and Steady State
Evidence of UV filter bioaccumulation is summarized from several types of studies: those that employ standard bioaccumulation test guidelines (e.g., OECD 305), inferential laboratory studies where tissue concentrations were measured but exposures are not fully quantified or evidence of steady state was reached (e.g., He et al., 2021b), and field studies where the abundance of UV filters in various aquatic organisms are determined (Vidal-Liñán et al., 2018). The latter two types of studies often do not quantify exposure in a way that makes bioaccumulation or bioconcentration factor calculation possible or meaningful. Most often field studies inadequately assess exposure, both temporally relevant to the organism’s life history (usually a single grab sample) and spatially if it is mobile (e.g., fish). For field studies to be meaningful, understanding where an organism has been and for how long is required. Proper quantification of highly dynamic exposures (due to tidal activity, diurnal shifts from wastewater inputs, or currents) are needed along with measurements of the diversity and quantity of food (prey) consumed by sampled biota. This is especially true for more hydrophobic UV filters as they will accumulate on prey surfaces. These studies are useful, though, to understand the prevalence and distribution of UV filter chemicals throughout the ecosystem and they can set the stage for deeper experimental evidence.
Several UV filters on the U.S. market are also registered in the European Union under the REACH regulation. In the REACH context, chemical entities are more critically assessed for bioaccumulation potential if the log Kow exceeds 3.0. Formal bioaccumulation studies are required if the confirmed (measured) log Kow exceeds 4.0–4.5. As several UV filters marketed in the United States meet these criteria, controlled laboratory studies of their bioaccumulation in select species (i.e., fish) are available through the REACH registered substance portal.3
The standard bioaccumulation assay known as the OECD Test Guideline 305 (OECD, 2012) is the most commonly used bioaccumulation study design and is nearly identical to EPA Test Guideline OCSSP 850.1730 (EPA, 2016b). A population of fish is exposed to one or two concentrations of test chemical at 1/100 the LC50 (lethal concentration to 50 percent of the population) or 1/10 the chronic no-observed-effect-concentration (NOEC) so that toxicity does not conflate the bioaccumulation estimate (these values are described further in Chapter 6). High log Kow compounds (> 4.5) that possess low solubility are preferably presented to the fish via food; otherwise exposure is via the water. The duration of the study is dictated by the time to achieve steady state. Because lipophilicity is an important factor in interpreting bioaccumulation, the preferred expression of experimental tissue concentrations is lipid normalized wet weight. Studies can either be performed to empirical steady state followed by depuration to understand the toxicokinetics of the compound, or first order uptake and depuration rate constants can be experimentally determined with the ratio deriving the BCF estimate. Preferentially, both methods can be
___________________
3 See https://echa.europa.eu/information-on-chemicals/registered-substances.
performed and used to confirm their respective findings. A few additional studies were available that did not match the guidelines but did meet criteria fundamental to making sound estimates of accumulation including measuring exposures throughout, low levels of observed toxicity, and full description of modeling.
Table 5.4 summarizes high quality bioaccumulation studies on several freshwater species. No data are currently available for marine or estuarine taxa. Various weight normalization metrics were used including wet weight (WW), dry weight (DW), and lipid normalization (Table 5.3). Therefore, direct comparisons across studies may contain this uncertainty. The highest measured BCFs were for octocrylene and homosalate at approximately 1,000 L/kg with all others measurably below. This suggests a relatively low potential for UV filters to be transferred and biomagnified through the food chain and is also consistent with the limited BMF values for avobenzone, octocrylene, and octinoxate. A study by Zhou et al. (2019a) compared tissue levels of octinoxate and its major biotransformation product 3,5-dichloro-2-hydroxyacetophenone (3,5DCl2HAcP). The 3,5DCl2HAcP biotransformation product had BAFs approximately two to six times that of the parent depending on the exposure concentration: BAFs for octinoxate were 176–190 L/kg versus the metabolite at 353–1,131 L/kg (estimated from graphical summaries). In all cases, the chemicals would not be considered bioaccumulative. UV filters with low log Kow values have not been well assessed, although some data are available for ensulizole (measured log Kow of 0.038). Studies by Grabicová et al. (2013) on rainbow trout and Falfushynska et al. (2021) on the mussel Mytilus edulis indicate bioaccumulation of this UV filter is low and, in many instances, tissue concentrations were nondetectable. Some cautions must remain with these estimates as they do not include marine organisms and not all UV filters are represented. In addition, the values for homosalate and padimate O, as examples, seem unlikely low given their high log Kows, but this may also be reflective of the species choice (crayfish, Procambarus clarkii, for homosalate; Crucian carp, Carassius carassius, and midge, Chironomus riparius, for padimate O). Of additional interest in the table is a representative value for nano-titanium dioxide (TiO2) with BCFs in the range of 19–352 L/kg, depending on the tissue and exposure concentration. Such values are fairly low for a metal, but may be associated with the relative high degree of insolubility of nano-TiO2.
Federici et al. (2007) exposed rainbow trout (Oncorhynchus mykiss) to TiO2 for 14 days and assayed its presence in water, gills, brain, liver, and muscle under the assumption that steady state was achieved within the exposure period. Tissue-specific BCFs (normalized to dry weight, not accounting for lipid) ranged from a low of 200 L/kg to 352 L/kg. Kinetic BCFs using Daphnia magna exposed in the presence and absence of food demonstrated that BCFs were 100 times lower when food was present (1,232 L/kg versus 118,063 L/kg). Depuration (i.e., elimination of the compound from the body by all physiological and physical processes) was more rapid and complete in the presence of food indicating egestion of food-bound TiO2 was important.
When possible, it is informative to compare both the measured and predicted BCF values of UV filters to gain insights into the possible over and underestimation of bioconcentration of the chemicals. In a regulatory setting, high quality measured data are usually given precedence by eliminating many underlying assumptions of QSAR estimation methods. Table 5.5 shows that measured values are consistently lower than predicted values made in the absence of metabolism being considered. As described in the preceding section, there is ample evidence for biotransformation of some UV filters (namely octocrylene and octinoxate). Some of the structurally similar benzophenone UV filters have indirect evidence of metabolism by comparing observed and measured BCFs (measured being lower). An exception to this appears to be avobenzone, which has previously been described as nonbiodegradable and apparently not well metabolized either. The QSAR prediction for avobenzone seems low and a non-biotransformed, high log Kow compound may deserve greater inspection.
Laboratory-Based Tissue Concentration Studies
Several studies have demonstrated the accumulation ability of UV filters; however, these studies are often insufficient to determine BCF or BAF (see Appendix D), limiting comparability to formal, standard bioaccumulation studies often for a regulatory purpose (e.g., OECD 305 or EPA 850.1730). Common reasons include: inadequate confirmation of the chemical exposure in the appropriate media (i.e., water, sediment, or soil); the study duration
TABLE 5.4 Compilation of High-Quality Bioaccumulation Studies from Literature and ECHA REACH Registered Compound Websites
| Compound | Fish Species | Duration | Route of Exposure | Bioaccumulation Estimate | Source |
|---|---|---|---|---|---|
| Avobenzone | Oncorhynchus mykiss (rainbow trout) | 10 d uptake 42 d depuration |
Diet | BMF: Steady state: 0.032 (WW); 0.122 (lipid normalized) BCF: 1,807 L/kg (lipid normalized) |
ECHAb |
| Ensulizole | Oncorhynchus mykiss (rainbow trout) | 42 d uptake 16 d depuration |
Water | BAF: 0–72.2 L/kg (weight normalization not specified) | Grabicová et al., 2013 |
| Octocrylene | Danio rerio (zebrafish) | 28 d uptake 16 d depuration |
Water | BAF: 830 L/kg at 0.1 mg/L (lipid normalized, kinetic); 887 L/kg at 1 mg/L (lipid normalized, kinetic) | Pawlowski et al., 2019; ECHAb |
| Octocrylene | Oncorhynchus mykiss (rainbow trout) | 14 d uptake 7 d depuration |
Diet | BMF: 0.009 (whole body WW) SS; 0.034 (whole body) kinetic | Pawlowski et al., 2019; ECHAb |
| Octocrylene | Oncorhynchus mykiss (rainbow trout) | 14 d uptake 14 d depuration |
Diet | BMF: 0.0038–0.0048 (lipid normalized) BAF: 1,105–1,350 L/kg at SS (WW, lipid normalized) | Saunders et al., 2020 |
| Octocrylenea | Procambarus clarkii (red swamp crayfish) | 42 d uptake 42 d depuration |
Water | BCF: 187 ± 131 L/kg, DW, lipid normalized | He et al., 2021b |
| Octocrylenea | Danio rerio (zebrafish) | 16 d uptake | Water | BAF: 41–136 L/kg; assumed SS, WW | Blüthgen et al., 2014 |
| Octinoxate | Oncorhynchus mykiss (rainbow trout) | 5 d uptake 9 d depuration |
Water | BAF: 433 L/kg (WW) at SS; 175 L/kg (WW at SS) for high and low | ECHAb |
| Octinoxate | Oncorhynchus mykiss (rainbow trout) | 14 d uptake 14 d depuration |
Diet | BMF: 0.001–-0.0027 (lipid normalized) BCF: 340–471 L/kg at SS (WW, lipid normalized) | Saunders et al., 2020 |
| Octinoxatea | Procambarus clarkii (red swamp crayfish) | 42 d uptake 42 d depuration |
Water | BAF: 1,050 ± 47 L/kg, DW, lipid normalized | He et al., 2021b |
| Octinoxate | Danio rerio (zebrafish) | 21 d uptake | Water | BAF: 176–190 L/kg WW | Zhou et al., 2019a |
| Oxybenzone | Oryzias latipes (Japanese medaka) | 10 weeks uptake | Water | BCF: 33–156 and 39–160 L/kg WW at SS for low and high exposures | ECHAb |
| Oxybenzonea | Procambarus clarkii (red swamp crayfish) | 42 d uptake 42 d depuration |
Water | BCF: 54 ± 23 L/kg, DW, lipid normalized | He et al., 2021b |
| Homosalatea | Procambarus clarkii (red swamp crayfish) | 42 d uptake 42 d depuration |
Water | BCF: 991 ± 569 L/kg, DW, lipid normalized | He et al., 2021b |
| Padimate Oa | Chironomus riparius (midge) | 10 d uptake 10 d depuration |
Sediment | BSAF: 0.10–4.0 ng/g (DW, lipid normalized) | Lu et al., 2018 |
| Padimate Oa | Carrasius carrasius (Crucian carp) | 28 d uptake 28 d depuration |
Water | BAF: 8.97–11.0 L/kg (liver); 3–9 L/kg (kidney, muscle, gill, brain, skin) (DW, lipid normalized) | Lu et al., 2018 |
| TiO2 | Oncorhynchus mykiss (rainbow trout) | 14 d uptake | Water | BAF: 19–208 L/kg (liver), 26–272 L/kg (muscle), 34–352 L/kg (brain), 20–200 L/kg (gills); DW normalized | Federici et al., 2007; ECHAb |
NOTE: BAF = bioaccumulation factor; BCF = bioconcentration factor; BMF = biomagnification factor; BSAF = biota-sediment accumulation factor; DW = dry weight; SS = steady state; WW = wet weight.
a Information was suitable to determine BCF/BAF.
b https://echa.europa.eu/information-on-chemicals/registered-substances?p_p_id=dissregisteredsubstances_WAR_dissregsubsportlet&p_p_lifecycle=1&p_p_state=normal&p_p_mode=view&_dissregisteredsubstances_WAR_dissregsubsportlet_javax.portlet.action=dissRegisteredSubstancesAction.
TABLE 5.5 Comparison of Log Kow Predicted and Measured Bioconcentration Values
| Compound | Log Kow | QSAR Predicted (L/kg) | Measured BCF (L/kg) |
|---|---|---|---|
| Avobenzone | 6.1 | 1,278 | 1,807 |
| Ensulizole | 0.038 | 0.5 | 0–72.2 |
| Octocrylene | 6.1 | 4,918 | 830–837 |
| 1105–1350 | |||
| 187 | |||
| 136 | |||
| Octinoxate | > 6 | 3,128 | 175–433 |
| 340–471 | |||
| 1050 | |||
| 176–190 | |||
| Oxybenzone | 3.45 | 22.81 | 33–160 |
| 54 | |||
| Homosalate | > 6 | 5,387 | 991 |
| Padimate O | 6.2 | 5,725 | 3–11 |
NOTE: Cells with multiple entries reflect all available estimates.
SOURCE: Committee generated using the BCFBAF module of EPI Suite™ 4.11.
was too short to achieve steady state; too few organisms were used to assess variability; the study lacked time course data to determine kinetic rates; exposures were excessively variable; and exposures were in the range of acute and chronic toxicity. Laboratory studies, in general, were substantially less abundant in literature than field studies of tissue concentrations (see Appendix D). A total of 532 studies of aquatic biota with unique organism–UV filter combinations in 43 different publications were reviewed. Many studies report on multiple organisms taking up anywhere from one to several different UV filters. Of the 532 studies, field-based studies in saltwater and freshwater and laboratory studies in saltwater and freshwater were 47.6, 44.4, 7.3 and 0.8 percent of the total, respectively.
The most commonly investigated UV filter in laboratory test systems was TiO2. Field studies of TiO2 and zinc oxide (ZnO) were absent, likely because these metals would be impossible to isolate as their respective chemical entities in field environments and they would be included in broader investigations of titanium or zinc presence in biota (all chemical species—or complexes, hydrides, carbonates, etc.—of the metal).
Included among the species assessed in the laboratory were the corals Pocillopora damicornis and Seriatopora caliendrum. These species were exposed to several different sunscreens (oxybenzone, sulisobenzone, dioxybenzone, and quantification of benzophenone-1 [an oxybenzone metabolite]) at concentrations that may induce acute and chronic responses (He et al., 2019c). Assuming the studies produced reliable steady state estimates of tissue concentrations, BCFs ranged from 10–6 to 10–8 L/kg, which seem low for the log Kows of these compounds with species that likely have a lower level of metabolism potential than fishes.
The mussel, Mytilus galloprovincialis, was the most common species assessed (Balbi et al., 2014; D’Agata et al., 2014; Della Torre et al., 2015; Gomez et al., 2012; Gornati et al., 2016; Hanna et al., 2013; Vidal-Liñán et al., 2018). All data presented for this taxon were normalized to dry weight but without lipid quantification, with a range of analytical techniques, and under a wide range of conditions (see Appendix D). Seven different UV filters were studied by the group of authors assessing M. galloprovincialis. TiO2 tissue concentrations ranged from 50–500
ng/g DW, which is a fairly narrow range considering whole soft tissue, digestive gland, gills, and mantle were assessed. Under several assumptions (e.g., tissue was at steady state, water exposure was steady, depuration was complete), one could calculate BCFs for oxybenzone and aminobenzoic acid to M. galloprovincialis as reported by Vidal-Liñán et al. (2018) yielding values of 102 and 82 L/kg DW, respectively. These are reasonable given their log Kows and comparisons to formal BCF studies given in Table 5.3.
Ziarrusta et al. (2018a) measured concentrations of oxybenzone in gilthead bream (Sparus aurata) bile, liver, plasma, muscle, and gill. As expected, bile concentrations, which are considered a route of elimination, were the highest (17,000 ng/g). The remaining tissues ranged from 129–1,400 ng/g DW, not lipid normalized. Assuming the bream were in steady state by 14 days exposure, putative BCFs would be 3,494, 26.5, 287.8, 123.3, and 30.8 L/kg (DW normalized) for bile, plasma, liver, muscle, and gill, respectively. These are within the range expected from formal BCF studies (those with the sole purpose of estimating the BCF under standard conditions) but should be viewed cautiously nonetheless.
Field-Based Tissue Concentration Studies
UV filters in aquatic organism tissues have been analyzed across the globe (see sampling locations in Figure 5.4). The majority of studies are from Europe and the coast and inland waters of mainland China. Studies are nearly equally distributed between freshwater and saltwater/estuarine environments with 236 (freshwater) and 253 (saltwater/estuarine) unique organism–UV filter combinations across 19 known peer-reviewed publications. These studies had various purposes ranging from demonstration of a novel analytical method or approach (e.g., Peng et al., 2015), to surveys of potential contamination of food for humans (Cuhna et al., 2018), to phenomenological investigations (e.g., occurrence, frequency; Langford et al., 2015). While documentation of tissue concentrations is an important and relevant endeavor, no study available from the literature adequately quantifies tissue burdens alongside exposure concentrations in aquatic food, water, or sediment in a manner conducive to calculating BAFs, BCFs, BMFs, or TMFs (trophic magnification factor). When bioaccumulation metrics are calculated, the exposure determinations are frequently poorly documented. When available, exposure concentrations were one time/one location values, which are inappropriate for mobile, long-lived organisms (e.g., Gago-Ferraro et al., 2013, 2015). In one instance, TMFs for a suite of UV filters (i.e., octinoxate, padimate O, homosalate, benzophenone-1 [metabolite], sulisobenzone, oxybenzone, octisalate, octocrylene, aminobenzoic acid, avobenzone) were postulated, with many well above one, indicating trophic magnification. However, the studies did not adequately describe fish diets and relied heavily on the sampling of a few (relative to gut analysis) potential prey items.
An appropriate description of diets would include temporal and spatial sampling of potential prey items from the environment across relevant size classes of fish and would extend to comparison with gut contents to reflect prey selectivity. Typically, this is a fairly involved investigation. Kidd et al. (2019) identified common shortcomings of TMF studies based on field evidence including prey item selection, lipid normalization, time-dependent sampling to address seasonality of food, and gross metabolic dynamics of top predators. Burkhard et al. (2012b) indicated that “TMFs, calculated from the slope of log-transformed concentrations of a contaminant versus trophic level (using stable N isotopes), can be used to understand whether a chemical does (TMF > 1) or does not (TMF < 1 or = 0) biomagnify through food webs.” This level of information (i.e., the study design and execution necessary to calculate TMFs) is not yet available for UV filters. That said, BCF studies with BAF or BCF below 2,000 L/kg also suggest trophic magnification remains unlikely.
Tissue concentration studies, even in the absence of the appropriate environmental exposure context, still provide useful information. These studies are indicative of exposure having occurred and can ultimately be related to laboratory determinations of critical tissue residues (concentrations in tissue determined to cause acute, sublethal, or chronic effects). A total of 649 unique organism–UV filter tissue concentration data were found in literature for both field and laboratory investigations (see Appendix D). Fish were the most studied group (52 percent) followed by invertebrates (35 percent). The diversity of biota investigated, for a relatively understudied group of compounds, was actually quite high. Eighteen invertebrate species, including six coral species, have been studied as were some
vertebrate species, including 31 fish species. Ten of the FDA-approved UV filters (i.e., aminobenzoic acid, avobenzone, dioxybenzone, homosalate, octinoxate, octisalate, octocrylene, oxybenzone, padimate O, sulisobenzone) as well as four of the more abundant metabolites (i.e., benzophenone-1, benzophenone-2, 4-hydroxybenzophenone, and 4,4'-dihydroxybenzophenone) were studied in various ways under field conditions. ZnO has not been studied in the field as bioaccumulation would have been confounded by other metal sources (natural and anthropogenic). The UV filters that were thus not assessed in field situations were: TiO2, ZnO, ecamsule, ensulizole, trolamine salicylate, cinoxate, and meradimate.
Due to the abundance of data for some UV filters, individual summaries by compound were considered useful. Octocrylene (n = 71 data points), oxybenzone (n = 63 data points), octinoxate (n = 55 data points), and padimate O (44 data points) have sufficient information to begin making meaningful comparisons. In addition, avobenzone (n = 6 studies) and homosalate (n = 15 studies) have been rigorously studied under laboratory conditions and are also considered more deeply below. All other UV filters were investigated approximately one dozen times or fewer.
Octocrylene
The laboratory-based BCFs of 830–880 L/kg (lipid normalized wet weight) were associated with tissue concentrations of ~900–8,600 ng/g (Pawlowski et al., 2019). The highest recorded level of octocrylene in fish from field studies was 2,400 ng/g lipid DW for brown trout (Salmo trutta) by Buser et al. (2006) from Swiss rivers (Figure 5.5). Multiple studies recorded UV filter tissue concentration levels from two to several hundred ng/g in fish (Balmer et al., 2005; Buser et al., 2006; Cunha et al., 2015; Langford et al., 2015; Sang and Leung, 2016; Yang et al., 2020) and on several occasions, levels were not detectable. The single highest value was found by Langford et al. (2015) in Atlantic cod (Gadus morhua) liver at 11,875 ng/g WW (not lipid normalized); unfortunately, relevant exposure levels were not available. Levels in field environments are indirectly supportive of metabolism of octocrylene by fish. Various bivalves (i.e., mussels, clams, and conch) had levels from ND to 3,992 ng/g DW,
although the upper value included octocrylene that may have been sorbed to sediment and present in the gut (Figure 5.5). Levels were generally in the low tens of ng/g to several hundred ng/g.
Several coral species (Favites abdita, Porites spp., Platygyra acuta, Pavona decussata, and Acropora valida) were evaluated with respect to octocrylene accumulation in wet and dry seasons of coastal Hong Kong (Tsui et al., 2017). Worst case accumulations were 12.4 ng/g WW for A. valida. Concentrations in Porites spp. collected from various sites (n = 19) around Oahu, Hawaii (at a 96 percent detection frequency) including from Waikiki Beach averaged 78.2 ng/g DW (not lipid normalized; Mitchelmore et al., 2019). Concentrations did not relate to the level of recreational activity as corals collected nearshore Waikiki Beach, where many people were in the water and on the beach, averaged 84.9 ± 148.0 ng/g DW compared to nearshore sites at Ka’a’awa, where there were very few people on the beach or in the water (i.e., Ka’a’awa was higher averaging 104.0 ± 18.8 ng/g DW). The source of octocrylene at this location is unclear.
Oxybenzone
Oxybenzone had a laboratory-based BCF of 33–160 L/kg (lipid normalized wet weight) with 3.3–16 ng/g as the tissue concentration at steady state. Most values from field studies on fish ranged from 0.1–100 ng/g. Langford et al. (2015) found up to 1,037 ng/g in Atlantic cod liver; however, relative to other analytically-driven studies LOQs (limits of quantitation) appear relatively high (up to 50 ng/g for biological samples; Figure 5.5) and may deserve additional field and laboratory investigation. Oxybenzone was below 10 ng/g (regardless of type of normalization) in 35 of 63 instances with five instances occurring above 100 ng/g. Oxybenzone was also studied by Tsui et al. (2017). Oxybenzone was consistently detected in both seasons and all species ranging from 2.8–31.8 ng/g WW. A detection frequency of 82 percent was seen in corals from Hawaii (Porites spp.) with an average
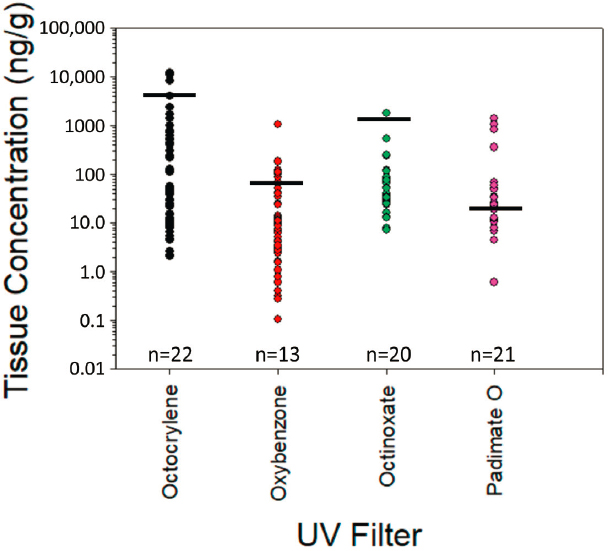
concentration across numerous (n = 19) sites in Hawaii including from Waikiki Beach reported at 60.7 ng/g DW (not lipid normalized; Mitchelmore et al., 2019). Corals collected from nearshore sites showed higher concentrations where more people were located (e.g., Waikiki Beach averaged 161.0 ± 88.9 ng/g DW) compared to a site with few people on the beach or in the water (i.e., Ka’a’awa averaged 37.7 ± 29.1 ng/g DW; Mitchelmore et al., 2019). Although concentrations were much lower in corals collected in deeper waters off Waikiki Beach (i.e., an average of 25.8 ± 12.5 ng/g DW), results suggest that the source of oxybenzone is nearshore and concentrations are much lower further from the shore, which concurrent sampling of seawater at these locations demonstrated (Mitchelmore et al., 2019). Interestingly, some of the highest coral concentrations were measured at sites offshore of Ka’a’awa, where no or little recreational activity was observed and there are no obvious sources of UV filters.
Octinoxate
Laboratory-based BCF determinations for octinoxate range was 175–1,050 L/kg (Saunders et al., 2020) which is a factor three to five times below that based on log Kow and no metabolism (Tables 5.3 and 5.4). The highest concentration for fish collected in the field is 241.7 ng/g (Gago-Ferrero et al., 2015) from a survey of 12 different fish species encompassing various trophic levels (Figure 5.5). Picot-Groz et al. (2014) recorded a single highest concentration of 1,765 ng/g for mussel (Mytilus galloprovincialis); however, gut contents were not evacuated so tissue concentrations would be confounded by the presence of the chemical in food and particles in the gut. Octinoxate was below 100 ng/g in 45 of 55 instances, although many organisms had several hundred ng/g including two dolphin species (Alonso et al., 2015). Octinoxate, an extremely high log Kow chemical, is apparently well metabolized based on the totality of the investigations. Only a 4 percent detection frequency was observed in corals (n = 67) collected around Oahu, Hawaii with the detections being up to 12.1 ng/g DW (observed in one coral sample; Mitchelmore et al., 2019).
Padimate O
A laboratory-based BCF for padimate O was determined to be 3–11 L/kg, a factor of approximately 500 times below that predicted solely on log Kow (Tables 5.3 and 5.4). Tissue concentrations from field-collected organisms was generally below 50 ng/g with a few notable exceptions (Figure 5.5). Picot-Groz et al. (2014) found 833 ng/g in soft tissue of mussel (Mytilus galloprovincialis); however, gut contents were not evacuated, likely confounding tissue concentration estimates. The highest values for padimate O were recorded in dolphins (Pontoporia blainvillei and Sotalia guianensis) off the coast of Brazil at ND to 1,385 ng/g in placental tissue and fetal dolphin blubber and muscle was far less at ND to 67.5 ng/g. Accumulation in corals (Favites abdita, Porites spp., and Pavona decussata) ranged from ND to 12.48 ng/g DW.
Avobenzone
Avobenzone is relatively poorly studied; however, a laboratory-based BCF study determined a value of 1,807 L/kg (Table 5.3). The highest measured tissue concentration in the study yielded 32.7 μg/g, which corresponds to 0.10 mmol/kg, which is below the known nonpolar and polar critical body burdens for acute and chronic toxicity (McCarty and Mackay, 1993; McCarty et al., 2013). The predicted BCF, absent metabolism, was about 1,200 L/kg strongly suggesting a lack of metabolism. A field study by Yang et al. (2020) summarized tissue concentrations across a wide range of trophic levels with the highest values derived from zooplankton (8.5 ng/g DW) and fish (8.1 ng/g DW). Values are presented as mixtures of taxa, so additional specificity is not possible. A 58 percent detection frequency was observed in corals from Oahu, Hawaii, with concentrations ranging from ND to 291.3 ng/g DW (not lipid normalized). Although more detections were observed for corals collected from the popular Waikiki Beach location (highest of 140.9 with an average at nearshore sites of 60.5 ± 24.5 ng/g DW), the highest concentration (291.3 ng/g DW) was measured in a coral from Ka’a’awa with no obvious source for UV filters into the environment (i.e., recreational activity) with corals from nearshore sites variable but averaging higher than Waikiki Beach at 92.0 ± 34.0 ng/g DW (Mitchelmore et al., 2019).
Homosalate
Bioaccumulation of homosalate was measured in the laboratory for the red swamp crayfish (Procambarus clarkii). BAFs were 991 ± 569 L/kg with tissue concentrations of around 5,000 ng/g DW (He et al., 2021b). Accumulations were from both food and water. Prediction for BCF to fish was 5,387 L/kg. He et al. (2017) investigated tissue concentrations of homosalate in several crayfish species collected from the field. Red swamp crayfish had up to 174 ng/g DW and virile crayfish (Orconectes virilis) had the highest level for the five species investigated at 399 ng/g DW (He et al., 2017). Of the remaining studies, Cuhna et al. (2018) measured the highest concentrations of 58.5 ng/g in a study of five different saltwater predator fishes (cod, mackerel, monkfish, plaice, and tuna). Homosalate was detected in all 67 corals sampled from Oahu, Hawaii and there were no trends with recreational activity sources or nearshore/offshore locations (Mitchelmore et al., 2019). Concentrations were similar across all sites. For example, concentrations detected in coral at nearshore locations at Waikiki Beach and Ka’a’awa were 329.8 ± 77.7 ng/g DW and 304.9 ± 101.4 ng/g DW (not lipid normalized), respectively (Mitchelmore et al., 2019). It is not clear from the homosalate data set whether laboratory predictions are truly indicative of field measurements and if homosalate is well metabolized. Given the high log Kow of homosalate, more research to understand the bioaccumulation potential of homosalate warranted.
EXPOSURE BEYOND AQUATIC ECOSYSTEMS
Contaminants present in aquatic ecosystems may pose a risk to non-aquatic organisms (Walters et al., 2008, 2020), especially those that rely heavily on aquatic animals as prey. The direct feeding of aquatic animals by terrestrial predators (e.g., birds feeding on fishes or wolves and bears feeding on salmon), may be a route for UV filters to be transferred to terrestrial ecosystems. UV filters have been detected in the tissues of birds of prey, presumably from consuming polluted prey (González-Rubio et al., 2020). For example, benzophenone UV filters were detected in tissues of Eurasian sparrowhawks and owls collected in France, and eagles collected in Greenland (González-Rubio et al., 2020). Additional work found UV filters in bird eggs (Molins-Delgado et al., 2017) and illustrated the potential for bioaccumulation of these compounds in terrestrial species. Given the potential impacts of trophic magnification and UV filter exposure across ecosystems, more research examining UV filters in terrestrial consumers that rely on aquatic prey will clarify its potential for trophic transfer and magnification.
FINDINGS AND KNOWLEDGE GAPS
Finding: UV filters exhibit a range of bioaccumulation potentials, driven primarily by the lipophilicity of the compound and the metabolism of the parent compound by biota.
Finding: High quality, laboratory-based bioaccumulation (BAFs) or bioconcentration factors (BCFs) available for avobenzone, octocrylene, octinoxate, oxybenzone, homosalate, padimate O, and titanium dioxide reveal a low to moderate bioaccumulation potential. For all other UV filters, reliable laboratory-based BAFs or BCFs are not available.
Finding: BAF or BCF studies for the most lipophilic UV filters indicate a low likelihood of trophic magnification, although some have measurable BCFs. Additional research, for example determination of critical body burdens, may clarify if accumulations of UV filters contribute to long-term stress of biota.
Knowledge Gap: Currently available field studies of tissue concentrations lack comprehensive characterizations of UV filter exposure in water and sediment, leaving interpretation solely as presence and concentration of UV filters in tissues. While important and relevant, these findings would benefit from additional research by considering both tissue burdens with UV filters and spatial and temporal UV filter exposure concentrations in aquatic food, water, or sediment.
Knowledge Gap: Based on low BCFs for UV filters, trophic magnification (e.g., biomagnification) is not likely; however, additional research investigating biomagnification in food webs specifically is warranted.
Knowledge Gap: Future research needs include development of standard guidelines on bioconcentration under laboratory conditions for saltwater fish and invertebrates and improved studies of critical body residues in acute and chronic exposures to simultaneously understand both bioaccumulation and toxicity and to address mixture toxicity of UV filters simultaneously.
This page intentionally left blank.


















